Introduction
Primary testicular tumors in children are rare,
accounting for ~1% of all pediatric solid tumors, with an annual
incidence of 0.5-2 cases per 100,000 boys (1,2).
Testicular teratomas in neonates are even more uncommon, with an
estimated incidence of 0.015-0.06 cases per million live births
(3). A large cohort study has
shown that yolk sac tumors are the most common prepubertal
testicular neoplasm, comprising ~42% of cases, with >97%
classified as malignant. Notably, ~75% of these cases require
radical orchiectomy (4). However,
current treatment approaches remain limited by the lack of
standardized management guidelines and the potential risk of
overtreatment in benign cases (5).
In the present study, the rare case of a fetal testicular teratoma
diagnosed prenatally at 28 weeks of gestation is reported. A
comprehensive perioperative assessment, including intraoperative
frozen section analysis and histological evaluation of the
peritumoral tissue, enabled successful testis-sparing management,
thereby avoiding unnecessary orchiectomy and preserving bilateral
testicular function and future fertility potential.
Case report
A male infant was delivered vaginally at 39 weeks
after a fetal ultrasound performed at 28 weeks revealed a cystic
mass superior to the fetal bladder, suspected to be a teratoma.
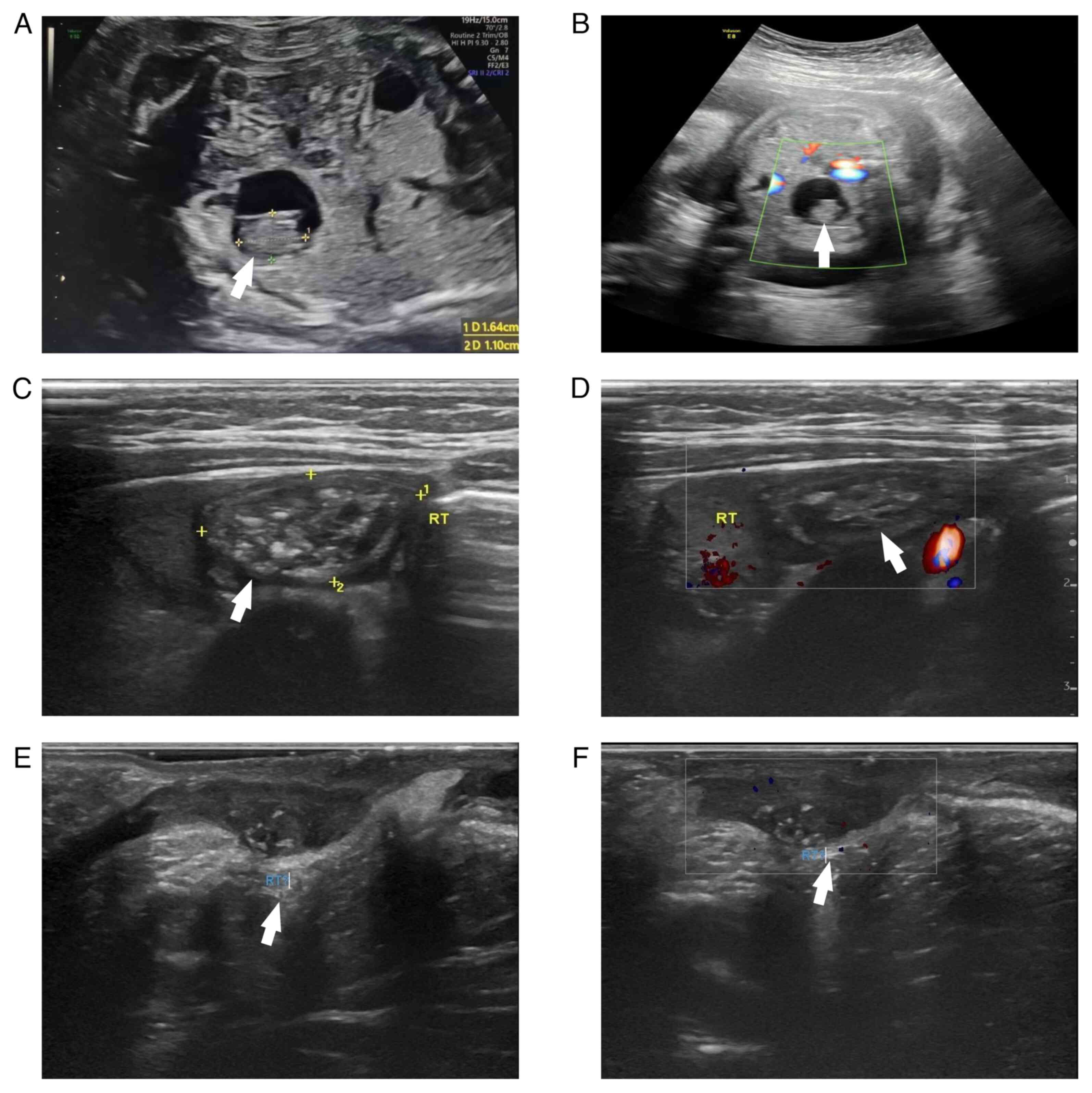
Prenatal ultrasound of the fetus in a 31-year-old primigravida
demonstrated a well-defined anechoic area with a thin wall and good
sound transmission in the right-superior bladder region, showing a
1.6x1.1 cm irregular hyperechoic focus with punctate echogenic foci
and scattered vascular signals on color Doppler flow imaging (CDFI)
(Fig. 1A and B); this lesion had not been observed on
earlier scans. Postnatal examination revealed an empty right
hemiscrotum without palpable abdominal or inguinal masses.
