Introduction
The hair follicle is one of the skin appendages, a
mini organ that forms early in embryonic development as a result of
epithelial-mesenchymal cell interactions. The occurrence and
maintenance of hair follicle is related to more than 20 types of
cells (1). The hair follicle is
one of the few organs in the body with the ability to undergo
cycles of degeneration and regeneration throughout life (2). It has been reported that multipotent
stem cells, isolated from individual follicles, are similar to bone
marrow mesenchymal stem cells since they express surface markers of
mesenchymal stem cells and can differentiate into adipocytes,
chondrocytes, osteoblasts, glial cells, melanocytes, smooth muscle
cells and endothelial cells (3–5).
In addition, hair follicle allografts have not
demonstrated immune rejection, suggesting that the engrafted hair
follicles have low immunogenic properties (6). Therefore, hair follicle stem cells
appear to be highly appropriate seed cells for tissue engineering
and clinical application.
Self-renewal and multipotent differentiation are
characteristics of stem cells. Since numerous undifferentiated stem
cells are required for clinical applications, several laboratories
have supplemented their expansion medium with growth factors to
accelerate stem cell proliferation. Fibroblast growth factors
(FGFs), are a family of growth factors involved in angiogenesis,
wound healing, and embryonic development. The FGFs are
heparin-binding proteins, and interactions with cell-surface
associated heparan sulfate proteoglycans are essential for FGF
signal transduction (7).
FGFs are key players in the processes of
proliferation and differentiation of a wide variety of cells and
tissues. Acidic fibroblast growth factor (aFGF) functions as a
modifier of endothelial cell migration and proliferation, as well
as an angiogenic factor. It acts as a mitogen for a variety of
mesoderm- and neuroectoderm-derived cells in vitro (8,9).
Basic fibroblast growth factor (bFGF) is a critical
component of human embryonic stem cell culture medium and is
necessary for the cells to remain in an undifferentiated state,
although the mechanisms by which it does this are poorly defined.
It is necessary in mouse-feeder cell dependent culture systems, as
well as in feeder and serum-free culture systems (10,11).
Epidermal growth factor (EGF) is known to enhance
migration and cell proliferation of bone marrow-derived mesenchymal
stem cells while maintaining differentiation potential (8). Numerous studies have demonstrated
that EGF and bFGF increase proliferation and modulate the
differentiation potential of human adipose-derived stromal/stem
cells (12–15).
Based on these observations, we hypothesized that
the presence of aFGF, EGF and bFGF would enhance the proliferation
and multipotent potential of human hair follicle-derived
mesenchymal stem cells (HF-MSCs). The current study examined the
effect of aFGF, EGF and bFGF with respect to HF-MSC proliferation
and multipotent differentiation.
Materials and methods
Isolation and cultivation of human hair
follicle stem cells
Human hair follicle stem cells were isolated from
adult occipital and temporal hair follicles. The hair follicles
were drawn off by cropped tweezers. After extensive washing with
phosphate-buffered saline (PBS) containing antibiotic-antimycotic
(Hyclone, USA), the hair follicles were transferred each into a
single well of a 96-well culture plate, and cultured in 100 μl of
Dulbecco’s modified Eagle’s medium/F12 (DMEM/F-12) containing 10%
fetal bovine serum (FBS; Gibco, USA) and 1% antibiotic/antimycotic,
supplemented with 10 ng/ml EGF and 10 ng/ml bFGF to allow for cell
migration onto the tissue culture plastic. The wells populated with
cells originating from the dermal sheath or papilla and which had
the morphological appearance of mesenchymal cells were selected,
pooled and expanded. Expanded cells were cultured in DMEM/F-12
containing 10% FBS and 1% antibiotic/antimycotic and supplemented
with 2 ng/ml bFGF. Prior to the proliferation assay, the cells were
cultured in stromal medium (DMEM/F-12, 10% FBS) for 7 days.
Adipogenic, osteogenic and chondrogenic
differentiation of human hair follicle stem cells
Conditions to induce the differentiation of human
hair follicle stem cells into adipocytes, chondrocytes, and
osteoblasts were employed as previously described (2). Cells were grown in the adipogenic
medium [high glucose DMEM (Gibco), 10% FBS, 0.5 μM IBXM, 1 μM
dexamethasone, 10 μM insulin, 200 μM indomethacin (Sigma, USA)] for
2 weeks, and then stained using Oil Red O (2).
