Introduction
Arginine is one of the most common 20 natural amino
acids and is semi-essential for the human body, carrying a unique
long carbon-containing side chain with a complex guanidinium group
at the end. Characterization of the interaction of this amino acid
with proteins has revealed that, compared to other amino acids,
arginine is unique in that it enhances the solubility of proteins
and small organic compounds (1).
An additional novel property of arginine is its ability to
inactivate a variety of viruses, depending on the concentration,
incubation pH and temperature (2–5).
The systematic characterization of the effect of arginine on DNA
and RNA viruses revealed that arginine or its derivative was
effective in inactivating several enveloped viruses of different
families, including human herpesvirus 1 (HHV-1, a member of the
Herpesvirus family), influenza A virus (a member of the
Orthomyxovirus family) and Sendai virus (a member of the
Paramyxovirus family). However, it was incapable of inactivating
non-enveloped poliovirus (a member of the Picornavirus family).
Unexpectedly, one enveloped virus, i.e., Newcastle disease virus
(NDV; a member of the Paramyxovirus family), was observed not to be
inactivated by these reagents, indicating that not all enveloped
viruses were sensitive to arginine (6).
Virus inactivation using various solvent systems is
a critical technology for the reduction of virus load on
contaminated surfaces and in pharmaceutical products (7). However, this type of technology is
not normally applicable to the therapeutic treatment of viral
diseases due to its strong tissue toxicities. In this regard,
arginine, being a natural metabolite, may be applied as a more
effective virucidal agent in vivo, in particularly against
superficial virus infections at the body surface, such as upper
respiratory infections and herpetic keratitis (8,9).
In addition to its virucidal effects, arginine affects the
multiplication of HHV-1 at concentrations far below the
concentration at which it exerts virucidal activity and
cytotoxicity (10). Thus,
arginine may suppress HHV-1 infection through both virucidal and
antiviral activities. In fact, a preliminary study reported that
arginine significantly inhibited the development of herpetic
keratitis in a rabbit eye model (Naito et al, unpublished
data). In the present study, we examined the virucidal and
antiviral activities of arginine against human herpesvirus 2
(HHV-2, also known as herpes simplex virus type 2 or HSV-2), which
is responsible for genital infection, the most common causes of
genital ulcer disease worldwide. HHV-2 establishes latent infection
in the neuronal cells in ganglia after initial infection, remaining
dormant in the infected cells for a lifelong period and causes an
asymptomatic virus shedding or symptomatic vesicle and ulcer
formation on the mucosal surfaces of the genital organs during
recurrences (11). Considering
in vivo application of arginine against genital herpes, we
have characterized the virucidal effects of arginine against HHV-2
near body temperature (5). Based
on observation of the excellent virucidal activities of arginine,
we then examined arginine for its ability to suppress HHV-2 genital
infection using a mouse model.
Materials and methods
Cells and viruses
HEp-2 and Vero cells were grown in Eagle’s minimum
essential medium (MEM) containing 5% fetal bovine serum (FBS).
HHV-2, strain 186, was used throughout the experiments. HHV-2 was
propagated in Vero cells cultured in MEM supplemented with 0.5%
FBS. The virus was stored at −80°C until use. The amount of virus
was measured by a plaque assay on Vero cells as previously
described (12,13).
Reagents
L-arginine hydrochloride (simply described as
arginine) was obtained from Ajinomoto Co., Inc. Aqueous solutions
containing arginine or NaCl were prepared in 20 mM acetic acid. The
pH of the arginine solutions was adjusted to the indicated values
with HCl; 20 mM acetic acid is insufficient to titrate arginine
solution to the desired pH. The pH of the NaCl in 20 mM acetic acid
and aqueous citrate solutions was adjusted to the indicated pH with
NaOH. The pH meter was routinely calibrated using pH calibration
standards.
Assay for virucidal activity
All the starting materials were stored on ice prior
to the virus inactivation experiments. A 190 μl aliquot of
the solutions to be evaluated was placed in 2.0-ml plastic tube on
ice and received 10 μl of virus preparation [approximately
108 plaque-forming units (PFU)/ml)]. This was
immediately followed by vigorous mixing and the sample mixture was
incubated at the indicated temperatures. After the incubation,
aliquots of these virus samples were 100-fold diluted with
Dulbecco’s phosphate-buffered saline (PBS) without Ca2+
and Mg2+ containing 0.5% FBS to terminate the virucidal
action of the solutions. This dilution step helped stabilize the
infectivity of the surviving viruses after the inactivation
treatment. The viruses were further diluted with ice-cold PBS
containing 0.5% FBS and the number of infectious viruses in the
treated preparation was measured by a plaque assay on Vero cells.
All the experiments were conducted in duplicate or triplicate.
Virus yields in the presence of
arginine
The effect of arginine on the multiplication of
HHV-2 was examined as follows. The monolayered HEp-2 cells in 35-mm
dishes were infected with the virus at an indicated multiplicity of
infection (MOI). The infected cells were further incubated at 37°C
for the indicated period in the serum-free MEM containing 0.1%
bovine serum albumin (BSA) and in the indicated concentrations of
arginine. At the end of the incubation, the amounts of progeny
virus in the infected cultures were determined as previously
described (14). Briefly, after
two or three cycles of freezing and thawing the infected cells
along with the culture media, the number of infectious viruses in
the lysate was measured by a plaque assay.
Determination of cytopathic effects and
the cell death
The cytopathic effects (CPEs) were determined by
microscopic observation of the cells; approximate amounts of
rounded cells on monolayers were estimated under a phase-contrast
microscope. The extent of cell death in the cultures was determined
as follows. The monolayerd cells were trypsinized to obtain a
single cell suspension. After the addition of MEM containing 5% FBS
to the suspension to neutralize trypsin and stabilize the cells,
the numbers of living and dead cells were measured by a
dye-exclusion method with trypan blue (15).
