Introduction
Diabetes is a metabolic disorder characterized by
hyperglycemia resulting from abnormalities in insulin secretion and
action (1). Chronic hyperglycemia
leads to microvascular (e.g. neuropathy, and nephropathy) and
macrovascular (mainly cardiovascular) complications that arise due
to the increased reactive oxygen species production and reduced
antioxidants (2).
Herbal products have been widely used throughout
history for the treatment of several diseases. Since the
characterization and exact mechanisms of action of these natural
products remain unclear, researchers are trying to evaluate their
beneficial effects on human health as well as their possible
adverse effects (3). Despite the
presence of several treatments for diabetes such as insulin
analogs, sulphonylureas, biguanides, dipeptidyl peptidase-4
inhibitors, thiazolidine, and α-glucosidase inhibitors, patients
prefer to use botanicals due to the increased cost and side effects
of these medications (2).
Many plants, such as cinnamon and ginseng reduce
glucose and lipid levels through the stimulation of insulin
secretion, delay of gastric emptying, inhibiting glucosidase
activity, increasing GLUT4 expression (4), and the activation of AMP-activated
protein pathway (5), and
inhibiting gluconeogenesis (6).
Several medicinal plants are used for the treatment of diabetes,
such as ginger, garlic, fenugreek and cumin (7,8).
Fenugreek (Trigonella foenum-graecum), is a
historically used herbal medicinal plant that is popular in Africa,
India, South, and Central Asia (9). It is traditionally used to treat
several conditions, such as diabetes and obesity. It possesses
antioxidant, antihyperlipidemic, antibacterial, antifungal,
anti-inflammatory, and galactagogic properties (10).
Fenugreek's pharmacological effects are attributed
to a range of bioactive compounds such as polyphenols, steroids,
lipids, alkaloids, saponins, flavonoids, hydrocarbons,
carbohydrates, galactomannan fiber, and amino acids. Several
scientific groups examined its antidiabetic effect. A previous
study showed that fenugreek increased glucose uptake in HepG2 cells
is due to the overexpression of the glucose transporter (GLUT-2)
(12) and sterol regulatory
element-binding protein (SREBP1C) mRNA levels (11). Another report by Pradeep and
Srinivasan (13), demonstrated
that when combined with 3% onion, better fenugreek antidiabetic
results were seen. A potential fenugreek-based drug
(Fenfuro®) was compared to Metformin in a clinical
trial. Results showed that Fenfuro combined with Metformin gave
better results than Metformin alone (14).
Diosgenin saponin is considered the most bioactive
substance of fenugreek. It has antioxidative effects and plays a
pivotal role in improving the diabetic status by several mechanisms
(1,15). The mechanisms include β-cell
renewal and insulin secretion stimulation. Besides, diosgenin
elevates the mRNA transcription levels of CCAAT/enhancer-binding
protein (C/EBPδ) and peroxisome proliferator-activated receptor-γ
(PPAR-γ) (10,12).
Other components in fenugreek include;
4-hydroxyisoleucine, which is an amino acid that enhances insulin
secretion, decrease plasma triglycerides, and total cholesterol
levels (1). Galactomannan is a
carbohydrate that represents 45-60% of the seed of fenugreek. It
has been shown to block the carbohydrate and lipid hydrolyzing
enzymes in the digestive system, resulting in lowering the
postprandial glucose level (1).
Although the detailed mechanisms of action of the
fenugreek antidiabetic activity are yet to be identified, many
studies suggest that antioxidant activity plays a significant role
in hepatoprotection. Another possibility would be that fenugreek
reverses protein glycation caused by hyperglycemia (16). Further investigations into the
molecular mechanisms of actions and active components of the plant
are needed.
In many parts of the world, fenugreek is commonly
consumed as a drink. In the current study, we attempt to compare
different routes of administration of fenugreek at a clinically
feasible dose (100 mg/kg) in a diabetic rat model induced by
streptozocin (STZ).
Materials and methods
Animals
All experiments were conducted in compliance with
the guidelines established by the NIH for Animal Care and Use and
were approved by the Institutional Animal Care and Use Committee
(IACUC) of the October University for Modern Sciences and Arts
(2018). Male Sprague Dawley rats weighing (175-200 g), were
obtained from Theodore Bilarz Research Institute (Cairo, Egypt).
Rats were randomly divided into the following groups: Group 1:
diabetic (DM) rats receiving 100 mg/kg every other day (EOD) of
fenugreek extract (HERB-PHARM). Group 2: DM rats receiving daily
(ED) fenugreek 100 mg/kg IP. Group 3: DM rats receiving oral
fenugreek 100 mg/kg daily. Group 4: untreated diabetic group. Group
5: healthy nondiabetic rat group.
Diabetes model
Animals received Streptozocin (STZ; 75 mg/kg in
sterile citrate buffer) intraperitoneally. Diabetes was confirmed
one week following STZ injection, by blood glucose levels. Rats
showing fasting glucose levels at or above 270 mg/dl (>15
mmol/l) were included in the study (3). At the end of the experiment, the
histological examination of the rat pancreatic tissues confirmed
DM.
Sample collection
At the end of the 4 weeks of treatment, rats were
anesthetized by ketamine/xylazine (ketamine 80-100 mg/kg, xylazine
10-12.5 mg/kg IP). Blood was collected by cardiac puncture, and
rats were dissected and tissue samples (pancreas, kidney and liver)
were collected for biochemical and histological analysis. There was
no sample size difference; animals were added to replace lost
animals due to mortality.
Biochemical analysis
The collected serum was divided into aliquots to
assess the liver, and kidney functions as well as serum glucose and
the lipid profile, as previously described (3).
Antioxidant activity assays
Frozen liver tissue samples (0.2 g) were homogenized
in phosphate-buffered saline. The suspension was centrifuged at
4400 rpm, and the supernatants collected and tested for the
antioxidant enzyme levels.
Catalase enzyme activity
Catalase enzyme activity was measured according to
the method originally described by Aebi (17). Briefly, the assay is based on
catalase reaction with a known quantity of hydrogen peroxide
(H2O2), and the reaction is stopped after 1
min by a catalase inhibitor. The remaining
H2O2 reacts with 4-aminophenazone and
3,5-dichloro-2-hydroxy-benzene sulfonic acid to form a chromophore.
The absorbance is measured at 510 nm using a spectrophotometer.
Glutathione peroxidase
Glutathione peroxidase was measured based on the
method described initially by Paglia and Valentine (18). The assay principle is based on the
indirect measurement of the activity of cellular glutathione
peroxidase enzyme. Oxidized glutathione (GSSG) is produced by
reduction of an organic peroxide by cellular glutathione. The rate
of decrease in the absorbance at 340 is directly proportional to
the glutathione peroxidase activity in the sample.
Glutathione S-transferase (GST)
enzymatic activity
The measured enzyme activity is based on the method
designed by Habig et al (19). The action of GST enzyme is to
catalyze the conjugation of reduced glutathione (GSH) with 1-chloro
2,4-dinitrobenzene (CDNB) via the -SH group of glutathione. This
results in the production of the conjugate,
S-(2,4-dinitrophenyl)-L-glutathione, which can be detected. The
absorbance is read at 340 nm.
Peroxidase activity
Peroxidase activity is based on the enzyme
inhibition (20) by the addition
of sulfite so that it is inactive when the hydrogen peroxide is
added. Freshly prepared 1% o-phenylenediamine and 0.3% hydrogen
peroxide are added to liver tissue homogenate. The reaction is
stopped after 5 min by adding sodium bisulfite. The absorbance was
measured at 430 nm. The enzyme activity is expressed as the change
in absorbance at 430 nm (∆OD430)/min/mg protein.
