Introduction
Calcifying fibrous tumour (CFT) is a very rare,
non-cancerous lesion that can develop in various body parts
(1). Its key microscopic features
are spindle-shaped cells, collagen, chronic inflammation and
distinctive dystrophic calcification (2). Despite its benign nature, CFT can
grow in an invasive manner and appear as a poorly defined mass on
scans. This makes it difficult to tell apart from other tumours,
often leading to misdiagnosis and unnecessary treatment.
Diagnosing CFT is challenging for two main reasons.
First, on imaging like CT scans, it typically shows a mass with
coarse calcifications. On MRI, it often appears dark on both T1-
and T2-weighted images due to its fibrous content (3,4).
However, these features are not unique and overlap with other
conditions, including cancers. Second, even a tissue biopsy can be
misleading if it doesn't sample the characteristic calcified areas,
potentially leading to a misdiagnosis of a more aggressive
tumour.
Due to its rarity, reported cases of CFT are limited
worldwide. While they have been found in numerous locations, this
report presents, to the best of our knowledge, the first documented
case of a CFT located in the diaphragm of a living patient.
Previous reports of diaphragmatic involvement have been
exceptionally scarce and just identified post-mortem (5).
This unique case describes the patient's journey,
from clinical presentation and imaging to pathological findings,
treatment and outcome. By detailing this process and comparing it
with existing literature, we aim to improve the recognition of this
rare tumour and help guide its diagnosis and management, ultimately
preventing overtreatment.
Case report
A 45-year-old immunocompetent female patient
presented with a pulmonary nodule that was incidentally detected
during routine health screening 2 months prior to admission (the
examination was conducted in an external hospital and no report
could be retrieved). The asymptomatic patient denied any
respiratory symptoms, including cough, sputum production,
haemoptysis, dyspnoea or systemic manifestations, such as fever,
night sweats or weight loss. Initial surveillance over a two-month
period at a referring institution demonstrated that the left lower
lobe nodule slightly increased in size from 1.6 to 1.7 cm in
maximal diameter on serial computed tomography (CT) imaging
(baseline images unavailable for comparison). No therapeutic
interventions were initiated during the observation period. For
further evaluation, contrast-enhanced chest CT was performed in
