Introduction
Type 1 diabetes mellitus (T1DM) is an increasingly
prevalent systemic disease in the world. It is well known that this
disease has detrimental effects on bone remodeling, leading to a
series of bone complications including osteopenia, osteoporosis,
increased risk of fracture, as well as impaired bone healing and
regeneration (1–3). All of the above problems may
significantly affect the quality of life of patients. Despite
numerous studies conducted, to date, little is known about the
detailed pathogenesis of these effects of diabetes, and few
effective therapies exist for healing these bone disorders
(4).
Bone marrow stromal cells (BMSCs) are a major source
of osteoblasts and are crucial for bone remodeling and repair
through direct regeneration such as cell differentiation or
maturation, or indirect mechanisms via paracrine effects such as
increased vascularization (5). In
addition, due to their easy availability and multipotent potential,
BMSCs are an attractive candidate for tissue engineering
applications, which have been successfully used to enhance bone
repair (6,7). Previous studies have demonstrated
that diabetes has direct detrimental effects on many types of
cells, including impaired proliferation and function of adipose
tissue resident mesenchymal stem cells and bone mesenchymal stem
cells (8–10). Recently, impairments of BMSCs were
reported to be responsible for osteopenia in diabetes which might
represent a cellular mechanism (11). However, to date, the effects of
diabetes on the osteogenic potential of BMSCs and the possible
related mechanisms remain unclear.
T1DM is characterized by hypoinsulinemia and
insulin-like growth factor 1 (IGF-1) deficiency. Both insulin and
IGF-1 are important for the normal development of mineralized
skeleton and bone remodeling. Moreover, the imbalance between
insulin and the IGF system may contribute to the bone disorders in
diabetes (12,13). In particular, delivery of insulin
or IGF-1 enhanced bone repair in diabetic rats (14,15). Insulin and IGF-1 may function in
the process of bone formation through insulin receptor (IR), IGF-1
receptor (IGF-1R) and a common hybrid receptor, which could
activate the insulin receptor substrate (IRS) proteins that
regulate a variety of signaling pathways controlling cell
proliferation, differentiation, apoptosis and metabolism (16). In vitro, insulin and IGF-1
promote osteoblasts to express IR or IGF-1R, and enhance cell
proliferation and differentiation via the extracellular
signal-regulated kinase (ERK) pathway (17,18). Conversely, mice lacking IR in
osteoblasts resulted in reduced trabecular bone and mineralization
(19). Mice lacking IGF-1R in
osteoblasts or pre-osteoblastic cells also showed decreased bone
mass and mineral deposition rates (20,21). Whether abnormalities in insulin
and IGF-1 signaling may explain altered osteogenic potential of
BMSCs derived from diabetic rats has not been determined.
Therefore, in the present study, we aimed to
investigate and compare osteoblastic differentiation of BMSCs
derived from diabetic rats in vitro, and bone formation
capacity in vivo of BMSCs derived from normal and diabetic
rats. We also determined whether alterations in osteogenic
potential of diabetic BMSCs are related to parallel decreases in
expression of IGF-1, IGF-1R, IR and IRS-1 via the ERK signaling
pathway. Systematic evaluation of these questions may provide a
comprehensive understanding of bone complications associated with
diabetes and the possible relevance for future cell therapies for
these diseases.
Materials and methods
Animal models
All animal experiments were approved by the Animal
Research Committee of the Ninth People’s Hospital affiliated to
Shanghai Jiao Tong University, School of Medicine. Sixteen
4-week-old male Wistar rats and 4 4-week-old male nude Balb/c mice
were used in the study. Rats were randomly divided into 2 groups.
Diabetes was induced in 8 rats via a single intraperitoneal
injection of streptozotocin (65 mg/kg) (Sigma, St. Louis, MO, USA)
dissolved in 0.01 M citrate buffer (pH 4.5). Another 8 age-matched
normal rats that received no injection served as the controls.
Diabetes in the rats was confirmed when blood glucose
concentrations were higher than 16.7 mmol/l tested by a blood
glucose meter (Accu-Chek Performa; Roche Diagnostics, Indianapolis,
IN, USA) 1 week post-injection (22). The blood glucose concentrations
were tested on the same day before injection, 1, 4, 8 and 12 weeks
post-injection. Body weights were measured on the same day before
injection, 1 and 12 weeks post-injection. All animals were fed a
regular diet and given water ad libitum.
Isolation and culture of rat BMSCs
After euthanasia, BMSCs were harvested from both the
tibia and femur bone marrow of the normal and diabetic rats, and
cultured in low glucose Dulbecco’s modified Eagle’s medium (DMEM,
low glucose; Gibco-BRL, Grand Island, NY, USA) supplemented with
10% fetal bovine serum (Hyclone, Logan, UT, USA), containing 100
U/ml penicillin, 100 U/ml streptomycin and 2 mM L-glutamine (Sigma)
as previously described (23).
After BMSCs were incubated for 24 h, the medium was changed to
discard non-adherent cells. Then the medium was refreshed every 3
days. When confluence reached ∼80%, cells were passaged for
expansion. The following experiments used cells at passage 2 or 3.
To study the osteoblastic differentiation of BMSCs, osteogenic
medium contained 10-8 M dexamethasone, 50 μg/ml L-2-ascorbic
acid and 10 mM β-glycerophosphate were used.
Cell proliferation assay
To measure the proliferation of cells, MTT assay was
used to assess the amount of viable cells (24). Briefly, cells were seeded at a
density of 5×103 cells/well on a 96-well plate. On the
next day, the medium was replaced by fresh medium, and cells were
cultured for 1, 3, 5 and 7 days. MTT (5 mg/ml) was added into each
well and incubated for 4 h. After removing the medium, DMSO was
used to dissolve the formazan crystals and the absorbance value was
measured using a microplate reader (Bio-Tek, Winooski, VT, USA) at
490 nm.