Histological analysis
Formaldehyde fixed tissue samples were
paraffin-embedded, and 5 µm sections were cut and stained with
hematoxylin and eosin. Slides were examined and photographed using
a BX51 light microscope with an Olympus digital camera (DP20)
(Olympus).
Statistical analysis
The statistical analyses were performed using
GraphPad Prism software, version 7.04 (GraphPad, Inc.). Data are
presented as mean ± standard deviation. One-way ANOVA and Tukey
test were used. P<0.05 was considered to indicate a
statistically significant difference.
Results
Blood glucose levels
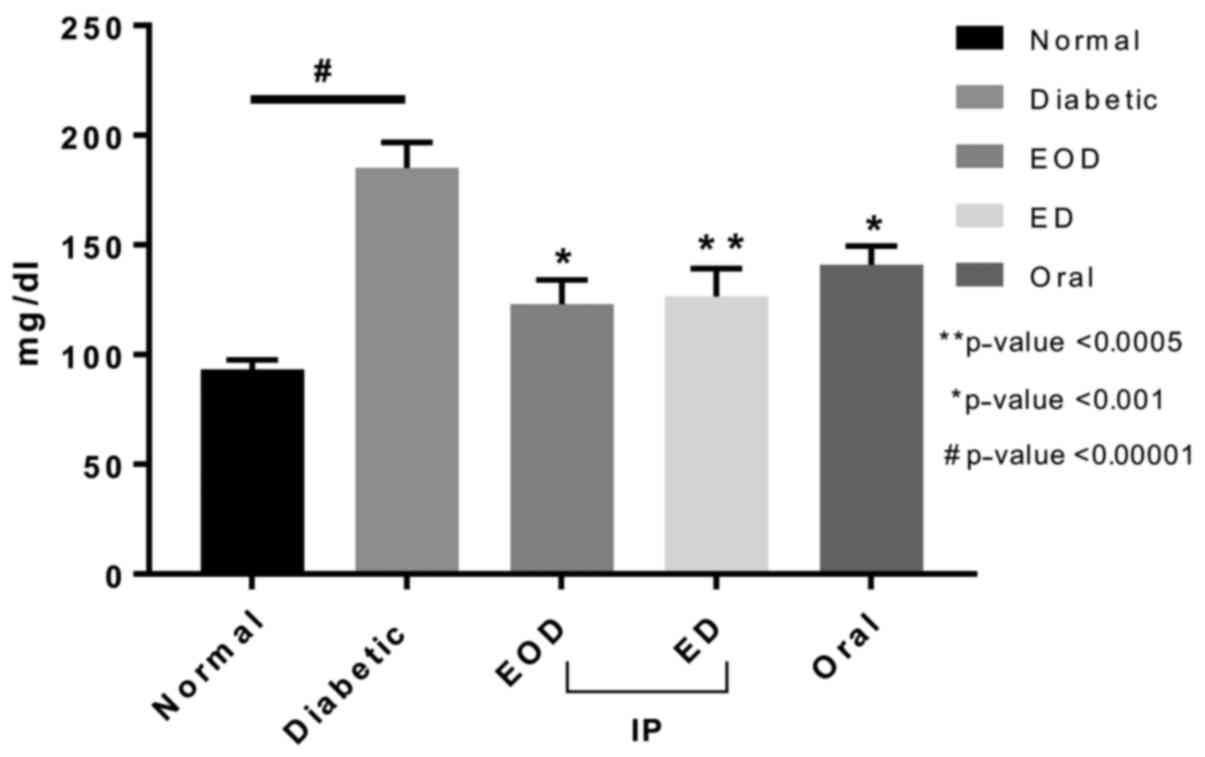
Fasting blood glucose levels of rats treated with
fenugreek extract were significantly reduced after treatment
compared to the diabetic control group in all treated groups
(Fig. 1). There was no significant
difference between treatment groups.
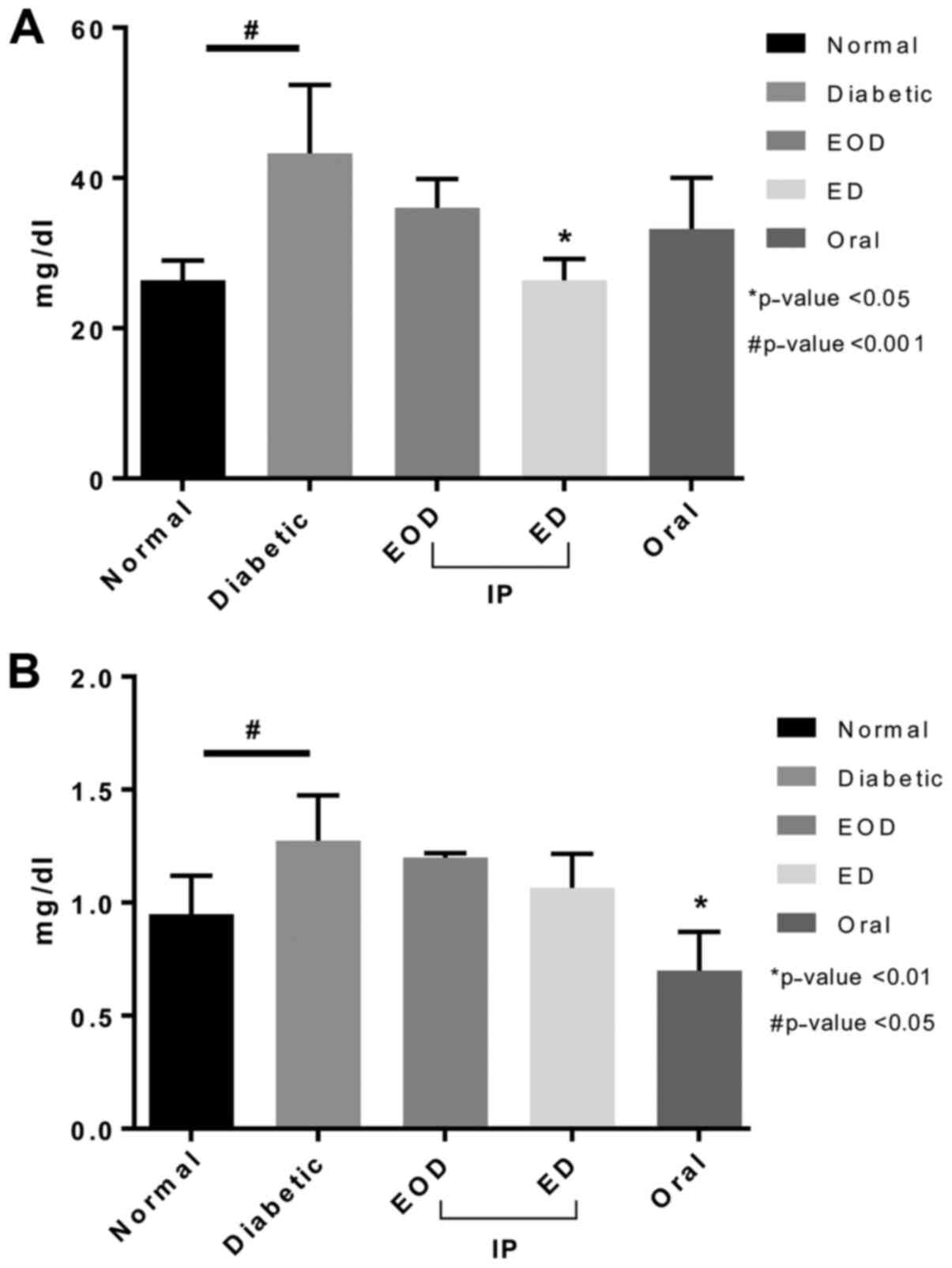
Renal function
Urea levels increased in diabetic rats (Fig. 2). No significant reduction was seen
after fenugreek treatment except with the daily injection group
(Fig. 2). Creatinine levels also
increased in untreated DM rats. Fenugreek injection did not reduce
creatinine levels. Only after oral treatment, a significant
decrease in creatinine was seen (Fig.
