Introduction
In the US, the incidence of prostate cancer (PC)
ranks first among men, while the mortality from PC ranks second
after lung cancer. In Europe, approximately 260,000 individuals are
diagnosed with PC every year (1),
and PC accounts for 9% of cancer-related deaths among men (2). The frequency of PC varies from
country to country; it has been reported to be lowest in the Far
East, particularly in mainland China and Japan (3). In Japan, however, the frequency in
2015 is expected to increase to 4.6 times that in 1985 (4), and a recent study revealed that PC
screening would reduce mortality from PC by 20% (5). PC will thus become an increasingly
important disease in men. Patients with PC have only vague symptoms
in the early phase of the disease; it is not rare for patients who
present with a chief complaint of bone pain or neurological
symptoms to have PC with bone metastases at the time of diagnosis
(6). Most PC is
androgen-dependent. Patients with metastatic PC are rarely cured
and most of them are treated by endocrine therapy. In the majority
of such patients, however, resistance to endocrine therapy develops
within several years. Endocrine resistance is considered to be
acquired through abnormalities in the androgen receptor as well as
a mechanism independent of the androgen receptor (7). At present, however, the
characteristics of patients in whom endocrine-therapy resistance is
likely to develop have not been clarified, and no effective therapy
has been established for endocrine therapy-resistant PC.
The present study retrospectively assessed the
potential significance of various clinical data (serum biochemical
data and pathological findings) in predicting the outcome of
prostate cancer with bone metastasis (M1b PC) after endocrine
therapy.
Subjects and methods
Of the 454 patients who were diagnosed with prostate
cancer at our hospital between January 1998 and December 2006, 104
with bone metastasis confirmed by bone scintigraphy and with a
Karnofsky performance scale of ≥70% were targeted for the present
study.
All subjects were treated with endocrine therapy. In
all subjects, the prostate-specific antigen (PSA) level was
confirmed to have decreased to ≤4 ng/ml after the initiation of
treatment, and none of them received chemotherapy with anticancer
drugs after recurrence. The last observation was performed on May
31, 2009. The baseline characteristics of the subjects are
presented in Table I. The
prostate-specific antigen level was measured using a Tandem-R PSA
kit (Hybritech, San Diego, CA, USA). The day of determination of
the stage was defined as the first day of observation.
Histopathological grading was performed using the Gleason score
(GS) (8), and the clinical stage
was determined based on the International Union Against Cancer
(UICC) classification (9).
Metastatic spread to bone was assessed by bone scintigraphy and
classified according to the extent of disease (EOD) grade (10).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Patient, n | 104 |
| Age (years) | |
| Range | 54–91 |
| Average (±SD) | 74.2±7.4 |
| Median | 74 |
| Serum PSA
(ng/ml) | 10–100,060.0 |
| Average (±SD) | 920.1±1759.3 |
| Median | 268.7 |
| Follow-up period
(months) | 4–122 |
| Average (±SD) | 46.9±29.1 |
| Median | 43 |
| Treatment | |
| MAB | 93 (89.4%) |
| LH-RH agonist
monotherapy | 2 (1.9%) |
| Orchiectomies | 1 (0.9%) |
| Orchiectomies +
antiandrogen | 8 (7.7%) |
| Outcome | |
| Alive | 50 (48.0%) |
| Cancer-related
death | 45 (43.2%) |
| Other cause of
death | 9 (8.6%) |
| Gleason score | |
| 7 | 18 (17.3%) |
| 8 | 31 (29.8%) |
| 9 | 49 (47.1%) |
| 10 | 6 (5.8%) |
| EOD grade | |
| 1 | 39 (37.5%) |
| 2 | 41 (39.4%) |
| 3 | 15 (14.4%) |
| 4 | 8 (7.7%) |
| X | 1 (0.9%) |
| T classification | |
| T1 | 4 (3.8%) |
| T2 | 25 (24.0%) |
| T3 | 24 (23.0%) |
| T4 | 49 (47.1%) |
| Tx | 2 (1.9%) |
| N classification | |
| N0 | 57 (54.8%) |
| N1 | 44 (42.3%) |
| Nx | 3 (2.8%) |
The parameters investigated were T classification, N
classification, GS, pre-treatment PSA, EOD grade, alkaline
phosphatase (ALP), lactate dehydrogenase (LDH), calcium (Ca) and
hemoglobin (Hb) levels and platelet (PLT) count.