Real-time PCR analysis
Total RNA was extracted at day 7 using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) and was transcribed with
PrimeScript RT reagent kit (Takara, Kyoto, Japan). cDNA
amplification and detection were performed using the Bio-Rad iQ5
real-time PCR system (Bio-Rad, Hercules, CA, USA) using SYBR Premix
Ex Taq kit (Takara) and specific primers (Table I). The relative gene expression
level was normalized to the internal control (β-actin) based on the
2−ΔΔCt method.
 | Table ISequences of the primers used for
real-time PCR. |
Table I
Sequences of the primers used for
real-time PCR.
| Genes | Forward primer | Reverse primer |
|---|
| β-actin |
CACCCGCGAGTACAACCTTC |
CCCATACCCACCATCACACC |
| Runx2 |
ATCCAGCCACCTTCACTTACACC |
GGGACCATTGGGAACTGATAG |
| OCN |
GCCCTGACTGCATTCTGCCTCT |
TCACCACCTTACTGCCCTCCTG |
| ALP |
GGCTCTGCCGTTGTTTC |
GGGTTGGGTTGAGGGACT |
| IR |
ACATTGCCCTGAAGACCAAC |
AACAGCATGAATCCCAGGAG |
| IGF-1 |
CCGCTGAAGCCTACAAAGTC |
GGGAGGCTCCTCCTACATTC |
| IGF-1R |
GTGCTGTACGCCTCTGTGAA |
TTGCAGCCTCATTCACTGTC |
| IRS-1 |
TGTGCCAAGCAACAAGAAAG |
ACGGTTTCAGAGCAGAGGAA |
ALP staining and ALP activity assay
For ALP staining, after being fixed for 15 min at
4°C, BMSCs at days 7 and 14 in osteogenic medium were treated with
a BCIP/NBT solution (Beyotime, Shanghai, China) in the dark, and
areas that stained purple were regarded as positive. ALP activities
were determined using p-nitrophenyl phosphate (Sigma) as described
previously (25).
Alizarin red staining for mineralization
measurement
After culturing in the osteogenic medium for 21
days, BMSCs were fixed in 70% ethanol and stained with 40 mM
Alizarin Red S solution. Then the stain was desorbed with 10%
cetylpyridinium chloride (Sigma) for 1 h, and the Alizarin Red
concentration of dye extracts was determined by absorbance at 590
nm (Bio-Tek).
Western blot analysis
For western blotting, cells at day 7 were lysed with
a protein extraction reagent containing protease inhibitor
cocktail, phosphatase inhibitor cocktail and phenylmethanesulfonyl
fluoride (PMSF) (Kangchen, Shanghai, China). The protein
concentration was measured according to the BCA protein assay kit
manufacturer’s protocol (Beyotime). Equal protein samples were
separated on SDS-polyacrylamide gel electrophoresis (PAGE) and then
electro-transferred to polyvinylidene difluoride membranes. The
membranes were blocked with primary antibodies: rabbit anti-rat
IGF-1R, rabbit anti-rat IRS-1 (both from Bioworld), rabbit anti-rat
ERK, rabbit anti-rat phosphorylated-ERK (both from Cell Signaling
Technology, Inc.) and mouse anti-rat β-actin (Sigma) and incubated.
Finally, the blots were visualized with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit or anti-mouse IgG (Beyotime)
using the ECL Plus reagents (Amersham Pharmacia Biotech, Arlington
Heights, IL, USA) using UVItec Alliance 4.7 gel imaging system. The
relative integrated density of each protein band was analyzed by
NIH imageJ 1.34s.
Surgical implantation of BMSCs
The porous calcium phosphate cement (CPC) scaffolds
(4-mm diameter, 2-mm height) used in the study were purchased from
Rebone Biomaterial Co., Ltd. (Shanghai, China). Average diameter of
the scaffold pores was 400 μm and average porosity was 70%.
BMSCs from diabetic or normal rats were seeded at a density of
2×107 cells/ml in 20 μl on the CPC scaffolds. The
implants were then subcutaneously implanted into 4 nude mice at the
intrascapular area. Each mouse received the following 3 groups of
complexes: CPC scaffold group (n=4), CPC/ normal BMSCs (n=4) and
CPC/diabetic BMSCs (n=4).
Histological and immunohistochemical
analysis
Four weeks after surgery, the implants were
harvested, fixed, decalcified, embedded in paraffin, sectioned
(4-μm) and stained with hematoxylin and eosin (H&E).
Photomicrographs of each section were captured with a light
microscope (Olympus, Tokyo, Japan) and histomorphological analysis
was performed using the automated image analysis system, Image Pro
5.0 (Media Cybernetics, Silver Spring, MD, USA). The percentages of
new bone area were calculated by the mean value of the 3 parallel
sections randomly selected from serial sections of each sample cut
at cross-sectional directions. The mean value of the 3 measurements
was calculated for each implant, and were further used to calculate
mean values for each group. New bone formation in each section was
defined as the percentage of new bone area in the observed
areas/the entire implant (26).
Immunohistochemical analyses were
performed as described previously (27)
Briefly, the paraffin sections were dewaxed and
rehydrated, then immersed in H2O2 to quench
peroxidase. After blocking, the sections were incubated with
primary mouse monoclonal antibodies against OCN (Abcam, Cambridge,
UK) overnight at 4°C. Then the sections were incubated with
HRP-conjugated rabbit anti-mouse IgG (Beyotime) for 1 h at room
temperature. Finally, a brown color was produced by using the
diaminobenzidine (DAB; Beyotime, Jiangsu, China) kit and sections
were counter-stained with hematoxylin. Photomicrographs of each
section were captured with a light microscope (Olympus).
Statistical analysis
Results are expressed as means ± standard deviation
(SD). Comparisons were performed by independent samples t-test
using SPSS 11.0 (SPSS, Inc., Chicago, IL, USA), and P<0.05 was
considered to indicate a statistically significant result.