2).
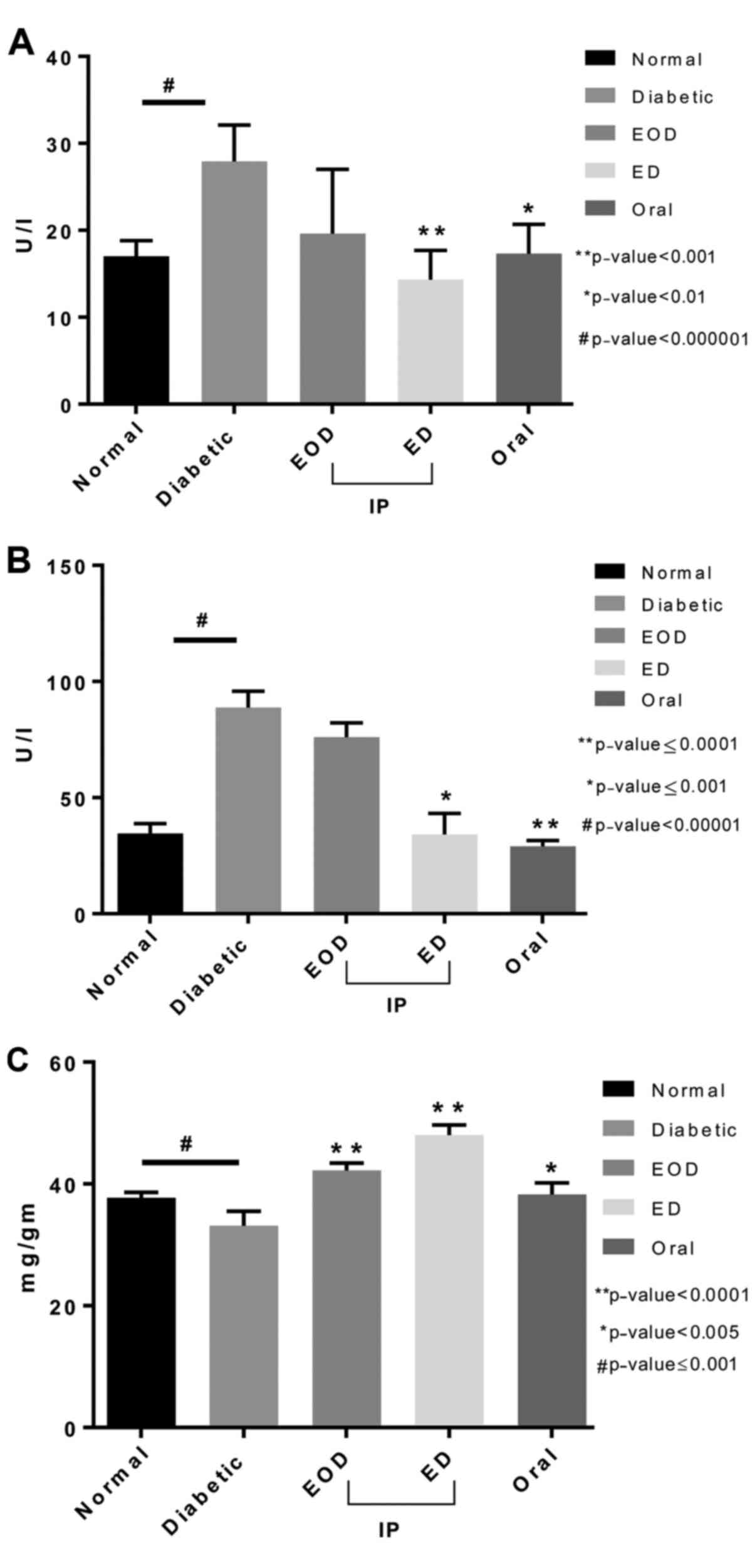
Liver functions and lipid profile
Alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) levels increased in diabetic rats. Fenugreek
treatment in both the oral and daily injection (ED) groups
significantly decreased both AST and ALT levels compared to the
diabetic control group. Protein levels significantly increased in
all treated groups compared to untreated diabetic rats. ED
injections showed the highest value and significance compared to
the diabetic control (Fig. 3).
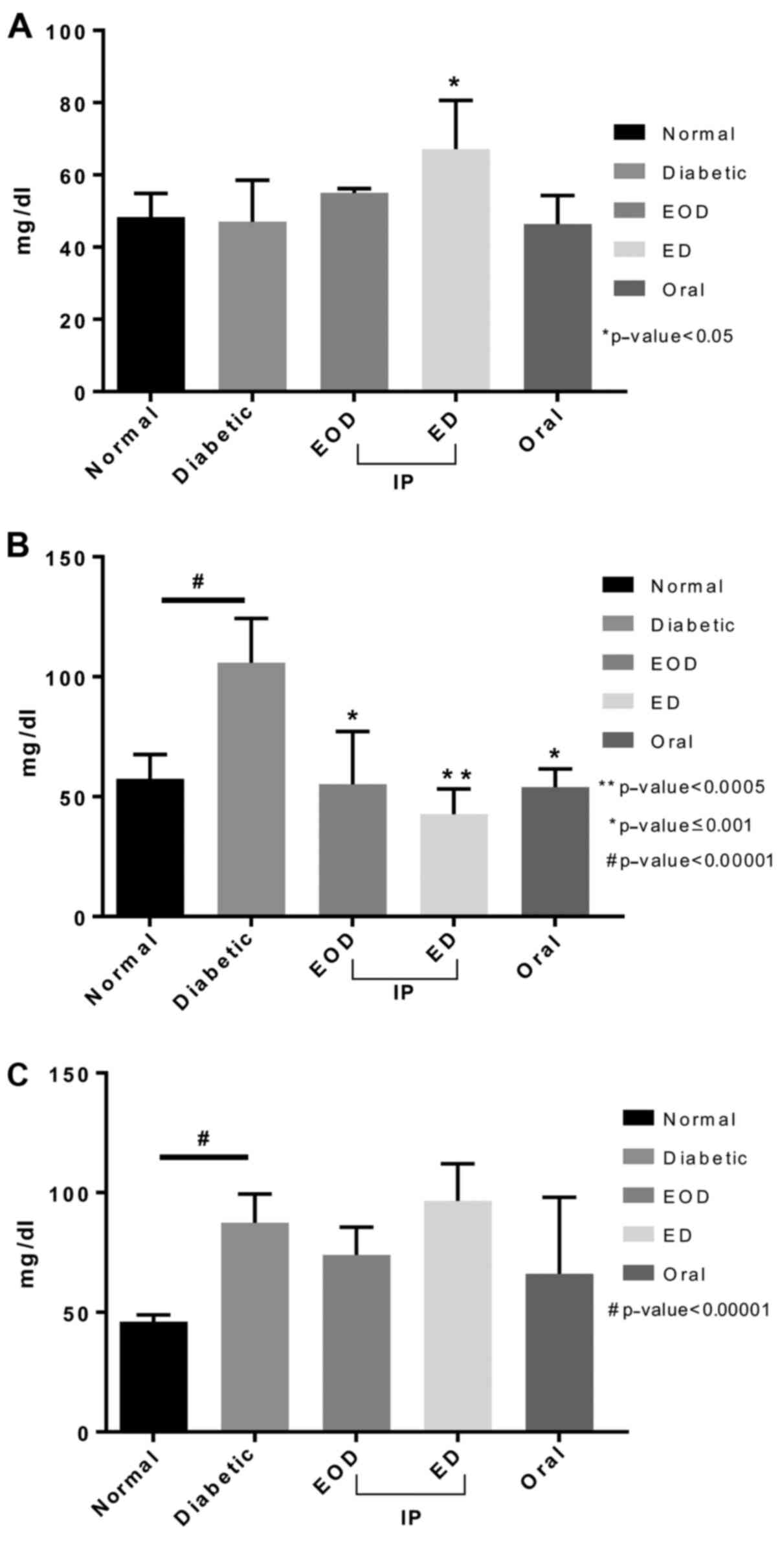
High-density lipoprotein (HDL) levels did not
increase in diabetic rats compared to normal. Only after daily
fenugreek injection, a significant increase was seen.
