Contents
Introduction
Mechanism of action and functions of microRNAs
microRNAs and cancer
microRNAs and thyroid carcinoma
Conclusion
Introduction
Gene expression in normal cells is highly regulated
by complex gene regulatory networks. Disruption of these networks
may lead to cancer. Previous studies have shown the presence of
small regulatory RNAs, known as microRNAs (miRNAs), that represent
a class of highly conserved small endogenous RNAs made up of 20–22
nucleotides single-stranded non-coding proteins. These miRNAs are
involved in the regulation of proliferation differentiation and
apoptosis by interfering with protein expression (1–3),
usually resulting in translational repression or target degradation
and gene silencing (4).
Accumulating evidence has shown that miRNAs are associated with
cancer because of their deregulation (5–7).
Many miRNAs are expressed in a tissue-specific manner and exhibit
expression profiles that are different between normal and
neoplastic tissues and between tumors with distinct biological
properties (8,9). Several research lines have been
developed to understand the role of miRNAs in cancer development
and in clinical practice, as tumor biomarkers and therapeutic
targets. The deregulated expression of miRNAs has been recognized
in multiple cancer types, including thyroid cancer. Thyroid cancer
represents an attractive model for studying the events involved in
epithelial cell multistep carcinogenesis as these events comprise a
broad spectrum of lesions with different degrees of malignancy.
Most thyroid carcinomas originate from thyroid follicular cells and
are subdivided into well-differentiated papillary thyroid carcinoma
(PTC) and follicular thyroid carcinoma (FTC). Both PTCs and FTCs
may progress into poorly differentiated carcinoma or may completely
lose differentiation to give rise to anaplastic carcinoma (ATC).
Less than 5% of cells within the thyroid gland are C-cells that
give rise to medullary thyroid carcinoma (MTC) (10). The aim of this review was to
highlight the latest findings on miRNA expression profiles in
various subtypes of thyroid cancer.
Mechanism of action and functions of
microRNAs
The miRNAs that were first discovered, lin-4
(11,12) and let-7 (13,14) were identified genetically in
Caenorhabditis elegans. Subsequently, hundreds of
non-protein-coding miRNAs were identified across species, showing
that they are highly conserved (2,15–17). Both cloning and bioinformatic
approaches have shown that the human genome contains a larger
number of miRNAs than previously appreciated (18). It has been suggested that the
total number may be >800 or 1,000 (3). miRNA genes located in the introns
and/or exons of protein-coding genes or in the intergenic regions
between protein-coding genes, may form polycistronic clusters or
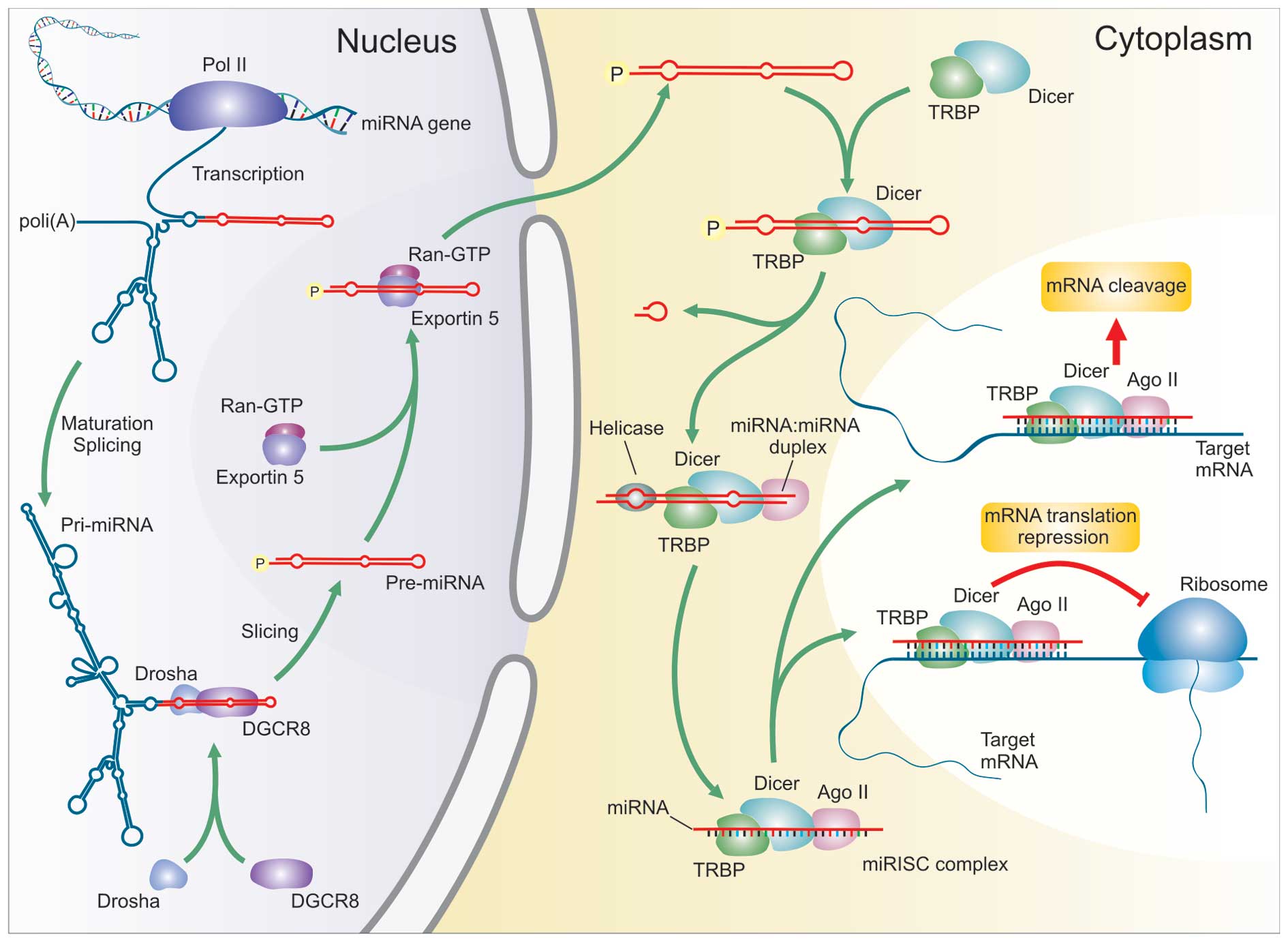
exist individually (16,19,20). miRNA biogenesis consists of a
stepwise-process that starts with a primary transcript RNA
(pri-miRNA) transcribed from a miRNA gene by RNA polymerase II and
thus processed into a stem-loop structure of ∼70–100 nt [precursor
miRNA (pre-miRNA)] by a double-strand (ds)-RNA-specific
ribonuclease, Drosha, with the help of its binding partner DGCR8
(21–23). These pre-miRNAs are transported
into the cytoplasm via an exportin-5-RanGTP dependent mechanism
(24–26). In the cytoplasm, they are digested
by a second, dsRNA-specific ribonuclease known as Dicer, with the
help of transactivating response RNA-binding protein (TRBP) and
argonaute 2 AGO2 (27–29). The released 17–25 nt mature miRNA
is bound by a complex termed miRNA-associated RNA-induced silencing
complex (miRISC) (30). miRNAs
can direct the RISC to downregulate gene expression by using one of
2 post-transcriptional mechanisms: mRNA cleavage or translational
repression (Fig. 1). According to
the prevailing model, the selection of post-transcriptional
mechanisms is determined by the identity of the target. Once
incorporated into a cytoplasmic RISC, the miRNA will specify
cleavage if the mRNA has sufficient complementarity to the miRNA or
it will repress productive translation if the mRNA does not have
sufficient complementarity to be cleaved but has a suitable
constellation of miRNA complementary sites (31,32). A single miRNA can regulate
multiple target genes, while a single gene can be targeted by
multiple miRNAs, suggesting that the miRNAome and mRNAome
interaction is a complicated network (20). This enormous variability suggests
that miRNAs may be involved in almost all physiological processes
of the cell, defining a regulatory control of approximately 1/3 of
the messenger RNA of the whole human genome (33).
