Introduction
Persistent hepatitis C virus (HCV) infection results
in reactive oxygen species (ROS) in the liver (1,2), and
the oxidative stress induced by HCV infection leads to lipid
peroxidation (3), eventually
progressing to hepatic steatosis (4,5).
Hepatic steatosis is usually observed, not only in
patients with chronic hepatitis C (CH-C), but also in those with
alcoholism (6), obesity and
diabetes mellitus (DM) (7), and
patients who have undergone treatment with tetracycline or
corticosteroid (8). Previous
studies have found hepatic steatosis in approximately 30–70% of
patients with CH-C (9–16). The degree of hepatic steatosis in
the majority of these patients was mild to moderate. The prevalence
of severe hepatic steatosis, occupying more than 50% of
hepatocytes, appears to be relatively low in patients with
CH-C.
The clinical significance of hepatic steatosis in
patients with CH-C has been well established. Obesity (10–13,15),
insulin resistance (14,15), hepatic fibrosis (9,12,13,16)
and HCV genotype 3 (10–13,16)
are independently associated with hepatic steatosis upon
multivariate analysis. Hepatic steatosis also appears to be a
predictive hallmark of poor response to antiviral treatments in
patients with CH-C (16,17). In addition, hepatic steatosis may
be a possible indicator of progression to hepatocellular carcinoma
(18,19).
Persistent HCV infection often evokes autoimmune
phenomena, such as the production of numerous types of
autoantibodies and/or concomitant autoimmune diseases (20,21).
Recent studies have revealed the identification of oxidatively
modified autoantigens, including oxidized low-density lipoprotein
(ox-LDL), from the sera of patients with systemic lupus
erythematosus (SLE) (22) and type
1 DM (23). The main purpose of
this study was to investigate whether an immune response to ox-LDL
may be involved during the process of hepatic steatosis in patients
with CH-C.
Materials and methods
Study population
Forty-two patients who had detectable serum HCV-RNA
by polymerase chain reaction (PCR) and showed histological findings
compatible with CH-C were randomly selected for participation in
this study. Administration of pegylated interferon (PEG-IFN) alone
or PEG-IFN plus ribavirin was carried out for 24 or 48 weeks in all
of the enrolled patients after percutaneous liver biopsy. The
evaluation of the antiviral treatment was carried out in 34 of the
42 patients with CH-C.
Clinical assessments
Age at entry, gender and the prevalence of type 2 DM
were examined in the enrolled patients. Obesity was evaluated by
body mass index (BMI) which was calculated in accordance with the
formula: weight (kg) divided by height2
(m2).
Laboratory assessments
Anti-ox-LDL levels were determined using
commercially available enzyme-linked immunosorbent assay (ELISA)
kits (Biomedica, Vienna, Austria). Biochemical tests, including
analysis of alanine aminotransferase (ALT), total choresterol
(T-Cho) and triglyceride (TG) levels, were carried out before the
antiviral treatment. Insulin resistance was determined by the
Homeostasis Model for Assessment of Insulin Resistance (HOMA-IR)
method using the following equation: HOMA-IR = fasting insulin
(μU/ml) x fasting glucose (mg/dl)/405. Quantitative detection of
serum HCV-RNA was performed by the Amplicor-HCV monitor assay
(Roche Molecular Diagnostics, Tokyo, Japan) (24). The HCV genotype was determined by
the HCV-RNA genotyping assay system (Home Brew SRL Inc., Tokyo,
Japan) (25). Sustained viral
response (SVR) was defined as an absence of HCV-RNA in serum 24
weeks after the completion of the treatment. No biochemical or
virological response to the treatment was regarded as non-SVR. As
immunoserological assessments, antinuclear antibody (ANA) and serum
immunoglobulin G (IgG) levels were measured. ANA was tested at a
serum dilution of 1:40 by the indirect immunofluorescence method
using HEp-2 cells as substrates. Positive reactions were titered by
double dilution to the end point.