Triglycerides, on the other hand, significantly increased in
diabetic rats. All treatment groups showed a significant decrease
in triglyceride levels compared to diabetic rats injected daily,
which showed the highest significance. Cholesterol levels increased
following induction of diabetic and were not decreased after
fenugreek treatment (Fig. 4).
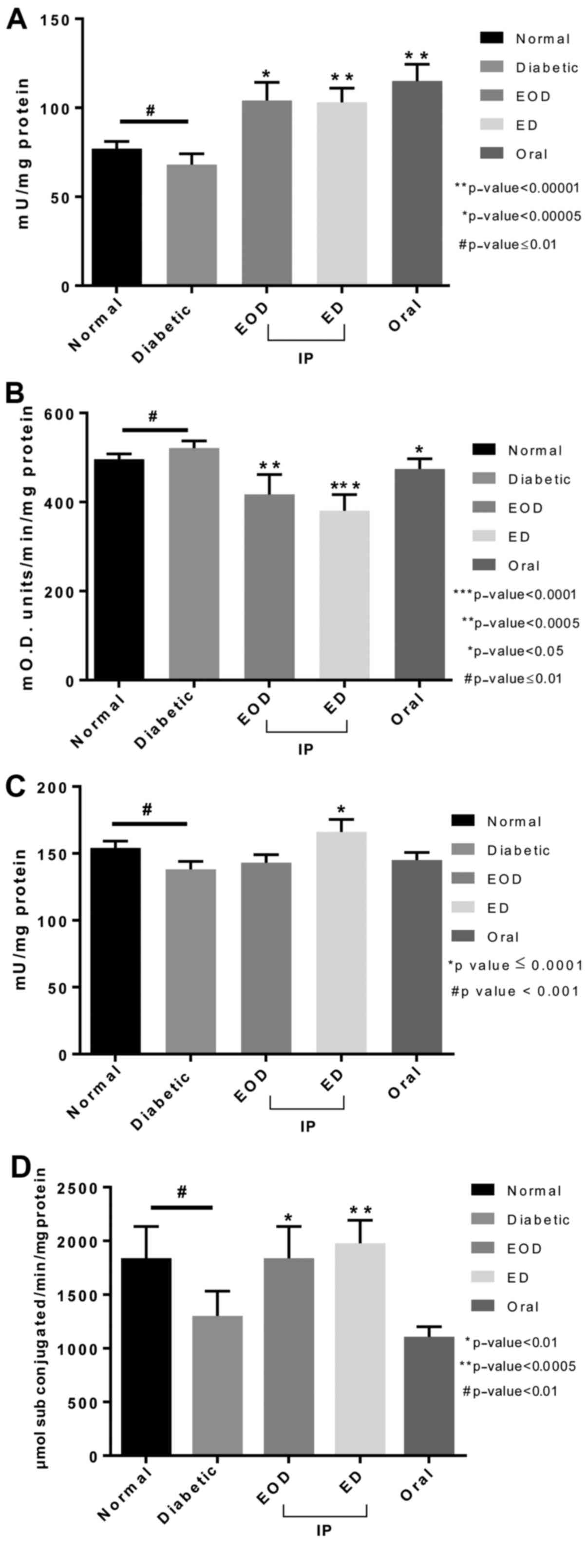
Antioxidant enzymes
Catalase enzyme in the liver tissue of untreated
diabetic rats was slightly lower compared to the normal rats.
Catalase levels increased significantly in all treatment groups
compared to both normal and untreated diabetic rats, with oral
fenugreek showing the highest levels (Fig. 5A). Quantitative determination of
peroxidase activity in liver tissue showed that the peroxidase
antioxidant enzyme levels were increased in diabetic rats compared
to normal. All treated groups showed a significant reduction in
peroxidase levels compared to diabetic untreated rats. The daily
injection group showed the highest and most significant reduction
(Fig. 5B).
Glutathione peroxidase levels were changed slightly
in diabetic untreated rats. Only following daily injections, there
was a significant increase in glutathione peroxidase levels
(Fig. 5C). Glutathione
s-transferase enzyme was significantly reduced in untreated
diabetic rats. Both EOD and daily injections treated groups showed
a significant increase in the enzyme levels compared to the
diabetic group with the daily injection group significantly higher
than the EOD injection group (Fig.
5D).
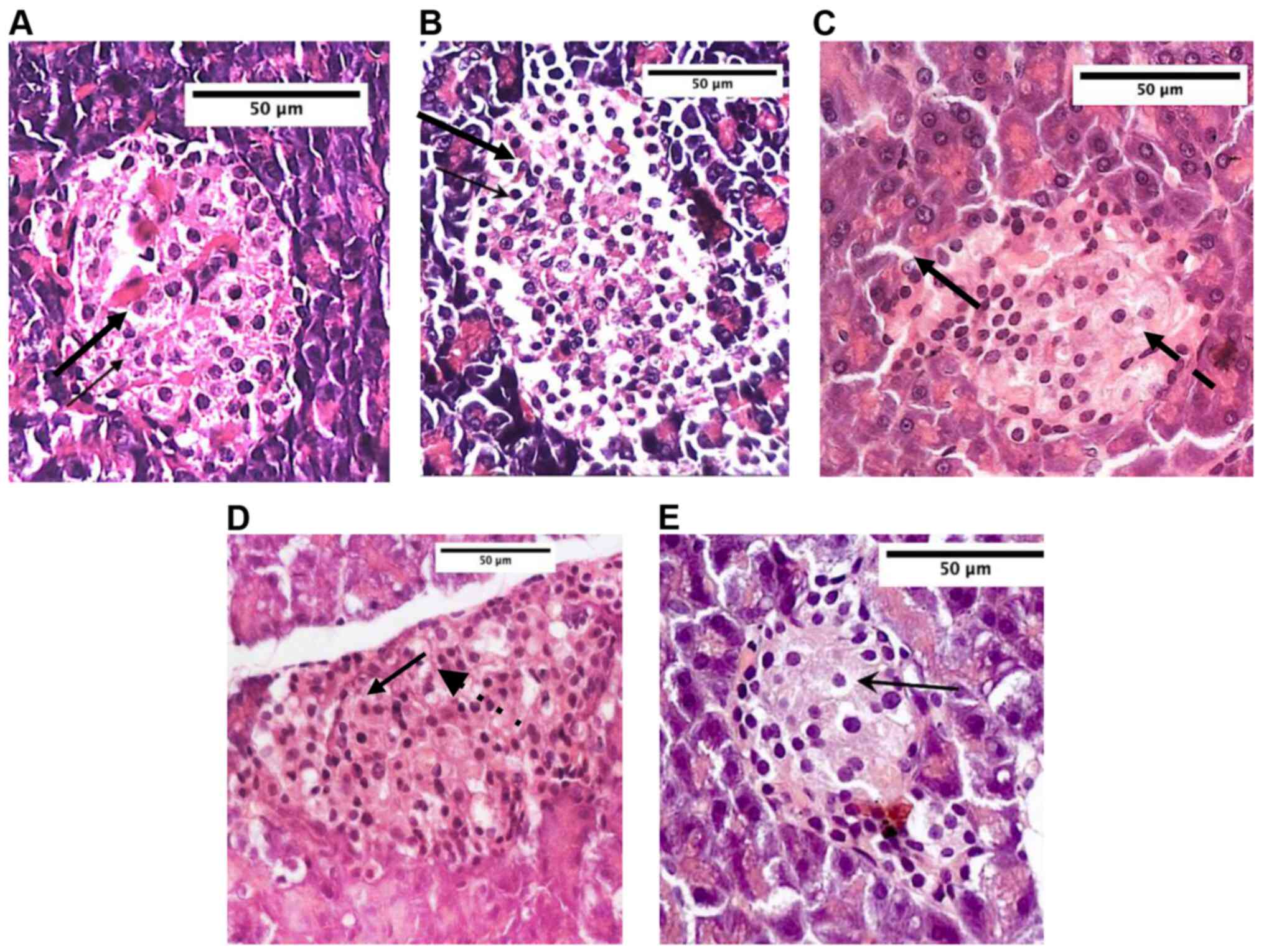
Histological changes in the
pancreas
Normal rats showed a typical healthy architecture of
the pancreatic islets of Langerhans, with no signs of cell injury
observed. Untreated DM rats showed severe alteration in both the
acini and islets of the pancreas, most of the cell nuclei were
thickened and appeared larger, indicating karyo-pyknosis of the
cells; also, numerous vacuoles were observed (Fig. 6A and B).