microRNAs and cancer
Cell proliferation, differentiation and apoptosis
are essential processes in cell and tissue homeostasis and their
alteration is a fundamental step in the initiation and progression
of malignant disease. Evidence has shown that miRNA mutations or
misexpression correlate with various human cancers, indicating that
miRNAs function as tumor suppressors and oncogenes (33). Genome-wide studies have
demonstrated that miRNA genes are frequently located at
cancer-associated genomic regions or in fragile sites. They are
also present in the minimal regions of loss of heterozygosity, in
the minimal regions of amplifications or in the common breakpoint
regions, suggesting that miRNAs may be a new class of genes
involved in human tumorigenesis (34). Analysis of miRNA expression
profiles in tumor samples and normal tissues have displayed
different patterns of overexpression or downregulation (8). A general finding is that global
miRNA expression levels are lower in tumor tissues than normal
tissues independent of cell type. In addition, poorly
differentiated tumors present a lower global level of miRNA
expression compared to more differentiated tumors. These data are
consistent with the hypothesis that high global miRNA expression
levels are associated with cellular differentiation (35).
Previously, a series of bioinformatics prediction
programs, based on mathematical algorithms, have been developed to
properly identify the hypothetical mRNA targets for each specific
miRNAs. Interestingly, predominant miRNA targets are transcription
factors or kinases and researchers are currently attempting to
dissect the target genes of miRNAs and their signaling pathways
involved in cancer (36). The
deregulation of miRNAs participates in the activation of cell
proliferation or in the inactivation of the apoptotic signaling
pathway in conjunction with other genetic changes leading to cancer
pathogenesis (37). Several miRNA
signaling pathways have been carefully studied: i) the human RAS
gene contains multiple let-7 complimentary binding sites, allowing
the let-7 miRNA family to regulate RAS expression (38); ii) miR-19 has been demonstrated to
bind the 3′ UTR of phosphatase and tensin homologue (PTEN) mRNA
in vitro suggesting that overexpression of mir-19 in tumor
cells could be an alternative mechanism by which the PI3K/Akt
signaling pathway is activated (39); iii) the mir-17-92 cluster is
overexpressed in multiple malignancies and, in a mouse B-cell
lymphoma model, it has been demonstrated to act with c-myc
expression to accelerate tumor development (40); iv) miRNAs also interfere with the
apoptotic process by blocking the expression of critical
apoptosis-related genes (8,41–43); v) KIT is an important tyrosine
kinase receptor in cell differentiation and growth. Inhibiting the
KIT signaling via miRNAs may contribute to uncontrolled cell growth
in certain type of cells (44);
vi) miR-106a and miR-20a may negatively regulate retinoblastoma 1
(RB1) and transform growth factor (TGF)-β signaling (45); g) miR-372 and miR-373 are
potential oncogenes by numbing the p53 pathway and allowing
tumorigenic growth in the presence of wild-type p53 (46).
Moreover, the scientific community is focusing on
miRNA-expression signatures that may represent a powerful tool for
cancer diagnosis, for defining a treatment strategy for patients,
for prediction of the prognosis and for monitoring treatment
response (33).
microRNAs and thyroid carcinoma
A number of studies have analyzed miRNA expression
in different types of thyroid tumor, demonstrating that miRNA
deregulation occurs in cancer tissues compared to their normal
counterparts. Moreover, miRNA expression profiles presented high
variability among the different histotypes of thyroid cancer
(Table I).
 | Table I.miRNAs in thyroid tumors. |
Table I.
miRNAs in thyroid tumors.
| Thyroid tumor
type | miRNAs | Author/(Refs.) |
|---|
| FTC | miR-191 | Colamaio et
al (52) |
| FTC | miR-192, miR-197,
miR-328 and miR-346 | Weber et al
(47) |
| FTC, PTC other
thyroid variants | PTC: miR-31,
miR-122a, miR-146b, miR-155, miR-187, miR-205, miR-221, miR-222 and
miR-224 | Nikiforova et
al (51) |
| Conventional FA:
miR-190, miR-205, miR-210, miR-224, miR-328, miR-339 and
miR-342 |
| Oncocytic FA:
miR-31, miR-183, miR-203, miR-221, miR-224 and miR-33. |
| Conventional FTC:
miR-146b, miR-155, miR-187, miR-221, miR-222 and miR-224 |
| Oncocytic FTC:
miR-183, miR-187, miR-197, miR-221, miR-222 and miR-339 |
| Poorly
differentiated carcinomas: miR-129, miR-146b, miR-183, miR-187,
miR-221, miR-222 and miR-339 |
| ATC: miR-137,
miR-155, miR-187, miR-205, miR-214, miR-221, miR-222 and
miR-224 |
| MTC: miR-9,
miR-10a, miR-124a, miR-127, miR-129, miR-137, miR-154, miR-224,
miR-323 and miR-370 |
| PTC | miR-21, miR-146,
miR-181, miR-221, miR-222 | He et al
(59) |
| PTC | miR-15a, miR-34a,
miR-34c, miR-96, miR-99a, miR-100, miR-107, miR-125b, miR-127,
miR-128b, miR-130b, miR-135b, miR-139, miR-141, miR-142-3p,
miR-145, miR-146, miR-148, miR-149, miR-154, miR-181a, miR-185,
miR-200a, miR-200b, miR-211, miR-213, miR-216, let7d, miR-218,
miR-299, miR-302b, miR-302c, miR-323 and miR-370 | Cahill et al
(60) |
| PTC | left-7b | Ricarte-Filho et
al (61) |
| PTC | miR-181b, miR213,
miR 220, miR-221 and miR-222 | Pallante et
al (62) |
| PTC | miR-19b-1,2,
miR-21, miR-30a-5p, miR-30c miR-31, miR-34a, miR-130b, miR-145sh,
miR-172, miR-181a, miR-181b, miR-213, miR-218, miR-221, miR-222,
miR-223, mir-224, miR-292-as, miR-300 and miR-345 | Tetzlaff et
al (64) |
| PTC | miR-146b, miR-221
and miR-222 | Chen et al
(65) |
| PTC | miR-21, miR-146b,
miR-181b, miR-213, miR-220, miR-221 and miR-222 | Sheu et al
(66–68) |
| PTC | miR-146b, miR-221
and miR-222 | Chou et al
(69) |
| PTC | miR-221 and
miR-222 | Visone et al
(70) |
| ATC | miR-17-3p,
miR-17-5p, miR-18a, miR-19a, miR-19b, miR-20a and miR-92-1 | Takakura et
al (73) |
| ATC | miR-21, miR-26a,
miR-138, miR-146b, miR-219, miR-221, miR-222 and miR-345 | Mitomo et al
(76) |
| ATC | miR-30 and
miR-200 | Braun et al
(78) |
| ATC | miR-200 | Park et al
(79) |
| ATC | miR-26a,
miR-30a-5p, miR-30d and miR-125b | Visone et al
(80) |
| ATC | miR-146a | Pacifico et
al (81) |
| MTC | miR-19, miR-183 and
miR-375 | Abraham et
al (82) |
Follicular thyroid carcinoma
FTC belongs to the well-differentiated thyroid
carcinoma group with a usually more aggressive behavior than PTC.