Murine experiments
Seven-week-old female BALB/c mice were infected with
HHV-2 as follows. The mice, whose vaginal walls were scratched,
were infected with 6×105 PFU HHV-2 in 10 μl by
vaginal instillation. For the treatment with test solutions, the
mice were treated with 20 μl of the indicated solutions by
vaginal instillation at 2, 12 and 24 h post-infection (h p.i.) and
then once every day for 20 days. In case of acycloguanosine, it was
administrated daily intraperitoneally at a dose of 0.05 mg/200
μl/head. The number of mice that survived and the symptoms
of these infected mice were examined daily. All animal experiments
were performed in compliance with the principles of laboratory
animal care (NIH publication No. 86, revised 1985) and with the
guidelines of Teikyo University School of Medicine Animal
Experiments Committee.
Results
Effect of arginine on the inactivation of
HHV-2
Previously we observed that arginine and arginine
derivatives are effective in inactivating a variety of enveloped
viruses, although a number of them, such as NDV, are not sensitive
to arginine (6). When HHV-2 was
incubated with mildly acidic arginine solution, this virus was also
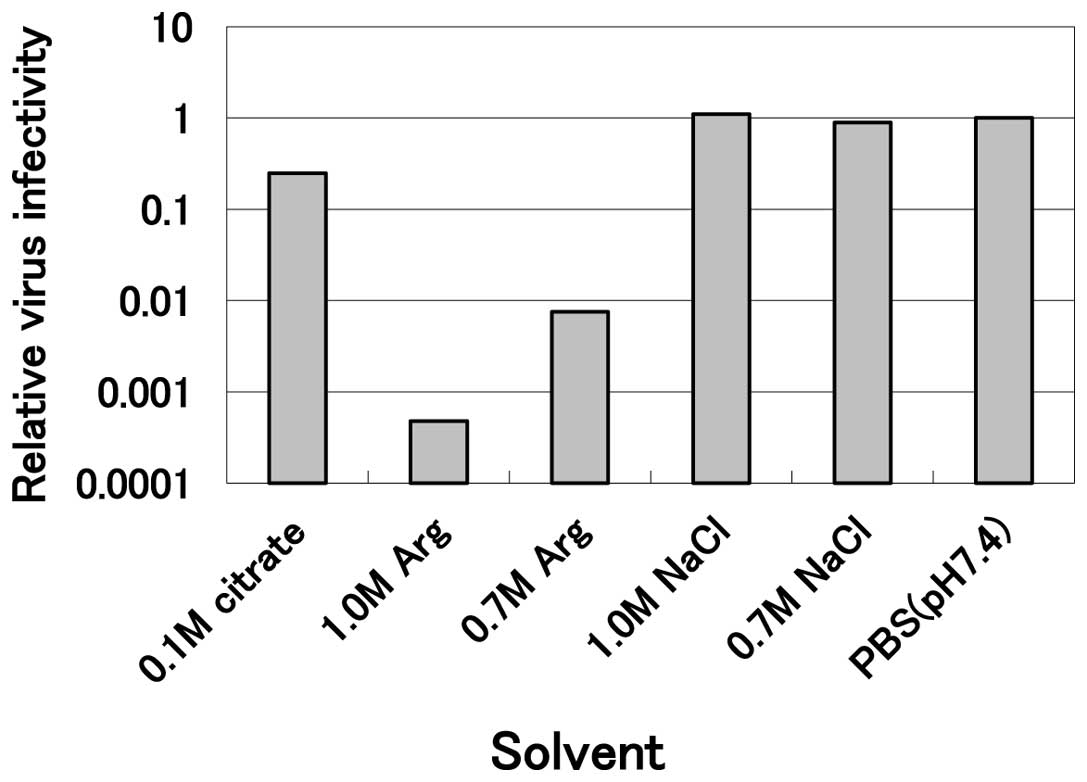
sensitive to arginine (Fig. 1).
Incubation of the virus with 1.0 M arginine at pH 4.3 at 30°C for
10 min reduced the infectivity to less than one-thousandth the
level of the PBS control, while 0.1 M citrate at the same pH
revealed marginal inactivation (0.25 relative to the PBS control)
and 0.7 and 1.0 M sodium chloride solutions at the same pH were not
effective. These results clearly indicate that mildly acidic
arginine effectively inactivates HHV-2 and that neither the acidic
pH nor the high salt concentration was effective. This inactivation
by arginine was concentration-dependent, since 0.7 M arginine
solution reduced the infectivity to only one-hundredth the level of
the PBS control (Fig. 1). A
comparison with other viruses indicates that HHV-2 is the most
sensitive virus examined thus far (data not shown).
