Introduction
It is now recognized that soybeans (Glycine
max) and soybean foods, such as tofu and soymilk, can
contribute to the better health of the elderly, young people, and
pregnant woman (1). The soybean,
a legume with a high protein content (~35–40%) is excellent at
meeting dietary protein needs. Moreover, the importance of soybeans
is proven by clinical and preclinical studies of
hypocholesterolemia, diabetes, obesity, renal dysfunction and
cardiovascular disorders (2,3).
Soybeans contain many known components, such as phytoalexins, that
are incredibly useful. Genistein, a soybean isoflavone, has been
highlighted over the last few years as a potentially beneficial
agent due to its wide-ranging effects on breast cancer,
osteoporosis, coronary heart disease, diabetes and menopausal
discomfort (4–6). Glyceollin, a phytoalexin found in
soybeans and grapes, is produced as an immune response to pathogens
(7). Glyceollins are isolated as
a mixture of glyceollin I, II, and III (Fig. 1A), from germinated soybean from
Rhyzopus sp. by an elicitor (8). These compounds have been
investigated in relation to their anti-estrogenic activity through
the inhibition of estrogen receptor α and β compared with
well-known phytoestrogenic chemicals such as enterolactones and
genistein (9–11). It also has been recognized that
glyceollins can suppress human breast and ovarian carcinoma
tumorigenesis (12) and may
modulate potential estrogenic properties in the breast through an
anti-estrogenic effect (13);
however, researchers have yet to elucidate the molecular mechanisms
of their biological activity.
On the other hand, it has been revealed that NF-κB
plays a pivotal role in molecular inflammation by controlling the
expression of various genes that encode pro-inflammatory cytokines,
chemokines and inducible enzymes, such as inducible nitric oxide
synthase (iNOS) and cyclooxygenase-2 (COX-2), in mammalian immune
cells (14). NF-κB is
ubiquitously located in the cytoplasm of non-stimulated cells
because of interactions with inhibitory proteins such as IκBs
(15); but, it responds to
pro-inflammatory stimuli, when IκBs are rapidly phosphorylated and
degraded by the 26S proteasome (16,17), which results in the dissociation
of free NF-κB dimers of p65 and p50, which translocate to the
nucleus, and then activate the transcription of target genes. The
IκBα kinase (IKK) complex contains two catalytic subunits: i)
IKKα/β and ii) a regulatory subunit, IKKγ (18–20). Activation of IKK is mediated by
phosphorylation through various upstream kinases, such as the
NF-κB-inducing kinase, NF-κB-activating kinase, Akt, and protein
kinase Cζ, that are involved in cellular signaling in response to
pro-inflammatory stimuli (21).
Following activation, the NF-κB heterodimer activates the
transcription of target genes, including the genes encoding the
pro-inflammatory cytokines, such as interleukin (IL)-1, -6, -8, -18
and tumor necrosis factor-α (TNF-α) as well as iNOS, COX-2 and cell
adhesion molecules (22–24). In turn, the products regulated by
NF-κB, such as TNF-α and IL-1β, also lead to the activation of
NF-κB. This fact means that there is a complex regulatory loop that
amplifies and perpetuates inflammatory responses; therefore, it has
become a biological target for new types of anti-inflammatory
treatment.
Based on this reason, we first hypothesized that
glyceollins, overproduced by an elicitor Rhyzopus sp., can
ameliorate immune cells by an NF-κB signaling pathway. To prove
this hypothesis, we compared whether glyceollins have the potential
to possess potent anti-inflammatory effects by inhibiting
LPS-induced iNOS and COX-2 expression, as well as various
inflammatory cytokines like TNF-α, IL-1β, IL-18 in RAW 264.7 cells.
We further examined whether glyceollins inhibit the LPS-induced
inflammation process by blocking an NF-κB signaling pathway.
Materials and methods
Materials and cell culture
LPS (purified from Escherichia coli 055:B5)
was purchased from Sigma-Aldrich (St. Louis, MO) and fetal bovine
serum (FBS) was purchased from Invitrogen (Carlsbad, CA).
Antibodies against iNOS, COX-2, IKKα/β, NF-κB p65, IκBα and
phospho-IκBα (Ser-32/36) were obtained from Cell Signaling
Technology (Beverly, MA). RAW 264.7 cells were cultured in a
RPMI-1640 medium supplemented with 10% of FBS, 2 mM of L-glutamine,
100 U/ml of penicillin, and 100 μg/ml of streptomycin at 37˚C in a
5% CO2 humidified incubator. We measured cell viability
by counting, with a stain of 0.2% trypan blue solution.
Preparation of glyceollin mixture by
elicitation
Glyceollins I, II and III (Fig. 1A, B and C) were semi-purified from
elicited soybeans, with a slight modification, as previously
described (8,11). In brief, we used soybeans (AGA,
variant no. 3, a gift from KNU Soyventure, Daegu, Korea) that
overproduce isoflavones to the rate of 10 mg/g of soybeans. The
soybeans were elicited by a Rhyzopus sp. for de novo
synthesis of glyceollins, which produced three kinds of isomers
(data not shown). The cultures of Rhyzopus sp. were grown at
25˚C in a culture room on potato dextrose agar media and the
inoculums were harvested after 5 days. We first washed the soybeans
by dipping in a 65% ethanol solution for 1 min; thereafter, we
washed them with deionized water before the soaking process.
Soybean seeds were soaked in sterile, deionized water for 6 h, and
then placed into a Sanyo MLR-351H growth chamber (Carlsbad, CA).
After saturation with distilled water, the seeds were cut into 4
pieces by a knife. A spore suspension (100 μl) of Rhyzopus
sp. was dispersed on the cut surfaces of the seeds. The seeds (20
g) were incubated at 26˚C for 4 days, extracted with 50 ml of 80%
(v/v) ethanol for 1 h, then cooled and centrifuged at 20,000 × g
for 10 min. The extracts were filtered by a membrane filter (0.45
μm) from Sartorius (Aubagne Cedex, France). Each crude extract was
freeze-dried and dissolved in dimethyl sulfoxide. The extract was
100 μg/ml, and was used for further purification. For purification,
we used a high-performance liquid chromatography system, the HPLC,
PerkinElmer Series 200 (Waltham, MA). A Brownlee Choice C18
(150×4.6 mm) reverse-phase column and guard column were used. The
injection volume and column temperature were 10 and 30 μl,
respectively. Detection was monitored at 260 and 285 nm, and the
flow rate was 1.0 ml/min at the following solvent condition (A,
acetonitrile, B, 1% acetate in water; 0 to 45% A for 10.2 min; 45
to 90% A for 6 min, holding at 90% A for 3.6 min). The glyceollins
were separated by thin-layer chromatography (TLC) for use in
further experiments because the concentration was low and the
amounts that we needed were small. The glyceollin phase was
separated by open-column chromatography (OCC). A methanolic extract
was fractionated on a Silica gel-60 (<0.063 mm: 0.063 mm, 8:2)
developed in methylene chloride:methanol (97–99:1–3, v/v) solution.
