Introduction
Severe acute pancreatitis (SAP) occurs in up to 20%
of patients with acute pancreatitis (AP) and it has a high
mortality rate (1,2). In general, SAP develops in two
phases. The first two weeks after the onset of symptoms are
characterized by the systemic inflammatory response syndrome
(SIRS). Release of proinflammatory mediators is thought to
contribute to the pathogenesis of SIRS associated pulmonary,
cardiovascular, and renal insufficiency (3). These systemic manifestations of a
disease initially limited to the pancreas are thought to be
mediated by a variety of pro-and anti-inflammatory mediators
released from the pancreas and various other sources during the
course of the disease. Cytokines and chemokines play a crucial role
in SAP (4). If we prevent or
decrease the release of inflammatory mediators, the prognosis of
SAP will be better.
Fractalkine (FKN) is the unique member of the CX3C
chemokine subfamily. It exists in two forms, each mediating
distinct biological actions. The membrane-anchored protein, which
is primarily expressed on the inflamed endothelium, serves as an
adhesion protein promoting the retention of monocytes and T cells
in inflamed tissue. The soluble form more closely resembles a
conventional chemokine and strongly induces chemotaxis. Based on
this function, FKN acts as both an adhesion molecule and a
chemoattractant. Thus, FKN plays a crucial role in the initiation
and progression of inflammation (5–7).
Several studies have reported that FKN plays an important role in
inflammatory diseases, including rheumatoid arthritis (8), atherosclerosis (9), acute hepatitis (10) and kidney diseases (11). Our previous studies proved that
FKN was overexpressed in the SAP rat model. We hypothesized that
the SAP prognosis would be better after suppressing FKN
overexpression.
RNA interference (RNAi) was first identified in 1998
and subsequently in mammalian cells as a post-transcriptional gene
silencing mechanism (12). RNAi
is a mechanism for RNA-guided regulation of gene expression in
which double-stranded ribonucleic acid (dsRNA) results in rapid
destruction of mRNA containing the identical sequence as the dsRNA
(13). RNA interference mediated
by siRNA is an important defense mechanism that binds to
complementary target mRNA, while specifically targeting these
sequences for degradation, resulting in the inhibition of protein
expression to prevent and/or treat disease. siRNA has already been
demonstrated as a potent therapy for targeting a wide variety of
diseases (14–16). In addition, siRNA technology has
been extensively tested against inflammation diseases in animal
models (17). RNAi has developed
within a decade into a tool for functional molecular genetics,
target gene validation in drug discovery, and a novel therapeutic
strategy (18,19).
In this study, we used siRNA to target FKN and
observed its influence on the biological functions of
cerulein-stimulated AR42J cells. Furthermore, we employed siRNA to
target FKN overexpression and assessed its ability to suppress
inflammation development in SAP rats.
Materials and methods
Cell culture
The rat pancreatic acinar cell line, AR42J (ATCC,
Rockville, MD, USA), was cultured in DMEM (Gibco-BRL, Gaithersburg,
MD, USA) plus 10% fetal bovine serum (Gibco-BRL) and 1%
penicillin/streptomycin (Sigma, St. Louis, MO, USA) in standard
conditions (37°C and 5% CO2). The AR42J cells were
plated at a density of 3×105/ml in a 6-well culture
plate and allowed to attach for 12 h. The cells were stimulated
with cerulein (10−8 M) for 12 h.
Transfection with siRNA
The adenoviral constructs carrying siRNA against FKN
were designed and constructed by MingHong Co., Ltd., Shanghai,
China. The specific silencing of the rat FKN gene expression was
achieved by the siRNA technique. The FKN siRNA sequences were
5′-GCA ACA TCA CGT GCC ACAA-3′ and 5′-TTG TGG CAC GTG ATG TTGC-3′.
The cerulein-stimulated AR42J cells (3×105/well) were
transfected with siRNA (10 nmol/ml) in 6-well plates following the
manufacturer’s protocol.
Detection of pancreatic amylase and
lactate dehydrogenase (LDH) release by chromatometry
Cerulein-stimulated AR42J cells culture supernatant
was collected by centrifugation and analyzed according to the
operation manual of the pancreatic amylase test kit and LDH
detection kit (Jiancheng Biotech, Nanjing, China).
Colony formation assay in soft agar
The standard colony formation assay was performed as
described previously (20). The
cerulein stimulated AR42J cells were transfected without (mock) or
with siRNA targeting FKN. After 14 days of culture, crystal
violet-stained colonies (>50 cells/colony) were counted under a
dissecting microscope. The plating efficiency (PE) represents the
percentage of cells seeded that grow into colonies under a
specified culture condition. The survival fraction, expressed as a
function of irradiation, was calculated as follows: survival
fraction = colonies counted/(cells seeded x PE/100).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide (MTT)
analysis of FKN siRNA in cultured cells
Cerulein-stimulated AR42J cells were transfected
with FKN siRNA. Twelve hours after transfection, the cells were
split into 96-well culture plates and incubated for 24, 48 and 72
h. The viable cells were then determined with the MTT assay. For
the MTT assay, 20 μl MTT (Sigma) stock solution (5 mg/ml) was added
to each well on the plate. Cells were incubated for 4 h with MTT at
37°C and then lysed in 100 ml of dimethyl-sulfoxide (DMSO). The
intensity of the color developed, which is the reflection of number
of live cells, was determined by a microplate reader at a 570-nm
wavelength. All values were compared to the corresponding controls.
All assays were performed with 5 replicates.