Several studies have attempted to define the roles of miRNA
deregulation in this histotype.
In 2006, Weber et al (47) published data on deregulated miRNA
expression in FTC. It was investigated whether miRNAs were
differentially expressed in human FTC and follicular adenomas (FAs)
using 2 high-density expression arrays to identify miRNAs and their
target genes. In total, 45 primary thyroid samples (23 FTC, 20 FA
and 4 normal control thyroid samples) were analyzed. Four miRNAs
(miR-192, miR-197, miR-328 and miR-346) were observed to be
overexpressed in FTCs compared to FA. These miRNAs appeared to be
specific to the FTC phenotype. miR-197 and miR-346 were also
validated by real-time quantitative RT-PCR that confirmed their
overexpression. The effects of these 2 miRNAs have been
functionally studied using two cell lines of human thyroid cancer,
FTC133 and K5, a cell line of human papillary thyroid cancer,
NPA87, and a line of human embryonic kidney cells, HEK293T as the
control. The induced overexpression of miR-197 and miR-346
stimulated cell proliferation in vitro, while the
downregulation of miR-197 and miR-346 led to cell growth
suppression in both the FTC133 and K5 cell lines, but not in the
NPA87 cell line, confirming that the deregulation of miR-197 and
miR-346 contributes to FTC carcinogenesis, but not PTC
carcinogenesis. The targets of these 2 miRNAs were also detected.
EFEMP2 (fibulin 4), a protein involved in the stabilization and
organization of extracellular matrix structures that functions as
as tumor suppressor (48,49), was inhibited by the overexpression
of miR-346. The activin A receptor type 1 (ACVR1), a potent
inhibitor of cell growth in several human cells types, including
the thyroid epithelium (50), and
tetraspanin 3 (TSPAN3), whose exact biological role in tumors is
still unknown, were both downregulated as a consequence of the
overexpression of miR-197. The authors suggested that these 2
miRNAs may participate in the malignant transformation of
follicular tumors and that their target genes may provide new
molecular markers for distinguishing malignant from benign FTC
(FA).
Nikiforova et al (51) did not confirm the results
published by Weber et al, except for miR-197. miRNA
expression levels were detected in 60 surgically removed thyroid
neoplastic and non-neoplastic samples and in 62 fine-needle
aspiration (FNA) samples by RT-PCR using the TaqMan miRNA panel or
individual miRNA sequence-specific primers. miRNA expression levels
were also calculated relative to normal thyroid tissue. Various
histopathological types of thyroid tumors, including those derived
from the same cell type, showed significantly different profiles of
miRNA expression. Oncocytic tumors, conventional follicular tumors,
papillary carcinomas and MTCs formed distinct clusters on the
unsupervised hierarchical cluster analysis. A significant
correlation between miRNA expression patterns and somatic mutations
was observed in papillary carcinomas. A set of 7 miRNAs (miR-187,
miR-221, miR-222, miR-146b, miR-155, miR-224 and miR-197) that were
most differentially overexpressed in thyroid tumors vs.
hyperplastic nodules in the surgical samples was validated in the
FNA samples, displaying a high accuracy of thyroid cancer
detection. miR-187 is one of the most upregulated miRNAs in PTC and
FTC but remains unchanged in FA. The authors suggested that miR-187
may be a useful marker to discriminate FTC from FA. However, it is
not suitable for discriminating FTC from other carcinomas of
follicular origin.
The study by Colamaio et al (52) aimed to evaluate the expression and
the role of miR-191 in thyroid carcinogenesis. The expression of
miR-191 was analyzed by quantitative RT-PCR in tissue samples from
patients with FA (n=24), FTC (n=24), PTC (n=15), anaplastic thyroid
carcinoma (n=8), and in the follicular variant of PTC (n=6)
compared to the normal tissue of the thyroid gland. miR-191 was
downregulated in FA, FTC and the follicular variant of PTC. They
identified CDK6, a serine-threonine kinase involved in the control
of cell cycle progression, as a novel target of miR-191. Moreover,
the restoration of miR-191 expression in WRO cells (a follicular
thyroid cell line) reduced cell growth and the migration rate on
vitronectin. CDK6 overexpression, correlated with miR-191
downregulation, was established in FA and FTC, suggesting a role of
miR-191 downregulation (likely by targeting CDK6) in the generation
of the follicular histotype of thyroid carcinoma.
Papillary thyroid carcinoma
PTC is the most common malignancy in thyroid tissue,
accounting for ∼80% of all thyroid cancers (53). Genetically, PTC is characterized
by alterations in the RET/PTC-RAS-BRAF signaling pathway (54,55) through activating mutations in BRAF
and RET/PTC gene rearrangements (56,57). A strong inherited genetic
predisposition to PTC has been suggested by case-control studies
demonstrating a 3- to 8-fold risk in first-degree relatives, one of
the highest of all cancers. However, there are no identified genes
predisposing or contributing to PTC. The mechanisms may require the
interaction of 2 or more regulatory, rather than protein-encoding
genes (58).
He et al (59) identified a set of 5 miRNAs
(miR-146, miR-221, miR-222, miR-21 and miR-181) significantly
overexpressed in PTC compared to normal tissue. Inter alia miR-146,
miR-221 and miR-222, displayed 11- to 19-fold higher levels in PTC
than in normal tissues. The expression of miR-221 was detectable in
normal thyroid tissue adjacent to PTC tumors from all patients
suggesting, as the authors highlighted, that the increased
expression of miR-221 in normal thyroid tissue may be an early
genetic event in PTC carcinogenesis and may play a role as an
oncogene in the thyroid. The study analyzed the predicted targets
of the 3 most significantly overexpressed miRNAs (-221, -222 and
-146), by using publicly available algorithms, and found a group of
19 genes clustered together with miR-221 and miR-222, whose
expression levels were significantly lower in PTC tumors compared
to normal tissues. KIT was one of the genes displaying profound
mRNA under-expression in PTC tumors. This observation did not apply
to all tumors, emphasizing the complexity of these regulatory
pathways, which may be organ- or cell-specific. To date, the
biological significance of the loss of c-KIT in thyroid tumors is
not clear. Intriguingly multiple miRNAs have been predicted to
target KIT, including those overexpressed in PTC, and it is
possible that the multiple interaction opportunities provided by
networks or ‘signatures’ of miRNA deregulation create different
responses in target genes under different circumstances and
combinations.
Cahill et al (60) studied the effects of the RET
proto-oncogene on gene expression and the transcriptional
regulation of PTC cell lines carrying the ret/PTC1 rearrangement
and the expression of miRNAs in 2 human PTC cell lines carrying the
ret/PTC1 rearrangement, compared with normal thyroid cell lines.
Apart from alterations on the expression profiles of the coding
genes involved in cell differentiation and proliferation (CEBPB,
CCNG1, IFITM3 and HTRA), they analyzed miRNA expression profiles.
They found 20 miRNAs significantly overexpressed (miR-34a, miR-96,
miR-99a, miR-100, miR-125b, miR-128a, miR-128b, miR-130b, miR-139,
miR-141, miR-142-3p, miR-146, miR-148, miR-185, miR-200a, miR-200b,
miR-211, miR-213, miR-216 and let-7d) and 15 downregulated miRNAs
(miR-15a, miR-34c, miR-107, miR-127, miR-135b, miR-145, miR-149,
miR-154, miR-181, miR-218, miR-299, miR-302b, miR-302C, miR-323 and
miR-370) in tumor cell lines compared to normal thyroid
tissues.
In 2009, Ricarte-Filho et al (61) performed a functional study to
investigate the involvement of miRNA let-7f in PTC development.