The cells were cultured in the osteogenic medium
[high glucose-DMEM, 10% FBS, 50 μM ascorbate-2-phosphate, 0.1 μM
dexamethasone, 10 mM β-glycerolphosphate (Sigma)] for 2 and 4
weeks. Following differentiation for 2 weeks, the alkaline
phosphatase (ALP) enzymatic activity (2), Alizarin Red staining and the von
Kossa staining (2) was performed
after 4 weeks of culture to examine osteogenic differentiation.
For chondrogenic differentiation, cell spheres of
HF-MSCs were generated by adding 20 ml of 8×106 cell/ml
HF-MSCs drop wise in non-tissue-culture-treated 24-well plates.
After 4 h of incubation at 37°C and 10% CO2, the medium
was changed to the chondrogenic differentiation medium: high
glucose DMEM with 10% FBS, 6.25 μM insulin, 10 ng/ml transforming
growth factor-β1 (TGF-β1; Sigma), and 50 μM of
ascorbate-2-phosphate. The culture medium was replenished every 3
days for 2 weeks. At that time, cell spheres of HF-MSCs were fixed
in 10% buffered formalin phosphate (Fisher Scientific) and embedded
in paraffin. The tissue sections were used for Alcian blue staining
and collagen II immunohistochemistry (2).
Real-time PCR
After the differentiation of human hair follicle
stem cells into adipocytes, chondrocytes, and osteoblasts, the
total RNA was extracted from differentiated cells using TRI-Reagent
(Sigma), according to the manufacturer’s instructions. Total RNA
was reverse transcribed by RNA PCR kit (AMV) Ver.3.0 (Takara,
Dalian, China). For quantitative determination, the real-time PCR
was performed in the Applied Biosystems 7500 sequence detection
system (Applied Biosystems, Foster City, CA, USA) using
SYBR®-Green (Roche Diagnostics) as a double-strand
DNA-specific binding dye, according to the manufacturer’s
instructions. Samples were amplified using specific primers to ap2,
peroxisome proliferator-activated receptor γ2 (PPARγ2), ALP,
runt-related transcription factor 2 (RUNX2), osteocalcin (OC),
collagen II, SOX9 and β-actin. β-actin was used as internal
standard, and relative expression was calculated according to the
ΔCt method. The result of real-time PCR was represented as fold
increase with respect to the control sample (construct grown in
DMEM/F-12 containing 10% FBS and 1% antibiotic/antimycotic and
supplemented with 2 ng/ml bFGF). The PCR oligonucleotide primers
were (34,35): ap2 (F, 5′-AAAGAAGTAGG
AGTGGGCTTTGC-3′ and R, 5′-CCCCATTCACACTGAT GATCAT-3′); PPARγ2 (F,
5′-AGGCGAGGGCGATCTTG-3′ and R, 5′-CCCATCATTAAGGAATTCATGTCATA-3′);
SOX9 (F, 5′-TTCATGAAGATGACCGACGA-3′ and R, 5′-GTCCAG
TCGTAGCCCTTGAG-3′); collagen II (F, 5′-AGAGACCTG AACTGGGCAGA-3′ and
R, 5′-TGACACGGAGTAGCACC ATC-3′); ALP (F,
5′-CCAACGTGGCTAAGAATGTCATC-3′ and R, 5′-TGGGCATTGGTGTTGTACGTC-3′);
RUNX2 (F, 5′-TGGTTAATCTCCGCAGGTCAC-3′ and R, 5′-ACTGTGCT
GAAGAGGCTGTTTG-3′); OC (F, 5′-CCATGAGAGCCCTCA CACTCC-3′ and R,
5′-GGTCAGCCAACTCGTCACAGTC-3′); β-actin (F,
5′-CATGTACGTTGCTATCCAGGC-3′ and R,
5′-CTCCTTAATGTCACGCACGAT-3′).
