Introduction
Angiogenesis is the formation of new blood vessels
from pre-existing ones, and is required for tumor growth and
metastasis. Without new blood vessel formation, the tumor cannot
grow larger than 1–2 mm in diameter (1). To develop, tumors must make an
‘angiogenic switch’ by perturbing the local balance between pro-
and anti-angiogenic factors in the immediate environment of
endothelial cells through the overexpression of proangiogenic
growth factors, such as vascular endothelial growth factor (VEGF),
basic fibroblast growth factor (bFGF) and angiopoietin (Ang)
(2,3). As a general mechanism, oxidative
stress is a common hallmark of numerous tumors. Tumor growth
produces large amounts of reactive oxygen species (ROS) (4). Previous studies have shown that ROS
trigger ‘angiogenic switch’ responses by inducing VEGF expression
and matrix metalloproteinase activity (5). Thus, discovery of non-toxic
antiangiogenic agents from nature antioxidants targeting to inhibit
the production of proangiogenic growth factors, the initial step of
angiogenesis, is a promising strategy for cancer therapy and
prevention.
Colon cancer is the third most commonly diagnosed
cancer in men and women, with 103,170 new cases and 51,690 deaths
estimated to occur in 2012 in the United States (6). The incidence of colon cancer in
China is lower than that in the western countries, but it has
increased in recent years and become a substantial cancer burden in
China, particularly in the more developed areas (7). Epidemiological studies have shown
that the regular consumption of fruits and vegetables is associated
with a reduced risk of cancer (8). The beneficial effects may be partly
attributable to polyphenolic compounds which have antioxidant and
free radical scavenging properties (9).
Grapes are one of the most widely consumed fruits in
the world and are rich in polyphenols, of which about 60–70% are
found in grape seeds as dimers, trimers and other oligomers of
flavan-3-ols and commonly known as proanthocyanidins. Grape seed
proanthocyanidins (GSPs) are widely consumed as a dietary
supplement and possess chemopreventive and/or chemotherapeutic
effects in various cell culture and animal models (10,11). Pharmacokinetic studies have shown
that, in vivo, GSPs can hardly be absorbed or metabolized
during upper gastrointestinal tract transit, allowing these
chemicals to reach the colon at high concentrations (12–14). This metabolic characteristic
suggests that GSPs have a natural colon-targeting feature and are
more adaptive to acting as a chemopreventive and chemotherapeutic
agent for colon cancer. Epidemiological studies suggested an
inverse relationship between the dietary consumption of
proanthocyanidins and the risk of colorectal cancer (15,16). This is also supported by both
in vitro and experimental animal studies (17–25). These studies have shown that GSPs
inhibit growth and induce the apoptosis of some colon caner cell
lines in vitro and in vivo. Dietary-feeding of grape
seed extract also prevents azoxymethane-induced colonic aberrant
crypt foci formation in Fischer 344 rats (26), suggesting that grape seed extract
could inhibit the early steps of colon carcinogenesis.
In the present study, we report that GSPs inhibit
tumor-induced angiogenesis, and, thus, colon tumor growth by
inhibiting the expression of VEGF and Angl through scavenging ROS.
Our results provide a novel explanation for GSPs as an
angiopreventive agent against colon cancer.
Materials and methods
Materials
GSPs, consisting of at least 95% proanthocyanidins,
1.8% proanthocyanidin B2 and 60% oligomers were purchased from
Jianfeng Co. (Tianjin, China). Leibovitz’s L-15 medium, MCDB131
medium, epithelial growth factor (EGF), hydrocortisone,
sulforhodamine B (SRB) and 2′,7′-dichlorofluorescein diacetate
(DCFH-DA) were purchased from Sigma (St. Louis, MO, USA). Fetal
bovine serum (FBS) was purchased from Lanzhou National HyClone
Bio-Engineering Co., Ltd. (Lanzhou, China). Millicell cell culture
inserts were purchased from Millipore Corp. (Bedford, MA, USA).
VEGF and Ang1 ELISA kits were ordered from R&D Systems
(Minneapolis, MN, USA). Rabbit polyclonal anti-VEGF and rabbit
polyclonal anti-Ang1 were purchased from Boster Bio-engineering
Limited Co., Ltd. (Wuhan, China). MaxVision TM HRP-Polymer
anti-Mouse/Rabbit IHC kit and DAB kit were purchased from Maixin
Biological Technology, Ltd. (Fuzhou, China).
Cell culture
Human colon cancer SW620 cells were obtained from
the Cell Bank of the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China) and were cultured in Leibovitz’s L-15
medium supplemented with 10% FBS. Human microvascular endothelial
cells (HMEC-1) were cultured in MCDB131 medium supplemented with
1.18 mg/ml NaHCO3, 20% inactivated FBS, 10 ng/ml EGF and
1 μg/ml hydrocortisone. All cells were cultured in a highly
humidified atmosphere of 5% CO2 at 37°C. Fertilized
White Leghorn chicken eggs were obtained from the Lanzhou Institute
of Biological Products (Lanzhou, China) and were incubated at 37°C
in a humidified egg incubator.
Cell viability assay
The viability of SW620 cells was determined in
96-well plates by the SRB method with some modifications (27). Briefly, exponentially growing
cells were seeded in 96-well plates with the final volume 100
μl/well. After 24 h of incubation, cells were treated with
various concentrations of GSPs for the indicated times. The
cultures were then fixed at 4°C for 1h with ice-cold 50%
trichloroacetic acid to give a final concentration of 10%. Fixed
cells were rinsed 5 times with deionized water and stained for 10
min with 0.4% SRB dissolved in 0.1% acetic acid. The wells were
then washed 5 times with 0.1% acetic acid and left to dry
overnight. The absorbed SRB was dissolved in 150 μl
unbuffered 1% Tris base (pH 10.5). The absorbance of extracted SRB
at 515 nm was measured on a microplate reader.
