Introduction
Lung cancer is the major cause of cancer-related
mortality worldwide and is also one of the most frequent causes of
death in Korea (1). The high
mortality of lung cancer generally results from the difficulties in
early diagnosis and the lack of effective therapeutic methods.
Established therapeutic methods for lung cancer are surgical
resection, chemotherapy, and radiotherapy. The results remain
unsatisfactory, making it imperative that new diagnostic and
therapeutic methods be developed. Immunotherapy is one of the
alternative treatments for lung cancer, because various
immunotherapies seem to improve the prognosis of patients with lung
cancer (2).
An essential condition for the development of
effective immunotherapeutic strategies is the existence and
identification of tumor specific antigens that are either
exclusively or preferentially expressed in malignant compared to
normal tissues (3). Human tumor
antigens are classified in several categories, including
differentiation antigens (4),
mutated gene products (5),
overexpressed oncogenes (6), and
cancer/testis antigens (CT) (7,8).
CT antigens are immunogenic proteins expressed in
normal testis and in different types of tumors (3,9).
CT antigens are promising candidates for cancer immunotherapy and
the identification of novel CT antigens is a prerequisite for the
development of cancer vaccines (10,11). CT antigens have previously been
isolated by various methods. Examples of CT antigens are the T-cell
defined MAGE (12), BAGE
(13), and GAGE (14) antigens, as well as SSX-2 (15), NY-ESO-1 (16), SCP-1 (17), CT-7 (8), NY-SAR-35 (18), and NY-TLU-57 (19), all which have been defined using
SEREX (the serological identification of antigens by recombinant
expression cloning) (20). To
date, more than 100 CT antigens have been identified and their
expression studied in numerous cancer types (11). Only 19 protein products of CT
antigen families have been demonstrated to be able to elicit an
immune response in humans (3,11).
Though little is known about the biological function of CT
antigens, knowledge about their presence in lung cancer tissues can
have important implications for the understanding of the biology of
both lung cancer and cancer immunity. Previous analysis of the CT
antigens revealed that some of them are also expressed in lung
cancer (21,22). However, little is known about
analysis of a larger panel of CT antigens especially in lung cancer
tissues.
The present study analyzes the frequency of
expression of 13 CT antigens in 79 lung cancer tissues. The
correlation between CT antigen expression patterns and pathological
characteristics of lung cancer tissues was also studied.
Materials and methods
Lung cancer tissues
Human tumor tissues were obtained from lung cancer
operations performed at the Pusan National University Hospital,
Busan, Korea. Analyses were performed on tumor tissue samples of 79
patients with lung cancer confirmed pathologically. The tumor
samples consisted of 33 cases of adenocarcinoma, 24 of squamous
cell carcinoma, 6 of neuroendocrine carcinoma, 4 of pleomorphic
carcinoma, and 12 of others (3 adenosquamous carcinoma, 2
unclassified non-small cell carcinoma, 2 small cell carcinoma, 1
small round cell sarcoma, and 4 metastatic cancers). Among the 79
lung cancer patients from whom tissue samples were obtained, 68 had
available records, but 11 did not. Data for the 68 tissues
including gender, age, classification of TNM status, and stages
were obtained from the clinical and pathological records. Tumor
stage and progression were classified according to the
International Staging System (23).
Total-RNA extraction from lung cancer
tissues
Total cellular RNA was extracted from frozen tissue
specimens of 79 lung cancer samples, using TRI Reagent (Molecular
Research Center, Inc.). RNA extraction using TRIzol (Invitrogen
Life Technologies, Carlsbad, CA), a common protocol, was used.
Tumor tissues removed at surgery were snap-frozen and stored at
−70°C. Total-RNA was isolated from ~100 mg of each tissue sample
using 1 ml TRI Reagent (Molecular Research Center, Inc.), extracted
with chloroform, precipitated with isopropyl alcohol, washed with
ethanol and re-dissolved in RNase-free water. The amount of
isolated RNA was measured by a spectrophotometer (Ultrospec 2000,
Pharmacia Biotech) at 260 nm.
Reverse transcription (RT)-PCR
The cDNA preparations used as templates in the
RT-PCR reactions were prepared by using 500 ng of total-RNA in
conjunction with the SuperScript First Strand Synthesis kit
(Invitrogen Life Technologies).
The 13 primer sets for the 13 CT antigens and the
lengths of each PCR product are shown in Table I. Each 20 μl PCR mixture consisted
of 2 μl cDNA, 0.4 μl of 10 mM dNTP-mix (Solgent), 2 μl of 10X Taq
buffer (Solgent), gene specific forward and reverse primers, and
0.2 μl of TaqDNA polymerase (Solgent). For PCR, DNA polymerase
activation was performed for 5 min at 94°C, and then amplification
was performed in a 96-well Gene Amp PCR System 9700 for 35 cycles
as follows: 1 min at 94°C; 1 min at the respective annealing
temperature as indicated in Table
I; 1 min at 72°C and was concluded with a final extension step
of 10 min at 72°C. A 20 μl aliquot of each reaction was
size-fractionated on a 1.5% agarose gel, visualized by ethidium
bromide staining and assessed for products of the expected
size.