Determination of FKN, IL-8 and TNF-α
expression in AR42J cells by western blotting
The cerulein stimulated AR42J cells were randomly
allocated into 3 groups: the phosphate-buffered saline
(PBS)-treated group (25 μl), the negative siRNA and FKN siRNA
groups. The cells were washed twice with PBS and then homogenized
in RIPA buffer (Shanghai Biocolor BioScience and Technology Co.,
Shanghai, China). Following centrifugation at 12,000 x g at 4°C for
10 min, the supernatant was collected and stored at −80°C. The
protein concentration of each sample was determined by the BCA
protein assay. Each sample was adjusted up to the desired protein
content of 40 μg. Western blotting was performed as previously
described (21) with minor
modifications. The membrane was incubated with primary antibodies
against FKN, IL-8 and TNF-α (diluted, 1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C followed
by a peroxidase-conjugated anti-IgG (diluted, 1:2,000; Santa Cruz
Biotechnology, Inc.) secondary antibody for 1 h. GAPDH was
determined in a similar manner with anti-GAPDH antibody (diluted
1:2,000; Santa Cruz Biotechnology, Inc.) as an endogenous control
for other proteins. Western blotting was analyzed by scanning
densitomertry using the Bio-Image analysis system (Bio-Rad,
Baltimore, MD, USA) for quantification.
Animal model of SAP
Sprague-Dawley (SD) rats (220–250 g) were provided
by the Experimental Animal Center of Ruijin Hospital, Shanghai
Jiaotong University School of Medicine, Shanghai, China. The rats
were deprived of food but were allowed access to water for 12 h
before the operation. The rats were anaesthetized with an
intraperitoneal administration of sodium pentobarbital (30%, 0.15
ml/100 g). The biliopancreatic duct was cannulated through the
duodenum and the hepatic duct was closed by a small clamp. The SAP
rats were induced by retrograde perfusion of 5% sodium taurocholate
(Sigma) in a volume of 1.5 ml/kg, using a perfusion pump (22). The control rats received an
intraperitoneal injection of saline solution. The experiments were
conducted according to the Guidelines of the Shanghai Animal Use
and Care Committees and the National Animal Welfare Law.
In vivo SAP studies
FKN siRNA ((20 μg/100 g) was injected once 24 h
prior to the first injection of sodium taurocholate to the rats.
The injected taurocholate SAP rat models were randomly divided into
3 groups (n=5 for each group): mock (no siRNA), negative FKN siRNA,
FKN siRNA. The mock group used PBS instead of siRNA. SAP sats were
sacrificed under anesthesia 48 h after injecting of FKN siRNA.
Blood was collected by abdominal aorta puncture. After
centrifugation for 10 min at 2,500 x g/min, the supernatant of the
blood was placed into sterilized EP tubes and stored in a
refrigerator at −20°C. Meanwhile, pancreas and lung tissue frozen
in a refrigerator at −80°C until biochemical assays were
performed.
Animal model assessment
Tissue samples were fixed in 4% formaldehyde
overnight and subsequently dehydrated through a graded ethanol
series. After impregnation in paraffin wax, tissue samples were cut
into blocks (4 μm). Pancreas and lung tissue were stained with
hematoxylin-eosin and examined by light microscopy. Sections were
examined by an experienced histologist who was not aware of the
sample identity for tissue injury. For this study, 5 randomly
chosen microscopic fields were examined for each tissue sample and
the histological score of pancreatic injury was calculated by a
previously described method (23). Serum amylase activity was
determined by spectrophotometric assay using the AMS detection kit
(Jiancheng Biotech). Pancreatic tissue neutrophilic infiltration
was assessed by measuring myeloperoxidase (MPO) activity, using the
MPO detection kit (Jiancheng Biotech) (24).
Enzyme-linked immunosorbent assay
(ELISA)
Cell culture supernatants and the serum levels of
FKN, TNF-α and IL-8 were examined with ELISA assay kits (Mai Bio
Co., Ltd., Shanghai, China). Analyses were performed according to
the instructions of the manufacturer (24).
FKN expression in SAP by western blot
analysis
Pancreas and lung tissue proteins were extracted
with RIPA buffer. Western blot analysis was performed as previously
described.
FKN expression in SAP by RT-PCR
The total-RNA of pancreas and lung tissue was
extracted with TRIzol (Takara Biotechnology Co., Ltd. China)
reagent for each group. The cDNA was synthesized using the Prime
Script™ RT reagent kit (Takara Biotechnology Co., Ltd.), and the
reverse transcription was then performed on 1 μg RNA sample by
adding PrimeScript reagents. After reverse transcription, the
RT-PCR reactions were performed in 25 μl volumes. The primers were
as follows: the primer sequence of FKN (141 bp); forward, 5′-CTG
CCC TGA CTA GAA ATG GT-3′ and reverse, 5′-CAG TCG GTT CCA AAG TAA
GG-3′; The primer sequence of GAPDH (460 bp): forward, 5′-ACC ACA
GTC CAT GCC ATC AC-3′ and reverse, 5′-TCC ACC ACC CTG TTG CTG
TA-3′. The band densities were normalized to the GAPDH band
densities and the results were expressed as the ratio. The
densities of the cDNA bands were analyzed by scanning densitometry
using the Gel Doc 2000 software (Bio-Rad).
Immunohistochemical staining
analysis
Sections embedded in paraffin wax were dewaxed,
rehydrated in gradient alcohol, and endogenous peroxidase activity
was blocked with 3% H2O2 for 5 min.
