Introduction
Acute inflammatory diseases such as sepsis, acute
lung injury (ALI) and acute respiratory distress syndrome (ARDS)
are serious conditions induced by viruses and bacterial endotoxins
(1,2). The coronavirus disease of 2019
increased the incidence of these diseases and was reported to be
associated with hospital mortality rates of sepsis that may exceed
40% and increased ARDS mortality rates by 23 to 56% (3,4).
Additionally, chronic inflammatory diseases such as asthma affects
>350 million people globally and causes 0.4 million deaths
annually, posing a significant social burden (5).
Cytokine upregulation is a key feature of sepsis,
ALI and asthma. Immune cells contribute to the pathogenesis of
these diseases by secreting excessive amounts of cytokines
(6,7). Macrophage-released cytokines (such as
IL-6 and TNF-α), chemokines [such as monocyte chemoattractant
protein-1 (MCP-1)] and mediators [such as inducible nitric oxide
synthase (iNOS)] exacerbate systemic inflammation in septic
conditions (8,9). These molecules also promote pulmonary
inflammation in ALI (10). Airway
epithelial cells promote airway inflammation and lung damage in ALI
by secreting TNF-α (11) and
induce pulmonary inflammation in asthma by expressing IL-6(12). In addition, cell-derived MCP-1
promotes bronchitis by inducing immune cell recruitment (13-15).
MAPK activation is implicated in the formation of
inflammatory molecules. p38 activation is associated with IL-6 and
TNF-α expression and p38 inactivation ameliorates endotoxin-induced
sepsis and ALI (16).
Additionally, p38 activation contributes to airway inflammation and
is present in the epithelium of patients with asthma (17). ERK is activated in sepsis and ARDS
(18,19). IL-13 leads to ERK activation and
mucin gene expression in airway epithelial cells, influencing
asthma progression (20).
Furthermore, JNK activation occurs in airway epithelial cells and
macrophages (21,22) and is associated with lung
inflammation and oxidative stress in the lipopolysaccharide
(LPS)-induced ALI model (22). JNK
inhibition ameliorates endotoxin-induced sepsis and
ovalbumin-induced asthma in mice (21,23).
The NF-κB signaling pathway is activated in response
to a variety of stimuli, including bacterial endotoxins. This
activation has been shown to promote hyperinflammation by
increasing IL-6, TNF-α, MCP-1 and iNOS generation in both in
vitro and in vivo studies of sepsis and ALI (24,25).
Phorbol 12-myristate 13-acetate (PMA) has also been shown to
upregulate IL-6, TNF-α and MCP-1 generation as well as NF-κB
activation in A549 lung epithelial cells in an in vitro
study of asthma (21,26-28).
Heme oxygenase-1 (HO-1) decreases inflammatory
response in cell models such as RAW264.7 and A549 by decreasing the
formation of cytokines/chemokines and inhibiting the activation of
MAPK and NF-κB (29,30).
Bacterial endotoxin LPS increases cytokine and
MAPK/NF-κB activation in the RAW264.7 macrophage cell line;
therefore, LPS is used for in vitro studies on sepsis and
ALI (31,32). PMA is an established MAPK/NF-κB
activator that elevates cytokines and chemokines in the A549 airway
epithelial cell line and is used as an inducer for in vitro
studies of asthma (21,28). Based on the abilities of LPS and
PMA, both molecules were selected to induce an inflammatory
response in RAW264.7 and A549 cells in the present study.
The phenolic compound N-(p-Coumaroyl)
serotonin (CS) is an active component of safflower seeds and
exhibits various biological effects. For example, CS demonstrates
antioxidant effects and decreases atherosclerosis in vivo
(33). CS also ameliorates
vascular smooth muscle cell activation by suppressing calcium
release (34). Additionally,
Lazari et al (35) reported
that CS exhibits anticancer effects by inducing S-phase cell cycle
arrest in both U251 and T98 glioblastoma cell lines. To the best of
our knowledge, however, the anti-inflammatory effects of CS have
not been reported in RAW264.7 macrophages and A549 airway
epithelial cells. Therefore, the present study examined whether CS
exerts anti-inflammatory effects in these cell lines.
Materials and methods
Cell culture and viability assay
RAW264.7 macrophages were obtained from American
Type Culture Collection and cultured in DMEM (cat. no. LM001-05;
Welgene, Inc.) with 10% FBS (cat. No. 6000044; Thermo Fisher
Scientific, Inc.) at 37˚C in a CO2 incubator for 24 h.
To analyze cell viability, the cells were seeded into a 12-well
plate (1x105/ml) and incubated with 6.3, 12.5, 25.0 and
50.0 µM CS (Wuhan ChemFaces Biochemical Co., Ltd.) for 24 h at
37˚C. Cell viability was then determined using MTT assay, following
a previously described protocol (10). To detect cell viability, A549 cells
(American Type Culture Collection) were seeded into a 12-well plate
(1x105/ml) and incubated with CS (6.3, 12.5 and 25.0 µM)
for 24 h at 37˚C. After adding the MTT solution (Amresco, LLC) to
each well, the plate was incubated at 37˚C for 3 h. Subsequently,
DMSO (Sigma-Aldrich; Merck KGaA) was used to dissolve the purple
formazan, and the absorbance at 570 nm was measured using a
SPARK® 10M microplate reader (Tecan Group, Ltd.).
NO assay
To analyze the NO content, cells were seeded into a
12-well plate (1x105/ml) and incubated with CS (6.3,
12.5 and 25.0 µM) for 1 h at 37˚C. Cells were incubated for 18 h
with LPS (200 ng/ml) at 37˚C. NO levels were detected using a NO
assay as previously described (36).
Cytokines and chemokine ELISA
To measure the levels of cytokines and chemokines
secreted from RAW264.7 and A549 cells, 1x105/ml cells
were seeded into a 12-well plate, incubated with CS (6.3, 12.5 and
25.0 µM) for 1 h at 37˚C and maintained for 18 h at 37˚C with 200
ng/ml LPS or 50 nM PMA, respectively. The levels of IL-6, TNF-α and
MCP-1 in the cell culture media (CCM) were analyzed using ELISA
kits according to the manufacturers protocols of these kits [BD
OptEIA™ Mouse IL-6 ELISA Set (cat. no. 555240), BD
OptEIA™ Mouse TNF ELISA Set (cat. no. 558534), BD
OptEIA™ Human IL-6 ELISA Set (cat. no. 555220), BD
OptEIA™ Human TNF ELISA Set (cat. no. 555212) (BD
Biosciences); Mouse CCL2/JE/MCP-1 DuoSet ELISA (cat. no. DY479) and
Human CCL2/MCP-1 DuoSet ELISA (cat. no. DY279) (R&D Systems,
Inc.)].
Western blotting
To detect the expression of iNOS in RAW264.7 cells
and the levels of phosphorylated (p)-p38, p-ERK, p-JNK, p-p65 and
p-IκBα in RAW264.7 and A549 cells, the cell lysate was isolated
using RIPA lysis butter (0.15 M NaCl, 0.05 M Tris, 5 mM EDTA, pH
8.0) including protease (cat. no 11836153001; Roche Diagnostics)
and phosphatase inhibitors (cat. no.4906837001; Roche Diagnostics).
