1. Introduction
Since their initial discovery in the mid-1940s,
aminoglycoside (AG) antimicrobials have been used extensively to
treat severe infections. The first AG was streptomycin, which was
initially created to treat tuberculosis (1); gentamicin (1963), tobramycin (1967)
and amikacin (1972) are three of the other AGs that have been
discovered and are still widely used in modern medicine (2-4).
Due to the ability of AGs to specifically bind to the 30S ribosomal
subunit, which effectively prevents bacterial intracellular protein
synthesis, they demonstrate potent bactericidal activity (5). Due to their narrow therapeutic index
(a small range between the minimum effective dose and the minimum
toxic dose) and potential for ototoxicity and nephrotoxicity, AGs
must only be prescribed under strict guidelines and in accordance
with meticulous therapeutic drug monitoring (TDM) (6). Currently, patients with sepsis or
septic shock who are at high risk of multidrug-resistant (MDR)
Gram-negative bacilli infections are frequently treated empirically
with AGs in combination with another antimicrobial drug (mostly a
β-lactam) (6).
Antimicrobial resistance has emerged as a
significant global threat to healthcare. By 2050, it is predicted
that 10 million individuals per year could die from infections that
are antimicrobial resistant (AMR), according to the World Health
Organization (7). The different
resistance mechanisms produced by MDR bacteria against
new-generation and broad-spectrum antibiotics are quickly growing,
limiting the treatment options for infections (8). Particularly relevant to carbapenem
resistance mechanisms is the identification of five carbapenemase
genes (oxa-48, imp, ndm, vim and
kpc) in Gram-negative bacteria (GNB). These genes are all
carried by plasmids, which allows for a high rate of horizontal
transmission. Extended spectrum β-lactamases (ESBLs)-producing
Enterobacterales and carbapenemase-producing Acinetobacter
baumannii, Pseudomonas aeruginosa and Enterobacterales
were among the bacterial pathogens that indicated a strong ability
to be AMR. These infections are becoming more difficult to treat
empirically after being microbiologically confirmed (9).
The ESKAPE healthcare-associated infections are
caused by a group of GNB and Gram-positive bacterial pathogens
including Enterococcus faecium, Staphylococcus
aureus, Klebsiella pneumoniae, Acinetobacter
baumannii, Pseudomonas aeruginosa and
Enterobacter. These healthcare-associated infections are
extremely resistant to antibiotics, including AGs (10). The two main mechanisms of
Enterobacterales resistance to AGs are enzymatic drug modification
or target site modification (11,12).
The most common mechanism is enzymatic drug modification by
aminoglycoside-modifying enzymes (AMEs): N-acetyltransferases
(AACs), o-adenyl transferases (nucleotidyltransferases) and
o-phosphotransferases (APHs), are three major classes of AMEs, each
with several variants, and certain AGs are impacted by each
specific enzyme but not others. Gram-negative bacilli, especially
Klebsiella pneumoniae and Escherichia coli, usually
have the aac(60) gene
encoding the acetyltransferase enzyme. This modification reduces
the antibacterial activity of AGs by preventing it from attaching
to the bacterial ribosome. Notably, the microbe may create several
enzymes from the same or distinct classes, resulting in high-level
resistance (6).
ArmA and RmtB are the most expressed 16S rRNA
methyltransferases (16RMT) that modify (by methylation) the target
site of AGs (12). Nevertheless,
they are rarely encountered in clinical settings, with only 1.28,
0.14 and 0.05% isolates reported from Europe and parts of Asia
(13), the US (14) and Canada (15), respectively, that have been
recorded expressing ArmA and RmtB. Additionally, drug
impermeability with porin channel down-regulation or active drug
elimination by efflux pumps (AcrD) might lead to resistance
against AGs, especially with Acinetobacter baumannii and
Pseudomonas aeruginosa, explaining the higher minimum
inhibitory concentrations (MICs) frequently exhibited by these
bacterial pathogens (16,17).
The use of traditional AGs has been limited by AME
generation and the toxicity/benefit ratio experienced in patients
with infected with AME-producing bacteria (18). These factors highlight the need for
a next-generation AG antibiotics with unquestionable efficacy and
little toxicity. Plazomicin has exhibited notable bactericidal
action (observed in 2010) against most AG-resistant organisms with
lower toxicity (9,19-21).
The United States Food and Drug Administration (US FDA) approved
the plazomicin antibiotic (formerly known as ACHN-490) in June
2018(22). Plazomicin is a
semisynthetic antimicrobial that has been created to target MDR
Enterobacterales including carbapenemases, ESBLs and AME-producing
bacteria (23,24). These resistant bacteria have the
potential to cause dangerous bacterial infections that are a
problem all over the world, including ventilator-associated
pneumonia (VAP), healthcare-associated pneumonia (HAP) and blood
stream infection (BSI). Since the emergence of carbapenem-resistant
Enterobacterales (CRE), clinicians have had few alternatives for
treating MDR bacterial pathogens. Traditional AGs, such as
gentamicin, tobramycin and amikacin, have little effectiveness
against bacterial strains that can produce AMEs (22).
In the present review, the molecular
characteristics, chemical properties, mechanism of action,
antibacterial spectrum, pharmacokinetics, clinical therapeutic
indications, side effects, role in therapy and special
considerations of plazomicin are addressed.
2. Molecular characteristics, chemical
properties and mechanism of action
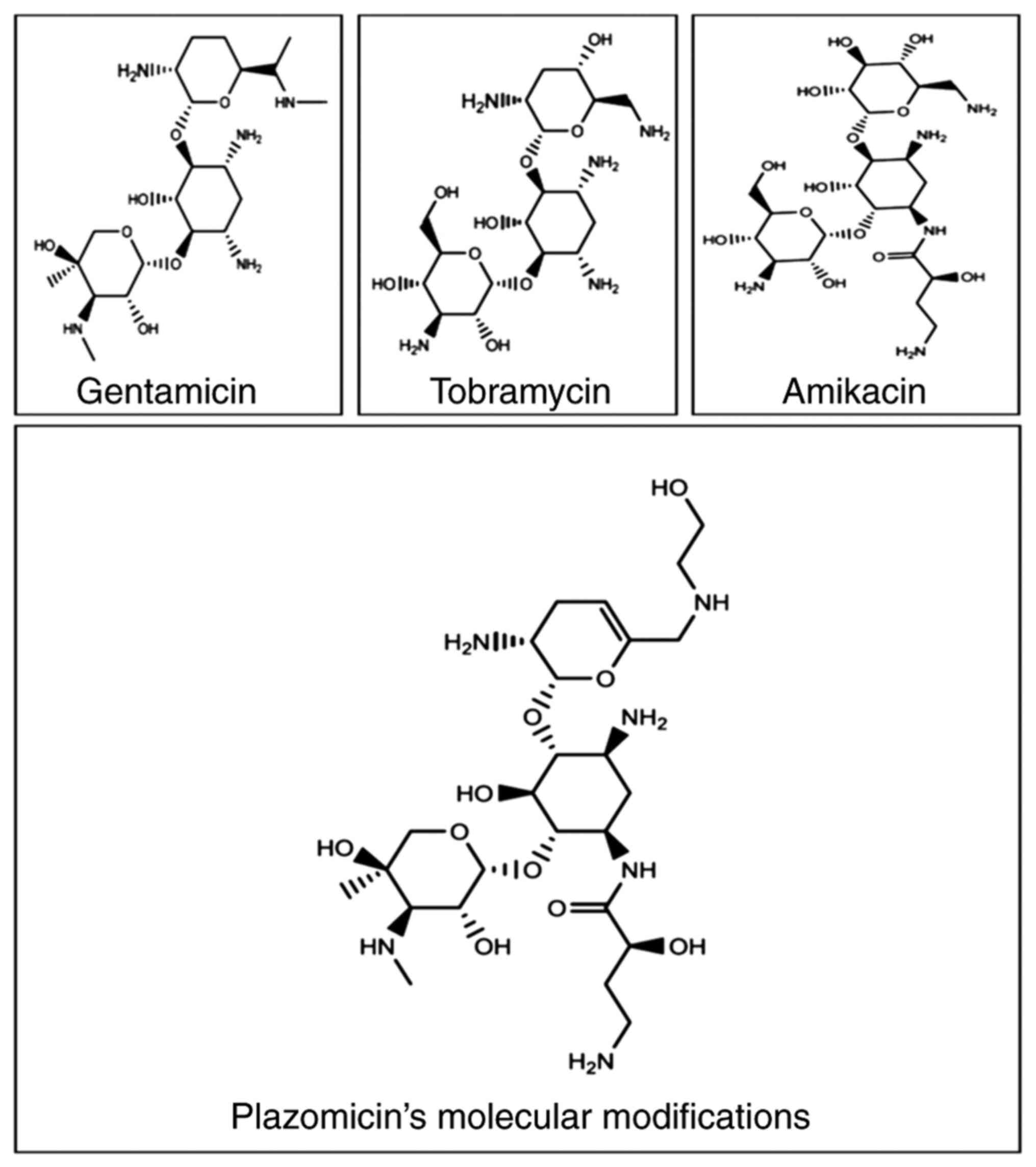
Being a semisynthetic antimicrobial, plazomicin
[6'-(hydroxylethyl)-1-(haba)-sisomicin] has a unique molecular
structure (Fig. 1) that makes it
resistant to being inactivated by a variety of enzymes produced by
MDR bacteria. Sisomicin (dehydro analog of gentamicin C1a) is the
source of plazomicin; sisomicin, similar to gentamicin, is
susceptible to several AMEs that GNB may produce (11). The majority of AMEs are inhibited
by sisomicin modifications when an N1 2(S)-hydroxy aminobutyryl and
a hydroxethyl group are added at the 6' position (25). The activity of plazomicin can be
improved against isolates producing AMEs due to these molecular
modifications, but not against 16RMT, which cause AGs (including
plazomicin) to have less affinity for the ribosomal target
(12). When compared with
sisomicin, the antibacterial effectiveness of plazomicin is only
slightly reduced by these molecular modifications; however, action
is restored in the presence of AMEs (26). Comparing plazomicin with
traditional AGs, the deleted or added domains (Table I) explain the resistance of
plazomicin to enzymatic inactivation by AMEs (9,27).