Effect of growth factors on human hair
follicle stem cell proliferation
Following culture in stromal medium (DMEM/F-12, 10%
FBS) for 7 days, the human hair follicle stem cells were harvested
by trypsin digestion and replated at a density of 10,000 cells/well
in 6-well plates in stromal medium (2). After 24 h to allow for adherence,
the stromal medium was converted to DMEM/F-12 containing 10% FBS
and 1% antibiotic/antimycotic and supplemented with EGF (0, 1.0,
2.0, 5.0, 10 or 20 ng/ml) or bFGF (0, 1.0, 2.0, 5.0, 10 or 20
ng/ml) or aFGF (0, 1.0, 3.0, 5.0, 10 or 20, 50 or 100 ng/ml). Cells
used in proliferation assays were maintained under these conditions
for 7 days.
Cell counting (n=3 donors)
Cell proliferation was determined on passages 9–11
human hair follicle stem cells after 7 days of conditioning with
varying concentrations of EGF, bFGF and aFGF. Cells from individual
wells of a 6-well plate were harvested by digestion with 0.25%
trypsin/0.01% EDTA. An aliquot of cells was stained with trypan
blue, and total number of cells/well was measured using a
hemocytometer.
Cell cycle analysis
After the cell counting, the optimal concentration
of each individual growth factor was determined. The cells were
cultured in stromal medium (DMEM/F-12, 10% FBS) in the presence of
each individual optimal concentration of EGF, bFGF and aFGF,
respectively, for 7 days followed by trypsinization with 1 ml of
trypsin. Five milliliters PBS was added to the digest and the cells
were spun down at 800 rpm for 5 min. Five milliliters of 70% EtOH
(cold) were added to the cell pellets. The cells were vortexed
gently and incubated in EtOH at 4°C overnight. Next day, the cells
were spun down at 800 rpm for 5 min and the cell pellets were
washed once in PBS for 1 min and incubated with 150 μl RNAse (5
μg/ml) at 37°C, 5% CO2 for 45 min. At the end of the
incubation, 350 μl of propidium iodine (PI; Dingguo, Beijing,
China) were added and the cells were incubated at 4°C for 30 min
and analyzed immediately following the completion of the incubation
to prevent cells from clumping.
Proliferating cell nuclear antigen (PCNA)
immunohistochemistry
Cells were seeded in a 24-well tissue culture plate
at a density of 5,000 cells/well and were cultured in the DMEM-F12
medium deprived of any growth factors for 7 days. Subsequently,
individual growth factors (EGF, bFGF or aFGF) at optimal
concentrations were added to the culture medium and the cells were
cultured for another 7 days. At the end of the cultivation, the
medium was aspirated and the cells were washed 3 times with PBS,
fixed in 4% paraformaldehyde at room temperature (RT) for 10 min.
Following fixation, the cells were washed 3 times with PBS and
permeabilized in 0.1% Triton X-100 at RT for 10 min, blocked with
10% FBS/PBS for 30 min. They were then incubated with mouse
anti-human PCNA antibody (1:200 dilution, at RT for 30 min;
Millipore) in blocking solution (0.01% Triton X-100 in 10% FBS) at
4°C overnight. After 3 washes in PBS, the cells were incubated with
Alexa Fluor 488-conjugated goat anti-mouse antibody (1:200
dilution, at RT for 60 min in dark; Abcam), washed once with PBS
and incubated with Hoechst 33342 (1:10,000, at RT, 2 min in dark;
Abcam) to stain the nuclei. Cells stained only with secondary
antibody served as the negative control.
Results
Isolation and cultivation of human hair
follicle stem cells
After being cultured for one week, mesenchymal cells
were observed migrating from the hair follicles. Cells from
individual wells were harvested by 0.25% trypsin in 0.01% EDTA.
