Introduction
Liver ischemia-reperfusion is a common problem in
many clinical conditions such as liver transplantation, hepatic
failure after shock, and liver surgery. Liver reperfusion injury
not only causes liver dysfunction, but also frequently induces
injury in extrahepatic organs, including the lung, the kidney and
the heart (1,2). Understanding the pathophysiological
process of liver reperfusion injury is clinically and
pathophysiologically important.
Ischemic preconditioning, which is defined as
multiple cycles of brief ischemia and reperfusion before a
prolonged ischemic insult, has been reported to exert protection in
several organs, resulting in increased tolerance toward organ
hypoxia. Ischemic preconditioning has been shown to attenuate the
tissue injury observed after reperfusion of the liver (3–5).
Several mediators, including adenosine, have been shown to play a
crucial role in the protective response of ischemic preconditioning
(3–6).
In the present study, we focused on adenosine and
its endogenous metabolic derivate, inosine, to observe their
effects in an in vitro model of liver I-R injury. For a long
time inosine was considered to be an inactive metabolite. However,
several studies have shown that it has immunomodulatory,
neuroprotective, cardioprotective and cytoprotective effects
(7–10). These effects have been attributed
to several independent mechanisms. First, inosine can bind to
A2A adenosine receptors activating several receptor
dependent intracellular signaling pathways (11,12). Second, previous studies showed in
kidney epithelial cells that inosine serves as an alternative
substrate for ATP generation during hypoxia (13,14). Third, inosine (but not adenosine)
can inhibit the activation of poly(ADP-ribose) polymerase enzyme
(PARP) preserving cells from a suicidal utilization of
NAD+ and ATP and, subsequently, cell death (15).
In this study, we evaluated the potential
cytoprotective effects of adenosine and inosine in a cell-based
model of liver I-R injury and pharmacologically characterized their
mode of action.
Materials and methods
Materials
Adenosine, inosine,
8-cyclopentyl-1,3-dipropylxanthine (CDPX), 8-(3-chlorostyryl)
caffeine (CSC), alloxazine, MRS 1523 and
erythro-9-(2-hydroxy-3-nonyl) adenine hydrochloride (EHNA) were
obtained from Sigma-Aldrich (St. Louis, MO, USA).
4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2,3-d]pyrimidine,
2HCl (ABT 702) was purchased from Calbiochem-Merck, Darmstadt,
Germany. The receptor antagonists and ABT 702 were dissolved in
dimethylsulfoxide (DMSO): dilutions were made in phosphate-buffered
saline (PBS, pH 7.4) to obtain a final 0.5% DMSO content in the
assay volume. EHNA was dissolved in distilled water. Adenosine and
inosine were dissolved in DMEM.
Cell culture
The human hepatocellular carcinoma-derived cell line
HepG2 was obtained from the European Collection of Cell Cultures
(Salisbury, UK) and maintained in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 4.5 g/l glucose and 10% fetal
bovine serum (Invitrogen, Carlsbad, CA, USA), 4 mM glutamine, 100
IU/ml penicillin and 100 μg/ml streptomycin. Five days prior
to the assay 10,000 cells/well were plated into 96-well tissue
culture plates and cultured at 37°C in a 5% CO2
atmosphere. Cells from passage numbers 9–25 were used for
subsequent assays.
In vitro liver ischemia-reperfusion
model
We developed a cell-based assay of liver
ischemia-reperfusion in HepG2 human liver epithelial cells. HepG2
cells were plated into 96-well tissue culture plates. Cells were
cultured for 5 days to form a confluent monolayer for the following
assay. Culture medium was replaced with DMEM containing no glucose
(Biochrom AG, Berlin, Germany) prior to induction of hypoxia.
Culture plates were placed in gas-tight incubation chambers
(Billups-Rothenberg Inc., Del Mar, CA, USA) and the chamber
atmosphere was replaced by flushing the chamber with 95%
N2: 5% CO2 mixture at 25 l/min flow rate for
5 min. The hypoxic chamber was sealed and incubated at 37°C for
various time periods. Following hypoxia, the culture medium was
removed and fresh DMEM containing 4.5 g/l glucose supplemented with
10% serum was added and the cells underwent re-oxygenation at 37°C
in a 5% CO2 atmosphere for various time periods
depending on the specific experimental protocol. Cells exposed to
hypoxia in complete culture medium served as controls (CTL), as no
reduction was detected in cell viability compared to cells
maintained in normal culture conditions (complete culture medium,
5% CO2 atmosphere, 37°C), if HepG2 cells were exposed to
oxygen depletion with culture medium containing 4.5 g/l glucose and
10% serum for 24 h.
Inosine and adenosine proved to be markedly
cytoprotective in our in vitro cell-based assay of liver I-R
injury. In various studies we tested different periods of hypoxia
(0-14-24 h) and subsequent re-oxygenation (0-4-24 h) in HepG2
cultures. Four groups were studied (n=24 for each group). The first
group received pretreatment with adenosine, while the second group
was pretreated with inosine prior to combined oxygen-glucose
deprivation (COGD) conditions (from 300–1,000 μM, applied 10
min before hypoxia). The third group (control) was subjected to
COGD with drug vehicle only. The fourth group was the negative
control group of the assay in which the cells were cultured in
glucose containing medium (4.5 g/l) during the entire assay period.
At the end of the experiments
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) viability and lactate dehydrogenase (LDH) cytotoxicity assays
were conducted as described below.
Pharmacological characterization of the
cytoprotective effects of adenosine and inosine in the in vitro
model of liver I-R
The concentration-dependence of the cytoprotective
effects was tested in HepG2 cells subjected to COGD with adenosine
or inosine pretreatment (1, 3, 10, 30, 100, 300, 1,000–3,000
μM, n=3 each). The effects of adenosine and inosine were
compared on the same 96-well tissue culture plate. A1
adenosine receptor antagonist CDPX (16,17), selective A2A antagonist
CSC (18,19), the selective A2B
adenosine receptor antagonist alloxazine (20), and the A3 adenosine
receptor antagonist MRS 1523 (21,22) were added 30 min prior to adenosine
or inosine (300 μM) in the indicated concentration prior to
COGD. The cytotoxicity of receptor antagonists was also tested
under normoxic conditions.
The adenosine deaminase inhibitor EHNA (23) and the adenosine kinase inhibitor
ABT 702 (24,25) were applied at 10 and 30 μM
prior to administering adenosine or inosine and COGD. The combined
administration of EHNA and ABT 702 was also investigated. In all
analyses, all relevant assay conditions (including adenosine
receptor antagonists assays, adenosine and inosine comparisons)
were represented on the same 96-well tissue culture plate to
prevent inter-plate assay variability. The experiments were
repeated independently at least three times.
MTT cell viability assay
To estimate the number of viable cells, MTT was
added to the cells at a final concentration of 0.5 mg/ml and
cultured at 37°C in a 5% CO2 atmosphere for 1 h
(26). The incubation medium was
removed and the converted formazan dye was dissolved in isopropanol
and measured at 570 nm with background measurement at 690 nm on a
PowerWave reader (BioTek Instruments,. Inc., Winooski, VT, USA). A
calibration curve was created by measuring the converting capacity
of MTT of serial dilutions of HepG2 cells. The viable cell count
was calculated using Gen5 data reduction software.
LDH cytotoxicity assay
Cell culture supernatant (30 μl) was mixed
with 100 μl freshly prepared LDH assay reagent to reach
final concentrations of 85 mM lactic acid, 1040 mM nicotinamide
adenine dinucleotide, 224 mM N-methylphenazonium methyl sulfate,
528 mM 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium
chloride and 200 mM Tris (pH 8.2). The changes in absorbance were
read kinetically at 492 nm for 15 min. LDH activity values are
shown as Vmax in mOD/min for kinetic assay (27).
Statistical analysis
Data are shown as the means ± SEM. One-way ANOVA was
used to detect differences between groups. Post hoc comparisons
were made using Tukey’s test. A value of P<0.05 was considered
to indicate statistically significant differences. All statistical
calculations were performed using GraphPad Prism 5 analysis
software.