With this design, plazomicin can be prescribed not only to treat
infections caused by traditional AG-resistant Enterobacterales, but
also to treat infections caused by Enterobacterales that resist
colistin (polymyxin B) and carbapenems (carbapenemases producers),
including the bacteria that produce ESBLs (23).
 | Table IRelationship between plazomicin
molecular structure and its resistance to inactivation by AMEs when
compared with traditional aminoglycosides (9). |
Table I
Relationship between plazomicin
molecular structure and its resistance to inactivation by AMEs when
compared with traditional aminoglycosides (9).
| Traditional
aminoglycosides inactivated by AMEs | Molecular of
plazomicin modifications | Plazomicin's
protection against AMEs granted from the molecular
modifications |
|---|
| Amikacin | Hydroxyl (-OH)
groups deletion |
O-phosphotransferase [APH (3')] |
| Amikacin and
tobramycin | in the C-3' and 4'
positions |
O-adenyltransferases [ANT (4')] |
| Amikacin,
gentamicin, and tobramycin | Unsaturated
hydroxyethyl group addition at the C-6' position | N-acetyltransferase
[AAC (6')] |
| Gentamicin and
tobramycin | Addition of
4-amino-2-hydroxybutanoic | N-acetyltransferase
[AAC (3')] |
| Gentamicin and
tobramycin | acid
(hydroxy-aminobutyric acid; HABA) |
O-adenyltransferases [ANT (2')] |
| Amikacin,
gentamicin, and tobramycin | at the C-1'
position. |
O-phosphotransferase [APH (2')] |
Due to the fact AGs are hydrophilic cationic
compounds, they are hypothesised to enter GNB via porin channels or
breakdown of the lipopolysaccharide outer membrane (28). AGs must be transported across the
cytoplasmic membrane, and the transport of plazomicin inside
bacterial cell cytoplasm is an aerobic energy-dependent process
that can be slowed down by a drop in pH and/or anaerobic condition.
AGs are not very effective in an anaerobic and/or acidic
environment due to the drop in their antibacterial action. The
effect of AGs relies on the proton motive force, which consists of
Δψ (electrical potential across membrane) and ΔpH (transmembrane
difference in hydrogen ion concentration) (29). The aminoacyl-transfer RNA
recognition site (A-site) of the 16S rRNA of the 30S ribosomal
subunit is the main intracellular target of action for AGs,
including plazomicin, where they interfere with bacterial protein
synthesis. Following their binding to the target site, several
intracellular steps (including severe translocation inhibition,
miscoding and complete collapse of the proteome) occur that prevent
translation, elongation of the nascent protein sequence and enhance
the bactericidal activity of AGs (30).
3. Antibacterial spectrum of plazomicin
Overall, the Gram-negative in vitro activity
of plazomicin is comparable with or superior to that of other AGs
(20,23). In nearly half of the cases
(46.97%), the bacterial pathogens tested for plazomicin activity
belong to the order Enterobacterales (9). The
Enterobacterales-susceptible/-resistant breakpoints for plazomicin
are ≤2/≥8, ≤2/≥8 and ≤4/≥8 µg/ml according to the Clinical and
Laboratory Standards Institute (CLSI 2023) (31), US FDA 2019(32) and United States Committee on
Antimicrobial Susceptibility Testing (USCAST 2019) (33), respectively. The discrepancies
between CLSI, US FDA and USCAST breakpoints were based on the most
recent pharmacokinetic/pharmacodynamic data (34).
Between 2017 and 2021, 9,809 Enterobacterales
isolates (1/patient) were obtained in 37 medical centers in USA,
and the antimicrobial susceptibility was assessed using the broth
microdilution method, and CLSI 2023 and US FDA 2019 criteria were
used to calculate susceptibility rates. Plazomicin had strong
activity against isolates that produced ESBLs (98.9%), MDR (94.8%)
and CRE (94.0%), and it was active against 96.4% of the total
isolates. Plazomicin was markedly more effective against AMR
Enterobacterales compared with gentamicin, tobramycin or amikacin.
AMEs-encoding genes were detected in 801 (8.2%) isolates, whereas
11 (0.1%) isolates included 16RMT-encoding genes; plazomicin
inhibited 97.3% of the AMEs and none (0.0%) of the 16RMT producers
(Table II) (34,35).
 | Table IIPlazomicin and comparator
antimicrobial activity against Enterobacterales (34). |
Table II
Plazomicin and comparator
antimicrobial activity against Enterobacterales (34).
| A, Enterobacterales
(n=9,809)a |
|---|
| | US FDAb | CLSI
(2023)c |
|---|
| Plazomicin and
comparator antimicrobials | MIC50,
µg/ml | MIC90,
µg/ml | Resistant, % | Susceptible, % | Resistant, % | Susceptible, % |
|---|
| Plazomicin | 0.50 | 1.00 | 0.8 | 96.4 | 0.8 | 96.4 |
| Amikacin | 2.00 | 4.00 | 0.2 | 99.4d | 1.4 | 94.6 |
| Tobramycin | 0.50 | 4.00 | 6.1 | 90.6d | 9.4 | 88.0 |
| Gentamicin | 0.50 | 2.00 | 7.7 | 91.5d | 8.5 | 90.6 |
| Meropenem | 0.03 | 0.06 | 1.0 | 98.7d | 1.0 | 98.7 |
| Levofloxacin | 0.06 | 8.00 | 16.4 | 80.3d | 16.4 | 80.3 |
| Colistin | 0.25 | >8.00 | - | - | 15.3 | 84.7e |
| B,
Extended-spectrum β-lactamases producer-Enterobacterales
(n=1,011)f |
| | US FDAb | CLSI
(2023)c |
| Plazomicin and
comparator antimicrobials | MIC50,
µg/ml | MIC90,
µg/ml | Resistant, % | Susceptible, % | Resistant, % | Susceptible, % |
| Plazomicin | 0.50 | 1.00 | 0.9 | 98.9 | 0.9 | 98.9 |
| Amikacin | 4.00 | 8.00 | 1.3 | 96.9d | 8.4 | 79.7 |
| Tobramycin | 8.00 | >16.00 | 41.7 | 43.9d | 56.1 | 40.4 |
| Gentamicin | 1.00 | >16.00 | 42.2 | 55.7d | 44.3 | 54.5 |
| Meropenem | 0.03 | 0.12 | 5.5 | 93.5d | 5.5 | 93.5 |
| Levofloxacin | 8.00 | >16.00 | 67.2 | 23.2d | 67.2 | 23.2 |
| Colistin | 0.25 | 0.25 | - | - | 1.8 | 98.2 |
| C,
Carbapenem-resistant Enterobacterales (n=117)g |
| | US FDAb | CLSI
(2023)c |
| Plazomicin and
comparator antimicrobials | MIC50,
µg/ml | MIC90,
µg/ml | Resistant, % | Susceptible, % | Resistant, % | Susceptible, % |
| Plazomicin | 0.25 | 1.00 | 5.1 | 94.0 | 5.1 | 94.0 |
| Amikacin | 4.00 | 32.00 | 8.5 | 75.2d | 31.6 | 59.0 |
| Tobramycin | 16.00 | >16.00 | 61.5 | 29.9d | 70.1 | 27.4 |
| Gentamicin | 4.00 | >16.00 | 35.9 | 56.4d | 43.6 | 47.0 |
| Meropenem | - | - | 100.0 | 0.0 | 100 | 0.0 |
| Levofloxacin | 16.00 | >16.00 | 78.6 | 14.5d | 78.6 | 14.5 |
| Colistin | 0.25 | 0.50 | - | - | 8.6 | 91.4e |
| D,
Aminoglycoside-modifying enzymes producer-Enterobacterales
(n=801)h |
| | US FDAb | CLSI
(2023)c |
| Plazomicin and
comparator antimicrobials | MIC50,
µg/ml | MIC90,
µg/ml | Resistant, % | Susceptible, % | Resistant, % | Susceptible, % |
| Plazomicin | 0.50 | 1.00 | 1.4 | 97.3 | 1.4 | 97.3 |
| Amikacin | 4.00 | 16.00 | 2.0 | 94.3d | 13.1 | 68.4 |
| Tobramycin | 16.00 | >16.00 | 68.0 | 12.0d | 88.0 | 2.0 |
| Gentamicin | >16.00 | >16.00 | 83.3 | 15.2d | 84.8 | 14.0 |
| Meropenem | 0.03 | 0.25 | 7.7 | 91.4d | 7.7 | 91.4 |
| Levofloxacin | 8.00 | >16.00 | 63.4 | 29.0d | 63.4 | 29.0 |
| Colistin | 0.25 | 0.25 | - | - | 4.3 | 95.7e |
| E,
Multidrug-resistant Enterobacterales (n=844)i |
| | US FDAb | CLSI
(2023)c |
| Plazomicin and
comparator antimicrobials | MIC50,
µg/ml | MIC90,
