Introduction
In clinical practice, blood pressure measurement at
home is employed for non-office blood pressure monitoring, which is
not possible at outpatient clinics. However, ambulatory blood
pressure monitoring (ABPM), which facilitates the assessment of
24-h blood pressure changes, is necessary to measure the nocturnal
blood pressure, which may be associated with organ disorders
(1). In Japan, National Health
Insurance coverage of ABPM began in April 2008. In The Japanese
Society of Hypertension Guidelines for the Management of
Hypertension (JSH2009) (2), the
importance of 24-h blood pressure monitoring was also described as
an additional section. Therefore, ABPM may be increasingly applied
in hypertension treatment. However, in Japan few studies have
presented data on the effects of various anti-hypertensive agents
on 24-h blood pressure changes.
Irbesartan (3)
became commercially available in July 2008 as the sixth angiotensin
II type 1 receptor blocker (ARB) in Japan. Since this agent is
characterized by a half-life of 10.2-15.4 h (4) and strong binding to AT1
receptors (5), it may exhibit
continuous anti-hypertensive effects over 24 h (6). However, no data on 24-h blood
pressure involving Japanese outpatients have been published. In
this study, we investigated 24-h blood pressure changes in patients
receiving irbesartan to evaluate its anti-hypertensive/persistent
effects. In addition, we measured the urinary albumin and serum
creatinine levels.
Patients and methods
Among patients newly diagnosed with hypertension in
the three clinics participating in the study, those without a
history of anti-hypertensive therapy within 4 weeks before the
start of irbesartan administration, who were able to consult the
outpatient clinic every 2 weeks and who consented to participate in
this study, were registered as subjects. Physicians were prohibited
from additionally administering other anti-hypertensive agents. As
a rule, agents that had been prescribed (for other concomitant
disorders) were not changed during the study period.
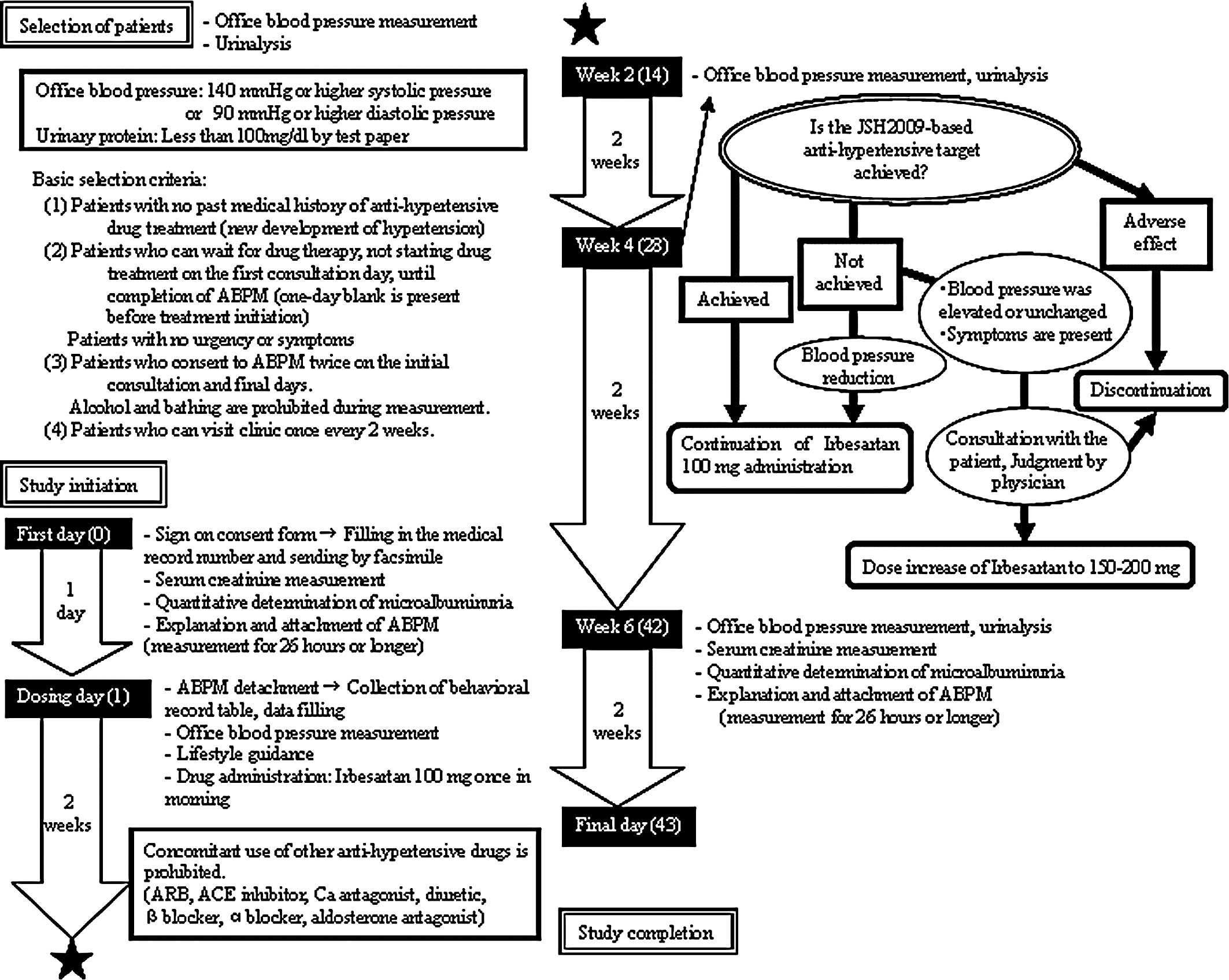
Thirty-two patients were enrolled between December
2008 and May 2009. As shown in the flow chart (Fig. 1), the criteria for registration
included systolic or diastolic office blood pressure of ≥140/90
mmHg and age ranging from 20 to 75 years, regardless of gender and
complications. Patients with a urinary protein level of ≥100 mg/dl
by urine test paper were excluded. The daily dose of irbesartan was
100 mg (once-a-day administration); however, if necessary, the dose
could be increased to 200 mg based on the attending physician’s