 | Table IPrimers used for RT-PCR. |
Table I
Primers used for RT-PCR.
| Genes | Primer
sequences | Annealing
temperature (°C) | References |
|---|
| NY-SAR-35 | F:
5′-CTTGGTGCGATCAGCCTTAT-3′ | | |
| R:
5′-TTGATGCATGAAAACAGAACTC-3′ | 55 | (18) |
| SCP-1 | F:
5′-GTACAGCAGAAAGCAAGCAACTGAATG-3′ | | |
| R:
5′-GAAGGAACTGCTTTAGAATCCAATTTCC-3′ | 60 | (18) |
| SSX-1 | F:
5′-CTAAAGCATCAGAGAAGAGAAGC-3′ | | |
| R:
5′-AGATCTCTTATTAATCTTCTCAGAAA-3′ | 60 | (18) |
| SSX-2 | F:
5′-GTGCTCAAATACCAGAGAAGATC-3′ | | |
| R:
5′-TTTTGGGTCCAGATCTCTCGTG-3′ | 65 | (18) |
| SSX-4 | F: 5′-AAA
TCGTCTATGGTATATGAAGCT-3′ | | |
| R:
5′-GGGTCGCTGATCTCTTCATAAAC-3′ | 60 | (18) |
| MAGE-1 | F:
5′-GCTGGAACCCTCACTGGGTTGCC-3′ | | |
| R:
5′-CGGCCGAAGGAACCTGACCCAG-3′ | 62 | (18) |
| MAGE-3 | F:
5′-GAAGCCGGCCCAGGCTCG-3′ | | |
| R:
5′-GGAGTCCTCATAGGATTGGCT-3′ | 62 | (18) |
| MAGE-4 | F:
5′-GAGCAGACAGGCCAACCG-3′ | | |
| R:
5′-AAGGACTCTGCGTCAGGC-3′ | 65 | (18) |
| MAGE-10 | F:
5′-GGAACCCCTCTTTTCTACAGAC-3′ | | |
| R:
5′-TCCTCTGGGGTGCTTGGTATTA-3′ | 60 | (18) |
| CT-7 | F:
5′-GACGAGGATCGTCTCAGGTCAGC-3′ | | |
| R:
5′-ACATCCTCACCCTCAGGAGGG-3′ | 60 | (18) |
| NY-TLU-57 | F:
5′-TCATATGCCTAGCTCTGTCAAAAG-3′ | | |
| R:
5′-TCCCGGGTCTGGCATCAATAAAAT-3′ | 60 | (19) |
| NY-ESO-1 | F:
5′-CCCCACCGCTTCCCGTG-3′ | | |
| R:
5′-CTGGCCACTCGTGCTGGGA-3′ | 60 | (19) |
| LAGE-1 | F:
5′-CTGCGCAGGATGGAAGGTGCCCC-3′ | | |
| R:
5′-GCGCCTCTGCCCTGAGGGAGC-3′ | 62 | (19) |
Immunohistochemistry
The M3H67 monclonal antibody (to MAGE-3) was
obtained from the Ludwig Institute for Cancer Research, New York
Branch at the Memorial-Kettering Cancer. Immunohistochemical
staining was performed according to the previously report (24). Briefly, paraffin sections were
applied to slides for immunohistochemistry and heated for 20 min at
60°C. Slides were deparaffinized and rehydrated in a series of
graded alcohols. Antigen retrieval was performed by placing the
slides in TE buffer (pH 9.0) and heating for 40 min in a steamer
and then allowed to cool. M3H67 of 1.0 μg/ml antibody was incubated
for 1 h in a room temperature. Sections were washed with PBS for 20
min and then slides were incubated with a secondary antibody for 1
h at 37°C, washed and incubated with the avidin-biotin complex
system for 1 h at 37°C. Sections were sequentially washed and then
slides were counterstained with hematoxylin. The extent of staining
was estimated and graded as follows: focal staining of single cells
or small clusters (>50% total) was considered positive, whereas
focal staining of single cells or small clusters (<50% total)
was considered negative.
Statistical analysis
Statistical analysis was performed with the SPSS
program (version 11.5; SPSS Inc., Chicago, IL). Pearson
χ2 test was used to compare the correlation between
disease stage, grade, and CT antigen expression. Statistical
significance was accepted at P<0.05.