Subsequently, a polyclonal FKN antibody (diluted 1:100, Santa Cruz
Biotechnology, Inc.) that could recognize the amino-terminal
sequences was applied overnight at 4°C, followed by the
UltraSensitive™ SP kit (Maixin-Bio, Fujian, China). At last,
binding was visualized by diaminobenzidine (DAB) and the sections
were counterstained with hematoxylin. Positive signals were
detected as cytoplasm and nucleus staining presenting yellow
color.
Statistics
All data are expressed as the mean ± standard
deviation (SD). Statistics were performed by the SPSS program 11.0
version (SPSS, Chicago, IL, USA). The one-way analysis of variance
(ANOVA) with Dunnett’s multiple comparison tests was used for
comparisons. P<0.05 indicated statistically significant
differences.
Results
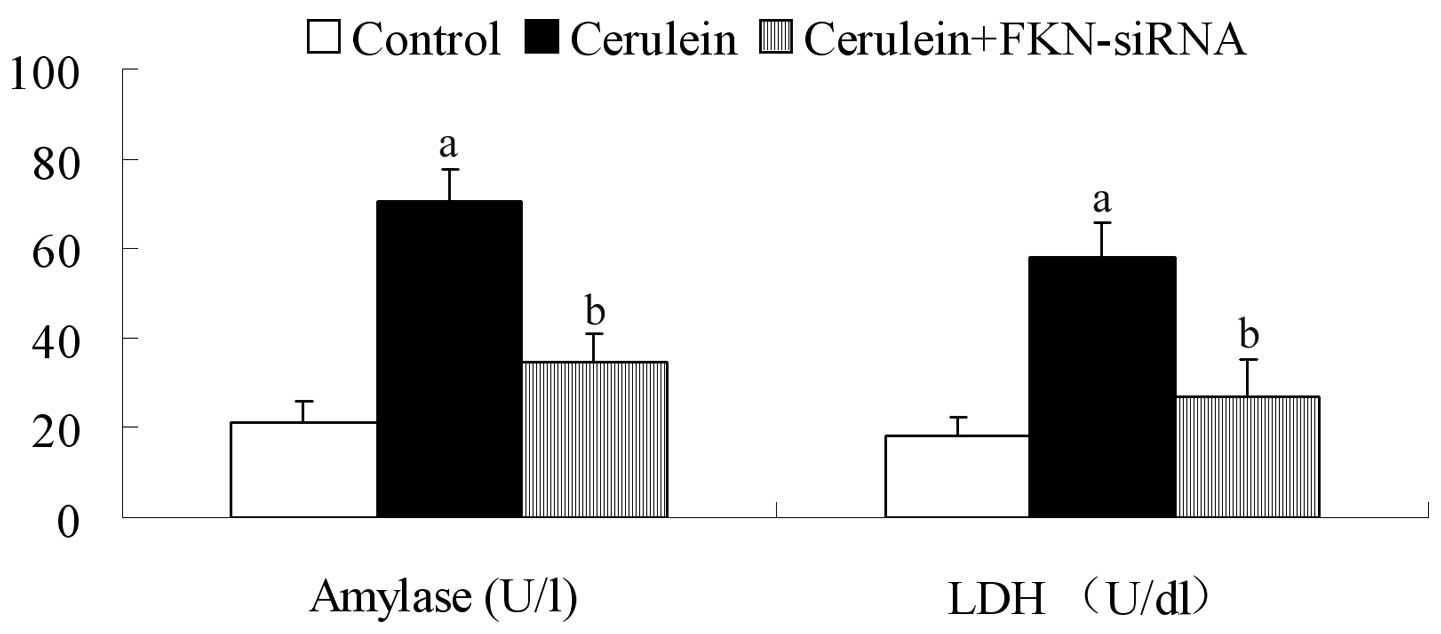
Release of amylase and LDH in
cerulein-stimulated AR42J cells
Amylase is synthesized and stored in acinar cells of
the pancreas. LDH is an enzyme of energy metabolism. Elevated
activities of these enzymes in the plasma are generally considered
markers of acute pancreatic injury. We found that in the control
group amylase and LDH release levels were relatively low. In
cerulein-stimulated AR42J cells, the release levels of the two
enzymes were significantly higher compared with the control group
(P<0.05). Furthermore, the levels of amylase and LDH were
decreased after FKN siRNA treatment (P<0.05) (Fig. 1).