Chick chorioallantoic membrane (CAM)
tumor formation assay (28,29)
Fertilized White Leghorn chicken eggs were incubated
under conditions of constant humidity at a temperature of 37°C. On
Day 10 of incubation, a small hole was punched over the air sac in
order to detach the CAM from the eggshell by gently exhausting, and
then a square window was opened on the broad side of the egg,
exposing the CAM. After the CAM was exposed, 40 μl
serum-free culture medium containing 1×106 SW620 cells
were deposited on the CAM. The window was sealed with tape and the
eggs were returned to the incubator. When the solid tumor began to
vascularize after implantation for two days, GSP or vehicle was
deposited locally each day. Five days later, CAM was photographed
in ovo under a stereomicroscope and tumors were resected and
weighed.
Enzyme-linked immunosorbent assay
(ELISA)
For the measurement of VEGF and Ang1 secretion,
confluent SW620 cells (90–100%) were cultured in serum-free media
for 24 h in the absence or presence of GSP. Cell-free culture
supernatants were harvested and used for the determination of VEGF
and Ang1 levels using a human VEGF or Ang1 ELISA kit according to
the manufacturer’s instructions. The concentration of the VEGF and
Ang1 in the samples was then determined by comparing the optical
density of the samples to the standard curve.
Preparation of tumor conditioned medium
(30)
Tumor conditioned medium was prepared from the SW620
cell culture as follows: SW620 cells were grown to subconfluency
(approximately 90%). After being washed twice with D-Hanks, cells
were incubated in MCDB131 medium supplemented with or without 100
μg/ml GSP for 24 h. The supernatant was then harvested,
centrifuged at 2000 × g at 4°C for 10 min, filter-sterilized
through 0.22-μm pore size filters and stored at −20°C prior
to use.
Cell migration assay
Cell migration was performed in millicell cell
culture inserts using a polycarbonate filter with a pore size of 8
μm. HMEC-1 cells (2×105) suspended in 0.4 ml
serum-free MCDB131 culture medium were added to the upper
compartment of cell culture inserts. The lower compartment
contained 0.6 ml conditioned medium. Following incubation for 24 h
at 37°C, the nonmigrated cells on the upper surface of the membrane
were removed with a cotton swab. The migrated cells on the lower
surface of the membrane were fixed with methanol and then stained
with 0.1% crystal violet. Images from randomly selected microscopic
fields were obtained under light microscopy. Each sample was
repeated three times.
Intracellular ROS staining
Intracellular ROS levels in both GSP-treated and
control cells were measured by DCFH-DA assay as previously
described (31). Briefly,
sub-confluent SW620 cells were treated with different
concentrations of GSP for 24 h. Following incubation, cells were
washed once with D-Hanks and stained with DCFH-DA (10 μM)
for 30 min. Subsequently, cells were washed twice with D-Hanks to
remove the excess dye, and then 100 μl of D-Hanks was added.
The images were visualized and photographed under a fluorescent
microscope. During the entire procedure with DCFH-DA, the plate was
kept out of light to avoid fading of the fluoroprobe.
Immunohistochemical assay for VEGF and
Angl expression
Following pretreatment with or without 100
μg/ml GSP for 2 h and further incubation with or without 100
μM H2O2 for 24 h, SW620 cells seeded
on glass coverslips were fixed with 4% paraformaldehyde. The
expression of both VEGF and Ang1 was detected with the
immunohistochemical staining kit according to the manufacturer’s
instructions.
Statistical analysis
Results are expressed as the means ± SD, and were
analyzed using the Student’s t-test. Values of P<0.05 were
considered to indicate statistically significant differences.
Results
Effect of GSPs on the cell viability of
SW620 cells
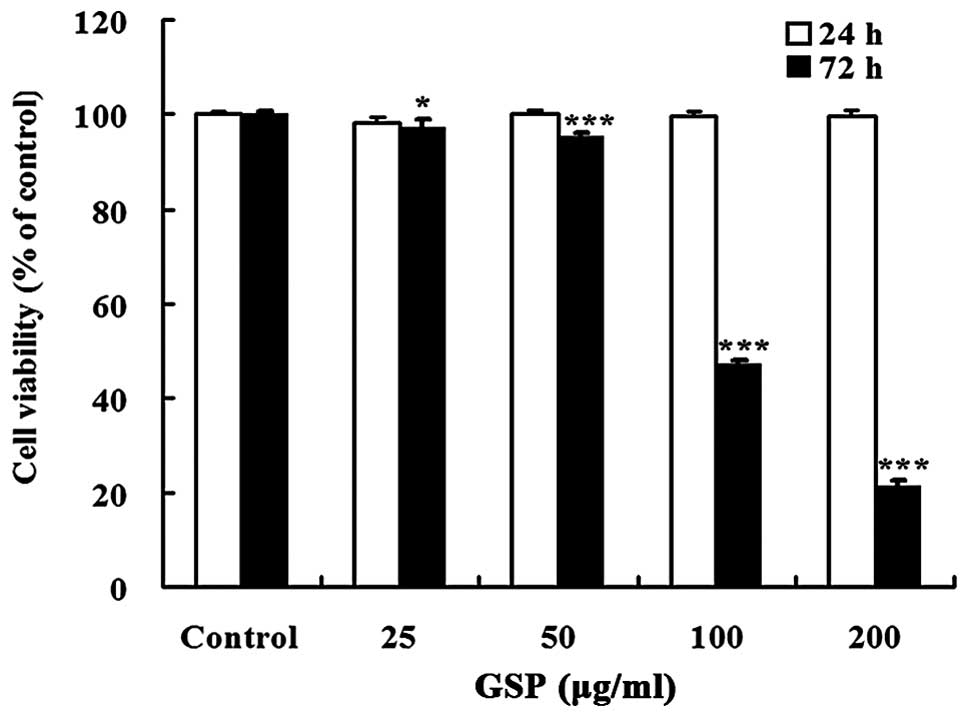
GSP treatment for 72 h significantly inhibited cell
viability in a dose-dependent manner with an IC50 value
of 116.44 μg/ml. However, treatment with GSP (25–200
μg/ml) for 24 h did not change cell viability (Fig. 1). Therefore, all subsequent
experiments were carried out with the treatment time not exceeding
24 h.