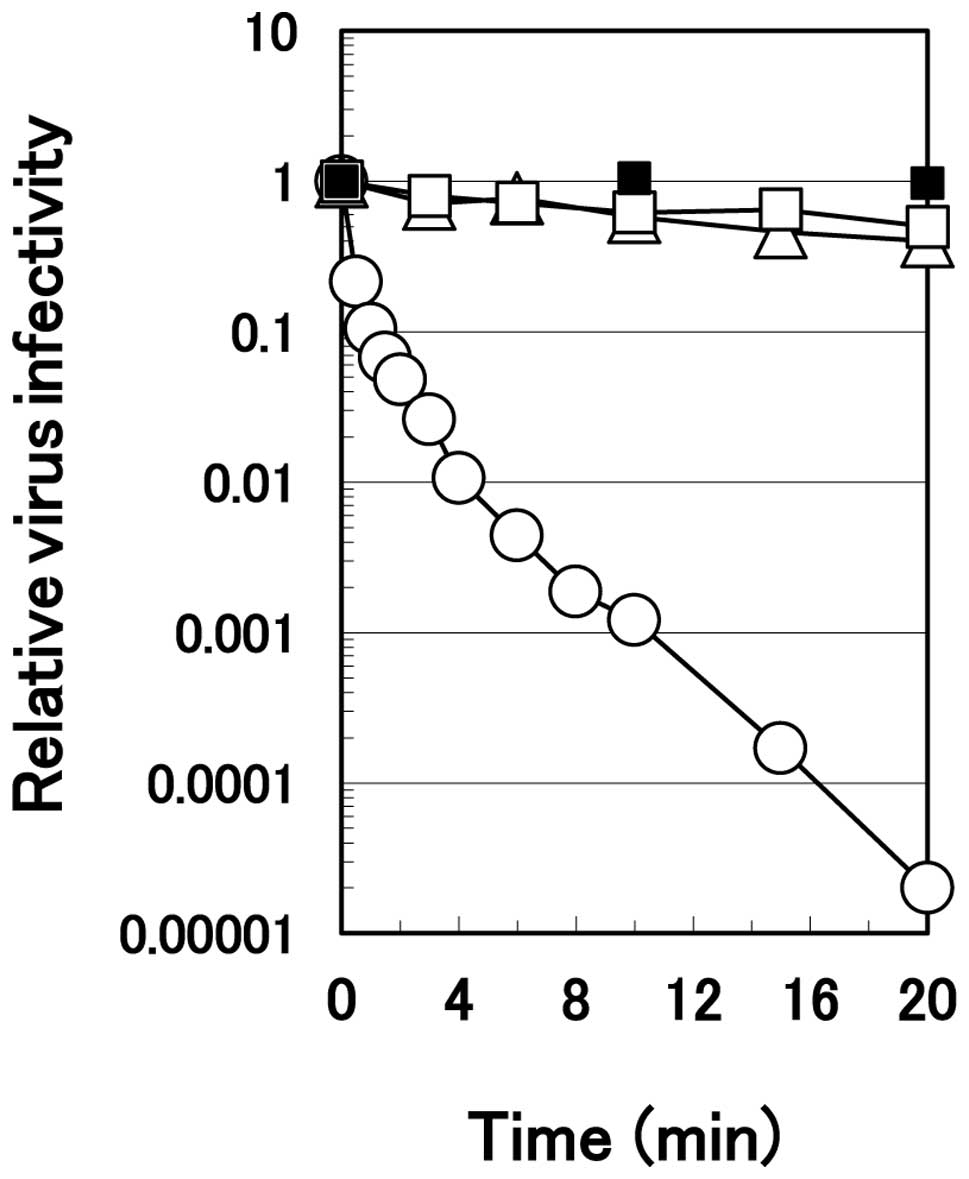
The rate of the virus inactivation was much faster
with arginine. HHV-2 revealed no noticeable inactivation in the PBS
during 20 min of incubation on ice (Fig. 2). In both 1.0 M sodium chloride
and 0.1 M citrate solutions at pH 4.3, the virus was inactivated
only slightly with time at the same temperature. Conversely, the
virus inactivation occurred rapidly in 1.0 M arginine solution at
pH 4.3; it decreased in monotone with time, reaching one-thousandth
the level of the PBS control at 10 min of the incubation on ice and
approximately 10−5 at 20 min of the incubation.
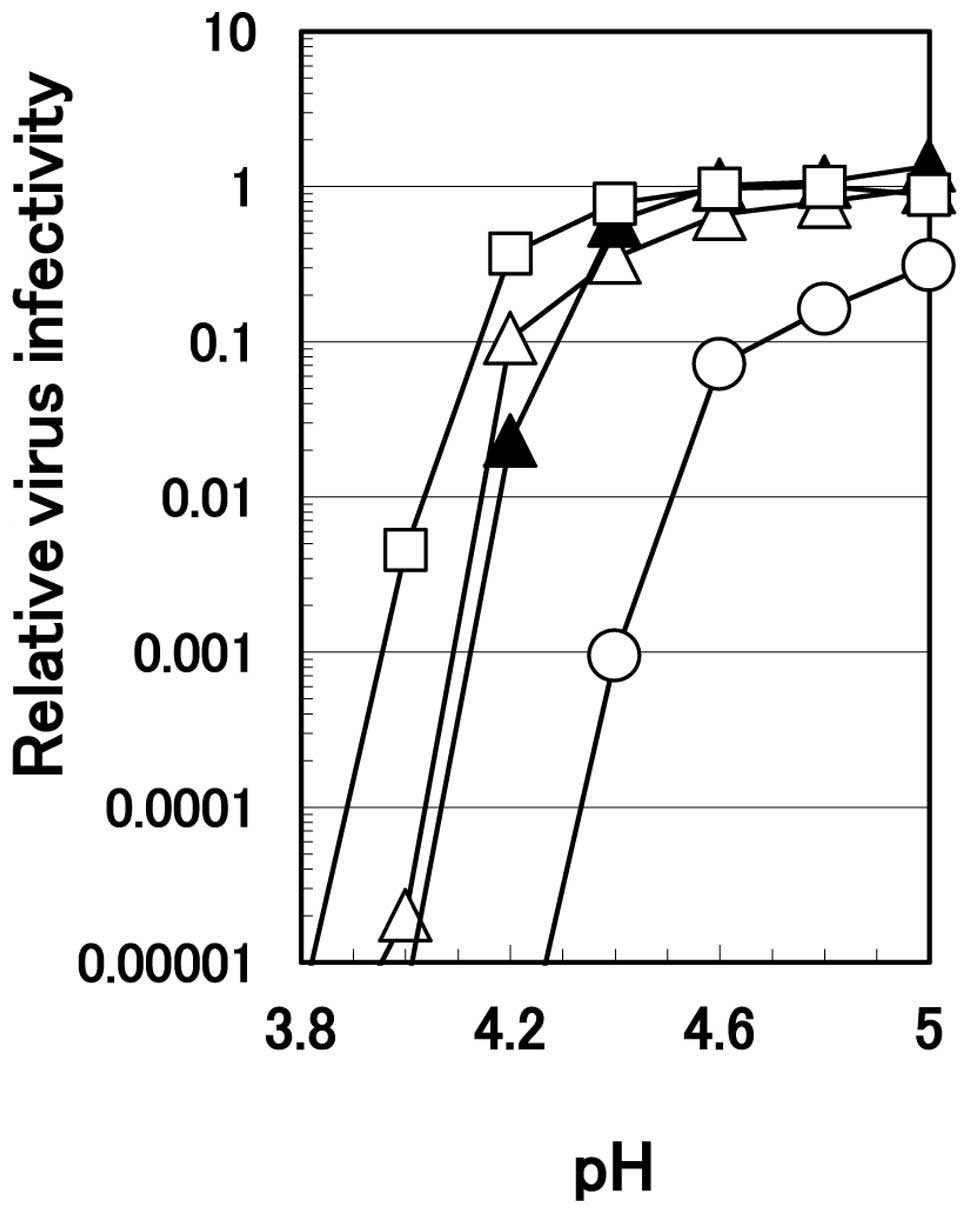
We previously demonstrated that the ability of
arginine and citrate to inactivate HHV-1 diminishes rapidly when
the pH of the solution was increased (2). The effect of pH on the inactivation
of HHV-2 by 0.1 M citrate or 0.7 M arginine is displayed in
Fig. 3. To compensate for the
difference in ionic strength between these two solutions, 0.6 M
sodium chloride was added to the series of 0.1 M citrate. At or
below pH 4.0, all three solutions equally and efficiently
inactivated HHV-2. Above pH 4.0, 0.1 M citrate solution by itself
or supplemented with 0.6 M sodium chloride lost the ability to
inactivate the virus to a much greater extent than 0.7 M arginine;
0.7 M arginine reduced virus infectivity below the detection level
even at pH 4.2. A higher concentration of citrate at 0.7 M was
least effective, possibly due to the salting-out effect of citrate
that protected the virus from the inactivation, as discussed
previously (5). Differences in
the degree of virus inactivation among different solutions
decreased at higher pH, e.g., above 4.6.