Histological assessments
Liver tissue specimens were obtained by liver biopsy
under the guidance of ultrasound using 16-gauge needles before
treatment. The tissue samples were fixed in 10% formalin and
embedded in paraffin. The tissue sections were stained with H&E
for morphological evaluation. The severity of hepatic steatosis was
graded on the basis of the classification proposed by Brunt and
colleagues (26). Briefly,
steatosis observed in none, <33, 33–66 or >66% of hepatocytes
was defined as grades 0, 1, 2 or 3, respectively. Fibrosis and
necroinflammation in the liver were evaluated in accordance with
the New Inuyama Classification system (27) and the histological activity index
(HAI) scores designed by Knodell et al (28). The staging of hepatic fibrosis was
classified from F0 to F4. F0 was defined as no fibrosis in the
tissue specimen, while F4 was defined as liver cirrhosis.
Statistical analyses
Data values are represented as the mean ± standard
deviation (SD). The Mann-Whitney U test was applied for comparison
of continuous variables. Linear regression analysis was used to
analyze the relationships between titers of anti-ox-LDL and serum
IgG, ALT levels or values of HOMA-IR. We used the Fisher's exact
test to compare the differences in frequencies. p-values of
<0.05 were considered to indicate a significant difference
between groups.
Results
Demographic features of the enrolled
patients with CH-C
Among the enrolled patients, 22 cases were male and
20 were female. HCV genotypes of the enrolled patients were 1b in
26 (62%) patients, 2a in 11 (26%) and 2b in 5 (12%) patients. The
ages at entry in the enrolled patients ranged from 23 to 76
years.
As shown in Table
I, 22 (52%) of the 42 patients with CH-C had no hepatic
steatosis (grade 0), while 12 (29%) patients had hepatic steatosis
of grade 1 and 8 (19%) of grade 2. None of the patients showed
grade 3 hepatic steatosis. There were no significant differences in
age or gender between CH-C patients with grade 0, 1 or 2 hepatic
steatosis. The prevalence of concurrent type 2 DM in CH-C patients
with grade 2 hepatic steatosis was higher than that in the other
groups, although no significant differences were apparent. The mean
BMI in the grade 2 group was significantly higher than that in the
grade 0 group (27.2±3.7 vs. 22.0±2.8, p=0.0004) and that in the
grade 1 group (27.2±3.7 vs. 23.3±2.6, p=0.0115). The severity of
hepatic steatosis was independent of HCV genotypes and loads of
HCV-RNA (data not shown).
 | Table I.Demographic characteristics of the
enrolled patients with CH-C. |
Table I.
Demographic characteristics of the
enrolled patients with CH-C.
| Hepatic steatosis
| |
|---|
| Grade 0 (n=22) | Grade 1 (n=12) | Grade 2 (n=8) | p-value |
|---|
| Age (years) | 57.8±13.3 | 59.2±10.0 | 63.4±9.1 | NS |
| Gender
(male/female) | 11/11 | 6/6 | 5/3 | NS |
| HCVgenotype
(1b/2a/2b) | (13/6/3) | (9/2/1) | (4/3/1) | NS |
| Loads of HCV-RNA
(KIU/ml) | 996±899 | 1555.0±1519 | 1,098.0±504 | NS |
| BMI
(kg/m2) |
22±2.8a |
23.3±2.6b |
27.2±3.7a,b |
p=0.0004a |
| | | |
p=0.0115b |
| Concurrent type 2
DM | 6 (27%) | 1 (8%) | 4 (50%) | NS |
Correlation of hepatic steatosis with
laboratory findings in patients with CH-C
Table II summarizes
the values of the biochemical and immunological parameters measured
in each group. There were no significant differences in serum ALT,
T-Cho or TG levels among the three groups. CH-C patients with grade
1 or 2 hepatic steatosis had significantly higher values of serum
IgG levels than those with grade 0 hepatic steatosis (1,868±382 vs.
1,465±196 mg/dl, p=0.0003; 1,999±340 vs. 1,465±196 mg/dl,
p<0.0001). The prevalence of ANA in CH-C patients with grade 2
hepatic steatosis was higher than ANA in the other groups, although
no significant differences were found among the three groups.
 | Table II.Laboratory data of the patients
according to hepatic steatosis group. |
Table II.