July 2024, at the outpatient clinic of Taihe Hospital (Shiyan,
China) and revealed a well-circumscribed solid pulmonary nodule
(1.7x1.3 cm) in the anteromedial basal segment of the left lower
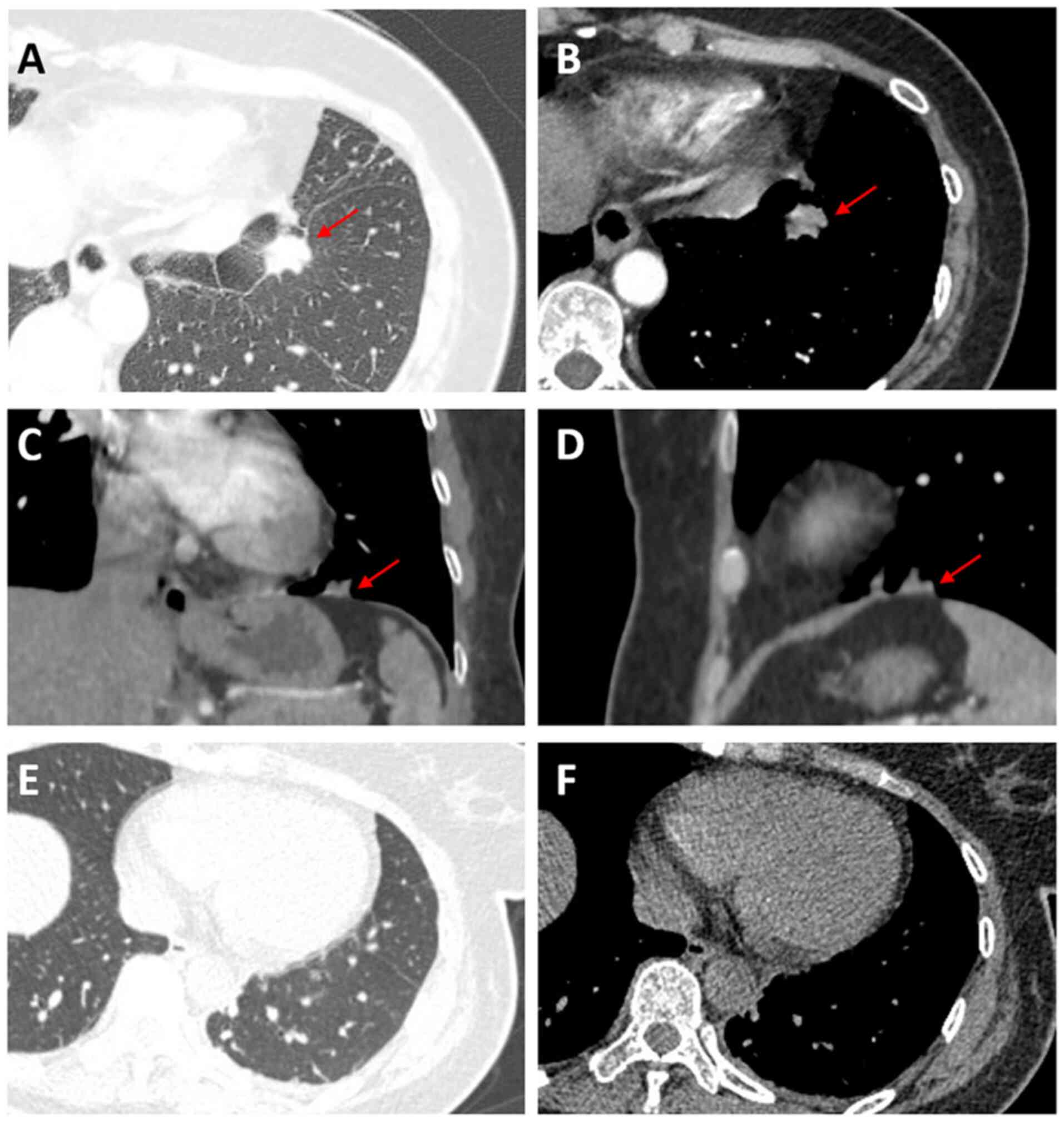
lobe with focal pleural thickening and retraction (Fig. 1A and B). Physical examination was within normal
ranges. It revealed a body temperature of 36.6˚C (normal range,
36.0-37.2˚C), a pulse of 90 bpm (normal range, 60-100 bpm), a
respiratory rate of 20 bpm (normal range, 12-20 breaths/min), a
blood pressure of 122/87 mmHg (normal range, systolic 120-129 mmHg
and diastolic 80-84 mmHg), a pulse oxygen saturation of 98% (normal
range, ≥95%, without oxygen supplementation), clear breath sounds
in both lungs, no dry or wet rales and no positive physical signs
of other systems such as heart, abdomen, etc. Laboratory tests
after admission revealed a white blood cell count of
5.66x109/l (normal range, 4-10x109/l), a red
blood cell count of 5.54x10¹²/l (normal range, 4.3-5.8x10¹²/l), a
haemoglobin concentration of 109 g/l (normal range, 130-175 g/l)
and a platelet count of 209x109/l (normal range,
125-350x109/l). The erythrocyte sedimentation rate, IL-6
level, procalcitonin level, cardiac troponin I level, N-terminal
pro-brain natriuretic peptide level, coagulation profile, liver
function, renal function, electrolytes and thyroid function were
all within normal ranges, and respiratory tumour markers and
tuberculosis IgG antibodies were negative. Pulmonary function
tests, electrocardiogram, cardiac ultrasound and lower limb venous
ultrasound showed no abnormalities. Fiberoptic bronchoscopy
revealed inflammatory changes in the bronchi without neoplastic
lesions. Bronchoalveolar lavage fluid smears revealed no acid-fast
bacilli but a small number of Gram-positive cocci. A CT-guided lung
biopsy planned for two days after admission was cancelled due to
the high risk associated with the lesion's proximity to the heart.
A multidisciplinary consultation on four days after admission
concluded that compared to two months ago, the pulmonary nodule had
increased in size and exhibited imaging features such as
lobulation, fine spiculation and pleural retraction (Fig. 1A and B). Three-dimensional (3D) reconstruction
(Fig. 1C and D) demonstrated extensive connectivity
between the lesion base and the diaphragm, raising suspicion of
malignancy. Owing to the high risk of CT-guided percutaneous
fine-needle aspiration biopsy, surgical biopsy was recommended.