Protein was quantified using a BCA assay and protein samples (20-60
µg) were separated using 8-12% SDS-PAGE as previously described
(31). Proteins were transferred
onto PVDF membranes and each membrane was blocked with 5% skimmed
milk in 1X TBST for 1 h at room temperature. Subsequently,
membranes were incubated with the primary antibodies (Table I) diluted to 1:1,000 in 1% BSA for
24 h at 4˚C and the corresponding secondary antibodies [(goat anti
rabbit-HRP; cat. no. 111-035-003; Jackson Laboratory) and (goat
anti mouse-HRP; cat. no. 115-035-003; Jackson Laboratory)] diluted
to 1:2,000 in 1% BSA for 2 h at room temperature. Finally, each
membrane was exposed to ECL solution (cat. no. 32106; Thermo Fisher
Scientific, Inc.) to visualize the protein bands. Protein
semi-quantitative analysis of iNOS/β-actin, p-p38/p38, p-ERK/ERK,
p-JNK/JNK, p-p65/p65, p-IκB/IκBα and HO-1/β-actin was performed
using ImageJ version 1.52a software (National Institutes of
Health).
 | Table IPrimary antibodies used for western
blotting. |
Table I
Primary antibodies used for western
blotting.
| Primary
antibody | Dilution | Manufacturer | Cat. no. | Molecular weight,
kDaa | Host |
|---|
| iNOS | 1:1,000 | Enzo Life Sciences,
Inc. | ADI-KAS-NO001 | 130 | Rabbit |
| HO-1 | 1:1,000 | Invitrogen (Thermo
Fisher Scientific, Inc.) | PA5-27338 | 32 | Rabbit |
| p-p38 | 1:1,000 | Cell Signaling
Technology, Inc. | 9211S | 43 | Rabbit |
| p38 | 1:1,000 | Santa Cruz
Biotechnology, Inc. | sc-7972 | 38 | Mouse |
| p-ERK | 1:1,000 | Cell Signaling
Technology, Inc. | 9101S | 42,44 | Rabbit |
| ERK | 1:1,000 | Cell Signaling
Technology, Inc. | 9102S | 42, 44 | Rabbit |
| p-JNK | 1:1,000 | Cell Signaling
Technology, Inc. | 4668S | 46, 54 | Rabbit |
| JNK | 1:1,000 | Cell Signaling
Technology, Inc. | 9252S | 46, 54 | Rabbit |
| p-NF-κB p65 | 1:1,000 | Cell Signaling
Technology, Inc. | 3033S | 65 | Rabbit |
| NF-κB p65 | 1:1,000 | Santa Cruz
Biotechnology, Inc. | sc-8008 | 65 | Mouse |
| p-IκBα | 1:1,000 | Santa Cruz
Biotechnology, Inc. | sc-8404 | 41 | Mouse |
| IκBα | 1:1,000 | Invitrogen (Thermo
Fisher Scientific, Inc.) | MA5-15132 | 36 | Mouse |
| β-actin | 1:5,000 | Santa Cruz
Biotechnology, Inc. | sc-47778 | 43 | Mouse |
Immunocytochemistry
To determine the nuclear translocation of NF-κB p65
in RAW264.7 cells, cells were fixed with 10% formalin for overnight
at 4˚C, washed with ice-cold PBS, blocked with 5% BSA (Gibco;
Thermo Fisher Scientific, Inc.) blocking buffer for 30 min at room
temperature, incubated with the primary antibody (anti-NF-κB p65
subunit; Table I) for 24 h at 4˚C
and the corresponding secondary antibody (Alexa Fluor
488-conjugated goat anti-rabbit IgG; cat no. A-11034; Invitrogen;
Thermo Fisher Scientific, Inc.) diluted to 1:250 in 1% BSA for 1 h
at room temperature and stained with Antifade Mounting Medium with
DAPI (cat. no. H-1500; Vector Laboratories, Inc.) at room
temperature as previously described (31). Finally, cells were visualized using
a confocal microscope.
Statistical analysis
All data are expressed as the mean ± standard
deviation (n=3). Data were analyzed using an unpaired two-tailed
Student's t-test to assess the difference between two groups.
One-way ANOVA followed by Tukey's multiple comparison test was
performed to assess differences between >2 groups. Data were
analyzed using SPSS software (version 20.0; IBM Corp.). P<0.05
was considered to indicate a statistically significant
difference.
Results
CS ameliorates LPS-stimulated
inflammatory responses in RAW264.7 macrophages
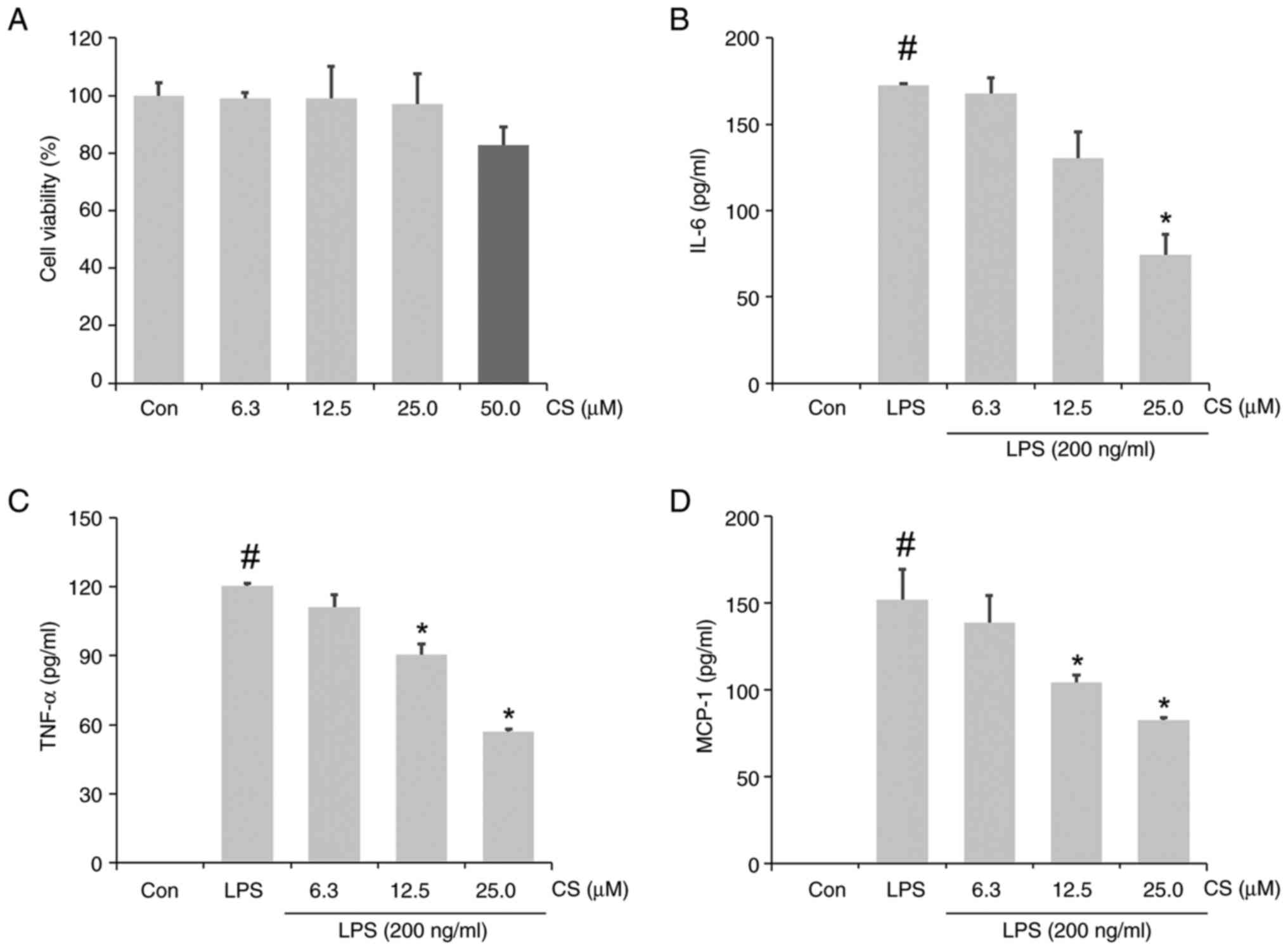
Viability of RAW264.7 cells was assessed following
treatment with CS (6.3, 12.5, 25.0 and 50.0 µM) by an MTT assay. No
notable changes in cell viability were observed following treatment
with ≤25.0 µM CS (Fig. 1A). Based
on this result, the anti-inflammatory effects of CS at 6.3, 12.5
and 25.0 µM on LPS-stimulated RAW264.7 cells were examined. ELISA
results confirmed the significant increase in IL-6, TNF-α and MCP-1
levels in the CCM of LPS-stimulated RAW264.7 cells (Fig. 1B-D) compared to the control group.
This increase was significantly inhibited in the CS pretreated-LPS
group. Overall, 25 µM CS had a notable inhibitory effect, resulting
in a 56.93% decrease in IL-6, 52.62% decrease in TNF-α and a 45.73%
decrease in MCP-1 levels.