Results
Expression of CT antigens in lung cancer
tissues
Expression of the 13 CT antigens (NY-SAR-35, SCP-1,
SSX-1, SSX-2, SSX-4, MAGE-1, MAGE-3, MAGE-4, MAGE-10, CT-7,
NY-TLU-57, NY-ESO-1 and LAGE-1) was assessed by RT-PCR in 79 lung
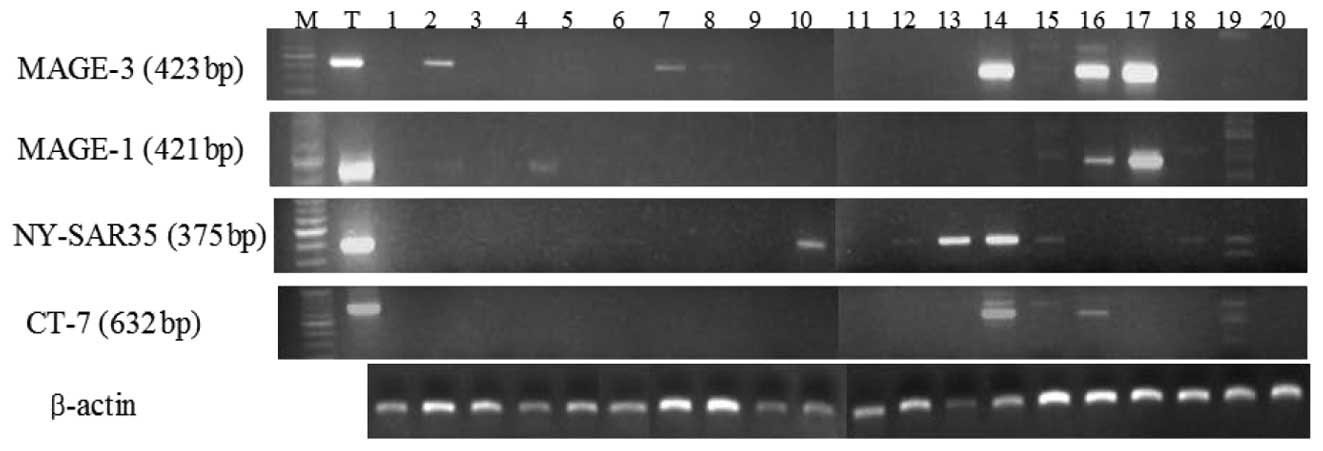
cancer tissue samples. Representative RT-PCR results from lung
cancer tissues are shown in Fig.
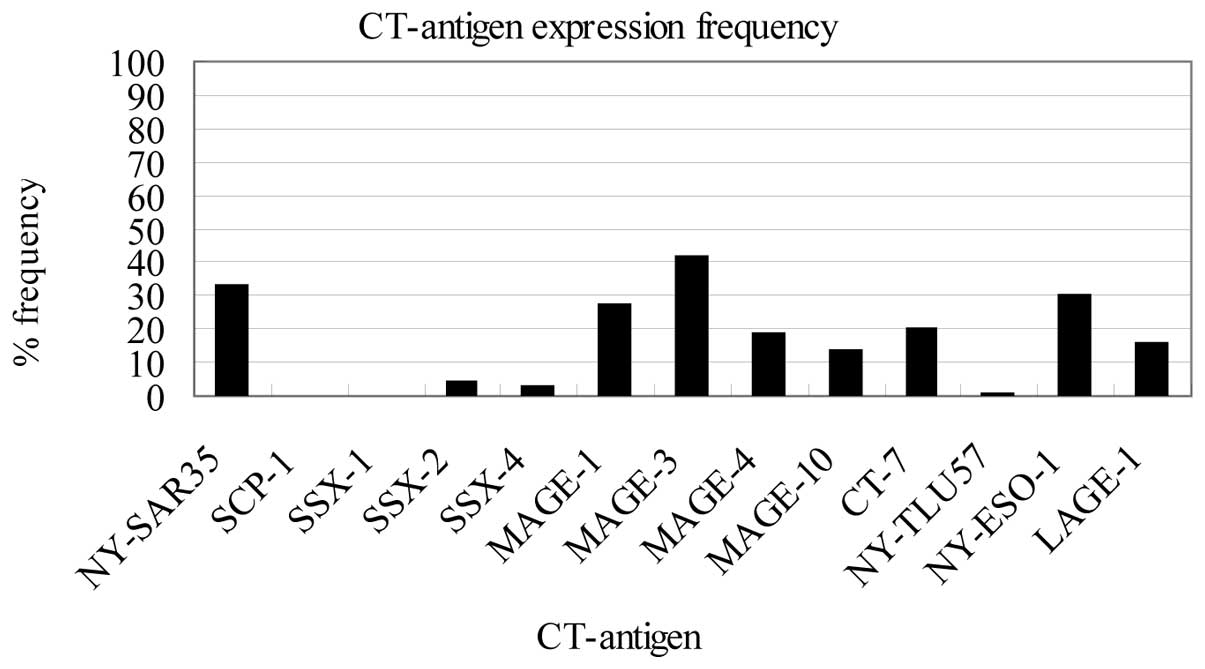
1. The most frequently expressed CT antigen was MAGE-3 (33/79,
42%), followed by NY-SAR-35 (26/79, 33%), NY-ESO-1 (24/79, 30%),
MAGE-1 (21/79, 27%), CT-7 (16/79, 20%), MAGE-4 (15/79, 19%), LAGE-1
(13/79, 16%), MAGE-10 (11/79, 14%), SSX-2 (3/79, 4%), SSX-4 (2/79,
3%), NY-TLU-57 (1/79, 1%), SCP-1 (0/79, 0%) and SSX-1 (0/79, 0%)
(Fig. 2). In previous studies,
the MAGE-3 antigen was expressed in between 30–50% of the lung
cancer tissues examined (25,26). In the present study, MAGE-3 was
detected in 42% of all lung cancer tissue samples. Among the 58 CT
antigen-positive lung cancer tissue samples, MAGE-3 and NY-SAR-35
were respectively expressed in 57 and 45% of the samples. These
findings indicate that MAGE-3 and NY-SAR-35 are attractive targets
for antigen-specific immunotherapy in Korean lung cancer
patients.