µg/ml | Resistant, % | Susceptible, % | Resistant, % | Susceptible, % |
| Plazomicin | 0.25 | 1.00 | 2.5 | 94.8 | 2.5 | 94.8 |
| Amikacin | 4.00 | 16.00 | 2.0 | 94.0d | 13.3 | 71.0 |
| Tobramycin | 16.00 | >16.00 | 59.1 | 20.1d | 79.9 | 15.2 |
| Gentamicin | >16.00 | >16.00 | 59.0 | 36.4d | 63.6 | 33.8 |
| Meropenem | 0.03 | 4.00 | 12.0 | 85.5d | 12.0 | 85.5 |
| Levofloxacin | 8.00 | >16.00 | 72.8 | 16.5d | 72.8 | 16.5 |
| Colistin | 0.25 | 0.50 | - | - | 8.6 | 91.4e |
| F, Extensively drug
resistant Enterobacterales (n=84)j |
| | US FDAb | CLSI
(2023)c |
| Plazomicin and
comparator antimicrobials | MIC50,
µg/ml | MIC90,
µg/ml | Resistant, % | Susceptible, % | Resistant, % | Susceptible, % |
| Plazomicin | 0.25 | 1.00 | 6.0 | 94.0 | 6.0 | 94.0 |
| Amikacin | 8.00 | >32.00 | 11.9 | 65.5d | 44.0 | 41.7 |
| Tobramycin | >16.00 | >16.00 | 86.9 | 0.0d | 100.0 | 0.0 |
| Gentamicin | 8.00 | >16.00 | 48.8 | 40.5d | 59.5 | 29.8 |
| Meropenem | 16.00 | >32.00 | 88.1 | 2.4d | 88.1 | 2.4 |
| Levofloxacin | >16.00 | >16.00 | 95.2 | 0.0d | 95.2 | 0.0 |
| Colistin | 0.25 | 0.50 | - | - | 7.1 | 92.9e |
| G,
Amikacin-non-susceptible Enterobacterales based on 2023 CLSI
criteria (n=528) |
| | US FDAb | CLSI
(2023)c |
| Plazomicin and
comparator antimicrobials | MIC50,
µg/ml | MIC90,
µg/ml | Resistant, % | Susceptible, % | Resistant, % | Susceptible, % |
| Plazomicin | 1.00 | 4.00 | 6.4 | 86.4 | 6.4 | 86.4 |
| Amikacin | 8.00 | 32.00 | 3.6 | 89.4d | 26.5 | 0.0 |
| Tobramycin | 8.00 | >16.00 | 49.4 | 46.0d | 54.0 | 42.2 |
| Gentamicin | 2.00 | >16.00 | 31.8 | 66.9d | 31.1 | 66.9 |
| Meropenem | 0.03 | 0.50 | 8.7 | 90.7d | 8.7 | 90.7 |
| Levofloxacin | 4.00 | >16.00 | 54.4 | 42.6d | 54.4 | 42.6 |
| Colistin | 0.25 | >8.00 | - | - | 16.5 | 83.5e |
Plazomicin use can be an effective method for
treating carbapenems-resistant bacterial infections, particularly
in countries where the prevalence of AMR is rising, including
countries in Europe and Asia (9).
Plazomicin was found to be more effective compared with the other
tested AGs and comparable with ceftazidime/avibactam and
merpenem/vaborbactam when tested against ESBLs-producing
Klebsiella pneumoniae, ESBL-producing Escherichia
coli, CRE and colistin-resistant Enterobacterales (Table III) (22). Alternatively, it was reported that
plazomicin displayed low activity against Acinetobacter
species (mostly A. baumannii) (17,23),
Pseudomonas aeruginosa (24) and Stenotrophomonas
maltophilia (22) with an MIC
to inhibit 90% of tested strains (MIC90) of ≥16, ≥16 and
>64 µg/ml, respectively. Moreover, plazomicin has been tested by
Castanheira et al (13)
against 99 Acinetobacter species isolated from European
nations (13), and the results
showed that it had a MIC90 of >128 µg/ml and a
susceptibility of 40.0%. This low activity was not superior to
those of other comparator drugs, including gentamicin, amikacin,
levofloxacin and meropenem, which displayed susceptibilities
ranging from 34-41%. A total of 90% of these Acinetobacter
species strains were susceptible to colonistin. Furthermore, the
authors tested plazomicin against 60 Enterobacterales isolates that
are producers of 16RMT, but it displayed no activity with
MIC50/MIC90 of >128/>128 µg/ml
(13).
 | Table IIIPlazomicin and comparator
antimicrobial in vitro MIC90 against some
resistant Gram-negative bacterial isolates (22). |
Table III
Plazomicin and comparator
antimicrobial in vitro MIC90 against some
resistant Gram-negative bacterial isolates (22).
| | MIC90,
µg/ml (% susceptible) |
|---|
| Organism | Plazomicin | Amikacin | Tobramycin | Gentamicin | Meropenem |
Meropenem/vaborbactam |
Ceftazidime/avibactam |
|---|
| ESBLs-producing
Escherichia coli (n=343) | 1.00 (100.0) | 8.00 (98.8) | 32.00 (55.7) | >32.00
(67.1) | 0.06 (99.7) | 1.00 (100.0) | 0.50 (NA) |
| ESBLs-producing
Klebsiella pneumoniae (n=73) | 0.50 (98.6) | 8.00 (100.0) | 32.00 (37.0) | >32.00
(49.3) | 0.50 (93.2) | 0.50 (99.3) | 1.00 (NA) |
|
Carbapenem-resistant Enterobacterales
(n=110) | 1.00 (98.1) | 32.00 (23.6) | 64.00 (3.6) | 16.00 (81.8) | ≥16.00 (2.7) | 32.00 (79.6) | 2.00 (97.5) |
| Colistin-resistant
Enterobacterales (n=95) | 4.00 (93.7) | 32.00 (21.0) | 32.00 (8.0) | 64.00 (12.0) | 16.00 (12.0) | NA | 2.00 (99.5) |
Plazomicin had comparable anti-Gram-positive isolate
activity to other AGs (22,24)
(Table IV); methicillin-resistant
and methicillin-sensitive Staphylococcus aureus are both
susceptible with an MIC90 of 1 µg/ml and a
MIC90 ≤0.5 µg/ml, respectively. Plazomicin also
demonstrated strong efficacy against Staphylococcus
epidermidis. Plazomicin are not effective against streptococci;
its MIC90 values for Streptococcus agalactiae,
Streptococcus pneumoniae and Streptococcus pyogenes
are 64, 32 and 32 µg/ml, respectively. Plazomicin is similarly
ineffective against Enterococcus faecalis and
Enterococcus faecium strains with MIC90 >64
and 16 µg/ml, respectively (22).
No notable activity of plazomicin has been demonstrated against the
Clostridium species, other Gram-positive anaerobes and
Gram-negative anaerobes (specifically Bacteroides and
Prevotella species) (18).
 | Table IVPlazomicin and comparator
antimicrobial in vitro MIC90 against some
Gram-positive bacterial isolates (22). |
Table IV
Plazomicin and comparator
antimicrobial in vitro MIC90 against some
Gram-positive bacterial isolates (22).
| | MIC90,
µg/ml (% susceptible) |
|---|
| Organism | Plazomicin | Amikacin | Tobramycin | Gentamicin | Vancomycin |
|---|
|
Methicillin-susceptible Staphylococcus
aureus (n=3,009) | 1.00 (NA) | 4.00 (NA) | ≤0.50 (NA) | ≤0.50 (98.6) | 1.00 (100.0) |
|
Methicillin-resistant Staphylococcus
aureus (n=687) | 1.00 (NA) | 32.00 (NA) | >64.00 (NA) | ≤0.50 (96.2) | 1.00 (99.7) |
|
Methicillin-susceptible Staph.
epidermidis (n=339) | 0.25 (NA) | 4.00 (NA) | 16.00 (NA) | >32.00
(69.3) | 2.00 (100.0) |
|
Methicillin-resistant Staph.
epidermidis (n=25) | 0.50 (NA) | 16.00 (NA) | >64.00 (NA) | >32.00
(20.0) | 2.00 (100.0) |
Recently, it was reported that plazomicin was
effective in monotherapy (9).
Plazomicin in combination with numerous antibiotics appears to
inhibit the development of AMR without posing any hazards (36). The synergy of plazomicin with other
antibiotics (such as carbapenems, ceftazidime and
piperacillin/tazobactam) used to treat complicated infections is
notable; it can be utilized in combination to treat severe
Gram-negative infections caused by MDR Enterobacterales, including
isolates that are resistant to β-lactams and AGs. Carbapenems in
combination with plazomicin showed synergism against
Acinetobacter baumannii (22).
Plazomicin has also been shown to have in
vitro synergistic activity against Pseudomonas
aeruginosa when combined with cefepime, imipenem, doripenem or
piperacillin-tazobactam, as well as against methicillin-resistant
Staphylococcus aureus, heteroresistant
vancomycin-intermediate Staphylococcus aureus (hVISA), VISA
and vancomycin-resistant Staphylococcus aureus when combined
with ceftobiprole or daptomycin (37).