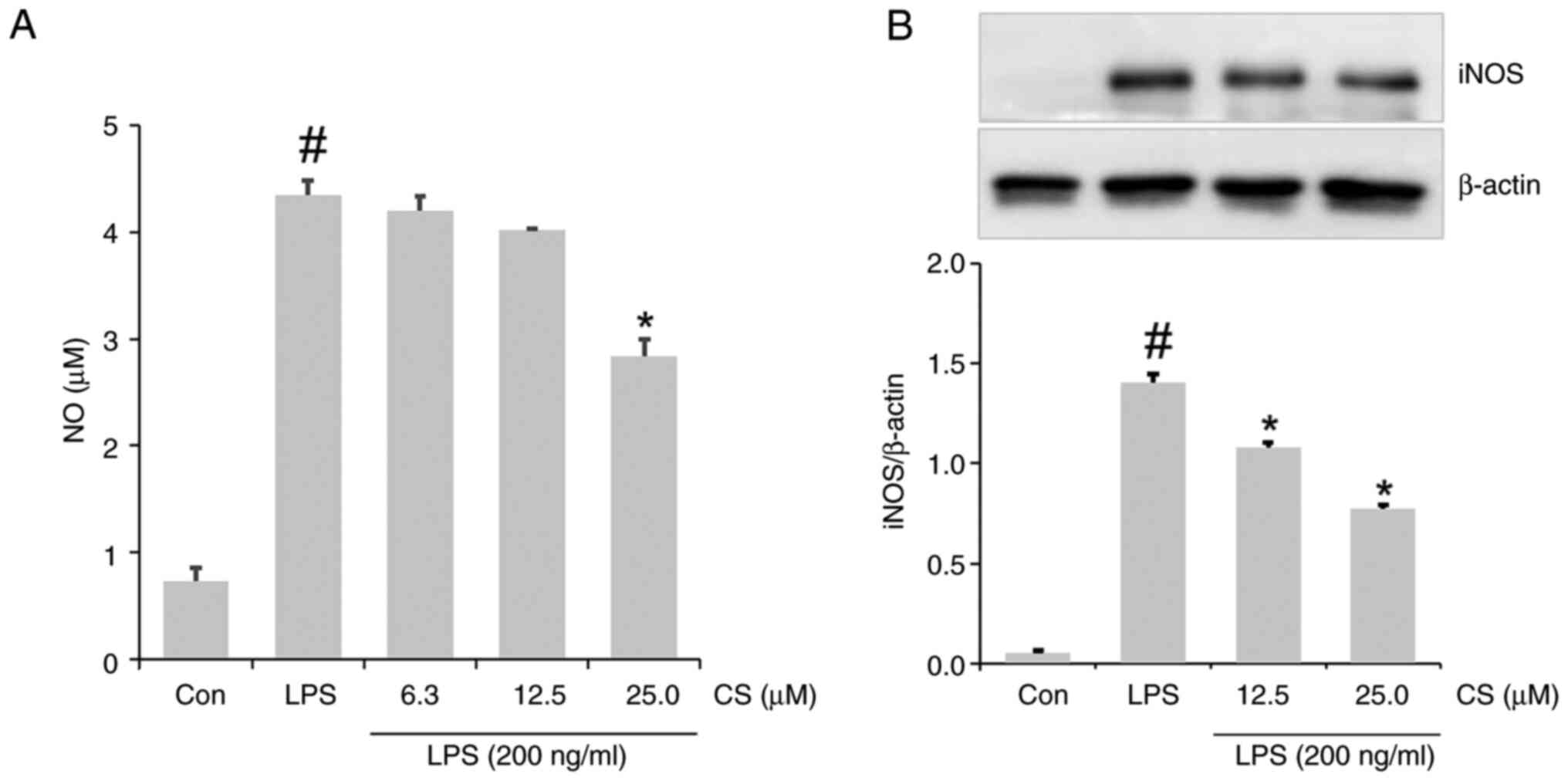
The notable elevation in NO formation in
LPS-stimulated RAW264.7 cells was decreased by the addition of CS
(Fig. 2A). Western blotting
revealed increased expression of iNOS in the lysate of
LPS-stimulated RAW264.7 cells. This expression was attenuated in
the lysate of the CS-pretreated LPS group (Fig. 2B).
The inhibitory effects of dexamethasone (DEX), which
is a synthetic pregnane corticosteroid on inflammatory molecules,
were demonstrated in an experimental study of ALI and asthma
(10,13), Notably, the present study confirmed
that the inhibitory effect of 25 µM CS on LPS-induced MCP-1 and NO
was similar to that of 20 µM DEX (Table II).
 | Table IIInhibitory effect of CS and DEX on
MCP-1 and NO in lipopolysaccharide-stimulated RAW264.7 cells. |
Table II
Inhibitory effect of CS and DEX on
MCP-1 and NO in lipopolysaccharide-stimulated RAW264.7 cells.
| Treatment | MCP-1 inhibition
rate (%) | NO inhibition rate
(%) |
|---|
| 25 µM CS | 33.9±5.95 | 33.4±0.57 |
| 20 µM DEX | 35.5±4.02 | 31.9±2.17 |
CS ameliorates LPS-stimulated MAPK
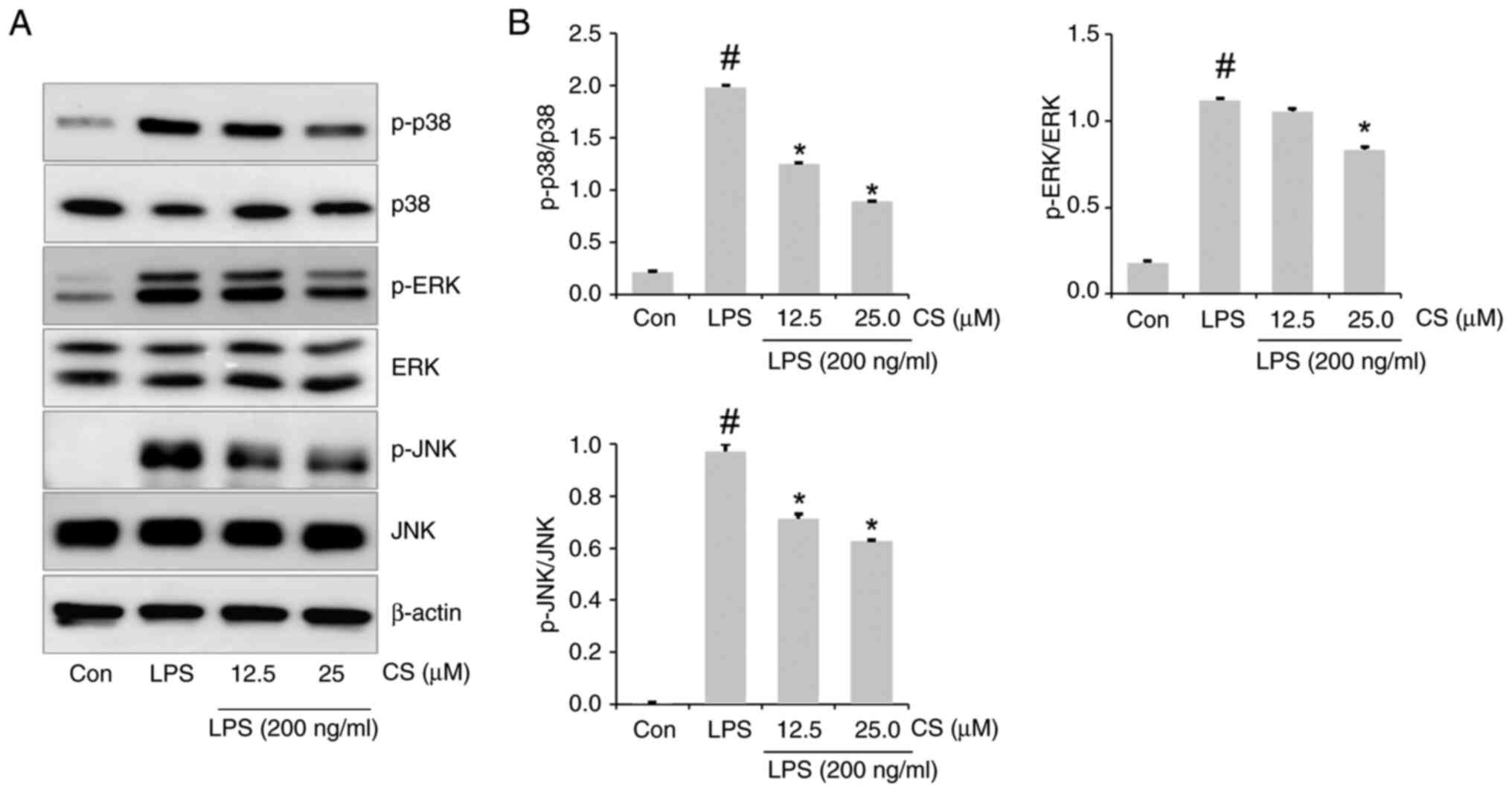
activation in RAW264.7 cells
To elucidate the underlying mechanisms of CS, the
levels of p38, ERK and JNK activation were investigated via western
blotting. LPS significantly upregulated not only p-p38 and p-ERK
expression but also p-JNK expression in RAW264.7 cells (Fig. 3A and B). However, this upregulation in
LPS-stimulated RAW264.7 cells was inhibited by CS pretreatment.
