Introduction
Melanogenesis is a physiological process resulting
in the synthesis of melanin pigments, which are secreted by
melanocytes in the basal layer of the epidermis. Melanin is
principally responsible for skin color and plays an important role
in the prevention of skin injury under normal physiological
conditions (1). However, abnormal
pigmentations such as freckles, age spots and melasma could
indicate skin problems (1,2).
The pigmentary disorders are caused by various factors, including
UV radiation, inflammation, estrogens and genetic disorders
(1). Melanin synthesis is
mediated by melanocyte-specific enzymes such as tyrosinase,
tyrosinase-related protein (TRP)-1, and TRP-2 or dopachrome
tautomerase (Dct) (3,4). On the sequential pathway to melanin
formation, tyrosinase is a rate-limiting enzyme that catalyzes
tyrosine to 3,4-dihydroxyphenylalanine (L-DOPA) and further
oxidizes it to dopaquinone (5).
Therefore, melanin production mainly depends on the expression and
activation of tyrosinase (6). The
modulation of melanogenesis is one of the significant strategies to
treat abnormal skin pigmentations through medication and cosmetics
(7).
To satisfy the desire for decreased melanogenesis,
several cosmetic companies are developing melanogenesis inhibitors
and discovering skin-whitening cosmetic preparations. In cosmetic
preparations, inhibitors such as kojic acid, arbutin, ascorbic acid
and licorice extracts have been used as whitening ingredients
(8). In particular, tyrosinase
inhibitors may not only be clinically useful for the treatment of
some dermatological diseases associated with melanin
hyperpigmentation, but they may also be important in cosmetics for
depigmentation (9). A great deal
of interest has recently focused on deriving tyrosinase inhibitors
from a natural source. Several chemical compounds have been
reported from plant origins as tyrosinase inhibitors, such as
ellagic acid (10),
oxyresveratrol (11),
chlorophorin and norartocarpanone (12) and the most common natural
antibrowning agent is ascorbic acid (13). However, the effect of ascorbic
acid against enzymatic oxidation is temporary since it is
chemically oxidized to the nonfunctional form, dehydroascorbic acid
(13). These problems prompted us
to search for safer and more effective melanin formation inhibitors
from natural sources.
Marine organisms are rich sources of structurally
novel and biologically active metabolites with valuable industrial
potential. Therefore, in recent years, numerous marine resources
have attracted attention as researchers search for bioactive
compounds to develop new cosmetics, drugs and health food. In
particular, some efforts have sought to develop new depigmentation
agents using biologically active compounds obtained from marine
organisms (14). Among the marine
organisms, the starfish has gradually increased in fish or
shellfish farms and can cause serious damage to fish, shellfish,
ark shell, abalone, little clam, scallop, that inhabit farms of
coastal area (15). It is known
that a starfish has very strong reproduction-power, i.e., cutting
part of the body as well as the whole body can develop into new
starfish. Starfish grow in the sea to the east of Korea; they are
known to cause serious damage to shellfish farms, and are known as
Asterias amurensis and Asterina pectinifera (A.
pectinifera) (16). Some
crude extracts of Acanthaster planci, Asterias
forbesi and A. pectinifera among starfish are active
against influenza B virus in embryonated chicks. It was reported
that some extracts obtained from A. pectinifera had
antimicrobial and anticancer activities (17) and also exhibited antimicrobial
capacities against B. subtilis and S. aureus
(18,19). Moreover, we previously showed that
the methanolic extract from A. pectinifera had strong
anti-inflammatory activity (20).
However, little is known about the inhibitory effects of starfish
extracts on melanin synthesis via tyrosinase activity.
In this study, we found the candidate fraction which
contained potential bioactive compounds such as tyrosinase
inhibitor from A. pectinifera extracts and investigated the
inhibitory activity of the candidate fraction on melanin
biosynthesis through tyrosinase activity in melan-a cells.
Materials and methods
Chemicals and reagents
L-Tyrosine, L-DOPA, mushroom tyrosinase, phorbol
12-myristate 13-acetate (TPA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
ascorbic acid, arbutin and other chemical reagents were purchased
from Sigma (St. Louis, MO, USA). RPMI-1640, penicillin/streptomycin
solution and trypsin were obtained from Gibco (Gaithersburg, MD,
USA).
Preparation of A. pectinifera
extracts
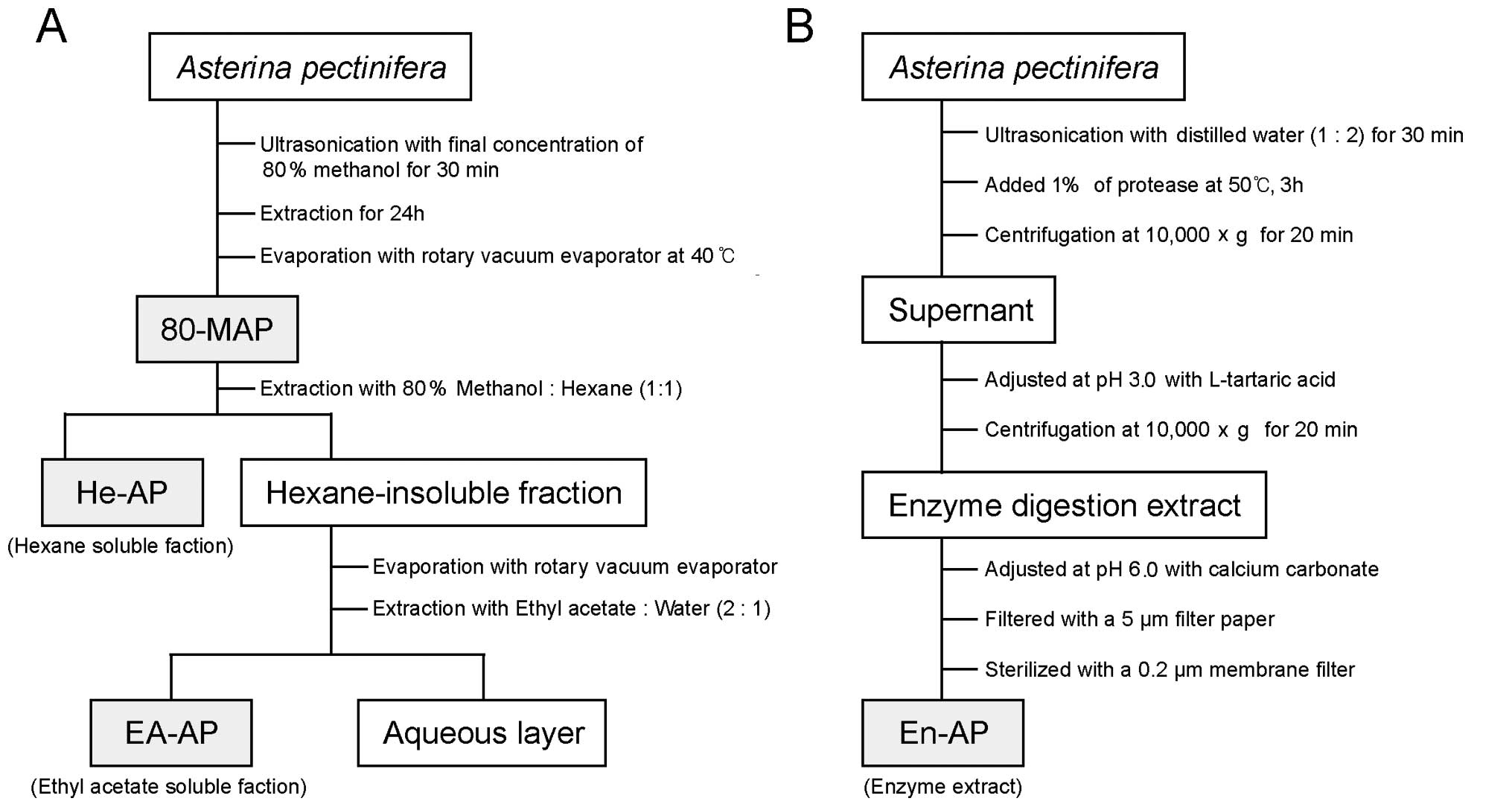
For the 80% methanolic extract (80-MAP), the
powdered A. pectinifera (100 g d.w.) was first soaked with
80% methanol at room temperature and was ultrasonicated (Sonic
Dismembrator; Fisher Scientific Inc., Pittsburgh, PA, USA) for 30
min. Then, it was incubated at room temperature for 24 h and
methanolic extracts were filtered by Whatman filter paper (Whatman
Lab Sales Ltd., UK) (particle retention, 20–25 μm) and the
filtrates were made into powder by the vacuum rotary evaporator
(Tokyo Rikakikai Co., Ltd., Tokyo, Japan) at 40°C (Fig. 1A). To give a hexane-soluble
fraction (He-AP), hexane (n-C6H14, 1:1
ratio, w/v) was added into 80-MAP for 24 h at room temperature
(Fig. 1A). The hexane-insoluble
residue was partitioned between ethyl acetate-water (2:1 ratio,
w/v) to give an ethyl acetate soluble fraction (EA-AP) and a water
soluble fraction. The ethyl acetate fraction was dried in a rotary
evaporator at 40°C to yield a dry extract. The 80-MAP, He-AP and
EA-AP were dissolved in dimethyl sulphoxide (DMSO; Sigma). The
enzyme extract (En-AP) of A. pectinifera was treated with
protease (Protamex™; Novo Nordisk Co., Bagsvaerd, Denmark) in a
dried powder of A. pectinifera. The powdered A.