Results
Characterization of an in vitro liver
ischemia-reperfusion model in HepG2 cells
To develop a reproducible in vitro liver
ischemia reperfusion model on HepG2 liver epithelial cells, we
tested different periods (12, 14 and 24 h) of COGD, followed by a
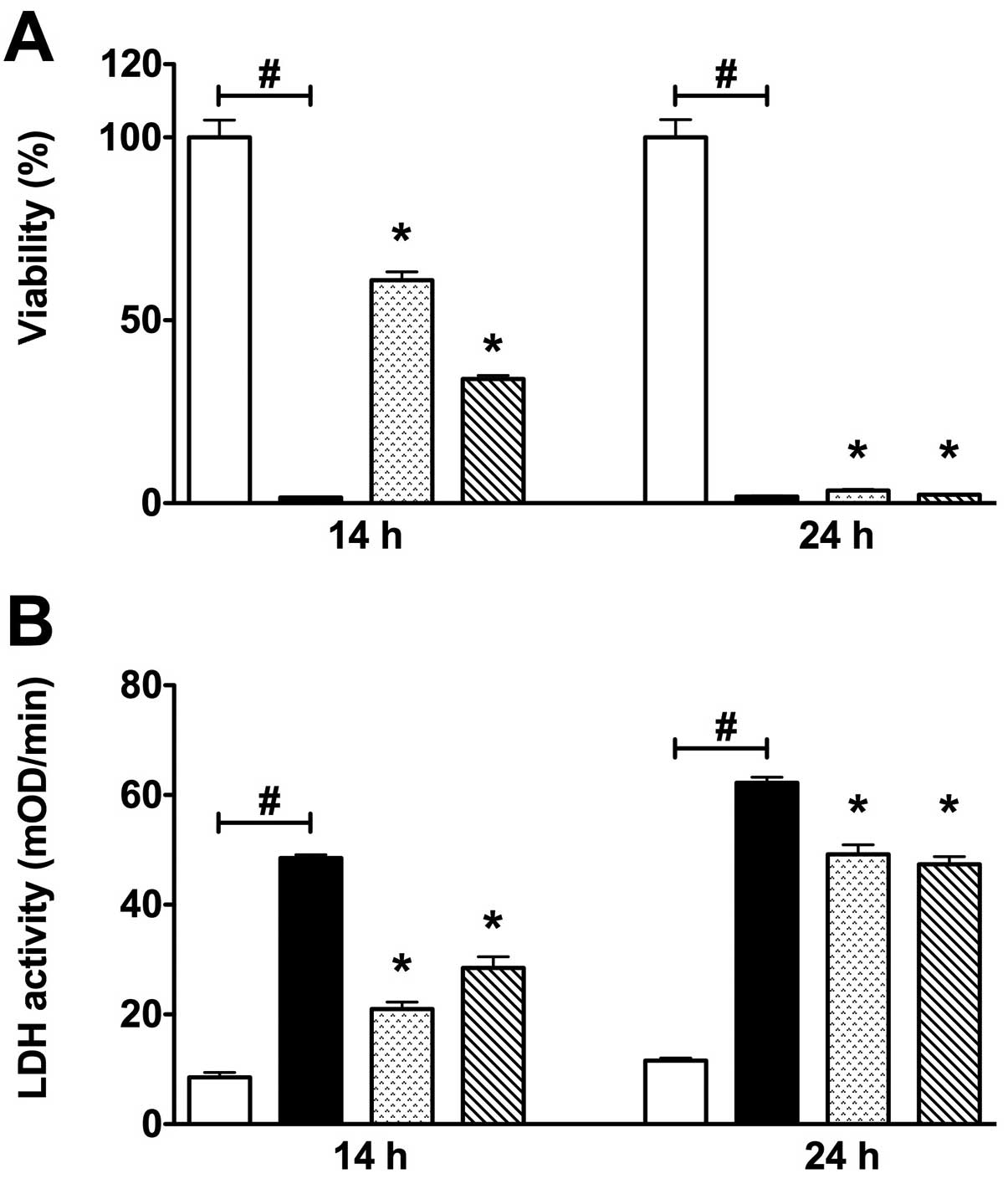
subsequent re-oxygenation period of 4 h (Fig. 1). Twelve hours of hypoxia combined
with 4 h of re-oxygenation did not induce a significant decline of
the cell viability (data not shown). However, 14 h of hypoxia
combined with 4 h re-oxygenation induced a significant decline of
the cell viability. Furthermore, 24 h of hypoxia followed by a 4 h
re-oxygenation period markedly reduced cellular viability in all
groups, as detected by MTT viability assay. Both 14 and 24 h of
COGD were associated with a significant elevation of LDH activity
detected in the cell culture supernatant (Fig. 1).
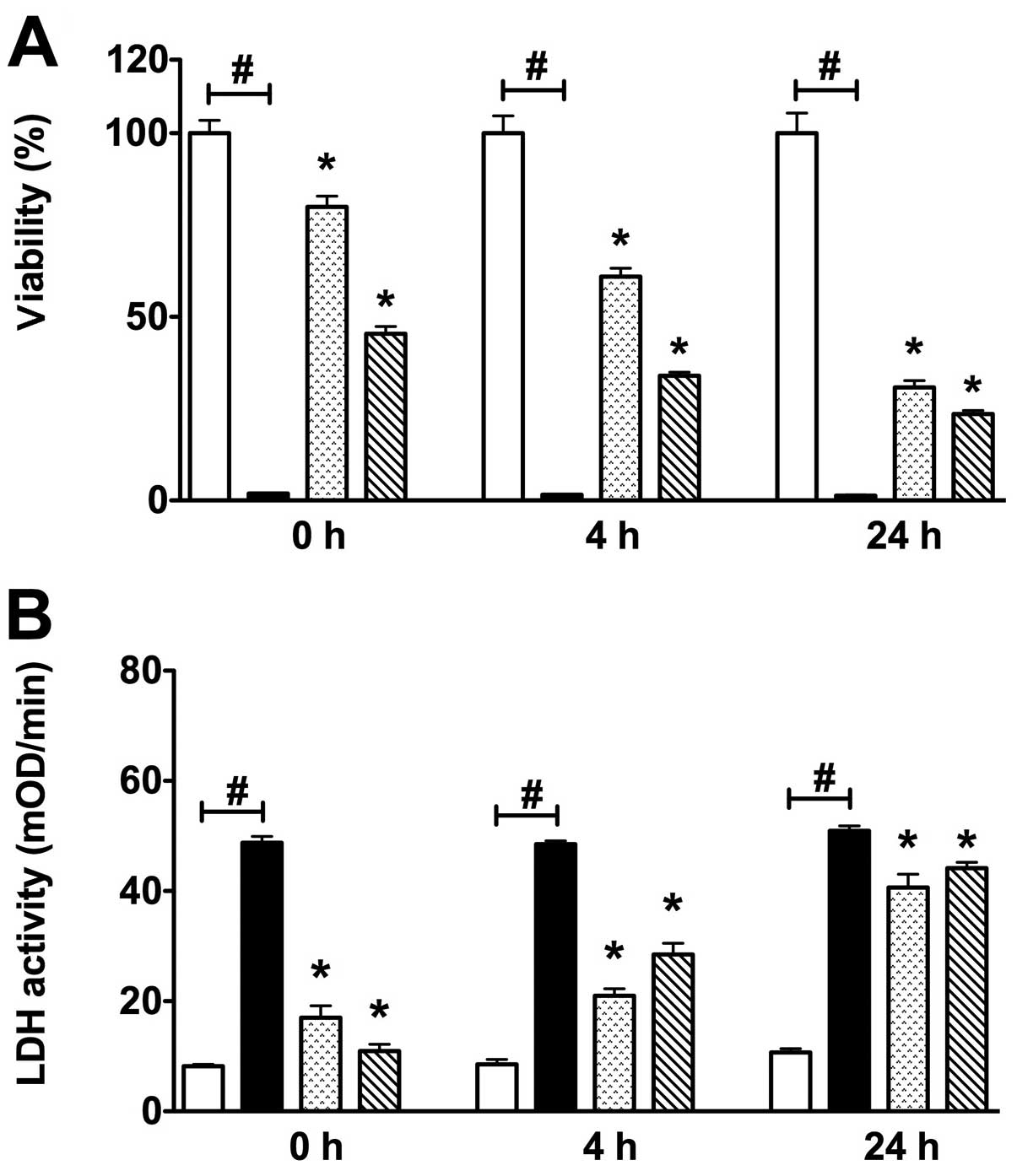
Various re-oxygenation periods (0-4-24 h) were also
investigated after 14 h-long COGD. Increased cell injury was
detected through the progression of the re-oxygenation period, as
measured by both MTT and LDH assays (Fig. 2). Overall, these data indicate
that in the current assay a substantial degree of cell injury
occurs during the hypoxic period.
Effects of adenosine and inosine in an in
vitro model of liver ischemia-reperfusion
Adenosine and inosine significantly protected
against the loss of cell viability of HepG2 cultures during 14 and
24 h of the COGD group, and reduced LDH release from the cells
(Fig. 3). Although adenosine and
inosine significantly protected HepG2 cultures from 14 h hypoxia
injury, during the progression of the re-oxygenation phase a
progressive reduction in cell viability and enhanced LDH enzyme
release were detected in all groups, as assessed by the MTT and LDH
assays. These findings are consistent with the hypothesis that an
extended hypoxia results in further cell damage during the
reoxygenation (reperfusion) phase. Thus our in vitro model
clearly demonstrates the main aspects of the in vivo liver
ischemia-reperfusion injury, with subsequent secondary injury
occurring in the reperfusion phase.