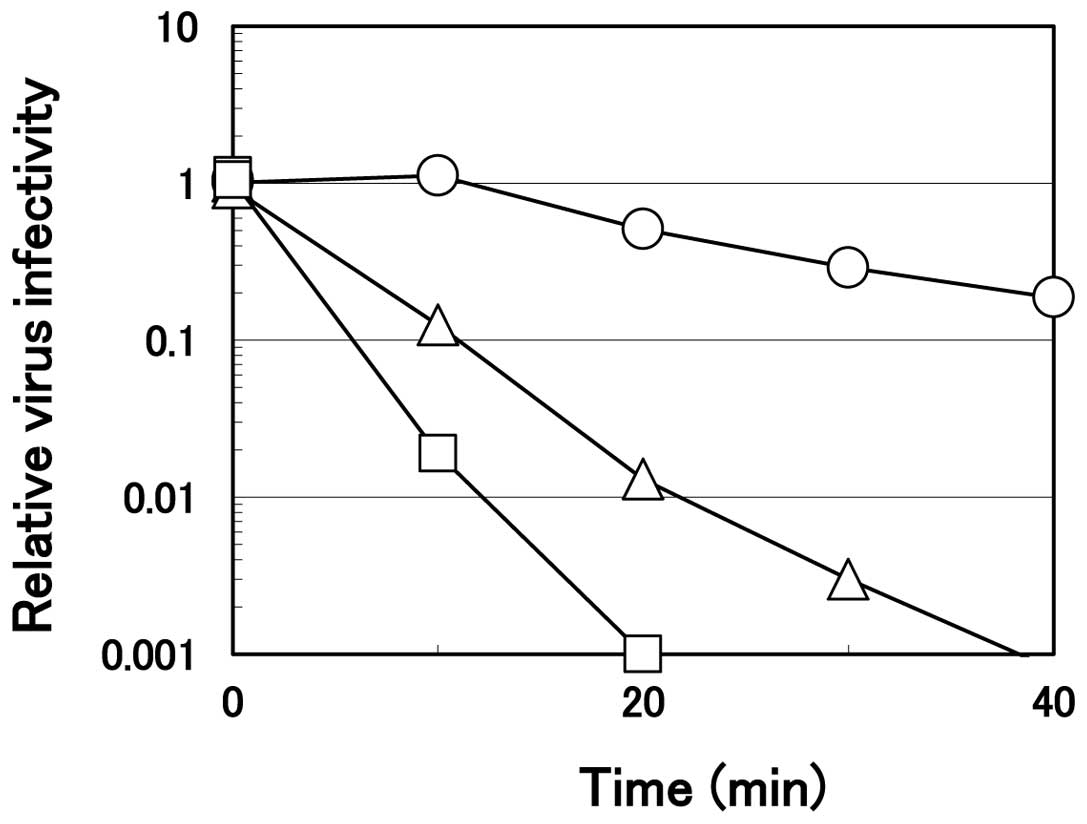
Virucidal effect of arginine at a neutral
pH
As described above, virucidal activity of arginine
was much weaker at a higher pH. However, even at a neutral pH, a
prolonged incubation time resulted in significant inactivation. The
time course of the virus inactivation on ice by arginine at pH 7.0
is displayed in Fig. 4.
Consistent with the results shown in Fig. 1, the ability depended an arginine
concentration. HHV-2 was inactivated quickly with arginine solution
at 1.2 M, reaching one-thousandth within 20 min of the incubation
while the virus infectivity decreased slightly, although
significantly (only 50–60% of the PBS control), in 0.75 M arginine
solution. At 1.0 M, arginine at pH 7.0 inactivated the virus more
effectively compared to that at 0.75 M, but less effectively
compared to that at 1.0 M arginine at pH 4.3 (Fig. 2), reaching one hundredth the level
of the PBS control at 20 min of the incubation. These results
clearly reveal that, even at a neutral pH, arginine inactivates
HHV-2, provided that higher arginine concentration and a longer
incubation time were employed than those at an acidic pH.