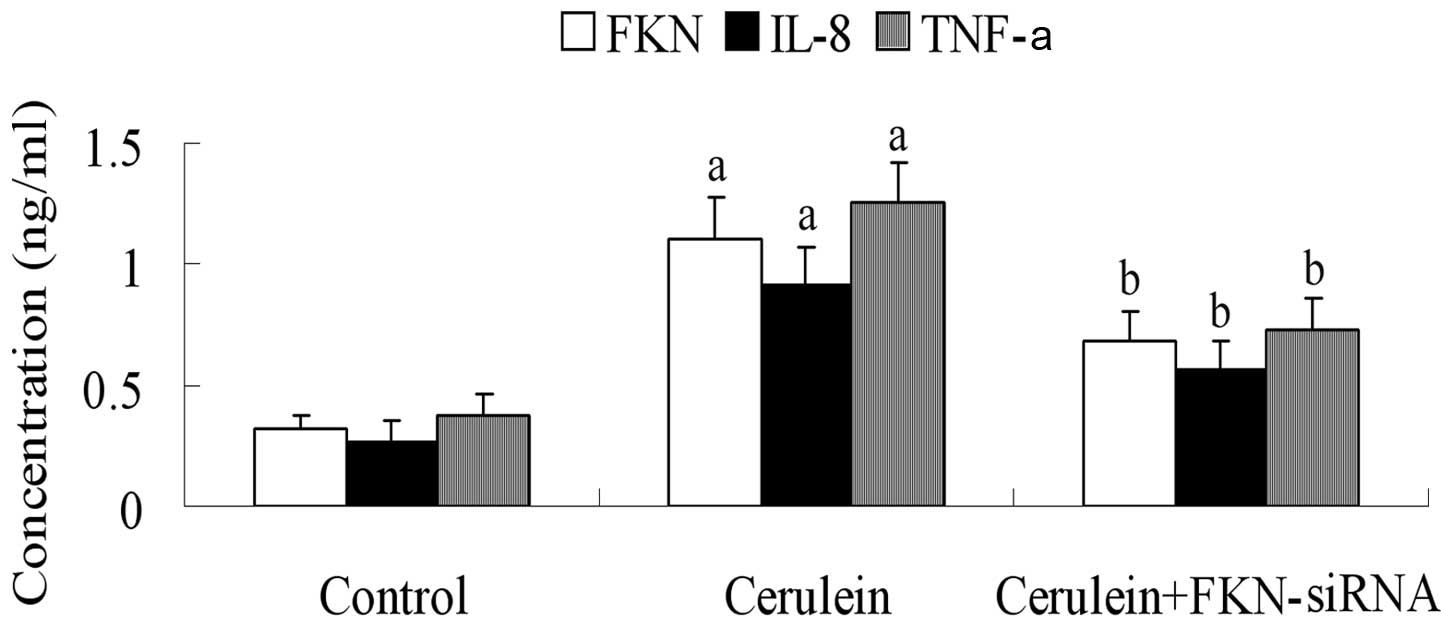
FKN, IL-8 and TNF-α levels in
cerulein-stimulated AR42J cells
To determine the release of FKN, IL-8 and TNF-α in
cerulein-stimulated AR42J cells, we used ELISA to examine cell
culture supernatants after FKN siRNA treatment. FKN was
significantly decreased after FKN siRNA treatment (P<0.05)
(Fig. 2). At the same time, the
results showed that FKN siRNA inhibited the expression of IL-8 and
TNF-α.
Effect of FKN siRNA on cell growth
To determine whether FKN siRNA actually affected
growth of cerulein-stimulated AR42J cells, we examined the growth
curves of the cells in response to FKN siRNA treatment. FKN siRNA
did not decrease the cell number as compared with the
cerulein-stimulated AR42J cells (Fig.
3A). FKN siRNA promoted cerulein-stimulated AR42J cell growth
on soft agar (Fig. 3B).
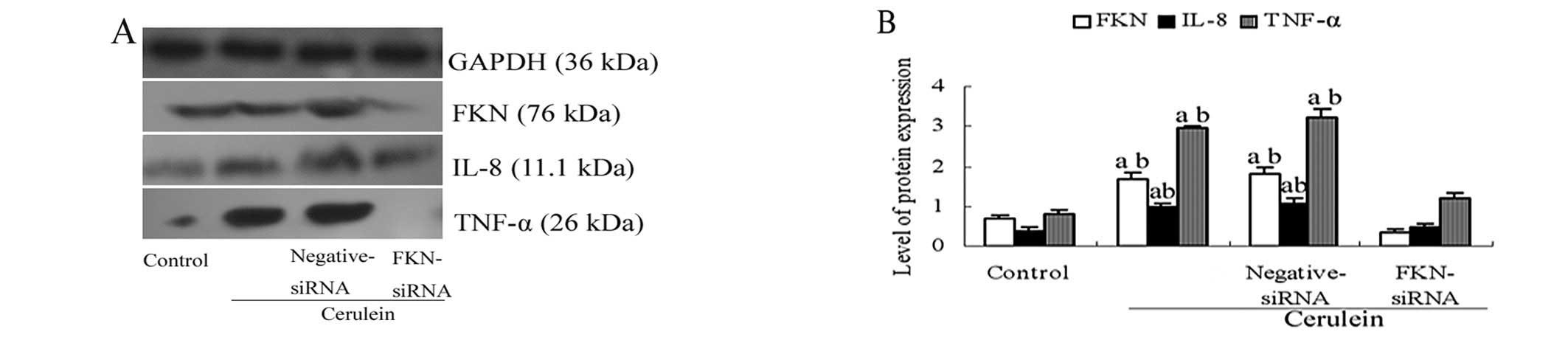
FKN overexpression is suppressed in
cerulein-stimulated AR42J cells by siRNA targeting
To address if FKN can serve as a novel therapeutic
target for SAP, we first used siRNA to deplete FKN expression in
cerulein-stimulated AR42J cells. The siRNA was transfected into
cerulein-stimulated AR42J cells. The protein levels of FKN were
reduced by FKN siRNA (Fig. 4).
Furthermore, the inhibitory effect of the FKN siRNA affected the
expression of IL-8 and TNF-α (Fig.
4). We found that the expression of IL-8 and TNF-α protein were
reduced after transfection with FKN siRNA. These data indicate that
FKN siRNA can effectively suppress the overexpression of FKN.