Preoperative evaluations, including non-contrast brain CT, adrenal
ultrasound, carotid ultrasound and coronary CT angiography,
revealed no abnormalities; abdominal ultrasound showed gallbladder
wall thickening. On the eighth day after admission, the patient was
administered a prophylactic antibiotic regimen of intravenous
cefradine (1 g, BID) at a rate of 40 drops per minute for two days
to prevent postoperative infection. On the subsequent day,
video-assisted thoracoscopic surgery was performed. Intraoperative
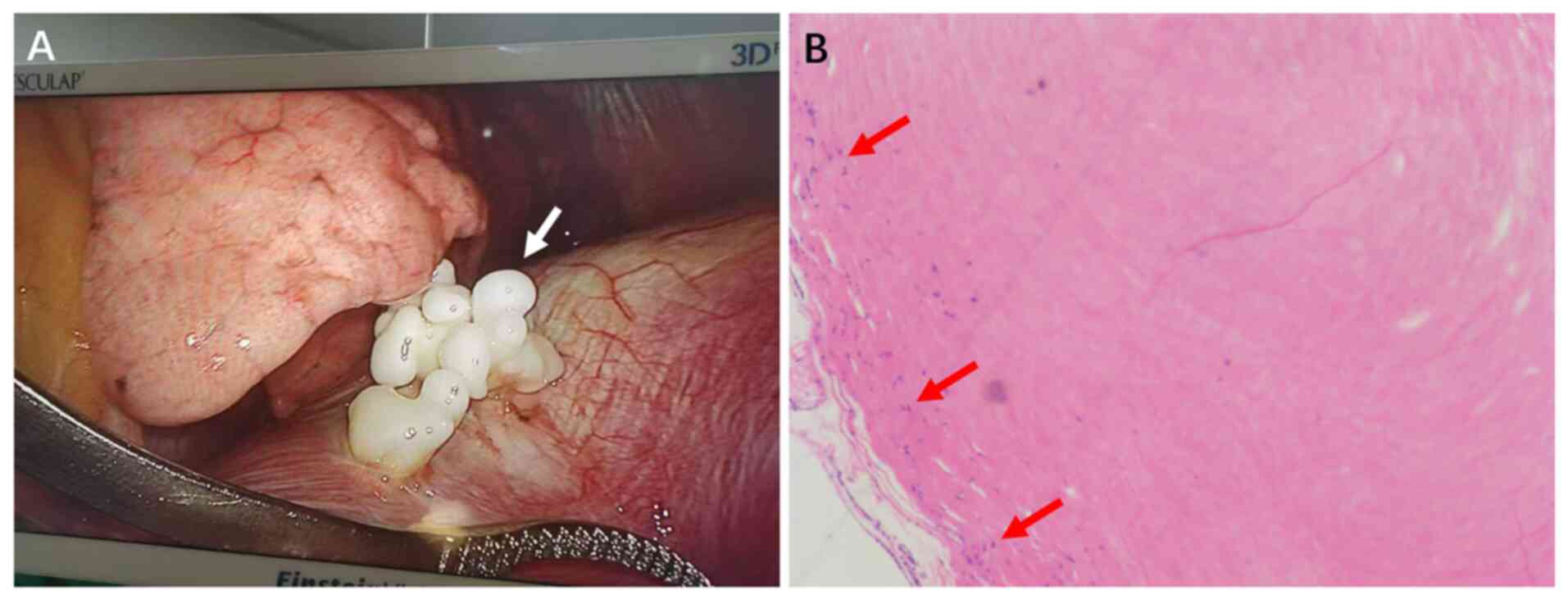
findings included multiple white, firm, coral-like nodules observed
on the diaphragmatic pleural surface without pleural effusion or
adhesions. The largest nodule was approximately 2 cm (Fig. 2A). Biopsy samples were fixed in 10%
neutral buffered formalin at room temperature for 4-6 h, dehydrated
using an increasing alcohol series, embedded in paraffin and
sectioned into 3-µm slices. Haematoxylin and eosin staining was
performed at room temperature for 40 min and sections imaged using
a CX31 light microscope (Olympus Corp.). Pathological examination
(Fig. 2B) of the diaphragmatic
nodule revealed fibrous tissue hyperplasia with hyaline
degeneration. Biopsy samples were fixed in 10% neutral buffered
formalin at room temperature for 4-6 h, dehydrated using an
increasing alcohol series, embedded in paraffin and sectioned into
3-µm slices. Hematoxylin and eosin staining was performed at room
temperature for 40 min and sections were imaged using a CX31 light
microscope (Olympus Corp.).
Postoperative chest CT performed one day
postoperatively revealed postresection changes in the left
diaphragm (Fig. 1E and F). Attempted supplemental
immunohistochemistry (IHC) studies on the specimen from the first
surgery were non-diagnostic due to tissue detachment artefacts. The
patient was discharged on the second day after surgery, and the
patient underwent two serial follow-up CT scans of the chest
performed at six-month intervals, both of which demonstrated no
evidence of recurrence. Subsequent follow-up with annual CT
surveillance is planned.