GSPs inhibit tumor-induced angiogenesis
and tumor growth in a xenografted chick chorioallantoic membrane
(CAM) model
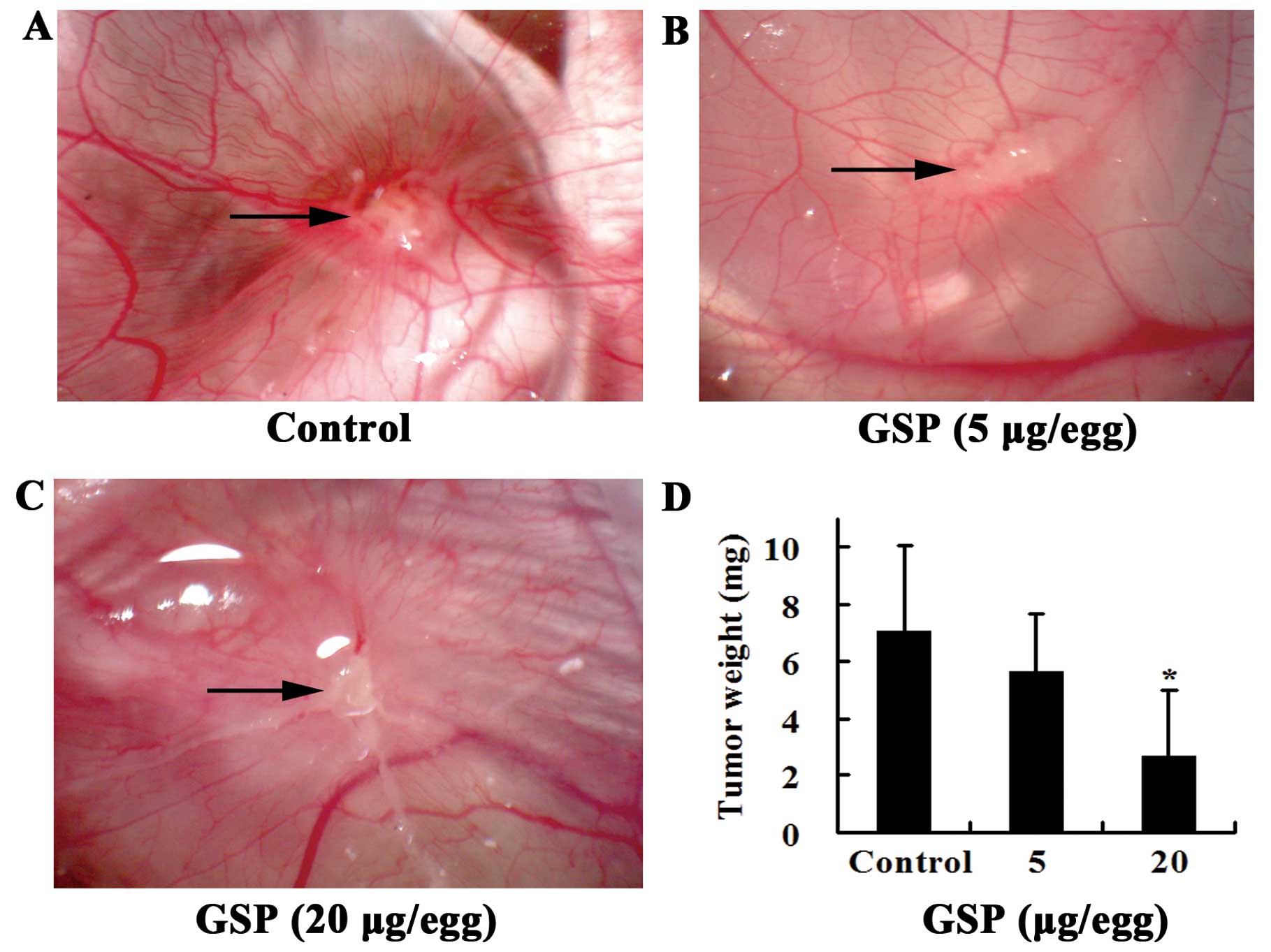
SW620 cells inoculated on chick CAM formed solid,
avascular tumor within the first day. Two days after inoculation,
the tumor became vascularized and grew rapidly. Five days later,
numerous vessels developed radially around the tumor. Vessels at
the tumor surface were clearly visible in the control group
(Fig. 2A). Local treatment of the
tumor from Day 3 to Day 7 (the day when SW620 cells were inoculated
was designated as Day 1) with GSPs markedly inhibited tumor-induced
angiogenesis. The tumor appeared white (Fig. 2B and C). The growth of the tumor
was also inhibited by GSP treatment (Fig. 2D). During the experiment, some
chick embryos from both the control and the GSP-treated groups died
before the end of the experiment and were not included in the
results shown. However, there was no significant difference in the
incidence of embryonic death between GSP-treated and control
groups, indicating that the inhibitory effect of GSP on
tumor-induced angiogenesis was not related to toxic effects.