pectinifera (100 g d.w.) was ultrasonicated with distilled
water (1:2 ratio, w/v) for 30 min and was supplemented with 1% of
protease (to dried weight of sample). The enzyme reactant was
incubated at 50°C for 3 h and centrifuged at 10,000 × g for 20 min.
The supernatant was adjusted at pH 3.0 with L-tartaric acid until
the supernant was a transparent solution. Following centrifugation
at 10,000 × g for 20 min, the supernant was readjusted to pH 6.0
with calcium carbonate. The sample was filtered with Whatman filter
paper (particle retention, 5 μm) and was then filtered with 0.2 μm
membrane filter (Advantec MFS, Inc., Dublin, CA, USA) (Fig. 1B). The stock solutions were
diluted appropriately with buffer or media at the time of testing
and the final concentration of DMSO in test wells was 1% for
cell-free assay and 0.1% for cell-based assay.
Mushroom tyrosinase assay
In vitro mushroom tyrosinase assay was
performed with L-tyrosine and L-DOPA as substrate for tyrosinase
activity. Inhibitory activity of each extract against tyrosinase
catalysed oxidation of L-tyrosine was determined according to the
methods of Chang et al (21) in the presence of each crude
extract of A. pectinifera. A volume of 40 μl of 1.5 mM
substrate (L-tyrosine) dissolved in 0.1 M phosphate buffer (pH 6.8)
and 120 μl of 0.1 M phosphate buffer, were mixed with 20 μl of
different concentrations from each extracted sample (80-MAP, He-AP,
EA-AP and En-AP). Then, 20 μl of mushroom tyrosinase (2,000 U/ml in
phosphate buffer) were added to initiate the reaction. The assay
mixture was incubated at 37°C for 15 min. The increase in
absorbance at 475 nm caused by the formation of dopachrome was
monitored using a microplate reader (Opsys MR; Dynex Technologies,
Ltd., Frankfurt, Germany). The inhibitory effect of each extract on
mushroom tyrosinase in L-DOPA oxidation was determined according to
Masamoto et al (22) with
some modifications. A volume of 100 μl of 0.1 M phosphate buffer
was mixed with 20 μl of different concentrations from each
extracted sample (80-MAP, He-AP, EA-AP and En-AP). Then, 20 μl of
mushroom tyrosinase (2,000 U/ml in phosphate buffer) were added to
initiate the reaction. The mixture was incubated at 37°C for 5 min
and added to 40 μl of L-DOPA (4 mM in 0.1 M phosphate buffer). The
mixture was incubated for 10 min at 37°C and the absorbance at 475
nm of the reaction mixture was recorded. Ascorbic acid (500 μg/ml)
as a positive control was used for assay. The percentage inhibition
of tyrosine or L-DOPA oxidation was calculated as follows: %
inhibition = 100 - (B/A × 100), where A = ΔOD475 in 10
min without sample, and B = ΔOD475 in 10 min with tested
sample.
Cell cultures and treatment
Murine melan-a melanocytes were originally derived
from C57BL/6 J (black, a/a) mice and were obtained from David
Kallenberg (St. George’s University of London, UK). Melan-a cells
were cultured in RPMI-1640 medium containing 10% heat-inactivated
FBS, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 200 nM
of phorbol 12-myristate 13-acetate (TPA) at 37°C in 10%
CO2. The culture medium was changed every 2 days. The
cells were harvested by trypsinization when they were approximately
70% confluent, counted with a haemocytometer and seeded at the
appropriate numbers into wells of cell culture plates for further
experiments.
Cell viability assay
The number of viable cells was determined by the
ability of mitochondria to convert MTT to formazan dye. Melan-a
cells were cultured overnight in 96-well plates, at a density of
2×104 cells/200 μl in each well. The next day, the cells
were coincubated with various concentrations of EA-AP and En-AP
from A. pectinifera for 24 h. Following incubation, the
medium was removed and the cells were supplemented with 10 μl of 10
mg/ml MTT into each well. Following incubation for another 4 h at
37°C in a humidified 10% CO2 atmosphere, the MTT was
removed, and cells were lysed with 150 μl DMSO. The absorbance was
measured at 550 nm using a microplate reader.
Measurement of melanin content
Determination of the amount of melanin content was
performed using a modified method of Hosoi et al (23). Briefly, melan-a cells were seeded
onto a 24-well plate at a density of 1×105 cells/well.
Following overnight incubation, the medium was replaced with a
medium containing EA-AP and En-AP at different concentrations and
incubated for a further 72 h. Arbutin (250 μg/ml) as a positive
control was used for the assay. The medium was then removed, the
cells washed twice with phosphate-buffered saline (PBS) and
harvested by trypsinization using 0.25% trypsin/0.02% EDTA in PBS.
The harvested cells were pelleted and solubilized in 1 N NaOH.
After centrifugation at 3,000 × g for 10 min, the optical density
at 450 nm of the resulting supernatant was measured by a microplate
reader. The melanin contents per well were calculated, and were
expressed as a percentage of the control.
Cellular tyrosinase assay
Cellular tyrosinase activity was measured using the
method of Pomerantz (24) with a
slight modification. Cells were pretreated with TPA 200 nM for 72 h
and harvested. The cells were then washed with sodium PBS (pH 6.8)
and lysed with M-PER mammalian protein extraction reagent (Pierce,
Rockford, IL, USA). The lysates were then clarified by
centrifugation at 13,000 × g for 15 min at 4°C. The, protein
concentration was determined by the Bradford method (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using bovine serum albumin
(BSA, St. Louis, MO, USA) as the standard. These proteins were used
as a tyrosinase source. A volume of 100 μl of 0.1 M phosphate
buffer was mixed with 20 μl of different concentrations from EA-AP
and En-AP. Then, 20 μl of the reaction mixture consisting of 40 μg
protein (adjusted to 100 μl with 0.1 M PBS, pH 6.8) was added to
initiate the reaction. The mixture was incubated at 37°C for 5 min
and added to 40 μl of L-DOPA (4 mM in 0.1 M phosphate buffer). The
mixture was incubated for 10 min at 37°C and the absorbance at 475
nm of the reaction mixture was recorded. Ascorbic acid (500 μg/ml)
as a positive control was used for assay. The percentage inhibition
of tyrosine or L-DOPA oxidation was calculated as follows: %
inhibition = 100 - (B/A × 100), where A = ΔOD475 in 10
min without sample, and B = ΔOD475 in 10 min with tested
sample.