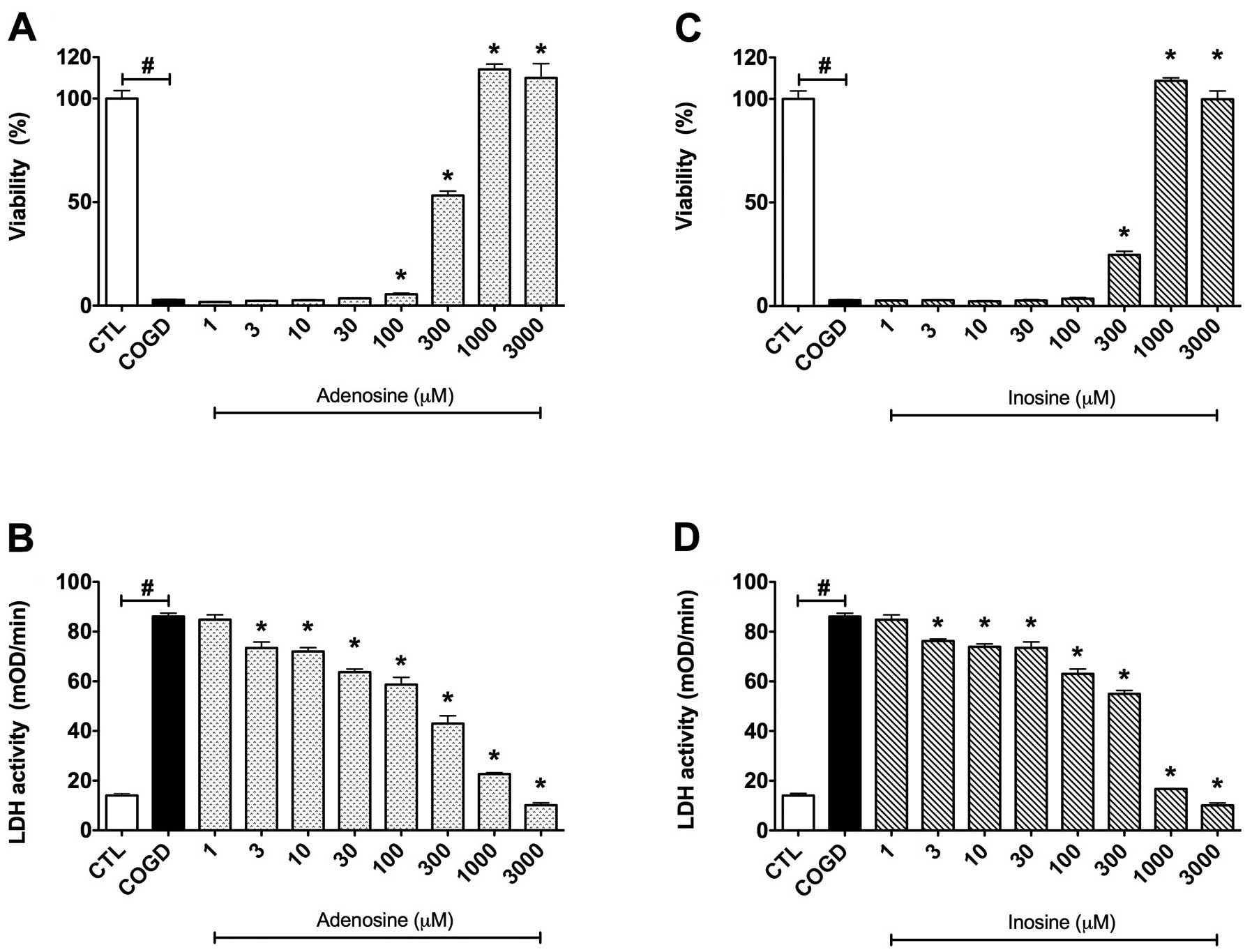 | Figure 3.Dose-response effects of (A and B)
adenosine and (C and D) inosine on percent viability values by MTT
assay and LDH activities in mOD/min in HepG2 cultures exposed to a
14 h-long combined oxygen-glucose deprivation (COGD) and a
subsequent 4 h-long re-oxygenation. Each group, except the control
(CTL) group, was incubated in glucose-free medium under anaerobic
conditions for a 14 h-long period and following a 4 h-long
re-oxygenation phase by normalized glucose and oxygen levels in the
cell culture medium and atmosphere. Data are shown as the means ±
SEM. White bar is control (CTL, n=16) group in the control
conditions of the assay, black bar is COGD group (n=32) during COGD
without any pharmacological pretreatment, dotted bar shows the
adenosine pretreatment group at 1, 3, 10, 30, 100, 300, 1,000 and
3,000 μM (ADE) and ruled bar represents the inosine
pretreatment group at 1, 3, 10, 30, 100, 300, 1,000 and 3,000
μM (INO). ADE and INO groups (n=3) were also under COGD
conditions. #P<0.05 compared to the CTL group and
*P<0.05 compared to the COGD group. |
Pharmacological characterization of the
effects of adenosine and inosine in an in vitro liver
ischemia-reperfusion model
For subsequent in-depth characterization of the
effects of adenosine and inosine, we selected 14 h of hypoxia and 4
h of re-oxygenation periods as the standard assay conditions.
First, we established a dose-response comparison between the
cytoprotective effects of adenosine and inosine, by testing each
compound in a concentration range of 1 μM – 3 mM. Adenosine
and inosine showed cytoprotective effects already at 300 μM,
and reached their maximum cytoprotective effect at 1,000 μM
(Fig. 3). Adenosine and inosine
partially attenuated cellular LDH release, starting already at the
concentration of 3 μM (Fig.
3).
We next evaluated the potential involvement of
adenosine receptors in the protective effects of adenosine and
inosine. Cells were pretreated with the adenosine receptor
antagonists CDPX, CSC, alloxazine, and MRS 1523 prior to
administration of cytoprotective concentrations (300 μM) of
adenosine or inosine. None of the adenosine receptor antagonists
affected the cytoprotective effects of adenosine and inosine during
COGD and following a 4 h-long re-oxygenation period (Fig. 4). These data suggest that
adenosine and inosine exert their cytoprotective effects by
receptor-independent pathways.
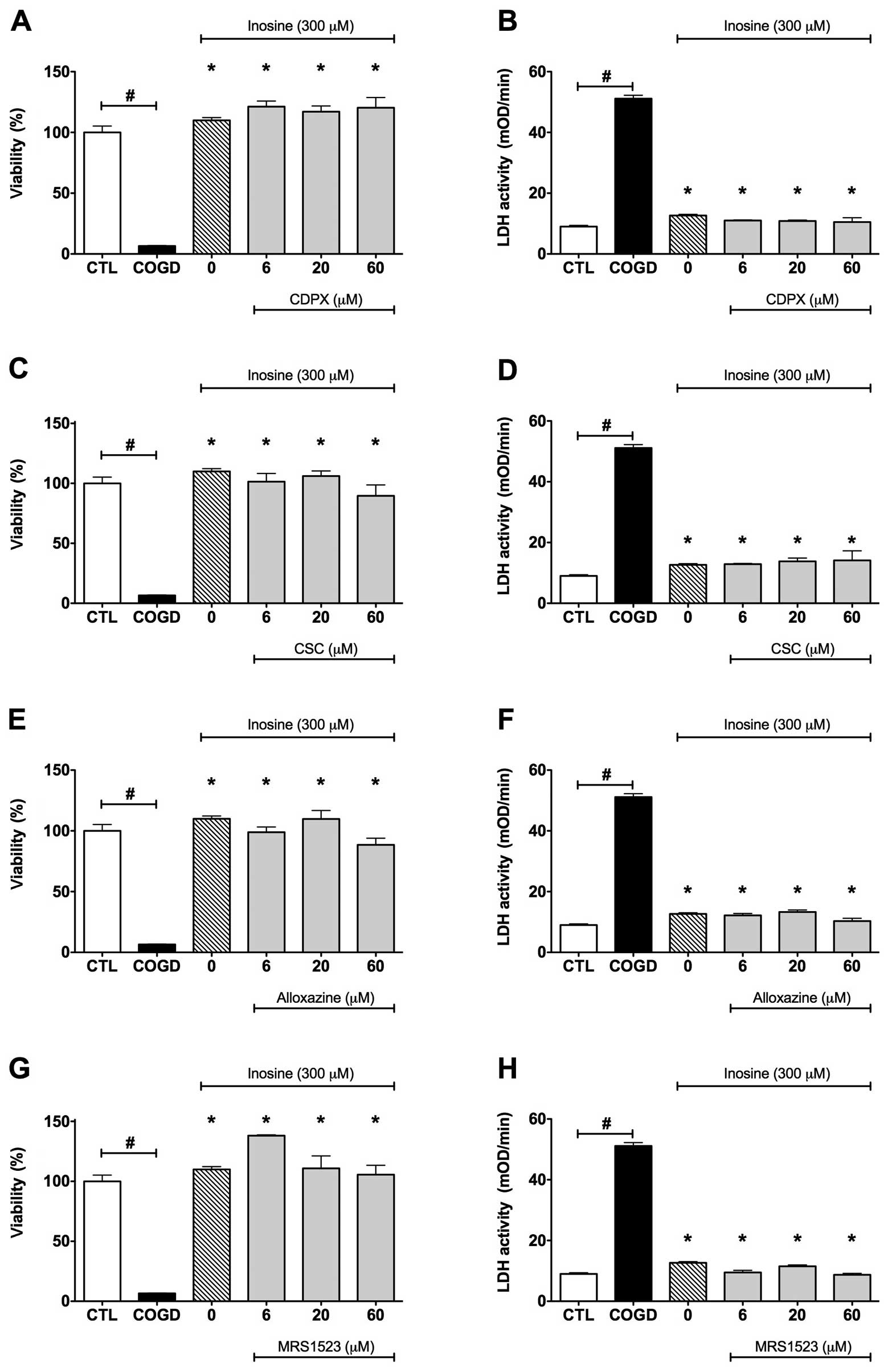 | Figure 4.Effect of adenosine receptor
antagonists on the cytoprotective effects of inosine. Confluent
HepG2 cultures were subjected to combined oxygen-glucose
deprivation (COGD, n=32) for 14 h followed by a 4-h-long
re-oxygenation period. (A and B) The A1 adenosine
receptor antagonist, CDPX, (C and D) the A2A adenosine
receptor antagonist, CSC, (E and F) the A2B adenosine
receptor antagonist, alloxazine, and the A3 adenosine
receptor antagonist, (G and H) MRS 1523 were applied in the
indicated concentrations (n=3) 30 min prior to the inosine or
adenosine pretreatment (INO or ADE, n=12) and were present
throughout the COGD period. Viability was measured by the MTT assay
(A, C, E and G) and LDH activities (B, D, F and H) were measured
from the cell culture supernatant. Controls (CTL, n=16) were
exposed to hypoxia in complete culture medium. Data are shown as
the means ± SEM. #P<0.05 compared to CTL and
*P<0.05 compared to COGD. None of the adenosine
receptor antagonists affected the cytoprotective effects of
adenosine (data not shown) and inosine during COGD. |
The adenosine deaminase inhibitor EHNA (10
μM) almost fully reversed the protective effect of 300–1,000
μM adenosine during COGD and following a 4 h-long
re-oxygenation period (Fig. 5).