Survival curves were prepared by the Kaplan-Meier
method. To identify predictive factors for the outcome of M1b PC,
the subjects who had died of causes other than PC were counted as
closed cases in the calculation of the cause-specific survival
rate, and the significance of differences was assessed with the
log-rank test. For univariate and multivariate analyses, Cox
proportional hazard analysis was employed. All statistical analyses
were performed with the Statistical Package for Social Sciences
(SPSS, Chicago, IL, USA) version 10.0 for Windows. Probability (P)
values of <0.05 were considered statistically significant.
Results
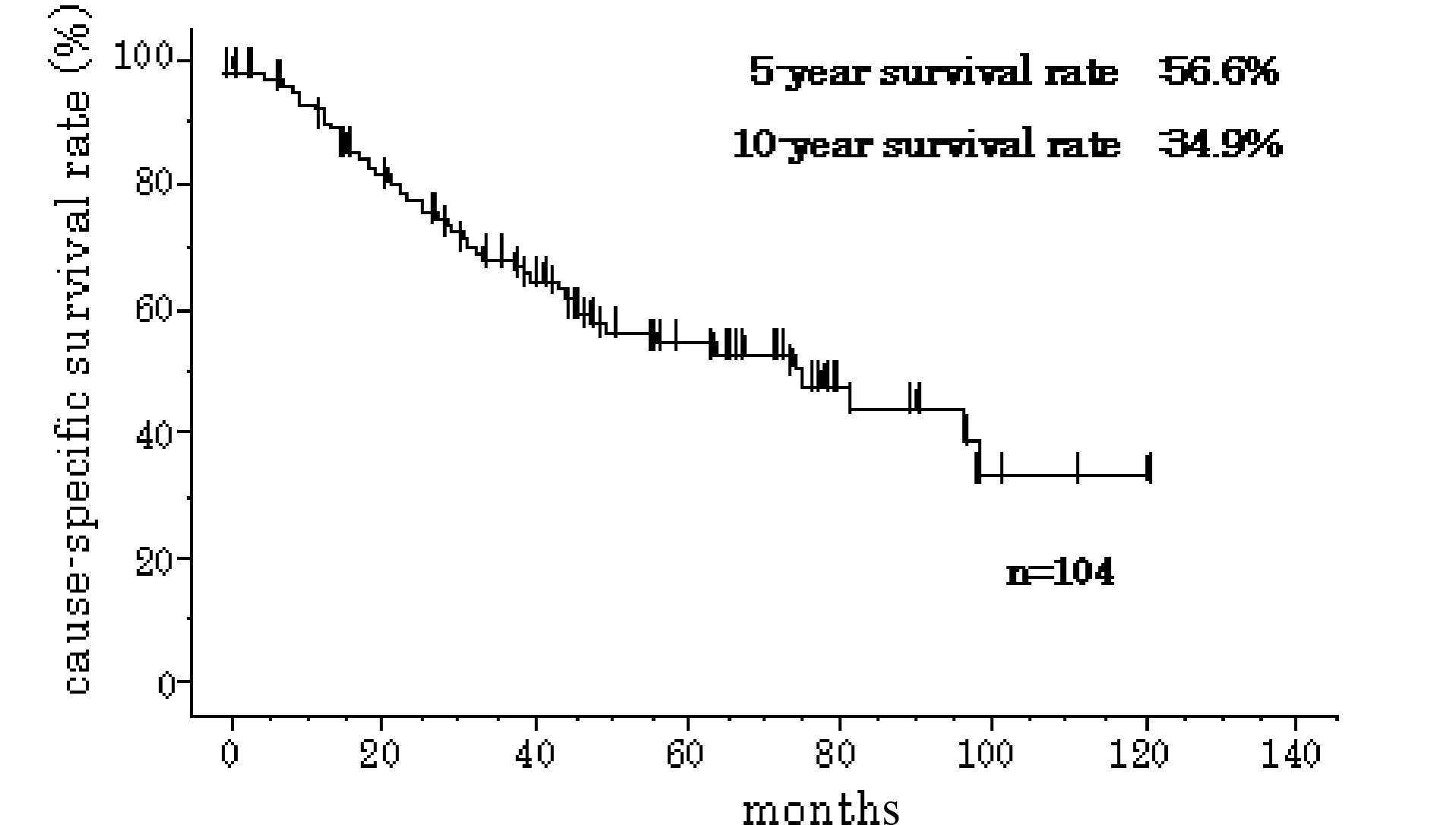
In the 104 subjects, the 5-year cause-specific
survival rate was 56.6% and the 10-year cause-specific survival
rate was 34.9% (Fig. 1).