evaluation. The blood pressure and pulse were measured along with
examination for general symptoms at consultations before the start
and after 2, 4 and 6 weeks of administration. A blood test and
urinalysis were conducted to determine the serum creatinine and
urinary albumin levels before and 6 weeks after the start of
administration. At these points, an ABPM device was worn for ≥26 h
from the time of consultation until the following day. The blood
pressure was measured at 1-h intervals. For each patient, the mean
of the values obtained between 9:00 pm and 5:00 am was regarded as
the nocturnal blood pressure. The mean of the values obtained
between 8:00 am and 6:00 pm was regarded as the daytime blood
pressure. As an index of blood pressure changes early in the
morning, differences in the mean blood pressure between two
intervals: 3:00–5:00 am and 7:00–9:00 am were calculated. The
estimated glomerular filtration rate (eGFR) was calculated using
the formula [eGFR (ml/min/1.73 m2) (7) = 194 x Cr−1.094 x
age−0.287 (female: x 0.739)] as established by the
Japanese Society of Nephrology. Patients showing an eGFR of <60
ml/min/1.73 m2 or a urinary albumin level of ≥30 mg/g•Cr
before the start of irbesartan administration were regarded as
having nephropathy. The study objective and method were fully
explained to the subjects, and written informed consent was
obtained.
Statistical analysis
Various parameters were expressed as the mean ±
standard deviation, excluding those with notes. To compare the mean
24-h blood pressure and pre-/post-treatment values, the Student’s
t-test was employed. When comparing data on repetitive blood
pressure/pulse measurements to the pre-treatment values,
Bonferroni’s correction was performed, considering test
multiplicity. P<0.0167 (≈0.05÷3) was regarded as significant.
Concerning other items, P<0.05 was regarded as significant. With
respect to the urinary albumin level, significance was tested after
routine logarithmic transformation, as a log-normal distribution
was assumed.
Patient backgrounds
This study was completed in 30 of the 32 subjects.
Two patients did not consult the outpatient clinic as scheduled,
dropping out of the study. There was no discontinuation related to
adverse reactions. The backgrounds of the 30 patients are shown in
Table I. They consisted of 11
males and 19 females with a mean age of 61.3 years. The mean
systolic and diastolic blood pressures determined at the outpatient
clinic were 169.9±18.5 and 92.4±11.8 mmHg, respectively.
 | Table I.Patient characteristics at
baseline. |
Table I.