A sample of OCC extract was fractionated on aluminum-backed Silica
gel-60 TLC plates developed in hexane:acetone (4:1.5, v/v).
Glyceollins were visualized with 20% aqueous sulfuric acid spray
reagent, and UV light at 254 nm. A single glyceollin band (Rf=0.2)
was confirmed by a standard glyceollin, and further purified by
preparative TLC. The concentration of glyceollin isomers in the
fraction was up to ~90% as analyzed by preparative HPLC. The
relative ratio of glyceollin I, II and III in the fraction was
17:2:1. Thereafter, we designated the fraction as glyceollins.
Cell viability assay
Cell viability was measured by the MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay (25). Cells were seeded on
96-well plates at a concentration of 5×104 cells/well.
After seeding, cells were incubated with MTT solution (1 mg/ml) for
2 h. The medium was discarded and the formazan product was
solubilized with dimethyl sulfoxide. Viability was assessed by
measuring absorbance at 570 nm with a Bio-Rad microplate reader
(Hercules, CA).
Nitrite quantification
RAW 264.7 cells were treated with 1 μg/ml LPS plus
sample for 16 h. Nitric oxide synthesis can be determined by
assaying nitrite in the culture supernatant because nitrite is a
stable reaction product of NO (26). Briefly, cell-free culture media
were reacted with a 1:1 mixture of Griess reagent (1%
sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride and
2.5% phosphoric acid) at room temperature for 10 min. The OD of the
assay sample was measured spectrophotometrically at a wavelength of
570 nm. Nitrite concentration was calculated from a standard curve
prepared using NaNO2 under the same assay
conditions.
Western immunoblot analysis
Proteins were separated by SDS-PAGE and
immunoblotted onto a nitrocellulose membrane in a buffer containing
20% methanol, 25 mM of Tris, and 192 mM of glycine, as described
elsewhere (27). The membranes
were then blocked with 5% non-fat dry milk and incubated with the
primary antibody overnight. Subsequently, membranes were washed in
Tween-Tris buffer saline (TTBS), incubated for 4 h with horseradish
peroxidase-conjugated goat anti-mouse or goat anti-rabbit (1:4,000)
antibodies, and finally developed using an enhanced ECL system (KPL
Inc., Gaithersburg, MD). The membranes were then reprobed with a
β-actin antibody as a control.
Reverse transcription-polymerase chain
reaction
Total-RNA was extracted from RAW 264.7 cells using
an easy-BLUE total-RNA extraction kit from Intron Biotechnology
(Sungnam, Korea) according to the manufacturer's protocols and was
quantified by measuring absorbance at 260 nm. RNA was
reverse-transcribed using 2.5 μM oligo(dt) primers, 1 mM dNTPs, and
Moloney murine leukemia virus (MMLV) reverse transcriptase
(Promega, Madison, WI), and the resulting cDNAs were amplified with
SuperTherm DNA polymerase SR Product (Kent, UK). β-actin primers
were used to standardize the amount of RNA in each sample. PCR
products were resolved on 1% agarose gels and visualized by
ethidium bromide staining (28).
Measurement of prostaglandin E2
production
RAW 264.7 cells were subcultured in 6-well plates
treated with the indicated dose of glyceollins for 2 h in the
presence or absence of LPS (1 μg/ml) for 16 h (29). The culture media was collected for
determination of prostaglandin E2 (PGE2) concentration
by an enzyme immunoassay kit from Cayman (Ann Arbor, MI).
Nuclear extracts
RAW 264.7 cells were incubated with glyceollins and
LPS as indicated (30). The cells
were harvested in PBS containing 2% serum, washed twice with
ice-cold PBS, and resuspended in 500 μl of buffer A (10 mM of
HEPES, pH 7.9, 5 mM of MgCl2, 10 mM of KCl, 1 mM of
ZnCl2, 0.2 mM of EGTA, 1 mM of
Na3VO4, 10 mM of NaF, 0.5 mM of
dithiothreitol, 0.5 mM of PMSF and protease inhibitors). After the
cells had been incubated on ice for 10 min and lysed by adding 50
μl of 20% Nonidet P-40, to a final concentration of 2%, their
nuclei were harvested by centrifugation. The nuclear pellets were
then resuspended in 60 μl of extraction buffer (10 mM HEPES, pH
7.9, 5 mM MgCl2, 300 mM NaCl, 0.2 mM EGTA, 25% glycerol,
1 mM Na3VO4, 10 mM NaF, 0.5 mM
dithiothreitol, 0.5 mM PMSF and protease inhibitors), and incubated
on ice for 15 min. Nuclear debris was then removed by
centrifugation (13,000 rpm, 10 min), and the nuclear extracts were
subjected to gel shift analysis. Protein concentrations were
determined using a Bradford method (31).
Electrophoretic mobility shift assay
RAW 264.7 cells, in 10-cm diameter dishes
(107 cells/dish), were pretreated with or without
glyceollins for 2 h and then incubated with LPS (1 μg/ml) for 16 h.
For the gel shift assay, a consensus sequence for the NF-κB DNA
binding site, sc-2505 from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA), was used. The mutant binding sequence for NF-κB was
identical to sc-2505 except for a G→C substitution in the NF-κB-DNA
binding motif (sc-2511, Santa Cruz Biotechnology, Inc.).
Beforehand, the NF-κB probe was biotin-end labeled by the biotin 3′
end DNA labeling kit from Thermo Scientific Pierce (Rockford, IL).