Each variable was constructed as follows: T
classification, T1–3 vs. T4; N classification, N0 vs. N1; GS, 7 vs.
≥8 (since there was no subject with GS ≤6); pre-treatment PSA
level, <192 vs. ≥192 (since a significant difference in survival
rate was observed between these groups); EOD grade, 1+2 vs. 3+4;
ALP, LDH and Ca levels, normal values vs. high values (defined as
at least 1.15 times higher than the upper limit of normal); PLT and
Hb levels, normal values vs. low values (defined as not more than
0.85 times lower than the lower limit of the normal).
The log-rank test identified the following factors
with statistically significant differences: pre-treatment PSA ≥192,
N1, GS ≥8, EOD grade 3+4, high LDH, high ALP and low Hb. Univariate
analysis identified the same factors with statistically significant
differences. The hazard ratio was the highest at 5.612 for GS ≥8
(Table II). Multivariate Cox
proportional hazard analysis was performed on the factors
identified with significant differences by univariate analysis, and
determined the factors GS ≥8 and high LDH with statistically
significant differences with a hazard ratio of 4.967 and 2.728,
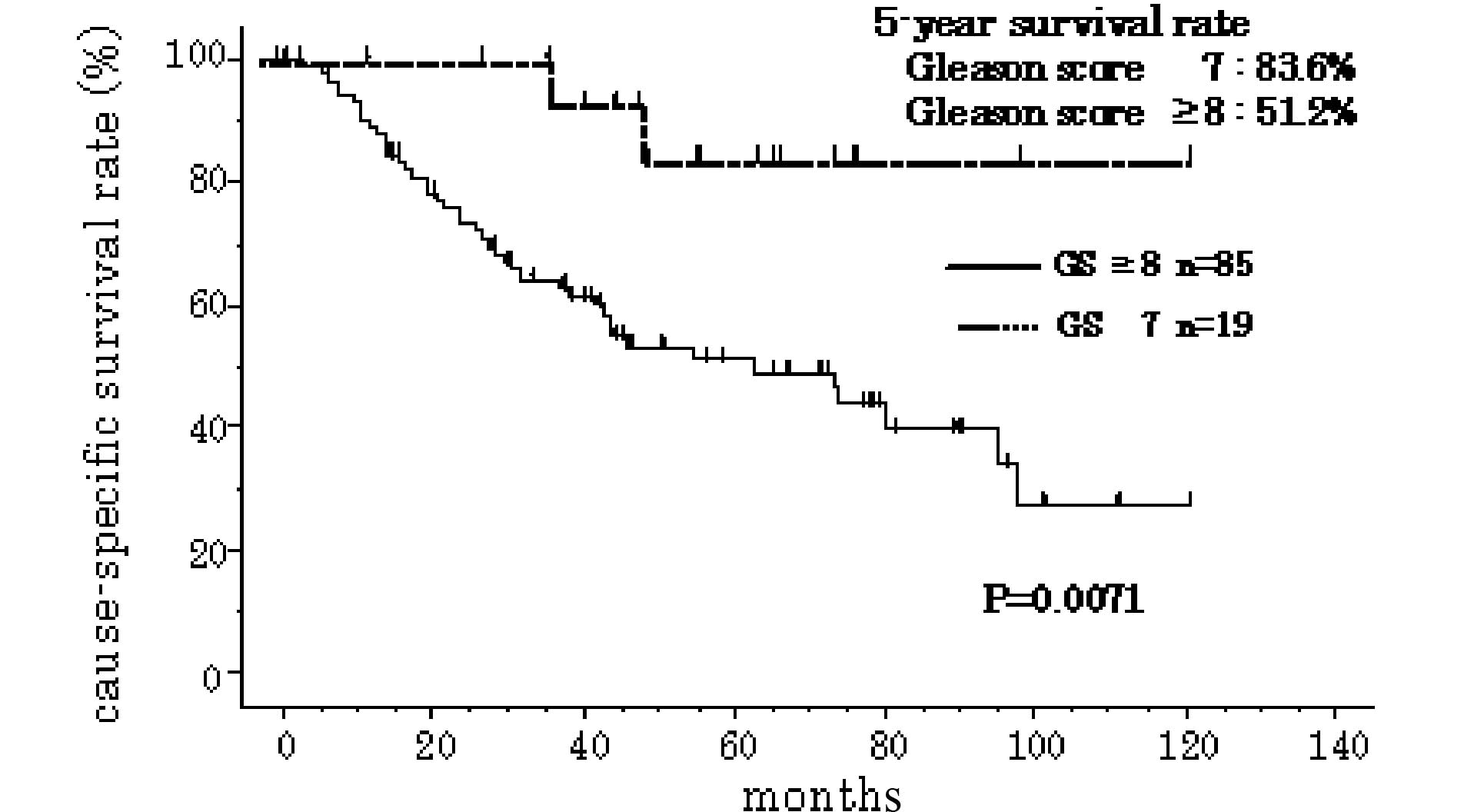
respectively (P=0.029 and 0.004, respectively) (Table III). The 5-year cause-specific