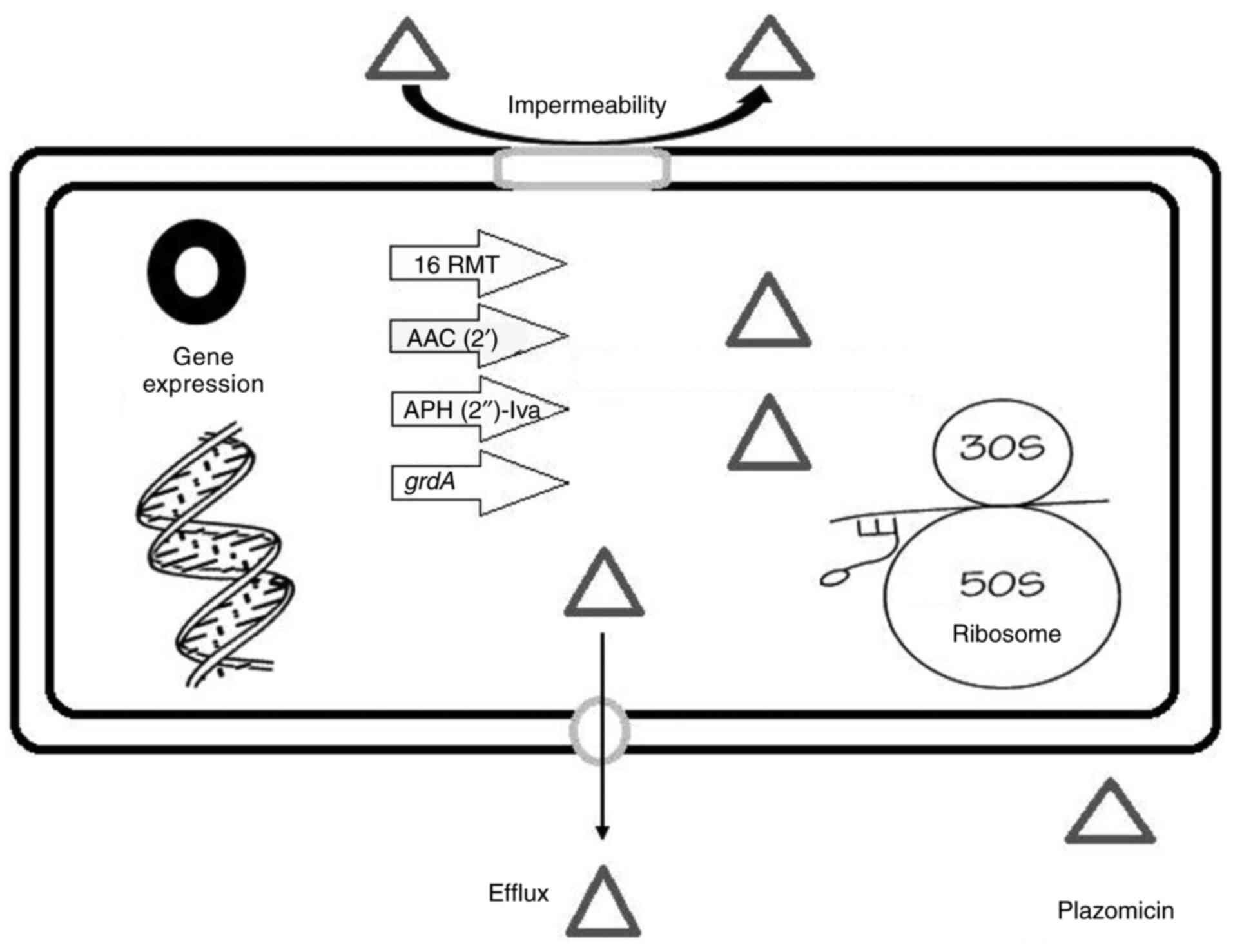
4. Bacterial resistance to plazomicin
Fig. 2 shows a
schematic representation of the resistance determinants of
plazomicin. Clark and Burgess (38) reported that the identification of
plazomicin-resistant bacteria was uncommon, and the expression of
16RMT was confirmed among the plazomicin-resistant isolates.
High-level resistance to classical AGs (gentamicin, tobramycin and
amikacin) as well as plazomicin is caused by acquired 16RMT such as
ArmA and RmtC (18). Plazomicin is
ineffective against bacteria that express 16RMT isolated mainly in
East Asia and occasionally co-expressed with the New Delhi
metallo-β-lactamase (NDM). Furthermore, the bacterial phenotypes
co-expressing 16RMT with NDM have occasionally been observed in the
US, other nations in Europe and Asia have reported a more
widespread frequency (9).
Co-expression of 16RMT with OXA-48-carbapenemases is not uncommon.
Thus, plazomicin should be used with caution when treating
Enterobacterales that express NDM or OXA-48, and it is advisable to
use commercially available rapid detection kits capable of
detecting carbapenemases production, particularly NDM, prior to
empirical administration of plazomicin (9).
As previously established, structural changes in
plazomicin shield it against all clinically notable AMEs. The AAC
(2')-I is an AME that is known to have anti-plazomicin activity
among GNB, and it is chromosomally expressed in some isolates of
Providencia stuartii (17).
Notably, the AAC (2')-I encoding gene appears not to have
transferred to any other bacterial species (17). APH (2')-Iva is another known AME
with anti-plazomicin activity; however, this enzyme has only been
found in the Enterococcus species for which plazomicin would
not be considered a recommended therapy option (25).
Bacterial resistance against plazomicin (as in
Pseudomonas aeruginosa and Acinetobacter) can also be
generated by additional AG resistance mechanisms, such as efflux
pumps or drug impermeability with down-regulation of porin
channels. Therefore, traditional AGs can be more effective against
Pseudomonas aeruginosa or Acinetobacter baumannii
compared with plazomicin regardless of the absence or presence of
AMEs (18).
Salmonella enterica strains with the
gentamicin resistance gene (grdA) are extremely resistant to
plazomicin (MIC >256 µg/ml) according to a study from the USA
(39). Compared with the
previously identified plazomicin-resistant AMEs AAC
(2')-Ia and APH (2')-Iva, these results show
that grdA confers markedly greater resistance to plazomicin
(39). Healthcare professionals
should continue to use plazomicin susceptibility testing because
tests for identifying the genetic type of bacterial resistance are
not always easily available (22).
5. Pharmacokinetics and dosing
As with other AGs, plazomicin has modest plasma
protein binding (~20%) and first-order linear elimination and is
mainly excreted by the kidneys (glomerular filtration) without
undergoing plasma or hepatic metabolism (9). Plazomicin must be taken parenterally
because, similar to other AGs, it is poorly absorbed. The volume of
distribution (Vd) of the AGs is ~25% of body weight, which is
comparable with the volume of extracellular fluid, and they do not
penetrate most cells. Adults with cUTIs and healthy adults both had
mean Vd values of 18 and 31 liters, respectively, for plazomicin.
In patients with cUTI, the mean maximum serum concentration was 51
mg/l and the area under the concentration time curve was 226 mg h/l
after receiving a single intravenous (IV) dosage of plazomicin (15
mg/kg). Plazomicin neither induces nor inhibits the cytochrome P450
drug-metabolizing enzymes, it is not metabolized to any
considerable level and in patients with normal renal function, the
typical serum elimination half-life of plazomicin is ~3.5 h
(22).
Assessing the effectiveness of plazomicin against
respiratory infections, particularly in patients with resistant
bacteria, requires an understanding of its distribution in lung
tissue. Plazomicin can infiltrate the lungs to a comparable extent
to amikacin even without lung inflammation (40). Plazomicin showed little to no
affinity for melanin; therefore, it is unlikely that it will be
retained in pigmented skin tissues (18). Plazomicin is supplied as a single
use, fliptop, 10-ml vial containing 500 mg plazomicin (50 mg/ml),
and sterilely compounded products are stable for 24 h at room
temperature (41). The US FDA has
approved a single daily IV infusion dose of 15 mg/kg, given over 30
min in 0.9% sodium chloride or lactated Ringers. After the initial
dose, TDM should be used to adjust the dosing interval by 1.5 folds
to keep the plasma trough concentration (the concentration of drug
in the blood immediately before the next dose is given) <3 µg/ml
(38). Plazomicin's renal
clearance is comparable with its overall body clearance; following
a single 15 mg/kg IV injection, 56 and 97.5% of the unmodified
plazomicin is excreted in urine within the first 4 h and a week,
respectively, with <0.2% recovered in stool (42).
To reduce toxicity, a reduction in the dose of
plazomicin is recommended in patients suffering from moderate
(creatinine clearance <60 ml/min) and severe (creatinine
clearance <30 ml/min) renal impairment to 10 mg/kg every 24 h
and 10 mg/kg every 48 h, respectively (22). The following formula should be used
to determine the adjusted body weight (ABW): For patients whose
total body weight (TBW) is >25% greater than IBW, ABW=ideal body
weight (IBW) + 0.4 (TBW-IBW) (41).
6. Clinical therapeutic indications
The US FDA has approved plazomicin for cUTIs and
acute pyelonephritis (AP) treatment in patients aged 18 years and
older when these infections are caused by susceptible bacteria with
few or no other therapeutic options (41,43).