GSPs inhibit expression of VEGF and Ang1
in SW620 cells
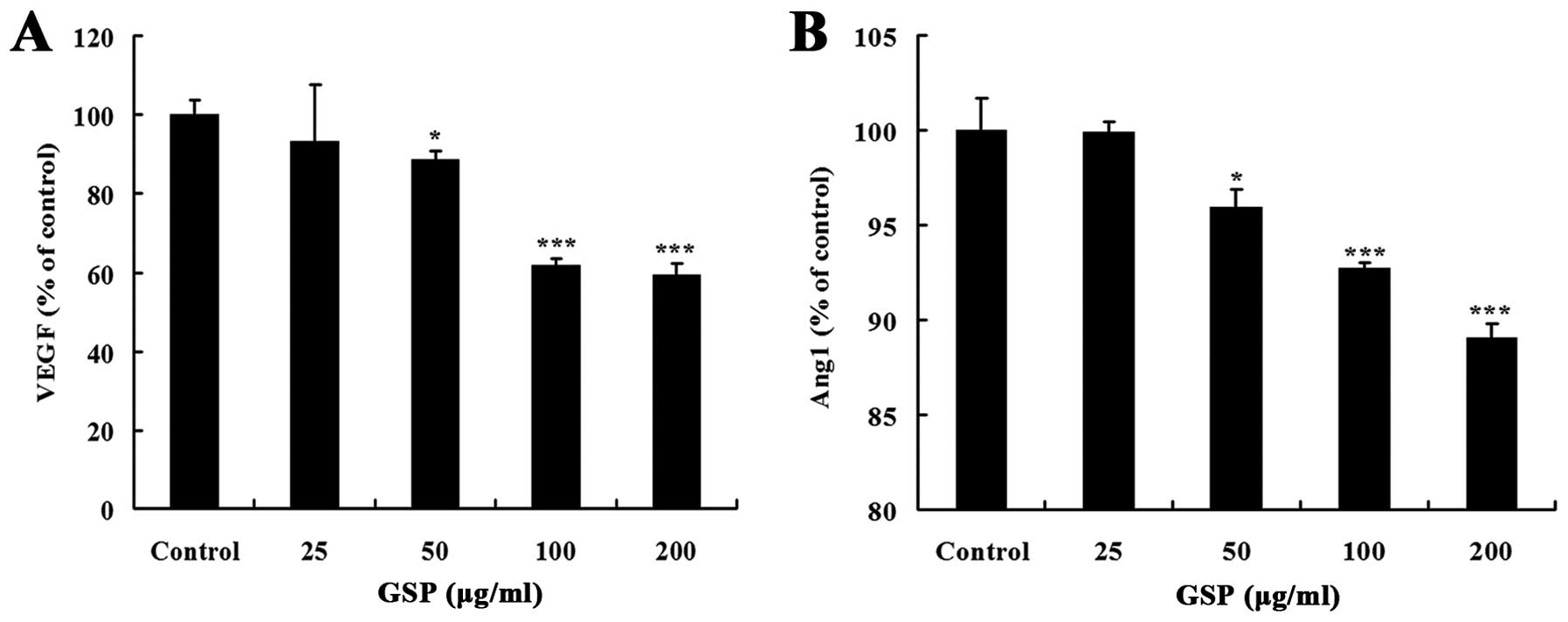
Solid tumors secrete various proangiogenic factors,
such as VEGF and Ang1, to activate the nearest endothelial cells in
the host tissue for neoangiogenesis (32). In the present study, we
investigated whether GSPs could inhibit proangiogenic attributes of
colon cancer SW620 cells. GSP treatment for 24 h inhibited both
VEGF and Ang1 expression in a dose-dependent manner (Fig. 3). The inhibitory effect on VEGF
expression was stronger than on Ang1 expression. These results
suggest that the inhibitory effect of GSPs on tumor-induced
angiogenesis is partly through suppressing the expression of
angiogenic factors that initiate tumor angiogenesis in SW620
cells.
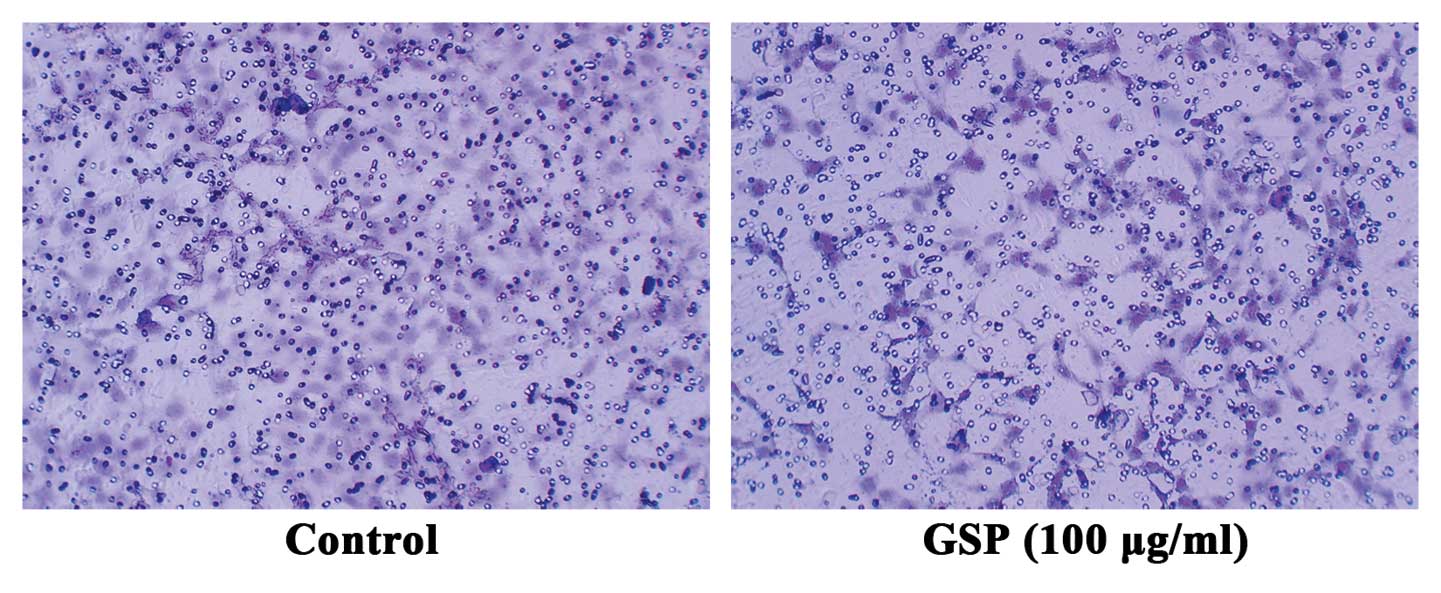
Conditioned medium from GSP-treated SW620
cells inhibits cell migration of HMEC-1 cells
Both VEGF and Ang1 are chemotactic factors specific
for endothelial cells. To further verify that GSPs inhibit
tumor-induced angiogenesis by suppressing the expression of both
VEGF and Ang1 in tumor cells, we conducted endothelial cell
migration assay to examine the effect of conditioned medium from
either GSP-treated or untreated SW620 cells on endothelial cell
migration. Conditioned medium collected from SW620 cells induced
endothelial cell migration (Fig.