Patient characteristics at
baseline.
| Demographics | |
| Male/female | 11/19 |
| Age (years) | 61.3±13.3 |
| Office blood
pressure | |
| SBP (mmHg) | 169.9±18.5 |
| DBP (mmHg) | 92.4±11.8 |
| Renal parameters | |
| Serum creatinine
(mg/dl) | 0.671±0.173 |
| eGFR (ml/min/1.73
m2) | 81.2±18.8 |
| Urinary albumin
(μg/g•Cr) | 60.8±155.5 |
The mean urinary albumin and serum creatinine levels
were 60.8±155.5 mg/g•Cr and 0.671±0.173 mg/dl, respectively. The
eGFR was 81.2±18.8 ml/min/1.73 m2. Of the 30 patients,
10 showed abnormal urinary albumin levels (microalbuminuria 9;
manifest albuminuria 1). In 3 patients, the eGFR was <60
ml/min/1.73 m2. The distribution of the urinary albumin
level/eGFR-stratified patients is shown in Table II. A total of 12 patients had
nephropathy, showing an eGFR of <60 ml/min/1.73 m2 or
a urinary albumin level of ≥30 mg/g•Cr.
 | Table II.Patient distribution: baseline urinary
albumin (U-Alb) and estimated glomerular filtration rate
(eGFR). |
Table II.
Patient distribution: baseline urinary
albumin (U-Alb) and estimated glomerular filtration rate
(eGFR).
| eGFR | ≥90 | 60–90 | ≤60 | Total |
|---|
| U-Alb | | | | |
| ≤30 | 5 | 13 | 2 | 20 |
| 30–300 | 2 | 6 | 1 | 9 |
| ≥300 | - | 1 | - | 1 |
| Total (n) | 7 | 20 | 3 | 30 |
Results
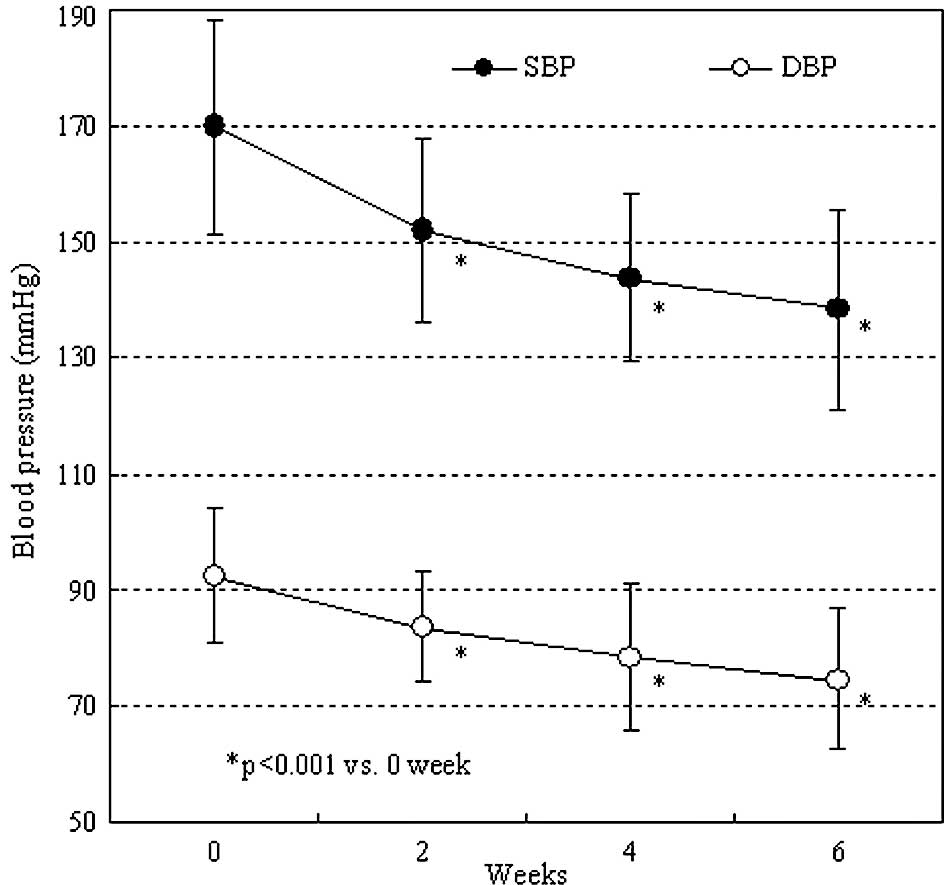
Both the systolic and diastolic office blood
pressures were significantly decreased after 2 weeks of irbesartan