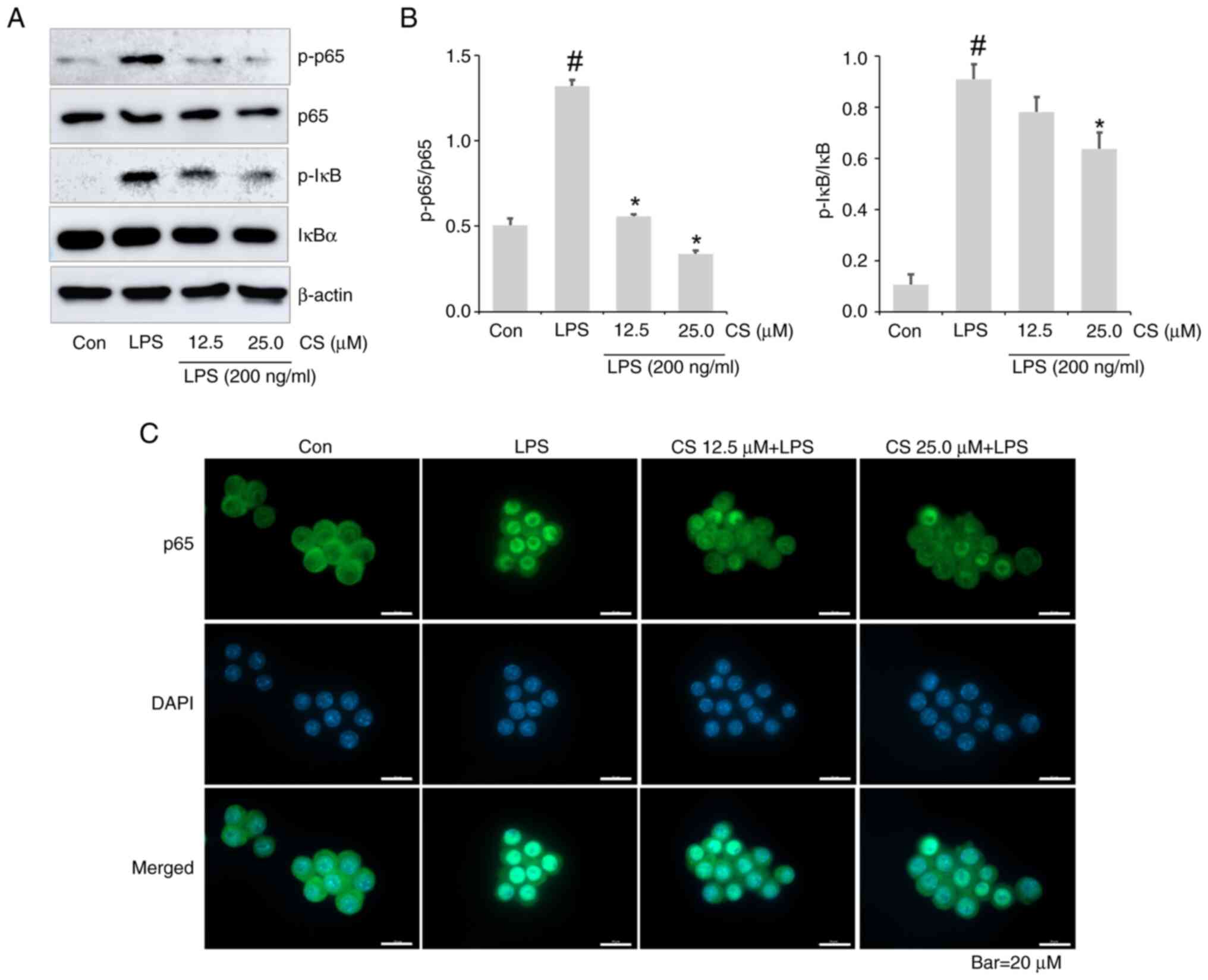
CS ameliorates LPS-stimulated NF-κB
activation in RAW264.7 cells
As NF-κB signaling pathways are associated with the
expression of inflammatory molecules (24,25),
the abrogative effects of CS on LPS-induced NF-κB activation were
explored. Increased expression of p-NF-κB and p-IκB was confirmed
in the lysate of the LPS-treated group. However, this was
effectively inhibited by CS (Fig.
4A and B). Furthermore,
immunocytochemistry demonstrated that CS resulted decreased nuclear
translocation of NF-κB p65 in LPS-stimulated RAW264.7 cells
(Fig. 4C).
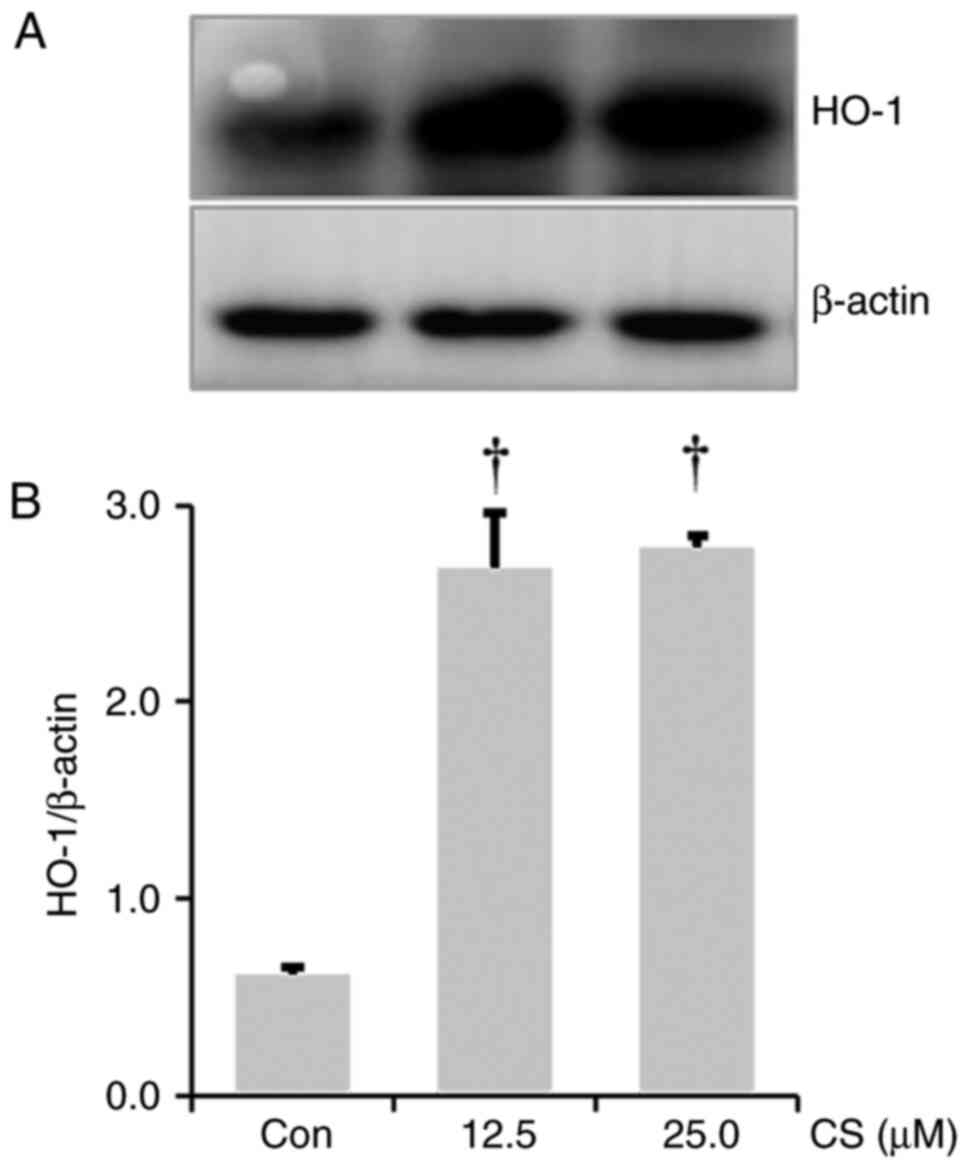
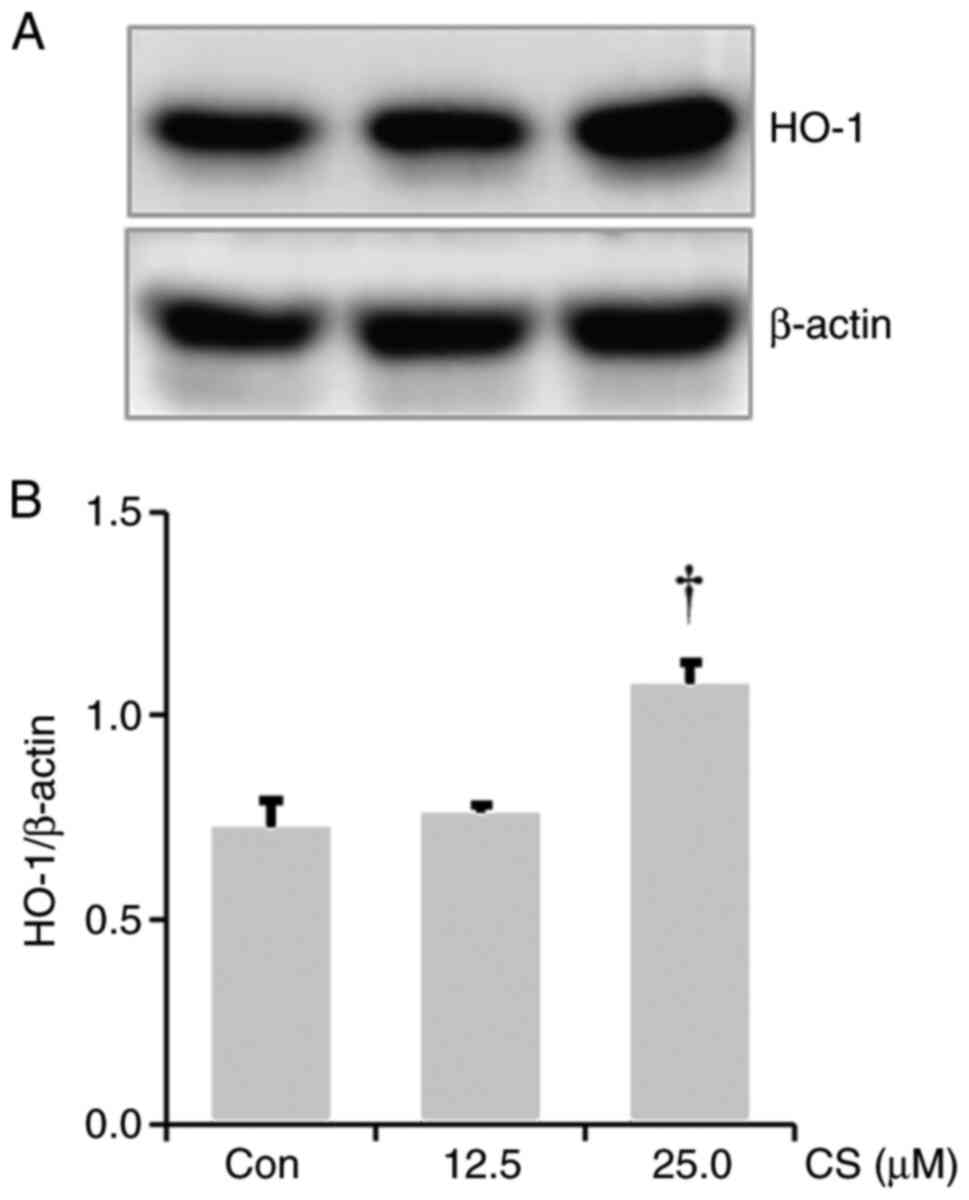
CS results in HO-1 upregulation in
RAW264.7 cells
As HO-1 induction exhibits anti-inflammatory effects
(29,30), the effect of CS on HO-1
upregulation was investigated. A significant increase in HO-1