FKN siRNA treatment is able to suppress
inflammation in vivo
To determine whether FKN siRNA could suppress
inflammation in vivo, we established the SAP rat model and
treated tha rats with FKN siRNA. All SAP rats were sacrificed under
anesthesia at 48 h after treatment with FKN siRNA or sham
operation. Serum amylase levels and MPO activity are shown Table I. Serum amylase and MPO activity
were significantly elevated compared with the control group
(P<0.05). The histological score of pancreatic injury indicated
there were no remarkable pathologic changes in control rats. In the
SAP groups, interstitial edema, inflammatory cell infiltration,
focal necrosis and interstitial hemorrhage were observed. The
histological score of pancreatic injury was significantly elevated
compared with the control group (P<0.05). More importantly, we
observed serum amylase levels and MPO activities were decreased
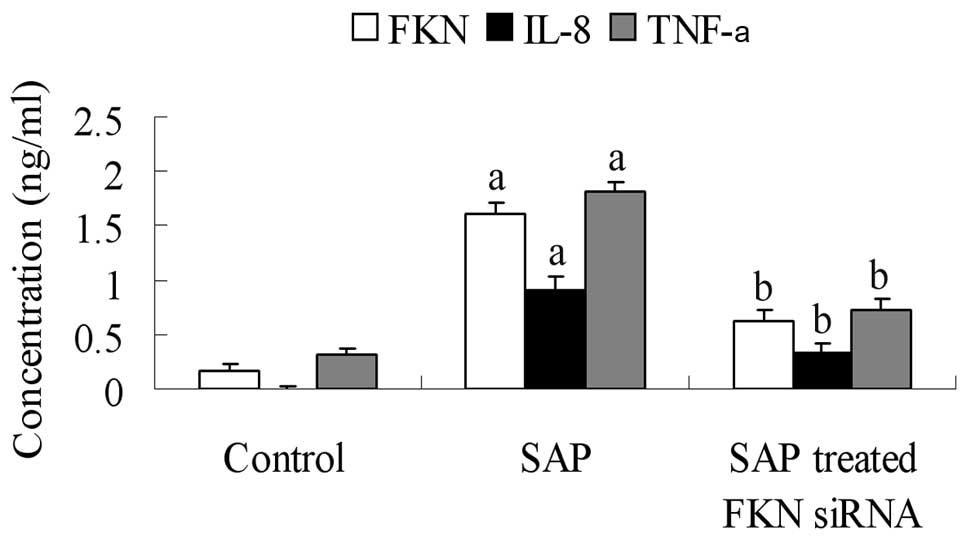
after FKN siRNA treatment. We used ELISA to detect the serum levels
of FKN, TNF-α and IL-8. The values of serum FKN, TNF-α and IL-8 in
each group are shown in Fig. 5.
Serum FKN levels were significantly greater in the SAP compared
with the control group (P<0.05). Serum TNF-α and IL-8 levels
were both elevated in the SAP compared with the control group
(P<0.05). The values of serum FKN, TNF-α and IL-8 were decreased
after induction with FKN siRNA compared with the SAP group
(P<0.05). In addition, western blotting and RT-PCR analysis
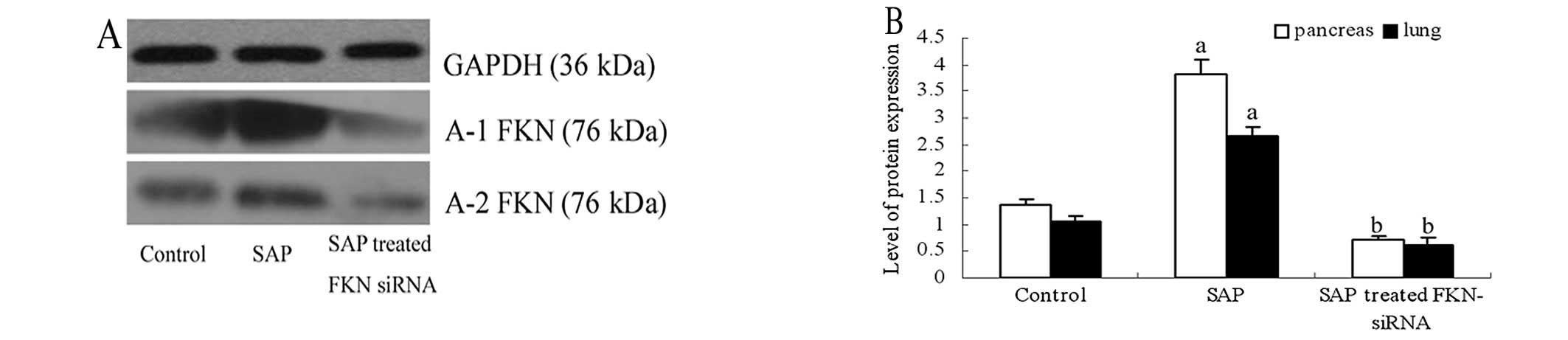
showed that FKN protein and mRNA levels of pancreas and lung
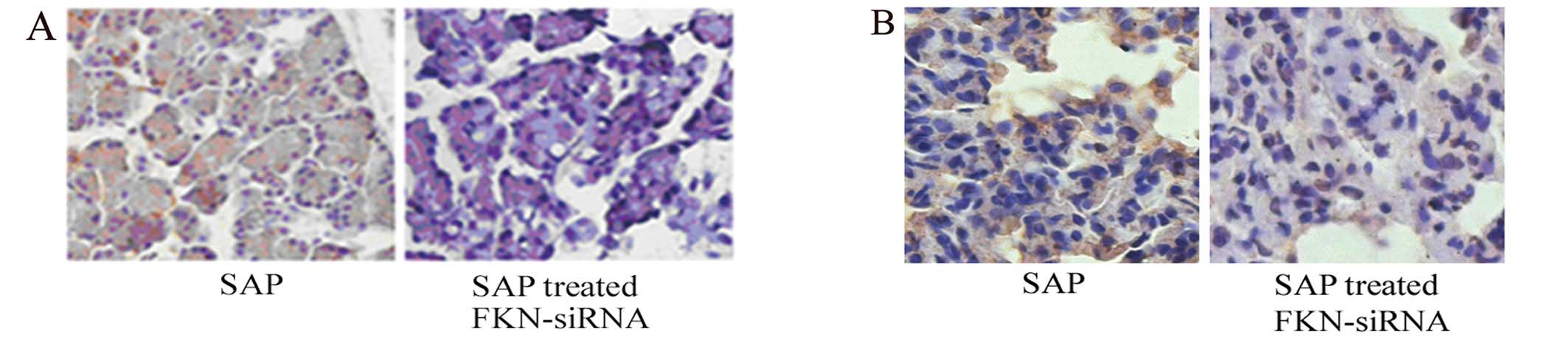
tissues were also decreased in the SAP rat model (Figs. 6 and 7). Furthermore, immunohistochemistry
showed that FKN decreased after injection of FKN-siRNA in SAP rat
model (Fig. 8). These results
showed that treatment with FKN siRNA can inhibit inflammation
development in SAP rats, indicating that FKN siRNA may serve as a
novel therapeutic agent for treating SAP.