Laboratory data of the patients
according to hepatic steatosis group.
| Hepatic steatosis
| |
|---|
| Grade 0 (n=22) | Grade 1 (n=12) | Grade 2 (n=8) | p-value |
|---|
| ALT (IU/l) | 81±64 | 69±37 | 112±66 | NS |
| TG (mg/dl) | 89±40 | 89±35 | 118±35 | NS |
| T-Cho (mg/dl) | 157±39 | 162±29 | 162±39 | NS |
| IgG (mg/dl) |
1,465±196a,b |
1,868±382a |
1,999±340b |
p=0.0003a |
| | | |
p<0.0001b |
| Prevalence of
ANA | 5 (23%) | 4 (33%) | 4 (50%) | NS |
Correlation of hepatic steatosis with
anti-ox-LDL
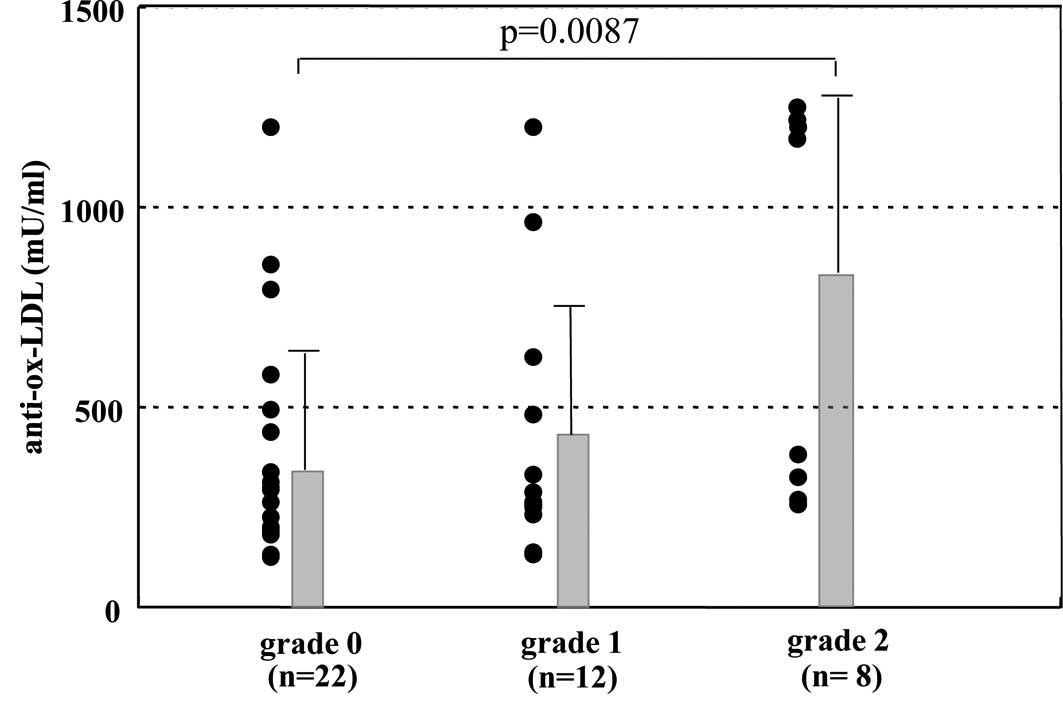