RNA isolation and real-time PCR
Melan-a cells (1×105 cells/ml) were
plated on 100 mm culture dishes and incubated in the presence of
TPA 200 nM. Then, the cells were treated with various
concentrations of EA-AP and En-AP for 24 h. Arbutin (250 μg/ml) as
a positive control was used for the assay. The cells were harvested
and washed twice with ice-cold PBS. Total cellular RNA was prepared
using TRIzol solution (Invitrogen, Paisley, UK) according to the
manufacturer’s instructions. RNA was then precipitated with
isopropanol and dissolved in diethylpyrocarbonate-treated distilled
water. First-strand cDNA was generated with the oligo(dT) adaptor
primers by reverse transcriptase (Takara Bio, Inc., Otsu, Japan).
Each specific primer (accession no. of tyrosinase, Mm.238127;
accession no. of TRP-1, Mm.30438; accession no. of Dct, Mm.19987;
and accession no. of GADPH, Mm.304088) was designed using primer
express software from TaqManR Gene expression array
(Applied Biosystems, Carlsbad, CA, USA). GAPDH was used as the
invariant control. The real-time PCR reaction (10 μl) contained 10
ng of reverse transcribed RNA, 200 nM each of forward and reverse
primers, and a PCR master mixture. The reaction was performed using
the CFX96 real time system (Bio-Rad, Hercules, CA, USA). All
reactions were conducted in triplicate.
Immunoblotting
Melan-a cells (1×105 cells/ml) were
plated on 100 mm culture dishes and incubated in the presence of
TPA 200 nM. The cells were treated with various concentrations of
EA-AP and En-AP for 72 h. Arbutin (250 μg/ml) as a positive control
was used for the assay. The cells were harvested and washed twice
with ice-cold PBS. Then, the cells were resuspended in 200 μl
ice-cold solubilizing buffer (300 mM NaCl, 50 mM Tris-HCl, pH 7.6,
0.5% Triton X-100, 1 ml protease inhibitor cocktail) and incubated
at 4°C for 40 min. The lysates were centrifuged at 14,000 × g for
20 min. Protein concentrations of cell lysates were determined by
the Bradford method. Equal amounts of protein were subjected to
7.5–15% SDS-PAGE for tyrosinase, TRP-1, Dct and β-actin (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), respectively, and
transferred to a nitrocellulose membrane. Immunostaining with
antibodies was performed using Super-Signal West Pico enhanced
chemiluminescence substrate and detected with LAS-3000PLUS (Fuji
Photo Film Co., Kanagawa, Japan).
Statistical analysis
The data are expressed as the means ± standard
deviation (SD). The evaluation of statistical significance was
performed using Student’s t-test or one-way analysis of variance
(ANOVA) using the Statistical Package for the Social Sciences
(SPSS) statistical software for Windows, version 18.0 (SPSS,
Chicago, IL, USA). P<0.05 was considered to indicate
statistically significant differences.
Results
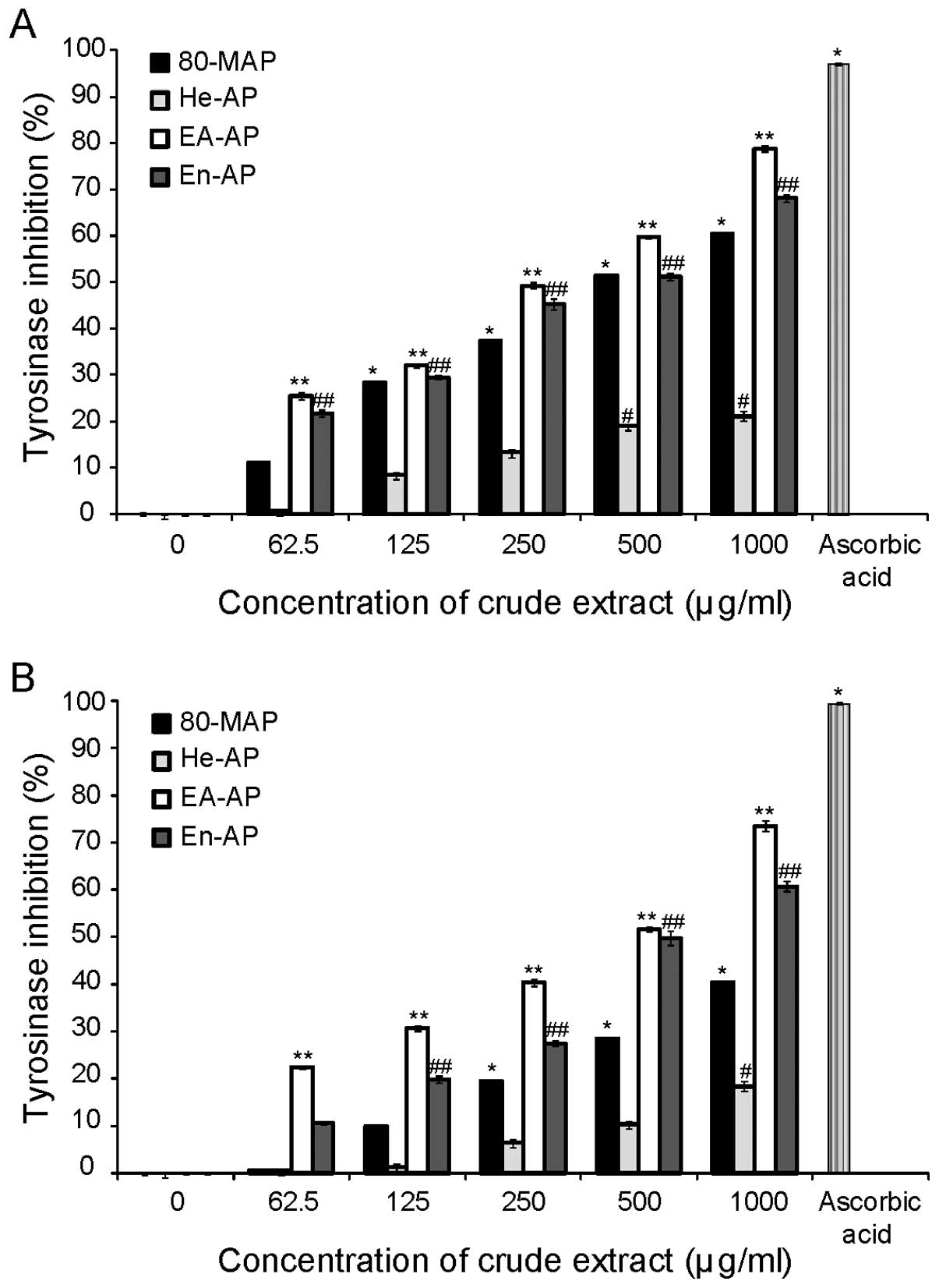
A. pectinifera extracts inhibit mushroom
tyrosinase activity
To investigate whether A. pectinifera
extracts showed any direct inhibitory effect against the key enzyme
in the whole melanogenesis, in vitro cell-free mushroom
tyrosinase assay was carried out. The effects of each extract
(80-MAP, He-AP, EA-AP and En-AP) on mushroom tyrosinase activity
are shown in Fig. 2. We observed
the inhibitory effect of all extracts on the oxidation of tyrosine
and L-DOPA by mushroom tyrosinase in a dose-dependent manner.
However, 80-MAP and He-AP showed less inhibitory activity of
mushroom tyrosinase than EA-AP or En-AP. A positive control,
ascorbic acid, showed strong tyrosinase inhibition, as expected.