At 1 month of age, ultrasound (April 2025,
Children's Hospital of Chongqing Medical University, Chongqing,
China) showed a testis-like structure in the right lower abdomen
measuring ~2.8x1.8x1.0 cm. Within this structure, an abnormal
echogenic lesion ~1.8x1.6x1.0 cm in size was identified, with a
heterogeneous echotexture (mixed hyperechoic, isoechoic and small
anechoic areas) and detectable blood flow on CDFI, findings
consistent with an intra-abdominal undescended right testis
containing a teratoma (Fig. 1C and
D). Notably, tumor marker analysis
showed an elevated serum ferritin level of 473 ng/ml (reference
range, 28-365 ng/ml), while α-fetoprotein (AFP; 531 ng/ml,
reference range 24-4,800 ng/ml), β-human chorionic gonadotropin
(<0.3 mIU/ml, reference range <10 mIU/ml), carcinoembryonic
antigen (2.63 ng/ml, reference range <5.09 ng/ml), and
neuron-specific enolase (35.3 ng/ml; reference range <35.6
ng/ml) were all within normal limits.
Laparoscopy confirmed an intra-abdominal right
testis located ~1 cm from the internal inguinal ring. A 2.5x1.5 cm
cystic-solid mass was identified in the mid-to-lower portion of the
testis, with a thin, intact capsule and soft consistency; a small
amount of residual testicular tissue was noted at the upper pole.
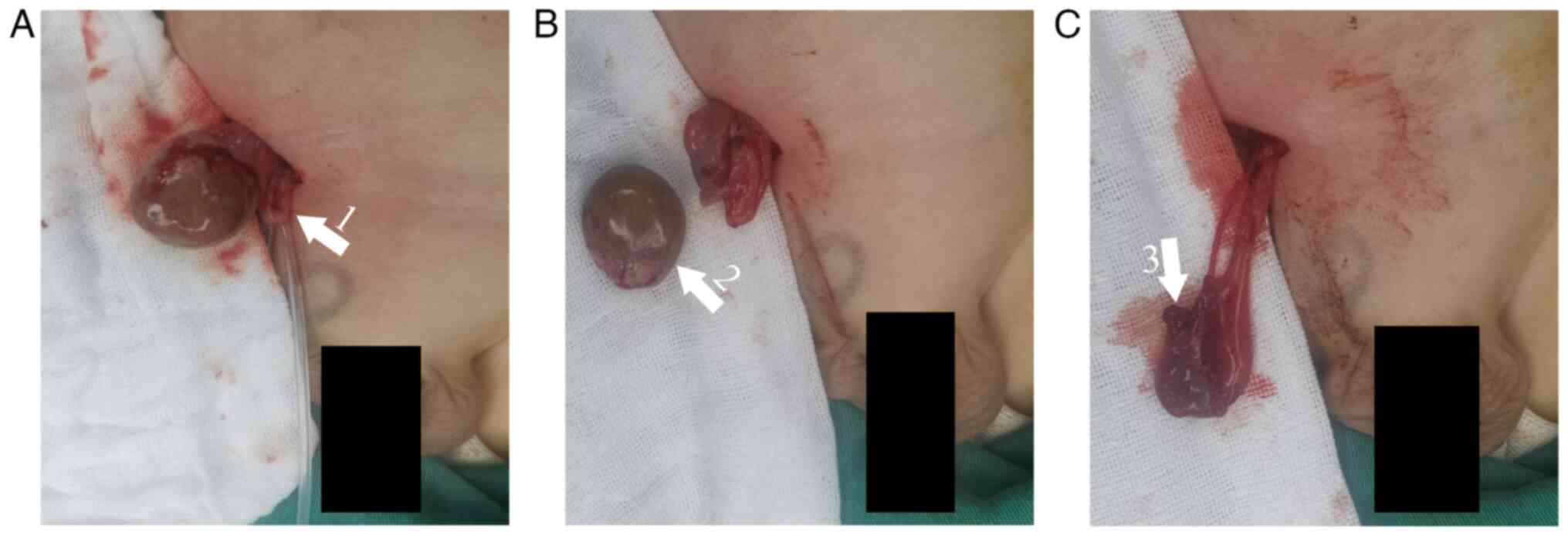
The testis was delivered through a right lower abdominal incision.