Literature review
Only PubMed (https://pubmed.ncbi.nlm.nih.gov/) was used for the
literature search and 200 case reports (199 in English and 1 in
French) published between 1946 and 2024 were manually searched,
using ‘calcifying fibrous tumour’ and ‘calcifying fibrous
pseudotumour’ as search terms. Multiple reports have reported more
than one patient, resulting in a total of 215 cases being included.
In the search process, review articles that only conducted
retrospective analyses and duplicate reports were excluded, and
only the first published cases with sufficient case descriptions
were included for analysis (Fig.
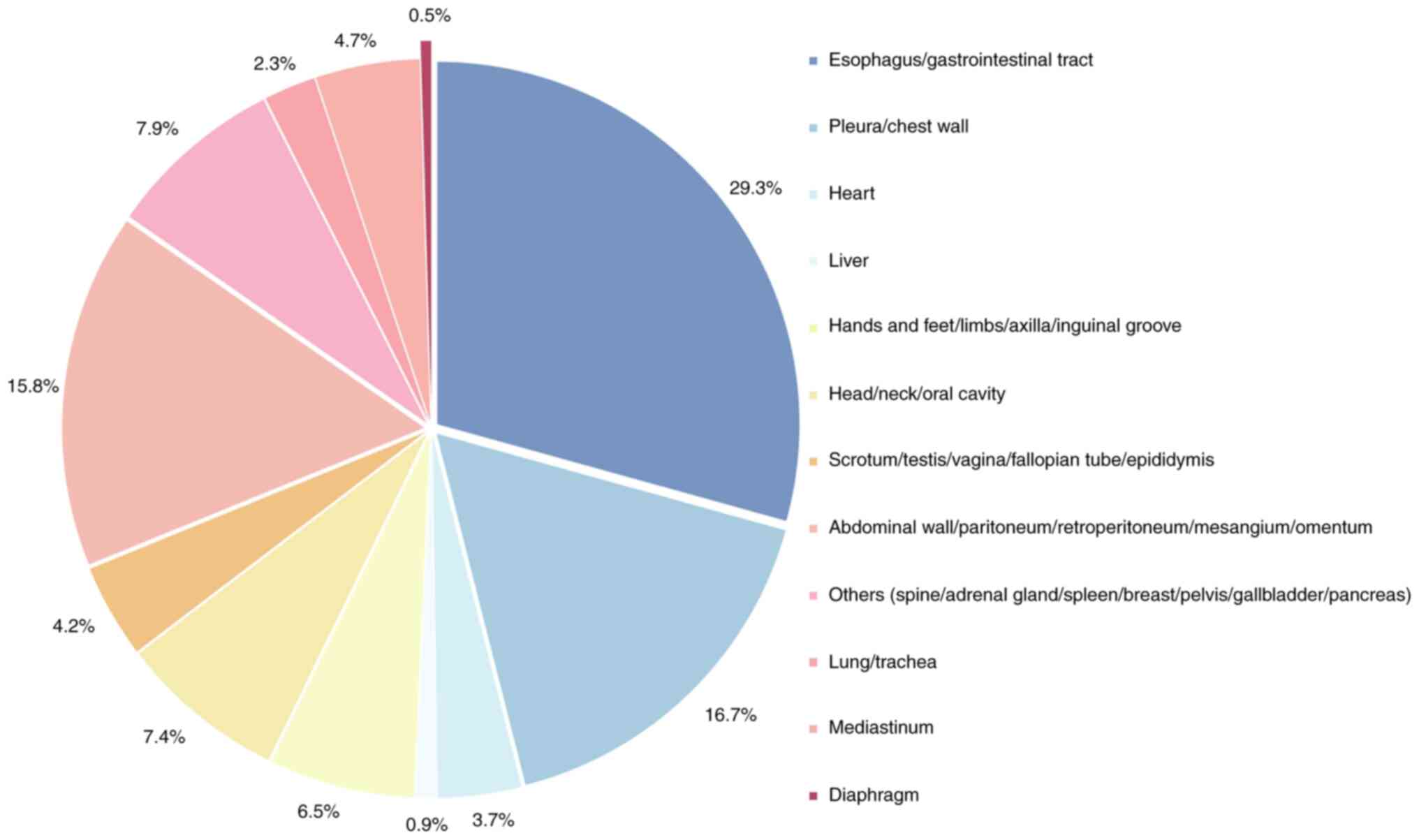
3). CFT can arise in multiple anatomical sites, most frequently
the gastrointestinal tract and pleura, and less commonly the liver
or reproductive tract. According to statistical data, only one case
of a solitary CFT localized to the diaphragm has been documented,
highlighting its exceptional rarity in clinical practice. For
statistical purposes, only the most characteristic disease location
was recorded in instances of multifocal involvement (excluding the
current case). To better characterize chest calcifying fibrous
tumour (CFT), seven published cases with the closest clinical
association were analysed to enable comparison (Table I) (6-9).