The levels of anti-ox-LDL in each group are shown in
Fig. 1. The mean titer of
anti-ox-LDL in patients with grade 2 hepatic steatosis was
significantly higher than that in patients with grade 0 hepatic
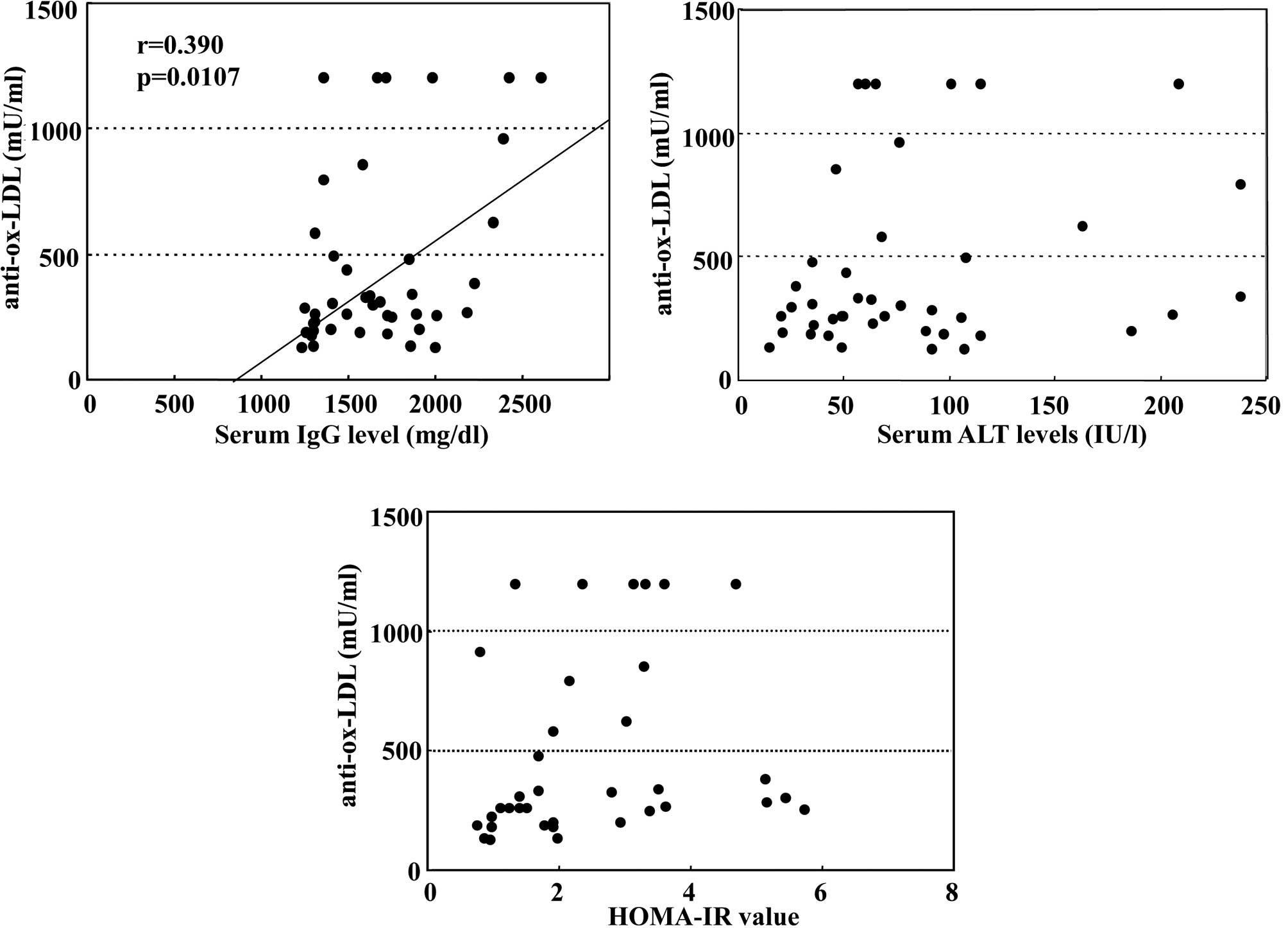
steatosis (754±479 vs. 361±274 mU/ml, p=0.0165). Fig. 2A demonstrates the close correlation
between titers of anti-ox-LDL and serum IgG levels (r=0.390,
p=0.0107). However, the titers of this autoantibody were not
associated with serum ALT levels (r=0.208, p=0.1865) or values of
HOMA-IR (r=0.192, p=0.2627), as shown in Fig. 2B and C, respectively.