Briefly, EMSA binding reactions were performed by incubating 20 μg
of nuclear extract with the annealed oligos with the LightShift
EMSA kit from Thermo Scientific Pierce, according to the
manufacturer's instructions. The reaction mixture was subjected to
electrophoresis on a 4% native gel in a 0.5xTBE buffer. After
transfer, the membrane was immediately cross-linked for 15 min on a
UV transilluminator equipped with 312 nm bulbs. A chemiluminescence
detection method utilizing a luminol/enhancer solution and a stable
peroxide solution from Thermo Scientific Pierce was used as
described by the manufacturer's manual, and the membranes were
exposed to X-ray films for 2–5 min before developing (32).
Statistical analysis
Statistical differences between mean values ± SD
were determined by the Dunnett's multiple range test. The
significance was set at P<0.05 (33).
Results
Effect of glyceollins on cell
viability
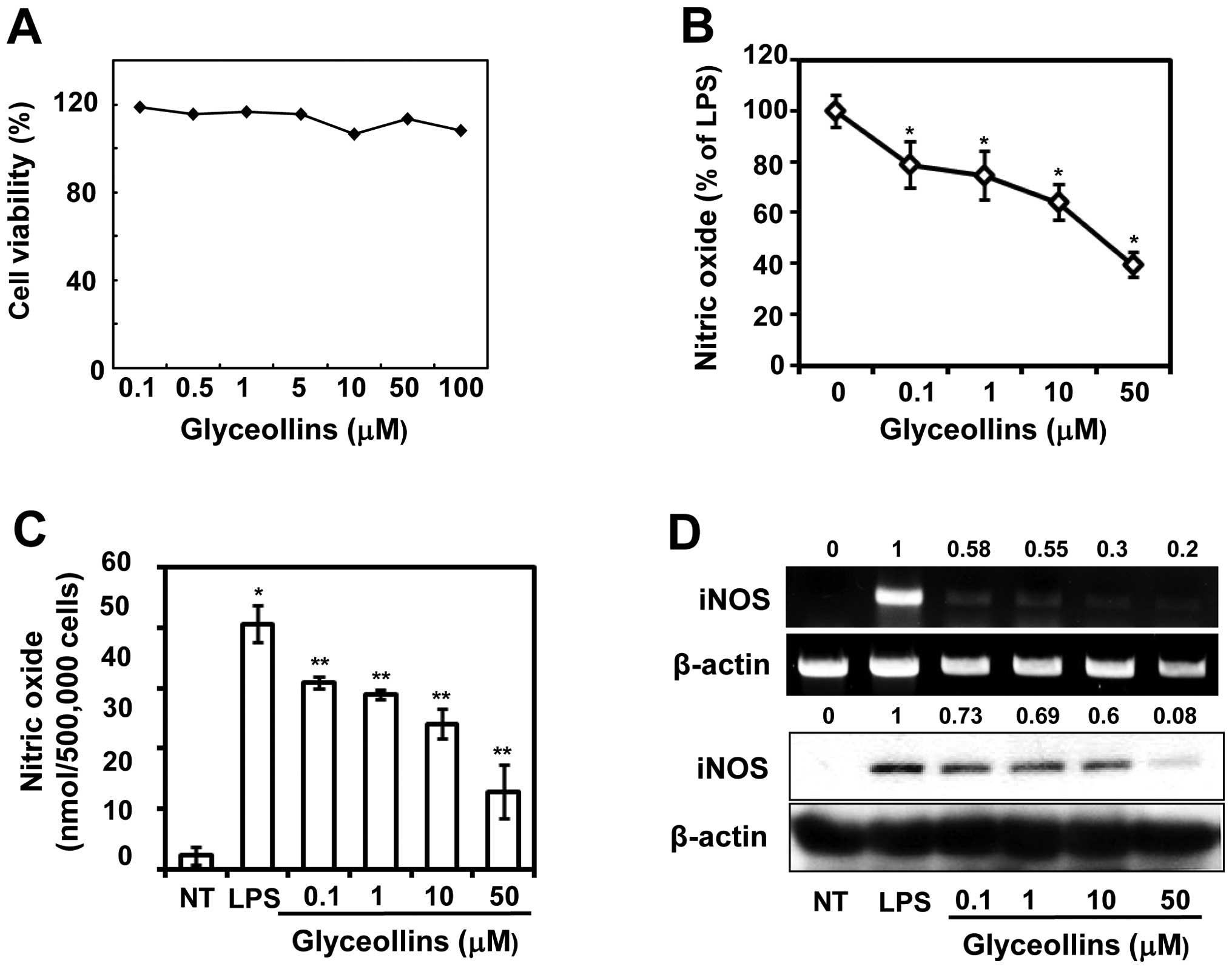
To examine whether glyceollins exhibit cytotoxicity
in cells, we first measured whether glyceollins affect cell
proliferation in RAW 264.7 cells. The result showed that, at
concentrations up to 100 μM, glyceollins had no toxic effect on
cell viability (Fig. 1A). Major
and/or minor fractions did not have any morphological changes in
microscopic observation (data not shown). Therefore, we decided to
investigate whether glyceollins have potential in reducing iNOS and
COX-2 expressions, which could be a landmark for the assessment of
molecular inflammation.
Effects of glyceollins on NO production
and expression of iNOS in LPS-stimulated RAW 264.7 cells
To assess the inhibitory effect of glyceollins on
LPS-induced NO production in RAW 264.7 cells, the cells were
treated with LPS (1 μg/ml) for 16 h after treatment in the presence
or absence of glyceollins (0.1, 1, 10 or 50 μM) for 2 h. The amount
of nitrite, a stable metabolite of NO, was used as the indicator of
NO production in the medium. During the 16 h of incubation, RAW
264.7 cells produced up to 6.4±0.02 μM of nitrite in the resting
state. When LPS (1 μg/ml) was added, NO production was dramatically
increased up to 56.2±0.01 μM (Fig.