4). However, endothelial cell migration was suppressed by
conditioned medium collected from 100 μg/ml GSP-treated
SW620 cells. These results further verified that the inhibitory
effect of GSP on tumor-induced angiogenesis is mediated by reducing
the expression of proangiogenic factors in SW620 cells.
GSPs inhibit the expression of VEGF and
Ang1 by reducing ROS production
Excessive ROS production has been confirmed to have
a vital role in the induction of VEGF or Ang1 expression in many
tumor cell lines. To investigate whether the antioxidant activity
is part of the mechanisms by which GSPs suppress the expression of
angiogenic factors in SW620 cells, we first used fluorescent probes
DCFH-DA to detect the effect of GSPs on ROS production in SW620
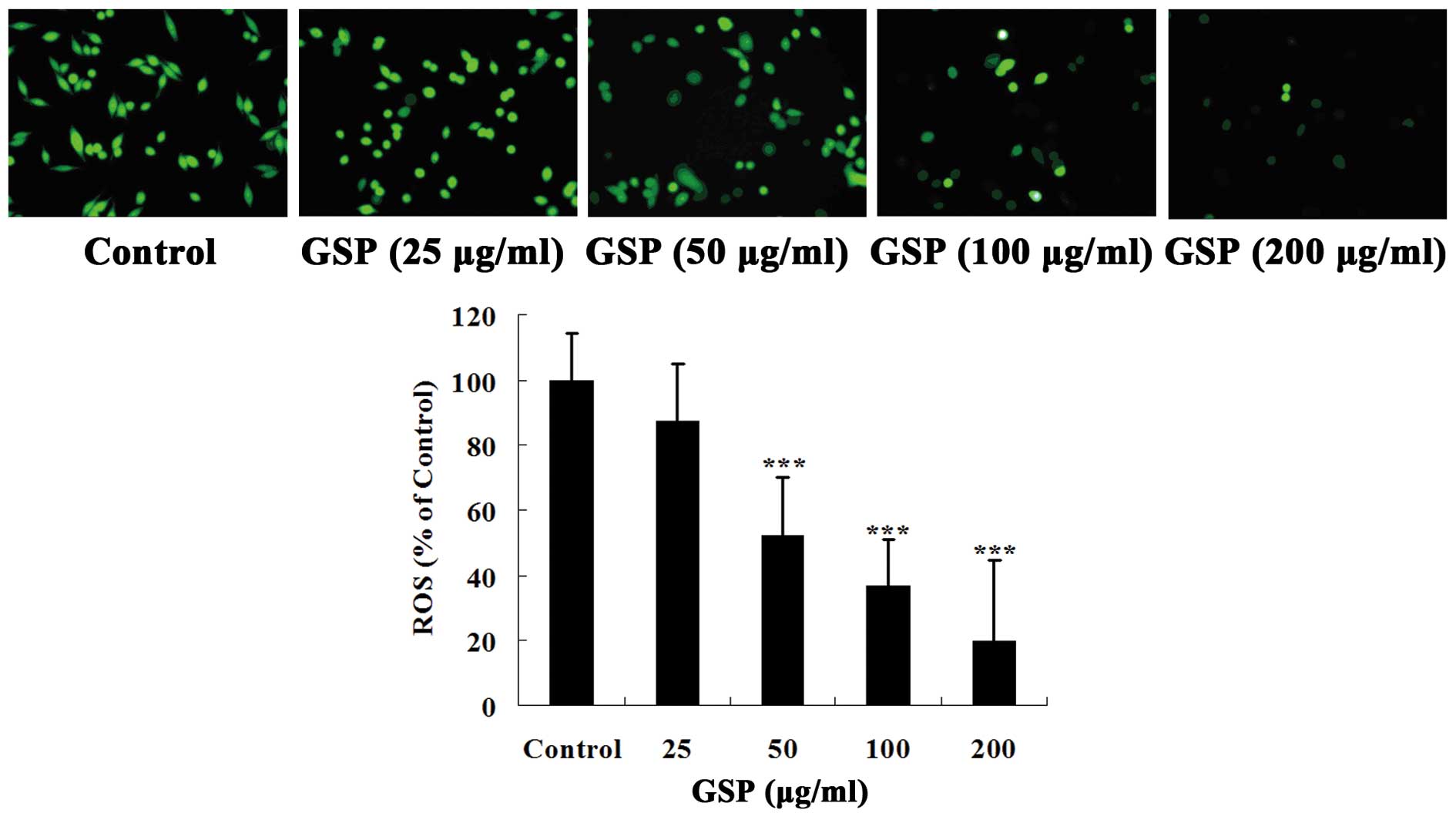
cells. As shown in Fig. 5, colon
cancer SW620 cells had a high level of ROS. Pretreatment with GSPs
significantly inhibited ROS production in a concentration-dependent
manner. Thus, our data suggest that GSPs can significantly reduce
the high levels of intracellular produced ROS. To further verify
that the antioxidant activity is part of the mechanisms by which
GSPs suppress the expression of angiogenic factors, the effects of
GSPs on H2O2-induced VEGF and Ang1 expression