Relationship between titers of
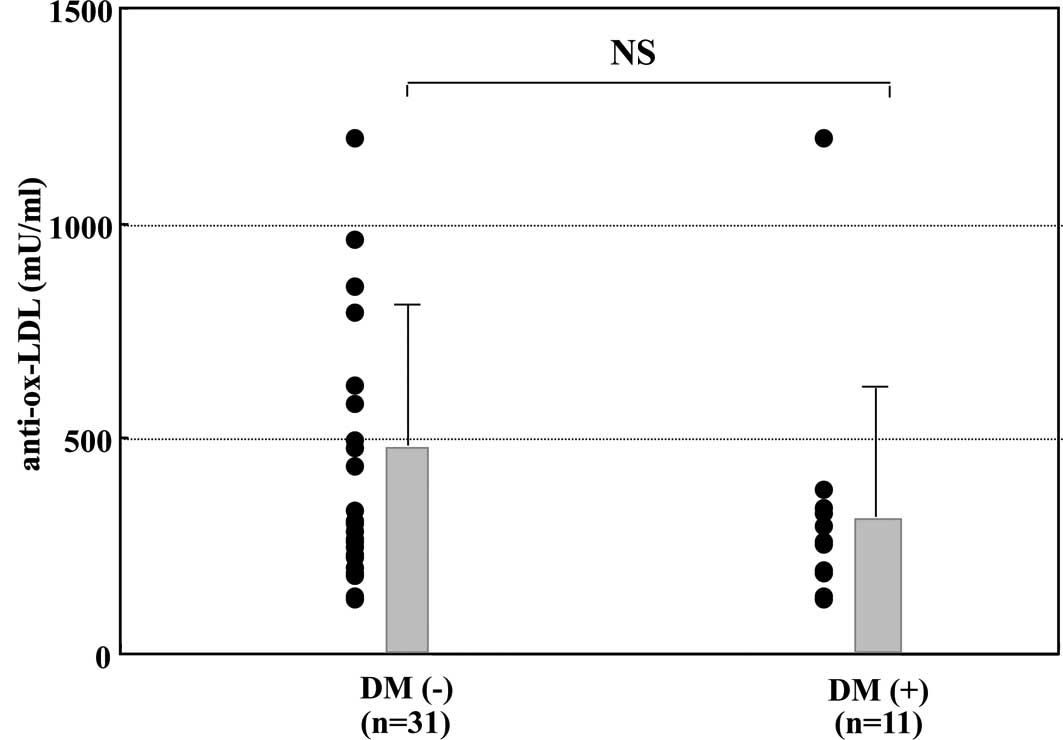
anti-ox-LDL and concurrent type 2 DM
The levels of anti-ox-LDL in CH-C patients with type
2 DM were compared to those in CH-C patients without type 2 DM. As
shown in Fig. 3, there was no
significant difference in the levels of the autoantibodies between
CH-C patients with type 2 DM and those without type 2 DM (337±298
vs. 497±376 mU/ml, p=0.2518).
Relationship between the titers of
anti-ox-LDL and the effect of antiviral treatment
The effect of antiviral treatment was evaluated in
20 patients with CH-C of genotype 1b and 14 patients with CH-C of
genotype 2a/2b. Among patients with CH-C of genotype 1b, 2 (25%) of
8 patients with grade 0 hepatic steatosis and 3 (38%) of 8 patients
with grade 1 hepatic steatosis acquired SVR, while none (0%) of
those with grade 2 hepatic steatosis did. The frequency of F3 in
patients with CH-C of genotype 1b who acquired SVR was
significantly lower than those who exhibited non-SVR (20 vs. 80%,
p=0.0176), which suggests that hepatic fibrosis affected the
outcome of antiviral treatment in patients with CH-C of genotype 1b
(Table III). However, there was no
significant difference in the levels of anti-ox-LDL between
patients who acquired SVR and those who showed non-SVR (273±118 vs.
521±424 mU/ml, p=0.2204) (Table
III). On the other hand, the levels of anti-ox-LDL were also
independent of the effect of antiviral treatment in CH-C patients
of genotype 2a/2b (477±376 mU/ml in the SVR group vs. 554±232 mU/ml
in the non-SVR group, p=0.2424).
 | Table III.Efficacy of the antiviral treatment
in patients with CH-C of genotype 1b. |
Table III.