On the other hand, EHNA did not influence the cytoprotective effect
of inosine in same assay conditions (Fig. 5). The adenosine kinase inhibitor
ABT 702 (30 μM) significantly reversed the protective effect
of 300 and 1,000 μM adenosine and inosine during COGD and
following a 4 h-long re-oxygenation period. We also tested both
enzyme inhibitors in combined administration (Fig. 7). Notably, ABT 702, on its own,
appeared to have a mild cytoprotective effect in our assay
(Figs. 6 and 7).
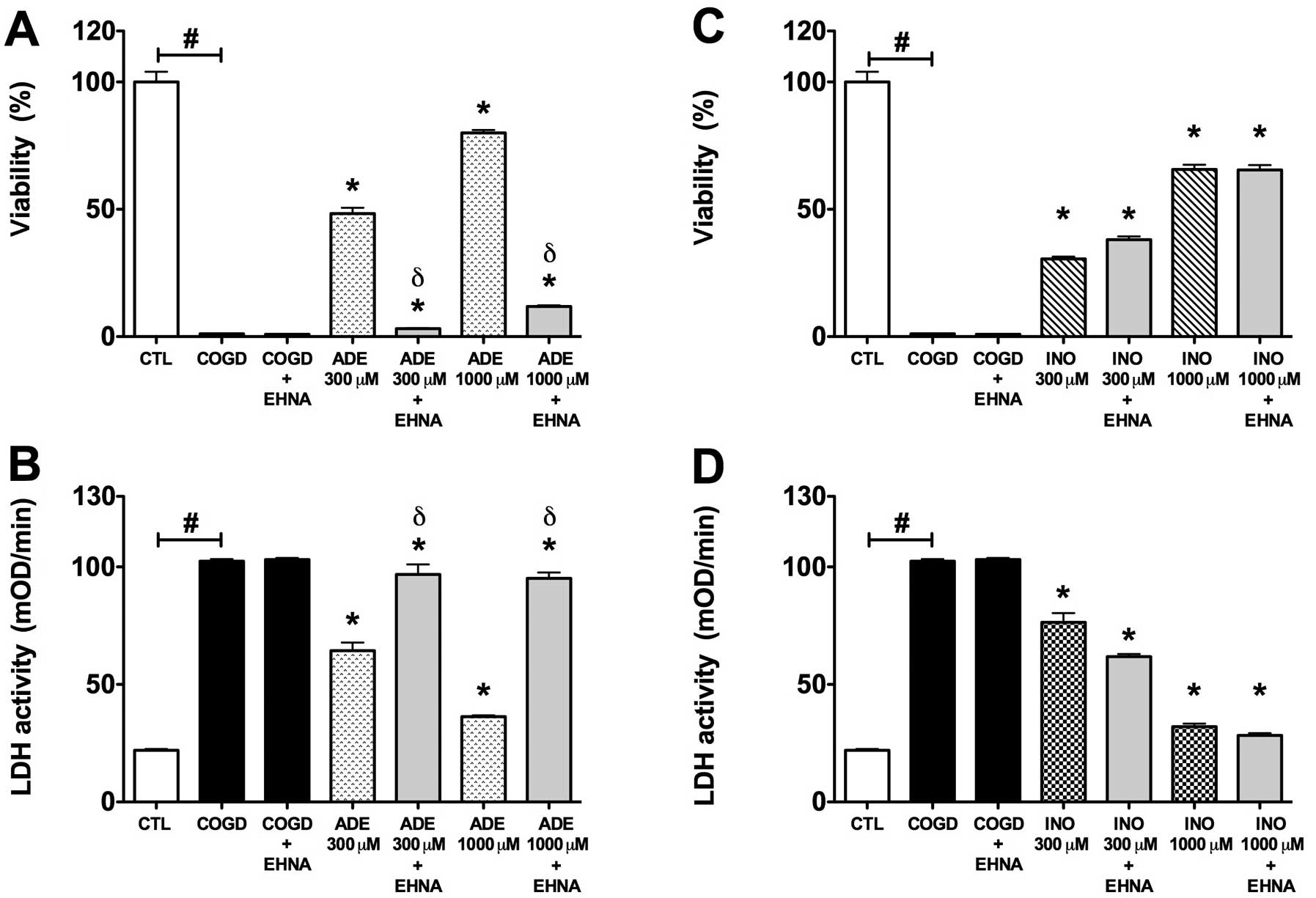 | Figure 5.Effect of adenosine deaminase
inhibitor (EHNA) on the cytoprotective action of 300–1,000
μM adenosine (ADE) and inosine (INO) in HepG2 cultures
exposed to a 14 h-long hypoxia period and a subsequent 4 h
re-oxygenation. Data are shown as the means ± SEM. (A and C)
Percent viability values by MTT assay and (B and D) LDH activities
in mOD/min are shown. Seven groups were studied both of the
adenosine and inosine. The COGD group during COGD (black bar,
n=16), a COGD group plus 10 μM EHNA during COGD (black bar,
n=16), groups pre-treated with 300 or 1,000 μM adenosine
(ADE) and inosine (INO) during COGD (ADE, dotted bar; INO, ruled
bar, n=3) and finally groups pre-treated with 300 or 1,000
μM adenosine and inosine plus 10 μM EHNA during COGD
(ADE 300–1,000 μM + EHNA and INO 300–1,000 μM + EHNA
are grey bar, n=3). The white bar is the control (CTL, n=16) group
and represents the negative control of the assay.