After incising the tunica albuginea, the mass was completely
excised (Fig. 2A and B). A portion of both peritumoral tissue
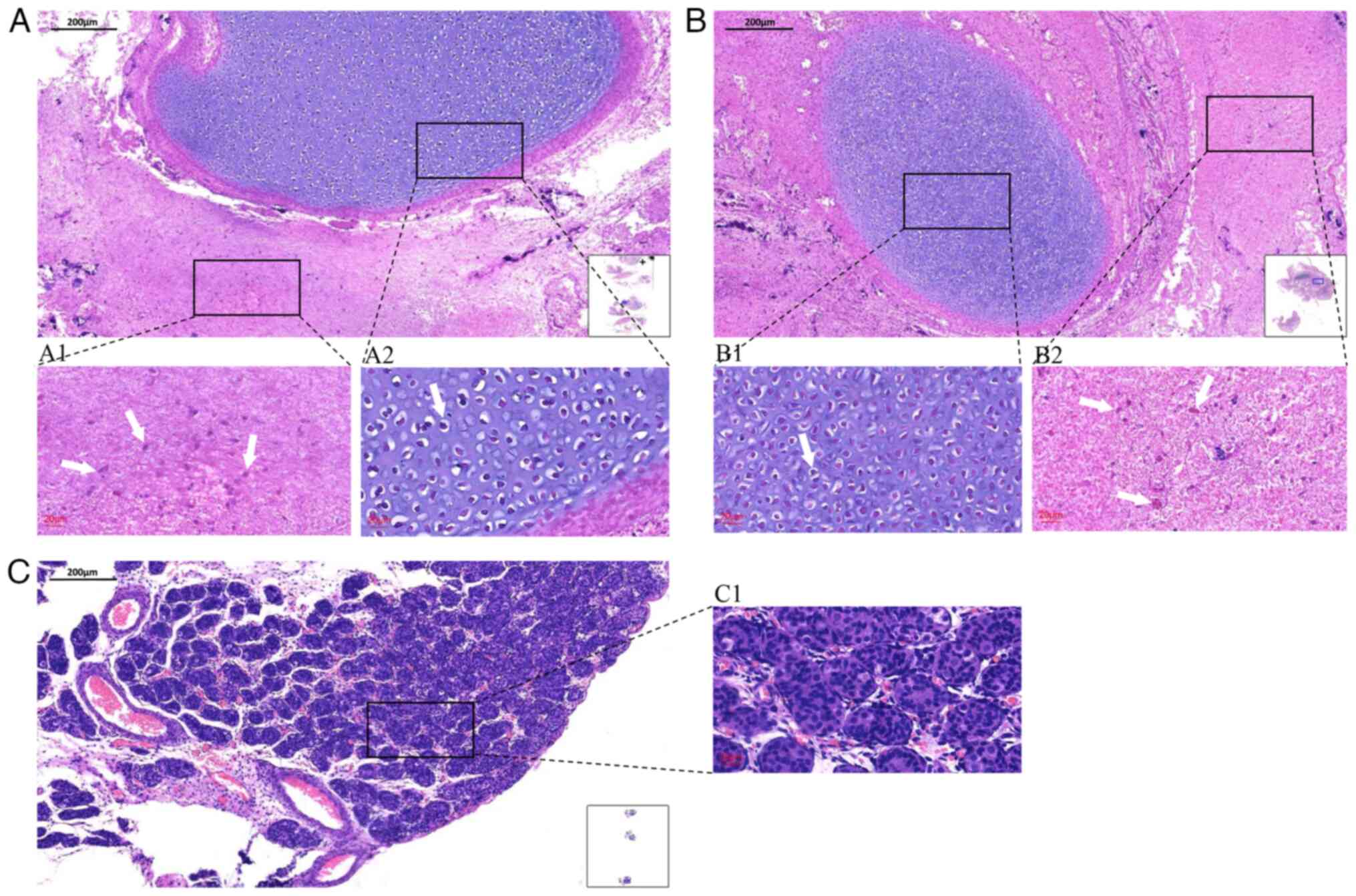
and the mass was sent for pathological examination. Intraoperative
frozen section analysis (5-µm-thick sections, cut at -25˚C, stained
with hematoxylin for 1 min and eosin for 15 sec at room
temperature, and examined by light microscopy) revealed mature
elements from all three germ layers, including abundant glial
tissue and cartilage (Fig. 3A).