They showed that let-7f induction in TPC-1 cells, a human PTC cell
line that spontaneously harbors the RET/PTC1 oncogene, causes a
marked reduction in cell proliferation and induced the expression
of molecular markers characteristic of thyroid differentiation
(TITF1) suggesting that let-7 miRNA is an essential regulator of
thyroid carcinogenesis.
Pallante et al (62) also analyzed genome-wide miRNA
expression profiles in human thyroid PTCs using a microarray (miRNA
Chip microarray) containing hundreds of human precursor and mature
miRNA oligonucleotide probes. Using this approach, they found an
aberrant miRNA expression profile that clearly differentiates PTCs
from normal thyroid tissues. In particular, a significant increase
in miRNAs, miR-221, -222 and -181b, was detected in PTCs in
comparison with normal thyroid tissue. Functional studies,
performed by blocking miR-221 function and by overexpressing
miR-221 in human PTC-derived cell lines, suggested a critical role
of miR-221 overexpression in thyroid carcinogenesis. However in
2010, Chiang et al did not confirm miR-220 as a miRNA in
their detailed study on mammalian miRNA expression profiles
(63).
Tetzlaff et al (64) analyzed global miRNA expression
profiles through microarray chips in a series of samples of PTC in
formalin-fixed paraffin-embedded tissue (FFPE) and compared them to
the expression profiles of benign proliferative multinodular goiter
(MNG). The analysis revealed a series of 13 miRNAs upregulated and
a series of 26 miRNAs downregulated. The validation by RT-PCR, in
an independent set of tumor tissues, was performed only for miR-21,
miR-31, miR-221 and miR-222. miR-221 and miR-222 were upregulated
in PTC compared to the MNG group. The authors highlighted their
ability to procure sufficient miRNA from FFPE tissue to describe a
series of miRNAs with deregulated expression, demonstrating that
FFPE tissues are suitable resources for such miRNA expression
analyses when fresh tissues are not available or for performing
retrospective studies.
Chen et al (65) analyzed the expression of a
selected group of miRNAs. The results showed that miR-146b was
consistently overexpressed in both classical papillary carcinoma
and follicular variants, whereas all other groups displayed lower
expression at a similar level. Follicular lesions with partial
features of papillary carcinoma all showed low miR-146b levels
similar to other non-papillary carcinoma groups, suggesting that
they are biologically distinct from papillary carcinoma. miR-221
and miR-222 also showed a higher expression in papillary carcinoma,
but with substantial overlaps between the other groups blocking
their use as biomarkers for PTC lesions. When these analyses were
applied to 40 FNA samples of various lesions, only miR-146b and
miR-222 persisted as distinguishing markers for papillary
carcinoma. They concluded that miRNAs, particularly miR-146b, may
potentially be adjunct markers for diagnosing PTC in both FNA and
surgical pathology specimens. Moreover they found that the
overexpression of miR-146b is common both in classic and follicular
variants of PTC, regardless of the BRAF mutation status.
The effect of BRAF mutations on miRNA expression
profiles in PTCs has also been analyzed by Sheu et al
(66). Differences were not
recognized in the expression profiles of 5 miRNAs (miR-21,
miR-146b, miR-181b miR-221 and miR-222) between PTCs with BRAF
V600E mutation and wild-type PTCs.
Recently, Sheu et al (67) explored whether miRNA upregulation
could be assigned to distinct histomorphological variants of PTC,
particularly the follicular variant and other encapsulated
follicular thyroid tumors. The authors concluded that the analysis
of a set of 5 selected miRNAs distinguished common variants of PTC
from FA/MNG but failed to be a useful diagnostic method in
individual and doubtful cases, especially in the differential
diagnosis of encapsulated follicular thyroid tumors.
In another study, Sheu et al (68) compared the expression patterns of
miRNAs (-146b, -181b, -21, -221, -222), in PTC and in hyalinizing
trabecular thyroid tumors (HTTs). Moreover, the 2 common genetic
alterations characteristic of PTC, the V600E mutation of BRAF gene
and the rearrangements of RET/PTC 1 and 3, were determined in all
HTTs. All miRNAs were significantly upregulated in PTC, while all
miRNAs in HTT, normal thyroid tissue, adenomas and MNGs were
downregulated. All HTTs lacked BRAF mutations and RET/PTC
rearrangements. These results did not support the concept that a
high proportion of HTTs represents a variant of PTC and the authors
suggested that HTTs lacking both a miRNA expression pattern
characteristic of PTC and RET/PTC rearrangements be re-designated
as ‘hyalinizing trabecular adenomas’.
Chou et al (69) evaluated the expression of
miR-146b, miR-221 and miR-222 in 100 cases of PTC, with distinct
clinicopathogenetic characteristics, and 16 paired normal controls.
The tumor samples were categorized into low- and high-risk groups
on the basis of the tumor-node-metastasis staging system. miR-221,
miR-222 and miR-146b were significantly associated with
extrathyroidal invasion and the expression levels of miR-221 and
miR-146b were significantly higher in the high-risk PTC group. The
miR-146b expression levels in PTCs with BRAF mutation were
significantly higher than those without this mutation.
Visone et al (70), after identifying the upregulation
of miR-221 and miR-222 in human thyroid papillary carcinomas,
searched for their molecular targets. Through a bioinformatic
approach, it was reported that the gene, CDKN1B (p27kip1, protein
name), an important regulator of the cell cycle able to inhibit the
initiation of th S phase, was the candidate target of the
miR-221/222 cluster. They reported that an enforced expression of
the miR-221 and miR-222 induced the thyroid papillary carcinoma
cell line (TPC-1) to progress to the the S phase of the cell cycle.
Moreover, the negative regulation of p27(Kip1) by miR-221 and
miR-222 may also play a role in vivo since an inverse
correlation between miR-221 and miR-222 upregulation and
downregulation of the p27(Kip1) protein levels in human thyroid
papillary carcinomas was reported. By contrast, inhibition of the
expression of miR-221 and miR-222 through specific antisense
oligonucleotides 2′-OMe-221 and 2′-O-Me-222 increased the p27(Kip1)
protein levels. As the authors proposed, these results strongly
suggest the involvement of miR-221 and miR-222 in the cell cycle
control through the modification of the p27(Kip1) protein level in
the cell.
Anaplastic thyroid carcinoma
ATCs are highly aggressive and fatal tumors with a
mean survival of less than 8 months after diagnosis (71). Various treatment patterns
including radiation and chemotherapy have been used in ATC, but
they are mostly unsuccessful (72). Therefore, the identification of
miRNAs involved in proliferation or apoptosis in ATC cells has
important therapeutic implications and may lead to the
establishment of a novel therapy for ATC.
Takakura et al (73) investigated the role of those
miRNAs specifically deregulated in ATC focusing on the miR-17-92
cluster, composed of 7 miRNAs (miR-17-5p, miR-17-3p, miR-18a,
miR-19a, miR-20a, miR-19b and miR-92-1). They first assessed the
overexpression of the miR-17-92 cluster in different ATC cell lines
and then investigated the functional role of these miRNAs through
cell transfection with miRNA inhibitors. The suppression of
miR-17-3p caused complete growth arrest, presumably due to
caspase-3 and -9 activation, resulting in apoptosis. The miR-17-5p
or miR-19a inhibitor also induced strong growth reduction, but only
the miR-17-5p inhibitor led to cellular senescence. The miR-17-5p
and miR-19a targets were identified in the RB1 and PTEN genes as
their expression increased after transfection with the miR-17-5p
and miR-19a inhibitors.