Regarding chest CFT, most patients present with ipsilateral chest
pain or are incidentally diagnosed. Chest CT typically shows
nodules with punctate enhancement at the margins, and the nodules
generally appear as milky-white, lobulated solid masses. These
features are highly consistent with those observed in the present
case. However, unlike most chest CFTs, pathological examination in
this case revealed fibrous tissue and inflammatory cell
infiltration but no typical calcified bodies. Literature review
revealed only one previously reported case of primary diaphragmatic
CFT, which was incidentally discovered during autopsy following
traumatic death (5). To the best
of our knowledge, the current study presents the first documented
case of primary diaphragmatic CFT successfully managed with
complete surgical resection (R0). Notably, the morphological
characteristics of the gross specimen of the present case are
highly consistent with those of CFT reported by Mylapalli et
al (5) and Kashizaki et
al (10,11), suggesting that the appearance of
this characteristic gross specimen may become an important basis
for the diagnosis of CFT.
 | Table ISummary of the characteristics of
seven previously reported cases of calcifying fibrous tumours that
are most similar to the present case. |
Table I
Summary of the characteristics of
seven previously reported cases of calcifying fibrous tumours that
are most similar to the present case.
| Author/year | Age, years/sex | Symptoms | Location | Chest CT/X-ray | Gross appearance | Pathological
features | Therapy | Prognosis | (Refs.) |
|---|
| Pinkard et al,
1996 | 23, F | Chest pain | Pleural | Multiple
pleural-based masses with central areas of increased
attenuation | Well-demarcated,
unencapsulated, lobulated masses; 1.5-12.5 cm | The calcifications
had a laminated appearance typical of psammoma bodies | Surgical
excision | NED | (9) |
| Pinkard et al,
1996 | 28, F | Incidental
finding | Pleural | Heterogeneous
extra-parenchymal pleural-based mass; central area of increased
attenuation | Smooth, lobulated
firm, whorled mass; 6.5 and 1.2 cm in maximum diameter | The calcifications
had a laminated appearance typical of psammoma bodies | Surgical
excision | NED | (9) |
| Pinkard et al,
1996 | 34, M | Chest pain | Pleural | Heterogeneous
extra-parenchymal pleural-based mass in left anteroinferior
cardio-phrenic angle; central area of increased attenuation | Attached by a short
pedicle to parietal pleura; 4.0 cm | The calcifications
had a laminated appearance typical of psammoma bodies | Surgical
excision | NA | (9) |
| Mylapalli et
al, 2024 | 48, M | Incidental
finding | Diaphragm | NA | Nodule-papillary
pearly white coral-like outgrowth; 2.0 cm in maximum diameter | Dense hyalinized
fibrocollagenous bundles with perivascular foci of scanty
lymphoplas-macytic infiltration | NA | NA | (5) |
| Mito et al,
2005 | 54, M | Incidental
finding | Pleural | Several nodular
lesions, no calcifications were detected | Solid, white, fibrous
matrix; 1.0x1.5x0.8 cm, 0.5x0.5x0.5 cm | Bland spindle cells,
scant lymphoplasmacytic infiltrate and small calcification | Surgical
excision | NED | (8) |
| Jang et al,
2004 | 31, F | Incidental
finding | Pleural | Well demarcated
calcifying mass | Smooth and lobulated;
8x7x5 cm | Psammomatous
calcifi-cations and areas of inflammatory infiltrate | Surgical
excision | NED | (7) |
| Jia et al,
2021 | 38, M | Chest pain | Pleural | Subpleural mass
with dystrophic calcification | Firm, pearly white
masses; 5.0 cm in maximum diameter | Inflammatory
lymphocytic infiltration, minute psammomatous calcifications | Incomplete
resection | NA | (6) |
| Guo et al,
2025 | 45, F | Incidental
finding | Diaphragm | Lobulated solid
nodule | Pearl-white,
lobulated mass with coral-like nodularity | Hyalinized collagen
bundles with sparse lymphocytic infiltration | Surgical
excision | NED | Present case |
Discussion
CFT represents a rare benign fibroblastic
proliferation (12,13); it was initially described by
Rosenthal and Abdul-Karim (13)
1988 as a ‘calcifying fibrous tumour of childhood’ on the basis of
paediatric cases. A subsequent clinicopathological analysis of 10
cases by Fetsch et al (14)
in 1993 led to the revised nomenclature ‘calcifying fibrous
pseudotumor’. In 2013, the World Health Organization Classification
of Tumours of Soft Tissue and Bone formally defined CFT as a rare
benign fibroblastic disorder (15).