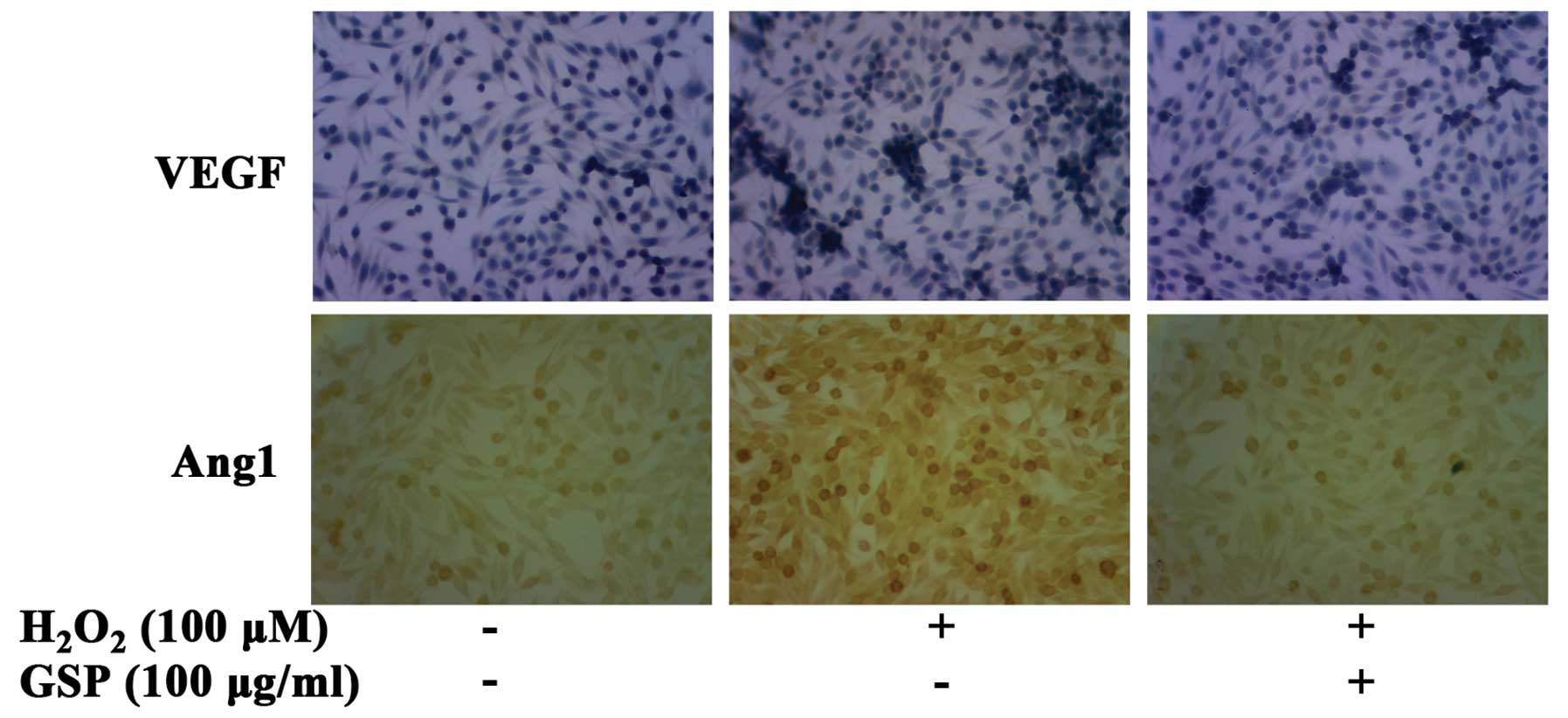
were examined with the immunohistochemical assay. As shown in
Fig. 6, 100 μM of
H2O2 markedly induced both VEGF and Ang1
expression in SW620 cells. However, the expression levels of VEGF
and Ang1 induced by H2O2 were suppressed by
pretreatment with 100 μg/ml GSP.
Discussion
Tumor growth and metastasis are dependent on
angiogenesis. Without new blood vessel formation, the tumor remains
dormant and cannot grow larger than 2–3 mm in diameter. Tumors that
grow beyond this size trigger angiogenesis by producing
proangiogenic factors. Among these molecules, VEGF and Ang1 are the
prime proangiogenic factors for sustaining tumor growth. Therefore,
inhibition of both VEGF and Ang1 production, the initial step of
tumor angiogenesis, is a promising strategy for cancer
chemoprevention and therapy. In the current study, GSPs inhibited
the colon tumor-induced angiogenesis and, thus, the growth of colon
tumor xenograft on the chick CAM without any apparent sign of
toxicity. Previous studies have demonstrated that the
chemopreventive effects of GSPs for colon cancer are associated
with their growth inhibitory and apoptosis-inducing effects. Our
results, provide another mechanism by which GSPs inhibit colon
tumor growth.