Efficacy of the antiviral treatment
in patients with CH-C of genotype 1b.
| SVR (n=5) | Non-SVR (n=15) | p-value |
|---|
| Age (years) | 58.4±12.5 | 64.7±7.7 | NS |
| ALT (IU/l) | 59.0±33.0 | 81.0±39 | NS |
| Hepatic steatosis
(grade 0/1/2) | 2/3/0 | 6/5/4 | NS |
| Loads of HCV-RNA
(KIU/ml) | 878±971 | 1,249±1,140 | NS |
| Hepatic fibrosis
(F0/F1/F2/F3) | 0/4/0/1 | 0/3/0/12 | p=0.0176 |
| HAI score | 10±4.6 | 11.8±3.9 | NS |
| Anti-ox-LDL
(mU/ml) | 273±118 | 521.0±424 | NS |
Discussion
In this study, we demonstrated that the degree of
hepatic steatosis was significantly associated with the elevation
of serum IgG levels (Table II) and
anti-ox-LDL levels (Fig. 1) in
patients with CH-C, which indicated that these autoantibodies were
produced during the process of hepatic steatosis. These autoimmune
responses were independent of HCV genotypes and loads of HCV-RNA,
which suggests that host factors may contribute to the autoimmune
phenomena. Accumulation of ox-LDL in the liver tissues during the
process of hepatic steatosis has been postulated as a possible
mechanism of autoantibody production in patients with CH-C.
Persistent HCV infection exerts the formation of ROS in the liver
(1,2). Oxidative stress caused by HCV
infection facilitates lipid peroxidation (3) and consequently promotes hepatic
steatosis (4,5). We speculated that oxidative
modification of LDL induced immunogenic epitopes in the LDL
molecule and that ox-LDL accumulated in the liver during the
process of hepatic steatosis. The accumulation of ox-LDL may
trigger the production of anti-ox-LDL. However, there were previous
reports in which no correlation (29) or inverse correlation (30) was found between the levels of
ox-LDL and anti-ox-LDL. Therefore, further studies are required to
confirm this speculation on the production of anti-ox-LDL in
patients with CH-C.
Recently, Vidali and colleagues demonstrated that
the levels of circulating IgG against malondialdehyde (MDA)-albumin
adducts were significantly higher in CH-C patients with hepatic
steatosis than in those without hepatic steatosis (31). This result implies that the
autoimmune response to MDA, which is a product of polyunsaturated
fatty acid peroxidation, is involved in the process of hepatic
steatosis in patients with CH-C.
On the other hand, recent studies have found that
10–30% of patients with non-alcoholic fatty liver disease (NAFLD)
or non-alcoholic steatohepatitis (NASH) have autoantibodies,
including ANA and/or smooth muscle antibodies (SMA), with elevated
serum IgG levels (32,33). These findings suggest that hepatic
steatosis or steatohepatitis are strictly associated with
autoimmune reactions.
By contrast, Czaja and colleagues previously
reported that CH-C patients with hepatic steatosis had lower serum
concentrations of IgG and a lower frequency of ANA than those of
CH-C patients without steatosis (34). However, our data in the present
study are in direct opposition to their results (Table II). This difference may have been
derived from the genotypes of HCV or the genetic backgrounds of
patients with CH-C.
The association of insulin resistance with
autoimmune disorder has been widely discussed. Patients with SLE
frequently exhibit insulin resistance (35). Loria and colleagues showed that
high titers of ANA are favorable indicators of insulin resistance
in patients with NAFLD (32). We
investigated the correlation between the levels of anti-ox-LDL and
insulin resistance in patients with CH-C, but observed no such
correlation (Fig. 2).
Previous reports have found that the emergence of
non-organ-specific autoantibodies, including ANA, SMA or parietal
cell autoantibodies at baseline or an increase in their titers
during antiviral treatments, appear to be a predictive indicator of
a poor response in patients with CH-C (36,37).
Therefore, we examined the relationship between the levels of
anti-ox-LDL and the outcomes of antiviral treatments in the CH-C
patients (Table III). However, the
levels of anti-ox-LDL did not affect the outcomes of antiviral
treatments in the patients with CH-C.