 | Table I.Serum amylase, pancreatic MPO
activity, and histological score of pancreatic injury in the SAP
rat model. |
Table I.
Serum amylase, pancreatic MPO
activity, and histological score of pancreatic injury in the SAP
rat model.
| Control | SAP | SAP treated
FKN-siRNA |
|---|
| Serum amylase
(U/dl) | 7.86±0.084 | 72±2.88a | 14.83±2.85b |
| Pancreatic MPO
activity (U/g) | 0.046±0.0038 | 1.13±0.064a | 0.32±0.023b |
| Histological score
of pancreatic injury | 0.4±0.24 | 10.4±0.89a | 4.2±0.36b |
Discussion
Chemokines are small secreted proteins that
stimulate the directional migration of leukocytes, playing a key
role in the inflammatory response and infectious diseases (25). In a variety of pathological
conditions, FKN may cause excessive attraction and activity of
cytotoxic lymphocytes, which might lead to vascular and tissue
damage (26). FKN is aberrantly
expressed in many inflammatory diseases (8–11).
Moreover, studies in animal models have shown that FKN inhibition
may delay the initiation and progression of lupus nephritis
(27). Cockwell et al
(28) have previously reported
increased mRNA levels of FKN in renal biopsies from patients with
antineutrophil cytoplasmic antibody-positive vasculitic
glomerulonephritis. Bjerkeli et al (29) have reported that increased
CX3CL1/CX3CR1 interaction could be involved in the pathogenesis of
the granulomatous vasculitis characterizing Wegener’s
granulomatosis patients. This study was aiming to prove that FKN
may be involved in the pathogenesis of SAP. Furthermore, the
present study clearly demonstrated a promising therapeutic
potential of FKN siRNA for the treatment of FKN-overexpressing
SAP.
FKN displays distinctive biological activities
compared to the other members of the chemokine family. It is a
unique membrane-bound molecule expressed on endothelial cells,
possessing a chemokine domain and an extended mucin-like stalk that
allows it to function as both a chemoattractant and an adhesion
molecule (30). The expression of
FKN on the endothelium is generally low in healthy individuals in
the absence of an inflammatory insult, but the expression of both
the membrane-bound and the secreted form is greatly induced by
inflammatory cytokines (5).
Membrane-bound FKN can act as an adhesion molecule to mediate firm
adhesion by binding with its receptor on leukocytes (7,30).
This membrane-bound form can be cleaved by metalloproteinases
(31) to create circulating
soluble FKN, a potential chemoattractant. TNF-α and IL-8 have been
recognized to be important factors in the progression of SAP
(4,32). TNF-α can lead to injured
pancreatic duct cells, pancreatic acinus ischemia, necrosis and
inflammation (32). Many cell
lines in the presence of activated neutrophils can release IL-8.
IL-8 acts as the main secondary mediator of TNF-α-induced
neutrophil activation (33,34). Our studies demonstrated the
markedly elevated levels of FKN, IL-8 and TNF-α in
cerulein-stimulated AR42J cells. These could potentially reflect
pancreatic acinar cell-related inflammation in the SAP lesions.
Moreover, the increased expression of FKN in the pancreas, as shown
by both western blotting and RT-PCR, could facilitate migration of
leukocyte subsets into the SAP lesions through FKN.
The adenovirus-mediated method of transferring siRNA
has shown this method to be a more efficient delivery mechanism
(35,36). In this study, we utilized an
adenovirus-mediated method to transfer siRNA, used FKN siRNA to
target FKN overexpression and assessed its ability to suppress
inflammation in cerulein-stimulated AR42J cells. This study
revealed that FKN siRNA effectively inhibited FKN overexpression.
TNF-α and IL-8, as primary inflammatory factors, directly injured
cells of multiple organs, and caused ischemia, hemorrhage,
necrosis, inflammation and edema (33,37). FKN siRNA also effectively
inhibited TNF-α and IL-8 overexpression in cerulein-stimulated
AR42J cells and in the SAP rat model.
Recently, various approaches have been adapted to
chemically modify the siRNAs to increase their nuclease resistance
as well as intracellular uptake (38,39). Thus, it will be of great interest
to test if chemical modifications of our FKN siRNA will improve its
therapeutic efficacy, especially for systematic treatment in the
rat. In this study, we used FKN siRNA to target FKN overexpression
and assessed its ability to suppress inflammation in the SAP rat
model. Our results revealed that FKN siRNA effectively inhibited
the FKN overexpression in cerulein-stimulated AR42J cells.
Depletion of FKN promoted growth of FKN-overexpressing cells and
blocked their proliferation. We also showed that FKN siRNA
inhibited inflammation in the SAP rat model. Thus, our study
clearly demonstrates the therapeutic potential of FKN siRNA for
treating FKN-overexpressing SAP.