#P<0.05 compared to CTL; *P<0.05
compared to the COGD group; and δP<0.05 compared to
the 300–1,000 μM ADE groups. |
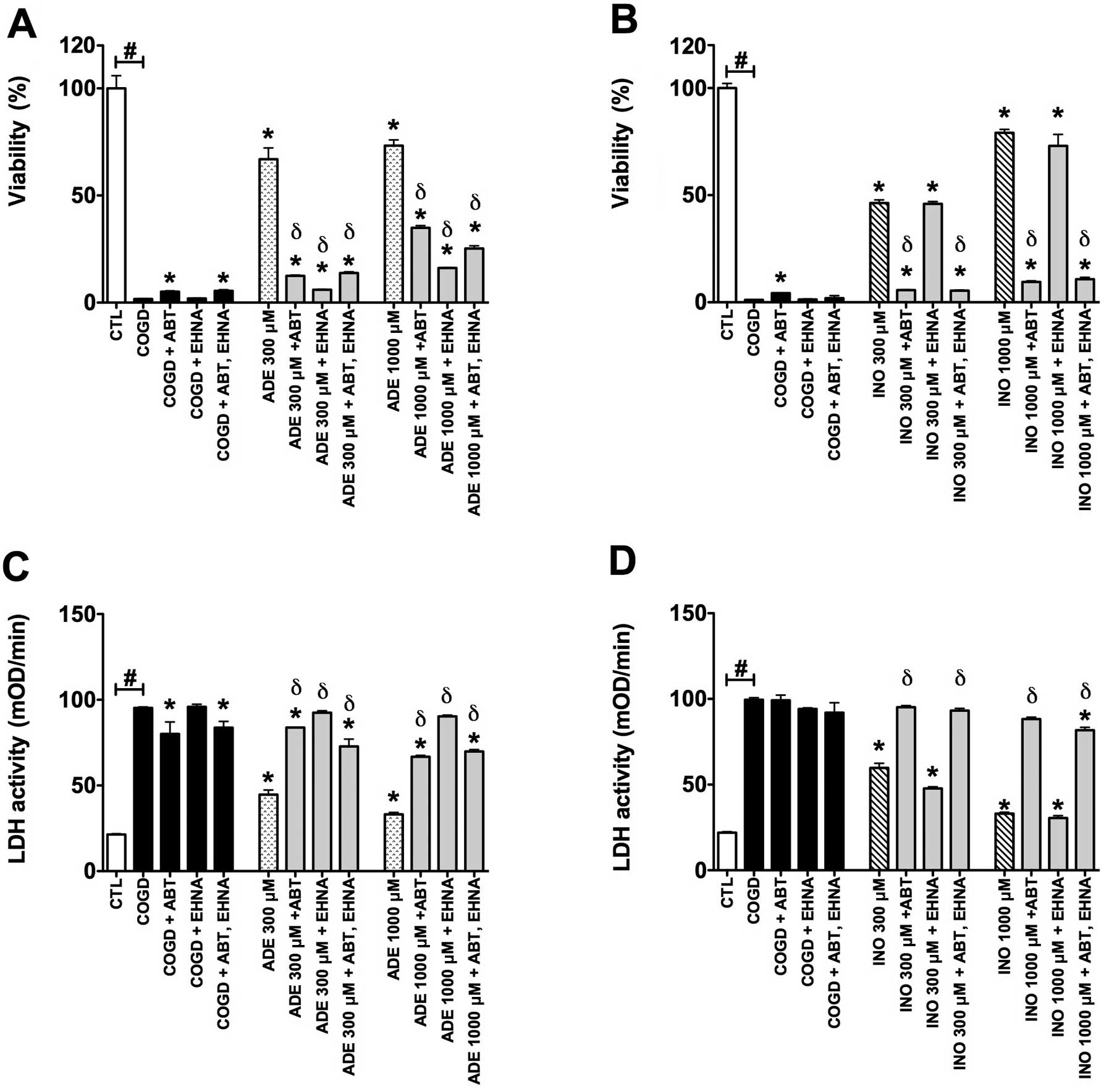 | Figure 7.Collective effect of adenosine
deaminase inhibitor (EHNA) and adenosine kinase inhibitor (ABT 702)
on the cytoprotective actions of 300–1,000 μM adenosine
(ADE) and inosine (INO) in HepG2 cultures exposed to a 14 h-long
hypoxia period and a subsequent 4 h re-oxygenation. Data are shown
as the means ± SEM. (A and C) Percent viability values by MTT assay
and (B and D) LDH activities in mOD/min are shown. Seven groups
were studied both of the adenosine and inosine. The COGD group
during COGD (black bar, n=32), a COGD group plus 30 μM ABT
702 during COGD (black bar, n=3), a COGD group plus 10 μM
EHNA during COGD (black bar, n=3), a COGD group plus 30 μM
ABT 702 and 10 μM EHNA in combination during COGD (black
bar, n=3), groups pre-treated with 300–1,000 μM adenosine
(ADE) and inosine (INO) during COGD (ADE, dotted bar; INO, ruled
bar, n=3) and finally groups pre-treated with 300 or 1,000
μM adenosine and inosine plus 30 μM ABT 702 or 10
μM EHNA during COGD (ADE/INO 300/1,000 μM + ABT
702/EHNA or in combination are grey bars, n=3). The white bar is
the control (CTL, n=16) group and represents the negative control
of the assay, subjected to hypoxia in complete culture medium.
#P<0.05 compared to CTL; *P<0.05
compared to the COGD group; and δP<0.05 compared to
the 300–1,000 μM ADE and INO groups. |
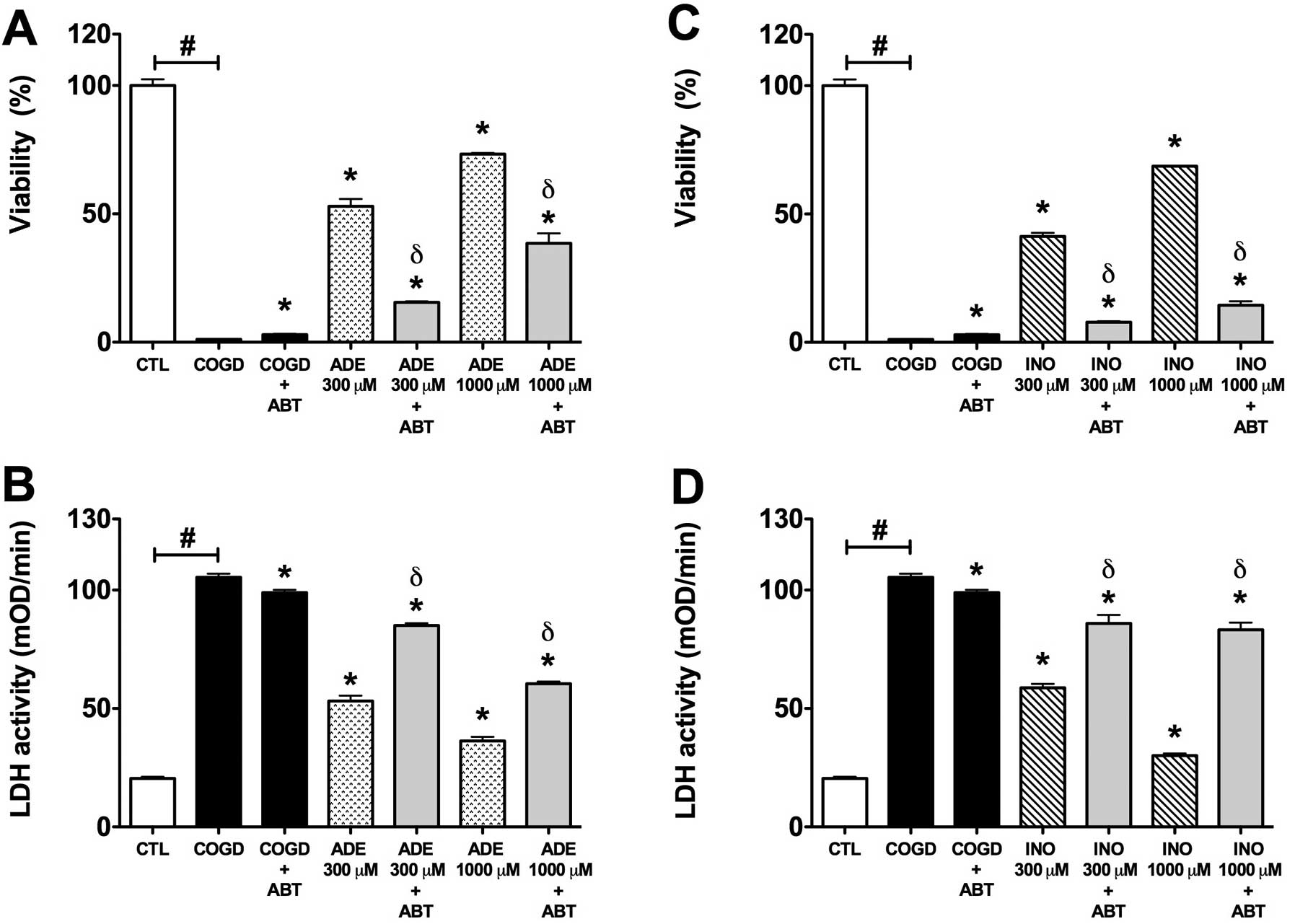 | Figure 6.Effect of adenosine kinase inhibitor
(ABT 702) on the cytoprotective action of 300–1,000 μM
adenosine (ADE) and inosine (INO) in HepG2 cultures exposed to a 14
h-long hypoxia period and a subsequent 4 h re-oxygenation. Data are
shown as the means ± SEM. (A and C) Percent viability values by MTT
assay and (B and D) LDH activities in mOD/min are shown. Seven
groups were studied both of the adenosine and inosine. The COGD
group during COGD (black bar, n=16), a COGD group plus 30 μM
ABT 702 during COGD (black bar, n=16), groups pre-treated with 300
or 1,000 μM adenosine (ADE) and inosine (INO) during COGD
(ADE, dotted bar; INO, ruled bar, n=3) and finally groups
pre-treated with 300 or 1,000 μM adenosine and inosine plus
30 μM ABT 702 during COGD (ADE 300–1,000 μM + ABT and
INO 300–1,000 μM + ABT are grey bars, n=3). The white bar is
the control (CTL, n=16) group and represents the negative control
of the assay. #P<0.05 compared to CTL;
*P<0.05 compared to the COGD group; and
δP<0.05 compared to the 300–1,000 μM ADE and
INO groups. |
Discussion
The present study utilizes a cell-based model of
liver ischemia-reperfusion injury in cultured HepG2 cells subjected
to combined oxygen-glucose deprivation followed by re-oxygenation.