In rats receiving oral fenugreek extract, the
pancreas showed no signs of protection, with destructive and
degenerative changes in the pancreatic islets in almost all tissue
sections, with many cells demonstrating pyknotic nuclei with
variable records of cytoplasmic vacuolation (Fig. 6C). Treatment with fenugreek
injections (ED and EOD) (Fig. 6D
and E) showed no histological
signs of improvement with changes in cellular components of
pancreatic islets with numerous cells demonstrating pyknotic
nuclei, and cytoplasmic vacuolation.
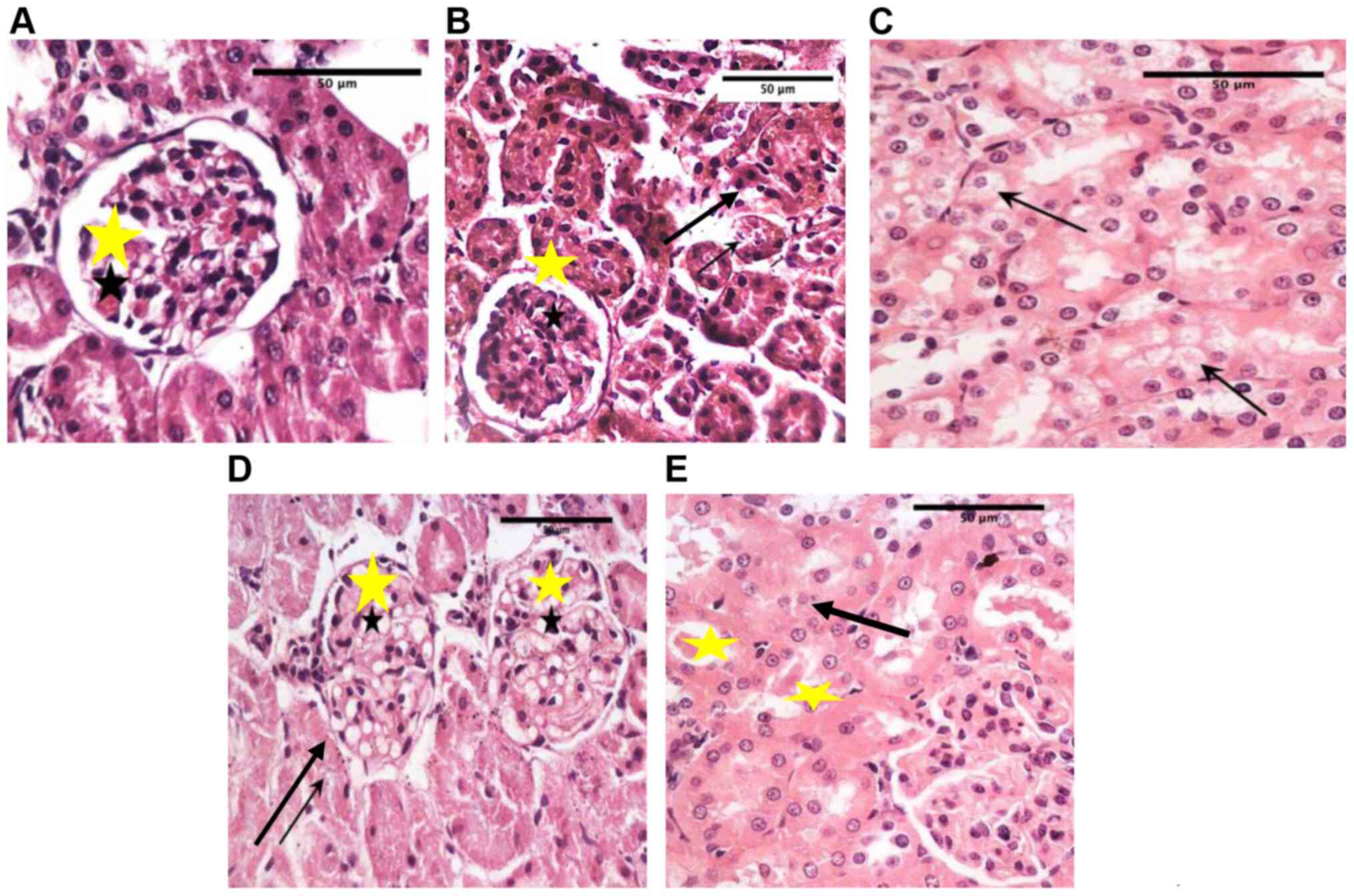
Histological changes in the
kidneys
Normal rats demonstrated normal histological
features of the renal cortex and medulla with intact corpuscles,
while diabetic rats showed wide areas of damage with intraluminal
cast formation and many degenerated tubular cells and congested
inter-tubular blood vessels. (Fig.
7A and B). Oral fenugreek
extract showed focal areas of degenerative vacuolar changes in
lining cells and or pyknotic nuclei (Fig. 7C). Fenugreek injections showed
pronounced damage of renal corpuscles, severe vacuolar changes in
mesangial cells, and severe necrotic changes in tubular lining
cells, with most cells losing cellular details (Fig. 7D and E).