The EA-AP and En-AP showed less inhibitory activity of mushroom
tyrosinase than ascorbic acid at the maximum concentration. In
addition, the IC50 of EA-AP and En-AP was 250 and 500
μg/ml for L-tyrosine and 500 μg/ml for L-DOPA, respectively
(Table I). Ascorbic acid
substantially inhibited the enzyme activity with an IC50
value of 63 μg/ml for L-tyrosine and 175 μg/ml for L-DOPA (Table I) and also showed inhibitory
activity >95% at 500 μg/ml when compared to untreated control in
L-tyrosine and L-DOPA. Taken together, our results demonstrated
that EA-AP and En-AP among A. pectinifera extracts have the
highest inhibitory effect on mushroom tyrosinase activity.
 | Table IThe inhibitory activity
(IC50) of mushroom tyrosinase on Asterina
pectinifera extracts in mushroom tyrosinase. |
Table I
The inhibitory activity
(IC50) of mushroom tyrosinase on Asterina
pectinifera extracts in mushroom tyrosinase.
| Extract | Tyrosinase
inhibition L-tyrosine (μg/ml) | Tyrosinase
inhibition L-DOPA (μg/ml) |
|---|
| 80-MAP | 500 | >1,000 |
| He-AP | >1,000 | >1,000 |
| EA- AP | 250 | 500 |
| En- AP | 500 | 500 |
| Ascorbic acid | 63 | 175 |
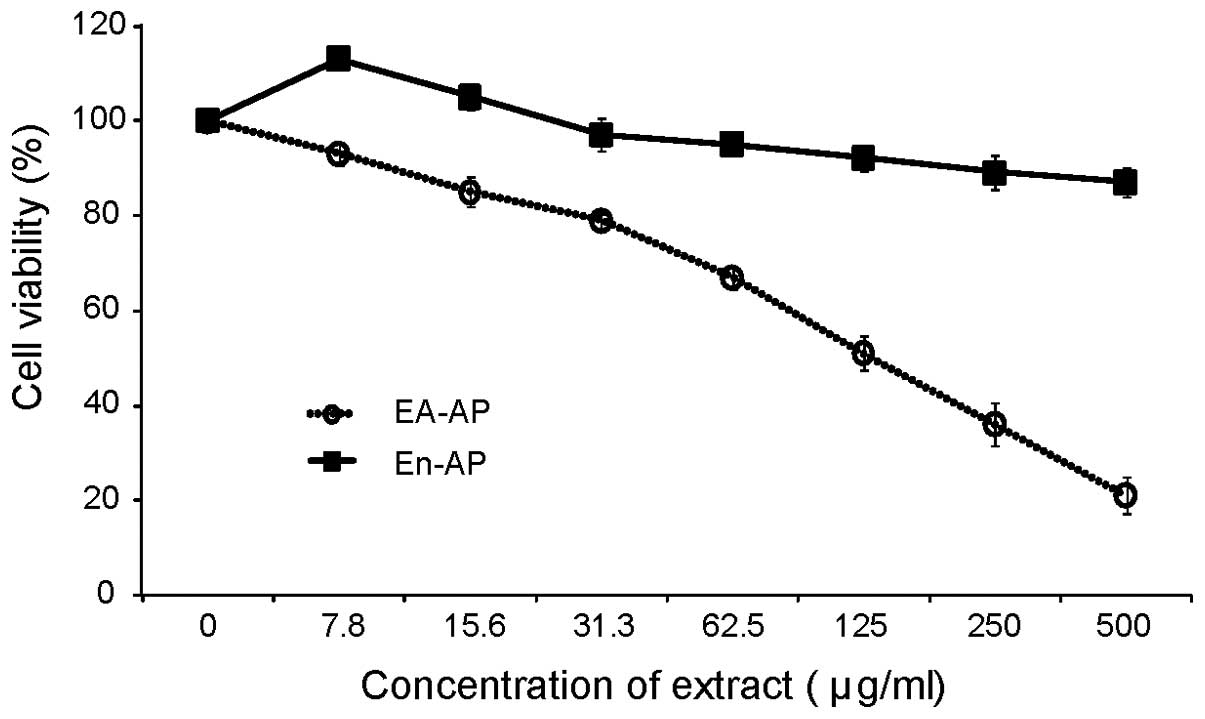
EA-AP and En-AP induce cell cytotoxicity
in melan-a cells
Based on the results of the enzymatic assay, we
determined EA-AP and En-AP among A. pectinifera extracts for
effective anti-melanogenic activity. Thus, to exclude the
possibility that inhibitory effects of EA-AP and En-AP on
melanogenesis might be caused by the inhibition of melan-a cell
growth, we compared the number of cell growth in the presence and
absence of these extracts. We observed the cell viability with MTT
assay after treatment of EA-AP and En-AP with different
concentrations. After treatment, 50% of cell viability presenting
concentration (IC50 values) of EA-AP was 125 μg/ml while
the IC50 of En-AP was over 500 μg/ml at tested maximum
concentrations (Fig. 3 and
Table II). A positive control,
the IC50 of arbutin, was 1,041 μg/ml and it induced no
cytotoxicity at below 500 μg/ml (data not shown). These results
showed that EA-AP induced more cell cytotoxicity than En-AP.
Therefore, in this study, we tested further experiments such as
melanin synthesis, cellular tyrosinase activity, related gene and
protein expression in melan-a cells at concentrations below
IC50 of EA-AP and En-AP.
 | Table IIEffects of EA- AP and En- AP on
melanin production and cellular tyrosinase activity of melan-a
cells. |
Table II
Effects of EA- AP and En- AP on
melanin production and cellular tyrosinase activity of melan-a
cells.