survival rate was 83.6 and 51.2%, respectively, in subjects with GS
7 and GS ≥8 (Fig. 2), while it was
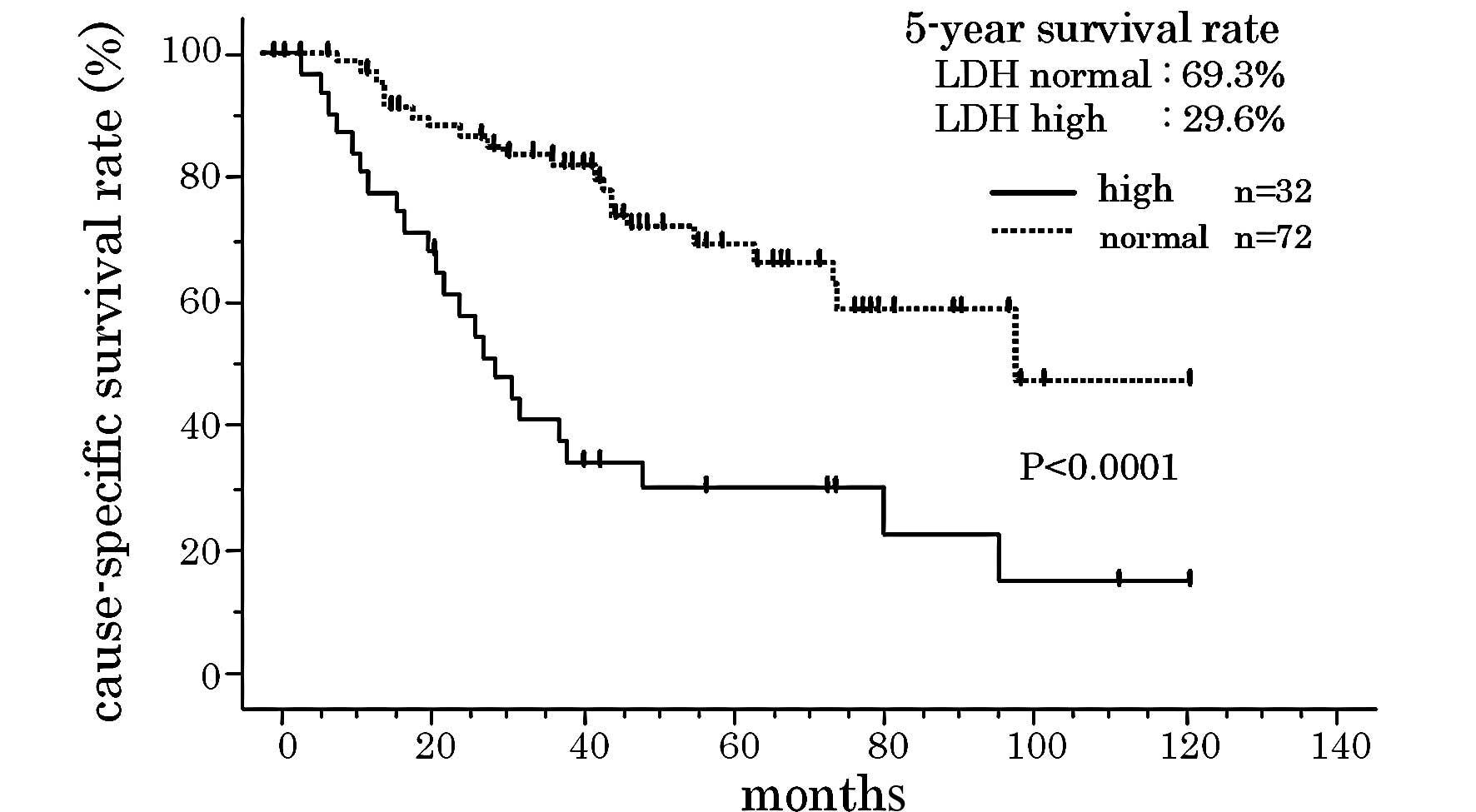
69.3 and 29.6%, respectively, in subjects with normal and high LDH
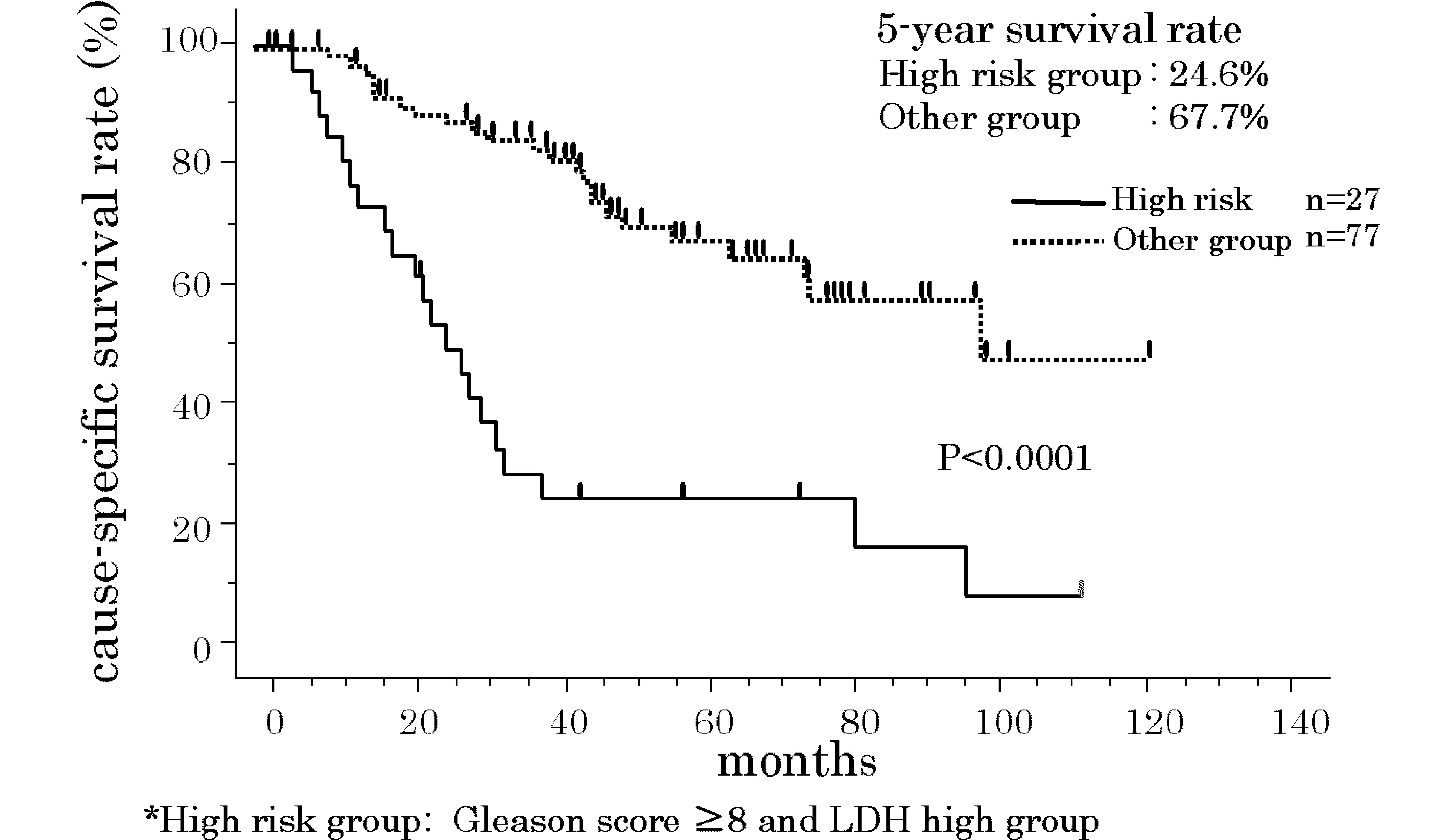
(P=0.0071 and <0.0001, respectively) (Fig. 3). When subjects with GS ≥8 and high
LDH were classified as the high-risk group, the 5-year
cause-specific survival rate was 24.6%. Outcome was significantly
poorer in this group than in the other group, which had a 5-year
cause-specific survival rate of 67.7% (P<0.0001) (Fig. 4).
 | Table II.Results of the univariate Cox
proportional hazard analysis method and log-rank test. |
Table II.