According to earlier literature, the predilection
sites of CFT were predominantly concentrated in soft tissues of the
extremities, trunk and head/neck regions. However, with an
increasing number of case reports, it is now evident that CFT can
arise in virtually any anatomical location (12). The most frequent sites include the
gastrointestinal tract, pleura, peritoneum and mesentery, which
aligns with the trends summarized in the present study. A
literature review by Chorti et al (1) reported a broad age range for CFT
onset (1-85 years), with a slight female predominance.
CFT typically lacks distinctive clinical symptoms.
However, when critical organs such as the lungs, spleen or pancreas
are involved, it may not only induce compressive symptoms but also
lead to functional impairments of the affected organs (16).
On CT images, thoracic CFT typically manifests as
well-circumscribed solitary masses or multiple solid soft-tissue
lesions. The lesions demonstrate either broad-based sessile
attachment or pedunculated growth to the chest wall, exhibiting
characteristic punctate to amorphous calcifications with a
predominantly peripheral distribution (3,4).
Enhanced CT revealed mild heterogeneous enhancement with preserved
homogeneity of the non-enhancing tumour parenchyma and no
radiologic evidence of peritumoral infiltration (3,17).
Magnetic resonance imaging findings of CFT lack
specificity, generally presenting as masses with isointensity on
T1-weighted (T1W) sequences and hypointensity on T2W sequences,
with calcified regions showing marked signal voids. However, few
reports describe CFT cases lacking detectable calcifications,
suggesting potential heterogeneity in imaging manifestations
(18). When CFT involves the
diaphragm or pleura, tumour margins are frequently obscured by
adhesions and traction to adjacent pleural tissues, posing
challenges for radiological diagnosis and surgical planning. In the
present case, 3D reconstruction was utilized to improve the
anatomical assessment of tumour-adjacent tissue relationships. 3D
imaging demonstrated extensive connectivity between the tumour base
and the diaphragm, offering vital anatomical guidance for precise
intraoperative resection. However, since this technology increases
costs without proven prognostic benefits, it should remain a
selective rather than standard preoperative tool.
CFT demonstrates distinct histopathological
features: Solid, firm, lobulated masses with well-defined margins;
smooth surfaces lacking encapsulation; and homogeneous grey-white
fibrous cut surfaces devoid of haemorrhage or necrosis (17,19).
Microscopically, spindle cells embedded in abundant collagenous
stroma are characteristic and accompanied by dystrophic
calcifications or psammoma bodies, with scattered lymphoplasmacytic
infiltrates in the interstitium (1,2). IHC
aids in differential diagnosis. Reported IHC profiles show diffuse
positivity for vimentin and focal CD34 expression in spindle cells,
whereas desmin, S-100 protein, STAT6 and anaplastic lymphoma kinase
(ALK) are typically negative (7,20).
In the current case, the gross specimen presented as white, hard,
coral-like nodules. Microscopic examination revealed prominent
fibrosis and scattered lymphocytes, although typical calcified
nodules were absent, which is potentially attributable to sampling
limitations in pathological sectioning or indication of early
disease progression. Despite technical challenges (such as the
tissue detachment in paraffin sections compromising IHC
interpretability), the diagnosis of CFT was confirmed through an
integrative analysis of morphological, pathological and
radiological features, consistent with the diagnostic frameworks
established by Mylapalli et al (5) and Kashizaki et al (5,10).
CFT generally has a favourable prognosis and the
literature seldom emphasizes the necessity of differentiating it
from malignant neoplasms. However, in the present case, the
thoracic CFT presented on axial CT as a solid nodule with
spiculated margins, lobulation and suspected pleural retraction.
Compared with prior imaging, the lesion demonstrated slight
interval growth, sharing overlapping radiological features with
those of primary pulmonary malignancies. Thus, distinguishing
thoracic CFT, particularly subpleural lesions, from primary lung
malignancies is imperative in imaging interpretation.