| Extract | Cytotoxicity
IC50 (μg/ml)a | Melanin synthesis
IC50 (μg/ml)b | Cellular tyrosinase
activity IC50 (μg/ml)b |
|---|
| EA-AP | 125 | 31.3 | 31.3 |
| En-AP | >500 | 250 | 250 |
| Arbutin | 1,041 | 63 | - |
| Ascorbic acid | - | - | 250 |
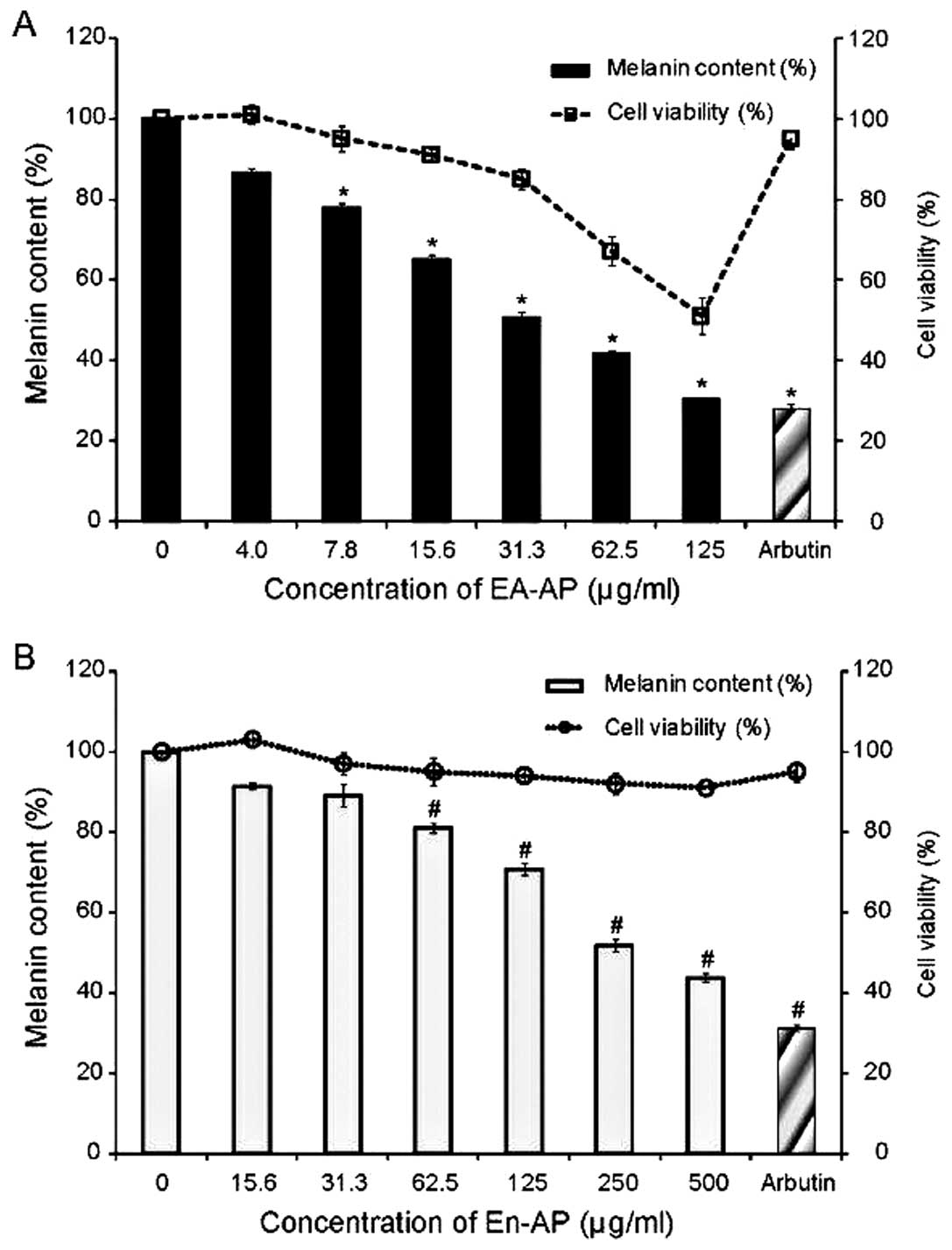
EA-AP and En-AP reduce melanin synthesis
and cellular tyrosinase activity in melan-a cells
To evaluate the inhibitory effect of EA-AP and En-AP
on melanin content and cellular tyrosinase activity, melan-a cells
were treated with different concentrations of these extracts. The
inhibitory effect of EA-AP and En-AP on melanin content is shown in
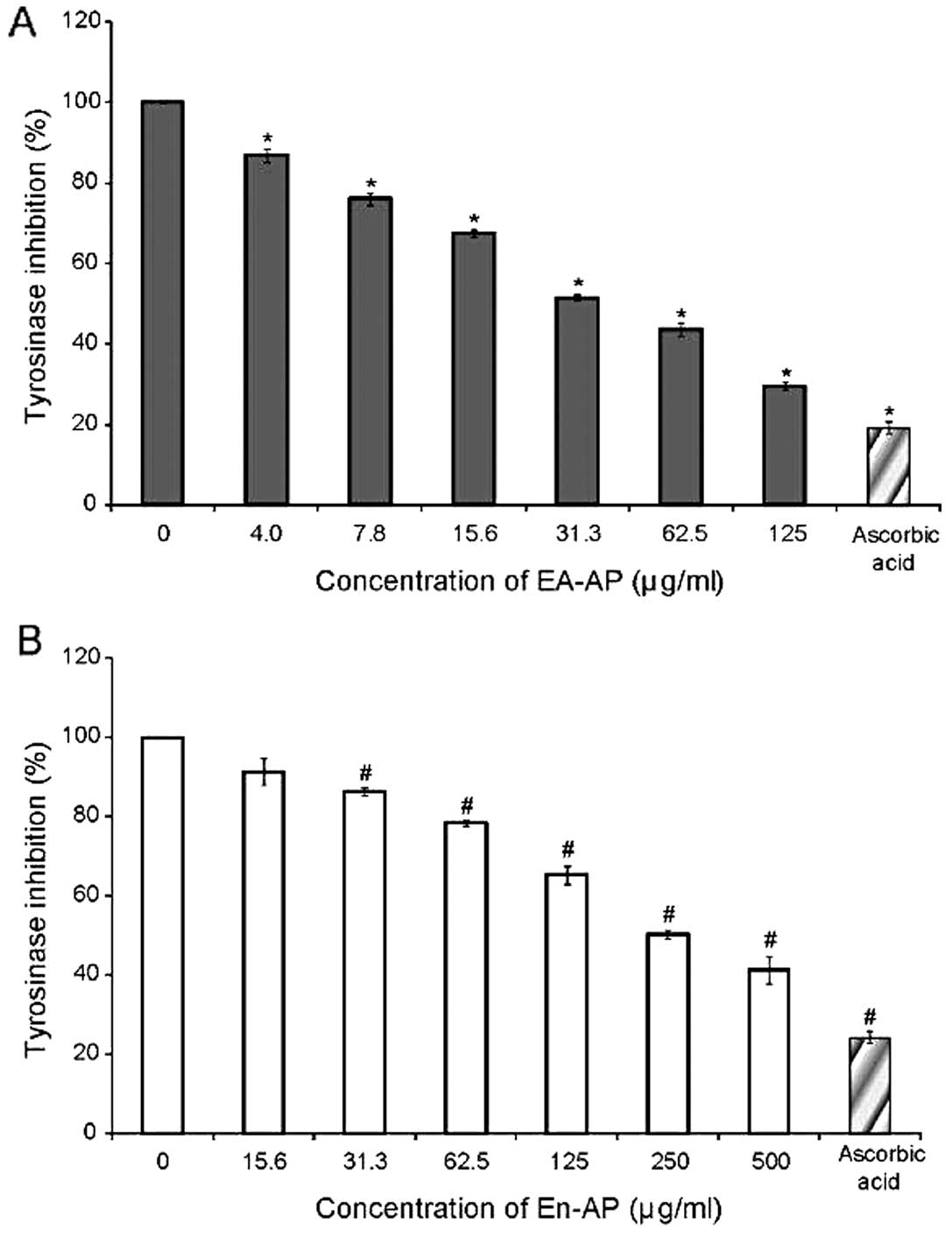
Fig. 4. The levels of melanin in
the melan-a cells were reduced significantly as a result of the
EA-AP (Fig. 4A) and En-AP
treatment (Fig. 4B) when compared
to the TPA-treated control group. In addition, to measure cellular
tyrosinase activity, instead of using mushroom tyrosinase, cell
lysates prepared from the melan-a cells treated with TPA were used
as a tyrosinase source. The EA-AP and En-AP showed an inhibitory
effect on cellular tyrosinase activity in a dose-dependent manner
(Fig. 5). The 50% inhibitory
concentration of melanin secretion and cellular tyrosinase activity
in melan-a cells was approximately 31.3 μg/ml of EA-AP and 250
μg/ml of En-AP (Table II).
Moreover, the effective concentration of En-AP was only slightly
cytotoxic to melan-a cells while EA-AP induced cytotoxicity in a
dose-dependent manner. Arbutin and ascorbic acid as a positive
control showed a significant reduction of the melanin secretion and
cellular tyrosinase activity (P<0.05) (Figs. 4 and 5), respectively. These results suggest
that the inhibitory effect of EA-AP and En-AP on melanin synthesis
appear to be well-correlated with the measurement of mushroom
tyrosinase activity and cellular tyrosinase activity.