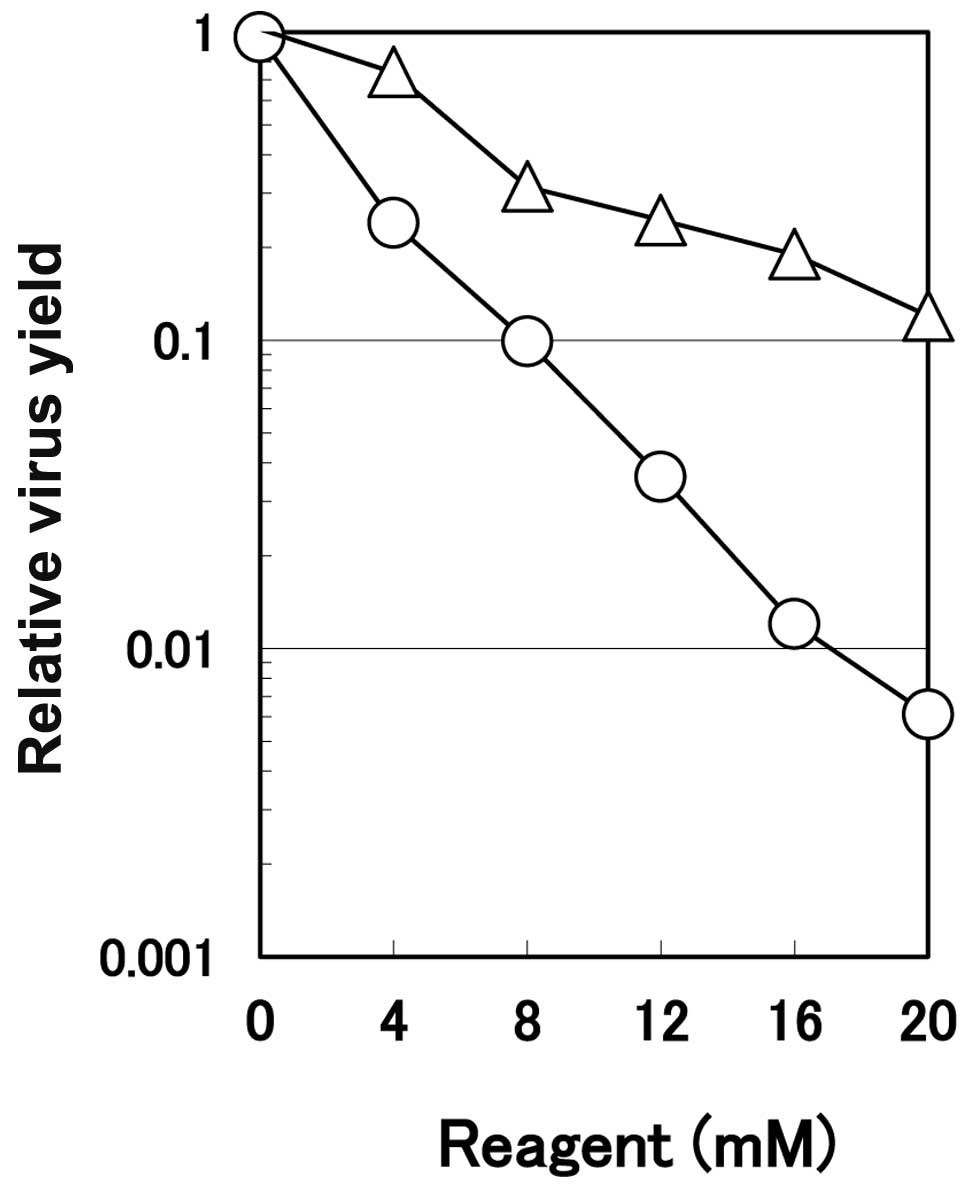
Suppression of virus multiplication by
arginine
From the results described above, it is evident that
arginine directly inactivates HHV-2 in the test tube. Next, we
examined the effects of arginine on the virus multiplication in
infected cells by incubating HHV-2-infected HEp-2 cells with
arginine at various concentrations. The progeny virus yield as a
function of arginine concentration (note that the arginine
concentration is mM, not M as in the virus inactivation
experiments) is displayed in Fig.
5. Arginine reduced the virus yield dose-dependently in the
infected cells, reaching one-hundredth the yield of the control
untreated culture at 16 mM. As a control of ionic strength of
arginine, the effects of sodium chloride were examined. As shown in
Fig. 5, sodium chloride also
concentration-dependently inhibited the multiplication of HHV-2,
but to a much smaller extent than the level achieved by arginine.
Although HHV-2 is closely related to HHV-1, HHV-2 is more sensitive
to arginine than is HHV-1; only 1-log reduction of the relative
virus yield was observed for HHV-1 in the presence of arginine at
25 mM (10). Although the
arginine concentration is not within a physiological level, it is
considered to be within a tolerable range for the infected cells,
as described in the following section (i.e., cytotoxic effects of
arginine).
It should be noted that the antiviral activity of
arginine described above has no relation to the observed direct
virucidal activity of arginine, as observed at higher
concentrations required to inactivate the virus (Figs. 1–4). Thus, these antiviral effects of
arginine are not mediated by virucidal activity, but rather by
other factors.
Cytotoxic effects of arginine
Arginine induced notable morphological alterations
in HEp-2 cells which included both rounding and shrinkage of the
cells, but without cell detachment from the dishes. CPEs were
enhanced with increasing concentrations of arginine and, at the
concentration of 20 mM, arginine induced the CPEs in a small, but
significant population of uninfected HEp-2 cells. However, we
previously demonstrated, that upon incubation of HEp-2 cells with
arginine for 26 h, the cell death was induced only by a small
(2–5%) fraction of the total cell population independently of
arginine concentrations (0–40 mM), a magnitude similar to the cell
death observed in the sodium chloride-treated cells at the same
concentrations (10). These
results suggest that the observed decrease in the virus yield by
arginine is unlikely due to the degeneration (cell death) of the
infected cells by arginine. This suggestion is further confirmed by
the observations that the infection with HHV-2 completely abolished
arginine-induced CPEs: namely, arginine does not induce CPEs in
HEp-2 cells infected with HHV-2, possibly due to the expression of
anti-apoptotic genes of HHV-2 in the infected cells (16).