A general review of plazomicin data has clearly highlighted two
clinical indications (38) as
presented in Table V: cUTI
(approved by the US FDA after passing the phase 2 clinical trial
NCT01096849(44) and the
Evaluating Plazomicin In cUTI (EPIC) trial (45) and serious CRE infections including
BSI, VAP and HAP (up to date, it is not approved by the US FDA
after premature termination of the Combating Antibiotic-Resistant
Enterobacteriaceae (CARE) trial (46).
 | Table VSummary of plazomicin clinical trials
in phase II and III. |
Table V
Summary of plazomicin clinical trials
in phase II and III.
| Trial | Phase | Indication | Primary
outcome | Results, n (%) | (Refs.) |
|---|
| P2-01 (trial no.
NCT01096849) | II | cUTI | Microbiological
recurrence at TOC visit (1 month following the final dose of the
study drug) | Plazomicin 9
(6.5) | Levofloxacin 34
(23.5) | (44) |
| | | | Serum creatinine
increases ≥0.5 mg/dl | 5 (3.4) | 0 (0.0) | |
| EPIC (trial no.
NCT02486627) | III | cUTI | Composite
curea on 5th day of
therapy | Plazomicin 168
(88.0) | Meropenem 180
(91.4) | (45) |
| | | | | Statistical
difference, 3.4% (95% CI, -10.0-3.1%) in favor of plazomicin | |
| | | | Composite
curea at TOC visit
(after IV therapy initiation by 15-19 days) | 156 (81.7) | 138 (70.1) | |
| | | | | Statistical
difference, 11.6% (95% CI, 2.7-20.3%) in favor of plazomicin | |
| CARE (trial no.
NCT01970371) | III | Serious CRE
infections (including BSI, HAP and VAP) | Disease-related
complications or all-cause mortality at 28 days in the
microbiologic modified intent-to-treat population (those with a
confirmed CRE infection who received at least one dosage of the
trial drug) |
Plazomicinb 4(24) |
Colistinb 10(50) | (46) |
| | | | | Statistical
difference, 26% (95% CI, -55-6%) in favor of plazomicin | |
Connolly et al (44) performed the phase 2 clinical trial
(trial no. NCT01096849) for comparing the efficacy and safety of
plazomicin (15 mg/kg/day) with levofloxacin (750 mg/day) by IV for
the treatment of cUTI and AP. In this multicenter, randomized,
double-blind research, 145 patients participated. In comparison
with the levofloxacin treatment group, the plazomicin treatment
group experienced a lower rate of microbiological recurrence (6.5
vs. 23.5%) 1 month following the final dose of the study drug.
Furthermore, 3.4% of the plazomicin therapy group experienced an
increase in serum creatinine of ≥0.5 mg/dl, although none of the
levofloxacin treatment groups did.
The EPIC study group (45) reported that plazomicin was
non-inferior when compared with meropenem for the treatment of cUTI
and AP. This study was a multicenter, international, randomized,
double-blind phase 3 trial. Plazomicin was demonstrated to be
non-inferior to meropenem regarding composite (microbiological and
clinical) cure on day 5 of therapy and at the test-of-cure (TOC)
visit, which occurred 15 to 19 days after the start of IV therapy.
In brief, after at least 4 days, the trial assessed the
effectiveness of IV plazomicin (15 mg/kg once daily) and IV
meropenem (1 g/8 h) for 7-10 days, followed by optional oral
levofloxacin 500 mg once a day, and there was no TDM performed. The
main goals were to show that plazomicin was non-inferior to
meropenem based on the variance in the clinical cure rates and
microbiological cure rates on days 5 and 15-19 after the start of
therapy. A 15% non-inferiority margin was applied during the
trials. Escherichia coli was the most prevalent uropathogen,
followed by Klebsiella pneumoniae. On day 5 of therapy,
plazomicin had a composite (microbiological and clinical) rate of
168 out of 191 cases (88%), whereas meropenem had a cure rate of
180 out of 197 cases (91.4%). Plazomicin and meropenem had
composite cure rates of 156 of 191 (81.7%) and 138 of 197 (70.1%),
respectively at the TOC visit. Furthermore, a rise in serum
creatinine of ≥0.5 mg/dl occurred in 3.7 and 3.0% of plazomicin and
meropenem treatment groups, respectively. Moreover, 100 and 81.8%
of patients taking meropenem and plazomicin, respectively,
experienced complete renal recovery.
In the CARE trial, plazomicin (15 mg/kg/day)-based
combinations were compared with colistin (5 mg/kg/day)-based
combinations with adjunctive meropenem or tigecycline in patients
with CRE-related BSI, HAP and VAP. Although preliminary findings
suggested that the plazomicin group had lower all-cause mortality
(at 28 days) and fewer major adverse events including
nephrotoxicity, the study was discontinued early due to the limited
number of the enrolled patients (a total of 39 individuals were
enrolled as the following; 29 patients having proven CRE-related
BSI, 8 patients having proven CRE-related HAP or VAP and
CRE-related infection was not proven in 2 patients) (46). Thus, the US FDA has not approved
plazomicin's use in case of bacterial infections causing BSI, HAP
and VAP as the findings lacked the strength required for accurate
hypothesis testing (22). Data
from the USA has demonstrated notable bactericidal activity against
a wide range of bacteria that are resistant to AGs, β-lactams,
fluoroquinolones and carbapenems, including the MDR isolates
(resistant to ≥1 agent in ≥3 different antibiotic classes)
(34). The data presented by
Alfieri et al (9) is
encouraging for the use of plazomicin monotherapy or in combination
with another antibiotic in three key scenarios (cUTI, BSI and VAP);
however, the research team recommended that data meta-analysis is
required to characterize the benefits.
7. Side effects and safety profile
The safety profile of traditional AGs reveals that
they have always had a wide range of adverse effects, particularly
when compared with the β-lactam antibiotics (9). Nephrotoxicity, ototoxicity and
neuromuscular inhibition are the three most reported toxicities of
AGs. Nephrotoxicity affects 3-11% of individuals, whereas
vestibular and cochlear toxicity affect 10 and 26% of patients,
respectively (Table VI) (6). The nephrotoxicity caused by AGs is
caused by drug accumulation in the cortical portion of the kidney.
It is a reversible process that relies on the capacity of tubular
epithelial cells to regenerate. As a result, in cases of patients
with poor kidney functions, a dose reduction must be considered
(9). Ototoxicity, on the other
hand, is the result of direct oxidative damage to the vestibular
organ, cochlea and its hairy cells and related cranial nerves. The
resulted harm is permanent; thus, it must be avoided. When used in
combination with AGs, aspirin, N-acetylcysteine, dexamethasone and
antioxidant compounds as N-methyl-D-aspartate receptor antagonists
have been shown to effectively reduce ototoxicity (9).
 | Table VIMain side effects of AGs (6). |
Table VI
Main side effects of AGs (6).
| | | Risk factors of
toxicity | | |
|---|
| Toxicity types | Effects of
toxicity | Clinical | Therapeutic | Potential treatment
of toxicity | Prevention of
toxicity |
|---|
| Renal | Acute injury of the
kidney with preserved diuresis; tubular necrosis | Age; chronic renal
disease; dehydration; hyperthermia | Previous treatment
with AG; cumulative dose; treatment duration >5 days | Dose adaptation
through TDM; stop AG when unnecessary | Avoid
co-nephrotoxic drugs; avoid cumulative risk factors; TDM |
|
Cochleovestibular | Cochlear-hearing
loss, tinnitus; vestibular-ataxia, vertigo, nystagmus | Past history of
hearing loss | Previous treatment
with AG; cumulative dose; treatment duration >5 days | Dose adaptation
through TDM; stop AG when unnecessary | Avoid
co-nephrotoxic drugs; avoid cumulative risk factors; TDM |
| Neuromuscular | Neuromuscular
block | Myasthenia gravis;
immediate postoperative period; respiratory acidosis | - | Anti-cholinesterase
drugs | Avoid
co-nephrotoxic drugs; avoid cumulative risk factors; TDM |
The US FDA added a black box warning for
nephrotoxicity, ototoxicity, neuromuscular blockade and pregnancy
risk when approving plazomicin (41,43).
Plazomicin is an AG, hence notable renal damage is to be expected;
however, the renal toxicity of plazomicin is near that of
meropenem, a rise in serum creatinine of ≥0.5 mg/dl from baseline
occurred in 3.7 and 3.0% of plazomicin and meropenem treatment
group, respectively. It is important to remember that the kidney
damage induced by plazomicin is reversible, with most patients
(81.8%) having complete renal function at the time of discharge in
the EPIC study (45). Both
plazomicin and meropenem groups reported the same adverse events in
the EPIC study including hypotension (1.0%), headache (1.3%),
nausea (1.3%), vomiting (1.3%), diarrhea (2.3%) and hypertension
(2.3%). Moreover, in the EPIC trial, there was only one instance of
Clostridoides difficile in the comparator group and none in
the plazomicin group. On the other hand, there was a single
mortality in the plazomicin group (a patient passed away on trial
day 18 from metastatic uterine cancer that had been discovered 48 h
after enrolment) (45).
Cochlear and vestibular function were assessed in a
study performed on healthy participants, at baseline and up to 6
months after starting plazomicin treatment, and revealed no signs
of ototoxicity, suggesting that plazomicin has a low potential for
ototoxicity (40). As the
plazomicin reports in the phase 3 trial were not based on cochlear
and vestibular examinations, adverse events with possible
ototoxicity seemed to be rare. Although there is a documented risk
of ototoxicity connected with AGs, the plazomicin phase 3 trials
were unable to identify any possible ototoxicity associated with
plazomicin treatment (22).