Co-expression of multiple CT antigen mRNA
in lung cancer tissues
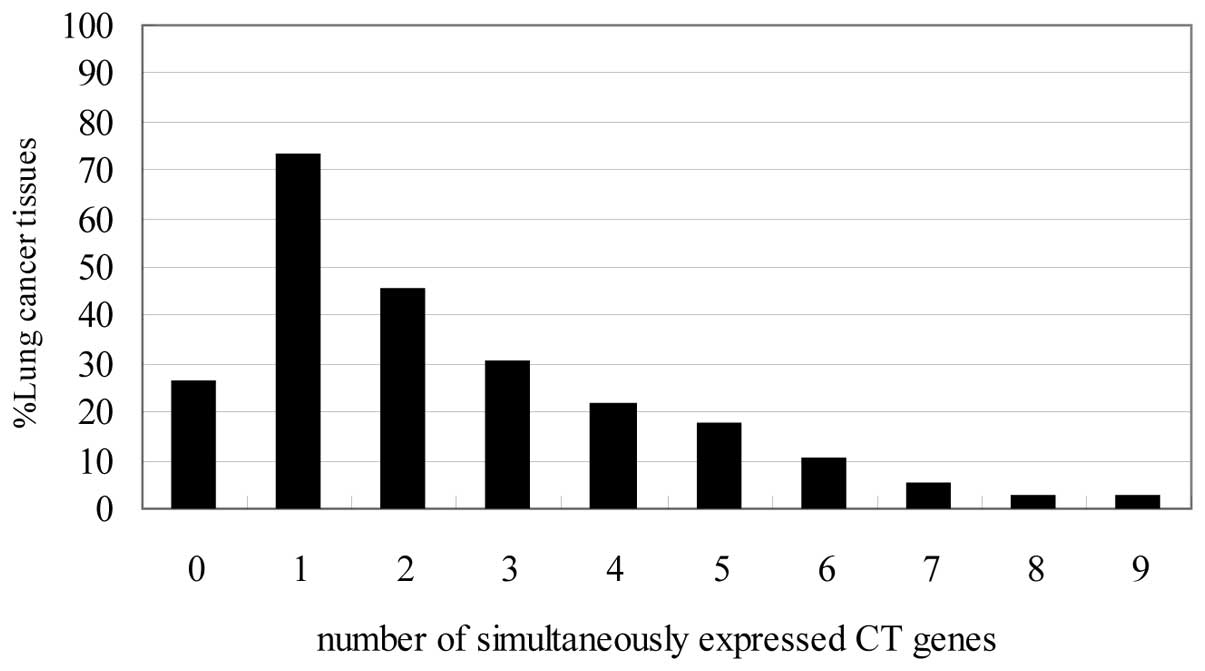
The percentages of co-expressed CT antigens in lung
cancer tissues are shown in Fig.
3. Fifty-eight of 79 lung cancer tissues (73%) were found to
express at least one of the CT antigens, whereas 21 (26%) did not
express CT antigens. Thirty-six cases (45%) expressed more than two
CT antigens and three or more CT antigens were expressed in 24
cases (30%). Seventeen specimens expressed ≥4 (21%), 14 specimens
expressed ≥5 (18%), 8 cases ≥6 (10%), 4 cases ≥7 (5%), 2 cases ≥8
(3%) and two tissues co-expressed 9 antigens. From these data, it
becomes evident that 73% of our patients with lung cancer tissues
would be eligible for antigen specific immunotherapeutic approaches
with at least one CT antigen.
Relationship between cancer/testis
antigen expression and tumor characteristics
The characteristics of the patients are summarized
in Table I. The following data of
the patients were entered in a prospective database: there were 49
men and 19 women, with ages ranging from 37 to 80 years (>60 vs.
≤ 60 years of ages); 22 patients were non-smokers, and 46 patients
were smokers; there were 26 patients with pT1, 35 with pT2 and 7
with pT3; there were 54 patients with pN0 or pN1 and 14 patients
with pN2 or pN3. In addition, there were 50 patients with clinical
stage I or II and 18 with clinical stage III or IV at the time of
diagnosis. We then investigated the possible correlation between CT
antigen expression and these clinical variables (Table II). The data included patients
whose tumors expressed at least one CT antigen. CT antigen
expression was found to be associated with male gender (P=0.001),
age (P<0.001), and smoking history (P=0.009). On the other hand,
no correlation was detected between CT antigen expression and other
clinical factors, such as pT status, pN status, tumor stages, and
histology history. Between the adenocarcinoma and squamous cell
carcinoma samples, expression of CT antigens in adenocarcinoma
samples had a tendency to be more frequent than in squamous cell
carcinoma. In addition, expression of individual MAGE-3 (P=0.003)
and NY-SAR-35 (P=0.036) were found to be associated with squamous
cell carcinoma and adenocarcinoma, respectively (Table III).
 | Table IISummary of patient characteristics
and expression of CT antigens. |
Table II
Summary of patient characteristics
and expression of CT antigens.