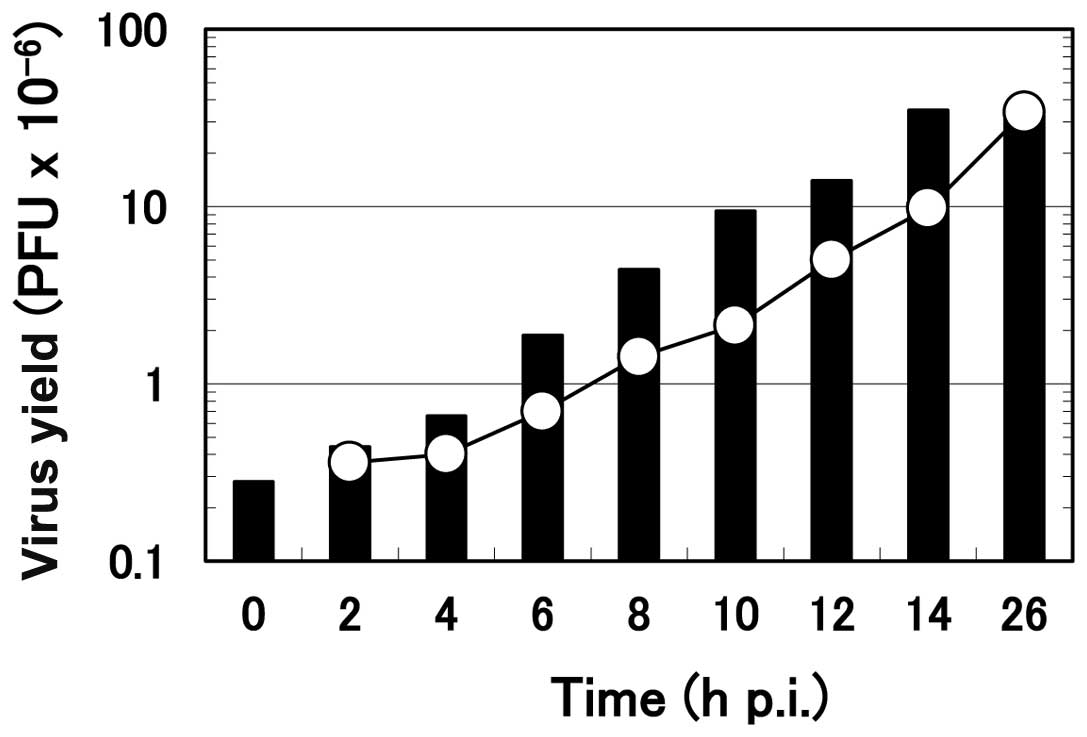
Effect of the time of addition of
arginine on the progeny virus yield
A previous characterization of the kinetics of the
viral multiplication process in HHV-infected Vero cells (17) revealed that viral DNA replication
occurs exclusively between 3 and 6 h p.i and a large amount of DNA
is accumulated in the infected cells when the replication of virus
DNA is completed. The formation of nucleocapsids as well as the
envelopment of the nucleocapsids begins at 5 h p.i. simultaneously
with the formation of infectious progeny virus and continues until
approximately 12 h p.i. In HEp-2 cells, the onset as well as the
completion of the formation of the progeny virus is slightly
delayed and a small but significant degree of the progeny virus
formation continues even after 12 h p.i., compared with those in
Vero cells (Koyama, unpublished data).
To examine the arginine-sensitive step in the
multiplication process of HHV-2 in HEp-2 cells, the ‘time of
addition’ experiment was performed. Arginine was added to the
infected culture at various times after the onset of HHV-2
multiplication, and the virus yield at 26 h p.i. was compared to
the amount of virus formed in the infected cells at the time of
addition of arginine (Fig. 6).
The formation of the progeny virus was almost immediately
suppressed by the addition when the infected cells received
arginine at 0, 2 or 4 h p.i., as noted in small additional
increases of the virus yield. In contrast, when arginine was added
at or after 6 h p.i., the suppression in the progeny virus
formation was evident but not complete; the formation of the
progeny virus continued for a short time after arginine addition.
For example, the amount of the infectious virus at 8 h p.i. was
1.42×106. When arginine was added at this time point and
cell culture was continued for 26 h, the final virus yield was
4.4×106, meaning that the progeny virus formation
continued after the addition to a significant extent. However, this
virus yield (4.4×106) was less than the virus yield
without arginine addition (35×106), although it was more
than 3-fold higher than the amount of the infectious virus formed
at 8 h p.i. (1.42×106). A similar result was obtained
from the previous ‘time of addition experiment’ with HHV-1
(10) and an additional
characterization of the one-step growth of HHV-1 revealed that the
formation of the progeny virus in the HHV-1-infected HEp-2 cells
continued normally for 3–4 h after the addition of arginine and
then stopped (10). Both the
current and the previous results involving HHV-1 agree with the
conclusion that the formation of the progeny virus remains
sensitive to arginine in the late stage (6, 8, 10 and 12 h p.i.) of
the infection, even after the completion of the viral DNA
replication and the formation of nucleocapsids. However, it should
be noted that our results do not exclude the possible
arginine-sensitive step in the early stages (2 and 4 h p.i.) of the
HHV infection.
Effect of arginine on the infection
through the genital route
The in vitro studies as described above
clearly indicate the antiviral and virucidal activities of arginine
against HHV-2 infection. Although both of these activities require
higher arginine concentration than the physiological level, we
carried out a pilot study to examine the potential application of
arginine as a therapeutic or preventive medicine in vivo.
Mouse genital herpes infection was used as a model disease for the
treatment with arginine, since arginine at high concentrations is
not tolerable for an in vivo systemic application but can be
used to treat superficial virus infection on the body surface.
BALB/c mice are very sensitive to HHV-2 following
infection through the vaginal route; the virus first multiplies at
the site of infection in the vagina, followed by invasion into the
central nervous system through sacral ganglia neuronal cells, and
finally causes lethal encephalitis at the brain stem. To examine
arginine as a possible treatment for genital herpes infection, we
examined the effects of acidic arginine on the establishment of
HHV-2 infection in a mouse model. To ensure the virus infection,
the vaginal walls of the mice were scratched before inoculation
with HHV-2. Two hours after the inoculation, the mice received 20
μl arginine solution by vaginal instillation according to
the schedule described in Materials and method. Considering a
potential dilution of the arginine solution by vaginal secretion,
arginine at slightly higher concentrations and a lower pH was used
in this in vivo study compared to those used in the in
vitro studies described above.