1B). In this condition, adding glyceollins inhibited
LPS-induced NO production in a concentration-dependent manner
corresponding to 10.6 and 58.7% inhibition at 0.1 and 50 μM,
respectively (Fig. 1C). To
further investigate whether the inhibitory effect of glyceollins on
NO production was associated with the inhibition of corresponding
gene expression, the protein and mRNA expressions of iNOS were
determined by semi-quantitative RT-PCR and western blot analysis,
respectively. In unstimulated RAW 264.7 cells, the iNOS mRNA and
protein expressions were almost undetectable (Fig. 1D, first bands of each set);
however, LPS treatment augmented the protein and mRNA expressions
of iNOS remarkably; pretreatment of the cells with different
concentrations of glyceollins also dramatically reduced LPS-induced
iNOS mRNA and protein expressions in a concentration-dependent
fashion (Fig. 1D, compare the
second and sixth bands of each set at 0 to 50 μM). The intensity
was a 5- and 12.5-fold decreased compared with that of LPS-treated
cells. The data suggest that glyceollins can downregulate
LPS-induced iNOS expression at the transcription level.
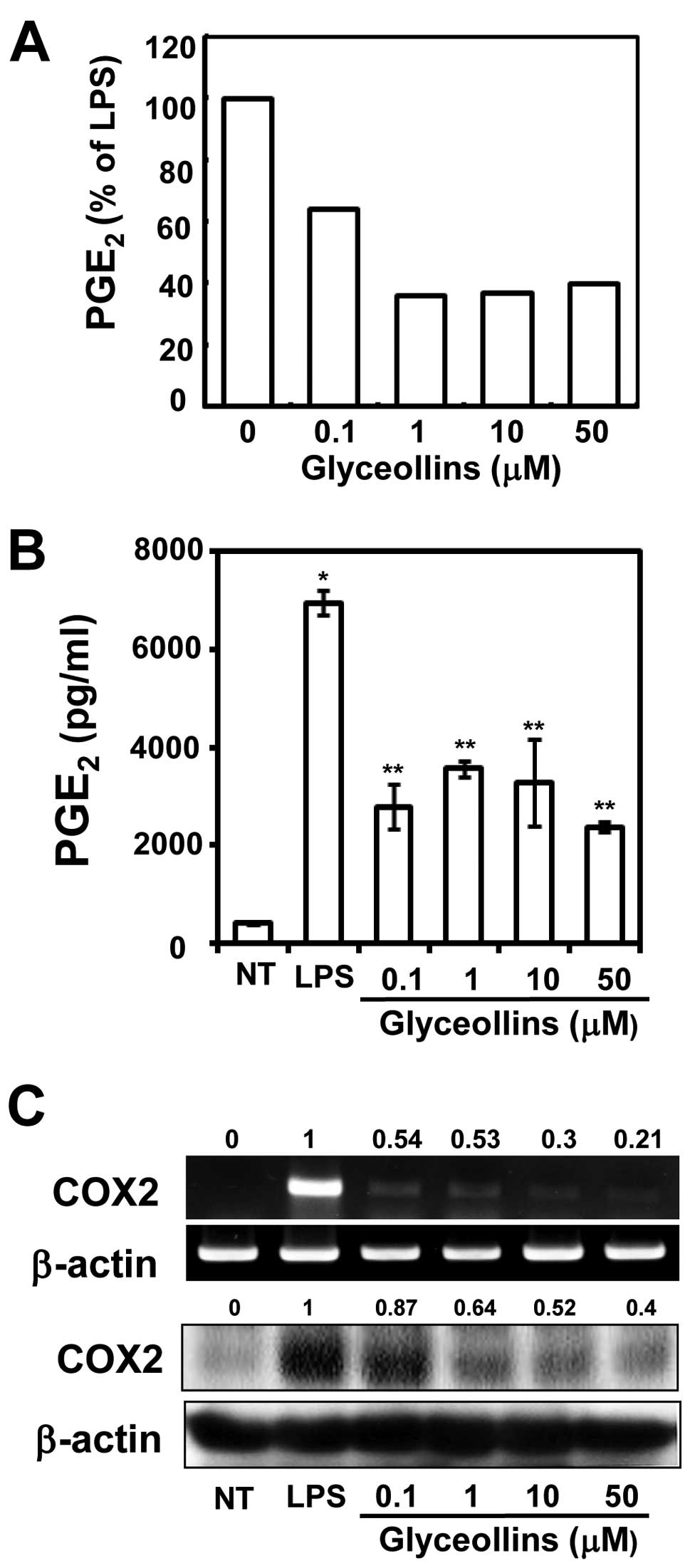
Effects of glyceollins on PGE2
production and COX-2 expression in LPS-stimulated RAW 264.7
cells
To examine whether glyceollins inhibit
PGE2 production, the cells were pre-incubated with
glyceollins for 2 h and then activated with 1 μg/ml of LPS for 16
h. As shown in Fig. 2A,
unstimulated RAW 264.7 cells mildly decreased PGE2
production when compared with treatment with glyceollins alone. In
Fig. 2B, LPS induced a 5.2-fold
increase in the biosynthesis of PGE2 as compared with
untreated cells, but glyceollins strongly inhibited the production
of PGE2 in a dose-dependent manner. Because the
biosynthesis of PGE2 is catalyzed by COX-1 and COX-2
enzymes, we next measured the effect of glyceollins on LPS-induced
COX-2 activities. We also detected that glyceollins inhibited the
COX-2 mRNA and protein expressions in a dose-dependent manner
(Fig. 2C, 5- and 2.5-fold,
respectively). The data suggest that glyceollins can downregulate
LPS-induced COX-2 expression at the transcription level. Inhibition
of COX-2 expression by glyceollins was responsible for the decrease
of PGE2 production.