| Characteristic | Number of
patients | Number of patients
expressing CT gene mRNAs | P-value |
|---|
| Age |
| >60 | 29 | 11 | <0.001 |
| ≤60 | 39 | 35 | |
| Gender |
| Male | 49 | 42 | 0.001 |
| Female | 19 | 8 | |
| Smoking
history |
| No | 22 | 11 | 0.009 |
| Yes | 46 | 38 | |
| pT status |
| pT1 | 26 | 19 | 0.678 |
| pT2 | 35 | 25 | |
| pT3 and 4 | 7 | 6 | |
| pN status |
| pN0 and 1 | 54 | 39 | 0.295 |
| pN 2 and 3 | 14 | 11 | |
| Pathological
stage |
| I and II | 50 | 36 | 0.635 |
| I and IV | 18 | 14 | |
| Histology |
|
Adenocarcinoma | 33 | 25 | 0.55 |
| Squamous cell
carcinoma | 24 | 16 | |
| Neuroendocrine
carcinoma | 6 | 6 | |
| Carcinoma with
pleomorphic | 4 | 3 | |
| Others | 12 | 8 | |
 | Table IIIThe expression of CT antigens
according to the histological classification of lung cancer
tissues. |
Table III
The expression of CT antigens
according to the histological classification of lung cancer
tissues.
| | Number of patients
expressing a CT gene |
|---|
| |
|
|---|
| Histological
type | No. of
patients | NY-SAR35 | SCP-1 | SSX-1 | SSX-2 | SSX-4 | MAGE-1 | MAGE-3 | MAGE-4 | MAGE-10 | CT-7 | NY-TLU57 | NY-ESO-1 | LAGE-1 |
|---|
| Adenocarcinoma | 33 | 10 | 0 | 0 | 1 | 1 | 4 | 8 | 3 | 3 | 6 | 0 | 8 | 4 |
| Squamous cell
carcinoma | 24 | 4 | 0 | 0 | 1 | 0 | 7 | 13 | 9 | 4 | 4 | 0 | 7 | 3 |
| Neuroendocrine
carcinoma | 6 | 4 | 0 | 0 | 1 | 0 | 4 | 5 | 1 | 2 | 3 | 1 | 3 | 3 |
| Sarcomatoid
carcinoma | 4 | 3 | 0 | 0 | 0 | 1 | 3 | 4 | 1 | 2 | 2 | 0 | 3 | 0 |
| Others | 12 | 4 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 1 | 0 | 2 | 3 |
| P-valuea | | 0.036 | | | | | 0.010 | 0.003 | 0.049 | 0.094 | 0.217 | | 0.236 | 0.072 |
MAGE-3 protein expression
Among the 79 lung cancer tissue specimens, 22 lung
carcinoma samples from which sufficient material was available were
investigated for the expression of the frequently expressed MAGE-3
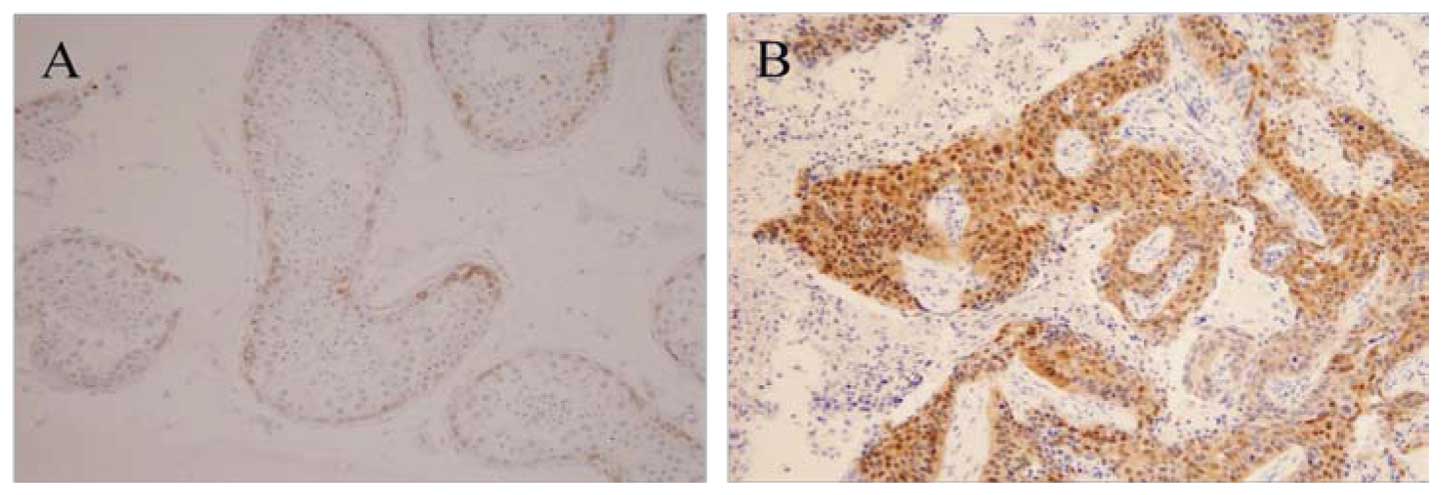
protein by immunohistochemistry. Representative
immunohistochemistry results of lung cancer tissues with the mAb
MAGE-3 are shown in Fig. 4.