It is becoming clear that PTEN plays a crucial role
in thyroid cancer. Germinal mutations are associated with Cowden’s
syndrome and the reduced expression of PTEN plays a crucial role in
thyroid cancer (74). Moreover,
it has been reported that PTEN inactivation is involved in highly
malignant or late-stage thyroid cancer, especially in the
anaplastic subtype (75).
Therefore, the overexpression of miR-19a and miR-19b may be
associated with the translational suppression of PTEN and the
induced cell growth in ATC (73).
By contrast, miR-18a inhibitor only moderately attenuated cell
growth. As proposed by the authors, these inhibitors may represent
valid therapeutic approaches for the treatment of ATC.
Mitomo et al (76) examined miRNA expression in ATC,
PTC cell lines and ATC, PTC tissue samples. They quantitatively
evaluated the expression of multiple miRNAs, 5 upregulated and 5
downregulated miRNAs previously reported to be differentially
expressed in comparison to normal thyroid tissues (61,66). miR-138 was the only one
significantly downregulated in ATC cell lines in comparison to PTC
cell lines. Then, they searched for target genes of miR-138 using
the miRBase and among 793 target genes they focused on the hTERT
(human telomerase reverse transcriptase gene) gene as the
overexpression of its protein has been reported in primary ATC in
comparison with PTC (77). They
demonstrated that the overexpression of miR-138 induced a reduction
in hTERT protein expression, and confirmed target specificity
between miR-138 and the hTERT 3′-untranslated region. These results
suggest that the loss of miR-138 expression may partially
contribute to the gain of hTERT protein expression in ATC, and that
further multiple miRNAs targeting hTERT mRNA may be involved in the
development of thyroid carcinoma. As proposed by the authors, the
upregulation of hTERT, subsequent to the downregulation of miR-138,
may be responsible for the malignant progression of
well-differentiated PTC in more aggressive ATC (77).
In 2010, Braun et al (78) identified 2 significantly decreased
miRNA families that unambiguously distinguish ATCs from PTCs and
FTCs: miR-200 and miR-30. The expression of these miRNAs in
mesenchymal ATC-derived cells reduced their invasive potential and
induced mesenchymal-epithelial transition (MET) by regulating the
expression of MET marker proteins. Supporting the role of TGF-β
signaling in modulating MET/epithelial-mesenchymal transition
(EMT), the expression of SMAD2 and TGF-βR1, upregulated in most
primary ATCs, was controlled by members of the miR-30 and/or
miR-200 families in ATC-derived cells. These findings identify
altered miRNA signatures as potent markers for ATCs that promote
de-/transdifferentiation (EMT) and invasion of these neoplasias.
Hence, TGFBR1 inhibition may have significant potential for the
treatment of ATCs and possibly other invasive tumors. The role of
the miR-200 family has been previously shown in 60 different human
cell lines as a regulator of the E-cadherin-vimentin system
(79).
Visone et al (80) analyzed miRNA expression profiles
in ATC samples compared to normal thyroid tissues and found a
significant downregulation of miR-26a, miR-30a-5p, miR-30d and
miR-125b in ATC compared to normal samples. They found an aberrant
miR expression profile that clearly differentiates ATC from normal
thyroid tissues and from PTCs previously analyzed. The induced
overexpression of miR-26a and miR-125b in 2 human ATC cell lines
caused the inhibition of cell growth, suggesting a role of these 2
miRNAs in the negative regulation of the cell cycle and their
downregulation in thyroid tumorigenesis. miR-26a influenced the
progression of the cell cycle by adjusting the negatively EZH2
oncogene expression, a gene involved in the epigenetic silencing
neoplastic development. Conversely, an effect on cell growth was
not observed after the overexpression of miR-30d and miR-30a-5p in
the same cells. These data indicate a miRNA signature associated
with ATC and suggest the miR deregulation as an important event in
thyroid cell transformation.
Pacifico et al (81) showed that NF-κB contributes to
anaplastic thyroid cancer by upregulating the expression of
miR-146a. Since the regulation of miRNA expression is controlled by
the RNA polymerase II-dependent transcription factors, NF-κB in the
ATC-derived FRO cell line was inactivated and its miRNA profile in
comparison with the parental counterpart was analyzed. miR-146a
resulted in the overexpression of human ATC specimens compared with
normal thyroid tissue. Moreover, the inhibition of miR-146a
expression in FRO cells decreased their oncogenic potential and
increased the susceptibility of the cells to chemotherapeutic
drug-induced apoptosis. The authors suggested that NF-κB
contributes to anaplastic thyroid cancer by upregulating the
expression of miR-146a.
Medullary thyroid carcinoma
Only 2 studies have analyzed the expression profile
of miRNAs in MTC.
Nikiforova et al (51) analyzed the expression of miRNAs in
surgically removed thyroid neoplastic and non-neoplastic samples
and in FNA samples, finding a group of 10 specific miRNAs (miR-9,
miR-10a, miR-124a, miR-127, miR-129, miR-137, miR-154, miR-224,
miR-323 and miR-370) upregulated in MTC.
In 2011, Abraham et al (82) analyzed miRNA expression in order
to identify potential prognostic biomarkers and therapeutic targets
in MTC management. miRNA microarray profiling was carried out on
tissue samples from patients with sporadic medullary thyroid cancer
(SMTC) and hereditary medullary thyroid cancer (HMTC). The
functional role of a selected miRNA was also investigated in
vitro in the human MTC cell line (TT cells). miRs-183 and -375
were overexpressed and miR-9 was underexpressed in SMTC vs. HMTC.
The overexpression of miRs-183 and -375 in MTC predicted lateral
lymph node metastases and was also associated with residual
disease, distant metastases and mortality. Knockdown of miR-183
expression in the TT cell line induced a significant decrease in
the viable cell count and upregulation of the LC3B protein, which
is associated with autophagy. This study indicated that miRNAs may
play a pivotal role in the biology of MTC and represent an
important class of prognostic biomarkers and therapeutic targets
warranting further investigation.
Conclusion
miRNAs mediate a recently recognized form of
translational inhibition that alters the levels of critical
proteins, thereby providing a mechanism for spatiotemporal control
of developmental and homeostatic events across a wide range of
plants and animals (4,85). Since abnormal proliferation and
apoptosis are a hallmark of human cancers, it is possible that
miRNA expression patterns may denote the malignant state. The
altered expression of a few miRNAs has been found in some tumor
types, and previous studies have shown that the altered expression
of specific miRNA genes contributes to the initiation and
progression of cancer (1,5,16,34,38,39,43,83–87). Therefore, miRNA expression
profiles offer the potential to inform cancer classification,
diagnosis and prognosis.
Thyroid cancers comprise a broad spectrum of lesions
with different degrees of malignancy representing a useful model to
study miRNA expression profiles. Several studies have demonstrated
that various histopathological types of thyroid tumor, derived from
the same cell, have distinct miRNA profiles, which further differ
within the same tumor type reflecting specific oncogenic mutations
in these tumors. These insights have an important implication on
the classification of thyroid tumors and refine their scheme of
progression. One of the main diagnostic problems in thyroid cancers
involves the pre-operative assessment of thyroid nodules. Thyroid
FNA is an important method for the pre-operative evaluation of
thyroid nodules, although in 10–20% of samples, the a diagnosis
cannot be reached (88).
Therefore, additional methods to improve the pre-operative
diagnosis are highly desirable and would result in a major impact
on clinical assistance. A recent study reported the possibility of
using circulating miRNAs (plasma, serum, urine or other bodily
fluids) as a new class of biomarkers for the diagnosis of cancer as
the expression profiles of miRNAs are specifically associated with
certain types of cancer (89).