Despite adequate mobilization of the spermatic cord, the testis
could only reach the mid-inguinal level. In accordance with the
family's request for testicular preservation and supported by a
temporary clamping test, which demonstrated well-sustained
perfusion without color change or signs of ischemia prior to
spermatic vessel ligation, a single-stage Fowler-Stephens
orchiopexy was performed (Fig.
2C). Final histopathology confirmed a mature teratoma
containing glial and cartilaginous components, without immature
elements or tumor infiltration in the adjacent tissue (Fig. 3B and C). At the 7 month follow-up, the infant
remained well without complications.
Discussion
Pediatric testicular tumors have two incidence
peaks. The first is before age 4, where one-third to half are
benign, advocating for testis-sparing surgery. The second is in
adolescents (15-18 years), where most germ cell tumors are
malignant; the most common type is mixed germ cell tumor, making
radical orchiectomy both diagnostic and therapeutic (6). Although teratomas account for ~10% of
all pediatric testicular tumors, their occurrence in undescended
testes is rare, especially when identified prenatally (7,8). The
management of intra-abdominal testicular tumors identified
postnatally in neonates generally follows Bolande's principle of
the ‘malignant transformation grace period’, which suggests tumors
originating in fetal or early neonatal life are often biologically
benign and may undergo spontaneous regression or
cytodifferentiation, typically before 6 months of age-reflecting a
transient period during which malignant transformation is
biologically restrained by the oncorepressive fetal milieu
(9,10). For mature teratomas, simple tumor
excision during the first month of life (or even antenatally) may
be sufficient without the need for radical orchiectomy.
Prenatal diagnosis of intra-abdominal testicular
tumors is typically achieved during routine fetal examinations
aimed at evaluating testicular position. Under normal embryological
development, the testes reach the area near the internal inguinal
ring by the fifth month of gestation and begin descending through
the inguinal canal by the seventh month. By the eighth month, they
typically reach the scrotum. If the penis is clearly visible but
the testes are not yet present in the scrotum, cryptorchidism
should be suspected (10-12).
This supports two potential mechanisms underlying the presence of
germ cell tumors in undescended testes during the prepubertal
period: i) A prenatal tumor that interferes with normal testicular
descent; and ii) inherent cryptorchidism that predisposes the
testis to malignant transformation (13-15).
Accordingly, some researchers recommend prenatal ultrasonographic
evaluation of fetal scrotal testes to improve diagnostic accuracy
(11).
When a prenatally detected calcified abdominal mass
appears distinct and clearly separated from the kidneys and spine,
the differential diagnoses should include neuroblastoma, teratoma,
meconium peritonitis, hamartoma and certain infectious masses
(11,16,17).
The concurrent presence of polyhydramnios, bowel dilatation and
ascites is highly suggestive of meconium peritonitis (18). Hamartomas typically present as
irregularly shaped masses, with sonographic characteristics that
vary depending on the predominant tissue component (such as liver,
lung and pancreas); they often present as heterogeneous, hypoechoic
or hyperechoic lesions, occasionally accompanied by ascites
(19).
Differentiating neuroblastoma from teratoma can be
challenging as both may present as solid tumors with or without
calcifications (20). Prenatally
diagnosed neuroblastoma is the most common malignant solid tumor in
the neonatal period; its typical prenatal ultrasonographic
appearance is cystic (53%), followed by hyperechoic (31%) and mixed
cystic-solid (16%) patterns (21).
Up to 93% of fetal neuroblastomas originate in the adrenal gland,
displacing the kidney inferiorly and laterally. Associated
complications include metastatic lesions, hepatomegaly, fetal
ascites and hydrops fetalis (22).
Teratomas typically exhibit a thick capsule, well-defined margins
and lack local invasion or distant metastasis (23). When diagnosis is uncertain,
magnetic resonance imaging can provide additional information.
Peripheral fat signals of the abdominal mass show high intensity on
both T1- and T2-weighted images, with the mass center appearing
hypointense on T1 and hyperintense on T2, indicating cystic and
solid components (11). Accurate
prenatal diagnosis facilitates timely referral to pediatric
surgeons, enabling timely detection and treatment of the tumor to
prevent complications such as torsion and malignant transformation
(13,24).