Expression of MAGE-3 protein was found in 12 of 22 tumor samples
(55%) while MAGE-3 mRNA expression was detected in 10/22 tumor
samples (45%). Expression of MAGE-3 antigen was observed in 7 of 10
MAGE-3 RT-PCR-positive tumor samples. We observed a correlation
between the expression of MAGE-3 protein and the histological type
of lung cancer tissues. The MAGE-3 protein was expressed in 8 of 10
squamous cell carcinomas as compared with 3 of 10
adenocarcinomas.
Discussion
CT antigens are immunogenic proteins expressed in
normal testis and in different types of tumors. Because of their
tissue-restricted expression, CT antigens are ideal candidates for
antigen-specific cancer immunotherapy. In general, CT antigens are
expressed in 20–40% of specimens from a given tumor type (3). The present study was undertaken to
evaluate the expression of CT antigens (NY-SAR-35, SCP-1, SSX-1,
SSX-2, SSX-4, MAGE-1, MAGE-3, MAGE-4, MAGE-10, CT-7, NY-TLU57,
NY-ESO-1, and LAGE-1) in lung cancer tissues. Moreover, the
prognostic role of CT antigen expression and their correlation with
a number of clinical pathological parameters were examined. A
number of studies have investigated the mRNA expression patterns of
individual CT antigens in lung cancer (19,25,27,28). In this study, the SEREX-defined
(33% NY-SAR-35 and 30% NY-ESO-1) and the CTL-defined (42% MAGE-3
and 27% MAGE-1) antigens were the most frequently expressed in lung
cancer tissues. As MAGE-3, NY-SAR-35, NY-ESO-1 and MAGE-1 were
expressed with a high percentage and specificity in lung cancer
tissues, their products might be ideal for antigen targets for lung
cancer immunotherapy (Fig.
2).
In addition, 58 of 79 lung cancer tissue specimens
(73%) were found to express at least one of the CT antigens.
According to our results, 73% of our lung cancer patients would be
eligible for specific immunotherapeutic approaches with at least
one CT antigen. A possibility for the high observation frequency of
CT antigen expression in lung cancer tissues could be due to the
characteristics of the patients. These results indicate that
expression of CT antigens was significantly associated with the age
and gender of the patients, whereas no significant correlation was
detected between CT antigen expression and other clinical factors
such as pT status, pN status, and tumor stages (Table II). In this study, the
differences in the expression of CT antigens in 79 lung cancer
tissues cannot be ascribed to the characteristics of the patients,
but rather reveal intrinsic differences in lung cancer tissues.
The highly homologous NY-ESO-1 and LAGE-1 (94%,
nucleotide identity and 88% amino acid identity) are highly
immunogenic, and a CTL response to an immunodominant,
HLA-A2-restricted NY-ESO-1/LAGE-1 epitope can commonly be detected
in cancer patients (29,30). We compared the expression of
NY-ESO-1 and LAGE-1 in tissues from lung cancer patients by RT-PCR
(Figs. 2 and 3). NY-ESO-1 gene expression was detected
in 24/79 (30%) more frequently than in two previous reports, in
which NY-ESO-1 was detected in 2/12 (17%) (16) and 3/15 (20%) (31) lung cancer tissues of Caucasian
origin. LAGE-1 gene expression was detected in 13/79 (16%)
specimens, less frequently than in a previous report (31) in which it was detected in 5/15
(33%) cases. Of the 79 lung cancer tissues, 24/79 (30%) were
NY-ESO-1-positive by RT-PCR and 13/79 (16%) were LAGE-1 positive by
RT-PCR. The expression of either NY-ESO-1 or LAGE-1 antigens was
observed in 29 of 79 (37%) of lung cancer tissue specimens. These
results suggested that NY-ESO-1 and LAGE-1 represent targets for
immunotherapy in a significant proportion of patients with lung
cancer.
Of particular interest was NY-SAR-35, which
represents a CT antigen as it appears to be a rare example of a
cell surface antigen (19), which
was highly expressed in lung cancer tissues. Expression of
NY-SAR-35 was found in 33% of all lung cancer tissues. NY-SAR-35
was detected in 45% of 58 CT antigen-positive lung cancer tissues.
Recently, we found that treatment with 5-aza-CdR can induce the
expression of NY-SAR-35, and that transcriptional silencing of
NY-SAR-35 is caused by hypermethylation of its promoter (32). These findings indicated that
NY-SAR-35 is an attractive target for antigen-specific
immunotherapy in lung cancer and that treatment with demethylating
agents, in combination with immunotherapy, could be a useful
therapeutic strategy for modulating the antigen expression.
To confirm the existence of MAGE-3 protein
expression, 22 lung carcinoma samples from which sufficient
material was available were immunohistochemically stained by a
specific MAGE-3 monoclonal antibody. Similarly to our previous
study (24), we found a
correlation with frequent expression of MAGE-3 protein and the
histological type of squamous cell carcinomas. The MAGE-3 protein
was expressed in 8 of 10 squamous cell carcinomas as compared with
3 of 10 adenocarcinomas. Expression of MAGE-3 protein was
demonstrated in 7 of 10 MAGE-3 RT-PCR-positive tumor samples. These
results indicate the discordance between RT-PCR and
immunohistochemistry positivity. As a note of caution, however, one
should keep in mind that MAGE-3 at the protein level may not be
detected in all cases expressing the respective mRNA. Whether this
is due to a lower sensitivity of the detection method used
(immunohistochemistry using CT antigen-specific antibodies) or
whether the mRNA is not translated into protein cannot be
determined at this point (27,33).