Results
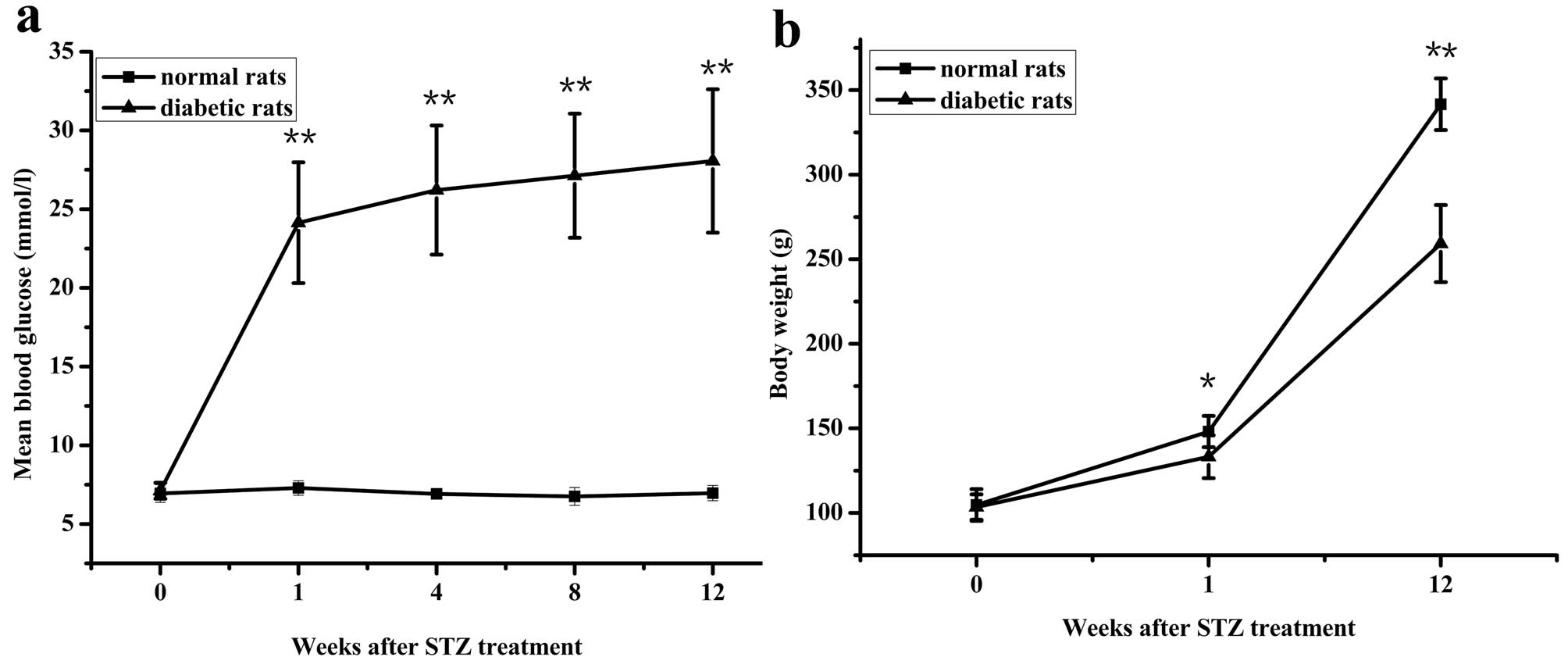
Experimental animal model
Rats treated with streptozotocin showed typical
symptoms of diabetes: high intake of food, polydipsia and polyuria.
Compared with normal rats, the diabetic rats exhibited
significantly higher blood glucose levels (Fig. 1a) and lower body weights (Fig. 1b).
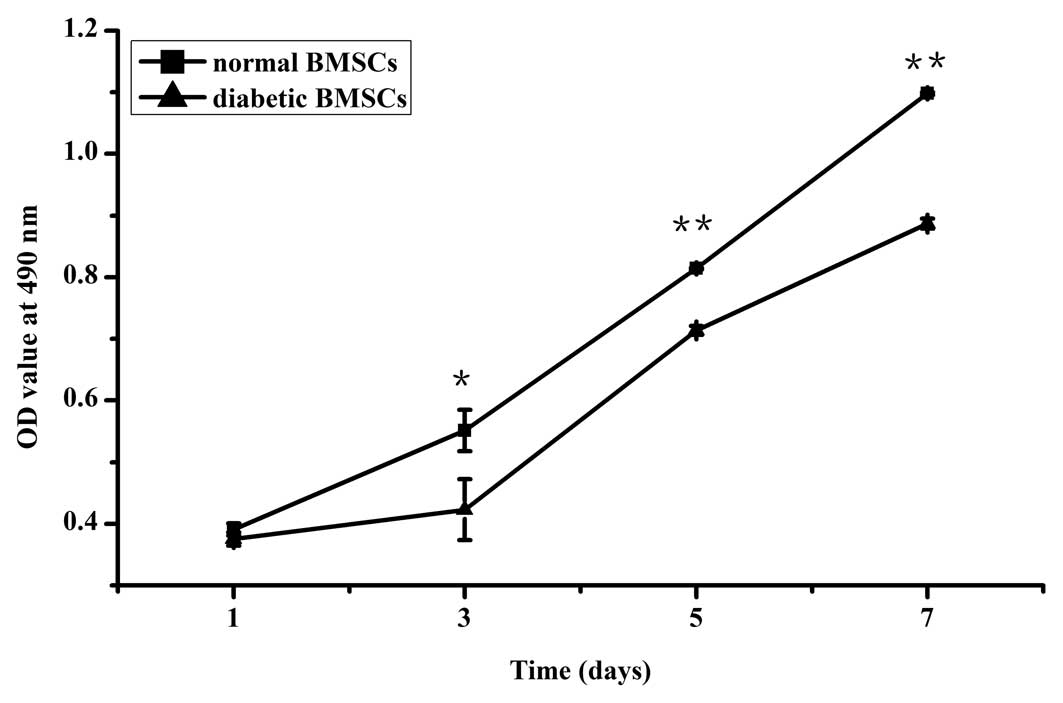
Cell proliferation
Proliferation of the diabetic BMSCs proceeded slower
than the normal BMSCs, with no significant difference at day 1 and
a significant difference at days 3, 5 and 7 (Fig. 2).