In conclusion, we demonstrated that hepatic
steatosis is strongly associated with the emergence of anti-ox-LDL
in patients with CH-C, which indicates that autoimmune responses to
ox-LDL are involved in the pathogenesis of hepatic steatosis in
patients with CH-C.
References
|
1.
|
Koike K and Miyoshi H: Oxidative stress
and hepatitis C viral infection. Hepatol Res. 34:65–73. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Okuda M, Li K, Beard MR, et al:
Mitochondrial injury, oxidative stress, and antioxidant gene
expression are induced by hepatitis C virus core protein.
Gastroenterology. 122:366–375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Farinati F, Cardin R, De Maria N, et al:
Iron storage, lipid peroxidation and glutathione turn over in
chronic anti-HCV positive hepatitis. J Hepatol. 22:449–456. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lonardo A, Adinolfi LE, Loria P, et al:
Steatosis and hepatitis C virus: mechanisms and significance for
hepatic and extrahepatic disease. Gastroenterology. 126:588–597.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Moriya K, Yotsuyanagi H, Shintani Y, et
al: Hepatitis C virus core protein induces hepatic steatosis in
transgenic mice. J Gen Virol. 78:1527–1531. 1997.PubMed/NCBI
|
|
6.
|
Devenyi P, Rutherdale J, Sereny G and Olin
JS: Clinical diagnosis of alcoholic fatty liver. Am J
Gastroenterol. 54:597–602. 1970.PubMed/NCBI
|
|
7.
|
Murthy VK and Shipp JC: Hepatic steatosis
in diabetes: increased synthesis of triglycerol and impaired
feedback regulation. Trans Assoc Am Physicians. 94:322–332.
1981.PubMed/NCBI
|
|
8.
|
Hill RB and Rude JFP: Cortisone-induced
lipaemia and hepatic steatosis in the male rat. Nature.
210:7331966. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hourigan LF, Macdonald GA, Purdie D, et
al: Fibrosis in chronic hepatitis C correlates significantly with
body mass index and steatosis. Hepatology. 29:1215–1219. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Adinolfi LE, Gambardella M, Andreana A,
Tripodi MF, Utili R and Ruggiero G: Steatosis accelerates the
progression of liver damage of chronic hepatitis C patients and
correlates with specific HCV genotype and visceral obesity.
Hepatology. 33:1358–1364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Monto A, Alonzo J, Watson JJ, Grunfeld C
and Wright TL: Steatosis in chronic hepatitis C: relative
contribution of obesity, diabetes mellitus, and alcohol.
Hepatology. 36:729–736. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hui JM, Kench J, Farrell GC, et al:
Genotype-specific mechanisms for hepatic steatosis in chronic
hepatitis C infection. J Gastroenterol Hepatol. 17:873–881. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Poynard T, Ratziu V, McHutchison J, et al:
Effect of treatment with peginterferon or interferon alfa-2b and
ribavirin on steatosis in patients infected with hepatitis C.
Hepatology. 38:75–85. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Fartoux L, Poujol-Robert A, Guechot J,
Wendum D, Poupon R and Serfaty L: Insulin resistance is a cause of
steatosis and fibrosis progression in chronic hepatitis C. Gut.
54:1003–1008. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Camma C, Bruno S, Marco VD, et al: Insulin
resistance is associated with steatosis in nondiabetic patients
with genotype 1 chronic hepatitis C. Hepatology. 43:64–71. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Soresi M, Tripi S, Franco V, et al: Impact
of liver steatosis on the antiviral response in hepatitis C
virus-associated chronic hepatitis. Liver Int. 26:1119–1125. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Westin J, Lagging M, Dhillon AP, et al:
Impact of hepatic steatosis on viral kinetics and treatment outcome
during antiviral treatment of chronic HCV infection. J Viral Hepat.
14:29–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ohta K, Hamasaki K, Toriyama K, et al:
Hepatic steatosis is a risk factor for hepatocellular carcinoma in
patients with chronic hepatitis C virus infection. Cancer.