Following centrifugation at 1,000 rpm for 5 min, the cells were
transferred into the wells of 24-well plates. The mesenchymal cells
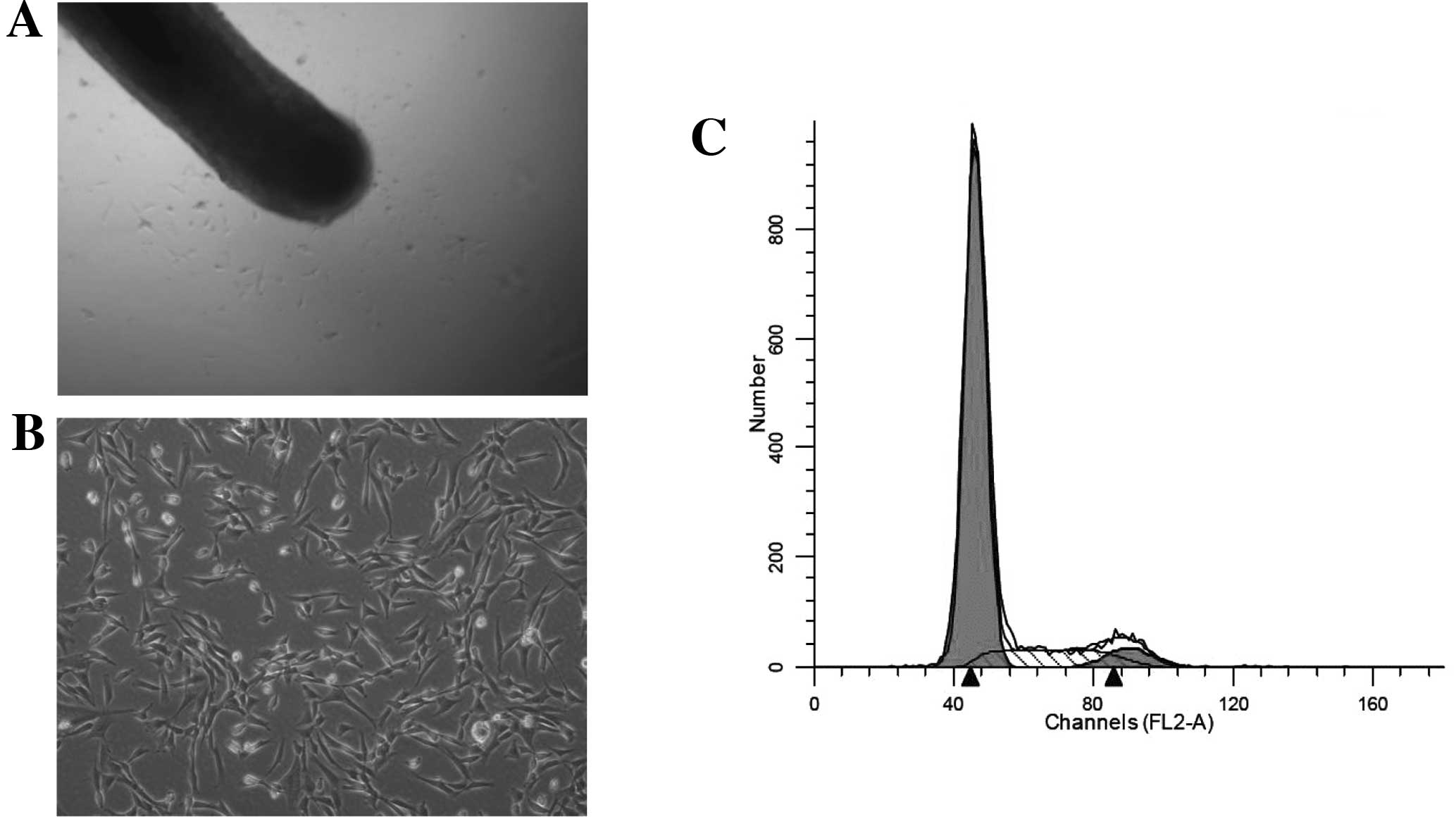
migrated out of the outer root sheath and dermal papilla (Fig. 1A) and had fibroblast-like cell
morphology (Fig. 1B).
Cell cycle analysis and multilineage
differentiation potential of HF-MSCs
Since the nuclear DNA content reflects the position
of a cell within a cell cycle, flow cytometric analysis of nuclear
DNA content was performed on cells following isolation. At passages
P9–11, 80% of the HF-MSCs were in the G0/G1 phase, and 14% of cells
were in the S phase (Fig. 1C).
This result suggested that the cells we obtained demonstrated the
slow cycling that characterizes stem cells.