Results of the univariate Cox
proportional hazard analysis method and log-rank test.
| Factors | Univariate hazard
ratio (95% Cl) | P-value | 5-year cause specific
survival rate (%) | 10-year cause
specific survival rate (%) | Log-rank test
P-value |
|---|
| Pre-treatment PSA
level (ng/ml) | | | | | |
| <192 | 1 | | 70.1 | 42.8 | |
| ≥192 | 1.98
(1.064–3.685) | 0.0311 | 45.8 | - | 0.0278 |
| T stage | | | | | |
| T1–3 | 1 | | 60.5 | 43.0 | |
| T4 | 1.285
(0.706–2.338) | 0.4123 | 54.4 | - | 0.3374 |
| N stage | | | | | |
| N0 | 1 | | 67.1 | 50.6 | |
| N1 | 2.206
(1.207–4.034) | 0.0102 | 41.3 | - | 0.0083 |
| Gleason score | | | | | |
| 7 | 1 | | 83.6 | 83.6 | |
| ≥8 | 5.612
(1.358–23.194) | 0.0172 | 51.2 | 27.1 | 0.0071 |
| EOD | | | | | |
| 1+2 | 1 | | 60.8 | 39.3 | |
| 3+4 | 1.978
(1.006–3.889) | 0.0479 | 37.3 | - | 0.0433 |
| LDH | | | | | |
| Normal | 1 | | 69.3 | 46.9 | |
| High | 3.307
(1.835–5.959) | <0.0001 | 29.6 | 14.8 | <0.0001 |
| ALP | | | | | |
| Normal | 1 | | 69.6 | 65.9 | |
| High | 2.903
(1.559–5.405) | 0.0008 | 41.7 | 13.7 | 0.0004 |
| Hb | | | | | |
| Normal | 1 | | 72.8 | 50.4 | |
| Low | 2.203
(1.168–4.155) | 0.0147 | 43.4 | - | 0.0122 |
| PLT | | | | | |
| Normal | 1 | | 57.4 | 38.9 | |
| Low | 1.027
(0.519–2.033) | 0.9392 | 52.2 | 27.9 | 0.9391 |
| Ca | | | | | |
| Normal | 1 | | 57.5 | 38.1 | |
| High | 1.414
(0.595–3.358) | 0.4328 | 51.1 | - | 0.4293 |
 | Table III.Results of the multivariate Cox
proportional hazard analysis method. |
Table III.
Results of the multivariate Cox
proportional hazard analysis method.
| Factors | Hazard ratio (95%
CI) | P-value |
|---|
| PSA (<192 vs.
≥192 ng/ml) | 1.315
(0.658–2.628) | 0.438 |
| N classification
(N0 vs. N1) | 1.489
(0.786–2.821) | 0.223 |
| Gleason score (7
vs. ≥8) | 4.967
(1.174–21.01) | 0.029 |
| EOD (1+2 vs.
3+4) | 1.232
(0.539–2.814) | 0.620 |
| LDH (normal vs.
high) | 2.728
(1.366–5.449) | 0.004 |
| ALP (normal vs.
high) | 1.829
(0.881–3.798) | 0.105 |
| Hb (normal vs.
low) | 1.037
(0.491–2.192) | 0.924 |
Discussion
Approximately 80% of patients with M1b PC respond to
endocrine therapy performed as initial treatment. However, the
5-year survival rate is known to be as low as 30% in patients with
M1b PC in Japan, since more than half of the patients become
resistant to endocrine therapy within several months to several
years (11).
Endocrine resistance is considered to be acquired
through abnormalities in the androgen receptor as well as a
mechanism not mediated by the androgen receptor. Abnormalities in
the androgen receptor include i) androgen receptor amplification
(which allows a small amount of androgen to react), ii) androgen
receptor gene mutations, iii) abnormalities in coactivators which
potentiate the transcriptional activity of the androgen receptor,
and iv) androgen receptor activation caused by abnormal production
of growth factors or cytokines. We previously reported that HER-2
overexpression in prostate biopsy specimens is an important
predictive factor for the acquisition of resistance to endocrine
therapy and outcome (12). On the
other hand, mechanisms not mediated by the androgen receptor
include i) evasion of apoptosis caused by abnormalities in
apoptosis-related genes and ii) the appearance and proliferation of
neuroendocrine cells. We also previously reported that
neuroendocrine cell differentiation in prostate biopsy specimens is
involved in the acquisition of resistance to endocrine therapy
(13). Debes and Tindall (7) suggested that these abnormalities do
not occur independently, but are involved in the acquisition of
endocrine resistance in a complicated manner, but this hypothesis
has not yet been verified.