Postnatal ultrasonography, particularly
high-resolution sonography, offers superior visualization of
cryptorchid testes and delineates their anatomical structure;
therefore, re-evaluation immediately after birth is recommended.
Normal testicular parenchyma exhibits a uniformly homogeneous
echotexture, whereas testicular teratomas, similar to teratomas at
other sites, appear as complex solid or cystic lesions with
well-defined margins (25). Mature
teratomas are typically not associated with abnormal elevations in
tumor markers; AFP is predominantly secreted by yolk sac tumors and
certain embryonal carcinomas, whereas increases in β-human
chorionic gonadotropin and lactate dehydrogenase isoform 1 suggest
endodermal sinus tumors, yolk sac tumors, immature teratomas,
non-seminomatous germ cell tumors and choriocarcinomas (26). Ferritin levels may be
physiologically elevated in neonates, making it an unreliable tumor
marker (27). Although testicular
teratoma may display characteristic ultrasonographic features and
laboratory tests can imply the histological subtype, intraoperative
frozen section examination remains critical for guiding surgical
management. A previous study has shown that testis-sparing surgery
(TSS) guided by intraoperative frozen section analysis represents a
viable alternative to orchiectomy (28).
In previously reported cases of prenatally diagnosed
cryptorchidism with teratoma, mass excision was performed without
attempting to preserve the testis (Table I), resulting in a partial loss of
fertility potential (10,11,13,15,17,23-25).
Almekaty et al (29)
analyzed 42 post-pubertal male patients who had undergone
unilateral orchiectomy and found that 45.2% developed postoperative
azoospermia; contralateral testicular abnormalities were identified
as an independent predictor and the azoospermic group showed
significantly lower serum testosterone levels. Although the primary
pathology may have contributed to this elevated rate, the
detrimental effect of unilateral orchiectomy on fertility is well
recognized. Given the excellent prognosis of mature teratomas and
the uncertain developmental status of the contralateral testis in
neonates, maintaining reproductive and endocrine function while
ensuring complete tumor removal should be paramount (30,31).
Furthermore, TSS can mitigate negative psychological effects during
childhood development, which is essential for long-term mental
health and social adaptation (32).
 | Table ICases of prenatal diagnosis of
intra-abdominal cryptorchidism associated with teratoma. |
Table I
Cases of prenatal diagnosis of
intra-abdominal cryptorchidism associated with teratoma.
| First author,
year | GA at diagnosis,
weeks | Laterality | Prenatal US
characteristics at diagnosis | Size of mass at
diagnosis, cm | Primary prenatal
diagnosis | Mass size at delivery
(cm) | Palpable abdominal
mass | Surgical procedure
and timing | Postoperative
follow-up | (Refs.) |
|---|
| Mboyo et al,
1997 | 31 | Left | Cystic and solid mass
on left side of bladder, empty left scrotum | 2.5x2.3 | Fetal retroperitoneal
teratoma/cystic neuroblastoma | 4.3x5.8 | Mass palpable | Excision 13 days
postpartum, | Persistent Grade III
vesicoureteric reflux at 1 year | (25) |
| Shih et al,
1997 | 36 | Right | Semisolid mass with
central echogenic part in front of right fetal kidney and anterior
to the bladder, undescended right testis | 5x4x3 | Fetal retroperitoneal
tumor | 5x4 | Mass palpable | Excision by
laparoscopy at 1 month | Normal outcome at 1
year | (10) |
| Siu et al,
2001 | 30 | Right | Cystic
intra-abdominal lesion with solid component anterior to the right
kidney and superior to the bladder, empty scrotal sacs | 2.4 | Cystic teratoma in
undescended testis | 2.5x2.7x1.9 | No mass | Excision by
laparoscopy at 1 month | Normal
postoperative outcome | (24) |
| Pramanik et
al, 2011 | 27 | Right | Intra-abdominal
mass in right iliac fossa | 2.1x1.9 | Intra-abdominal
mass | Unchanged | Mass palpable | Excision by
laparotomy at 5 months | Normal
postoperative outcome | (11) |
| Janda et al,
2014 | 22 | Left | Mass adjacent to
the bladder, subsequent onset of calcification in mass, absence of
vascular flow | 1.0x1.2 | Intra-abdominal
mass | 1.8x1.3x1.4 | No mass | Excision by
laparotomy at 19 days postpartum | Normal
postoperative outcome | (13) |
| Youssef et
al, 2016 | 32 | Left | Single unilo-cular
anechoic cyst with regular boundary located between the left kidney
and urinary bladder in retroperitoneal position, scarcely
vascularized small intracystic solid component, empty ipsilateral
hemiscrotum | 2.0x2.0 x2.2 | Cystic teratoma in
undescended testis | 3 | No mass | Excision by
laparoscopy 3 days postpartum, | Normal outcome at 1
year | (23) |
| Arkar et al,