Isolated HF-MSCs were cultured under specific
conditions and subsequently examined by functional differentiation
assays. We studied the potential of these cells to differentiate
into multiple cell lineages, in particular, chondrocytes,
adipocytes and osteocytes, as previously described in Materials and
methods.
Adipogenic differentiation was assessed by Oil Red O
staining. After 14 days of culture in adipogenic medium, the
HF-MSCs showed positive staining, with single adipocytic
multivacuolar cells secreting lipid droplets (Fig. 2A). The same cells maintained in
control medium (DMEM/F-12, 10% FBS and 2 ng/ml bFGF) exhibited
almost no lipid deposits (Fig.
2B).
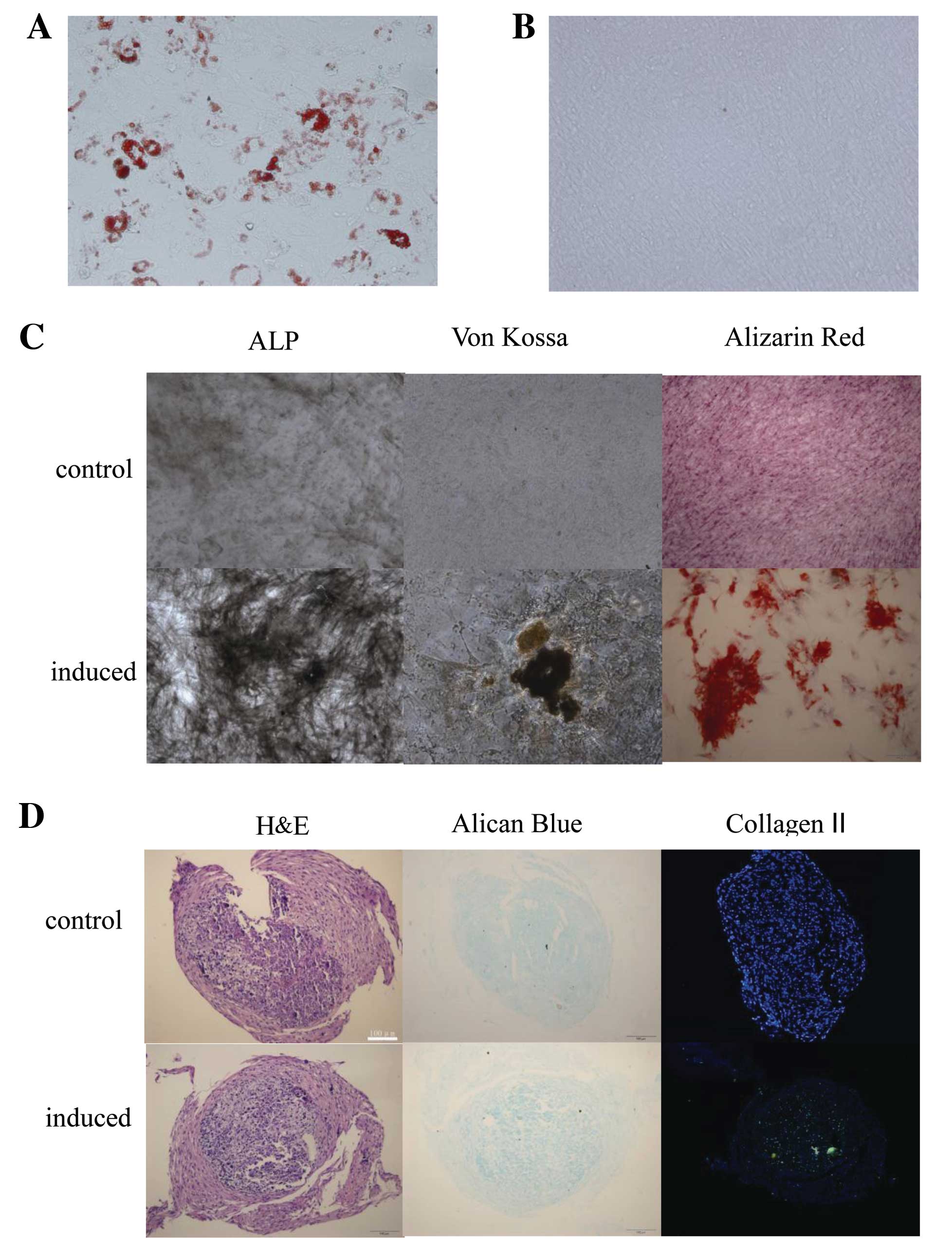 | Figure 2Human hair follicle-derived
mesenchymal stem cells (HF-MSCs) demonstrated adipogenic,
osteogenic and chondrogenic differentiation potential. HF-MSCs were
cultured in the (A and B) adipogenic, (C) osteogenic or (D)
chondrogenic differentiation medium or control medium. Oil Red O
staining of HF-MSCs that were treated with the (A) adipogenic or
(B) control medium. Magnification, (A and B) ×200, (C) ALP ×100,
von Kossa and Alizarin Red ×400. ALP, alkaline phosphatase. (E)
Adipogenic, osteogenic and chondrogenic differentiation was also
assessed by qRT-PCR. Expression levels of ap2 and PPARγ2 were
analyzed for adipogenic differentiation. Expression levels of OC,
RUNX2 and ALP were analyzed for osteogenic differentiation, and
expression levels of Col II and SOX9 were analyzed for chondrogenic