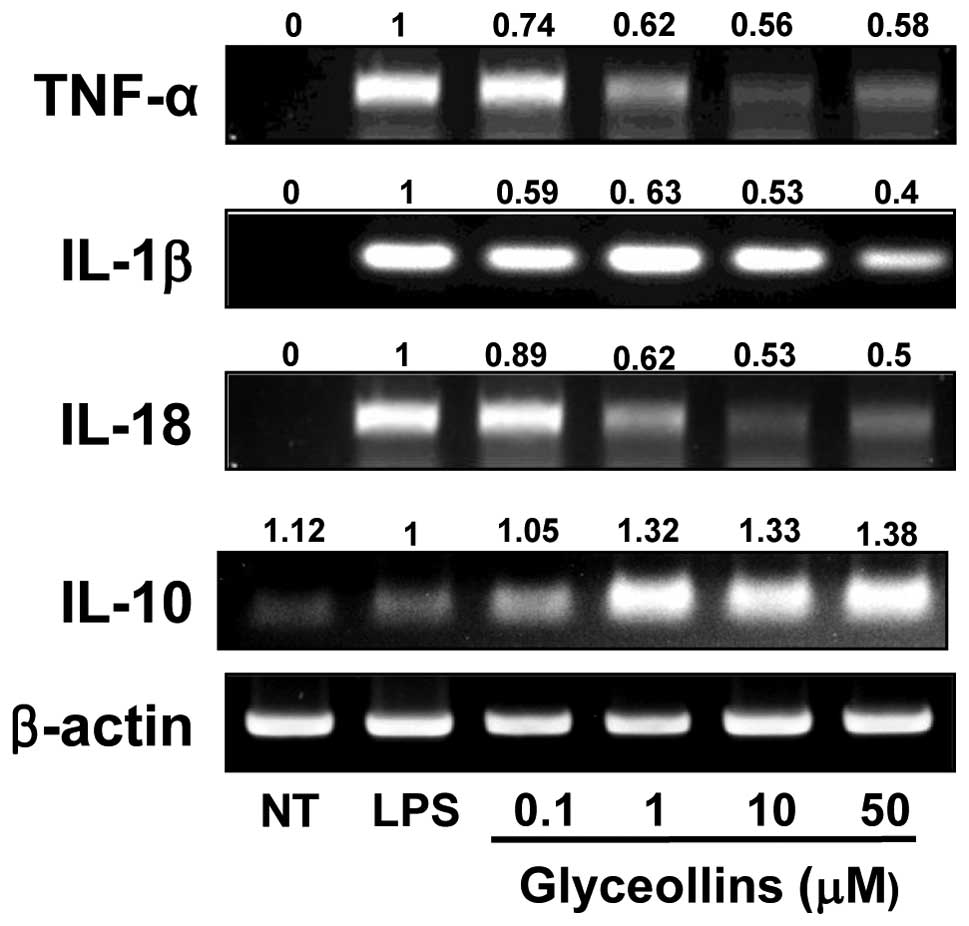
Effects of glyceollins on LPS-induced
TNF-α, IL-1β, IL-18 and IL-10 mRNA expression
Glyceollins were found to inhibit the
pro-inflammatory mediators, such as NO and PGE2, most
potently. Therefore, to test whether glyceollins could effectively
for regulate inflammatory and anti-inflammatory cytokines in RAW
264.7 cells, we examined the mRNA levels of TNF-α, IL-1β, IL-18 and
IL-10 by RT-PCR. Though IL-1β mRNA expression was only slightly
inhibited in a dose-dependent manner (Fig. 3), TNF-α and IL-18 mRNA expression
was significantly reduced by pretreatment of RAW 264.7 cells with
glyceollins (0.1, 1, 10 or 50 μM) (Fig. 3). In contrast, in terms of the
anti-inflammatory cytokines, the ability of glyceollins to induce
IL-10 increased up to 38±0.5% in the LPS-activated RAW 264.7 cells
(Fig. 3), while that of β-actin
was not altered.
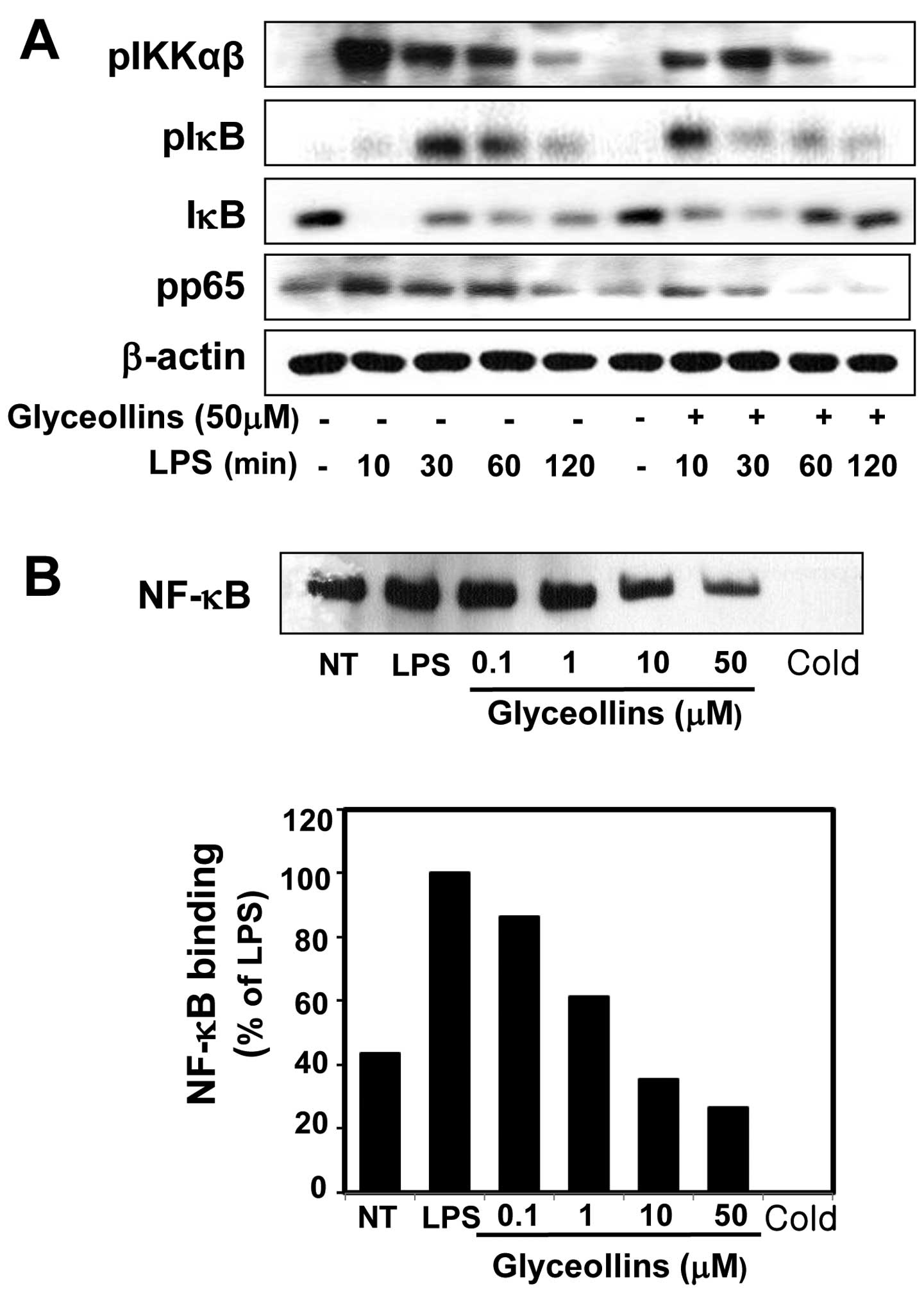
Inhibitory effects of glyceollins on IκBα
kinase activation and IκBα phosphorylation
Because the phosphorylation of IKK, and its
subsequent phosphorylation of IκBα, are key signals for the
activation of NF-κB, we examined the effect of glyceollins on
LPS-induced phosphorylation of IKK and the degradation of IκBα
protein by western blot analysis. A time-course experiment showed
that IκBα in the cytoplasm was almost completely degraded within 10
min, and that it recovered at 30 min, after LPS (1 μg/ml)
stimulation (Fig. 4A).