administration (P<0.001). After 6 weeks of administration, the
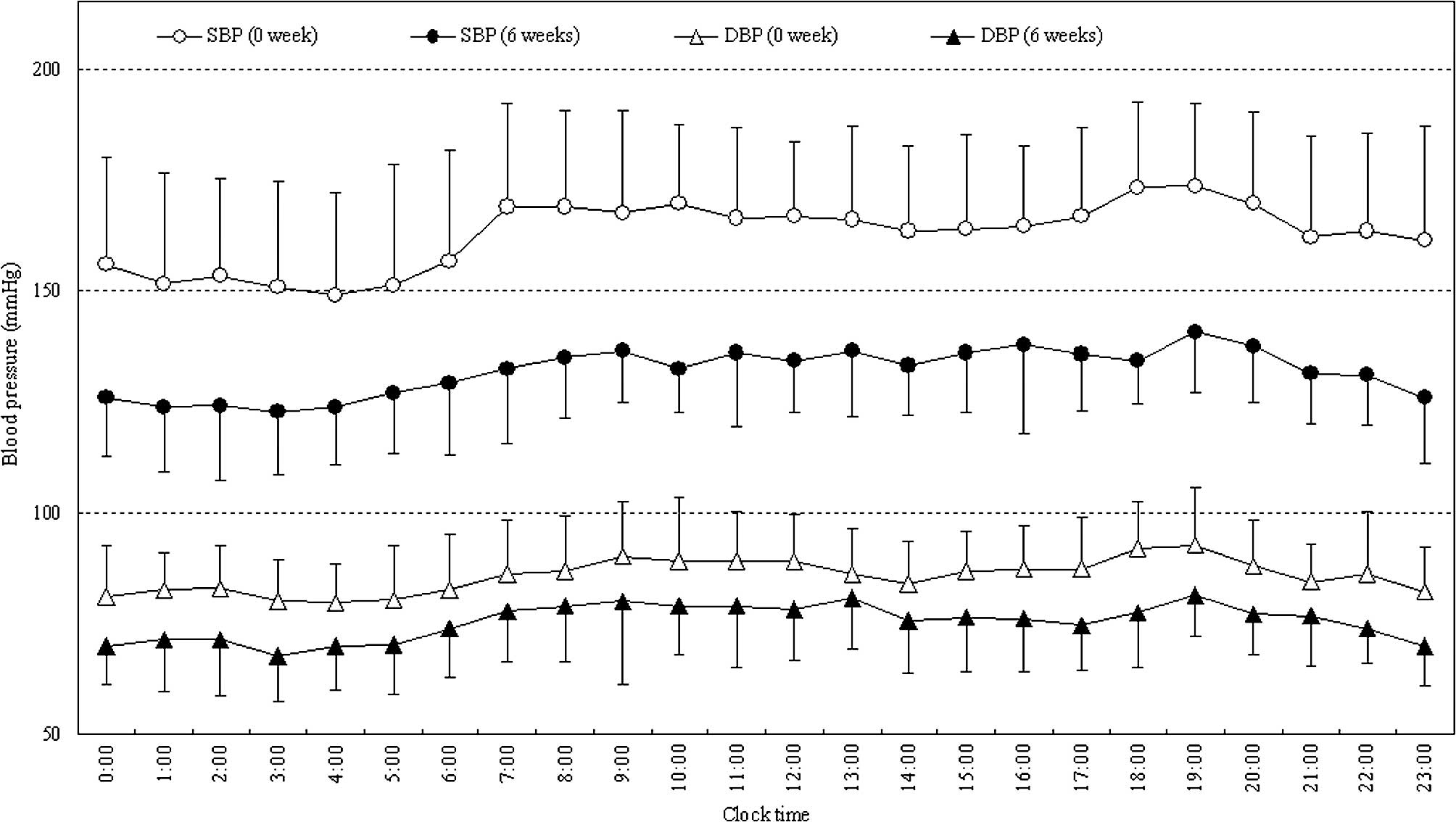
values were 138.8 and 74.4 mmHg, respectively (Fig. 2). As shown in Fig. 3, 24-h blood pressure control was
achieved by administration of irbesartan. The mean 24-h systolic
and diastolic blood pressures significantly decreased from
162.7±17.7 and 85.5±5.8 to 131.9±9.0 and 75.5±7.5 mmHg,
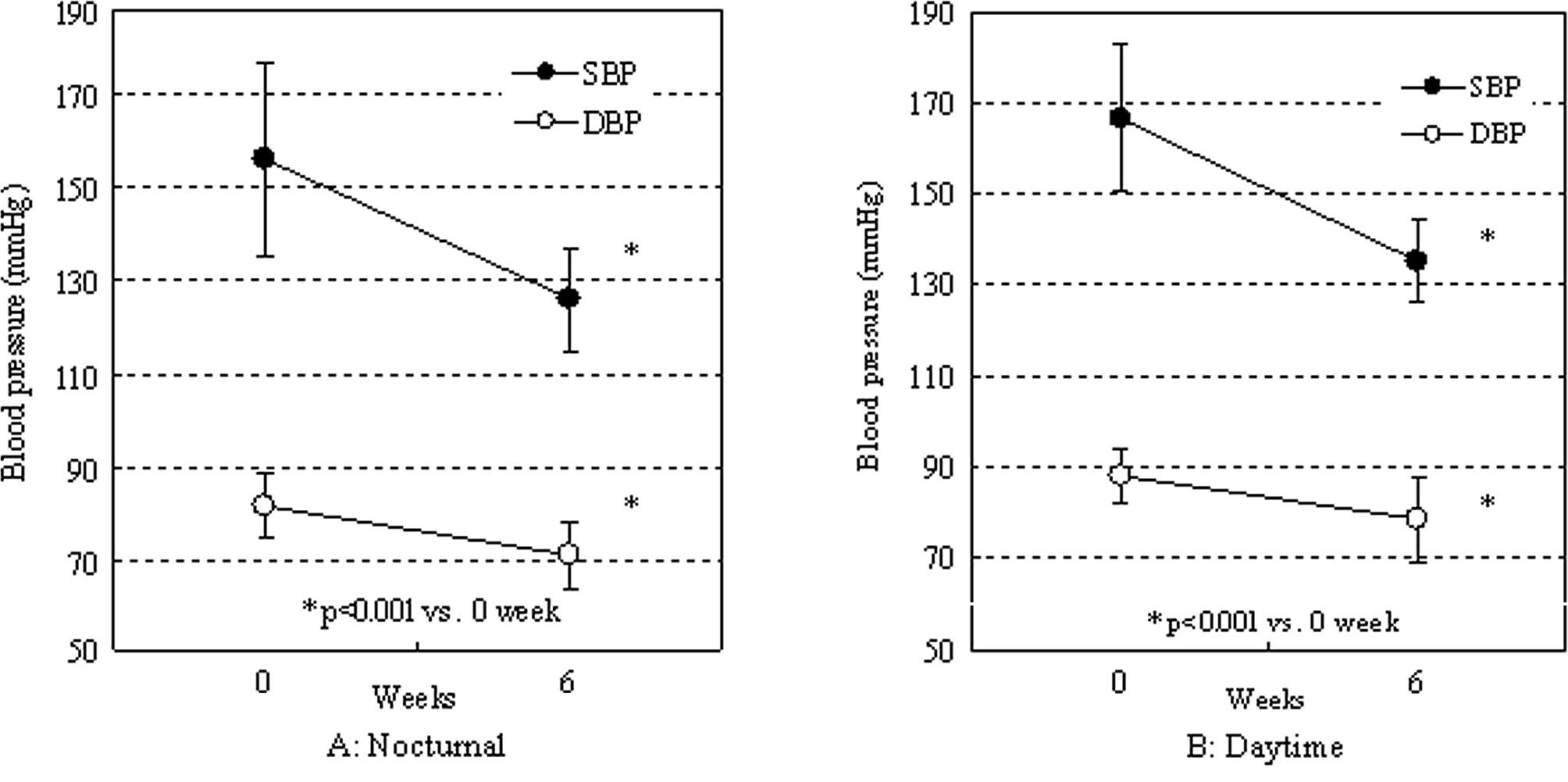
respectively. Both the nocturnal and daytime blood pressures
significantly decreased (Fig. 4A and
B). The rate of decrease was similar between the nocturnal and
daytime blood pressures (systolic: 29.8±20.1 vs. 31.5±19.4 mmHg;
diastolic: 10.8±6.7 vs. 9.7±6.8 mmHg, respectively).