From the data of our study, a high proportion
(58/79, 73%) of lung cancer tissues was positive for at least one
of these 13 CT antigens as targets will greatly increase the number
of candidates for CT antigen-based lung cancer immunotherapy.
However, our results showed no expression of CT antigens in 27%
(22/79) of lung cancer tissues. For these patients, it is necessary
to screen other CT antigens or tumor-specific antigens serving as
immune targets for lung cancer tissue immunotherapy. In conclusion,
this study demonstrated that lung cancer tissues frequently express
CT antigens and a high percent express more than one CT antigen,
suggesting that CT antigens are potential candidates for polyvalent
immunotherapy.
Acknowledgements
This study was supported by the Medical Research
Institute Grant (2006–23), Pusan National University.
References
|
1
|
DM ParkinF BrayJ FerlayP PisaniGlobal
cancer statistics, 2002CA Cancer J
Clin5574108200510.3322/canjclin.55.2.74
|
|
2
|
LJ OldCancer vaccines 2003: opening
addressCancer Immun3Suppl 2S12003
|
|
3
|
MJ ScanlanAJ SimpsonLJ OldThe
cancer/testis genes: review, standardization, and commentaryCancer
Immun41200414738373
|
|
4
|
PG CoulieV BrichardA Van PelT WolfelJ
SchneiderC TraversariS MatteiE De PlaenC LurquinJP SzikoraA new
gene coding for a differentiation antigen recognized by autologous
cytolytic T lymphocytes on HLA-A2 melanomasJ Exp
Med1803542199410.1084/jem.180.1.358006593
|
|
5
|
S LabrecqueN NaorD ThomsonG
MatlashewskiAnalysis of the anti-p53 antibody response in cancer
patientsCancer Res533468347119938339249
|
|
6
|
ML DisisE CalenoffG McLaughlinAE MurphyW
ChenB GronerM JeschkeN LydonE McGlynnRB LivingstonExistent T-cell
and antibody immunity to HER-2/neu protein in patients with breast
cancerCancer Res54162019947505195
|
|
7
|
T BoonPG CoulieB Van den EyndeTumor
antigens recognized by T cellsImmunol
Today18267268199710.1016/S0167-5699(97)80020-59190110
|
|
8
|
YT ChenAO GureS TsangE StockertE JagerA
KnuthLJ OldIdentification of multiple cancer/testis antigens by
allogeneic antibody screening of a melanoma cell line libraryProc
Natl Acad Sci USA9569196923199810.1073/pnas.95.12.69199618514
|
|
9
|
AJ SimpsonOL CaballeroA JungbluthYT ChenLJ
OldCancer/testis antigens, gametogenesis and cancerNat Rev
Cancer5615625200510.1038/nrc166916034368
|
|
10
|
RB ParmigianiF BettoniMD VibranovskiMH
LopesWK MartinsIW CunhaFA SoaresAJ SimpsonSJ de SouzaAA
CamargoCharacterization of a cancer/testis (CT) antigen gene family
capable of eliciting humoral response in cancer patientsProc Natl
Acad Sci USA1031806618071200610.1073/pnas.060885310317114284
|
|
11
|
OL CaballeroYT ChenCancer/testis (CT)
antigens: potential targets for immunotherapyCancer
Sci10020142021200910.1111/j.1349-7006.2009.01303.x19719775
|
|
12
|
P van der BruggenC TraversariP ChomezC
LurquinE De PlaenB Van den EyndeA KnuthT BoonA gene encoding an
antigen recognized by cytolytic T lymphocytes on a human
melanomaScience254164316471991
|
|
13
|
P BoelC WildmannML SensiR BrasseurJC
RenauldP CoulieT BoonP van der BruggenBAGE: a new gene encoding an
antigen recognized on human melanomas by cytolytic T
lymphocytesImmunity2167175199510.1016/S1074-7613(95)80053-07895173
|
|
14
|
B Van den EyndeO PeetersO De BackerB
GauglerS LucasT BoonA new family of genes coding for an antigen
recognized by autologous cytolytic T lymphocytes on a human
melanomaJ Exp Med1826896981995
|
|
15
|
O TureciU SahinI SchobertM KoslowskiH
ScmittHJ SchildF StennerG SeitzHG RammenseeM PfreundschuhThe SSX-2
gene, which is involved in the t(X;18) translocation of synovial
sarcomas, codes for the human tumor antigen HOM-MEL-40Cancer
Res564766477219968840996
|
|
16
|
YT ChenMJ ScanlanU SahinO TureciAO GureS
TsangB WilliamsonE StockertM PfreundschuhLJ OldA testicular antigen
aberrantly expressed in human cancers detected by autologous
antibody screeningProc Natl Acad Sci
USA9419141918199710.1073/pnas.94.5.19149050879
|
|
17
|