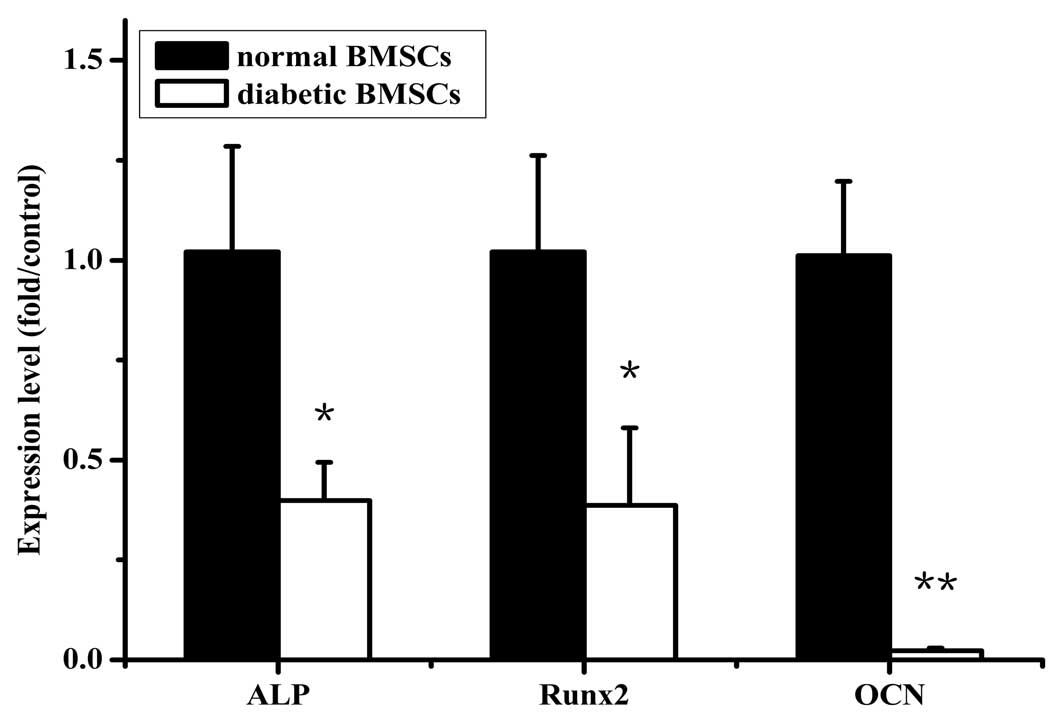
Real-time PCR analysis
The gene expression of osteogenic differentiation
markers, ALP, Runx2 and OCN, was evaluated at day 7 after cells
were incubated in osteogenic medium. The mRNA levels of ALP, Runx2
and OCN in the diabetic BMSCs were significantly reduced to ∼39, 38
and 2% of the control level, respectively (Fig. 3).
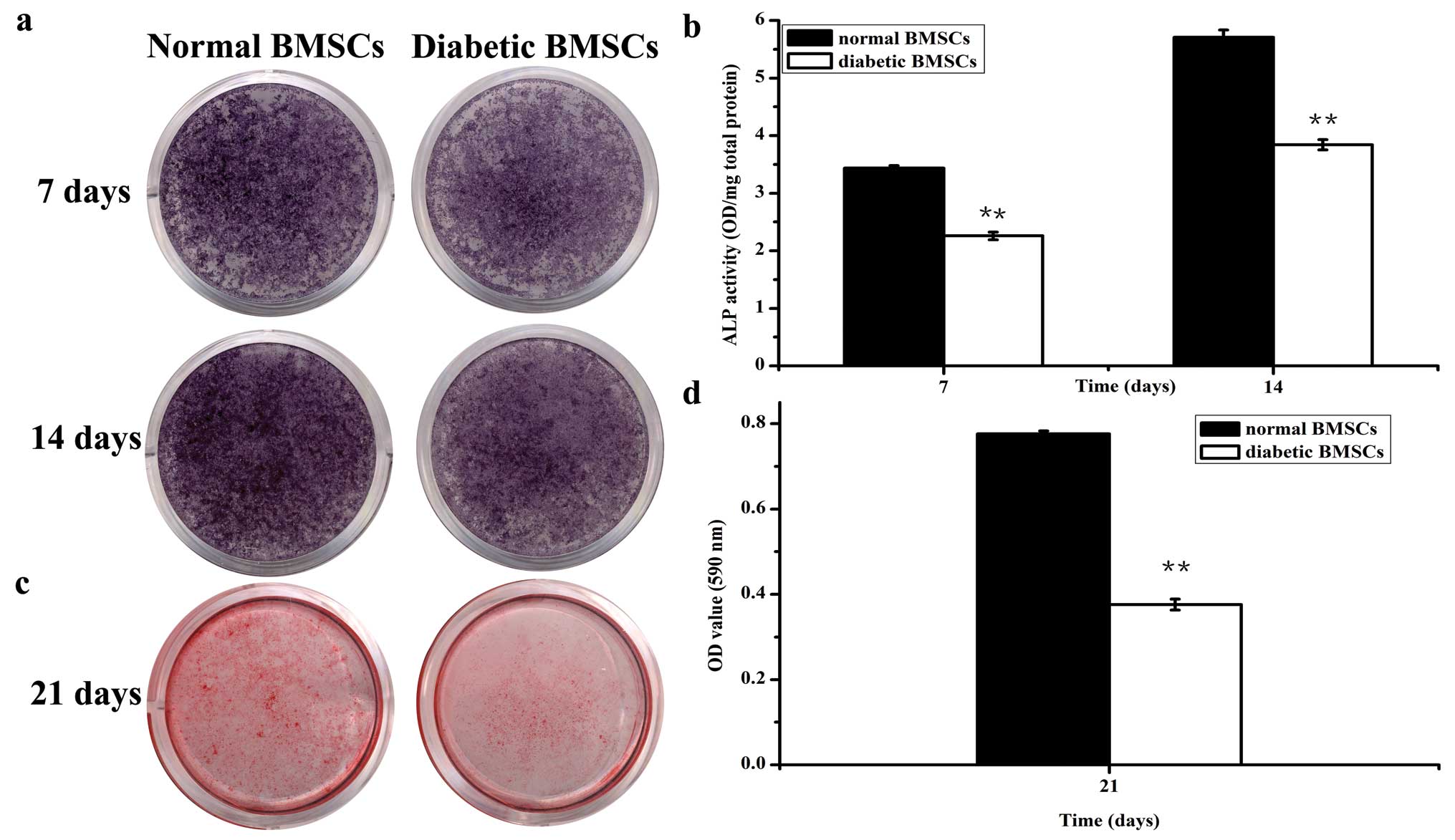
ALP activity and Alizarin Red assay
At days 7 and 14 after culture in osteogenic medium,
diabetic BMSCs demonstrated weaker ALP-positive staining compared
with the normal BMSCs (Fig. 4).