97:3036–3043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kumar D, Farrell GC, Kench J and George J:
Hepatic steatosis and the risk of hepatocellular carcinoma in
chronic hepatitis C. J Gastroenterol Hepatol. 20:1395–1400. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Pawlotsky JM, Yahia MB, Andre C, et al:
Immunological disorders in C virus chronic active hepatitis: a
prospective case-control study. Hepatology. 19:841–848. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Manns MP and Rambusch EG: Autoimmunity and
extrahepatic manifestations in hepatitis C virus infection. J
Hepatol. 31:S39–S42. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Vaarala O, Alfthan G, Jauhiainen M, et al:
Crossreaction between antibodies to oxidized low-density
lipoprotein and to cardiolipin in systemic lupus erythematosus.
Lancet. 341:923–925. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hsu RM, Devaraj S and Jialal I:
Autoantibodies to oxidized low-density lipoprotein in patients with
type 2 diabetes mellitus. Clin Chim Acta. 317:145–150. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lau JY, Davis GL, Kinffen J, et al:
Significance of serum hepatitis C virus RNA levels in chronic
hepatitis C. Lancet. 341:1501–1504. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Simmonds P, Alberti A, Alter HJ, et al: A
proposed system for nomenclature of hepatitis C viral genotypes.
Hepatology. 19:1321–1324. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Brunt EM, Janney CG and Bisceglie D:
Nonalcoholic steatohepatitis: a proposal for grading and staging
the histological lesions. Am J Gastroenterol. 94:2467–2474. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ichida F, Tsuji T, Omata M, et al: New
Inuyama classification: new criteria for histological assessment of
chronic hepatitis. Int Hepatol Commun. 6:112–119. 1996. View Article : Google Scholar
|
|
28.
|
Knodel RG, Ishak KG, Black WC, et al:
Formulation and application of a numerical scoring system for
assessing histological activity in asymptomatic chronic active
hepatitis. Hepatology. 1:431–435. 1981. View Article : Google Scholar
|
|
29.
|
Becarevic M, Singh S and Majkic-Singh N:
Oxidized LDL, anti-oxidized LDL and anti-annexin A5 antibodies in
primary antiphospholipid syndrome. Clin Lab. 54:97–101.
2008.PubMed/NCBI
|
|
30.
|
Shoji T, Nishizawa Y, Fukumoto M, et al:
Inverse relationship between circulating oxidized low-density
lipoprotein (oxLDL) and anti-oxLDL antibody levels in healthy
subjects. Atherosclerosis. 148:171–177. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Vidali M, Tripodi MF, Ivaldi A, et al:
Interplay between oxidative stress and hepatic steatosis in the
progression of chronic hepatitis C. J Hepatol. 48:399–406. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Loria P, Lonardo A, Leonardi F, et al:
Non-organ-specific autoantibodies in nonalcoholic fatty liver
disease: prevalence and correlates. Dig Dis Sci. 48:2173–2181.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Adams LA, Lindo KD and Angulo P: The
prevalence of autoantibodies and autoimmune hepatitis in patients
with nonalcoholic fatty liver disease. Am J Gastroenterol.
99:1316–1320. 2004. View Article : Google Scholar
|
|
34.
|
Czaja AJ, Carpenter HA, Santrach PJ and
Moore SB: Host- and disease-specific factors affecting steatosis in
chronic hepatitis C. J Hepatol. 29:198–206. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Escarcega RO, Garcia-Carrasco M,
Fuentes-Alexandro S, et al: Insulin resistance, chronic
inflammatory state and the link with systemic lupus
erythematosus-related coronary disease. Autoimmun Rev. 6:48–53.
2006. View Article : Google Scholar
|
|
36.
|
Gatselis NK, Georgiadou SP, Koukoulis GK,
et al: Clinical significance of organ- and non-organ-specific
autoantibodies on the response to anti-viral treatment of patients
with chronic hepatitis C. Aliment Pharmacol Ther. 24:1563–1573.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Wasmuth HE, Stolte C, Geier A, et al: The
presence of non-organ-specific autoantibodies is associated with a
negative response to combination therapy with interferon and
ribavirin for chronic hepatitis C. BMC Infect Dis. 4:1–8. 2004.
View Article : Google Scholar : PubMed/NCBI
|