Pretreatment with glyceollins prevented the induced degradation of
IκBα protein at 5 and 15 min; the recovery of IκBα, which is under
the control of NF-κB, was also suppressed (data not shown). IκBα
phosphorylation was also examined by western blot analysis. As
shown in Fig. 4A, the
phosphorylated form of IκBα was hardly detectable in the resting
RAW 264.7 cells; however, upon exposure to LPS (1 μg/ml) alone for
10 min, IκBα phosphorylation was initiated. At 30 min after LPS
stimulation, pretreatment of glyceollins moderately inhibited
LPS-mediated IκBα phosphorylation (Fig. 4A). Because IKK-α and β are the
upstream kinases of IκB in the NF-κB signaling pathway and are
activated via phosphorylation, we examined the effect of
glyceollins on LPS-induced IKKα/β activations by western blot
analysis using an a phospho-specific IKKα/β antibody. RAW 264.7
cells were pretreated with glyceollins (50 μM) for 2 h and then
stimulated with LPS (1 μg/ml) for various courses of time. As shown
in Fig. 4A, LPS was found to
strongly induce IKKα/β phosphorylation, whereas glyceollin
pretreatment significantly inhibited this phosphorylation. These
findings indicate that glyceollins suppressed IKK and IκBα
phosphorylation in LPS-induced RAW 264.7 cells.
Effects of glyceollins on LPS-induced
NF-κB activation
To investigate whether glyceollins affect the DNA
binding ability of the NF-κB complex in the RAW 264.7 cells, we
performed an electrophoretic mobility shift assay (EMSA). RAW 264.7
cells were pretreated with 0.1–50 μM of glyceollins for 2 h, and
then the cells were stimulated with LPS for 16 h. RAW 264.7 cells
with LPS strongly induced the DNA binding activity of NF-κB. In
contrast, pretreatment with glyceollins significantly suppressed
the induced the DNA-binding activity of NF-κB by LPS in a
dose-dependent manner (Fig. 4B).
Taken together, the above findings indicate that glyceollins
suppress NO production as well as expressions of iNOS, COX-2,
TNF-α, IL-1β and IL-18, at least in part via an NF-κB-dependent
mechanism.
Discussion
Phytoalexin is now a well-documented self-defense
biomaterial that is produced by plants, and plays a critical role
in exhibiting various biological events in animal and plant tissues
(7,8). Recently, it has been revealed that
these compounds have potential in ameliorating antioxidant,
antifungal and antidiabetic activities in vitro and in
vivo (34). In soybeans,
glyceollin, a family of lipophilic phytoalexins, as a secondary
metabolite, is often accumulated at sites infected by pathogens
like Phytophthora sojae to inhibit their growth (35). The compound is also known to be
induced by countless stress factors or physical stimuli, such as
freezing, UV light exposure, and/or microbes (34,36). Because bean, grape, and sunflower
seeds have been known to possess various biological activities,
many researchers have been focused on the isolation and
purification of active compounds; but this process was not
convenient to obtain enough material for experimentation. The
present approach of obtaining glyceollins deserves further study,
especially since the connection between the signaling pathways and
glyceollins has not been discovered so far. Although glyceollin
and/or their derivatives have been investigated in relation to
their biological activities, including anti-estrogenic activity
(9–13), their precise mechanisms of
signaling are scarcely understood at the present time.
Therefore, in the present study we first
hypothesized that glyceollins effectively protect against the
generation of pro-inflammatory mediators, specifically, NO and
PGE2, through the inhibition of iNOS and COX-2
expression levels, respectively. We subsequently confirmed that the
inhibition of NO production is concurrent with the suppression of
iNOS expression at the mRNA and protein levels as shown by RT-PCR
and western blot analysis (Fig.
1D). In addition, we investigated another mediator of
inflammation, COX-2, which acts as a rate-limiting enzyme in the
synthesis of prostaglandin-like PGE2. In line with our
prediction, glyceollins strongly inhibited PGE2
production and COX-2 mRNA and protein levels (Fig. 2). TNF-α plays a key role in the
induction of various genes, such as COX-2, through the activation
of NF-κB by T cells and macrophages (37). To alleviate the inflammatory
response in LPS-stimulated macrophages, it is also necessary to
hamper IL-1β and IL-18 production, which both enhance the body's
inflammatory response. These cytokines are known to be increased
through the NF-κB signaling pathway (38–40). On the other side, IL-10 is a
representative anti-inflammatory cytokine and has pleiotropic
effects during the immunoregulation and inflammation processes in
various immune cells (41). As
shown in our data, glyceollins are effective for
inflammation-related cytokines such as TNF-α, IL-1β, IL-18 or IL-10
expression using RT-PCR (Fig. 3).
We clearly found that glyceollins are potent inhibitors of TNF-α
production by RAW 264.7 macrophages with ~10 μM of an
IC50 value; this is similar to luteolin and quercetin,
which have been the most potent natural products so far at
inhibiting TNF-α release, with IC50 values of <1 μM
(42) and <5 μM, respectively
(data not published).