2016 | 36 | Left | The lesion, located
on the left lateral side of the urinary bladder, was round, cystic
with a solid component, and contained a few discrete
subcen-timeter-sized calcifications | 2.0x1.8 | Cryptorchid
testicular teratoma | 2.0x1.9 | No mass | Excision by
laparotomy 3 days postpartum | Normal
postoperative outcome | (17) |
| Yada et al,
2017 | 33 | Right | A mass in the right
lower quadrant | 3.0x2.0 | Intra-abdominal
mass | 3.0x2.0 | Mass palpable | Excision by
laparoscopy at 14 days postpartum | Normal outcome at 3
years | (15) |
| Present report,
2025 | 28 | Right | Single anechoic
area with thin wall and good sound transmission located in the
upper right bladder, showing irregular hyperechoic areas with small
punctate strong echoes within | 1.6x1.1 | Cryptorchid
testicular teratoma | 2.8x1.8x1.0 | No mass | Excision and
primary Fowler-Stephens orchidopexy at 1 month | Normal
postoperative outcome | - |
Although TSS aims to preserve endocrine and
reproductive function, the capacity of the residual tissue warrants
careful consideration. Most studies of radical orchiectomy report
postoperative reductions in serum testosterone or increased
compensated hypogonadism (33,34).
By contrast, reports show that small Leydig-cell remnants often
maintain normal testosterone, so clinically overt hypogonadism
after TSS is uncommon and overt testosterone deficiency is rare
(35,36). Spermatogenesis is more vulnerable
to the adverse effects of the testicular lesion; most patients
already have abnormal semen parameters preoperatively. In the
largest prospective series of benign lesions, oligo- and
asthenozoospermia were common before surgery but did not
significantly worsen postoperatively; however, radical orchiectomy
was frequently associated with measurable declines in semen quality
even without adjuvant therapy (37).
A potential limitation of the present study is the
relatively short 7 months follow-up period, which, although
adequate to detect early postoperative complications such as
testicular atrophy (typically apparent within 3 months) may not
completely exclude the possibility of late atrophy or tumor
recurrence, although these are rare in mature teratomas (38,39).
Nevertheless, the mid-term findings provide preliminary evidence
supporting the safety and feasibility of this testis-sparing
strategy. Long-term monitoring is ongoing and will be reported in
future updates.
In the present case, guided by the adequacy of
collateral circulation suggested on preoperative ultrasound and
confirmed by intraoperative clamping test, a single-stage
Fowler-Stephens orchiopexy was performed, although testicular
descent was only achievable to the mid-inguinal canal. Single-stage
Fowler-Stephens orchiopexy completes repair in a single operation,
avoiding a second general anesthetic and repeat-procedure risks,
shortening overall treatment time and limiting prolonged
intra-abdominal exposure of the testis, however, certain studies
report that, compared with two-stage FSO, single-stage procedures
generally have lower testicular survival/success rates and higher
atrophy rates (40,41). The present case highlights that
primary testicular tumors can be detected early, even in the late
fetal stage, and that TSS with selective removal of the teratoma is
a feasible option in infants. Intraoperative frozen section
analysis and histopathological examination of the peritumoral
tissue provided critical evidence supporting the safe application
of a testis-sparing procedure.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Chongqing Key
Project of Technology Innovation and Application (grant no.
2024TIAD-KPX0035) and the Joint Project of the Chongqing Health
Commission and Science and Technology Bureau (grant no.
2025ZDXM038).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HF, XL, SW, YH and GW analyzed clinical data,
performed the literature review, and drafted the manuscript. DZ
performed the surgical procedure. GP participated in data analysis.
HF and DZ were responsible for revising the manuscript. HF and DZ
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for the publication of all
clinical details and accompanying images was obtained from the
patient's legal guardian in accordance with institutional and
journal ethical guidelines.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hermann AL, L'Herminé-Coulomb A, Irtan S,
Audry G, Cardoen L, Brisse HJ, Vande Perre S and Pointe HDL:
Imaging of pediatric testicular and para-testicular tumors: A
pictural review. Cancers (Basel). 14(3180)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang K, Song J, Zhang Y, Chen X and Chao
M: Comparison of clinical characteristics of testicular tumor
between children and adult population: A retrospective analysis.