Experimental conditions similar to the current ones have previously
been used in multiple studies in cultured hepatocytes and Kupffer
cells to mimic conditions of ischemia, in order to study pathways
of cell death, signal transduction, free radical and oxidant
production and inflammatory responses (28–33). Our results demonstrate that both
adenosine and inosine exert cytoprotective effects in the current
model, in a concentration-dependent manner (when assessed by
measurement of LDH release and mitochondrial activity by the MTT
assay). Although in several experimental models the cytoprotective
effects of adenosine and inosine are known to be dependent on
activation of adenosine A2A receptors (11,12,34), the results of the current study
demonstrate that the protective effect of adenosine and inosine in
the current experimental conditions were not mediated by adenosine
receptor-dependent pathways, as evidenced by the failure of
specific adenosine receptor blockers to prevent the protective
effects. Although several reports suggest a role for cell surface
adenosine receptors in the reduction of liver reperfusion injury
in vivo (35–39), it is likely that the location of
these receptors is primarily on mononuclear cells involved in
pro-inflammatory/immune responses (as opposed to hepatocytes).
While adenosine receptors failed to play a role in
the cytoprotective effects of adenosine and inosine described in
the present study, the data suggest the involvement of
receptor-independent intracellular actions that are related to a
direct regulation of cellular bioenergetics. We utilized the
pharmacological inhibitor EHNA to inhibit adenosine deaminase, the
enzyme that is responsible for the intracellular conversion of
adenosine to inosine. EHNA significantly decreased the viability of
the adenosine-treated cells subjected to COGD and also
significantly increased the LDH release from the cells (Fig. 5). On the other hand, EHNA did not
reduce the protective effect of inosine. These data are consistent
with the hypothesis that, ultimately, an intracellular action,
mediated by inosine, is responsible for the protective effect of
adenosine. Our interpretation of the experimental findings is that,
similar to astrocytes and kidney epithelial cells subjected to
hypoxia and re-oxygenation (13,14,23), the conversion of adenosine to
inosine and its subsequent metabolism to ribose-phosphate, followed
by ATP generation via the pentose phosphate pathway are responsible
for the observed cytoprotective effects.
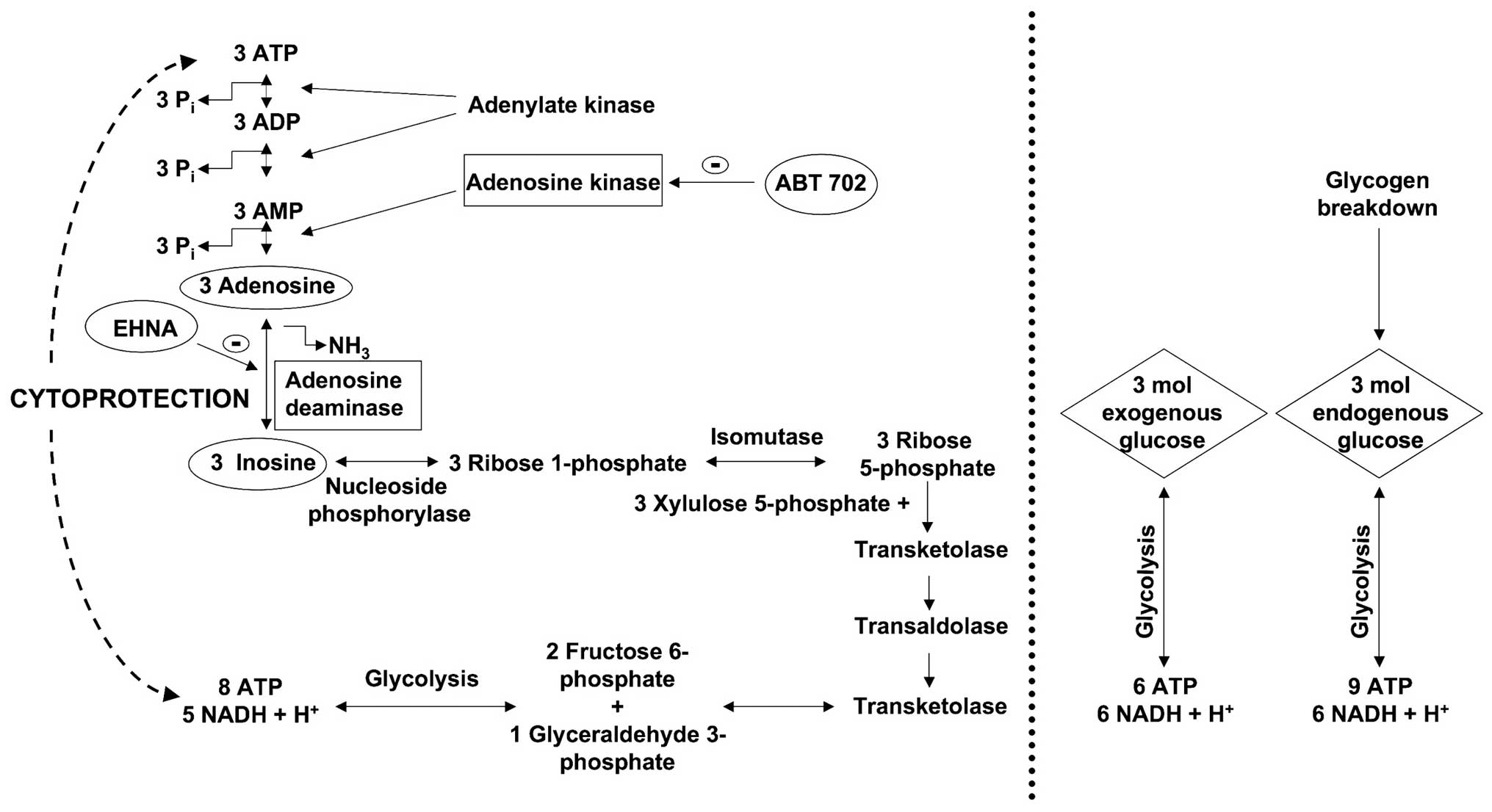
Haun et al (23), Jurkowitz et al (40) and Litsky et al (41) demonstrated that the ribose moiety
of the adenosine and inosine can be used as a precursor for
phosphorylated glycolytic intermediates in reactions catalyzed by
enzymes of the pentose phosphate pathway. The first reaction in
this pathway is the phosphorolysis of inosine and the formation of
ribose 1-phosphate or hypoxanthine, which is catalyzed by purine
nucleoside phosphorylase. Three ribose 1-phosphates are isomerized
to three D-ribose 5-phosphates, which then convert to two fructose
6-phosphates and one glyceraldehyde 3-phosphate, via transaldolases
and transketolases of the pentose phosphate pathway. These
phosphorylated intermediates enter the glycolytic pathway yielding
a net production of 8 moles ATP and 5 moles NADH + H+
from three molecules of ribose 1-phosphate (Fig. 8). All produced NADH +
H+ convert to NAD+ by lactate dehydrogenase
(LDH) in anaerobic conditions. However, after a while, lactate
accumulates resulting in cellular acidosis. For abolishing the
acidosis lactate degradation to pyruvate and its further
decomposition in the Krebs cycle at the presence of oxygen is
indispensable.
From the same molar amount (3 moles) of
extracellular (or exogenous) glucose: 6 moles of ATP and 6 moles of
NADH + H+ are produced during the glycolysis (Fig. 8). From the breakdown of glycogen,
3 moles of intracellular glucose-1 phosphate can produce a maximum
of 9 moles of net ATP and 6 moles of NADH + H+. On the
other hand, from 3 moles of adenosine or inosine, the cell can
produce net 8 moles of ATP and 5 moles of NADH + H+.
Thus, the cells tend to elevate the intracellular NAD+/NADH +
H+ ratio, thereby regenerating as many NADH +
H+ molecules as possible within a short time.
On the other hand, NAD+ molecules are
required to continue the glycolysis and lactate intermediates
inhibit the further regeneration if the NADH + H+
molecules thereby impairing glycolysis. During excessive hypoxia,
the accumulation of NADH + H+ occurs and, subsequently,
inhibition of several enzymes (citrate synthase,
isocitrate-dehydrogenase, α-ketoglutarate dehydrogenase) occurs,
thereby interfering with the Krebs cycle, which is controlled by
the ratio of the NADH/NAD+ and the ATP/ADP. Furthermore,
during massive anaerobic conditions, NADH + H+
accumulation also leads to an inhibition of glycolysis. In summary,
during COGD, adenosine and inosine may delay the accumulation of
NADH + H+ and they can serve as a source of energy to
maintain basal cellular function. In prolonged hypoxia, the
accumulation of NADH + H+ is inevitable and the
glycolysis and the Krebs cycle are both inhibited. Thus, long-term
absence of the terminal oxidation and the regeneration of NADH +
H+ to NAD+ result in severe cellular energy
imbalance.
The results of the present study also demonstrated
that the administration of the adenosine kinase inhibitor ABT 702
reversed all of the protective effects of adenosine and inosine.