differentiation. PPARγ2, peroxisome proliferator-activated receptor
γ2; ALP, alkaline phosphatase; OC, osteocalcin; RUNX2, runt-related
transcription factor 2. |
The osteogenic differentiation potential of HF-MSCs
was examined by testing ALP enzymatic activity, Alizarin Red
staining and the von Kossa staining. The cells demonstrated ALP
enzymatic activity and calcium deposition. The same cells
maintained in control medium had an absence of calcium deposits
(Fig. 2C).
The ability to undergo chondrogenic differentiation
was assessed by Alcian blue staining for proteoglycans. Collagen II
was detected by immunohistochemistry (Fig. 2D).
Adipogenic, osteogenic and chondrogenic
differentiation was also assessed by qRT-PCR. Expression levels of
ap2 and PPARγ2 were analyzed for adipogenic differentiation.
Expression levels of ALP, RUNX2 and OC were analyzed for osteogenic
differentiation, and expression levels of collagen II, SOX9 were
analyzed for chondrogenic differentiation. RNA extracted from
HF-MSCs following adipogenic, osteogenic and chondrogenic
differentiation showed expression of all these lineage-specific
genes (Fig. 2E) The expression of
the β-actin housekeeping gene was assessed in all samples to
analyze the integrity of the amplified cDNA.
Effect of growth factors on the
proliferation of human hair follicle stem cells
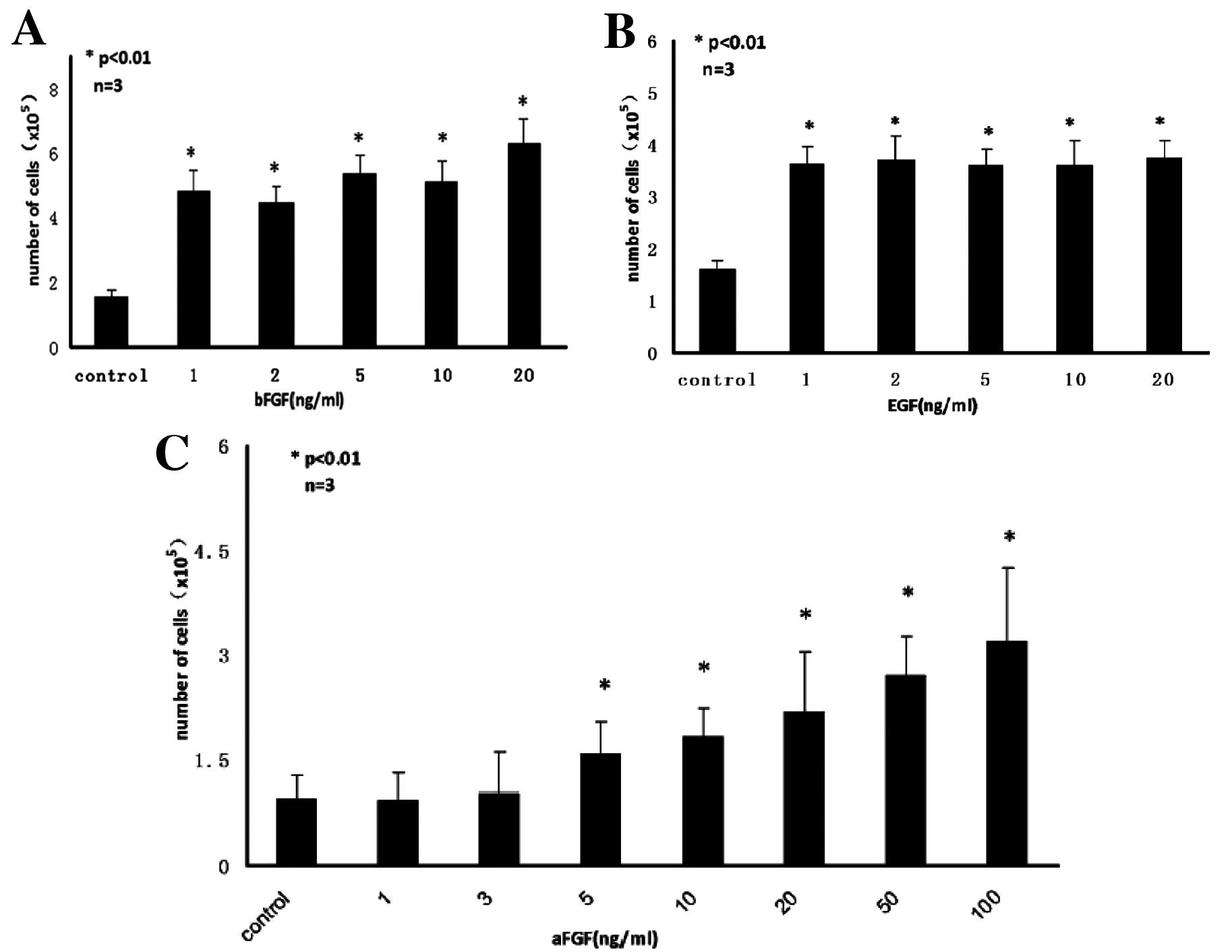
After 7 days of conditioning with varying
concentrations of bFGF, EGF and aFGF, the total number of human
hair follicle stem cells per well was determined using a