expression was observed in the lysate from the CS-treated RAW264.7
cells compared to the control group (Fig. 5A and B).
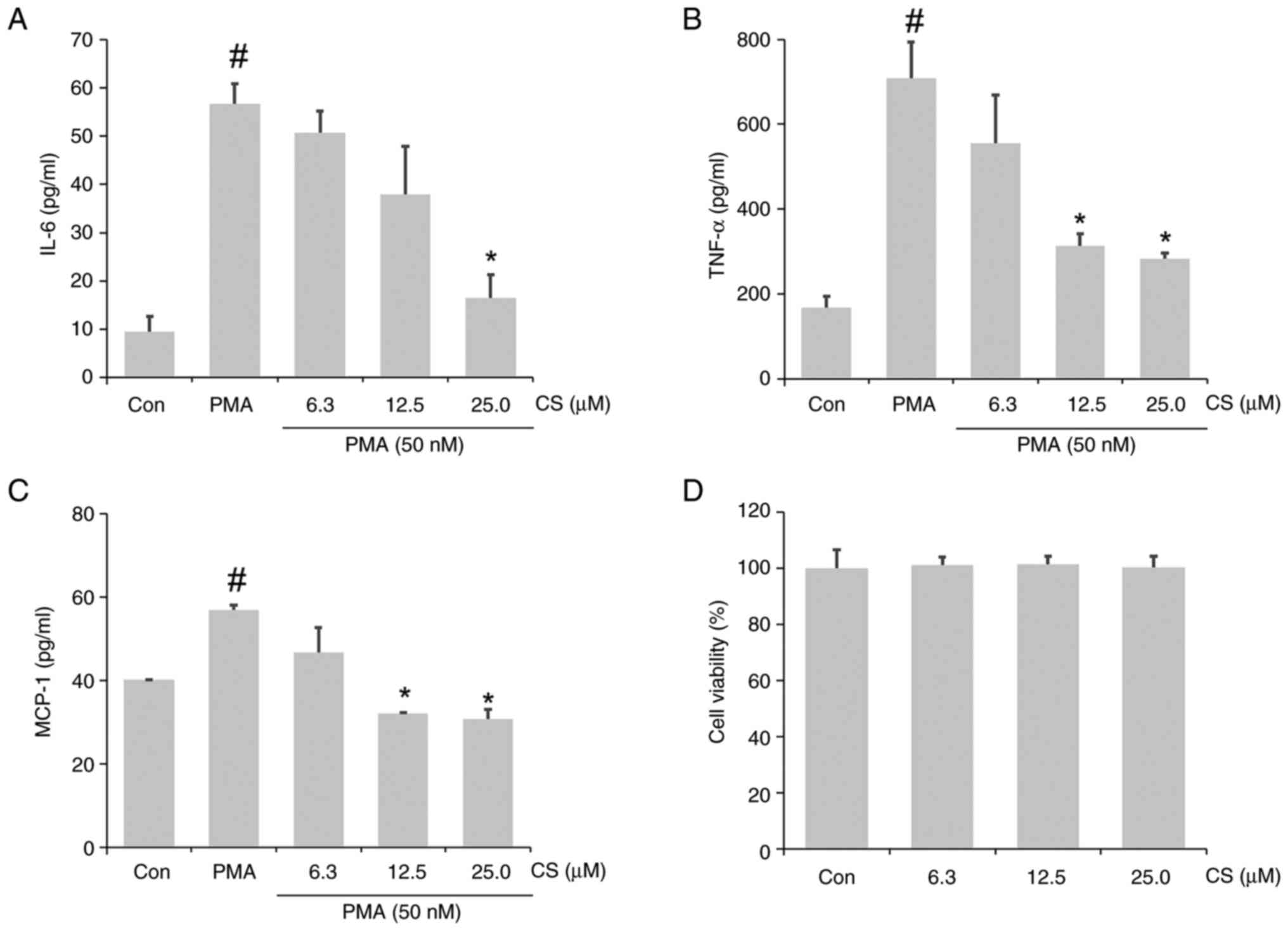
CS ameliorates the PMA-stimulated
inflammatory response in A549 cells
Based on the aforementioned anti-inflammatory
effects of CS against endotoxin stimulation in RAW264.7 cells, the
ameliorative effects of CS against PMA-induced inflammation in A549
airway epithelial cells were also investigated. Notable
upregulation of IL-6, TNF-α and MCP-1 was observed in the CCM of
PMA-stimulated A549 cells, which was mitigated by CS pretreatment
(Fig. 6A-C). No notable changes in
viability were observed in A549 cells within the treated
concentrations (Fig. 6D). In
general, 25 µM CS significantly suppressed cytokine and chemokine
secretion. The inhibition rates of IL-6, TNF-α and MCP-1 secretion
were 70.94, 60.01 and 46.05%, respectively.