These findings are consistent with the hypothesis that adenosine
kinase is responsible for producing AMP, ADP, and, eventually, ATP
from adenosine. When sufficient ADP molecules are present in the
cells, ATP can be created from ADP both via the adenosine
kinase-mediated route and via the pentose phosphate pathway. The
inhibition of the adenosine kinase pathway by ABT 702 results in
less ADP production in the adenosine kinase-mediated pathway, which
induces less ATP generation in the pentose phosphate shunt as well.
This mechanism explains that, i) ATP production of the pentose
phosphate pathway depends on the amount of ADP molecules derived
from the adenosine kinase mediated route, and ii) provides evidence
that administration of adenosine kinase inhibitor ABT 702 reversed
all of the protective effects of adenosine and inosine.
Furthermore, adenosine kinase inhibitor (ABT 702) administration
without exogenous adenosine or inosine affords a mild
cytoprotective effect, but prevents the cytoprotection provided by
exogenous adenosine or inosine (Figs.
6 and 7).
Our data and the above considerations, taken
together, indicate that adenosine and inosine may exert some of
their cytoprotective effects under our current experimental
conditions by stepping in as an emergency energy source, when
glucose is insufficient to support cellular functions. This
hypothesis is supported both by several reports in the literature
where cellular ATP levels were elevated in ischemic or hypoxic
cells treated with adenosine or inosine (23,40,42–44) as well as by our measurements
(13,14). Although we have not directly
measured the transport of adenosine or inosine into the cells,
previous studies have demonstrated that these purines can readily
enter the cells (23,40). We propose that the two processes,
i) degradation of adenosine and inosine via the pentose-phosphate
pathway, and ii) the phosphorylation of adenosine to AMP, are
required in a well-balanced parallel fashion. Our hypothesis is
that both pathways are necessary at the same time to support the
generation of ATP under hypoxic conditions. The first process
provides the energy, while the second one supplies the substrate
(e.g. adenosine, AMP, ADP) to convert it into a high energy
intermediate that conserves it in a ready-to-use form. Markedly,
the combined blockage of adenosine deaminase (by EHNA) and
adenosine kinase (by ABT 702) resulted in no further reduction in
cell viability after COGD (as compared to either inhibitor), which
supports that these enzymes take part in the same cytoprotective
mechanism (Fig. 7).
In conclusion, it is likely that the cytoprotective
effects of adenosine and inosine involve multiple, parallel and
interrelated mechanisms under the current experimental conditions.
During ischemia and inflammation, the concentration of purine
metabolites increases dramatically in the extracellular space. ATP
degrades into AMP and subsequently to adenosine, which may be
released from the cells and appears in the extra-cellular space.
Inosine can be formed from adenosine with an adenosine deaminase
enzyme, which occurs both intra- and extracellularly. Consistent
with these notions, there are several reports that demonstrate the
cytoprotective effect of endogenously formed adenosine in the
context of acute ischemic injury of the liver (45,46).
The current study shows that adenosine and inosine
are cytoprotective on HepG2 cultures exposed to combined
oxygen-glucose deprivation. This protective effect is not mediated
by a receptor-dependent pathway, but it is likely mediated by
maintenance of cellular bioenergetics due to the utilization of
adenosine and inosine as alternative substrates for ATP
generation.
While the therapeutic utilization of adenosine as a
hepatoprotective agent in vivo is difficult due to its short
half-life and adverse cardiovascular side-effect profile, inosine
may emerge as a potential candidate. Indeed, several recent studies
have demonstrated that administration of inosine can be protective
against various forms of ischemic conditions (7–10).
The current results may provide a mechanistic explanation to the
previously reported protective effect of inosine in vitro as
an adjuvant to organ storage solutions (47) or in vivo as a protective
agent in a rat model of hepatic reperfusion injury (48) and can stimulate further studies to
explore whether inosine has the potential to improve cellular
bioenergetics and to protect hepatocytes in various forms of liver
injury, including various forms of warm ischemia or cold ischemia
associated with liver transplantation.
Abbreviations:
|
ABT 702
|
4-amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin-3-yl)pyrido[2,3-d]pyrimidine,
2HCl;
|
|
CDPX
|
8-cyclopentyl-1,3-dipropylxanthine;
|
|
CSC
|
8-(3-chlorostyryl) caffeine;
|
|
COGD
|
combined oxygen-glucose
deprivation;
|
|
DMEM
|
Dulbecco’s modified Eagle’s
medium;
|
|
DMSO
|
dimethylsulfoxide;
|
|
EHNA
|
erythro-9-(2-hydroxy-3-nonyl) adenine
hydrochloride;
|
|
I-R injury
|
ischemia-reperfusion injury;
|
|
LDH
|
lactate dehydrogenase;
|
|
MTT
|
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium
bromide;
|
|
PBS
|
phosphate-buffered saline.
|
Acknowledgements
This study was supported by a grant
from the National Institutes of Health and by the Oszkar Asboth
project grant of the National Office for Research and Technology
(Budapest, Hungary). The technical assistance of Ms. Nora Nagy is
appreciated.
References
|
1.
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Frangogiannis NG: Chemokines in ischemia
and reperfusion. Thromb Haemost. 97:738–747. 2007.PubMed/NCBI
|
|
3.
|
Peralta C, Bartrons R, Riera L, Manzano A,
Xaus C, Gelpi E and Rosello-Catafau J: Hepatic preconditioning
preserves energy metabolism during sustained ischemia. Am J Physiol
Gastrointest Liver Physiol. 279:G163–G171. 2000.PubMed/NCBI
|
|
4.
|
Cavalieri B, Perrelli MG, Aragno M,
Mastrocola R, Corvetti G, Durazzo M, Poli G and Cutrin JC: Ischemic
preconditioning attenuates the oxidant-dependent mechanisms of
reperfusion cell damage and death in rat liver. Liver Transpl.
8:990–999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lee WY and Lee SM: Ischemic
preconditioning protects post-ischemic oxidative damage to
mitochondria in rat liver. Shock. 24:370–375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Carini R and Albano E: Recent insights on
the mechanisms of liver preconditioning. Gastroenterology.
125:1480–1491. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hasko G, Sitkovsky MV and Szabo C:
Immunomodulatory and neuroprotective effects of inosine. Trends
Pharmacol Sci. 25:152–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Spitsin S, Hooper DC, Leist T, Streletz
LJ, Mikheeva T and Koprowskil H: Inactivation of peroxynitrite in
multiple sclerosis patients after oral administration of inosine
may suggest possible approaches to therapy of the disease. Mult
Scler. 7:313–319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Szabo G, Stumpf N, Radovits T, Sonnenberg
K, Gero D, Hagl S, Szabo C and Bahrle S: Effects of inosine on
reperfusion injury after heart transplantation. Eur J Cardiothorac
Surg. 30:96–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Veres G, Radovits T, Seres L, Horkay F,
Karck M and Szabo G: Effects of inosine on reperfusion injury after
cardiopulmonary bypass. J Cardiothorac Surg. 5:1062010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gomez G and Sitkovsky MV: Differential
requirement for A2a and A3 adenosine
receptors for the protective effect of inosine in vivo. Blood.
102:4472–4478. 2003.PubMed/NCBI
|
|
12.
|
Rahimian R, Fakhfouri G, Daneshmand A,
Mohammadi H, Bahremand A, Rasouli MR, Mousavizadeh K and Dehpour
AR: Adenosine A2A receptors and uric acid mediate
protective effects of inosine against TNBS-induced colitis in rats.
Eur J Pharmacol. 649:376–381. 2010.
|
|
13.
|
Modis K, Gero D, Nagy N, Szoleczky P, Toth
ZD and Szabo C: Cytoprotective effects of adenosine and inosine in
an in vitro model of acute tubular necrosis. Br J Pharmacol.
158:1565–1578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Szoleczky P, Modis K, Nagy N, Dori Toth Z,
DeWitt D, Szabo C and Gero D: Identification of agents that reduce
renal hypoxiare-oxygenation injury using cell-based screening:
purine nucleosides are alternative energy sources in LLC-PK1 cells
during hypoxia. Arch Biochem Biophys. 517:53–70. 2012.
|
|
15.
|
Virag L and Szabo C: Purines inhibit
poly(ADP-ribose) polymerase activation and modulate oxidant-induced
cell death. FASEB J. 15:99–107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Bruns RF, Fergus JH, Badger EW, Bristol
JA, Santay LA, Hartman JD, Hays SJ and Huang CC: Binding of the
A1-selective adenosine antagonist
8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn
Schmiedebergs Arch Pharmacol. 335:59–63. 1987.
|
|
17.
|
Kim J, Kim M, Song JH and Lee HT:
Endogenous A1 adenosine receptors protect against
hepatic ischemia reperfusion injury in mice. Liver Transpl.