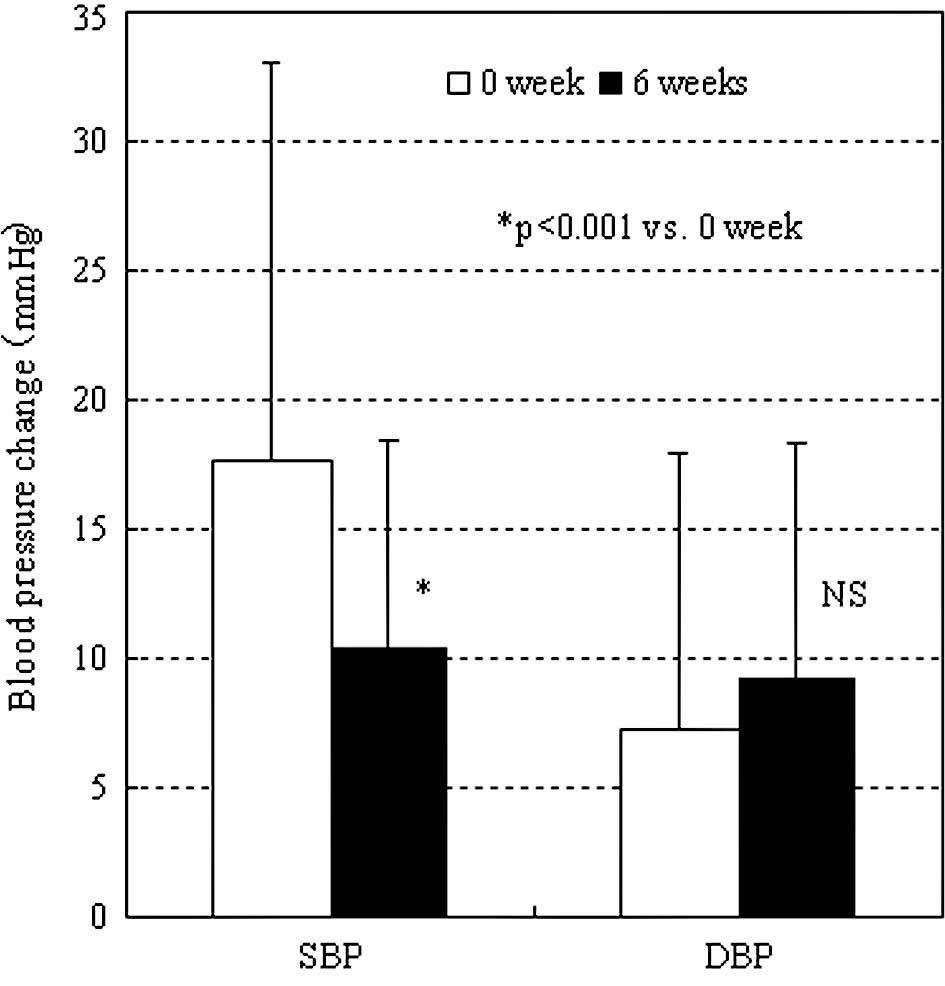
The rates of increase in the systolic blood pressure
early in the morning before and 6 weeks after the start of
administration were 17.6±15.5 and 10.5±10.8 mmHg, respectively,
showing a significant difference (Fig.
5) (P<0.05). There was no significant difference in the rate
of change in the diastolic blood pressure.
We compared changes in blood pressure-associated
parameters between the 12 patients with nephropathy and the other
18 patients. There were no marked differences. For influence on
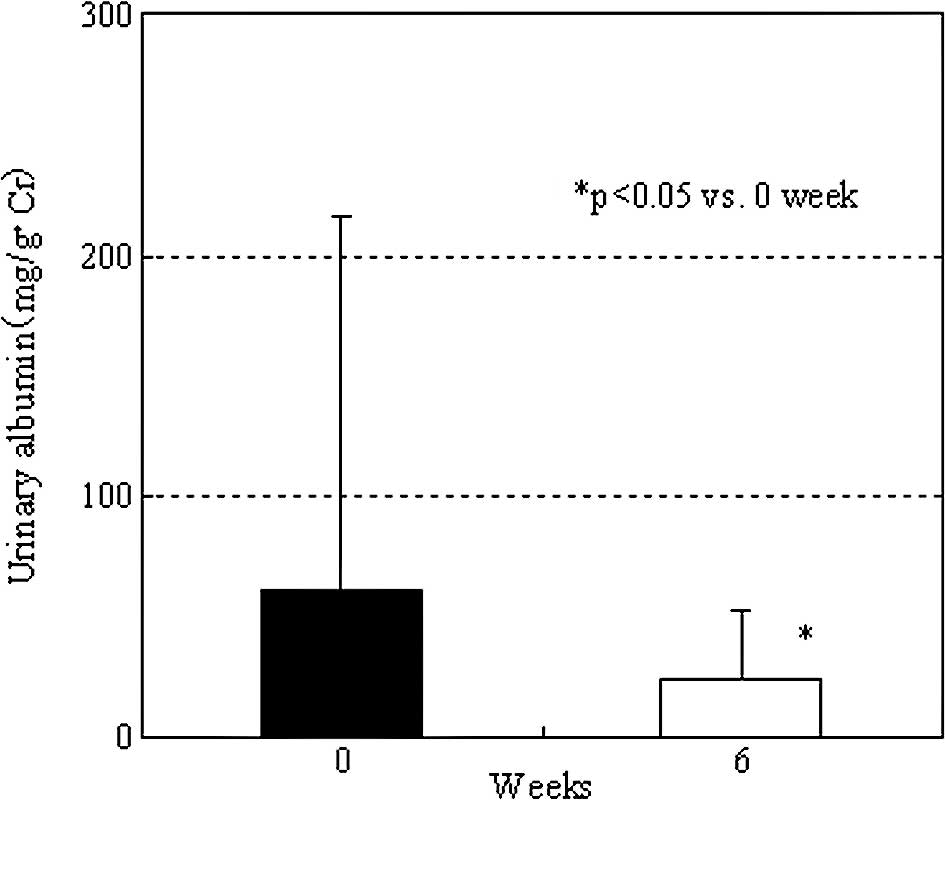
kidney-associated parameters, in 10 patients with abnormal urinary
albumin levels before irbesartan administration, the mean urinary
albumin level was significantly decreased from 152.9±252.3 (before
administration) to 45.9±38.6 mg/g•Cr after 6 weeks of
administration (P<0.05). In 5 patients, the urinary albumin
levels were normalized. In 20 patients, in whom the pre-treatment
urinary albumin levels were within the normal range, there was no
significant change (from 14.8±6.8 to 13.8±11.5 mg/g•Cr) (Fig. 6). However, in 1 patient the urinary
albumin level increased from 27.2 to 55.9 mg/g•Cr, showing
deterioration from the normal range to the microalbuminuria level.
In this patient, subsequent examination confirmed a decrease,
despite continuous therapy with irbesartan, suggesting a transient
change. After 6 weeks of administration, there was a slight
increase in the eGFR (83.8±23.1 ml/min/1.73 m2) in
comparison to that before irbesartan administration (81.2±18.8
ml/min/1.73 m2), although the difference was not
significant. There were no serious side effects requiring
discontinuation of the test agent.
Discussion
In humans, the daily heart rate is approximately
100,000 beats. The blood pressure changes on every beat depending
on the sympathetic nervous system and humoral factors. Recent
studies have indicated that the non-office blood pressure markedly
influences the prognosis, such as the onset of cardiovascular
events (8,9). Therefore, it may be important to
evaluate not only office, but also daytime/nocturnal blood