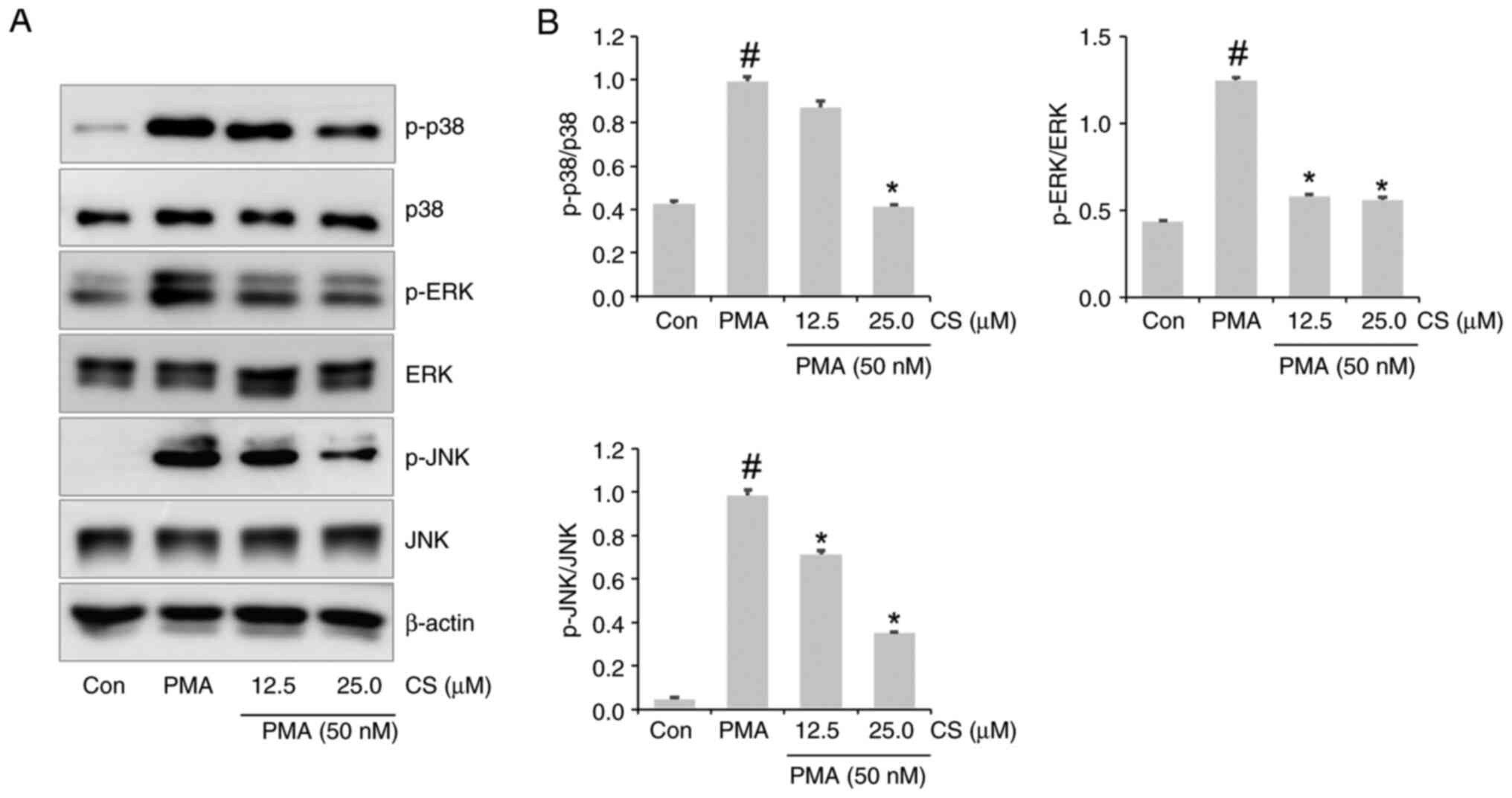
CS ameliorates PMA-stimulated MAPK
activation in A549 cells
p38, ERK and JNK phosphorylation were demonstrated
in the lysate of PMA-stimulated A549 cells (Fig. 7A and B). p38, ERK and JNK phosphorylation was
inhibited in the lysate of the 25 µM CS-pretreated PMA group
(Fig. 7A and B).
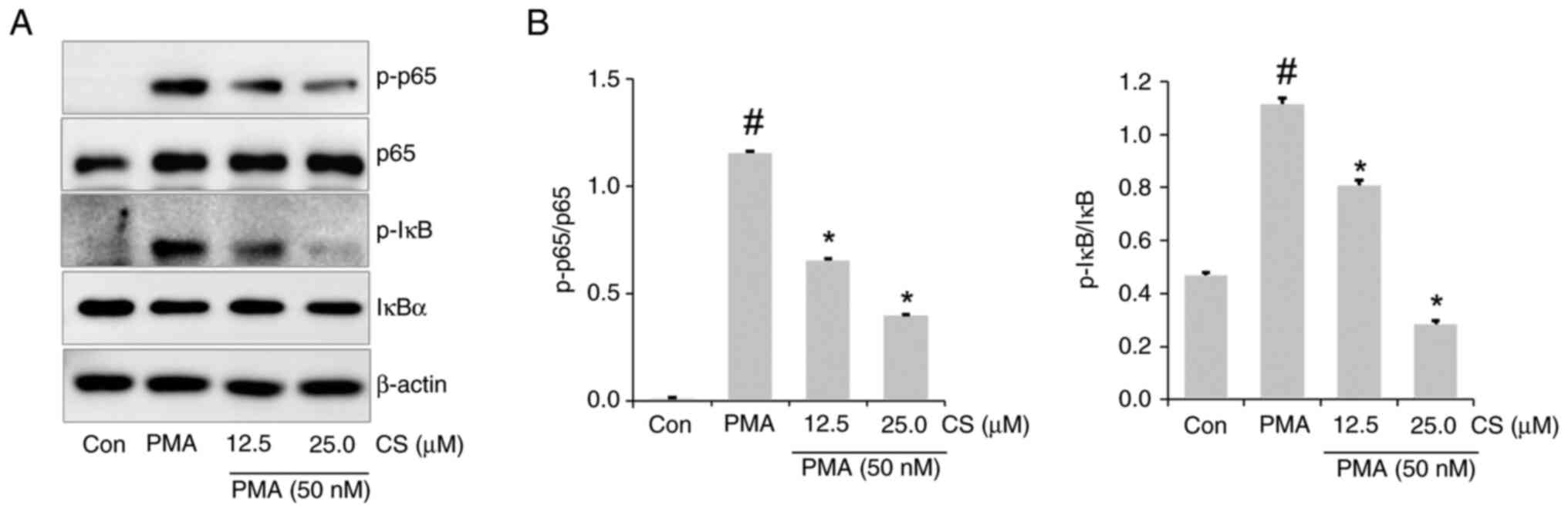
CS ameliorates PMA-stimulated NF-κB
activation in A549 cells
To determine whether the anti-inflammatory effects
of CS in A549 cells were associated with the NF-κB signaling
pathway, NF-κB and IκB activation levels in lysate were evaluated.