Accordingly, the ALP activity in the diabetic BMSCs significantly
decreased to ∼66 and 70% of the normal group, respectively
(Fig. 4). Furthermore, Alizarin
Red staining, as well as the quantitative analysis of
mineralization at day 21, indicated significantly lower calcium
deposition in diabetic BMSCs compared with the normal BMSCs
(Fig. 4).
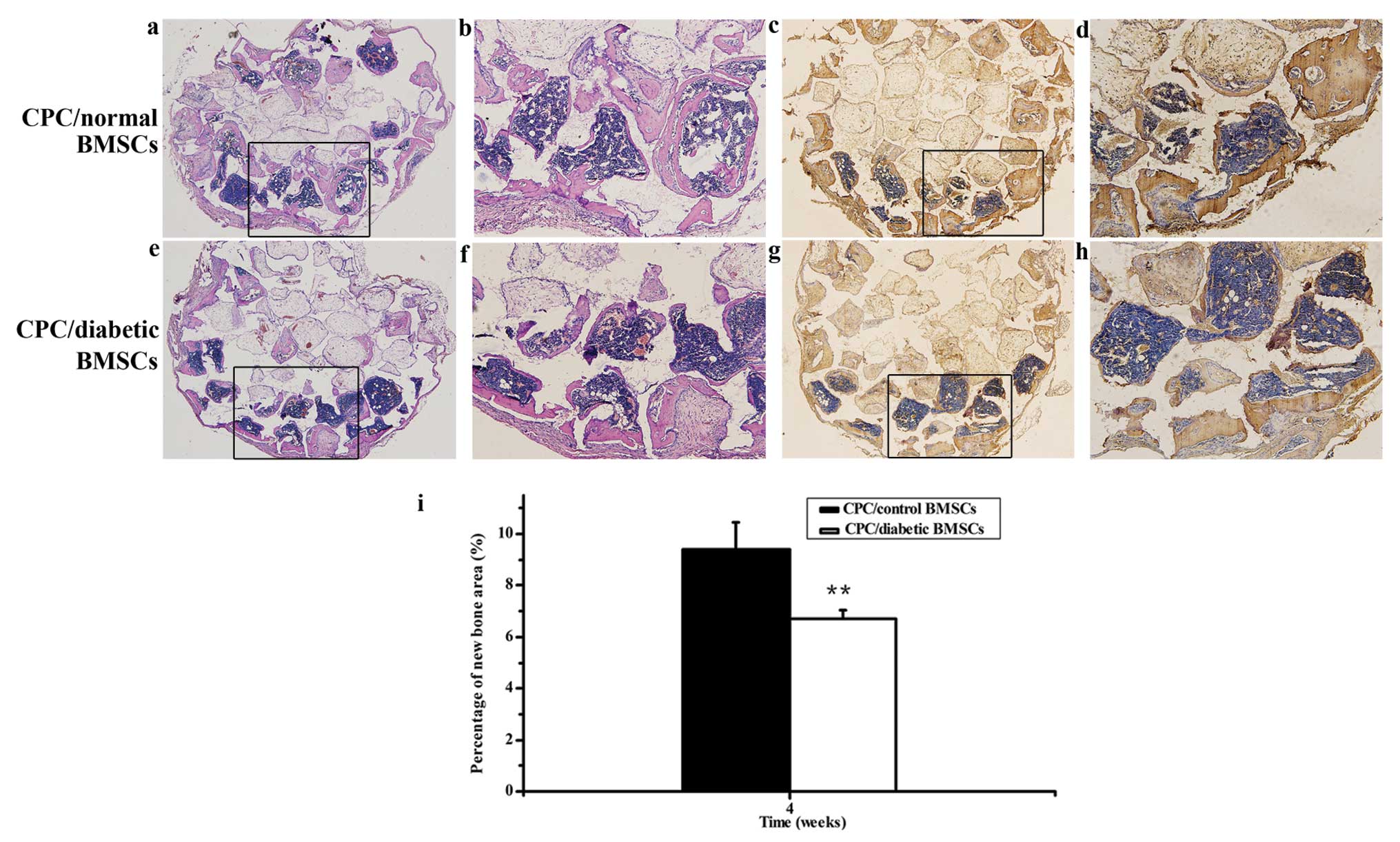
In vivo osteogenic potential of normal
BMSCs and diabetic BMSCs
At 4 weeks after implantation, no new bone formation
was noted in the CPC scaffold alone group (data not shown), whereas
less new bone formation was observed in the CPC/diabetic BMSC group
compared with the CPC/normal BMSC group. Histomorphometrical
analysis further revealed that the percentage of new bone area in
the CPC/diabetic BMSC group decreased to 71% of the normal value
(Fig. 5). Likewise, the
immunohistochemical staining of OCN displayed less intense staining
in the diabetic group compared to the normal group. Collectively,
these in vivo data further supplement the in vitro
data indicating that the osteogenic potential of diabetic BMSCs are
impaired compared with normal BMSCs.