hemocytometer. Proliferation analysis showed that both EGF and bFGF
at as low as 1 ng/ml and aFGF at above 5 ng/ml levels significantly
increased the proliferation of HF-MSCs. aFGF and bFGF acted in a
synergistic manner, but concentrations of EGF between 1 and 20
ng/ml had no significant effect on proliferation, relative to the
control without growth factor (Fig.
3A–C). The optimal concentration of the growth factors for
stimulation of HF-MSCs proliferation was 20 ng/ml bFGF, 1 ng/ml EGF
and 100 ng/ml aFGF. These concentrations were used in all further
experiments.
Consistent with proliferation analysis,
immunofluorescence staining showed that >95% of the HF-MSCs
cultured in the presence of aFGF, bFGF and EGF were positively
stained for PCNA (Fig. 3D).
Furthermore, flow cytometric analysis showed that up
to 70% of HF-MSCs cultured in the presence of aFGF, bFGF or EGF
remained at the G0/G1 phase, and that the proliferation index (PI)
significantly increased relative to controls (Fig. 3E). The results of PCNA and PI
analyses demonstrated that the addition of bFGF, EGF and aFGF to
the cell culture medium increased the proliferation of HF-MSCs.
To test whether the cells cultured in the presence
of growth factors could display the multilineage differentiation
potential, we replaced the culture medium after 7 days of
conditioning with 20 ng/ml bFGF, 1 ng/ml EGF, 100 ng/ml aFGF, with
induction medium. Indeed, we found that the cells could
differentiate into fat, bone, or cartilage cells under appropriate
differentiation conditions (Fig.
4).