It is well-recognized that NF-κB, a universal
transcription factor, plays a pivotal role in the various soluble
pro-inflammatory gene expressions and leukocyte adhesion molecules
(38,39). As a key step to activate NF-κB
functions, studies have been focused on the activation of the IKK
complex over the last several years. Many natural phytochemicals,
such as luteolin, quercetin, and resveratrol, suppress LPS-induced
iNOS and COX-2 expressions as well as inflammatory cytokine
expressions in macrophages by inhibiting the NF-κB signaling
pathway, such as phosphorylation of IKK, degradation of IκBα, and
nuclear translocation of NF-κB (43,44). Because the glyceollin-mediated
signaling pathway can provide us with useful information on the
function of the agent, we next studied the NF-κB-mediated signaling
pathway in RAW 264.7 cells. In our study, western blot analysis
revealed that glyceollins inhibited the LPS-induced phosphorylation
of IKK, a series of phosphorylations of IκBα and degradation of
p-IκB (Fig. 4A). We also
demonstrated that glyceollins inhibit LPS-induced NF-κB in RAW
264.7 cells by performing EMSA. Pretreatment with glyceollins
significantly suppressed the induced DNA-binding activity of NF-κB
by LPS in a dose-dependent manner (Fig. 4B). Therefore, the above results
suggest that glyceollins suppress the inflammation reaction through
an NF-κB-dependent signal transduction pathway. Up to now, no data
regarding a glyceollin-mediated signaling pathway has been
investigated. As a result, our data have the potential to help
develop prophylactic and therapeutic anti-inflammatory agents that
are both safe and original. Further study of the overall signal
transduction pathway promises to be rewarding because the
inhibitory mechanism by glyceollins can provide new data for
anti-inflammatory therapies.
This study describes a signaling pathway of the
anti-inflammatory effects elicited glyceollins. Our results suggest
that the glyceollins obtained from soybeans may exhibit increased
anti-inflammatory effects. Glyceollins have an appreciable
inhibitory activity against the overproduction of inflammatory
mediators such as iNOS, COX-2 and various cytokines. Not only can
they be used to investigate their effects in various biological
areas, but if we uncover the precise mechanisms by glyceollins in
immune cells, they can also be used to prevent immune diseases. Use
of traditionally fermented soybean products may prove rewarding
because we can naturally obtain their high levels of phytoalexins,
such as glyceollins.
Acknowledgements
This study was supported by a grant by the Ministry
of Knowledge and Economy, Republic of Korea, under contract no.
70000385 (Y.-H.K.), and partially by a Kyungpook National
University Research Fund (S.-H.L.).
Abbreviations:
|
NO
|
nitric oxide
|
|
iNOS
|
inducible nitric oxide synthase
|
|
COX-2
|
cyclooxygenase-2
|
|
PGE2
|
prostaglandin
|
|
IKK
|
IκBα kinase
|
|
TNF
|
tumor necrosis factor
|
|
IL
|
interleukin
|
References
|
1
|
N TorresI Torre-VillalvazoAR
TovarRegulation of lipid metabolism by soy protein and its
implication in diseases mediated by lipid disordersJ Nutr
Biochem17365373200610.1016/j.jnutbio.2005.11.00516481155
|
|
2
|
TB NgXJ YeJH WongEF FangYS ChanW PanXY
YeSC SzeKY ZhangF LiuHX WangGlyceollin, a soybean phytoalexin with
medicinal propertiesAppl Microbiol
Biotechnol5890599068201121336922
|
|
3
|
WO SongOK ChunI HwangHS ShinBG KimKS KimSY
LeeD ShinSG LeeSoy isoflavones as safe functional ingredientsJ Med
Food10571580200710.1089/jmf.2006.062018158825
|
|
4
|
CD AllredKF AllredYH JuTS GeoppingerDR
DoergeWG HelferichSoy processing influences growth of estrogen
dependent breast cancer
tumorsCarcinogenesis2516491657200510.1093/carcin/bgh17815131010
|
|
5
|
M MessinaSoy foods and soybean
phytoestrogens (isoflavones) as possible alternatives to hormone
replacement therapy (HRT)Eur J
Cancer367177200010.1016/S0959-8049(00)00233-111056326
|
|
6
|
DS LeeSH LeeGenistein, a soy isoflavone,
is a potent alpha-glucosidase inhibitorFEBS
Lett5018486200110.1016/S0014-5793(01)02631-X11457461
|
|
7
|
P JeandetAC Douillet-BreuilR BessisS
DebordM SbaghiM AdrianPhytoalexins from the Vitaceae: biosynthesis,
phytoalexin gene expression in transgenic plants, antifungal
activity, and metabolismJ Agric Food
Chem5027312741200210.1021/jf011429s11982391
|
|
8
|
SM BouéCH CarterKC EhrlichTE
ClevelandInduction of the soybean phytoalexins coumestrol and
glyceollin by AspergillusJ Agric Food
Chem82167172200010888516
|
|
9
|
ME BurowSM BouéBM Collins-BurowLI MelnikBN
DuongCH Carter-WientjesS LiTE WieseTE ClevelandJA
McLachlanPhytochemical glyceollins, isolated from soy, mediate
antihormonal effects through estrogen receptor α and βJ Clin
Endocrinol Metab8617501758200111297613
|
|
10
|
GN NikovNE HopkinsS BoueWL
AlworthInteractions of dietary estrogens with human estrogen
receptors and the effect on estrogen receptor-estrogen response
element complex formationEnviron Health
Perspect108867872200010.1289/ehp.0010886711017892
|
|
11
|
HJ KimHJ SuhJH KimS ParkYC JooJS
KimAntioxidant activity of glyceollins derived from soybean
elicited with Aspergillus sojaeJ Agric Food
Chem581163311638201010.1021/jf102829z21033668
|
|
12
|
VA SalvoSM BouéJP FonsecaS ElliottC
CorbittBM Collins-BurowTJ CurielSK SrivastavBY ShihCC
WientjesAntiestrogenic glyceollins suppress human breast and