According to previous findings, plazomicin is not ototoxic; thus,
it is safe, as well as practical and effective (47). Alfieri et al (9) reported that ototoxicity occurred in
<2% of patients in numerous trials, on average.
The clinical investigations of plazomicin to date
have not revealed any evidence of the AG class danger of
neuromuscular blocking leading to respiratory depression. However,
care should be taken when prescribing plazomicin to patients who
have a neuromuscular condition such as myasthenia gravis or to
individuals who are receiving neuromuscular blockers such as
succinylcholine because doing so could delay the neuromuscular
function recovery (22).
When provided to patients who are pregnant, AGs,
including plazomicin, can cross the placenta and cause fetal harm.
Streptomycin has been linked to multiple reports of complete,
irreversible, bilateral congenital deafness in young children who
were exposed in utero. Female patients should be informed of
the potential risk to the fetus if they use plazomicin while
pregnant or become pregnant while using plazomicin. To the best of
our knowledge, there is no data available on the presence of
plazomicin in human milk, its effects on breastfed infants or its
effects on milk production, but minimal levels are anticipated in
milk based on the excretion of other AGs; however, plazomicin has
been found in rat milk (41,43).
The National Institute of Child Health and Human Development
expects low plazomicin excretion in human milk (based on the
excretion of other AGs). Thus, the institute recommends the
inspection of any effects on the gastrointestinal flora of the
baby, such as diarrhea, candidiasis (such as thrush or diaper rash)
or infrequently, blood in the stool, which could be a sign of
antibiotic-associated colitis, when prescribing plazomicin to
lactating females (48). The
safety and effectiveness of plazomicin in patients <18 years
have not been demonstrated (41,43).
8. Role in therapy and special
considerations
Due to the potential for antimicrobials to lose
their effectiveness in treating infectious diseases, it is
imperative to consider newly developed antimicrobials and put
preventive measures in place to stop the emergence of AMR (49). The real-world therapeutic
experience will define the role of plazomicin as a monotherapy
and/or combination therapy. The therapeutic usage of AGs and
polymyxin antibiotics has increased because of the global spread of
MDR GNB. The majority of Enterobacterales that are resistant to the
traditional AGs can still be killed by plazomicin (20); thus, there are multiple potential
roles for plazomicin in therapy depending on its higher potency
(lower MICs) against numerous bacterial pathogens.
Plazomicin is effective in vitro against a
variety of isolates that produce ESBLs and carbapenemase enzymes
(19). However, with this class of
antibiotics, strong in vitro activity does not always
indicate clinical efficacy. A poor outcome was noticed while using
AGs in treating patients suffering from pneumonia and explained by
their low concentrations in alveolar lining fluid and inactivation
by the acidic pH within the inflamed lung tissue (50). Therefore, before accepting
plazomicin for extended usage, more clinical trials using
plazomicin in a variety of diseases, particularly pneumonias, are
required. However, due to the lack of therapeutic options for the
management of MDR bacterial infections, plazomicin may thus play a
special role in antibacterial therapy.
For patients with cUTI, AGs, including plazomicin,
were suggested over tigecycline (9,51).
Plazomicin offers certain dosage advantages compared with other
antimicrobials, including β-lactam/β-lactamase inhibitors, which
are effective against MDR GNB. Plazomicin can be administered
intravenously, once daily over a brief period of 30 min, and
hospitalized patients may benefit from this administration
schedule, but those undergoing outpatient parenteral antibiotic
therapy will especially benefit from it (52). However, plazomicin-associated
toxicity, especially nephrotoxicity and ototoxicity, is an area of
doubt, and it is important to monitor renal function. Notably, when
the benefits of plazomicin use as antibacterial therapy are weighed
against its side effects, the drug safety in comparison to
conventional AGs is notable (9).
In terms of drug-drug interactions, 90% of metformin
is eliminated via renal tubular secretion through three
transporters (multidrug and toxin extrusion 2-K, multidrug and
toxin extrusion 1 and organic cation transporter 2). Although
plazomicin selectively inhibits these transporters with variable
degrees, there are no noticeable changes in the blood metformin
levels when plazomicin was administered concurrently with
metformin, according to a study assessing the possibility of
interaction (53). Furthermore,
another study investigating the effect of plazomicin intake on the
heart found no discernible changes in the QT interval length
(54). Similar to other AGs, the
major drug interaction of concern would likely be additive toxicity
when combined with other nephrotoxic drugs (18).
As with other antimicrobials created for the
treatment of MDR GNB, plazomicin has a high acquisition cost
(55). The actual market entry
price of plazomicin per treatment course was $4,955.11 at the time
of commercialization in 2018(56).
The price of the recently approved antimicrobials with anti-CRE
activity against cUTIs, such as ceftazidime-avibactam (February
2015) and meropenem-vaborbactam (August 2017), was taken into
consideration while setting the price of plazomicin. In 2019, the
cost of purchasing plazomicin at wholesale was $945.00 a day, based
on a dosage of a patient weighing 75 kg, and ceftazidime-avibactam
and meropenem-vaborbactam were $1,076 and $990, respectively,
whereas colistin ($56.00 per day) and polymixin B ($21.78-45.00)
were markedly less expensive (57).
As a result, healthcare professionals may have
concerns about the cost of plazomicin therapy. Antimicrobial
stewardship will be necessary to avoid this cost and the cost of
its TDM. On the other hand, clinicians should also consider that
plazomicin monotherapy can improve the prognosis of patients with
MDR bacteria, reduce the likelihood of infection progression to
septic shock and shorten the hospitalization duration (58). For patients with severe infections,
plazomicin therefore may enable the reduction of the overall cost
of treatment (59). The additional
hospital expenses per patient for antibiotic-resistant
healthcare-associated infections among U.S. patients were estimated
by Achaogen to be >$15,000(60).
As mentioned before, cUTIs are the focus of most
plazomicin related therapeutic data (9). The scarcity of data from clinical
trials other than cUTIs is the main issue with non-extensive
therapeutic use of plazomicin (59). Randomized controlled trials were
identified by a recent systematic literature review that aimed to
evaluate the relative effectiveness of different treatment options
for cUTIs and AP. The study concluded that plazomicin demonstrated
notably higher relative efficacy vs. carbapenems for both composite
outcome and microbiological eradication at TOC (61).
The role of certain AG in therapy should be
connected to the susceptibility of the organism producing the
infection rather than the infection site (62). A study from Egypt concluded that
plazomicin displayed the most potent in vitro activity
against carbapenem-resistant Gram-negative intensive care unit
(ICU) and non-ICU isolates when compared with meropenem-vaborbactam
(carbapenem combined with a β-lactamase inhibitor) and omadacycline
(a semisynthetic minocycline derivative), with plazomicin
inhibiting 82.22% of CRE isolates. The tested samples in the study
included non-ICU samples (urine and surgical wound swabs), and ICU
samples (urine, blood, sputum, endotracheal aspirates,
bronchoalveolar lavage, peritoneal fluid aspirate, pericardial
fluid aspirate, pleural fluid aspirates, CSF, infected burn wounds
swabs and surgical wound samples) (63).
Most likely, plazomicin will be utilized either
alone to treat MDR cUTIs or in combination to treat severe CRE
infections, especially those having a variety of AMEs (38). Moreover, plazomicin has been used
in the treatment of patients with infections including the UTIs,
BSIs and VAPs if caused by plazomicin susceptible organisms. The
European Medicines Agency approved the drug because of these
encouraging results against the susceptible bacteria, but it is not
yet available in the market (6).
Despite their hydrophilicity, AGs effectively
penetrate bone and joint tissues (64). Plazomicin was discovered to be
active and superior to traditional AGs against CRE isolates from
skin and soft tissue infections (SSTIs) (65); however, the relevance of plazomicin
in the treatment of SSTIs, bone infections and diabetic foot
infections is still understudied (66). In the US, plazomicin showed little
or no activity against Achromobacter infections (including
respiratory, blood, genitourinary and wound infections), with
MIC50/MIC90 of >4/>4 µg/ml (67).
Plazomicin research has been limited by the fact
that it has only been used for conditions that affect the urinary
system and by the fact that varied ethnicities and patients who are
seriously unwell, such as those who have bacteremia and pneumonia,
are underrepresented (22).
According to the aforementioned information, plazomicin is a
reasonably expensive drug that should be used sparingly, but it can
enable patients with serious infections to save money on their
entire course of treatment (9).
9. Conclusions and recommendations
Plazomicin is a promising semisynthetic
antimicrobial that has been created to target MDR bacterial
pathogens, especially GNBs. Except for the approved use of
plazomicin to treat cUTIs, there is a gap in the literature
regarding its clinical uses for treatment of severe complicated
infections such as BSIs, HAPs and VAPs after premature termination
of the CARE trial. For the treatment of cUTIs, it is considered the
best treatment option against bacterial pathogens with poor
sensitivity to carbapenems and other alternatives. It has been
demonstrated to be non-inferior to meropenem, it is a convenient
treatment choice for outpatient antibiotic therapy settings (given
IV, once daily, with a short 30 min administration duration) and it
has low frequency side effects. Due to the rising prevalence of
highly resistant GNB and the absence or limited alternative
treatment options, plazomicin is a valuable non-β-lactam treatment
option against MDR bacterial infections such as BSIs, HAPs and
VAPs. This costly new antibacterial drug should be properly
exploited, prescribed when necessary and sparingly to maintain its
effectiveness over time. To help improve understanding of its
clinical and therapeutic importance in various bacterial illnesses,
a meta-analysis on this trending issue is advised. Clinicians using
plazomicin should recognize that the setting of clinical practice
is distinct from that of clinical trials. Thus, clinicians are
urged, as always, to review their local antibiograms and adhere to
the optimal guidelines which include proper plazomicin dosing and
administration with TDM, to achieve maximum effectiveness and least
toxicity especially for patients with a history of inner ear or
kidney diseases who should always be treated with caution.