14:845–854. 2008.PubMed/NCBI
|
|
18.
|
Lee HT and Emala CW: Systemic adenosine
given after ischemia protects renal function via A(2a) adenosine
receptor activation. Am J Kidney Dis. 38:610–618. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yasuda N, Inoue T, Horizoe T, Nagata K,
Minami H, Kawata T, Hoshino Y, Harada H, Yoshikawa S, Asano O,
Nagaoka J, Murakami M, Abe S, Kobayashi S and Tanaka I: Functional
characterization of the adenosine receptor contributing to
glycogenolysis and gluconeogenesis in rat hepatocytes. Eur J
Pharmacol. 459:159–166. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Rose’Meyer RB, Harrison GJ and Headrick
JP: Enhanced adenosine A(2B) mediated coronary response in
reserpinised rat heart. Naunyn Schmiedebergs Arch Pharmacol.
367:266–273. 2003.PubMed/NCBI
|
|
21.
|
Di Sole F, Cerull R, Babich V, Casavola V,
Helmle-Roth C and Burckhardt G: Short- and long-term A3
adenosine receptor activation inhibits the
Na+/H+ exchanger NHE3 activity and expression
in opossum kidney cells. J Cell Physiol. 216:221–233. 2008.
|
|
22.
|
Ohana G, Bar-Yehuda S, Arich A, Madi L,
Dreznick Z, Rath-Wolfson L, Silberman D, Slosman G and Fishman P:
Inhibition of primary colon carcinoma growth and liver metastasis
by the A3 adenosine receptor agonist CF101. Br J Cancer.
89:1552–1558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Haun SE, Segeleon JE, Trapp VL, Clotz MA
and Horrocks LA: Inosine mediates the protective effect of
adenosine in rat astrocyte cultures subjected to combined
glucose-oxygen deprivation. J Neurochem. 67:2051–2059. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Jarvis MF, Yu H, Kohlhaas K, Alexander K,
Lee CH, Jiang M, Bhagwat SS, Williams M and Kowaluk EA: ABT-702
(4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2,3-d]
pyrimidine), a novel orally effective adenosine kinase inhibitor
with analgesic and anti-inflammatory properties: I. In vitro
characterization and acute antinociceptive effects in the mouse. J
Pharmacol Exp Ther. 295:1156–1164. 2000.PubMed/NCBI
|
|
25.
|
Kowaluk EA, Mikusa J, Wismer CT, Zhu CZ,
Schweitzer E, Lynch JJ, Lee CH, Jiang M, Bhagwat SS, Gomtsyan A,
McKie J, Cox BF, Polakowski J, Reinhart G, Williams M and Jarvis
MF: ABT-702
(4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2,3-d]pyrimidine),
a novel orally effective adenosine kinase inhibitor with analgesic
and anti-inflammatory properties. II. In vivo characterization in
the rat. J Pharmacol Exp Ther. 295:1165–1174. 2000.PubMed/NCBI
|
|
26.
|
Jagtap P, Soriano FG, Virag L, Liaudet L,
Mabley J, Szabo E, Hasko G, Marton A, Lorigados CB, Gallyas F Jr,
Sumegi B, Hoyt DG, Baloglu E, VanDuzer J, Salzman AL, Southan GJ
and Szabo C: Novel phenanthridinone inhibitors of poly (adenosine
5′-diphosphate-ribose) synthetase: potent cytoprotective and
antishock agents. Crit Care Med. 30:1071–1082. 2002.
|
|
27.
|
Gero D, Modis K, Nagy N, Szoleczky P, Toth
ZD, Dorman G and Szabo C: Oxidant-induced cardiomyocyte injury:
Identification of the cytoprotective effect of a dopamine 1
receptor agonist using a cell-based high-throughput assay. Int J
Mol Med. 20:749–761. 2007.PubMed/NCBI
|
|
28.
|
Ohsaka Y, Ohgiya S, Hoshino T and Ishizaki
K: Phosphorylation of c-Jun N-terminal kinase in human
hepatoblastoma cells is transiently increased by cold exposure and
further enhanced by subsequent warm incubation of the cells. Cell
Physiol Biochem. 12:111–118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Laurens M, Defamie V, Scozzari G,
Schmid-Alliana A, Gugenheim J and Crenesse D:
Hypoxia-re-oxygenation-induced chemokine transcription is not
prevented by preconditioning or intermittent hypoxia, in mice
hepatocytes. Transpl Int. 18:444–452. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Roudier E, Bachelet C and Perrin A:
Pyruvate reduces DNA damage during hypoxia and after re-oxygenation
in hepatocellular carcinoma cells. FEBS J. 274:5188–5198. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Bhogal RH, Curbishley SM, Weston CJ, Adams
DH and Afford SC: Reactive oxygen species mediate human hepatocyte
injury during hypoxia/re-oxygenation. Liver Transpl. 16:1303–1313.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Kim JS, Wang JH and Lemasters JJ:
Mitochondrial permeability transition in rat hepatocytes after
anoxia/re-oxygenation: role of Ca2+-dependent
mitochondrial formation of reactive oxygen species. Am J Physiol
Gastrointest Liver Physiol. 302:G723–G731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Pillai VC, Snyder RO, Gumaste U,
Thekkumkara TJ and Mehvar R: Effects of transient overexpression or
knockdown of cytochrome P450 reductase on reactive oxygen species
generation and hypoxia re-oxygenation injury in liver cells. Clin
Exp Pharmacol Physiol. 38:846–853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Szabo C and Pacher P: The outsiders:
emerging roles of ectonucleotidases in inflammation. Sci Transl
Med. 4:146ps142012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Gallos G, Ruyle TD, Emala CW and Lee HT:
A1 adenosine receptor knockout mice exhibit increased
mortality, renal dysfunction, and hepatic injury in murine septic
peritonitis. Am J Physiol Renal Physiol. 289:F369–F376.
2005.PubMed/NCBI
|
|
36.
|
Day YJ, Marshall MA, Huang L, McDuffie MJ,
Okusa MD and Linden J: Protection from ischemic liver injury by
activation of A2A adenosine receptors during
reperfusion: inhibition of chemokine induction. Am J Physiol
Gastrointest Liver Physiol. 286:G285–G293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Ben-Ari Z, Pappo O, Sulkes J, Cheporko Y,
Vidne BA and Hochhauser E: Effect of adenosine A2A
receptor agonist (CGS) on ischemia/reperfusion injury in isolated
rat liver. Apoptosis. 10:955–962. 2005.PubMed/NCBI
|
|
38.
|
Lappas CM, Day YJ, Marshall MA, Engelhard
VH and Linden J: Adenosine A2A receptor activation
reduces hepatic ischemia reperfusion injury by inhibiting
CD1d-dependent NKT cell activation. J Exp Med. 203:2639–2648.
2006.
|
|
39.
|
León Fernández O, Pantoja M, Díaz Soto M,
Dranguet J, García Insua M, Viebhan-Hánsler R, Menéndez Cepero S
and Calunga Fernández J: Ozone oxidative preconditioning is
mediated by A1 adenosine receptors in a rat model of
liver ischemia/reperfusion. Transpl Int. 21:39–48. 2008.
|
|
40.
|
Jurkowitz MS, Litsky ML, Browning MJ and
Hohl CM: Adenosine, inosine, and guanosine protect glial cells
during glucose deprivation and mitochondrial inhibition:
correlation between protection and ATP preservation. J Neurochem.
71:535–548. 1998. View Article : Google Scholar
|
|
41.
|
Litsky ML, Hohl CM, Lucas JH and Jurkowitz
MS: Inosine and guanosine preserve neuronal and glial cell
viability in mouse spinal cord cultures during chemical hypoxia.
Brain Res. 821:426–432. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Mandel LJ, Takano T, Soltoff SP and
Murdaugh S: Mechanisms whereby exogenous adenine nucleotides
improve rabbit renal proximal function during and after anoxia. J
Clin Invest. 81:1255–1264. 1988. View Article : Google Scholar
|
|
43.
|
Takeo S, Tanonaka K, Miyake K and Imago M:
Adenine nucleotide metabolites are beneficial for recovery of
cardiac contractile force after hypoxia. J Mol Cell Cardiol.
20:187–199. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Weinberg JM and Humes HD: Increases of
cell ATP produced by exogenous adenine nucleotides in isolated
rabbit kidney tubules. Am J Physiol. 250:F720–F733. 1986.PubMed/NCBI
|
|
45.
|
Ajamieh HH, Candelario-Jalil E, Fernandez
OS and Gerbes AL: Ischaemic and pharmacological preconditionings
protect liver via adenosine and redox status following hepatic
ischaemia/reperfusion in rats. Clin Sci (Lond). 115:69–77. 2008.
View Article : Google Scholar
|
|
46.
|
Taniguchi M, Magata S, Suzuki T, Shimamura
T, Jin MB, Iida J, Furukawa H and Todo S: Dipyridamole protects the
liver against warm ischemia and reperfusion injury. J Am Coll Surg.
198:758–769. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Maggio AJ Jr, Das S, Smith RB and Kaufman
JJ: Renal preservation with inosine. Urology. 16:343–345. 1980.
View Article : Google Scholar
|
|
48.
|
Tilser I, Martinkova J and Chladek J: The
effect of metipranolol and inosine on total hepatic ischemia of
rats in vivo. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove.
36:25–29. 1993.PubMed/NCBI
|