ovarian carcinoma tumorigenesisClin Cancer
Res1271597164200610.1158/1078-0432.CCR-06-142617145841
|
|
13
|
CE WoodTB ClarksonSE ApptAA FrankeSM
BouéME BurowT McCoyJM ClineEffects of soybean glyceollins and
estradiol in postmenopausal female monkeysNutr
Cancer567481200610.1207/s15327914nc5601_1017176220
|
|
14
|
T HenkelU ZabelE FanningPA
BaeuerleIntramolecular masking of the nuclear location signal and
dimerization domain in the precursor for the p50 NF-κB
subunitCell681121113319921547506
|
|
15
|
M KarinM DelhaseThe IκB kinase (IKK) and
NF-κB: key elements of proinflammatory signalingSemin
Immunol1285982000
|
|
16
|
S GhoshM KarinMissing pieces in the NF-κB
puzzleCell109SupplS81S962002
|
|
17
|
MS HaydenS GhoshSignaling to NF-κBGenes
Develop18219522242004
|
|
18
|
E ZandiM KarinBridging the gap
composition, regulation and physiological function of the IκB
kinase complexMol Cell Biol1945474551199910373503
|
|
19
|
T WangX ZhangJJ LiThe role of NF-κB in the
regulation of cell stress responsesInt
Immunopharmacol2150915202002
|
|
20
|
F YangE TangK GuanCY WangIKK plays an
essential role in the phosphorylation of RelA/p65 on serine 536
induced by lipopolysaccharideJ
Immunol17056305635200310.4049/jimmunol.170.11.563012759443
|
|
21
|
HL PahlActivators and target genes of
Rel/NF-κB transcription factorsOncogene18685368661999
|
|
22
|
JT WuJG KralThe NF-κB/IκB signaling
system: a molecular target in breast cancer therapyJ Surg
Res1231581692005
|
|
23
|
R LangRL RutschmanDR GreavesPJ
MurrayAutocrine deactivation of macrophages in transgenic mice
constitutively overexpressing IL-10 under control of the human CD68
promoterJ
Immunol16834023411200210.4049/jimmunol.168.7.340211907098
|
|
24
|
LL CheshireAS Baldwin JrSynergistic
activation of NF-κB by tumor necrosis factor α and interferon via
enhanced IκBα degradation and de novo IκBα degradationMol Cell
Biol17674667541997
|
|
25
|
J KlostergaardA rapid extremely sensitive,
quantitative microassay for cytotoxic cytokinesLymphokine
Res430931719853876491
|
|
26
|
CJ LowensteinEW AlleyP RavalAM SnowmanSH
SnyderSW RussellWJ MurphyMacrophage nitric oxide synthase gene: two
upstream regions mediate induction by interferon gamma and
lipopolysaccharideProc Natl Acad Sci
USA9097309734199310.1073/pnas.90.20.97307692452
|
|
27
|
CH ParkDY NamHU SonSR LeeHJ LeeJC HeoTY
ChaJH BaekSH LeePolymer fraction of Aloe vera exhibits a
protective activity on ethanol-induced gastric lesionsInt J Mol
Med275115182011
|
|
28
|
MH PanYH ChangML TsaiCS LaiSY HoV
BadmaevCT HoPterostilbene suppressed lipopolysaccharide-induced
up-expression of iNOS and COX-2 in murine macrophagesJ Agric Food
Chem5675027509200810.1021/jf800820y18656926
|
|
29
|
N KusunokiK KitaharaF KojimaN TanakaK
KanekoH EndoT SuguroS KawaiAdiponectin stimulates prostaglandin
E(2) production in rheumatoid arthritis synovial
fibroblastsArthritis
Rheum6216411649201010.1002/art.2745020222108
|
|
30
|
KS AhnEJ NohHL ZhaoSH JungSS KangYS
KimInhibition of inducible nitric oxide synthase and cyclooxygenase
II by Platycodon grandiflorum saponins via suppression of
nuclear factor-κB activation in RAW 264.7 cellsLife
Sci7623152328200510.1016/j.lfs.2004.10.04215748625
|
|
31
|
MM BradfordA rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye bindingAnal
Biochem72248254197610.1016/0003-2697(76)90527-3942051
|
|
32
|
I AndújarMC RecioT BacelliRM GinerJL
RíosShikonin reduces oedema induced by phorbol ester by interfering
with IκBα degradation thus inhibiting translocation of NF-κB to the
nucleusBr J Pharmacol160376388201020423347
|
|
33
|
M SonA KimJ LeeCH ParkJC HeoHJ LeeSH
LeeEthanol extract of Lycoris radiata induces cell death in
B16F10 melanoma via p38-mediated AP-1 activationOncol
Rep244734782010
|
|
34
|
JD PaxtonBiosynthesis and accumulation of
legume phytoalexinsMycotoxins and PhytoalexinsRP SharmaDK
SalunkheCRC PressBoca Raton4854991991
|
|
35
|
LI Rivera-VargaAF SchmitthennerTL
GrahamSoybean flavonoid effects on metabolism by Phytophthora
sojaePhytochemistry32851857199310.1016/0031-9422(93)85219-H
|
|
36
|
S FengCL SawYK LeeD HuangFungal-stressed
germination of black soybeans leads to generation of
oxooctadecadienoic acids in addition to glyceollinsJ Agric Food
Chem5585898595200710.1021/jf071673517892258
|
|
37
|
E AndreakosTargeting cytokines in
autoimmunity: new approaches, new promiseExpert Opin Biol
Ther3435447200310.1517/14712598.3.3.43512783612
|
|
38
|
F ChenLM DemersX ShiUpstream signal
transduction of NF-κB activationCurr Drug Targets Inflamm
Allergy11371492002
|
|
39
|
D KulmsT SchwarzNF-κB and cytokinesVitam
Horm742833002006
|
|
40
|
K SukS KimU KimRegulation of IL-18
production by IFN gamma and PGE2 in mouse microglial
cells: involvement of NF-κB pathway in the regulatory
processesImmunol Lett777985200111377701
|
|
41
|
S MocellinMC PanelliE WangD NagorsenFM
MarincolaThe dual role of IL-10Trends
Immunol243643200310.1016/S1471-4906(02)00009-1
|
|
42
|
AT PaulVM GohilKK BhutaniModulating TNF-α
signaling with natural productsDrug Discov Today117257322006
|
|
43
|
DM MinichJS BlandDietary management of the
metabolic syndrome beyond macronutrientsNutr
Rev66429444200810.1111/j.1753-4887.2008.00075.x18667004
|
|
44
|
PA RuizD HallerFunctional diversity of
flavonoids in the inhibition of the proinflammatory NF-kappaB, IRF,
and Akt signaling pathways in murine intestinal epithelial cellsJ
Nutr136664671200616484540
|