pressures during activities/sleep (10,11).
The half-life of irbesartan at a clinical dose is
long (10.2–15.4 h) (4). In
addition, this agent exhibits stable, continuous, anti-hypertensive
and renoprotective effects over 24 h (6,12,13).
However, in Japan few studies have reported its efficacy. As
evidence regarding irbesartan involves doses employed in other
countries (150–300 mg/day) (3),
the anti-hypertensive and renoprotective effects of this agent at
doses approved in Japan, 50–200 mg/day, should be compared to those
of other ARBs.
In this study, we examined 24-h blood pressure
control using ABPM in Japanese patients with essential hypertension
who were treated with irbesartan and evaluated its efficacy. This
agent decreased both the nocturnal and daytime blood pressures and
reduced the rate of change in the systolic blood pressure early in
the morning, resulting in favorable blood pressure control. It also
significantly decreased the urinary albumin level, suggesting that
it exhibits renoprotective effects at doses approved in Japan.
According to Kario et al (14), blood pressure changes early in the
morning, morning surge, may be an important factor influencing the
prognosis of cardiovascular events. The approved doses differing
between Japan and other countries remain controversial, considering
the clinical efficacy. However, in this study, irbesartan at a
standard dose (100 mg/day, once a day) employed in Japan reduced
the daytime/nocturnal blood pressures and morning surge, suggesting
its potent effects as a long-acting anti-hypertensive agent.
A meta-analysis involving 36 international studies
showed that the effects on 24-h blood pressure changes depended on
the type of ARB (15). ARB
selection in anti-hypertensive treatment may be important for blood
pressure control. All ARBs approved in Japan, consisting of six
components, are administered once a day. Although there are marked
differences in the half-life and pharmacokinetics, few studies have
compared 24-h blood pressure control among the ARBs. Further
investigations are required.