NF-κB and IκB phosphorylation was increased in the lysate of
PMA-stimulated A549 cells compared to the control group, whereas
this was inhibited in the lysate of the CS-pretreated PMA group
(Fig. 8A and B).
CS results in HO-1 upregulation in
A549 cells
Increase in HO-1 expression was confirmed in the
lysate of the 25 µM CS-treated A549 cells compared to the control
group (Fig. 9A and B).
Discussion
Hyperinflammation leads to harmful effects, such as
organ damage (1). Therefore,
substances that modulate this are valuable as adjuvants or
therapeutics to prevent or improve the development of systemic or
pulmonary inflammatory disease. Similar to the suppression ability
of CS on IL-6 and TNA-α in a previous study (37), in the present study, CS inhibited
the secretion of IL-6, TNF-α and MCP-1 in both LPS-stimulated
macrophages and PMA-stimulated lung epithelial cells. In addition,
CS exerted a marked inhibitory effect on LPS-stimulated iNOS
expression in macrophages. Notably, 25 µM CS had a marked
inhibitory effect on cytokine formation in both cell lines. In
addition, the suppressive effects of 25 µM CS on chemokine and
mediators, such as MCP-1 and NO were comparable to those of 20 µM
DEX. These results suggested CS may prevent and improve the
development of systemic or bronchial inflammatory disease.
MAPK and NF-κB inactivation mitigates
hyperinflammation in various cell types, such as macrophages and
lung epithelial cells (30,38-40).
Therefore, MAPK and NF-κB pathways have been targeted as a
therapeutic strategy to improve inflammatory disease. Notably, the
present study demonstrated the inhibitory effect of CS on LPS- or
PMA-induced MAPK and NF-κB activation in vitro. These
observations suggested that CS may serve as an MAPK/NF-κB
inactivator in systemic or bronchial inflammatory disease.
There is a marked focus on HO-1 induction to
decrease the inflammatory response (30,31).
Notably, in the present study, 25 µM CS significantly elevated HO-1
expression in both RAW264.7 and A549 cell lines, indicating that CS
exerted not only anti-inflammatory, but also antioxidant
effects.
Serotonin and its derivative (N-feruloylserotonin)
decrease inflammatory responses both in vivo and in
vitro (41,42). Similar to previous results
(37,41,42),
the present study demonstrated the anti-inflammatory properties of
CS in vitro by evaluating its inhibitory ability on cytokine
and chemokine secretion.
In summary, the present study demonstrated that CS
ameliorated inflammation in both activated macrophages and lung
epithelial cells via MAPK and NF-κB inactivation. Furthermore, 25
µM CS and 20 µM DEX showed similar effects on reducing MCP-1 and NO
in activated-RAW264.7 cells. These results suggested CS may serve
as an adjuvant or therapeutic for sepsis, ALI and asthma. However,
further studies are required to determine whether CS affects the
activation of other pathways, such as STAT3. In addition, animal
studies are required to confirm the efficacy and mechanism of
CS.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the KRIBB Research
Initiative Program (grant no. KGM1202511), National Research
Foundation of Korea (NRF) grants funded by the Ministry of Science
and ICT (MSIT) (grant no. RS-2023-00279150), National Research
Foundation of Korea grants funded by the MSIT (grant no.
2022M3E5F4078558) and National Research Foundation of Korea grants
funded by the MSIT (grant no. RS-2023-00213076).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SHY, CHJ and SJP performed experiments. HJL, OKK and
JWL conceived and designed the study. SHY, CHJ, SJP, HJL, OKK and
JWL wrote the manuscript. SHY, CHJ, SJP, HJL, OKK and JWL confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee JW, Chun W, Lee HJ, Min JH, Kim SM,
Seo JY, Ahn KS and Oh SR: The role of macrophages in the
development of acute and chronic inflammatory lung diseases. Cells.
10(897)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marshall T, Dysert K, Young M and DuMont
T: Pathophysiology of sepsis. Crit Care Nurs Q. 48:88–92.
2025.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Koçak Tufan Z, Kayaaslan B and Mer M:
COVID-19 and sepsis. Turk J Med Sci. 51:3301–3311. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gallelli L, Zhang L, Wang T and Fu F:
Severe acute lung injury related to COVID-19 infection: A review
and the possible role for escin. J Clin Pharmacol. 60:815–825.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Batard T, Taillé C, Guilleminault L, Bozek
A, Floch VB, Pfaar O, Canonica WG, Akdis C, Shamji MH and Mascarell
L: Allergen immunotherapy for the prevention and treatment of
asthma. Clin Exp Allergy. 55:111–141. 2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saxena J, Das S, Kumar A, Sharma A, Sharma
L, Kaushik S, Kumar Srivastava V, Jamal Siddiqui A and Jyoti A:
Biomarkers in sepsis. Clin Chim Acta. 562(119891)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bhargava M and Wendt CH: Biomarkers in
acute lung injury. Transl Res. 159:205–217. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee JW, Bae CJ, Choi YJ, Kim SI, Kwon YS,
Lee HJ, Kim SS and Chun W: 3,4,5-Trihydroxycinnamic acid inhibits
lipopolysaccharide (LPS)-induced inflammation by Nrf2 activation in
vitro and improves survival of mice in LPS-induced endotoxemia
model in vivo. Mol Cell Biochem. 390:143–153. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kawamura A, Ito A, Takahashi A, Sawamoto
A, Okuyama S and Nakajima M: Benproperine reduces IL-6 levels via
Akt signaling in monocyte/macrophage-lineage cells and reduces the
mortality of mouse sepsis model induced by lipopolysaccharide. J
Pharmacol Sci. 156:125–133. 2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee JW, Seo KH, Ryu HW, Yuk HJ, Park HA,
Lim Y, Ahn KS and Oh SR: Anti-inflammatory effect of stem bark of
Paulownia tomentosa Steud. in lipopolysaccharide (LPS)-stimulated
RAW264.7 macrophages and LPS-induced murine model of acute lung
injury. J Ethnopharmacol. 210:23–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mukhopadhyay S, Hoidal JR and Mukherjee
TK: Role of TNFalpha in pulmonary pathophysiology. Respir Res.
7(125)2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rincon M and Irvin CG: Role of IL-6 in
asthma and other inflammatory pulmonary diseases. Int J Biol Sci.
8:1281–1290. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee YG, Jeong JJ, Nyenhuis S, Berdyshev E,
Chung S, Ranjan R, Karpurapu M, Deng J, Qian F, Kelly EAB, et al:
Recruited alveolar macrophages, in response to airway
epithelial-derived monocyte chemoattractant protein 1/CCl2,
regulate airway inflammation and remodeling in allergic asthma. Am
J Respir Cell Mol Biol. 52:772–784. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Park JM, Park JW, Lee J, Kim SH, Seo DY,
Ahn KS, Han SB and Lee JW: Aromadendrin inhibits PMA-induced
cytokine formation/NF-κB activation in A549 cells and
ovalbumin-induced bronchial inflammation in mice. Heliyon.