EA-AP and En-AP suppress
melanogenesis-related gene and protein expression
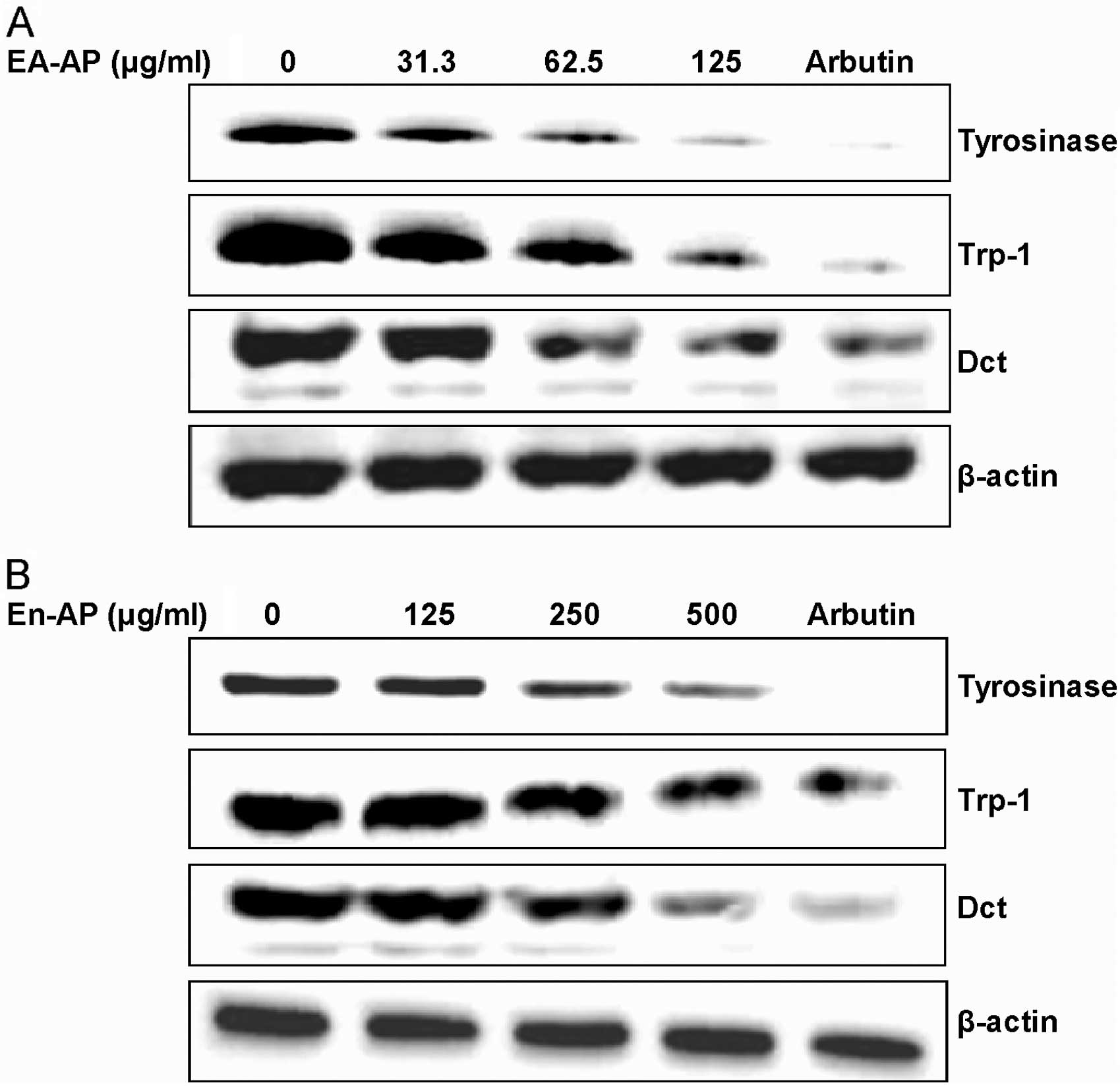
To investigate whether EA-AP and En-AP affect the
expression of melanogenesis-related proteins including tyrosinase,
TRP-1 and Dct, these proteins levels were examined in melan-a cells
in the presence of TPA using western blot analysis after treating
with various concentrations of EA-AP and En-AP for 72 h. Levels of
melanogenesis-related protein, tyrosinase, TRP-1 and Dct were
reduced after EA-AP (Fig. 6A) and
En-AP (Fig. 6B) treatment in a
dose-dependent manner. To examine whether the inhibition of
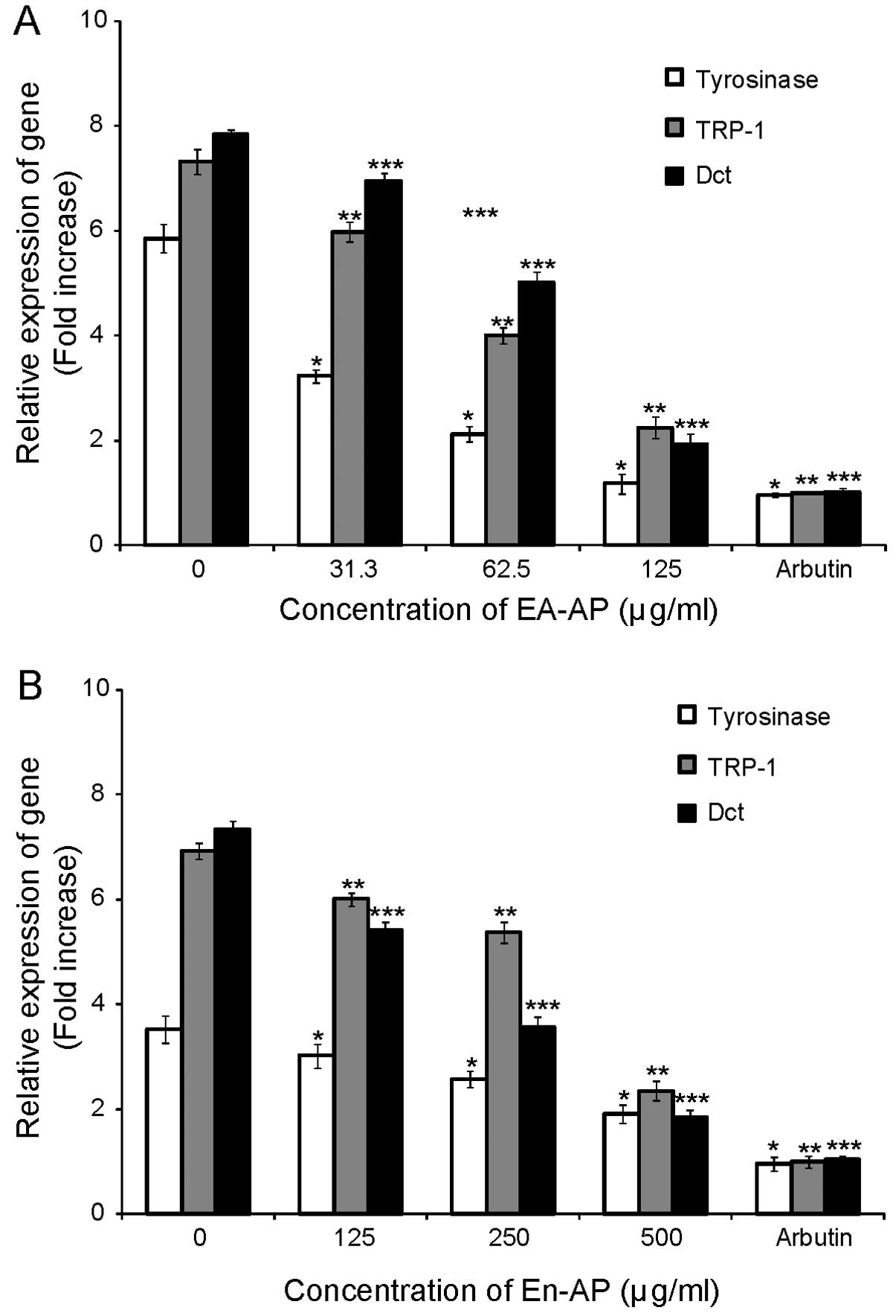
tyrosinase-related protein expression by EA-AP and En-AP was due to
decreased level transcription, we tested real time PCR using
specific primers. The mRNA level of tyrosinase, TRP-1 and Dct was
significantly decreased by treatment with EA-AP (P<0.05)
(Fig. 7A) and En-AP (P<0.05)
(Fig. 7B). Arbutin as a positive
control showed a significant reduction of gene and protein levels
of tyrosinase, TRP-1 and Dct in a dose-dependent manner while only
TPA-treated cells markedly increased gene and protein levels in
tyrosinase, TRP-1 and Dct (Figs.