Acute lung injury is the most common extrapancreatic
complication of SAP. The pathogenesis of lung injury is complex and
probably involves multiple mechanisms, but mounting evidences point
to the role of chemokines in the pathogenesis of SAP lung injury
(40,41). The expression of FKN on the
endothelium is generally low in healthy individuals in the absence
of an inflammatory insult, but the expression of both the
membrane-bound and the secreted form is greatly induced by
inflammatory cytokines (5). FKN
may induce pulmonary vascular inflammatory cell recruitment and
therefore, may promote inflammatory damage leading to abnormal
scarring and remodeling of pulmonary arteries (42). Furthermore, this study showed that
FKN was overexpressed in lung tissue and FKN siRNA inhibited the
lung injury in SAP. The protein and mRNA levels of FKN were
decreased after siRNA injection in lung tissue. We demonstrated
that this was sufficient to inhibit FKN expression in vivo
and as a result, led to SAP suppression. These results indicate
that FKN, which is overexpressed in SAP, may serve as a novel and
effective therapeutic target.
In conclusion, the present study validated systemic
adenoviral siRNA delivery into cerulein-stimulated AR42J cells and
the targeting of FKN in the SAP rat model. With foreseeable
improvements in siRNA delivery and specificity, chemokines, such as
FKN, may become attractive targets in the clinical therapy of
inflammatory diseases.
References
|
1.
|
CD JohnsonM Abu-HilalPersistent organ
failure during the first week as a marker of fatal outcome in acute
pancreatitisGut5313401344200410.1136/gut.2004.03988315306596
|
|
2.
|
Y TakeyamaSignificance of apoptotic cell
death in systemic complications with severe acute pancreatitisJ
Gastroenterol40110200510.1007/s00535-004-1505-815692783
|
|
3.
|
C JohnsonA KingsnorthC ImrieDouble blind,
randomised, placebo controlled study of a platelet activating
factor antagonist, lexipafant, in the treatment and prevention of
organ failure in predicted severe acute
pancreatitisGut486269200110.1136/gut.48.1.62
|
|
4.
|
J MayerB RauF GansaugeHG BegerInflammatory
mediators in human acute pancreatitis: clinical and
pathophysiological
implicationsGut47546552200010.1136/gut.47.4.54610986216
|
|
5.
|
H UmeharaET BloomT OkazakiY NaganoO
YoshieT ImaiFractalkine in vascular biology: from basic research to
clinical diseaseArterioscler Thromb Vasc
Biol243440200410.1161/01.ATV.0000095360.62479.1F12969992
|
|
6.
|
AM FongLA RobinsonDA SteeberFractalkine
and CX3CR1 mediate a novel mechanism of leukocyte capture, firm
adhesion, and activation under physiologic flowJ Exp
Med18814131419199810.1084/jem.188.8.14139782118
|
|
7.
|
T ImaiK HieshimaC HaskellIdentification
and molecular characterization of fractalkine receptor CX3CR1,
which mediates both leukocyte migration and
adhesionCell91521530199710.1016/S0092-8674(00)80438-99390561
|
|
8.
|
MV VolinJM WoodsMA AminMA ConnorsLA
HarlowAE KochFractalkine: a novel angiogenic chemokine in
rheumatoid arthritisAm J
Pathol15915211530200110.1016/S0002-9440(10)62537-011583978
|
|
9.
|
SJ LeeS NamkoongYM KimFractalkine
stimulates angiogenesis by activating the Raf-1/MEK/ERK- and
PI3K/Akt/eNOS-dependent signal pathwaysAm J Physiol Heart Circ
Physiol291H2836H2846200610.1152/ajpheart.00113.200616877565
|
|
10.
|
E EfsenC GrapponeRM DeFrancoUp-regulated
expression of fractalkine and its receptor CX3CR1 during liver
injury in humansJ
Hepatol373947200210.1016/S0168-8278(02)00065-X12076860
|
|
11.
|
S SegererE HughesKL HudkinsM MackT
GoodpasterCE AlpersExpression of the fractalkine receptor (CX3CR1)
in human kidney diseaseKidney
Int62488495200210.1046/j.1523-1755.2002.00480.x12110009
|
|
12.
|
SM ElbashirJ HarborthW LendeckelA YalcinK
WeberT TuschlDuplexes of 21-nucleotide RNAs mediate RNA
interference in cultured mammalian
cellsNature411494498200110.1038/3507810711373684
|
|
13.
|
GJ HannonRNA
interferenceNature418244251200210.1038/418244a12110901
|
|
14.
|
MS DuxburyE MatrosH ItoMJ ZinnerSW
AshleyEE WhangSystemic siRNA-mediated gene silencing: a new
approach to targeted therapy of cancerAnn
Surg240667674200415383794
|
|
15.
|
S FilleurA CourtinS AliSiRNA-mediated
inhibition of vascular endothelial growth factor severely limits
tumor resistance to antiangiogenic thrombospondin-1 and slows tumor
vascularization and growthCancer Res63391939222003
|
|
16.
|
E SongSK LeeJ WangRNA interference
targeting Fasprotects mice from fulminant hepatitisNat
Med9347351200310.1038/nm82812579197
|
|
17.
|
M SioudAdvances in RNA sensing by the
immune system: separation of siRNA unwanted effects from RNA
inferenceMethods Mol
Biol6293352201010.1007/978-1-60761-657-3_320387141
|
|
18.
|
RK LeungPA WhittakerRNA interference: from
gene silencing to gene- specific therapeuticsPharmacol
Ther107222239200510.1016/j.pharmthera.2005.03.00415908010
|
|
19.