References
|
1.
|
PS MeltzerCancer genomics: small RNAs with
big impactsNature435745746200510.1038/435745a15944682
|
|
2.
|
L HeGJ HannonMicroRNAs: small RNAs with a
big role in gene regulationNat Rev
Genet5522531200410.1038/nrg137915211354
|
|
3.
|
I BentwichA AvnielY KarovR AharonovS
GiladO BaradA BarzilaiP EinatU EinavE MeiriIdentification of
hundreds of conserved and nonconserved human microRNAsNat
Genet37766770200510.1038/ng159015965474
|
|
4.
|
DP BartelMicroRNAs: target recognition and
regulatory
functionsCell136215233200910.1016/j.cell.2009.01.00219167326
|
|
5.
|
MT McManusMicroRNAs and cancerSemin Cancer
Biol13253258200310.1016/S1044-579X(03)00038-5
|
|
6.
|
CM CroceGA CalinmiRNAs, cancer, and stem
cell divisionCell12267200510.1016/j.cell.2005.06.03616009126
|
|
7.
|
C SevignaniGA CalinLD SiracusaCM
CroceMammalian micro-RNAs: a small world for fine-tuning gene
expressionMamm
Genome17189202200610.1007/s00335-005-0066-316518686
|
|
8.
|
GA CalinCM CroceMicroRNA-cancer
connection: the beginning of a new taleCancer
Res6673907394200610.1158/0008-5472.CAN-06-080016885332
|
|
9.
|
JA ChanAM KrichevskyKS KosikMicroRNA-21 is
an antiapoptotic factor in human glioblastoma cellsCancer
Res6560296033200510.1158/0008-5472.CAN-05-013716024602
|
|
10.
|
RA DeLellisRV LloydPU HeitzC EngWorld
Health Organization Classification of Tumors. Pathology and
Genetics of Tumors of Endocrine OrgansIARC PressLyon2004
|
|
11.
|
B WightmanI HaG RuvkunPosttranscriptional
regulation of the heterochronic gene lin-14 by lin-4 mediates
temporal pattern formation in C.
elegansCell75855862199310.1016/0092-8674(93)90530-48252622
|
|
12.
|
RC LeeRL FeinbaumV AmbrosThe C.
elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14Cell758438541993
|
|
13.
|
BJ ReinhartFJ SlackM BassonAE
PasquinelliJC BettingerAE RougvieHR HorvitzG RuvkunThe
21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis
elegansNature403901906200010.1038/3500260710706289
|
|
14.
|
AE PasquinelliBJ ReinhartF SlackMQ
MartindaleMI KurodaB MallerDC HaywardEE BallB DegnanP
MüllerConservation of the sequence and temporal expression of let-7
heterochronic regulatory
RNANature4088689200010.1038/3504055611081512
|
|
15.
|
RC LeeV AmbrosAn extensive class of small
RNAs in Caenorhabditis
elegansScience294862864200110.1126/science.106532911679672
|
|
16.
|
M Lagos-QuintanaR RauhutW LendeckelT
TuschlIdentification of novel genes coding for small expressed
RNAsScience294853858200110.1126/science.106492111679670
|
|
17.
|
NC LauLP LimEG WeinsteinDP BartelAn
abundant class of tiny RNAs with probable regulatory roles in
Caenorhabditis
elegansScience294858862200110.1126/science.106506211679671
|
|
18.
|
X XieJ LuEJ KulbokasTR GolubV MoothaK
Lindblad-TohES LanderM KellisSystematic discovery of regulatory
motifs in human promoters and 30 UTRs by comparison of several
mammalsNature434338345200510.1038/nature0344115735639
|
|
19.
|
DP BartelMicroRNAs: genomics, biogenesis,
mechanism and
functionCell116281297200410.1016/S0092-8674(04)00045-514744438
|
|
20.
|
VN KimJW NamGenomics of microRNATrends
Genet22165173200610.1016/j.tig.2006.01.00316446010
|
|
21.
|
J HanY LeeKH YeomJW NamI HeoJK RheeSY
SohnY ChoBT ZhangVN KimMolecular basis for the recognition of
primary microRNAs by the Drosha-DGCR8
complexCell125887901200610.1016/j.cell.2006.03.04316751099
|
|
22.
|
Y LeeK JeonJT LeeS KimVN KimMicroRNA
maturation: stepwise processing and subcellular localizationEMBO
J2146634670200210.1093/emboj/cdf47612198168
|
|
23.
|
Y LeeC AhnJ HanH ChoiJ KimJ YimJ LeeP
ProvostO RadmarkS KimVN KimThe nuclear RNase III Drosha initiates
microRNA
processingNature425415419200310.1038/nature0195714508493
|
|
24.
|
E LundS GuttingerA CaladoJE DahlbergU
KutayNuclear export of microRNA
precursorsScience3039598200410.1126/science.1090599
|
|
25.
|
R YiY QinIG MacaraBR CullenExportin-5
mediates the nuclear export of pre-microRNAs and short hairpin
RNAsGenes Dev1730113016200310.1101/gad.115880314681208
|
|
26.
|
MT BohnsackK CzaplinskiD GorlichExportin 5
is a RanGTP-dependent dsRNA-binding protein that mediates nuclear
export of premiRNAsRNA10185191200410.1261/rna.516760414730017
|
|
27.
|
TP ChendrimadaRI GregoryE KumaraswamyJ
NormanN CoochK NishikuraR ShiekhattarTRBP recruits the Dicer
complex to Ago2 for microRNA processing and gene
silencingNature436740744200510.1038/nature0386815973356
|
|
28.
|
RI GregoryTP ChendrimadaN CoochR
ShiekhattarHuman RISC couples microRNA biogenesis and
post-transcriptional gene
silencingCell123631640200510.1016/j.cell.2005.10.02216271387
|
|
29.
|
G MeisterM LandthalerA PatkaniowskaY
DorsettG TengT TuschlHuman Argonaute2 mediates RNA cleavage
targeted by miRNAs and siRNAsMol
Cell15185197200410.1016/j.molcel.2004.07.00715260970
|
|
30.
|
SC HammondE BernsteinD BeachGJ HannonAn
RNA-directed nuclease mediates post-transcriptional gene silencing
in Drosophila
cellsNature404293296200010.1038/3500510710749213
|
|
31.
|
Y ZengEJ WagnerBR CullenBoth natural and
designed micro RNAs can inhibit the expression of cognate mRNAs
when expressed in human cellsMol
Cell913271333200210.1016/S1097-2765(02)00541-512086629
|
|
32.
|
Y ZengBR CullenSequence requirements for
microRNA processing and function in human
cellsRNA9112123200310.1261/rna.278050312554881
|
|
33.
|
A Esquela-KerscherFJ SlackOncomirs -
microRNAs with a role in cancerNat
Rev6259269200610.1038/nrc1840
|
|
34.
|
GA CalinC SevignaniCD DumitruT HyslopE
NochS YendamuriM ShimizuS RattanF BullrichM NegriniCM CroceHuman
microRNA genes are frequently located at fragile sites and genomic
regions involved in cancersProc Natl Acad Sci
USA10129993004200410.1073/pnas.030732310114973191
|
|
35.
|
J LuG GetzEA MiskaE Alvarez-SaavedraJ
LambD PeckA Sweet-CorderoBL EbertRH MakAA FerrandoMicroRNA
expression profiles classify human
cancersNature435834838200510.1038/nature0370215944708
|
|
36.
|
BP LewisIH ShihMW Jones-RhoadesDP BartelCB
BurgePrediction of mammalian microRNA
targetsCell115787798200310.1016/S0092-8674(03)01018-314697198
|
|
37.
|
SM HammondMicroRNAs as oncogenesCurr Opin
Genet Dev1649200610.1016/j.gde.2005.12.005
|
|
38.