References
|
1.
|
Chonan K, Kikuya M, Araki T, Fujiwara T,
Suzuki M, Michimata M, Hashimoto J, Ohkubo T, Hozawa A, Yamamoto N,
Miyawaki Y, Matsubara M and Imai Y: Device for the self-measurement
of blood pressure that can monitor blood pressure during sleep.
Blood Press Monit. 6:203–205. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ogihara T, Kikuchi K, Matsuoka H, et al:
The Japanese Society of Hypertension Guidelines for the Management
of Hypertension (JSH 2009). Hypertens Res. 32:3–107. 2009.
View Article : Google Scholar
|
|
3.
|
Croom KF and Plosker GL: Irbesartan: a
review of its use in hypertension and diabetic nephropathy. Drugs.
68:1543–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Marino MR, Langenbacher K, Ford NF and
Uderman HD: Pharmacokinetics and pharmacodynamics of irbesartan in
healthy subjects. J Clin Pharmacol. 38:246–255. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cazaubon C, Gougat J, Bousquet F,
Guiraudou P, Gayraud R, Lacour C, Roccon A, Galindo G, Barthelemy
G, Gautret B, Bernhart C, Perreaut P, Breliere J, Fur GL and Nisato
D: Pharmacological characterization of SR 47436, a new nonpeptide
AT1 subtype angiotensin II receptor antagonist. J Pharmacol Exp
Ther. 265:826–834. 1993.PubMed/NCBI
|
|
6.
|
Van den Meiracker AH, Admiraal PJ, Janssen
JA, Kroodsma JM, de Ronde WA, Boomsma F, Sissmann J, Blankestijn
PJ, Mulder PG, Man in’t Veld AJ and Schalekamp MADH: Hemodynamic
and biochemical effects of the AT1 receptor antagonist irbesartan
in hypertension. Hypertension. 25:22–29. 1995.PubMed/NCBI
|
|
7.
|
Matsuo S, Imai E, Horio M, Yasuda Y,
Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A:
Collaborators developing the Japanese equation for estimated GFR.
Revised equations for estimated GFR from serum creatinine in Japan.
Am J Kidney Dis. 53:932–935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bobrie G, Chatellier G, Genes N, Clerson
P, Vaur L, Vaisse B, Menard J and Mallion JM: Cardiovascular
prognosis of ‘masked hypertension’ detected by blood pressure
self-measurement in elderly treated hypertensive patients. JAMA.
291:1342–1349. 2004.
|
|
9.
|
Ohkubo T, Kikuya M, Metoki H, Asayama K,
Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H and Imai Y:
Prognosis of ‘masked’ hypertension and ‘white-coat’ hypertension
detected by 24-h ambulatory blood pressure monitoring 10-year
follow-up from the Ohasama study. J Am Coll Cardiol. 46:508–515.
2005.
|
|
10.
|
Harada K, Karube Y, Saruhara H, Takeda K
and Kuwajima I: Workplace hypertension is associated with obesity
and family history of hypertension. Hypertens Res. 29:969–976.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kario K, James GD, Marion R, Ahmed M and
Pickering TG: The influence of work- and home-related stress on the
levels and diurnal variation of ambulatory blood pressure and
neurohumoral factors in employed women. Hypertens Res. 25:499–506.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Parving HH, Lehnert H, Bröchner-Mortensen
J, Gomis R, Andersen S and Arner P; Irbesartan in Patients with
Type 2 Diabetes and Microalbuminuria Study Group: The effect of
irbesartan on the development of diabetic nephropathy in patients
with type 2 diabetes. N Engl J Med. 345:870–878. 2001. View Article : Google Scholar
|
|
13.
|
Lewis EJ, Hunsicker LG, Clarke WR, Berl T,
Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R and Raz I;
Collaborative Study Group: Renoprotective effect of the
angiotensin-receptor antagonist irbesartan in patients with
nephropathy due to type 2 diabetes. N Engl J Med. 345:851–860.
2001. View Article : Google Scholar
|
|
14.
|
Kario K, Pickering TG, Umeda Y, Hoshide S,
Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE and Shimada
K: Morning surge in blood pressure as a predictor of silent and
clinical cerebrovascular disease in elderly hypertensives: a
prospective study. Circulation. 107:1401–1406. 2003. View Article : Google Scholar
|
|
15.
|
Fabia MJ, Abdilla N, Oltra R, Fernandez C
and Redon J: Antihypertensive activity of angiotensin II AT1
receptor antagonists: a systematic review of studies with 24 h
ambulatory blood pressure monitoring. J Hypertens. 25:1327–1336.
2007. View Article : Google Scholar : PubMed/NCBI
|