Gene expression and protein levels
involved in the insulin and IGF-1 signaling pathway
A significant decrease in IR, IGF-1, IGF-1R and
IRS-1 gene expression was observed in diabetic BMSCs compared to
normal BMSCs (Fig. 6). Similarly,
the protein levels of IGF-IR and IRS-1 significantly decreased in
the diabetic BMSCs compared with normal BMSCs (Fig. 6). In addition, the decreased
amount of phosphorylated extracellular signal-regulated kinase
(p-ERK) protein was detected in diabetic BMSCs compared with
control BMSCs, while there was no significant difference in total
ERK expression between diabetic and normal BMSCs (Fig. 6).
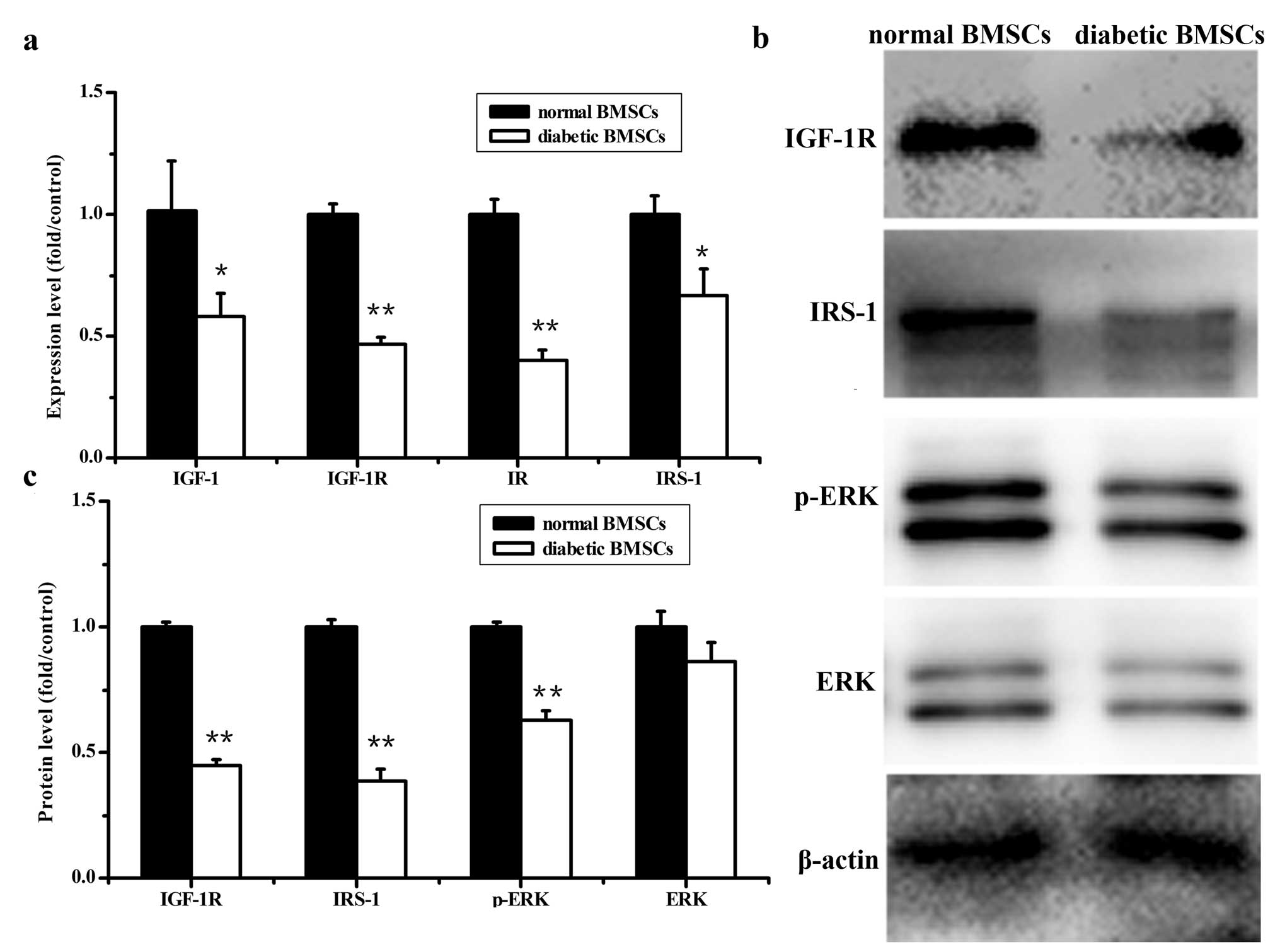 | Figure 6Gene expression and protein levels
involved in the insulin and IGF-1 signaling pathway in normal and
diabetic BMSCs. (a) mRNA levels of IR, IGF-1, IGF-1R and IRS-1, (b)
western blot analyses of IGF-1, IGF-1R, IRS-1, p-ERK and ERK. One
representative image from 3 independently performed experiments is
shown. (c) Levels of IGF-1, IGF-1R, IRS-1, p-ERK and ERK were
quantified by densitometry and expressed graphically
(**P<0.01, *P<0.05 vs. normal
BMSCs). |
Discussion
It is well documented that diabetes is associated
with a series of bone disorders. Previous research has demonstrated
that these disorders are primarily due to a defect in bone
formation rather than resorption (28). Moreover, previous studies have
focused on the dysfunction of osteoblasts, and mainly attributed
the defect in bone formation to suppressed differentiation,
proliferation and/or bone-forming capacity of osteoblast cells
(28). BMSCs mainly reside in the
bone marrow and differentiate into osteoblasts, chondrocytes and
many types of cells. They also play an important role in bone
remodeling and regeneration. However, investigations concerning the
role of alterations in BMSCs in bone disorders associated with
diabetes are scare.