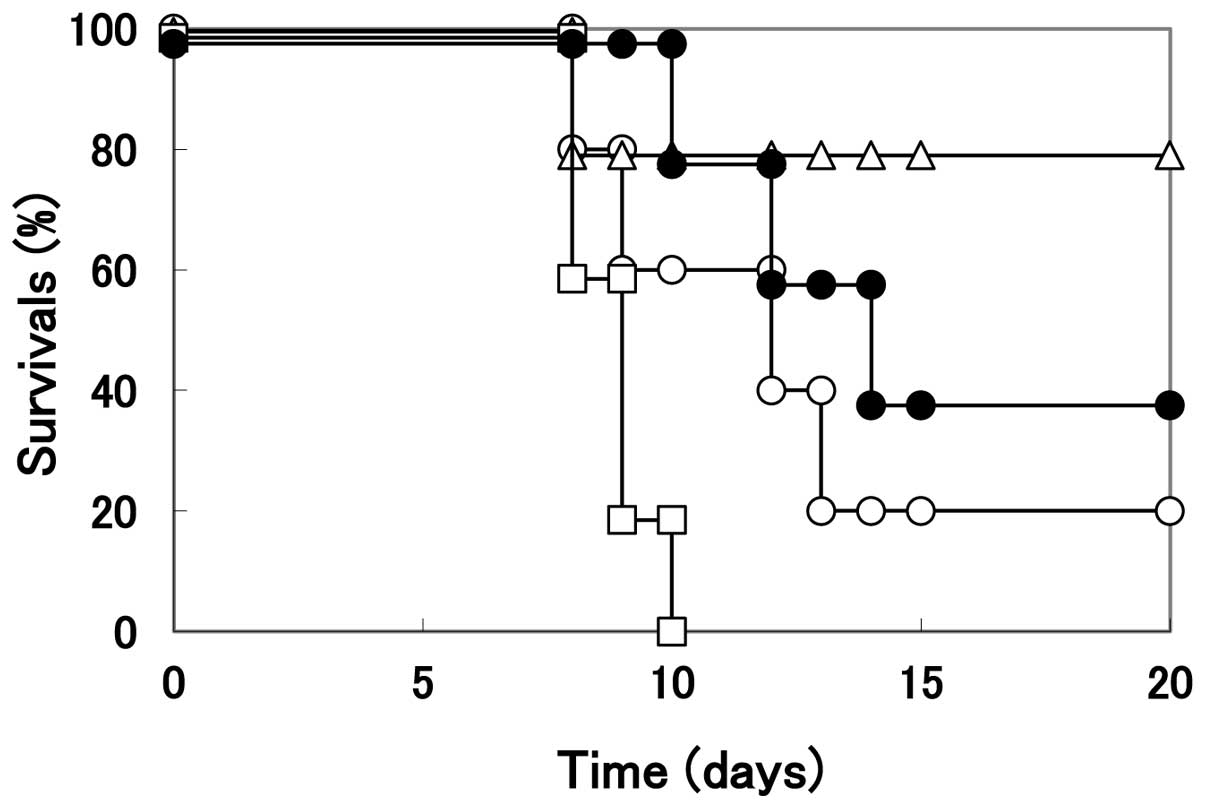
When the infected mice were treated with PBS
(control without arginine treatment), the mortality of mice was
noted at 8 days p.i. and the number of surviving mice was 1 out of
5 at 20 days p.i. (Fig. 7).
However, when the infected mice were treated with arginine solution
(pH 3.5), only one mouse died at 8 days p.i., with four mice
surviving until 20 days p.i. In contrast to the infected mice
treated with arginine, those treated with citrate buffer at the
same pH began to die at 8 days p.i., and all infected mice died by
10 days p.i.. Although acycloguanosine is known to be an effective
antiviral drug for human HSV infections, this medicine is toxic to
BALB/c mice and treatment with this compound may not rescue mice
from the infection. The number of surviving mice was almost similar
to those treated with PBS, although the mice treated with
acycloguanosine began to die at 10 days p.i. and the mice treated
with PBS began to die at 8 days p.i. It should be noted that no
apparent tissue damage was observed in the genital organs following
arginine treatment. These results clearly indicate that the
arginine treatment may effectively rescue mice from the infection
of HHV-2 through the vaginal route and suggest a potential use of
arginine as a preventive medicine against genital HHV-2
infection.
Discussion
The present study clearly showed the ability of
arginine to inactivate HHV-2 and suppress its growth. The
characterization of virus inactivation by arginine revealed the
following, i) at pH 4.3, 1.0 M arginine more efficiently
inactivated the virus than 0.1 M citrate or 1.0 M sodium chloride
(Figs. 1 and 2), indicating that neither acidic pH nor
ionic strength alone was sufficient for HHV-2 inactivation and ii)
while the inactivation was more efficient at an acidic pH (Fig. 3), arginine at a neutral pH was
capable of inactivating the virus, in particular at higher arginine
concentrations and with a prolonged incubation time (Fig. 4). iii) The rate of virus
inactivation was dependent on arginine concentrations (Figs. 1 and 4), pH (Fig. 2) and temperature (data not shown).
These characteristics of HHV-2 inactivation by arginine are similar
to those observed for HHV-1 (2,3),
although HHV-2 showed higher sensitivity to arginine than
HHV-1.
Although the target sites of arginine in its
virucidal effect have not yet been determined, the observed
requirement of a high concentration suggests that arginine does not
act on a specific site of the virion with high affinity, but likely
interacts with multiple sites of the virion with low affinity
(i.e., non-specific sites), affecting structures of virion
proteins, protein-protein interactions of envelope glycoproteins
and glycoprotein-lipid interactions on the viral envelope. Reported
characteristics of arginine (such as solubilization of inclusion
body or suppression of hydrophobic and aromatic interactions
between proteins) also support this notion (1,18).
Considering that antiviral drugs with a specific target inevitably
generate drug-resistant variants by the mutation of the
drug-binding site of the target protein molecule, the non-specific,
multiple target sites of arginine are unlikely to generate
resistant viruses and provide a unique and obvious advantage for
arginine over the conventional antiviral drugs.