To better understand the mechanism by which GSPs
inhibit the tumor-induced angiogenesis, we examined the effect of
GSPs on the expression of proangiogenic factors by colon tumor
cells. We found that GSP treatment exerted significant inhibitory
effects on both VEGF and Angl expression. The result was further
supported by the endothelial cell migration assay, which showed
that conditioned medium from GSP-treate d SW620 cells exhibited
greater inhibitory effects on endothelial cell migration than its
untreated counterparts. This conclusion is consistent with previous
findings that GSPs inhibit VEGF secretion from DU145 prostate
cancer cells (33). Among many
proangiogenic factors, both VEGF and Ang1 regulate different, but
complementary, aspects of blood vessel growth in tumors by binding
their receptor expressed on the endothelial cells. The former is
responsible for new blood vessel formation, while the latter
contributes to new blood vessel maturation and stabilization
(32). Our observations indicate
that GSPs inhibit both sprouting angiogenesis and maturation of
blood vessels.
Both VEGF and Ang1 expression is regulated by
several factors, including hypoxia-inducible factor-1 (HIF-1), in
response to hypoxia (34). HIF-1
is a heterodimeric that consists of a constitutively expressed
HIF-1β subunit and a HIF-1α subunit, the expression of which is
highly regulated. Under normal oxygen conditions, the HIF-1α
protein is hydroxylated by the prolyl hydroxylase enzymes (PHDs),
thereby facilitating ubiquitination and subsequent proteasomal
degradation (35). It has been
reported that ROS generated from mitochondria are required for the
stabilization of HIF-1α (36). A
previous study also showed that endogenous ROS regulate
tumor-induced angiogenesis and tumor growth through HIF-1α and VEGF
expression in ovarian cancer cells (37). To understand whether the
inhibition of GSPs on VEGF and Ang1 expression from SW620 cells may
be mediated through their ROS scavenging activity, resulting
blocking ROS/HIF-1α/VEGF or Ang1 pathway, we examined the effect of
GSPs on intracellular ROS levels at first. The results of our study
showed that GSPs significantly reduced ROS levels in SW620 cells.
Our results also showed that treatment with 100 μM hydrogen
peroxide stimulated both VEGF and Ang1 expression, while
pretreatment with GSPs inhibited both VEGF and Ang1 expression.
These results suggested that inhibition of VEGF and Ang1 expression
by GSPs might partially attribute to their anti-oxidative
activity.
It has been reported that GSPs, due to their
polymeric structure, are poorly absorbed along the gastrointestinal
tract and can reach the colon at concentrations of several hundred
micromoles per liter, allowing these chemicals to act locally.
These results, combined with our previous results that GSPs inhibit
angiogenesis by inhibiting VEGF receptor 2 and receptor of Ang1
(Tie2) phosphorylation (38),
indicate that GSPs are effective antiangiogenic agents by acting on
both tumor and endothelial cells. Therefore, the results of the
present study indicate that GSPs could be used as an effective,
non-toxic antiangiogenic agent for colon cancer.
Acknowledgements
This study was supported by a grant
from the National Natural Science Foundation of China (no. 30700142
to S.H.) and the Scientific Research Innovation Team of Northwest
University for Nationalities (no. BMUCXTD-2011-1).
References
|
1.
|
J FolkmanRole of angiogenesis in tumor
growth and metastasisSemin
Oncol291518200210.1053/sonc.2002.3726312516034
|
|
2.
|
D HanahanJ FolkmanPatterns and emerging
mechanisms of the angiogenic switch during
tumorigenesisCell86353364199610.1016/S0092-8674(00)80108-78756718
|
|
3.
|
G BergersLE BenjaminTumorigenesis and the
angiogenic switchNat Rev Cancer3401410200310.1038/nrc1093
|
|
4.
|
TP SzatrowskiCF NathanProduction of large
amounts of hydrogen peroxide by human tumor cellsCancer
Res5179479819911846317
|
|
5.
|
JL ArbiserJ PetrosR KlafterReactive oxygen
generated by Nox1 triggers the angiogenic switchProc Natl Acad Sci
USA99715720200210.1073/pnas.02263019911805326
|
|
6.
|
R SiegelD NaishadhamA JemalCancer
statistics, 2012CA Cancer J Clin621029201210.3322/caac.20138
|
|
7.
|
W ChenH ZengR ZhengS ZhangJ HeCancer
incidence and mortality in China, 2007Chin J Cancer
Res2418201210.1007/s11670-012-0001-6
|
|
8.
|
G BlockB PattersonA SubarFruit,
vegetables, and cancer prevention: a review of the epidemiological
evidenceNutr Cancer18129199210.1080/016355892095142011408943
|
|
9.
|
Y SurhMolecular mechanisms of
chemopreventive effects of selected dietary and medicinal phenolic
substancesMutat
Res248305327199910.1016/S1383-5742(99)00057-510518003
|
|
10.
|
V NandakumarT SinghSK
KatiyarMulti-targeted prevention and therapy of cancer by
proanthocyanidinsCancer
Lett269378387200810.1016/j.canlet.2008.03.04918457915
|
|
11.
|
M KaurC AgarwalR AgarwalAnticancer and
cancer chemopreventive potential of grape seed extract and other
grape-based productsJ
Nutr1391806S1812S200910.3945/jn.109.10686419640973
|
|
12.
|
JP SpencerF ChaudryAS PannalaSK SraiE
DebnamC Rice-EvansDecomposition of cocoa procyanidins in the
gastric milieuBiochem Biophys Res
Commun272236241200010.1006/bbrc.2000.274910872833
|
|
13.
|
LY RiosRN BennettSA LazarusC RémésyA
ScalbertG WilliamsonCocoa procyanidins are stable during gastric
transit in humansAm J Clin Nutr7611061110200212399286
|
|
14.
|
CG FragaPI OteizaDietary flavonoids: Role
of (−)-epicatechin and related procyanidins in cell signalingFree
Radic Biol Med518138232011
|
|
15.