Beyond primary lung cancer, CFT must be
differentiated from the following thoracic fibrous tumours: i)
Solitary fibrous tumour (SFT), typically arising from the pleura,
appears on CT as a solitary, well-demarcated, hypervascular
soft-tissue mass with homogeneous density. Larger SFTs may exhibit
hypodense or heterogeneous areas because of necrosis or
haemorrhage, facilitating differentiation from CFT. On IHC, STAT6
expression serves as the most specific and sensitive diagnostic
marker for SFT (21), whereas CFT
is STAT6-negative. ii) Inflammatory myofibroblastic tumour (IMT):
IMT often manifests on thoracic CT as a solitary, well-defined
lobulated mass, predominantly in the lower lung lobes, mimicking
CFT. However, IMT typically displays heterogeneous attenuation and
enhancement, with frequent ALK positivity on IHC, a feature rarely
observed in CFT (22,23). iii) Desmoid tumours: Desmoid
tumours on CT usually present as round or oval dense shadows with
minimal enhancement. Key discriminators include their infiltrative
growth pattern, ill-defined margins and high postoperative
recurrence rates. Definitive diagnosis relies on β-catenin
positivity via IHC (24).
With respect to management, individualized treatment
decisions are made on the basis of clinical evaluation. Given the
indolent growth of CFT and its potential for local compressive
effects, surgical resection is recommended for surgically eligible
young patients (clinical judgment) (25). Upon review of prior cases, CFT
demonstrates an unequivocally benign biological behaviour, with low
recurrence rates after complete excision, no evidence of malignant
transformation during long-term follow-up and a 100% survival rate
(1). For asymptomatic patients
with CFT with a high surgical risk (such as advanced age or severe
cardiopulmonary dysfunction), active surveillance rather than
intervention is advised. As reported in the present study, a
45-year-old female patient underwent thoracoscopic resection for a
solitary thoracic mass and was ultimately diagnosed with CFT.
Surveillance over one year has demonstrated no evidence of
recurrence. The present case underscores the clinical importance of
recognizing CFT to avoid misdiagnosis, inappropriate management and
related complications.
The precise aetiology and pathogenesis of CFT remain
elusive. It has been hypothesized that CFT may represent a late
sclerotic phase of IMT (23).
Another study has suggested associations with pathogenic mutations
in the ZN717, FRG1 and CDC27 genes, leading to copy number
variations in chromosomes 6 and 8 and altered coding regions.
However, the underlying mechanisms require further investigation
(6).
In summary, CFT is a benign tumour characterized by
a slow growth tendency and easy misdiagnosis as other pulmonary
nodules. Macroscopically, it typically presents as a white, firm,
coral-like nodule. Radiological findings reveal a
well-circumscribed, homogeneous mass with punctate calcifications.
The identification of characteristic calcified nodules through IHC
analysis serves as the gold standard for definitive diagnosis.
Surgical resection represents the primary intervention and is
associated with a favourable prognosis and a low rate of
recurrence. Despite its rarity, CFT warrants consideration in the
routine evaluation of pulmonary nodules because of its distinct
clinical implications. As a benign neoplasm with indolent
progression, CFT is frequently misclassified as granulomatous
inflammation or malignancy on initial assessment, a diagnostic
pitfall that may lead to overtreatment and substantial patient
distress. Therefore, more evidence from accumulating case reports
could aid in refining diagnostic and treatment approaches. When
thoracic CT reveals homogeneous nodules with peripheral punctate or
amorphous calcifications, CFT should be suspected. Through
comprehensive case analyses and a literature review, the present
study aimed to enhance the understanding of CFT and promote
diagnostic and therapeutic progress, thereby facilitating accurate
identification and more appropriate clinical management for these
patients. Since the present literature search only used PubMed, it
is expected that future studies will provide reviews with more
complete evidence in this area.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Innovation Development
Joint Fund of Hubei Provincial Science Foundation (grant no.
2025AFD181).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SG conceptualized the study and wrote the original
manuscript. LY, GC and YT searched the literature and obtained
case-related data. CX and WH analysed the data and relevant
literature. MW and HW edited the final draft and assisted with
language translation. JG provided surgical specimens. JG and MW
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Taihe Hospital (Shiyan, China; approval no. 2025KS127) and was
performed in accordance with the principles of good clinical
practice following the Tri-Council guidelines. Informed consent for
both the study and publication was obtained from the patient.
Patient consent for publication
The patient provided written informed consent for
the publication of case information and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chorti A, Papavramidis TS and
Michalopoulos A: Calcifying fibrous tumor: Review of 157 patients
reported in international literature. Medicine (Baltimore).
95(e3690)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang L, Wei JG, Fang SG, Luo RK, Xu ZG,
Li DJ and Kong LF: Calcifying fibrous tumor: A clinicopathological
analysis of 32 cases. Zhonghua Bing Li Xue Za Zhi. 49:129–133.
2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
3
|
Yu C, Wen X and Sun M: CT and MRI features
of calcified fibrous tumor: A case report. Asian J Surg.