In the present study, the 5-year cause-specific
survival rate was 56.6% and the 10-year cause-specific survival
rate was 34.9%. These favorable results may be attributable to the
short mean observation period of 47 months. Some patients with M1b
survive for a long time and, thus, it is sometimes difficult to
accurately predict the outcome. With regard to predictive factors
for the outcome of M1b PC, some studies recently identified:
performance scale, GS, response to endocrine therapy, anemia and
levels of serum albumin, LDH, ALP and PSA (14,15),
while another study showed that EOD grade and interleukin-6 were
good predictive factors (16).
Still, another study reported that serum cholesterol and
interleukin-6 levels are involved in cachexia (17). Thus, no consensus has been reached.
In the present study, log-rank test and univariate analysis
identified the factors: pre-treatment PSA ≥192, N1, GS ≥8, EOD
grade 3+4, high LDH, high ALP and low Hb with statistically
significant differences.
The presence or absence of lymph node metastasis and
GS are involved in the outcome of PC (18–20)
and are widely known to be clinically important indicators. During
the present study, no patient with bone metastasis had GS ≤6. This
finding suggests that active surveillance as recommended by Parker
(21) and Dall'Era et al
(22) is indicated for patients
with GS ≤6. During the present study, multivariate Cox proportional
hazard analysis identified the factors GS ≥8 and high LDH with
significant differences, and more than half of such patients died
within 2 years. Such patients have a very poor outcome and should
be classified as the high-risk group. The present study targeted
patients with a Karnofsky performance score of 70% or more who
could be treated on an outpatient basis, although hospital visits
were restricted due to pain, but patients could eat well and take
care of themselves. This may have allowed more accurate
identification of prognostic factors for M1b PC.
LDH is an intracellular enzyme widely distributed
throughout the tissues of the body. The serum LDH level increases
when any tissue is injured and LDH is released into the blood. It
is generally measured for screening during initial treatment, and
the fractionation of isozymes is useful for determining the injured
organ. The serum LDH level is known to become abnormally high in
the presence of diseases including acute myocardial infarction,
acute hepatitis, leukemia and malignant lymphoma. The serum LDH
level is known to become abnormally high in the presence of
testicular tumors and is used as an indicator of therapeutic
effect. However, only limited types of malignant tumors are
associated with high values in the early stage. Therefore, the
serum LDH level is generally used as a predictive factor of outcome
or an indicator of therapeutic effect or worsening of symptoms. Few
patients with M1b PC have increased LDH in the early stage. Some
studies showed that serum LDH level is a predictive factor for PC
with resistance to endocrine therapy (23,24),
while other studies reported the opposite (15,16).
Thus, no consensus has been reached. In the present study, however,
patients with high LDH had a very poor outcome since, in patients
with high LDH, cancer cells have great proliferative capacity and
thus a shorter cell cycle which results in increased necrotic
cells, and also because cancer cells potentiate the destruction of
normal tissue at sites of metastasis. Therefore, the LDH level may
be employed as an indicator of tissue destruction in patients with
M1b PC.
Patients with M1b PC with GS ≥8 and high
pre-treatment LDH may be effectively treated by endocrine therapy
combined with docetaxel as reported by Tannock et al
(25) and Petrylak et al
(26), but therapy with the latter
requires further study.
In conclusion, the present study revealed that
patients with M1b PC with GS ≥8 and high LDH had a very poor
outcome and thus should be treated as a high-risk group requiring
close follow-up.
References
|
1.
|
Bray F, Sankila R, Ferlay J and Parkin DM:
Estimates of cancer incidence and mortality in Europe in 1995. Eur
J Cancer. 38:99–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Black RJ, Bray F, Ferlay J and Parkin DM:
Cancer incidence and mortality in the European Union: cancer
registry data and estimates of national incidence for 1990. Eur J
Cancer. 33:1075–1107. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Parkin DM, Pisani P and Ferlay J: Global
cancer statistics. CA Cancer J Clin. 49:33–64. 1999. View Article : Google Scholar
|
|
4.
|
Hinotsu S: An international comparison of
epidemiologic factors of prostate cancer. Nippon Rinsho.