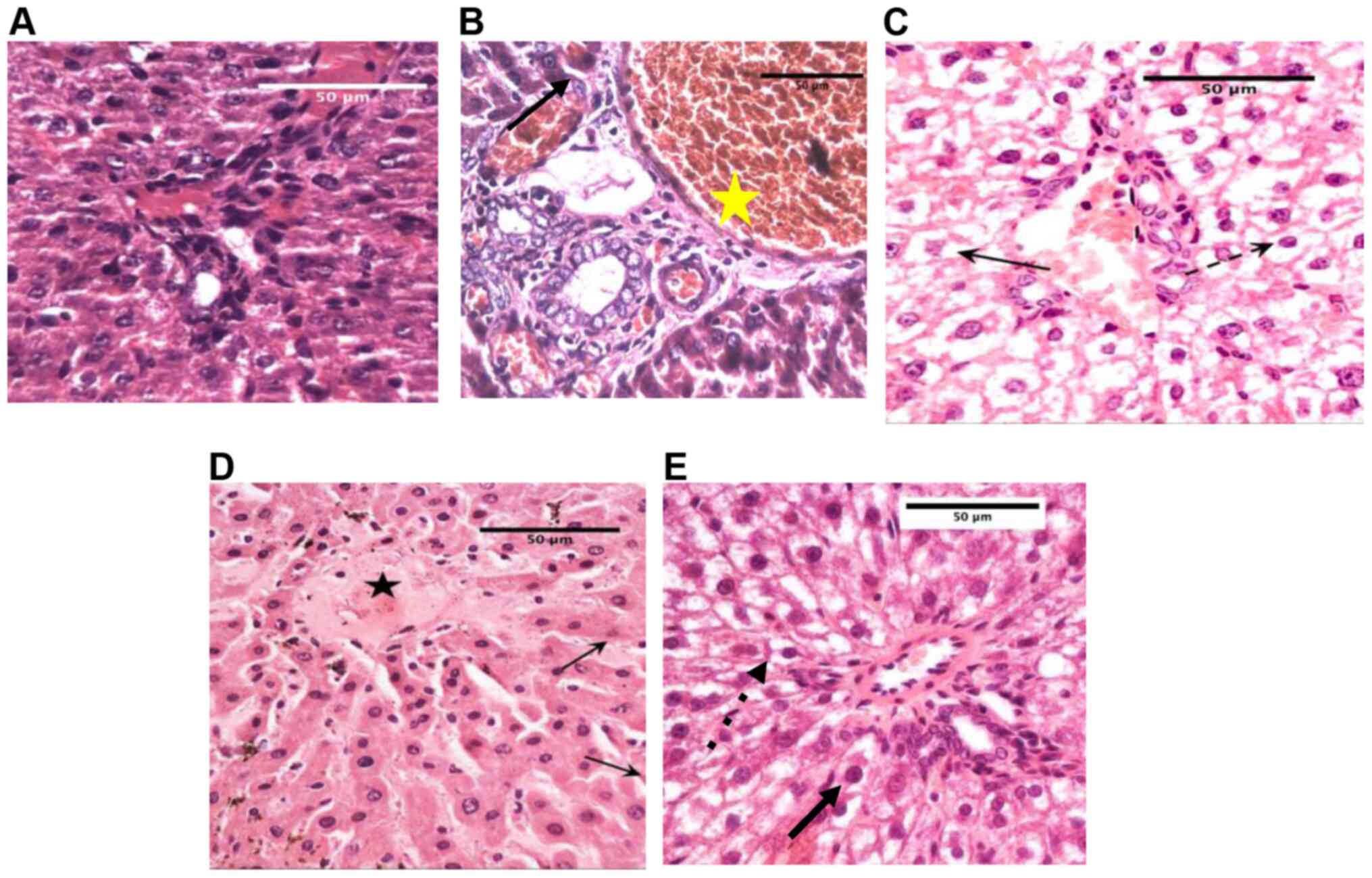
Histological changes in the liver
Hepatic tissue samples from normal rats showed
normal hepatocytes, blood vessels, and sinusoids, as shown in
Fig. 8A. Untreated diabetic rats
showed high numbers of degenerated hepatocytes with karyo-pyknosis,
and dilatation and congestion of blood vessels and sinusoids
(Fig. 8B). Oral fenugreek extract
treatment showed degenerating hepatocytes with shrunken pyknotic
nuclei and missing cellular details (Fig. 8C). Fenugreek injections showed
degenerating hepatocytes with shrunken pyknotic nuclei and loss of
cellular details (Fig. 8D and
E).
Discussion
In the current study, we used an STZ model to
compare different routes of administration of fenugreek (10). STZ have been used extensively in
diabetes research especially for the study of various therapeutic
approaches, including the use of plant extracts as supplements in
diabetes care (21-23).
Fenugreek is a plant widely consumed in different
parts of the world. Here we provide a comprehensive evaluation of
fenugreek seed extract treatment on the pancreas, liver, and
kidneys both biochemically and histologically. Induction of
diabetes was confirmed biochemically with elevated blood glucose
levels, as well as histologically with islets of the pancreas
showing signs of destruction on histological examination.
We attempted to test glycosylated hemoglobin
(HbA1c); unfortunately, the results obtained were unreliable (data
not shown). This might be due to the difficulty of measuring HbA1C
in Sprague-Dawley rats, as reported by other researchers (24,25).
Some researchers suggest that HbA1C should be estimated using other
tools such as ELISA, or high-performance liquid chromatography
(HPLC) (26).
Fenugreek seed extract administration reduced blood
glucose levels, possibly due to the high content of alkaloid
trigonelline and steroidal saponins in fenugreek, especially the
4-hydroxy- isoleucine compound that is said to be insulinotropic
(27). The current data confirm
previous reports (28) that showed
a dose of 50 mg/kg of orally administered fenugreek for 4 weeks in
STZ diabetic rabbits, produced similar effects.
The architecture of the pancreatic tissue correlates
with the stage of diabetes and its severity (29). In the current study after STZ
injection, diabetic rats showed damage to the pancreatic islet
cells and severe pathological changes to exocrine and endocrine
components, which is consistent with previous findings (30). Rats treated with fenugreek, showed
protection of pancreatic tissues, possibly due to the presence of
diosgenin which is postulated to have several antidiabetic effects,
such as the regeneration of pancreatic β-cells and enhancement of
insulin secretion in general (1,31).
Diosgenin also improved blood glucose levels maintenance and
preserved the pancreas, liver, and skeletal muscle tissues
(1). Previous report (32) on the effects of fenugreek oil on
the pancreas in an Alloxan induced diabetic rat model showed that
pancreatic cell damage and renal function were slightly reversed
after treatment with fenugreek oil.
Oral fenugreek showed a significant decrease in
creatinine levels, in contrast to EOD and ED injections. This is
consistent with previous reports (33), showing improved renal functions
with fenugreek administration. Histologically better protection was
achieved (33), possibly due to
the longer treatment period. Other researchers also reported
improvement in creatinine levels and improvement of the glomerular
base membrane in the kidneys of diabetic rats when fenugreek
extract was administered orally. These findings also support the
present study and show the potential of fenugreek as a drug for
diabetes and its renal complications (34,35).
Recent metabolomics studies on the effect of
fenugreek flavonoids on STZ showed a significant impact on liver,
kidney, and pancreas (36,37). The fenugreek flavonoids lowered
insulin resistance, improved glycolysis, and gluconeogenesis, and
protected kidneys and pancreatic islet cells from damage.
Serum levels of liver enzymes (ALT and AST)
increased in untreated diabetic rats and were significantly reduced
compared to diabetic rats after fenugreek treatment. This high
serum level is attributed to the injuries to the liver cells
(38). Fenugreek caused a
significant reduction in the liver enzyme levels, indicating a
protective effect of liver cells. These findings are consistent
with previous data that showed a protective effect of fenugreek as
a daily supplement (39-42).
Other studies reported that treatment with fenugreek aqueous seed
extract (43), using a dose of 25
mg/kg body weight for 60 days, significantly decreased blood
glucose and liver enzyme levels. However, no histological liver
protective role was reported.
The effects of fenugreek seed extract on lipid
profile in the current study showed a significant reduction in
triglyceride levels compared to diabetic rats. However, a
significant increase in HDL levels was demonstrated only after
daily injections, compared to diabetic rats, unlike other treatment
groups which did not show a substantial increase in HDL levels.