6 and 7). These results
indicate that the suppressive activity of EA-AP and En-AP on
melanogenesis is linked to the downregulation of tyrosinase
expression signaling pathways.
Discussion
In Asian countries, women are concerned with skin
whitening as having whiter skin is often seen to be a superior
standard of beauty (25). As a
number of women worry about skin pigmentation, effective agents for
the improvement of hyperpigmentation have been researched for skin
whitening products (26). These
foregoing attributes prompted the present hypothesis that a marine
natural product might be valuable as a cosmetic component to
improve the appearance of hyperpigmentation. We intended in this
study to find new whitening materials from A. pectinifera, a
marine organism that would also have significance insofar as we
would obtain bioactive materials by using starfish which would be
discarded after collection from the sea. Therefore, we investigated
the potential whitening effect of A. pectinifera extracts
(80-MAP, He-AP, EA-AP and En-AP) and also demonstrated the effect
of each extract on melanin biosynthesis through tyrosinase activity
which is a standard model for assessing regulators of melanogenesis
since tyrosinase is the key enzyme in melanogenesis, initiating a
cascade of reactions which convert tyrosine to the biopolimer
melanin (27).
Tyrosinase (polyphenol oxidase) plays rate-limiting
roles in the production of melanin by melanocytes. Melanin pigment,
responsible for visible skin color, is formed through a series of
oxidative reactions involving the amino acid, tyrosine. Tyrosinase
catalyses three main steps in melanogenesis; the hydroxylation of
L-tyrosine to L-dihydroxyphenylalanine (L-DOPA), the oxidation of
L-DOPA to dopaquinone, and the additional oxidation of
5,6-dihydroxyindole to indol-quinone (28). Almost all factors affecting
melanin production exert their action either directly or indirectly
via stimulation of tyrosinase and the most common target for
skin-lightening activities is tyrosinase inhibition (29). Indeed, recently, a great deal of
interest has been on deriving tyrosinase inhibitors from plant
origin and anti-melanogenesis activities of several herbal
medicines have been evaluated by their ability to inhibit
tyrosinase (30).
In this study, several fractions of powdered dried
A. pectinifera were extracted with solvents of different
polarity and tested for their possible anti-melanogenesis or
skin-whitening properties using the inhibition of mushroom
tyrosinase activity in a cell-free system as screening assays. Our
data revealed that the methanolic extract (80-MAP) and enzymatic
extract (En-AP) inhibited mushroom tyrosinase activity among A.
pectinifera extracts and particularly the separated ethyl
acetate fraction (EA-AP) from 80-MAP had a strong suppression of
tyrosinase activity. However, hexane fraction (He-AP) showed less
inhibitory activity than EA-AP (Fig.
2 and Table I). In addition,
EA-AP and En-AP inhibited cellular tyrosinase activity as well as
melanin production in melan-a cells (Figs. 4 and 5). These results suggest that EA-AP and
En-AP among A. pectinifera extracts may contain potential
skin-whitening materials that could induce reduction of
melanogenesis in melan-a cells by reduction of tyrosinase
activity.
Dooley (31)
previously speculated that a desirable skin-whitening agent should
inhibit melanin synthesis in melanosomes by acting specifically to
reduce the synthesis or activity of tyrosinase with little or no
cytotoxicity. In this study, En-AP appeared to have little
cytotoxic as well as anti-melanogenic activity among A.
pectinifera extracts while EA-AP induced some cytotoxicity
under TPA-stimulated melan-a cells (Fig. 3). Accordingly, EA-AP at higher
concentration levels (250 and 500 μg/ml) was not used further due
to its greater cytotoxicity on the melan-a cells. These data
demonstrate that En-AP contains safer materials for potential
skin-whitening activity than EA-AP due to the slight cytotoxic
effects of EA-AP at the higher concentrations. In mammals,
melanogenesis occurs in melanocytes after differentiation of the
nonpigmented precursors, melanoblasts. Three melanocyte-specific
enzymes, including tyrosinase, TRP-1 and Dct (TRP-2), are involved
in the enzymatic process that converts tyrosine to melanin pigments
(32). In particular, there are
two tyrosinase-related proteins, TRP-1 and Dct (TRP-2), which are
structurally related to tyrosinase and share approximately 40%
amino acid homology, suggesting that they originated from a common
ancestral gene (33). As TRP-1
forms a complex with tyrosinase, it is possible that TRP-1 plays a
role in tyrosinase activation and/or stabilization (34). TRP-1 also plays a role in
melanosomal biogenesis, as suppression of TRP-1 expression is
associated with structurally abnormal melanosomes (35). TRP-2 complexes with tyrosinase and
also with TRP-1 (36). TRP-2
converts DOPAchrome to the carboxylated derivative
dihydroxyindole-2-carboxylic acid (DHICA) during one of the later
stages of melanin biosynthesis (37). Indeed, TRP-1 and TRP-2 have been
demonstrated to increase tyrosinase stability (38) and are responsible for the
induction of melanin synthesis (29,39,40). Both EA-AP and En-AP reduced
melanogenic proteins, tyrosinase, TRP-1 and Dct (TRP-2) (Fig. 6), and also the inhibitory effects
of EA-AP and En-AP on melanogenesis likely resulted from suppressed
gene expression of tyrosinase, TRP-1 and Dct (TRP-2) (Fig. 7).
In conclusion, we suggest that EA-AP and En-AP from
A. pectinifera extracts inhibit melanin synthesis via
subsequent downregulation of tyrosinase-related proteins.
Therefore, EA-AP and En-AP may be useful candidates for the
development of potential therapeutic agents for hyperpigmentation
treatment, and may be an effective component in whitening and
lightening cosmetics. We will further separate single compounds
from EA-AP fraction of A. pectinifera and explore the
inhibition of melanin synthesis via MITF downregulation and related
signaling pathways.
Acknowledgements
We thank Dr Dorothy C. Bennett and Dr David
Kallenberg (St. George’s University of London) for their helpful
discussions and gifts of melan-a. This research was supported by
the 2012 National R&D Program through the Dongnam Institute of
Radiological and Medical Sciences (DIRAMS) funded by the Ministry
of Education, Science and Technology (50493-2012) and the
Technology Development Program for Agriculture and Forestry
(610003-03-1-SB110), Ministry for Food, Agriculture, Forestry and
Fisheries, Republic of Korea.
References
|
1
|
Pawelek JM, Chakraborty AK, Osber MP and
Bolognia JL: Ultraviolet light and pigmentation of the skin.
Cosmetics & Toiletries Magazine. 107:61–68. 1992.
|
|
2
|
Briganti S, Camera E and Picardo M:
Chemical and instrumental approaches to treat hyperpigmentation.
Pigment Cell Res. 16:101–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kobayashi T, Urabe K, Winder A, et al:
Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in
melanin biosynthesis. EMBO J. 13:5818–5825. 1994.PubMed/NCBI
|
|
4
|
del Marmol V and Beermann F: Tyrosinase
and related proteins in mammalian pigmentation. FEBS Lett.