Moreover, healthcare professionals should keep using plazomicin
susceptibility testing because tests for identifying the genetic
type of bacterial resistance are not always easily available.
Acknowledgements
Not applicable.
Funding
Funding: The present review was funded by the Deanship of
Graduate Studies and Scientific Research at Jouf University, Saudi
Arabia (grant no. DGSSR-2024-01-02130).
Availability of data and materials
Not applicable.
Authors' contributions
The present review was conceptualized by MA and AET;
the data was curated by MA, AET, IAT, HMS and GAB; funding was
acquired by MA; MA, AET, IAT, HMS and GAB were responsible for
resources and project administration; the project was supervised by
MA, AET, IAT, HMS and GAB; validation of references was performed
by MA, AET, IAT, HMS and GAB. Writing of the original draft was
completed by MA and AET and reviewing and editing of the manuscript
was performed by MA, AET, IAT, HMS and GAB. Data authentication is
not applicable. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schatz A, Bugie E and Waksman SA:
Streptomycin, a substance exhibiting antibiotic activity against
gram-positive and Gram-negative bacteria. 1944. Clin Orthop Relat
Res. 437:3–6. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Weinstein MJ, Luedemann GM, Oden EM and
Wagman GH: Gentamicin, a new broad-spectrum antibiotic complex.
Antimicrob Agents Chemother (Bethesda). 161:1–7. 1963.PubMed/NCBI
|
|
3
|
Kluge RM, Standiford HC, Tatem B, Young
VM, Greene WH, Schimpff SC, Calia FM and Hornick RB: Comparative
activity of tobramycin, amikacin, and gentamicin alone and with
carbenicillin against Pseudomonas aeruginosa. Antimicrob
Agents Chemother. 6:442–446. 1974.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reynolds AV, Hamilton-Miller JM and
Brumfitt W: Newer aminoglycosides-amikacin and tobramycin: An
in-vitro comparison with kanamycin and gentamicin. Br Med J.
3:778–780. 1974.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Degtyareva NN, Gong C, Story S, Levinson
NS, Oyelere AK, Green KD, Garneau-Tsodikova S and Arya DP:
Antimicrobial activity, AME resistance, and A-site binding studies
of anthraquinone-neomycin conjugates. ACS Infect Dis. 3:206–215.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Thy M, Timsit JF and de Montmollin E:
Aminoglycosides for the treatment of severe infection due to
resistant Gram-negative pathogens. Antibiotics (Basel).
12(860)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
World Health Organization (WHO): Deaths
due to AMR estimated to reach 10 million people by 2050. WHO,
Geneva, 2024. https://www.who.int/indonesia/news/detail/20-08-2024-deaths-due-to-amr-estimated-to-reach-10-million-people-by-2050--ministry-of-health-and-who-launch-national-strategy.
Accessed August 31, 2025.
|
|
8
|
Coppola N, Maraolo AE, Onorato L, Scotto
R, Calò F, Atripaldi L, Borrelli A, Corcione A, De Cristofaro MG,
Durante-Mangoni E, et al: Epidemiology, mechanisms of resistance
and treatment algorithm for infections due to carbapenem-resistant
Gram-negative bacteria: An expert panel opinion. Antibiotics
(Basel). 11(1263)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Alfieri A, Di Franco S, Donatiello V,
Maffei V, Fittipaldi C, Fiore M, Coppolino F, Sansone P, Pace MC
and Passavanti MB: Plazomicin against multidrug-resistant bacteria:
A scoping review. Life (Basel). 12(1949)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rice LB: Federal funding for the study of
antimicrobial resistance in nosocomial pathogens: No ESKAPE. J
Infect Dis. 197:1079–1081. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Ramirez MS and Tolmasky ME: Aminoglycoside
modifying enzymes. Drug Resist Updat. 13:151–171. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Doi Y, Wachino JI and Arakawa Y:
Aminoglycoside resistance: The emergence of acquired 16S ribosomal
RNA methyltransferases. Infect Dis Clin North Am. 30:523–537.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Castanheira M, Deshpande LM, Woosley LN,
Serio AW, Krause KM and Flamm RK: Activity of plazomicin compared
with other aminoglycosides against isolates from European and
adjacent countries, including Enterobacteriaceae molecularly
characterized for aminoglycoside-modifying enzymes and other
resistance mechanisms. J Antimicrob Chemother. 73:3346–3354.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Castanheira M, Davis AP, Serio AW, Krause
KM and Mendes RE: In vitro activity of plazomicin against
Enterobacteriaceae isolates carrying genes encoding
aminoglycoside-modifying enzymes most common in US Census
divisions. Diagn Microbiol Infect Dis. 94:73–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Walkty A, Karlowsky JA, Baxter MR, Adam
HJ, Boyd D, Bharat A, Mulvey MR, Charles M, Bergevin M and Zhanel
GG: Frequency of 16S ribosomal RNA methyltransferase detection
among Escherichia coli and Klebsiella pneumoniae
clinical isolates obtained from patients in Canadian hospitals
(CANWARD, 2013-2017). Diagn Microbiol Infect Dis. 94:199–201.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nikaido H and Takatsuka Y: Mechanisms of
RND multidrug efflux pumps. Biochim Biophys Acta. 1794:769–781.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aggen JB, Armstrong ES, Goldblum AA, Dozzo
P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias
RD, et al: Synthesis and spectrum of the neoglycoside ACHN-490.
Antimicrob Agents Chemother. 54:4636–4642. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eljaaly K, Alharbi A, Alshehri S, Ortwine
JK and Pogue JM: Plazomicin: A novel aminoglycoside for the
treatment of resistant Gram-negative bacterial infections. Drugs.
79:243–269. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Haidar G, Alkroud A, Cheng S, Churilla TM,
Churilla BM, Shields RK, Doi Y, Clancy CJ and Nguyen MH:
Association between the presence of aminoglycoside-modifying
enzymes and in vitro activity of gentamicin, tobramycin, amikacin,
and plazomicin against Klebsiella pneumoniae carbapenemase-
and extended-spectrum-β-lactamase-producing enterobacter species.
Antimicrob Agents Chemother. 60:5208–5214. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Thwaites M, Hall D, Shinabarger D, Serio
AW, Krause KM, Marra A and Pillar C: Evaluation of the bactericidal
activity of plazomicin and comparators against multidrug-resistant
Enterobacteriaceae. Antimicrob Agents Chemother. 62:e00236–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Karaiskos I, Lagou S, Pontikis K, Rapti V
and Poulakou G: The ‘old’ and the ‘new’ antibiotics for MDR
Gram-negative pathogens: For whom, when, and how. Front Public
Health. 7(151)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saravolatz LD and Stein GE: Plazomicin: A
new aminoglycoside. Clin Infect Dis. 70:704–709. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Castanheira M, Davis AP, Mendes RE, Serio
AW, Krause KM and Flamm RK: In vitro activity of plazomicin against
Gram-negative and gram-positive isolates collected from U.S.
Hospitals and comparative activities of aminoglycosides against
carbapenem-resistant Enterobacteriaceae and isolates carrying
carbapenemase genes. Antimicrob Agents Chemother. 62:e00313–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Walkty A, Karlowsky JA, Baxter MR, Adam HJ
and Zhanel GG: In vitro activity of plazomicin against
Gram-negative and gram-positive bacterial pathogens isolated from
patients in Canadian hospitals from 2013 to 2017 as part of the
CANWARD surveillance study. Antimicrob Agents Chemother.
63:e02068–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cox G, Ejim L, Stogios PJ, Koteva K,
Bordeleau E, Evdokimova E, Sieron AO, Savchenko A, Serio AW, Krause
KM and Wright GD: Plazomicin retains antibiotic activity against
most aminoglycoside modifying enzymes. ACS Infect Dis. 4:980–987.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sonousi A, Sarpe VA, Brilkova M, Schacht
J, Vasella A, Böttger EC and Crich D: Effects of the 1-N-(4-Amino-2
S-hydroxybutyryl) and 6'-N-(2-Hydroxyethyl) substituents on
ribosomal selectivity, cochleotoxicity, and antibacterial activity
in the sisomicin class of aminoglycoside antibiotics. ACS Infect
Dis. 4:1114–1120. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Armstrong ES and Miller GH: Combating
evolution with intelligent design: The neoglycoside ACHN-490. Curr
Opin Microbiol. 13:565–573. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Garneau-Tsodikova S and Labby KJ:
Mechanisms of resistance to aminoglycoside antibiotics: Overview
and perspectives. Medchemcomm. 7:11–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lebeaux D, Chauhan A, Létoffé S, Fischer
F, de Reuse H, Beloin C and Ghigo JM: pH-mediated potentiation of
aminoglycosides kills bacterial persisters and eradicates in vivo
biofilms. J Infect Dis. 210:1357–1366. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Parajuli NP, Mandava CS, Pavlov MY and
Sanyal S: Mechanistic insights into translation inhibition by
aminoglycoside antibiotic arbekacin. Nucleic Acids Res.