This suggests a better effect after the daily (ED) injection. No
effects on total cholesterol levels were observed in any of the
treatment groups. Cholesterol findings are somewhat in disagreement
with previous reports (44), where
a dose of 500 mg/kg for four weeks caused improvement in lipid
profile (HDL, cholesterol, and triglycerides). This difference
might be due to the lower dose used in the current study (100
mg/kg).
Catalase and glutathione-S-transferase (GST) enzymes
are crucial anti-oxidative enzymes in the liver. Our results show
that fenugreek treatment led to a significant increase in GST
enzyme levels in EOD and ED injection groups when compared to
diabetic rats, unlike the oral route, which did not show
significance. Concerning catalase, a significant increase in the
enzyme levels was observed in all groups compared to diabetic rats.
This indicates considerable potential for fenugreek as a booster
for antioxidant activity in the liver due to its high content of
diosgenin (1,31). Previous studies showed similar
results, therefore, confirming the antioxidant effect of fenugreek
(45).
There was a significant increase in peroxidase
activity within the liver tissue of untreated diabetic rats
compared to healthy rats. This might be due to the high oxidative
stress exerted on the liver tissue due to the induction of diabetes
by STZ or as a complication of hyperglycemia (46). In all treated groups, there was a
significant reduction in peroxidase activity compared to diabetic
rats.
Glutathione peroxidase levels were unchanged in the
present study except for the ED injection treated rats where a
significant increase was observed compared to normal and diabetic
rats. This suggests a better effect in this group (ED injection)
over other groups (EOD injection and oral).
In the current study, a reduction in total proteins
in the untreated diabetic group was reversed by fenugreek extract
treatment. This increase in protein levels could be attributed to
the hyperglycemic effect on the liver tissue, as a result of
glycation of the antioxidant enzymes, which eventually leads to
alteration and damage in cell structural proteins and enzymes
caused by the reactive oxygen species (46).
Fenugreek may also improve the body's use of sugar,
adjusting insulin release, and possibly lowers the absorption of
glucose from the intestine (47).
Oral intake of fenugreek is also associated with liver protection
and a better quality of life for diabetic patients (48). Fenugreek is also useful as a
digestive stimulant and has potent antibacterial and oxidant
activity (49).
In conclusion, fenugreek daily injection showed
better antidiabetic effects with better serum values than other
groups. Although in all groups, there was an improvement of the
antioxidant enzymes and other diabetic parameters, the histological
structure was not fully restored in the kidney, liver or the
pancreas. This can be due to the low dose tested and the relatively
short test period (one month).
Fenugreek seems to be a promising anti-diabetic
plant. Further work is needed to better identify the mechanism of
action and the effective dose range. Longer periods are recommended
to achieve histological improvement.
Acknowledgments
Not applicable.
Funding
This research did not receive any specific grant
from funding agencies in the public, commercial, or not-for-profit
sectors.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MEB, TIA and HE were involved in data investigation
and data curation. AMES was involved in data curation, and in the
writing of the original draft. DGS, MTB, SSAZ and HHH were involved
in data analysis and validation, and in the writing, reviewing and
editing of the manuscript. MKS and AA were involved in the
conceptualization, methodology, supervision, validation of the
study, and in the reviewing and editing of the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Institutional Animal
Care and Use Committee (IACUC) of the October University for Modern
Sciences and Arts (2018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
The Orcid ID numbers for the following authors are:
MTB, orcid.org/0000-0002-8570-7739; SSAZ,
orcid.org/0000-0001-6069-8760; AA, orcid.org/0000-0003-0486-348X;
MKS, orcid.org/0000-0002-2072-0975; AMES,
orcid.org/0000-0002-5169-1214.
References
|
1
|
Ota A and Ulrih NP: An overview of herbal
products and secondary metabolites used for management of type two
diabetes. Front Pharmacol. 8(436)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Choudhury H, Pandey M, Hua CK, Mun CS,
Jing JK, Kong L, Ern LY, Ashraf NA, Kit SW, Yee TS, et al: An
update on natural compounds in the remedy of diabetes mellitus: A
systematic review. J Tradit Complement Med. 8:361–376.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Essa R, El Sadek AM, Baset ME, Rawash MA,
Sami DG, Badawy MT, Mansour ME, Attia H, Saadeldin MK and
Abdellatif A: Effects of turmeric (Curcuma longa) extract in
streptozocin-induced diabetic model. J Food Biochem.
43(e12988)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bi X, Lim J and Henry CJ: Spices in the
management of diabetes mellitus. Food Chem. 217:281–293.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Governa P, Baini G, Borgonetti V, Cettolin
G, Giachetti D, Magnano AR, Miraldi E and Biagi M: Phytotherapy in
the management of diabetes: A Review. Molecules.
23(E105)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Adam SH, Giribabu N, Kassim N, Kumar KE,
Brahmayya M, Arya A and Salleh N: Protective effect of aqueous seed
extract of Vitis Vinifera against oxidative stress, inflammation
and apoptosis in the pancreas of adult male rats with diabetes
mellitus. Biomed Pharmacother. 81:439–452. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Deng R: A review of the hypoglycemic
effects of five commonly used herbal food supplements. Recent Pat
Food Nutr Agric. 4:50–60. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Medagama AB and Bandara R: The use of
complementary and alternative medicines (CAMs) in the treatment of
diabetes mellitus: Is continued use safe and effective? Nutr J.
13(102)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Basch E, Ulbricht C, Kuo G, Szapary P and
Smith M: Therapeutic applications of fenugreek. Altern Med Rev.
8:20–27. 2003.PubMed/NCBI
|
|
10
|
Nagulapalli Venkata KC, Swaroop A, Bagchi
D and Bishayee A: A small plant with big benefits: Fenugreek
(Trigonella foenum-graecum Linn.) for disease prevention and
health promotion. Mol Nutr Food Res. 61(1600950)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Naicker N, Nagiah S, Phulukdaree A and
Chuturgoon A: Trigonella foenum-graecum seed extract,
4-hydroxyisoleucine, and metformin stimulate proximal insulin
signaling and increase expression of glycogenic enzymes and GLUT2
in HepG2 cells. Metab Syndr Relat Disord. 14:114–120.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kumar A, Aswal S, Chauhan A, Semwal RB,
Kumar A and Semwal DK: Ethnomedicinal investigation of medicinal
plants of Chakrata region (Uttarakhand) used in the traditional
medicine for diabetes by Jaunsari tribe. Nat Prod Bioprospect.
9:175–200. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pradeep SR and Srinivasan K: Amelioration
of hyperglycemia and associated metabolic abnormalities by a
combination of fenugreek (Trigonella foenum-graecum) seeds
and onion (Allium cepa) in experimental diabetes. J Basic Clin
Physiol Pharmacol. 28:493–505. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Verma N, Usman K, Patel N, Jain A, Dhakre
S, Swaroop A, Bagchi M, Kumar P, Preuss HG and Bagchi D: A
multicenter clinical study to determine the efficacy of a novel
fenugreek seed (Trigonella foenum-graecum) extract
(Fenfuro™) in patients with type 2 diabetes. Food Nutr Res.
60(32382)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tomcik KA, Smiles WJ, Camera DM, Hügel HM,
Hawley JA and Watts R: Fenugreek increases insulin-stimulated
creatine content in L6C11 muscle myotubes. Eur J Nutr. 56:973–979.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Maritim AC, Sanders RA and Watkins JB III:
Effects of alpha-lipoic acid on biomarkers of oxidative stress in
streptozotocin-induced diabetic rats. J Nutr Biochem. 14:288–294.
2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Paglia DE and Valentine WN: Studies on the
quantitative and qualitative characterization of erythrocyte
glutathione peroxidase. J Lab Clin Med. 70:158–169. 1967.PubMed/NCBI
|
|
19
|
Habig WH, Pabst MJ, Fleischner G,
Gatmaitan Z, Arias IM and Jakoby WB: The identity of glutathione
S-transferase B with ligandin, a major binding protein of liver.