9(e22932)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jiang W, Wang JM, Luo JH, Chen Y, Pi J, Ma
XD, Liu CX, Zhou Y, Qu XP, Liu C, et al: Airway epithelial integrin
β4-deficiency exacerbates lipopolysaccharide-induced acute lung
injury. J Cell Physiol. 236:7711–7724. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fang W, Cai SX, Wang CL, Sun XX, Li K, Yan
XW, Sun YB, Sun XZ, Gu CK, Dai MY, et al: Modulation of
mitogen-activated protein kinase attenuates sepsis-induced acute
lung injury in acute respiratory distress syndrome rats. Mol Med
Rep. 16:9652–9658. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu W, Liang Q, Balzar S, Wenzel S, Gorska
M and Alam R: Cell-specific activation profile of extracellular
signal-regulated kinase 1/2, Jun N-terminal kinase, and p38
mitogen-activated protein kinases in asthmatic airways. J Allergy
Clin Immunol. 121:893–902.e2. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang T, Fu JN, Chen GB and Zhang X:
Plac8-ERK pathway modulation of monocyte function in sepsis. Cell
Death Discov. 10(308)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gong KQ, Mikacenic C, Long ME, Frevert CW,
Birkland TP, Charron J, Gharib SA and Manicone AM: MAP2K2 delays
recovery in murine models of acute lung injury and associates with
acute respiratory distress syndrome outcome. Am J Respir Cell Mol
Biol. 66:555–563. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qin Y, Jiang Y, Sheikh AS, Shen S, Liu J
and Jiang D: Interleukin-13 stimulates MUC5AC expression via a
STAT6-TMEM16A-ERK1/2 pathway in human airway epithelial cells. Int
Immunopharmacol. 40:106–114. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kwon OK, Lee JW, Xuezhen X, Harmalkar DS,
Song JG, Park JW, Hwang D, Min JH, Kim JH, Han HK, et al: DK-1108
exerts anti-inflammatory activity against phorbol 12-myristate
13-acetate-induced inflammation and protective effect against
OVA-induced allergic asthma. Biomed Pharmacother.
132(110950)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Du J, Wang G, Luo H, Liu N and Xie J:
JNK-IN-8 treatment alleviates lipopolysaccharide-induced acute lung
injury via suppression of inflammation and oxidative stress
regulated by JNK/NF-κB signaling. Mol Med Rep.
23(150)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nie Z, Xia X, Zhao Y, Zhang S, Zhang Y and
Wang J: JNK selective inhibitor, IQ-1S, protects the mice against
lipopolysaccharides-induced sepsis. Bioorg Med Chem.
30(115945)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang X, Kang Y, Li X, Huang Y, Qi R, Han
Y, Cai R, Gao Y and Qi Y: Potentilla discolor ameliorates
LPS-induced inflammatory responses through suppressing NF-κB and
AP-1 pathways. Biomed Pharmacother. 144(112345)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu Z, Wang K, Hu H, Hu Y, Huang J and Luo
Z: Sinensetin attenuates LPS-induced acute pulmonary inflammation
in mice and RAW264.7 cells by modulating NF-κB p65-mediated immune
resistance and STAT3-mediated tissue resilience. Int
Immunopharmacol. 148(114101)2025.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Park JW, Kim SM, Min JH, Kim MG, Kwon OK,
Hwang D, Oh JH, Park MW, Chun W, Lee HJ, et al:
3,4,5-Trihydroxycinnamic acid exerts anti-asthmatic effects in
vitro and in vivo. Int Immunopharmacol. 88(107002)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Patil RH, Babu RL, Naveen Kumar M, Kiran
Kumar KM, Hegde SM, Ramesh GT and Chidananda Sharma S: Apigenin
inhibits PMA-induced expression of pro-inflammatory cytokines and
AP-1 factors in A549 cells. Mol Cell Biochem. 403:95–106.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Park JW, Choi J, Lee J, Park JM, Kim SM,
Min JH, Seo DY, Goo SH, Kim JH, Kwon OK, et al: Methyl P-coumarate
ameliorates the inflammatory response in activated-airway
epithelial cells and mice with allergic asthma. Int J Mol Sci.
23(14909)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee JW, Park JW, Shin NR, Park SY, Kwon
OK, Park HA, Lim Y, Ryu HW, Yuk HJ, Kim JH, et al: Picrasma
quassiodes (D. Don) Benn. attenuates lipopolysaccharide
(LPS)-induced acute lung injury. Int J Mol Med. 38:834–844.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim SM, Ryu HW, Kwon OK, Hwang D, Kim MG,
Min JH, Zhang Z, Kim SY, Paik JH, Oh SR, et al: Callicarpa japonica
Thunb. ameliorates allergic airway inflammation by suppressing
NF-κB activation and upregulating HO-1 expression. J
Ethnopharmacol. 267(113523)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Park JW, Ryu HW, Ahn HI, Min JH, Kim SM,
Kim MG, Kwon OK, Hwang D, Kim SY, Choi S, et al: The
anti-inflammatory effect of Trichilia martiana C. DC. in the
lipopolysaccharide-stimulated inflammatory response in macrophages
and airway epithelial cells and in LPS-challenged mice. J Microbiol
Biotechnol. 30:1614–1625. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Song Y, Liu X, Yue H, Ji J, Dou H and Hou
Y: Anti-inflammatory effects of benzenediamine derivate FC-98 on
sepsis injury in mice via suppression of JNK, NF-κB and IRF3
signaling pathways. Mol Immunol. 67:183–192. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Katsuda SI, Suzuki K, Koyama N, Takahashi
M, Miyake M, Hazama A and Takazawa K: Safflower seed polyphenols
(N-(p-coumaroyl)serotonin and N-feruloylserotonin) ameliorate
atherosclerosis and distensibility of the aortic wall in Kurosawa
and Kusanagi-hypercholesterolemic (KHC) rabbits. Hypertens Res.
32:944–949. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Takimoto T, Suzuki K, Arisaka H, Murata T,
Ozaki H and Koyama N: Effect of N-(p-coumaroyl)serotonin and
N-feruloylserotonin, major anti-atherogenic polyphenols in
safflower seed, on vasodilation, proliferation and migration of
vascular smooth muscle cells. Mol Nutr Food Res. 55:1561–1571.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lazari D, Alexiou GA, Markopoulos GS,
Vartholomatos E, Hodaj E, Chousidis I, Leonardos I, Galani V and
Kyritsis AP: N-(p-coumaroyl) serotonin inhibits glioblastoma cells
growth through triggering S-phase arrest and apoptosis. J
Neurooncol. 132:373–381. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Vo VA, Lee JW, Chang JE, Kim JY, Kim NH,
Lee HJ, Kim SS, Chun W and Kwon YS: Avicularin inhibits
lipopolysaccharide-induced inflammatory response by suppressing ERK
phosphorylation in RAW 264.7 macrophages. Biomol Ther (Seoul).
20:532–537. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Takii T, Kawashima S, Chiba T, Hayashi H,
Hayashi M, Hiroma H, Kimura H, Inukai Y, Shibata Y, Nagatsu A, et
al: Multiple mechanisms involved in the inhibition of
proinflammatory cytokine production from human monocytes by
N-(p-coumaroyl)serotonin and its derivatives. Int Immunopharmacol.
3:273–277. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fang H, Chen J, Luo J, Hu J, Wang D, Lv L
and Zhang W: Abietic acid attenuates sepsis-induced lung injury by
inhibiting nuclear factor kappa-light-chain-enhancer of activated B
cells (NF-κB) pathway to inhibit M1 macrophage polarization. Exp
Anim. 71:481–490. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang S, Li F, Lu S, Ren L, Bian S, Liu M,
Zhao D, Wang S and Wang J: Ginseng root extract attenuates
inflammation by inhibiting the MAPK/NF-κB signaling pathway and
activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in
vivo. J Ethnopharmacol. 283(114739)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu QH, Zhang K, Feng SS, Zhang LJ, Li SY,
Wang HY and Wang JH: Rosavin alleviates LPS-induced acute lung
injury by modulating the TLR-4/NF-κB/MAPK signaling pathways. Int J
Mol Sci. 25(1875)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Abd Allah ESH, Makboul R and Mohamed AO:
Role of serotonin and nuclear factor-kappa B in the ameliorative
effect of ginger on acetic acid-induced colitis. Pathophysiology.
23:35–42. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Park CH, Han SW, Seong SH, Choi JS, Jeon
JP and Yokozawa T: N-Feruloylserotonin inhibits
lipopolysaccharide-induced inflammation via SIRT1-stimulated FOXO1
and NF-κB signaling pathways in RAW 264.7 cells. Cell Mol Biol
(Noisy-le-grand). 69:109–115. 2023.PubMed/NCBI View Article : Google Scholar
|