49:6880–6892. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Clinical and Laboratory Standards
Institute (CLSI): M100Ed33. Performance standards for antimicrobial
susceptibility testing. CLSI, Wayne, PA, 2023.
|
|
32
|
US Food and Drug Administration:
Antibacterial susceptibility test interpretive criteria 2019.
https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria.
Accessed June 6, 2025.
|
|
33
|
The United States Committee on
Antimicrobial Susceptibility Testing (USCAST): Breakpoint tables
for interpretation of MIC and zone diameter results. Version 5.3,
July 2019. https://app.box.com/s/cru62wluz4qq64vd5izk41wle9ss172x.
Accessed June 6, 2025.
|
|
34
|
Sader HS, Mendes RE, Kimbrough JH, Kantro
V and Castanheira M: Impact of the recent clinical and laboratory
standards institute breakpoint changes on the antimicrobial
spectrum of aminoglycosides and the activity of plazomicin against
multidrug-resistant and carbapenem-resistant enterobacterales from
United States medical centers. Open Forum Infect Dis.
10(ofad058)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Clinical and Laboratory Standards
Institute (CLSI): M100Ed32. Performance standards for antimicrobial
susceptibility testing. CLSI, Wayne, PA, 2022.
|
|
36
|
Earle W, Bonegio RGB, Smith DB and
Branch-Elliman W: Plazomicin for the treatment of
multidrug-resistant Klebsiella bacteraemia in a patient with
underlying chronic kidney disease and acute renal failure requiring
renal replacement therapy. BMJ Case Rep. 14(e243609)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ramirez MS and Tolmasky ME: Amikacin:
Uses, resistance, and prospects for inhibition. Molecules.
22(2267)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Clark JA and Burgess DS: Plazomicin: A new
aminoglycoside in the fight against antimicrobial resistance. Ther
Adv Infect Dis. 7(2049936120952604)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hikal AF, Zhao S, Foley S and Khan AA: The
newly identified grdA gene confers high-level plazomicin resistance
in Salmonella enterica serovars. Antimicrob Agents
Chemother. 69(e0160624)2025.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cass R, Kostrub CF, Gotfried M, Rodvold K,
Tack KJ and Bruss J: A double-blind, randomized, placebo-controlled
study to assess the safety, tolerability, plasma pharmacokinetics
and lung penetration of intravenous plazomicin in healthy subjects.
In: Proceedings of the 23rd European Congress of Clinical
Microbiology and Infectious Diseases (ECCMID). ECCMID, Berlin,
poster 1637, 2013.
|
|
41
|
U.S. Food and Drug Administration and
Center for Drug Evaluation and Research: Zemdri Approval Letter
Reference ID: 4282864. U.S. Food and Drug Administration, Silver
Spring, MD, USA, 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210303orig1s000lbl.pdf.
Accessed June 6, 2025.
|
|
42
|
Choi T, Seroogy JD, Sanghvi M and Dhuria
SV: 1400 Mass balance, metabolism, and excretion of
(14C)-plazomicin in healthy human subjects. Open Forum Infect Dis.
5 (Suppl 1)(S431)2018.
|
|
43
|
Zemdri (plazomicin) (package insert).
South Achaogen, Inc., San Francisco, CA, 2021. https://zemdri.com/assets/pdf/Prescribing-Information.pdf.
Accessed June 6, 2025).
|
|
44
|
Connolly LE, Riddle V, Cebrik D, Armstrong
ES and Miller LG: A multicenter, randomized, double-blind, phase 2
study of the efficacy and safety of plazomicin compared with
levofloxacin in the treatment of complicated urinary tract
infection and acute pyelonephritis. Antimicrob Agents Chemother.
62:e01989–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wagenlehner FME, Cloutier DJ, Komirenko
AS, Cebrik DS, Krause KM, Keepers TR, Connolly LE, Miller LG,
Friedland I and Dwyer JP: EPIC Study Group. Once-daily plazomicin
for complicated urinary tract infections. N Engl J Med.
380:729–740. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
McKinnell JA, Dwyer JP, Talbot GH,
Connolly LE, Friedland I, Smith A, Jubb AM, Serio AW, Krause KM and
Daikos GL: CARE Study Group. Plazomicin for infections caused by
carbapenem-resistant Enterobacteriaceae. N Engl J Med. 380:791–793.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jospe-Kaufman M, Siomin L and Fridman M:
The relationship between the structure and toxicity of
aminoglycoside antibiotics. Bioorg Med Chem Lett.
30(127218)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Drugs and Lactation Database
(LactMed®) (Internet). National Institute of Child
Health and Human Development, Bethesda, MD, 2006. https://www.ncbi.nlm.nih.gov/books/NBK513053/.
Accessed June 6, 2025.
|
|
49
|
Başaran SN and Öksüz L: Newly developed
antibiotics against multidrug-resistant and carbapenem-resistant
Gram-negative bacteria: Action and resistance mechanisms. Arch
Microbiol. 207(110)2025.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Edson RS and Terrell CL: The
aminoglycosides. Mayo Clin Proc. 74:519–528. 1999.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Paul M, Carrara E, Retamar P, Tängdén T,
Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S,
et al: European society of clinical microbiology and infectious
diseases (ESCMID) guidelines for the treatment of infections caused
by multidrug-resistant Gram-negative bacilli (endorsed by European
society of intensive care medicine). Clin Microbiol Infect.
28:521–547. 2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gilchrist M and Seaton RA: Outpatient
parenteral antimicrobial therapy and antimicrobial stewardship:
Challenges and checklists. J Antimicrob Chemother. 70:965–970.
2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Choi T, Komirenko AS, Riddle V, Kim A and
Dhuria SV: No effect of plazomicin on the pharmacokinetics of
metformin in healthy subjects. Clin Pharmacol Drug Dev. 8:818–826.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gall J, Choi T, Riddle V, Van Wart S,
Gibbons JA and Seroogy J: A phase 1 study of intravenous plazomicin
in healthy adults to assess potential effects on the QT/QTc
interval, safety, and pharmacokinetics. Clin Pharmacol Drug Dev.
8:1032–1041. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
No authors listed. Plazomicin (Zemdri)-a
new aminoglycoside antibiotic. Med Lett Drugs Ther. 60:180–182.
2018.PubMed/NCBI
|
|
56
|
Althobaiti H, Seoane-Vazquez E, Brown LM,
Fleming ML and Rodriguez-Monguio R: Disentangling the cost of
orphan drugs marketed in the United States. Healthcare (Basel).
11(558)2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Clancy CJ, Potoski BA, Buehrle D and
Nguyen MH: Estimating the treatment of carbapenem-resistant
Enterobacteriaceae infections in the United States using antibiotic
prescription data. Open Forum Infect Dis. 6(ofz344)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hawkey PM, Warren RE, Livermore DM,
McNulty CAM, Enoch DA, Otter JA and Wilson APR: Treatment of
infections caused by multidrug-resistant Gram-negative bacteria:
report of the British society for antimicrobial
chemotherapy/healthcare infection society/British infection
association joint working party. J Antimicrob Chemother. 73 (Suppl
3):iii2–iii78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Theuretzbacher U and Paul M: Developing a
new antibiotic for extensively drug-resistant pathogens: The case
of plazomicin. Clin Microbiol Infect. 24:1231–1233. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
United States Securities and Exchange
Commission (SEC 2019). Achaogen 10K fiscal year 2018. https://www.sec.gov/Archives/edgar/data/1301501/000156459019010412/akao-10k_20181231.htm.
Accessed September 05, 2025.
|
|
61
|
Wagenlehner F, Caballero VR, Maheshwari V,
Biswas A, Saini P, Quevedo J, Polifka J, Ruiz L and Cure S:
Efficacy of treatment options for complicated urinary tract
infections including acute pyelonephritis: A systematic literature
review and network meta-analysis. J Comp Eff Res.
14(e240214)2025.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Leibovici L, Vidal L and Paul M:
Aminoglycoside drugs in clinical practice: An evidence-based
approach. J Antimicrob Chemother. 63:246–251. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Abd El-Aziz Gadallah M, El-Sayed WM,
Hussien MZ, Elheniedy MA and Maxwell SY: In-vitro activity of
plazomicin, meropenem-vaborbactam, and omadacycline against
carbapenem-resistant Gram-negative isolates in Egypt. J Chemother.
13:205–218. 2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Thabit AK, Fatani DF, Bamakhrama MS,
Barnawi OA, Basudan LO and Alhejaili SF: Antibiotic penetration
into bone and joints: An updated review. Int J Infect Dis.
81:128–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Castanheira M, Deshpande LM, Hubler CM,
Mendes RE, Serio AW, Krause KM and Flamm RK: Activity of plazomicin
against Enterobacteriaceae isolates collected in the United States
including isolates carrying aminoglycoside-modifying enzymes
detected by whole genome sequencing. Open Forum Infect Dis. 4
(Suppl 1)(S377)2017.
|
|
66
|
Mougakou E, Mastrogianni E, Kyziroglou M
and Tziomalos K: The role of novel antibiotics in the management of
diabetic foot infection. Diabetes Ther. 14:251–263. 2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ray S, Flemming LK, Scudder CJ, Ly MA,
Porterfield HS, Smith RD, Clark AE, Johnson JK and Das S:
Comparative phenotypic and genotypic antimicrobial susceptibility
surveillance in Achromobacter spp. through whole genome
sequencing. Microbiol Spectr. 13(e0252724)2025.PubMed/NCBI View Article : Google Scholar
|