|
DH KimJJ RossiStrategies for silencing
human disease using RNA interferenceNat Rev
Genet8173184200710.1038/nrg200617304245
|
|
20.
|
L YulongG HongL Shiaw-YihJA GossFC
BrunicardiK LisiRNA-based targeting of cyclin E over-expression
inhibits breast cancer cell growth and suppresses tumor development
in breast cancer mouse modelPLoS
One5e12860201010.1371/journal.pone.001286020877462
|
|
21.
|
S YuberoL RamudoMA MansoI De
DiosMechanisms of dexamethasone-mediated chemokine down-regulation
in mild and severe acute pancreatitisBiochim Biophys
Acta179212051211200910.1016/j.bbadis.2009.10.00119818401
|
|
22.
|
P ChenY YuanS WangL ZhanJ XuCaptopril, an
angiotensin-converting enzyme inhibitor, attenuates the severity of
acute pancreatitis in rats by reducing expression of matrix
metalloproteinase 9Tohoku J Exp
Med20999101200610.1620/tjem.209.99
|
|
23.
|
HP GrewalA Mohey el DinL GaberM KotbAO
GaberAmelioration of the physiologic and biochemical changes of
acute pancreatitis using an anti-TNF-alpha polyclonal antibodyAm J
Sug167214218199410.1016/0002-9610(94)90076-08311136
|
|
24.
|
XP ZhangJ ZhangML MaPathological changes
at early stage of multiple organ injury in a rat model of severe
acute pancreatitisHepatobiliary Pancreat Dis
Int98387201020133235
|
|
25.
|
PM MurphyThe molecular biology of
leukocyte chemoattractant receptorsAnnu Rev
Immunol12593633199410.1146/annurev.iy.12.040194.0031138011292
|
|
26.
|
M BrueckmannM BorggrefeTherapeutic
potential of fractalkine: a novel approach to metastatic colon
cancerGut56314316200710.1136/gut.2006.10331717339240
|
|
27.
|
A InoueH HasegawaM KohnoAntagonist of
fractalkine (CX3CL1) delays the initiation and ameliorates the
progression of lupus nephritis in MRL/lpr miceArthritis
Rheum5215221533200510.1002/art.2100715880599
|
|
28.
|
P CockwellSJ ChakravortyJ GirdlestoneCO
SavageFractalkine expression in human renal inflammationJ
Pathol1968589200210.1002/path.101011748646
|
|
29.
|
V BjerkeliJK DamaB FevangJC HolterP
AukrustSS FrolandIncreased expression of fractalkine (CX3CL1) and
its receptor CX3CR1, in Wegener’s granulomatosis-possible role in
vascular inflammationRheumatology46142214272007
|
|
30.
|
JF BazanKB BaconG HardimanA new class of
membrane bound chemokine with a CX3C
motifNature385640644199710.1038/385640a09024663
|
|
31.
|
C HundhausenD MisztelaTA BerkhoutThe
disintegrin-like metalloproteinase ADAM10 is involved in
constitutive cleavage of CX3CL1 (fractalkine) and regulates
CX3CL1-mediated cell-cell
adhesionBlood10211861195200310.1182/blood-2002-12-377512714508
|
|
32.
|
XP ZhangL WangYF ZhouThe pathogenic
mechanism of severe acute pancreatitis complicated with renal
injury: a review of current knowledgeDig Dis
Sci53297306200810.1007/s10620-007-9866-517597411
|
|
33.
|
A KingsnorthRole of cytokines and their
inhibitors in acute
pancreatitisGut4014199710.1136/gut.40.1.19155566
|
|
34.
|
IA Al MoflehSevere acute pancreatitis:
pathogenetic aspects and prognostic factorsWorld J
Gastroenterol14675684200818205255
|
|
35.
|
TR BrummelkampR BernardsR AgamiStable
suppression of tumorigenicity by virus-mediated RNA
interferenceCancer
Cell2243247200210.1016/S1535-6108(02)00122-812242156
|
|
36.
|
C ChettyP BhoopathiP JosephS ChittiveluJS
RaoS LakkaAdenovirus-mediated siRNA against MMP-2 suppresses tumor
growth and lung metastasis in miceMol Cancer
Ther522892299200610.1158/1535-7163.MCT-06-016916985063
|
|
37.
|
AA SandbergA BorgstromEarly prediction of
severity in acute pancreatitis: is this
possible?JOP3116125200212221326
|
|
38.
|
J SoutschekA AkincB BramlageTherapeutic
silencing of an endogenous gene by systemic administration of
siRNAsNature432173178200410.1038/nature0312115538359
|
|
39.
|
BR GoyalMM PatelMK SoniSV
BhadadaTherapeutic opportunities of small interfering RNAFundam
Clin
Pharmacol23367386200910.1111/j.1472-8206.2009.00694.x19709318
|
|
40.
|
M SochorS RichterA SchmidtS HempelUT HoptT
KeckInhibition of matrix metalloproteinase-9 with doxycycline
reduces pancreatitis-associated lung
injuryDigestion806573200910.1159/00021208019494493
|
|
41.
|
C ChenS XuWX WangRosiglitazone attenuates
the severity of sodium taurocholate induced acute pancreatitis and
pancreatitis-associated lung injuryArch Med
Res407988200910.1016/j.arcmed.2008.11.00419237016
|
|
42.
|
F PerrosP DrofmullerR
SouzaFractalkine-induced smooth muscle cell proliferation in
pulmonary hypertensionEur Respir
J29937943200710.1183/09031936.0010470617182651
|