|
SM JohnsonH GrosshansJ ShingaraM ByromR
JarvisA ChengE LabourierKL ReinertD BrownFJ SlackRAS is regulated
by the let-7 microRNA
familyCell120635647200510.1016/j.cell.2005.01.01415766527
|
|
39.
|
GA CalinCG LiuC SevignaniM FerracinN
FelliCD DumitruM ShimizuA CimminoS ZupoM DonoMicroRNA profiling
reveals distinct signatures in B cell chronic lymphocytic
leukemiasProc Natl Acad Sci
USA1011175511760200410.1073/pnas.040443210115284443
|
|
40.
|
L HeJM ThomsonMT HemannE Hernando-MongeD
MuS GoodsonS PowersC Cordon-CardoSW LoweGJ HannonSM HammondA
microRNA polycistron as a potential human
oncogeneNature435828833200510.1038/nature0355215944707
|
|
41.
|
N YanaiharaN CaplenE BowmanM SeikeK
KumamotoM YiRM StephensA OkamotoJ YokotaT TanakaUnique microRNA
molecular profiles in lung cancer diagnosis and prognosisCancer
Cell9189198200610.1016/j.ccr.2006.01.02516530703
|
|
42.
|
A CimminoGA CalinM FabbriMV IorioM
FerracinM ShimizuSE WojcikRI AqeilanS ZupoM DonomiR-15 and miR-16
induce apoptosis by targeting BCL2Proc Natl Acad Sci
USA1021394413949200510.1073/pnas.050665410216166262
|
|
43.
|
MV IorioM FerracinCG LiuA VeroneseR
SpizzoS SabbioniE MagriM PedrialiM FabbriM CampiglioMicroRNA gene
expression deregulation in human breast cancerCancer
Res6570657070200510.1158/0008-5472.CAN-05-178316103053
|
|
44.
|
N FelliL FontanaE PelosiR BottaD BonciF
FacchianoF LiuzziV LulliO MorsilliS SantoroMicro-RNAs 221 and 222
inhibit normal erythropoiesis and erythroleukemic cell growth via
kit receptor down-modulationProc Natl Acad Sci
USA1021808118086200510.1073/pnas.050621610216330772
|
|
45.
|
S VoliniaGA CalinCG LiuS AmbsA CimminoF
PetroccaR VisoneM IorioC RoldoM FerracinA microRNA expression
signature of human solid tumors defines cancer gene targetsProc
Natl Acad Sci USA10322572261200610.1073/pnas.051056510316461460
|
|
46.
|
PM VoorhoeveC le SageM SchrierAJ GillisH
StoopR NagelYP LiuJ van DuijseJ DrostA GriekspoorA genetic screen
implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ
cell tumorsCell12411691181200610.1016/j.cell.2006.02.037
|
|
47.
|
F WeberRE TeresiCE BroelschA FrillingC
EngA limited set of human MicroRNA is deregulated in follicular
thyroid carcinomaJ Clin Endocrinol
Metab9135843591200610.1210/jc.2006-069316822819
|
|
48.
|
WS ArgravesLM GreeneMA CooleyWM
GallagherFibulins: physiological and disease perspectivesEMBO
Rep411271131200310.1038/sj.embor.740003314647206
|
|
49.
|
WM GallagherLM GreeneMP RyanV SierraA
BergerP Laurent-PuigE ConseillerHuman fibulin-4: analysis of its
biosynthetic processing and mRNA expression in normal and tumour
tissuesFEBS
Lett4895966200110.1016/S0014-5793(00)02389-911231014
|
|
50.
|
KM SchulteC JonasR KrebsHD RöherActivin A
and activin receptors in thyroid
cancerThyroid11314200110.1089/1050725015050060311272093
|
|
51.
|
MN NikiforovaGC TsengD StewardD DiorioYE
NikiforovMicroRNA expression profiling of thyroid tumors:
biological significance and diagnostic utilityJ Clin Endocrinol
Metab9316001608200810.1210/jc.2007-269618270258
|
|
52.
|
M ColamaioE BorboneL RussoM BiancoA
FedericoD CalifanoG ChiappettaP PallanteG TronconeS BattistaA
FuscomiR-191 down-regulation plays a role in thyroid follicular
tumors through CDK6 targetingJ Clin Endocrinol
Metab9619151924201110.1210/jc.2011-040821956418
|
|
53.
|
A JemalLX CleggE WardLA RiesX WuPM
JamisonPA WingoHL HoweRN AndersonBK EdwardsAnnual report to the
nation on the status of cancer, 1975–2001, with a special feature
regarding survivalCancer1013272004
|
|
54.
|
ET KimuraMN NikiforovaZ ZhuJA KnaufYE
NikiforovJA FaginHigh prevalence of BRAF mutations in thyroid
cancer: genetic evidence for constitutive activation of the
RET/PTC-RAS-BRAF signaling pathway in papillary thyroid
carcinomaCancer Res6314541457200312670889
|
|
55.
|
RM MelilloMD CastelloneV GuarinoV De
FalcoAM CiraficiG SalvatoreF CaiazzoF BasoloR GianniniM
KruhofferThe RET/PTC-RAS-BRAF linear signaling cascade mediates the
motile and mitogenic phenotype of thyroid cancer cellsJ Clin
Invest11510681081200510.1172/JCI20052275815761501
|
|
56.
|
Y CohenM XingE MamboZ GuoG WuB TrinkU
BellerWH WestraPW LadensonD SidranskyBRAF mutation in papillary
thyroid carcinomaJ Natl Cancer
Inst95625627200310.1093/jnci/95.8.62512697856
|
|
57.
|
MN NikiforovaET KimuraM GandhiPW
BiddingerJA KnaufF BasoloZ ZhuR GianniniG SalvatoreA FuscoBRAF
mutations in thyroid tumors are restricted to papillary carcinomas
and anaplastic or poorly differentiated carcinomas arising from
papillary carcinomasJ Clin Endocrinol
Metab8853995404200310.1210/jc.2003-03083814602780
|
|
58.
|
K CzeneP LichtensteinK
HemminkiEnvironmental and heritable causes of cancer among 9.6
million individuals in the Swedish Family-Cancer DatabaseInt J
Cancer99260266200210.1002/ijc.1033211979442
|
|
59.
|
H HeK JazdzewskiW LiS LiyanarachchiR NagyS
VoliniaGA CalinCG LiuK FranssilaS SusterThe role of microRNA genes
in papillary thyroid carcinomaProc Natl Acad Sci
USA1021907519080200510.1073/pnas.050960310216365291
|
|
60.
|
S CahillP SmythSP FinnK DenningR FlavinEM
O’ReganJ LiA PotratzSM GuentherR HenfreyEffect of ret/PTC 1
rearrangement on transcription and post-transcriptional regulation
in a papillary thyroid carcinoma modelMol
Cancer570200610.1186/1476-4598-5-7017156473
|
|
61.
|
JC Ricarte-FilhoCS FuziwaraAS YamashitaE
RezendeMJ da-SilvaET KimuraEffects of let-7 microRNA on cell growth
and differentiation of papillary thyroid cancerTransl
Oncol2236241200910.1593/tlo.0915119956384
|
|
62.
|
P PallanteR VisoneM FerracinA FerraroMT
BerlingieriG TronconeG ChiappettaCG LiuM SantoroM NegriniMicroRNA
deregulation in human thyroid papillary carcinomasEndocr Relat
Cancer13497508200610.1677/erc.1.0120916728577
|
|
63.
|
HR ChiangLW SchoenfeldJG RubyVC AuyeungN
SpiesD BaekWK JohnstonC RussS LuoJE BabiarzMammalian microRNAs:
experimental evaluation of novel and previously annotated
genesGenes Dev249921009201010.1101/gad.188471020413612
|
|
64.