In addition to these virucidal effects, arginine is
capable of inhibiting in vitro multiplication of HHV-2
(Fig. 5). Since in vitro
cell based assays do not contain immune cells, the results clearly
demonstrate the direct action of arginine on the growth suppression
of HHV-2. Although the primary arginine-sensitive step in the HHV-2
multiplication remains unclear, the time of addition experiments
demonstrated that the addition of arginine after the completion of
viral DNA replication (at 6 h p.i.) or even at the later stages of
the infection (such as 8–12 h p.i.) may still inhibit the normal
progeny virus formation to a significant extent, while earlier
addition of arginine nearly completely suppressed viral growth. It
remains unclear how arginine suppresses the progeny virus
production in the HHV-2-infected cells, although a proposal has
been made that arginine affects virus growth through its effects on
certain cellular enzymes (19,20).
Whatever the mechanism involved, arginine does have
antiviral activities against HHV-2 at moderate concentrations.
However, it is impossible to achieve such high serum arginine
concentrations and, thus, to use arginine as an antiviral drug
against systemic infections. However, such concentrations may be
readily achieved in topical applications against superficial viral
infections. HHV-2 and a number of other viruses cause serious
topical diseases on the body surface and hence antiviral activities
of arginine may be applied in these areas. Lack of the possibility
of generating drug-resistant variants as well as low cost offer
great advantages particularly for preventive use.
Acknowledgements
The authors thank Dr Daisuke Ejima
(Ajinomoto, Japan) for his helpful discussion.
References
|
1.
|
T ArakawaM UozakiAH KoyamaModulation of
small molecule solubility and protein binding by arginineMol Med
Rep3833836201021472322
|
|
2.
|
H YamasakiK TsujimotoAH KoyamaD EjimaT
ArakawaArginine facilitates inactivation of enveloped virusesJ
Pharm Sci9730673073200810.1002/jps.2122418186461
|
|
3.
|
H UtsunomiyaM IchinoseK TsujimotoY
KatsuyamaH YamasakiAH KoyamaD EjimaT ArakawaCo-operative thermal
inactivation of human herpes simplex virus and influenza virus by
arginine and NaClInt J
Pharm36699102200910.1016/j.ijpharm.2008.09.01218845231
|
|
4.
|
T ArakawaY KitaAH KoyamaSynergistic virus
inactivation effects of arginineBiotechnol
J4174178200910.1002/biot.20080017719156749
|
|
5.
|
K TsujimotoM UozakiK IkedaH YamasakiH
UtsunomiyaM IchinoseAH KoyamaT ArakawaSolvent-induced virus
inactivation by acidic arginine solutionInt J Mol
Med25433437201020127049
|
|
6.
|
Y KatsuyamaH YamasakiK TsujimotoAH KoyamaD
EjimaT ArakawaButyroyl-arginine as a potent virus inactivation
agentInt J
Pharm3619298200810.1016/j.ijpharm.2008.05.02018617337
|
|
7.
|
K BrorsonS KrejciK LeeE HamiltonK SteinY
XuBracketed generic inactivation of rodent retroviruses by low pH
treatment for monoclonal antibodies and recombinant
proteinsBiotechnol Bioeng82321329200310.1002/bit.1057412599259
|
|
8.
|
K IkedaH YamasakiY SuzukiAH KoyamaT
ArakawaNew strategy with acidic arginine solution for the treatment
of influenza A virus infection: ReviewExp Ther
Med1251256201022993536
|
|
9.
|
K IkedaH YamasakiS MinamiT NaitoH IrieT
ArakawaAH KoyamaVirucidal ability of arginine and its possible
application as an antiherpetic agent. In: From the Hallowed Halls
of HerpesvirologyJ BainesJ BlahoImperial College
PressLondon4354492011
|
|
10.
|
T NaitoH IrieK TsujimotoK IkedaT ArakawaAH
KoyamaAntiviral effect of arginine against herpes simplex virus
type 1Int J Mol Med23495499200910.3892/ijmm_0000015619288025
|
|
11.
|
B RoizmanDM KnipeRJ WhitleyHerpes simplex
virusesDM KnipePM HowleyFields Virology5th editionLippincott
Williams and WilkinsPhiladelphia250126012007
|
|
12.
|
AH KoyamaY MiwaSuppression of apoptotic
DNA fragmentation in herpes simplex virus type 1-infected cellsJ
Virol712567257119979032402
|
|
13.
|
AH KoyamaH AkariA AdachiF GoshimaY
NishiyamaInduction of apoptosis in HEp-2 cells by infection with
herpes simplex virus type 2Arch
Virol14324352441199810.1007/s0070500504739930199
|
|
14.
|
AH KoyamaT UchidaThe effect of ammonium
chloride on the multiplication of herpes simplex virus type 1 in
Vero cellsVirus
Res13271281198910.1016/0168-1702(89)90073-72554609
|
|
15.
|
AH KoyamaT ArakawaA AdachiAcceleration of
virus-induced apoptosis by tumor necrosis factorFEBS
Lett426179182199810.1016/S0014-5793(98)00338-X9599003
|
|
16.
|
S HataAH KoyamaH ShiotaA AdachiF GoshimaY
NishiyamaAntiapoptotic activity of herpes simplex virus type 2: A
role of US3 protein kinase geneMicrobe
Infect1601607199910.1016/S1286-4579(99)80059-810611736
|
|
17.
|
AH KoyamaT UchidaQuantitative studies on
the maturation process of herpes simplex virus type 1 in Vero
cellsVirus Res10281285198810.1016/0168-1702(88)90023-82842975
|
|
18.
|
D AroraN KhannaMethod for increasing the
yield of properly folded recombinant human gamma interferon from
inclusion bodiesJ
Biotechnol52127133199610.1016/S0168-1656(96)01636-79084211
|
|
19.
|
H TakadaC KishimotoY HiraokaH OchiaiOral
L-arginine prevents murine coxsackievirus B3 myocarditisInt J
Cardiol86272279200210.1016/S0167-5273(02)00279-612419566
|
|
20.
|
H AgawaK IkutaY MinamiyamaM InoueT
SairenjiDown-regulation of spontaneous Epstein-Barr virus
reactivation in the P3HR-1 cell line by
L-arginineVirology304114124200210.1006/viro.2002.170912490409
|