Proc Natl Acad Sci USA. 71:3879–3882. 1974.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vetter HF and Vullers R: Effectiveness of
enzyme substitution with pancynorm in chronic gastroduodenitis.
Munch Med Wochenschr. 100:1786–1787. 1958.(In German). PubMed/NCBI
|
|
21
|
Szkudelski T: The mechanism of alloxan and
streptozotocin action in B cells of the rat pancreas. Physiol Res.
50:537–546. 2001.PubMed/NCBI
|
|
22
|
Lenzen S: The mechanisms of alloxan- and
streptozotocin-induced diabetes. Diabetologia. 51:216–226.
2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ozkol H, Tuluce Y, Dilsiz N and Koyuncu I:
Therapeutic potential of some plant extracts used in Turkish
traditional medicine on streptozocin-induced type 1 diabetes
mellitus in rats. J Membr Biol. 246:47–55. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Little RR and Roberts WL: A review of
variant hemoglobins interfering with hemoglobin A1c measurement. J
Diabetes Sci Technol. 3:446–451. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Little RR and Sacks DB: HbA1c: How do we
measure it and what does it mean? Curr Opin Endocrinol Diabetes
Obes. 16:113–118. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pundir CS and Chawla S: Determination of
glycated hemoglobin with special emphasis on biosensing methods.
Anal Biochem. 444:47–56. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Haeri MR, Limaki HK, White CJ and White
KN: Non-insulin dependent anti-diabetic activity of (2S, 3R, 4S)
4-hydroxyisoleucine of fenugreek (Trigonella foenum graecum) in
streptozotocin-induced type I diabetic rats. Phytomedicine.
19:571–574. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Puri D, Prabhu KM and Murthy PS: Mechanism
of action of a hypoglycemic principle isolated from fenugreek
seeds. Indian J Physiol Pharmacol. 46:457–462. 2002.PubMed/NCBI
|
|
29
|
Murao K, Yu X, Imachi H, Cao WM, Chen K,
Matsumoto K, Nishiuchi T, Wong NC and Ishida T: Hyperglycemia
suppresses hepatic scavenger receptor class B type I expression. Am
J Physiol Endocrinol Metab. 294:E78–E87. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Noor Mohamad Zin NS, Hashim N, Samsulrizal
N and Azmi NS: The protective effect of Azadirachta excelsa
leaves extract and quercetin treatment on the learning and memory
impairments in relation with insulin and amylin levels in the brain
of streptozotocin-induced diabetic rats. J King Saud Univ Sci.
31:299–307. 2019.https://doi.org/10.1016/j.jksus.2018.05.017.
|
|
31
|
Kalailingam P, Kannaian B, Tamilmani E and
Kaliaperumal R: Efficacy of natural diosgenin on cardiovascular
risk, insulin secretion, and beta cells in streptozotocin
(STZ)-induced diabetic rats. Phytomedicine. 21:1154–1161.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hamden K, Jaouadi B, Carreau S, Aouidet A,
El-Fazaa S, Gharbi N and Elfeki A: Potential protective effect on
key steroidogenesis and metabolic enzymes and sperm abnormalities
by fenugreek steroids in testis and epididymis of surviving
diabetic rats. Arch Physiol Biochem. 116:146–155. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jin Y, Shi Y, Zou Y, Miao C, Sun B and Li
C: Fenugreek prevents the development of STZ-induced diabetic
nephropathy in a rat model of diabetes. Evid Based Complement
Alternat Med. 2014(259368)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xue WL, Li XS, Zhang J, Liu YH, Wang ZL
and Zhang RJ: Effect of Trigonella foenum-graecum
(fenugreek) extract on blood glucose, blood lipid and
hemorheological properties in streptozotocin-induced diabetic rats.
Asia Pac J Clin Nutr. 16 (Suppl 1):422–426. 2007.PubMed/NCBI
|
|
35
|
Xue W, Lei J, Li X and Zhang R: Trigonella
foenum graecum seed extract protects kidney function and morphology
in diabetic rats via its antioxidant activity. Nutr Res.
31:555–562. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jiang W, Gao L, Li P, Kan H, Qu J, Men L
and Liu Z and Liu Z: Metabonomics study of the therapeutic
mechanism of fenugreek galactomannan on diabetic hyperglycemia in
rats, by ultra-performance liquid chromatography coupled with
quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt
Technol Biomed Life Sci. 1044-1045:8–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jiang W, Si L, Li P, Bai B, Qu J, Hou B,
Zou H, Fan X, Liu Z, Liu Z, et al: Serum metabonomics study on
antidiabetic effects of fenugreek flavonoids in
streptozotocin-induced rats. J Chromatogr B Analyt Technol Biomed
Life Sci. 1092:466–472. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Brent JA and Rumack BH: Role of free
radicals in toxic hepatic injury. II. Are free radicals the cause
of toxin-induced liver injury? J Toxicol Clin Toxicol. 31:173–196.
1993.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Elmnan A, Balgees A and Mangara JL: Effect
of fenugreek (Trigonella foenm greacum) seed dietary levels
on lipid profile and body weight gain of rats. Pak J Nutr.
11:1004–1008. 2012.
|
|
40
|
Garcia TS, Rech TH and Leitão CB:
Pancreatic volume in diabetes mellitus. Pancreas.
46(e51)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Garcia TS, Rech TH and Leitão CB:
Pancreatic size and fat content in diabetes: A systematic review
and meta-analysis of imaging studies. PLoS One.
12(e0180911)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Raju J and Bird RP: Alleviation of hepatic
steatosis accompanied by modulation of plasma and liver TNF-alpha
levels by Trigonella foenum graecum (fenugreek) seeds in Zucker
obese (fa/fa) rats. Int J Obes. 30:1298–1307. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sushma N and Devasena T: Aqueous extract
of Trigonella foenum graecum (fenugreek) prevents
cypermethrin-induced hepatotoxicity and nephrotoxicity. Hum Exp
Toxicol. 29:311–319. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sharma MS and Choudhary PR: Hypolipidemic
effect of fenugreek seeds and its comparison with atorvastatin on
experimentally induced hyperlipidemia. J Coll Physicians Surg Pak.
24:539–542. 2014.PubMed/NCBI
|
|
45
|
Marzouk M, Soliman AM and Omar TY:
Hypoglycemic and antioxidative effects of fenugreek and termis
seeds powder in streptozotocin-diabetic rats. Eur Rev Med Pharmacol
Sci. 17:559–565. 2013.PubMed/NCBI
|
|
46
|
Shivakumar A, Jashmitha BG and Dhruvaraj
MR: Role of peroxidase in clinical assays: A short review. J Clin
Nutr Diet. 3(2)2017.https://doi.org/10.4172/2472-1921.100048.
|
|
47
|
Gaddam A, Galla C, Thummisetti S,
Marikanty RK, Palanisamy UD and Rao PV: Role of fenugreek in the
prevention of type 2 diabetes mellitus in prediabetes. J Diabetes
Metab Disord. 14(74)2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Herrera T, Navarro Del Hierro J, Fornari
T, Reglero G and Martin D: Acid hydrolysis of saponin-rich extracts
of quinoa, lentil, fenugreek and soybean to yield sapogenin-rich
extracts and other bioactive compounds. J Sci Food Agric.
99:3157–3167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Srinivasan K: Plant foods in the
management of diabetes mellitus: Spices as beneficial antidiabetic
food adjuncts. Int J Food Sci Nutr. 56:399–414. 2005.PubMed/NCBI View Article : Google Scholar
|