|
MT TetzlaffA LiuX XuSR MasterDA BaldwinJW
TobiasVA LivolsiZW BalochDifferential expression of miRNAs in
papillary thyroid carcinoma compared to multinodular goiter using
formalin fixed paraffin embedded tissuesEndocr
Pathol18163173200710.1007/s12022-007-0023-7
|
|
65.
|
YT ChenN KitabayashiXK ZhouTJ Fahey IIIT
ScognamiglioMicroRNA analysis as a potential diagnostic tool for
papillary thyroid carcinomaMod
Pathol2111391146200810.1038/modpathol.2008.10518587330
|
|
66.
|
SY SheuF GrabellusS SchwertheimS HandkeK
WormKW SchmidLack of correlation between BRAF V600E mutational
status and the expression profile of a distinct set of miRNAs in
papillary thyroid carcinomaHorm Metab
Res41482487200910.1055/s-0029-121555819370505
|
|
67.
|
SY SheuF GrabellusS SchwertheimK WormM
Broecker-PreussKW SchmidDifferential miRNA expression profiles in
variants of papillary thyroid carcinoma and encapsulated follicular
thyroid tumoursBr J
Cancer102376382201010.1038/sj.bjc.660549320029416
|
|
68.
|
SY SheuE VogelK WormF GrabellusS
SchwertheimKW SchmidHyalinizing trabecular tumour of the
thyroid-differential expression of distinct miRNAs compared with
papillary thyroid
carcinomaHistopathology56632640201010.1111/j.1365-2559.2010.03526.x20459574
|
|
69.
|
CK ChouRF ChenFF ChouHW ChangYJ ChenYF
LeeKD YangJT ChengCC HuangRT LiumiR-146b is highly expressed in
adult papillary thyroid carcinomas with high risk features
including extrathyroidal invasion and the BRAF
mutationThyroid20489494201010.1089/thy.2009.002720406109
|
|
70.
|
R VisoneL RussoP PallanteI De MartinoA
FerraroV LeoneE BorboneF PetroccaH AlderCM CroceA FuscoMicroRNAs
(miR)-221 and miR-222, both overexpressed in human thyroid
papillary carcinomas, regulate p27 protein levels and cell
cycleEndocr Relat Cancer14791798200710.1677/ERC-07-012917914108
|
|
71.
|
KB AinAnaplastic thyroid carcinoma:
behavior, biology, and therapeutic
approachesThyroid8715722199810.1089/thy.1998.8.7159737368
|
|
72.
|
L ViniC HarmerManagement of thyroid
cancerLancet
Oncol3407414200210.1016/S1470-2045(02)00787-812142170
|
|
73.
|
S TakakuraN MitsutakeM NakashimaH NambaVA
SaenkoTI RogounovitchY NakazawaT HayashiA OhtsuruS
YamashitaOncogenic role of miR-17-92 cluster in anaplastic thyroid
cancer cellsCancer
Sci9911471154200810.1111/j.1349-7006.2008.00800.x18429962
|
|
74.
|
P BruniA BocciaG BaldassarreF TrapassoM
SantoroG ChiappettaA FuscoG VigliettoPTEN expression is reduced in
a subset of sporadic thyroid carcinomas: evidence that PTEN-growth
suppressing activity in thyroid cancer cells is mediated by
p27kip1Oncogene1931463155200010.1038/sj.onc.1203633
|
|
75.
|
T FriskT FoukakisT DwightJ LundbergA HöögG
WallinC EngJ ZedeniusC LarssonSilencing of the PTEN
tumor-suppressor gene in anaplastic thyroid cancerGenes Chromosomes
Cancer357480200210.1002/gcc.1009812203792
|
|
76.
|
S MitomoC MaesawaS OgasawaraT IwayaM
ShibazakiA Yashima-AboK KotaniH OikawaE SakuraiN
IzutsuDownregulation of miR-138 is associated with overexpression
of human telomerase reverse transcriptase protein in human
anaplastic thyroid carcinoma cell linesCancer
Science99280286200810.1111/j.1349-7006.2007.00666.x
|
|
77.
|
Y ItoH YoshidaC TomodaT UrunoY TakamuraA
MiyaK KobayashiF MatsuzukaK KumaA MiyauchiTelomerase activity in
thyroid neoplasms evaluated by the expression of human telomerase
reverse transcriptase (hTERT)Anticancer Res25509514200515816620
|
|
78.
|
J BraunC Hoang-VuH DralleS
HüttelmaierDownregulation of microRNAs directs the EMT and invasive
potential of anaplastic thyroid
carcinomasOncogene2942374244201010.1038/onc.2010.16920498632
|
|
79.
|
SM ParkAB GaurE LengyelME PeterThe miR-200
family determines the epithelial phenotype of cancer cells by
targeting the E-cadherin repressors ZEB1 and ZEB2Gen
Dev22894907200810.1101/gad.164060818381893
|
|
80.
|
R VisoneP PallanteA VecchioneR CirombellaM
FerracinA FerraroS VoliniaS ColuzziV LeoneE BorboneSpecific
microRNAs are downregulated in human thyroid anaplastic
carcinomasOncogene2675907595200710.1038/sj.onc.121056417563749
|
|
81.
|
F PacificoE CrescenziS MelloneA IannettiN
PorrinoD LiguoroF MoscatoM GriecoS FormisanoA LeonardiNuclear
factor-{kappa}B contributes to anaplastic thyroid carcinomas
through upregulation of miR-146aJ Clin Endocrinol
Metab95142114302010
|
|
82.
|
D AbrahamN JacksonJS GundaraJ ZhaoAJ GillL
DelbridgeBG RobinsonSB SidhuMicroRNA profiling of sporadic and
hereditary medullary thyroid cancer identifies predictors of nodal
metastasis, prognosis, and potential therapeutic targetsClin Cancer
Res1747724781201110.1158/1078-0432.CCR-11-0242
|
|
83.
|
V AmbrosThe functions of animal
microRNAsNature431350355200410.1038/nature0287115372042
|
|
84.
|
GA CalinM FerracinA CimminoG Di LevaM
ShimizuSE WojcikMV IorioR VisoneNI SeverM FabbriA microRNA
signature associated with prognosis and progression in chronic
lymphocytic leukemiaN Engl J
Med35317931801200510.1056/NEJMoa05099516251535
|
|
85.
|
MZ MichaelSM O’ConnorNG Van Holst
PellekaanGP YoungRJ JamesReduced accumulation of specific microRNAs
in colorectal neoplasiaMol Cancer Res1882891200314573789
|
|
86.
|
J TakamizawaH KonishiK YanagisawaS TomidaH
OsadaH EndohT HaranoY YatabeM NaginoY NimuraReduced expression of
the let-7 microRNAs in human lung cancers in association with
shortened postoperative survivalCancer
Res6437533756200410.1158/0008-5472.CAN-04-063715172979
|
|
87.
|
M BoeriC VerriD ConteL RozP ModenaF
FacchinettiE CalabròCM CroceU PastorinoG SozziMicroRNA signatures
in tissues and plasma predict development and prognosis of computed
tomography detected lung cancerProc Natl Acad Sci
USA10837133718201110.1073/pnas.110004810821300873
|
|
88.
|
G PopoveniucJ JonklaasThyroid nodulesMed
Clin North Am96329349201210.1016/j.mcna.2012.02.002
|
|
89.
|
K ZenCY ZhangCirculating microRNAs: a
novel class of biomarkers to diagnose and monitor human cancersMed
Res Rev32326348201210.1002/med.2021522383180
|