In this study, we studied the proliferation and
osteogenic potential of BMSCs derived from diabetic rats. Our
results demonstrated that there is a decline in proliferation of
diabetic BMSCs compared with normal controls, consistent with a
previous study (10).
Subsequently, we studied the osteoblastic differentiation of BMSCs
in vitro. Real-time PCR was conducted to detect the mRNA
expression of osteogenic markers, ALP, Runx2 and OCN, in normal and
diabetic BMSCs. ALP, a membrane bound enzyme, is a marker of early
bone differentiation. Runx2 is a key transcriptional regulator of
osteoblastic differentiation and its expression is essential for
normal bone formation. OCN, a marker of late-stage osteoblast
differentiation, is a product of osteoblasts and accumulates in the
extracellular matrix of bone. As shown in the present study, these
osteogenic markers, as well as alkaline phosphatase activity and
mineralization were significantly reduced in diabetic BMSCs
compared with normal BMSCs. Our data clearly reveal impaired
osteogenic potential of diabetic BMSCs, which helps to explain the
well-documented correlation between the incidence of diabetes and
bone disorders. It also suggests that BMSCs could be a potential
target in the treatment of these disorders.
To investigate the bone regenerative ability of
diabetic BMSCs in vivo, we used a well-established ectopic
bone formation model. In vivo implantation experiments were
carried out for the first time, in which osteogenic differentiation
of diabetic BMSCs in vitro was recapitulated in vivo.
In nude mice, both types of BMSCs underwent osteoblastic
differentiation and formed new bone. Histomorphological analysis
demonstrated that the new bone area from the diabetic BMSCs was
reduced to 71% of the normal controls. BMSCs are attractive cell
sources for bone tissue engineering, which is effective to enhance
bone repair. These results further confirmed the impaired
osteogenic potential of diabetic BMSCs, and demonstrated a
possibility of cell-based therapy in bone disorders correlated with
diabetes, but efforts to compensate for their deficiency should be
considered.
Better understanding of the underlying mechanism of
diabetes on the osteogenic potential of BMSCs may be helpful to
devise successful strategies for therapy. Previous studies have
shown that hyperglycemia is an important cause of bone disorder in
diabetes (3). In this study, we
showed impaired osteogenic potential of diabetic BMSCs in a low
glucose culture environment suggesting that the impairment of BMSCs
may be durable, and the underlying reasons are not only related to
hyperglycemia.
T1DM is also characterized by hypoinsulinemia and
insulin deficiency, which can be associated with bone disorders. In
bone from type 1 diabetic rats, decreased expression of IGF-1 and
IGF-1R was detected (29).
Consistently, in this study, the insulin and IGF-1 signaling system
in BMSCs derived from diabetic rats exhibited multiple
abnormalities, including decreased expression of IR, IGF-1, IGF-1R,
IRS-1 and p-ERK compared with control BMSCs. Insulin and IGF-1
signaling are important in the maintenance of normal bone
metabolism. Mice lacking IR or IGF-1R in osteoblasts exhibited
reduced trabecular bone and mineralization (19,20). In vitro, the IR-deficient
osteoblasts showed impaired proliferation, differentiation and
decreased expression of Runx2 and OCN (18). In addition, IRS-1 deficiency in
osteoblasts was found to impair osteoblast proliferation and
differentiation (30). Therefore,
abnormalities in insulin and IGF-1 signaling in diabetic BMSCs are
potential contributors to reduced osteogenic potential.
The impaired insulin and IGF-1 signaling would
contribute to inhibition of the phosphorylation of ERK. Insulin and
IGF-1 activate IR, IGF-1R and subsequently IRS-1, which activates
the ERK pathway to regulate cell proliferation, differentiation,
apoptosis and metabolism. Both insulin and IGF-1 have been shown to
stimulate osteoblast proliferation and differentiation through the
activation of ERK signaling pathways (17,18). However, the expression levels of
IR, IGF-1, IGF-1R and IRS-1 were reduced in diabetic BMSCs in our
study, which led to inhibition of the phosphorylation of ERK. It is
well known that the ERK pathway is crucial for the regulation of
cell proliferation, osteoblast differentiation and skeletal
development (31). An important
function for the ERK pathway has been established in osteoblasts
that involves stimulation of Runx2 (32). Thus, inhibition of the
phosphorylation of ERK may also contribute to decreased
proliferation and impaired osteogenic potential of diabetic
BMSCs.
In summary, the present study demonstrated that the
osteogenic potential of BMSCs derived from diabetic rats was
impaired, which may be partially related to impaired insulin and
IGF-1 signaling. These findings may advance our understanding of
bone complications in diabetes. These results suggest that
restoring the function of BMSCs in diabetic patients is essential
to treat bone complications and that diabetic BMSCs could be an
alternative cell source for bone regeneration in diabetes,
including the use of additional growth factors targeting insulin
and IGF-1 signaling to compensate for their deficiency.
Acknowledgements
This study was jointly supported by
the National Basic Research Program of China (973 Program, No.
2012CB933604), the National Science Fund for Distinguished Young
Scholars of China (No. 81225006) and the National Natural Science
Foundation of China (No. 81170939).
References
|
1
|
Hofbauer LC, Brueck CC, Singh SK and
Dobnig H: Osteoporosis in patients with diabetes mellitus. J Bone
Miner Res. 22:1317–1328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janghorbani M, Van Dam RM, Willett WC and
Hu FB: Systematic review of type 1 and type 2 diabetes mellitus and
risk of fracture. Am J Epidemiol. 166:495–505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Retzepi M and Donos N: The effect of
diabetes mellitus on osseous healing. Clin Oral Implants Res.
21:673–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yaturu S: Diabetes and skeletal health. J
Diabetes. 1:246–254. 2009. View Article : Google Scholar
|
|
5
|
Khosla S, Westendorf JJ and Mödder UI:
Concise review: Insights from normal bone remodeling and stem
cell-based therapies for bone repair. Stem Cells. 28:2124–2128.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mauney JR, Volloch V and Kaplan DL: Role
of adult mesenchymal stem cells in bone tissue engineering
applications: current status and future prospects. Tissue Eng.