65:171–177. 2007.
|
|
5.
|
Schröder FH, Hugosson J, Roobol MJ, et al:
Screening and prostate-cancer mortality in a randomized European
study. N Engl J Med. 360:1320–1328. 2009.PubMed/NCBI
|
|
6.
|
Ito K, Kubota Y, Yamamoto T, et al: The
change of mass screening system for prostate cancer in Gunma
Prefecture: present state, and problems for 18 years. Jpn J Urol
Surg. 13:997–1001. 2000.
|
|
7.
|
Debes JD and Tindall DJ: Mechanisms of
androgen-refractory prostate cancer. N Engl J Med. 351:1488–1490.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: The 2005 International Society of Urological Pathology
(ISUP) consensus conference on Gleason grading of prostatic
carcinoma. Am J Surg Pathol. 29:1228–1242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sobin LH and Wittekind Ch: UICC TNM
Classification of Malignant Tumors. 6th edition. Wiley-Liss Inc;
New York: pp. 184–187. 2002
|
|
10.
|
Soloway MS, Hardeman SW, Hickey D, Raymond
J, Todd B, Soloway S and Moinuddin M: Stratification of patients
with metastatic prostate cancer based on extent of disease on
initial bone scan. Cancer. 61:195–202. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kumamoto Y, Tsukamoto T, Umehara T, et al:
Clinical studies on endocrine therapy for prostatic carcinoma (2):
prognosis of patients with prostatic carcinoma given endocrine
therapy, and analyses on causes of death and side effects of
endocrine therapy. Hinyokika Kiyo. 36:285–293. 1990.
|
|
12.
|
Nishio Y, Yamada Y, Kokubo H, et al:
Prognostic significance of immunohistochemical expression of the
HER-2/neu oncoprotein in bone metastatic prostate cancer. Urology.
68:110–115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kokubo H, Yamada Y, Nishio Y, et al:
Immunohistochemical study of chromogranin A in stage D2 prostate
cancer. Urology. 66:135–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Smaletz O, Scher HI, Small EJ, et al:
Nomogram for overall survival of patients with progressive
metastatic prostate cancer after castration. J Clin Oncol.
20:3972–3982. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Halabi S, Small EJ, Hayes DF, Vogelzang NJ
and Kantoff PW: Prognostic significance of reverse transcriptase
polymerase chain reaction for prostate-specific antigen in
metastatic prostate cancer: a nested study within CALGB 9583. J
Clin Oncol. 21:490–495. 2003. View Article : Google Scholar
|
|
16.
|
Nakashima J, Tachibana M, Horiguchi Y, Oya
M, Ohgashi T, Asakura H and Murai M: Serum interleukin 6 as a
prognostic factor in patients with prostate cancer. Clin Cancer
Res. 6:2702–2706. 2000.PubMed/NCBI
|
|
17.
|
Kuroda K, Nakashima J, Kanao K, et al:
Interleukin 6 is associated with cachexia in patients with prostate
cancer. Urology. 69:113–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Murphy GP and Whitmore WF Jr: A report of
the workshops on the current status of the histologic grading of
prostate cancer. Cancer. 44:1490–1494. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bostwick DG: Grading prostate cancer. Am J
Clin Pathol. 102(Suppl 1): 38–56. 1994.
|
|
20.
|
Kambara T, Oyama T, Segawa A, Fukabori Y
and Yoshida KI: Prognostic significance of global grading system of
Gleason score in patients with prostate cancer with bone
metastasis. BJU Int. Nov 13–2009.(E-pub ahead of print).
|
|
21.
|
Parker C: Active surveillance: towards a
new paradigm in the management of early prostate cancer. Lancet
Oncol. 5:101–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Dall'Era MA, Konety BR, Cowan JE, et al:
Active surveillance for the management of prostate cancer in a
contemporary cohort. Cancer. 112:2664–2670. 2008.
|
|
23.
|
Halabi S, Small EJ, Kantoff PW, et al:
Prognostic model for predicting survival in men with
hormone-refractory metastatic prostate cancer. J Clin Oncol.
21:1232–1237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Scher HI, Kelly WM, Zhang ZF, et al:
Post-therapy serum prostate-specific antigen level and survival in
patients with androgen-independent prostate cancer. J Natl Cancer
Inst. 91:244–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Tannock IF, de Wit R, Berry WR, et al:
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Petrylak DP, Tangen CM, Hussain MH, et al:
Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|