|
RL PriorL GuOccurrence and biological
significance of proanthocyanidins in the American
dietPhytochemistry6622642280200510.1016/j.phytochem.2005.03.02515904940
|
|
16.
|
M RossiE NegriM ParpinelProanthocyanidins
and the risk of colorectal cancer in ItalyCancer Causes
Control21243250201010.1007/s10552-009-9455-320012183
|
|
17.
|
KW SingletaryB MelineEffect of grape seed
proanthocyanidins on colon aberrant crypts and breast tumors in a
rat dual-organ tumor modelNutr
Cancer39252258200110.1207/S15327914nc392_1511759289
|
|
18.
|
H NomotoM IigoH HamadaS KojimaH
TsudaChemoprevention of colorectal cancer by grape seed
proanthocyanidin is accompanied by a decrease in proliferation and
increase in apoptosisNutr
Cancer498188200410.1207/s15327914nc4901_1115456639
|
|
19.
|
M KaurRP SinghM GuR AgarwalC AgarwalGrape
seed extract inhibits in vitro and in vivo growth of human
colorectal carcinoma cellsClin Cancer
Res1261946202200610.1158/1078-0432.CCR-06-146517062697
|
|
20.
|
AM EngelbrechtM MattheyseB
EllisProanthocyanidin from grape seeds inactivates the
PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell
lineCancer
Lett258144153200710.1016/j.canlet.2007.08.02017923279
|
|
21.
|
M KaurR MandairR AgarwalC AgarwalGrape
seed extract induces cell cycle arrest and apoptosis in human colon
carcinoma cellsNutr Cancer60Suppl
1S2S11200810.1080/0163558080238129519003575
|
|
22.
|
CP HsuYH LinCC ChouMechanisms of grape
seed procyanidin-induced apoptosis in colorectal carcinoma
cellsAnticancer Res29283289200919331163
|
|
23.
|
B VelmuruganRP SinghN KaulR AgarwalC
AgarwalDietary feeding of grape seed extract prevents intestinal
tumorigenesis in APC min/+ miceNeoplasia1295102201020072658
|
|
24.
|
S DinicolaA CucinaA
PasqualatoApoptosis-inducing factor and caspase- dependent
apoptotic pathways triggered by different grape seed extracts on
human colon cancer cell line Caco-2Br J
Nutr104824832201010.1017/S000711451000152220540818
|
|
25.
|
M KaurA TyagiRP SinghRA SclafaniR AgarwalC
AgarwalGrape seed extract upregulates p21 (Cip1) through
redox-mediated activation of ERK1/2 and posttranscriptional
regulation leading to cell cycle arrest in colon carcinoma HT29
cellsMol Carcinog50553562201110.1002/mc.20739
|
|
26.
|
B VelmuruganRP SinghR AgarwalC
AgarwalDietary-feeding of grape seed extract prevents
azoxymethane-induced colonic aberrant crypt foci formation in
fischer 344 ratsMol Carcinog49641652201020564341
|
|
27.
|
V VichaiK KirtikaraSulforhodamine B
colorimetric assay for cytotoxicity screeningNat
Protoc111121116200610.1038/nprot.2006.17917406391
|
|
28.
|
M HagedornS JaverzatD GilgesA MeyreB de
LafargeA EichmannA BikfalviAccessing key steps of human tumor
progression in vivo by using an avian embryo modelProc Natl Acad
Sci USA10216431648200510.1073/pnas.040862210215665100
|
|
29.
|
M BalkeA NeumannC KerstingMorphologic
characterization of osteosarcoma growth on the chick
chorioallantoic membraneBMC Res
Notes358201010.1186/1756-0500-3-5820202196
|
|
30.
|
PW HewettIdentification of tumour-induced
changes in endothelial cell surface protein expression: an in vitro
modelInt J Biochem Cell
Biol33325335200110.1016/S1357-2725(01)00020-611312103
|
|
31.
|
H WangJA JosephQuantifying cellular
oxidative stress by dichlorofluorescein assay using microplate
readerFree Radical Biol Med27612616199910490282
|
|
32.
|
P SaharinenL EklundK PulkkiP BonoK
AlitaloVEGF and angiopoietin signaling in tumor angiogenesis and
metastasisTrends Mol
Med17347362201110.1016/j.molmed.2011.01.01521481637
|
|
33.
|
RP SinghAK TyagimS DhanalakshmimR
AgarwalmC AgarwalmGrape seed extract inhibits advanced human
prostate tumor growth and angiogenesis and up-regulates
insulin-like growth factor binding protein-3Int J
Cancer108733740200410.1002/ijc.1162014696100
|
|
34.
|
MM HickeyMC SimonRegulation of
angiogenesis by hypoxia and hypoxia-inducible factorsCurr Top Dev
Biol76217257200610.1016/S0070-2153(06)76007-017118268
|
|
35.
|
WG Kaelin JrPJ RatcliffeOxygen sensing by
metazoans: the central role of the HIF hydroxylase pathwayMol
Cell30393402200810.1016/j.molcel.2008.04.00918498744
|
|
36.
|
EL BellTA KlimovaJ EisenbartThe Qo site of
the mitochondrial complex III is required for the transduction of
hypoxic signaling via reactive oxygen species productionJ Cell
Biol17710291036200710.1083/jcb.20060907417562787
|
|
37.
|
C XiaQ MengLZ LiuY RojanasakulXR WangBH
JiangReactive oxygen species regulate angiogenesis and tumor growth
through vascular endothelial growth factorCancer
Res671082310830200710.1158/0008-5472.CAN-07-078318006827
|
|
38.
|
SS HuangNG YangYY LiuGrape seed
proanthocyanidins inhibit angiogenesis via the downregulation of
both vascular endothelial growth factor and angiopoietin
signalingNutrition
Res32530536201210.1016/j.nutres.2012.05.01222901561
|