O TureciU SahinC ZwickM KoslowskiG SeitzM
PfreundschuhIdentification of a meiosis-specific protein as a
member of the class of cancer/testis antigensProc Natl Acad Sci
USA9552115216199810.1073/pnas.95.9.52119560255
|
|
18
|
SY LeeY ObataM YoshidaE StockertB
WilliamsonAA JungbluthYT ChenLJ OldMJ ScanlanImmunomic analysis of
human sarcomaProc Natl Acad Sci
USA10026512656200310.1073/pnas.043797210012601173
|
|
19
|
SY LeeB WilliamsonOL CaballeroYT ChenMJ
ScanlanG RitterCV JongeneelAJ SimpsonLJ OldIdentification of the
gonad-specific anion transporter SLCO6A1 as a cancer/testis (CT)
antigen expressed in human lung cancerCancer
Immun413200415546177
|
|
20
|
U SahinO TureciH SchmittB CochloviusT
JohannesR SchmitsF StennerG LuoI SchobertM PfreundschuhHuman
neoplasms elicit multiple specific immune responses in the
autologous hostProc Natl Acad Sci
USA921181011813199510.1073/pnas.92.25.118108524854
|
|
21
|
K TajimaY ObataH TamakiM YoshidaYT ChenMJ
ScanlanLJ OldH KuwanoT TakahashiT MitsudomiExpression of
cancer/testis (CT) antigens in lung cancerLung
Cancer422333200310.1016/S0169-5002(03)00244-714512184
|
|
22
|
JR TsaiIW ChongYH ChenMJ YangCC SheuHC
ChangJJ HwangJY HungSR LinDifferential expression profile of MAGE
family in non-small-cell lung cancerLung
Cancer56185192200710.1016/j.lungcan.2006.12.00417208331
|
|
23
|
LH SobinP HermanekRV HutterTNM
classification of malignant tumors. A comparison between the new
(1987) and the old
editionsCancer6123102314198810.1002/1097-0142(19880601)61:11%3C2310::AID-CNCR2820611127%3E3.0.CO;2-X3284634
|
|
24
|
SH KimS LeeCH LeeMK LeeYD KimDH ShinKU
ChoiJY KimY Park doMY SolExpression of cancer-testis antigens
MAGE-A3/6 and NY-ESO-1 in non-small-cell lung carcinomas and their
relationship with immune cell
infiltrationLung187401411200910.1007/s00408-009-9181-319795170
|
|
25
|
P WeynantsB LetheF BrasseurM MarchandT
BoonExpression of mage genes by non-small-cell lung carcinomasInt J
Cancer56826829199410.1002/ijc.29105606128119772
|
|
26
|
S LucasC De SmetKC ArdenCS ViarsB LetheC
LurquinT BoonIdentification of a new MAGE gene with tumor-specific
expression by representational difference analysisCancer
Res5874375219989485030
|
|
27
|
C FischerF GudatP StulzC NoppenC SchaeferP
ZajacM TrutmannT KocherM ZuberF HarderHigh expression of MAGE-3
protein in squamous-cell lung carcinomaInt J
Cancer7111191121199710.1002/(SICI)1097-0215(19970611)71:6%3C1119::AID-IJC34%3E3.0.CO;2-59185722
|
|
28
|
MJ ScanlanNK AltorkiAO GureB WilliamsonA
JungbluthYT ChenLJ OldExpression of cancer-testis antigens in lung
cancer: definition of bromodomain testis-specific gene (BRDT) as a
new CT gene, CT9Cancer
Lett150155164200010.1016/S0304-3835(99)00385-710704737
|
|
29
|
D RimoldiV Rubio-GodoyV DutoitD LienardS
SalviP GuillaumeD SpeiserE StockertG SpagnoliC ServisEfficient
simultaneous presentation of NY-ESO-1/LAGE-1 primary and nonprimary
open reading frame-derived CTL epitopes in melanomaJ
Immunol16572537261200010.4049/jimmunol.165.12.725311120859
|
|
30
|
F van RheeSM SzmaniaF ZhanSK GuptaM
PomtreeP LinRB BatchuA MorenoG SpagnoliJ ShaughnessyNY-ESO-1 is
highly expressed in poor-prognosis multiple myeloma and induces
spontaneous humoral and cellular immune
responsesBlood10539393944200515671442
|
|
31
|
B LetheS LucasL MichauxC De SmetD
GodelaineA SerranoE De PlaenT BoonLAGE-1, a new gene with tumor
specificityInt J
Cancer76903908199810.1002/(SICI)1097-0215(19980610)76:6%3C903::AID-IJC22%3E3.0.CO;2-19626360
|
|
32
|
JH ParkMH SongCH LeeMK LeeYM ParkL OldSY
LeeExpression of the human cancer/testis antigen NY-SAR-35 is
activated by CpG island hypomethylationBiotechnol
Lett3310851091201110.1007/s10529-011-0559-y21318630
|
|
33
|
A MischoB KubuschokK ErtanKD PreussB
RomeikeE RegitzC SchormannD de BruijnA WadleF NeumannProspective
study on the expression of cancer testis genes and antibody
responses in 100 consecutive patients with primary breast cancerInt
J Cancer118696703200610.1002/ijc.2135216094643
|