BMC Cancer. 24(1287)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grady RW, Ross JH and Kay R:
Epidemiological features of testicular teratoma in a prepubertal
population. J Urol. 158:1191–1192. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Maizlin II, Dellinger M, Gow KW, Goldin
AB, Goldfarb M, Nuchtern JG, Langer M, Vasudevan SA, Doski JJ,
Raval MV and Beierle EA: Testicular tumors in prepubescent
patients. J Pediatr Surg. 53:1748–1752. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kooij CD, Hulsker CCC, Kranendonk MEG,
Zsiros J, Littooij AS, Looijenga LHJ, Klijn AJ and
Mavinkurve-Groothuis AMC: Testis sparing surgery in pediatric
testicular tumors. Cancers (Basel). 12(2867)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Karmazyn B, Weatherly DL, Lehnert SJ, Cain
MP, Fan R, Jennings SG, Ouyang F and Kaefer M: Characteristics of
testicular tumors in prepubertal children (age 5-12 years). J
Pediatr Urol. 14:259.e1–259.e6. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Strader CH, Weiss NS, Daling JR, Karagas
MR and McKnight B: Cryptorchism, orchiopexy, and the risk of
testicular cancer. Am J Epidemiol. 127:1013–1018. 1988.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Isaacs H Jr: Perinatal (fetal and
neonatal) germ cell tumors. J Pediatr Surg. 39:1003–1013.
2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bolande RP: Models and concepts derived
from human teratogenesis and oncogenesis in early life. J Histochem
Cytochem. 32:878–884. 1984.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shih HH, Teng RJ, Yau KI, Lin HH, Hsieh FJ
and Chen CC: Mature teratoma arising from an intra-abdominal
undescended testis presenting as a fetal abdominal mass. Ultrasound
Obstet Gynecol. 10:209–211. 1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pramanik DD, Bhatnagar V, Subbarao KC,
Sharma MC, Agarwala S and Gupta AK: Antenatally detected mature
teratoma in an undescended testis. Eur J Pediatr Surg. 21:209–210.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Achiron R, Pinhas-Hamiel O, Zalel Y,
Rotstein Z and Lipitz S: Development of fetal male gender: Prenatal
sonographic measurement of the scrotum and evaluation of testicular
descent. Ultrasound Obstet Gynecol. 11:242–245. 1998.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Janda GM, Najdzionek JS, Kozielski R,
Greenfield SP and Williot PE: Early prenatal detection of an
intra-abdominal cryptorchid testicular teratoma. Urology.
83:214–216. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wood HM and Elder JS: Cryptorchidism and
testicular cancer: Separating fact from fiction. J Urol.
181:452–461. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yada K, Ishibashi H, Mori H and Shimada M:
Laparoscopic resection of prenatally detected intra-abdominal
testicular teratoma: Report of a neonatal case. J Pediatr Surg Case
Rep. 23:43–45. 2017.
|
|
16
|
Bajaber AO, Almarshad MA, Aldraihem AI and
Aljabr AA: Abdominopelvic tumors of infancy: A pictorial essay.
Pediatr Radiol. 55:437–458. 2025.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Arkar RR, Umap RA and Jadhav S: Prenatal
diagnosis of cryptorchid testicular teratoma. Indian J Radiol
Imaging. 26:67–69. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shinar S, Agrawal S, Ryu M, Van Mieghem T,
Daneman A, Ryan G, Zani A, Chiu P and Chitayat D: Fetal meconium
peritonitis-prenatal findings and postnatal outcome: A case series,
systematic review, and meta-analysis. Ultraschall Med. 43:194–203.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Varlas V, Neagu O, Moga A, Bălănescu R,
Bohiltea R, Vladareanu R and Balanescu L: Fetal pancreatic
hamartoma associated with hepatoblastoma-an unusual tumor
association. Diagnostics (Basel). 12(758)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kwasniewicz P, Wieczorek-Pastusiak J,
Romaniuk-Doroszewska A and Bekiesinska-Figatowska M: Congenital
tumors-magnetic resonance imaging findings with focus on rare
tumors. Cancers (Basel). 16(43)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Heling KS, Chaoui R, Hartung J, Kirchmair
F and Bollmann R: Prenatal diagnosis of congenital neuroblastoma.
Analysis of 4 cases and review of the literature. Fetal Diagn Ther.
14:47–52. 1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Acharya S, Jayabose S, Kogan SJ, Tugal O,
Beneck D, Leslie D and Slim M: Prenatally diagnosed neuroblastoma.