381:165–168. 1996.PubMed/NCBI
|
|
5
|
Lerner AB, Fitzpatrick TB, Calkins E and
Summerson WH: Mammalian tyrosinase; preparation and properties. J
Biol Chem. 178:185–195. 1949.PubMed/NCBI
|
|
6
|
Slominski A, Tobin DJ, Shibahara S and
Wortsman J: Melanin pigmentation in mammalian skin and its hormonal
regulation. Physiol Rev. 84:1155–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones K, Hughes J, Hong M, Jia Q and
Orndorff S: Modulation of melanogenesis by aloesin: a competitive
inhibitor of tyrosinase. Pigment Cell Res. 15:335–340. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maeda K and Fukuda M: In vitro
effectiveness of several whitening cosmetic components in human
Melanocyte. J Soc Cosmet Chem. 4:361–368. 1991.
|
|
9
|
Shiino M, Watanabe Y and Umezawa K:
Synthesis of N-substituted N-nitrosohydroxylamines as inhibitors of
mashroom tyrosinase. Bioorg Med Chem. 9:1233–1240. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimogaki H, Tanaka Y, Tamai H and Masuda
M: In vitro and in vivo evaluation of ellagic acid on melanogenesis
inhibition. Int J Cosmetic Sci. 22:291–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim DS, Kim SY, Chung JH, Kim KH, Eun HC
and Park KC: Delayed ERK activation by ceramide reduces melanin
synthesis in human melanocytes. Cell Signal. 14:779–785. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimizu K, Kondo R, Sakai K, Lee SH and
Sato H: The inhibitory components from Artocarpus incisus on
melanin biosynthesis. Planta Med. 64:408–412. 1998. View Article : Google Scholar
|
|
13
|
Komthong P, Igura N and Shimoda M: Effect
of ascorbic acid on the odours of cloudy apple juice. Food Chem.
100:1342–1349. 2007. View Article : Google Scholar
|
|
14
|
Cha SH, Ko SC, Kim D and Jeon YJ:
Screening of marine algae for potential tyrosinase inhibitor: those
inhibitors reduced tyrosinase activity and melanin synthesis in
zebrafish. J Dermatol. 38:354–363. 2010. View Article : Google Scholar
|
|
15
|
Park MS and Kim BY: Feeding behaviour of
the starfish, Asterias amurensis (LUTKEN). Bull Fish Res Dev
Agency. 34:1–174. 1985.
|
|
16
|
Park SW, Kim TH and Oh HK: A study on the
development of the extermination gear for starfish, Asterias
amurensis and its efficiency. J Korean Soc Fish Technol.
33:166–172. 1997.
|
|
17
|
Seo JK: Conformation and biological
activity of Mastoparan B and its analogs: isolation and
characterization of the biological substances from Inshore Hagfish
(Eptatretus burgeri) skin and starfish (Asterina
pectinifera). Thesis. Pukyong National University; 1997
|
|
18
|
Cho SY, Kang HJ, Joo DS, Jeon JK, Oh MH
and Kim JS: Seperation and identification of nature antimicrobial
agent from starfish. In: 47th Conference of Korean Society; J Food
Science Nutr. pp. 695–701. 2000
|
|
19
|
Cho SY, You BJ, Chang MH, Lee SJ, Sung NJ
and Lee EH: Screening for antimicrobial compounds in unused marine
resources by the paper disk method. Korean J Food Sci Technol.
26:261–265. 1994.(In Korean).
|
|
20
|
Jo WS, Choi YJ, Kim HJ, Nam BH, Lee GA,
Seo SY, Lee SW and Jeong MH: Methanolic extract of Asterina
pectinifera inhibits LPS-induced inflammatory mediators in
murine macrophage. Toxicol Res. 6:1–82. 2010.
|
|
21
|
Chang TS, Ding HY, Tai SSK and Wu CY:
Mushroom tyrosinase inhibitory effects of isoflavones isolated from
soygerm koji fermented with Aspergillus oryzae BCRC 32288.
Food Chem. 105:1430–1438. 2007. View Article : Google Scholar
|
|
22
|
Masamoto Y, Ando H, Murata Y, Shimoishi
YTM and Takahata K: Mushroom tyrosinase inhibitory activity of
esculetin isolated from seeds of Euphorbia lathyris L. Biosci
Biotechnol Biochem. 67:631–634. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hosoi J, Abe E, Suda T and Kuroki T:
Regulation of melanin synthesis of B16 mouse melanoma cells by 1α,
25-dihydroxyvitamin D3 and retinoic acid. Cancer Res.
45:1474–1478. 1985.
|
|
24
|
Pomerantz HS: Tyrosine hydroxylation
catalyzed by mammalian tyrosinase: an improved method of assay.
Biochem Biophys Res Commun. 16:188–194. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li EPH, Min HJ, Belk RW, Kimura J and Bahl
S: Skin lightening and beauty in four Asian cultures. Adv Consum
Res. 35:444–449. 2008.
|
|
26
|
Oh EY, Jang JY, Choi YH, Choi YW and Choi
BT: Inhibitory effects of 1-O-methyl-fructofuranose from
Schisandra chinensis fruit on melanogenesis in B16F0
melanoma cells. J Ethnopharmacol. 132:219–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Virador VM, Kobayashi N, Matsunaga J and
Hearing VJ: A standardized protocol for assessing regulators of
pigmentation. Anal Biochem. 270:207–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hearing VJ and Tsukamoto K: Enzymatic
control of pigmentation in mammals. FASEB J. 5:2902–2909.
1991.PubMed/NCBI
|
|
29
|
Busca R and Ballotti R: Cyclic AMP a key
messenger in the regulation of skin pigmentation. Pigment Cell Res.
13:60–69. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Momtaz S, Mapunya BM, Houghton PJ, Edgerly
C, Hussein A, Naidoo S and Lall N: Tyrosinase inhibition by
extracts and constituents of Sideroxylon inerme L. stem
bark, used in South Africa for skin lightening. J Ethnopharmacol.
119:507–517. 2008.PubMed/NCBI
|
|
31
|
Dooley TP: Topical skin depigmenting
agents: current products and discovery of novel inhibitors of
melanogenesis. J Dermatol Treat. 7:188–200. 1997.
|
|
32
|
Levy C, Khaled M and Fisher DE: MITF:
master regulator of melanocyte development and melanoma oncogene.
Trends Mol Med. 12:406–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sturm RA, O’ Sullivan BJ, Box NF, et al:
Chromosomal structure of the human TYRP1 and TYRP2 loci and
comparison of the tyrosinase-related protein gene family. Genomics.
29:24–34. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kobayashi T, Imokawa G, Bennett DC and
Hearing VJ: Tyrosinase stabilization by Tyrp1 (the brown locus
protein). J Biol Chem. 273:31801–31805. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Setaluri V: The melanosome: dark pigment
granule shines bright light on vesicle biogenesis and more. J
Invest Dermatol. 121:650–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu H and Park HY: Protein kinase
C-beta-mediated complex formation between tyrosinase and TRP-1.
Biochem Biophys Res Commun. 311:948–953. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pawelek JM and Chakraborty AK: The
enzymology of melanogenesis. The Pigmentary System: Physiology and
Pathophysiology. Nordlund JJ, Boissy RE, Hearing VJ, King RA and
Ortonne JP: Oxford University Press; New York: pp. 391–400.
1998
|
|
38
|
Manga P, Sato K, Ye L, Beermann F, Amoreux
ML and Orlow SJ: Mutational analysis of the modulation of
tyrosinase by tyrosinase-related proteins 1 and 2 in vitro. Pigment
Cell Res. 13:364–374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hamid MA, Sarmidi MR and Park CS:
Mangosteen leaf extract increases melanogenesis in B16F1 melanoma
cells by stimulating tyrosinase activity in vitro and by
up-regulating tyrosinase gene expression. Int J Mol Med.
29:209–217. 2012.PubMed/NCBI
|
|
40
|
Kim DS, Jeong YM, Park IK, et al: A new
2-imino-1,3-thiazoline derivative, KHG22394, inhibits melanin
synthesis in mouse B16 melanoma cells. Biol Pharm Bull. 30:180–183.
2007. View Article : Google Scholar : PubMed/NCBI
|