11:787–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Breitbart EA, Meade S, Azad V, et al:
Mesenchymal stem cells accelerate bone allograft incorporation in
the presence of diabetes mellitus. J Orthop Res. 28:942–949.
2010.PubMed/NCBI
|
|
8
|
Cramer C, Freisinger E, Jones RK, et al:
Persistent high glucose concentrations alter the regenerative
potential of mesenchymal stem cells. Stem Cells Dev. 19:1875–1884.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan M, Akhtar S, Mohsin S, N Khan S and
Riazuddin S: Growth factor preconditioning increases the function
of diabetes-impaired mesenchymal stem cells. Stem Cells Dev.
20:67–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin P, Zhang X, Wu Y, et al:
Streptozotocin-induced diabetic rat-derived bone marrow mesenchymal
cells have impaired abilities in proliferation, paracrine,
antiapotosis, and myogenic differentiation. Transplant Proc.
42:2745–2752. 2010. View Article : Google Scholar
|
|
11
|
Stolzing A, Sellers D, Llewelyn O and
Scutt A: Diabetes induced changes in rat mesenchymal stem cells.
Cells Tissues Organs. 191:453–465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fulzele K and Clemens TL: Novel functions
for insulin in bone. Bone. 50:452–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
AboElAsrar MA, Elbarbary NS, Elshennawy DE
and Omar AM: Iusulin-like growth factor-1 cytokines cross-talk in
type 1 diabetes mellitus: relationship to microvascular
complications and bone mineral density. Cytokine. 59:86–93. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gandhi A, Beam HA, O’Connor JP, Parsons JR
and Lin SS: The effects of local insulin delivery on diabetic
fracture healing. Bone. 37:482–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thaller SR, Lee TJ, Armstrong M, Tesluk H
and Stern JS: Effect of insulin-like growth factor type 1 on
critical-size defects in diabetic rats. J Craniofac Surg.
6:218–223. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taniguchi CM, Emanuelli B and Kahn CR:
Critical nodes in signaling pathways: insights into insulin action.
Nat Rev Mol Cell Biol. 7:85–96. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Shen X, Wan C, Zhao Q, Zhang L,
Zhou Q and Deng L: Effects of insulin and insulin-like growth
factor 1 on osteoblast proliferation and differentiation:
differential signalling via Akt and ERK. Cell Biochem Funct.
30:297–302. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Zhang X, Wang W and Liu J: Insulin
stimulates osteoblast proliferation and differentiation through ERK
and PI3K in MG-63 cells: cell biochemistry and function. Cell
Biochem Funct. 28:334–341. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fulzele K, Riddle RC, DiGirolamo DJ, et
al: Insulin receptor signaling in osteoblasts regulates postnatal
bone acquisition and body composition. Cell. 142:309–319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M, Xuan S, Bouxsein ML, et al:
Osteoblast-specific knockout of the insulin-like growth factor
(IGF) receptor gene reveals an essential role of IGF signaling in
bone matrix mineralization. J Biol Chem. 277:44005–44012. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xian L, Wu X, Pang L, Lou M, et al: Matrix
IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem
cells. Nat Med. 18:1095–1101. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Retzepi M, Lewis MP and Donos N: Effect of
diabetes and metabolic control on de novo bone formation following
guided bone regeneration. Clin Oral Implants Res. 21:71–79. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang X, Zhao J, Wang S, et al: Mandibular
repair in rats with premineralized silk scaffolds and
BMP-2-modified bMSCs. Biomaterials. 30:4522–4532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeng D, Xia L, Zhang W, et al: Maxillary
sinus floor elevation using a tissue-engineered bone with
calcium-magnesium phosphate cement and bone marrow stromal cells in
rabbits. Tissue Eng Part A. 18:870–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lü K, Zeng D, Zhang Y, et al: BMP-2 gene
modified canine bMSCs promote ectopic bone formation mediated by a
nonviral PEI derivative. Ann Biomed Eng. 39:1829–1839.
2011.PubMed/NCBI
|
|
26
|
Wang XJ, Huang H, Yang F, Xia LG, Zhang
WJ, Jiang XQ and Zhang FQ: Ectopic study of tissue-engineered bone
complex with enamel matrix proteins, bone marrow stromal cells in
porous calcium phosphate cement scaffolds, in nude mice. Cell
Prolif. 44:274–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia L, Xu Y, Chang Q, et al: Maxillary
sinus floor elevation using BMP-2 and Nell-1 gene-modified bone
marrow stromal cells and TCP in rabbits. Calcif Tissue Int.
89:53–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCabe LR: Understanding the pathology and
mechanisms of type 1 diabetic bone loss. J Cell Biochem.
102:1343–1357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hie M, Iitsuka N, Otsuka T and Tsukamoto
I: Insulin-dependent diabetes mellitus decreases osteoblastogenesis
associated with the inhibition of Wnt signaling through increased
expression of Sost and Dkk1 and inhibition of Akt activation. Int J
Mol Med. 28:455–462. 2011.
|
|
30
|
Ogata N, Chikazu D, Kubota N, et al:
Insulin receptor substrate-1 in osteoblast is indispensable for
maintaining bone turnover. J Clin Invest. 105:935–943. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stokoe D, Macdonald SG, Cadwallader K,
Symons M and Hancock JF: Activation of Raf as a result of
recruitment to the plasma membrane. Science. 264:1463–1467. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ge C, Yang Q, Zhao G, Yu H, Kirkwood KL
and Franceschi RT: Interactions between extracellular
signal-regulated kinase 1/2 and p38 MAP kinase pathways in the
control of RUNX2 phosphorylation and transcriptional activity. J
Bone Miner Res. 27:538–551. 2012. View
Article : Google Scholar : PubMed/NCBI
|