Cancer. 80:304–310. 1997.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Youssef A, Salsi G, Curti A, Bellussi F,
Elbarbary NA, Locatelli F, Lima M, Pilu G and Rizzo N: Prenatal
ultrasonographic features of mature cystic teratoma in undescended
testicle. Ultrasound Obstet Gynecol. 47:527–529. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Siu SS, Leung TN, Leung TY, Ng SW, Yeung
CK and Lau TK: Prenatal diagnosis of intra-abdominal mature
testicular teratoma. J Ultrasound Med. 20:1257–1260.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mboyo A, Foulet A, Hocine S, Cheve MT,
Plat M and Weil D: Teratoma in an undescended testis detected
prenatally. J Urol. 158:200–201. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lakhoo K: Neonatal teratomas. Early Hum
Dev. 86:643–647. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hisano T, Okada J, Tsuda K, Iwata S,
Saitoh S and Iwata O: Control variables of serum ferritin
concentrations in hospitalized newborn infants: An observational
study. Sci Rep. 13(8424)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Steiner H, Höltl L, Maneschg C, Berger AP,
Rogatsch H, Bartsch G and Hobisch A: Frozen section analysis-guided
organ-sparing approach in testicular tumors: Technique,
feasibility, and long-term results. Urology. 62:508–513.
2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Almekaty K, Zahran MH, Eid A, Ralph D and
Rashed A: Azoospermia and sperm retrieval in post-pubertal
testicular torsion; benefits and limitations. Urology. 171:121–126.
2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Templeman CL, Hertweck SP, Scheetz JP,
Perlman SE and Fallat ME: The management of mature cystic teratomas
in children and adolescents: A retrospective analysis. Hum Reprod.
15:2669–2672. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Miao X, Li Y, Zhou T and Lv M:
Testis-sparing surgery in children with testicular tumors: A
systematic review and meta-analysis. Asian J Surg. 44:1503–1509.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rodriguez-Wallberg KA, Ahlgren J, Smedby
KE, Gorman JR, Hellman K, Henriksson R, Ståhl O, Wettergren L and
Lampic C: Prevalence and predictors for fertility-related distress
among 1010 young adults 1.5 years following cancer
diagnosis-results from the population-based Fex-Can Cohort study.
Acta Oncol. 62:1599–1606. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Flores Martinez J, Ljubetic BM, Liso N and
Mulhall JP: (107) Identifying predictors of low testosterone levels
in men with testicular cancer after radical orchiectomy. J Sex Med.
21(qdae167.105)2024.
|
|
34
|
Kim GY, Conduit C, O'Haire S, Chong CY,
Baenziger O, Lewin J, Thomas B, Lawrentschuk N, Stockler MR, Olver
I, et al: Association between low total serum testosterone and body
mass index in Australian survivors of testicular cancer: A
retrospective analysis. Basic Clin Androl. 34(14)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Giannarini G, Dieckmann KP, Albers P,
Heidenreich A and Pizzocaro G: Organ-sparing surgery for adult
testicular tumours: A systematic review of the literature. Eur
Urol. 57:780–790. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ory J, Blankstein U, Gonzalez DC, Sathe
AA, White JT, Delgado C, Reynolds J, Jarvi K and Ramasamy R:
Outcomes of organ-sparing surgery for adult testicular tumors: A
systematic review of the literature. BJUI Compass. 2:306–321.
2021.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Pozza C, Pofi R, Tenuta M, Tarsitano MG,
Sbardella E, Fattorini G, Cantisani V, Lenzi A, Isidori AM and
Gianfrilli D: TESTIS UNIT. Clinical presentation, management and
follow-up of 83 patients with Leydig cell tumors of the testis: A
prospective case-cohort study. Hum Reprod. 34:1389–1403.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yu C, Long C, Wei Y, Tang X, Liu B, Shen
L, Dong X, Lin T, He D, Wu S and Wei G: Evaluation of
Fowler-Stephens orchiopexy for high-level intra-abdominal
cryptorchidism: A systematic review and meta-analysis. Int J Surg.
60:74–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhou G, Sun F, Yu X, Huang R, Liu X,
Ouyang Y, Yang Z and Li S: Clinical characteristics and long-term
management of prepubertal testicular teratomas: A retrospective,
multicenter study. Eur J Pediatr. 182:1823–1828. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pakkasjärvi N and Taskinen S: Surgical
treatment of cryptorchidism: Current insights and future
directions. Front Endocrinol (Lausanne). 15(1327957)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fung ACH, Tsang JTW, Leung L, Chan IHY and
Wong KKY: Comparative outcomes of single-stage versus two-stage
laparoscopic fowler-stephens orchidopexy: A systematic review snd
meta-analysis. Eur J Pediatr Surg. 35:28–35. 2025.PubMed/NCBI View Article : Google Scholar
|