48:872–873. 2025.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miyashita S, Ryu Y, Takata H, Asaumi Y,
Sakatoku M, Seike T, Okamura T, Inamura K, Kawai H, Okuno N and
Terahata S: Imaging findings of gastric calcifying fibrous tumour.
Bjr Case Rep. 2(20160064)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mylapalli JL, Jangir H, Sharma M,
Prabhakar V, Sandhu PR, Subramanian A, Barwad A and Lalwani S:
Post-mortem detection of a calcifying fibrous pseudotumor at a rare
site-a case report. Indian J Pathol Microbiol. 67:669–671.
2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jia B, Zhao G, Zhang ZF and Sun BS:
Multiple calcifying fibrous tumor of the pleura: A case report.
Thoracic Cancer. 12:2271–2274. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jang KS, Oh YH, Han HX, Chon SH, Chung WS,
Park CK and Paik SS: Calcifying fibrous pseudotumor of the pleura.
Ann Thorac Surg. 78:e87–e88. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mito K, Kashima K, Daa T, Kondoh Y, Miura
T, Kawahara K, Nakayama I and Yokoyama S: Multiple calcifying
fibrous tumors of the pleura. Virchows Arch. 446:78–81.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pinkard NB, Wilson RW, Lawless N, Dodd LG,
McAdams HP, Koss MN and Travis WD: Calcifying fibrous pseudotumor
of pleura. A report of three cases of a newly described entity
involving the pleura. Am J Clin Pathol. 105:189–194.
1996.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kashizaki F, Kano T, Kameda Y and Osawa H:
Calcifying fibrous tumors resembling coral. Intern Med.
63:3111–3112. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takabatake K, Arita T, Kuriu Y, Shimizu H,
Kiuchi J, Takaki W, Konishi H, Yamamoto Y, Morimura R, Shiozaki A,
et al: Calcifying fibrous tumor of the ileum resected by
single-port laparoscopic surgery: A case report. Surg Case Rep.
8(64)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Larson BK and Dhall D: Calcifying fibrous
tumor of the gastrointestinal tract. Arch Pathol Lab Med.
139:943–947. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rosenthal NS and Abdul-Karim FW: Childhood
fibrous tumor with psammoma bodies. Clinicopathologic features in
two cases. Arch Pathol Lab Med. 112:798–800. 1988.PubMed/NCBI
|
|
14
|
Fetsch JF, Montgomery EA and Meis JM:
Calcifying fibrous pseudotumor. Am J Surg Pathol. 17:502–508.
1993.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris C: Pathology and Genetics of Tumours of the Lung,
Pleura, Thymus and Heart. In: International Agency for Research on
Cancer. IARC Press, Lyon, 2007.
|
|
16
|
Abd Kahar NN and Al-Mukhtar A: Calcifying
fibrous tumour-a rare cause of anaemia. J Surg Case Rep.
2021(rjaa573)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shibata K, Yuki D and Sakata K: Multiple
calcifying fibrous pseudotumors disseminated in the pleura. Ann
Thorac Surg. 85:e3–e5. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee HJ, Jeong JS and Lee YC: Calcifying
fibrous tumour of the pleura without gross calcification. Respirol
Case Rep. 12(e01365)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Suh JH, Shin OR and Kim YH: Multiple
calcifying fibrous pseudotumor of the pleura. J Thorac Oncol.
3:1356–1358. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lisowska H, Marciniak M, Cianciara J and
Pawełczyk K: A rare case of calcifying fibrous pseudotumor of the
pleura with an accompanying vascular anomaly in the pulmonary
ligament. Kardiochir Torakochirurgia Pol. 15:59–61. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kazazian K, Demicco EG, de Perrot M,
Strauss D and Swallow CJ: Toward better understanding and
management of solitary fibrous tumor. Surg Oncol Clin N Am.
31:459–483. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Narla LD, Newman B, Spottswood SS, Narla S
and Kolli R: Inflammatory pseudotumor. Radiographics. 23:719–729.
2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sigel JE, Smith TA, Reith JD and Goldblum
JR: Immunohistochemical analysis of anaplastic lymphoma kinase
expression in deep soft tissue calcifying fibrous pseudotumor:
Evidence of a late sclerosing stage of inflammatory myofibroblastic
tumor? Ann Diagn Pathol. 5:10–14. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Prendergast K, Kryeziu S and Crago AM: The
evolving management of desmoid fibromatosis. Surg Clin N Am.
102:667–677. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Azam F, Chatterjee M, Kelly S, Pinto M,
Aurangabadkar A, Latif MF and Marshall E: Multifocal calcifying
fibrous tumor at six sites in one patient: A case report. World J
Surg Oncol. 12(235)2014.PubMed/NCBI View Article : Google Scholar
|