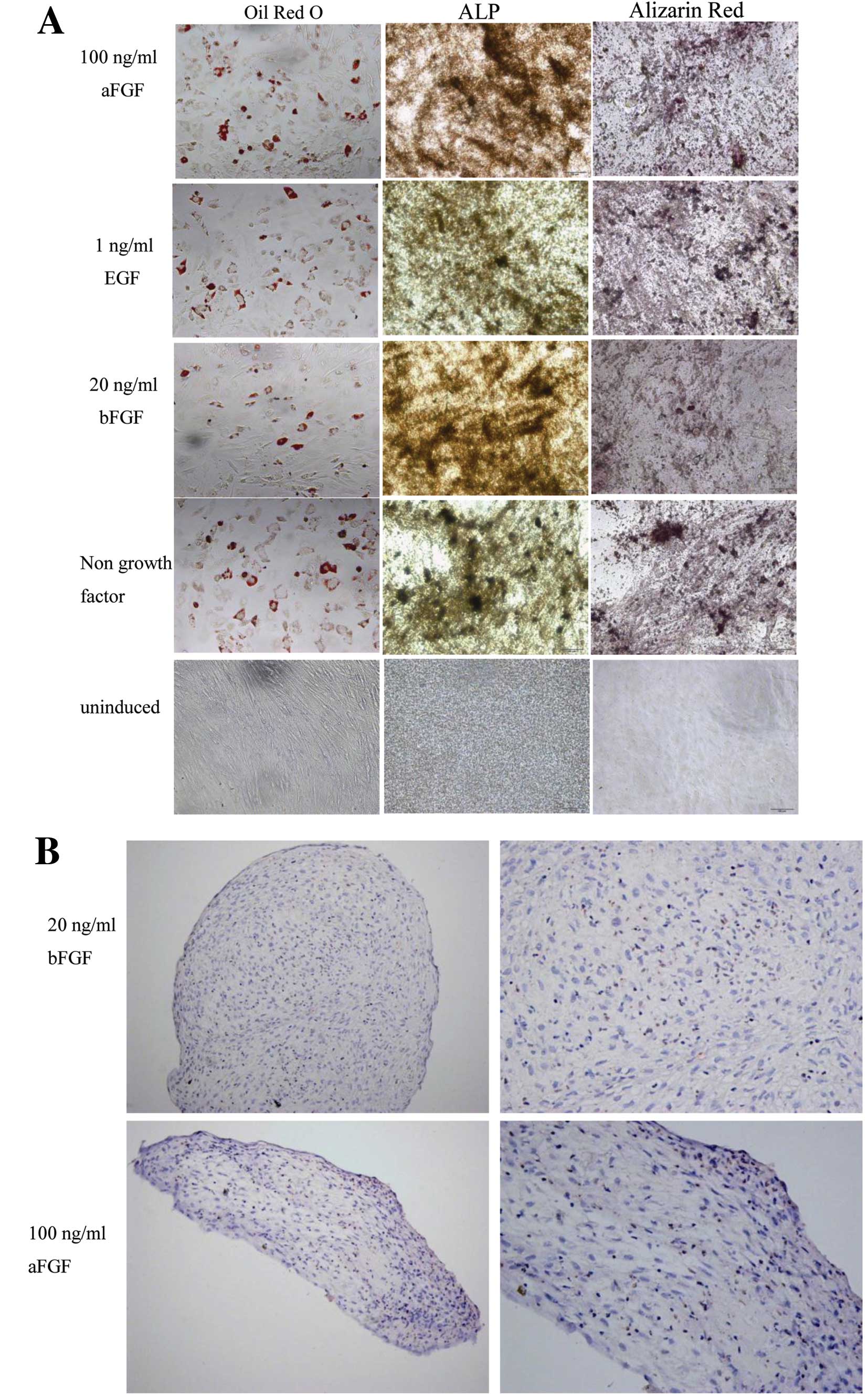 | Figure 4Human hair follicle-derived
mesenchymal stem cells (HF-MSCs) cultured with growth factors
retained their adipogenic, osteogenic and chondrogenic
differentiation potential. After 7 days of conditioning with 20
ng/ml bFGF, 1 ng/ml EGF, 100 ng/ml aFGF and control without growth
factors, the medium was changed to differentiation medium. HF-MSCs
were cultured in the (A) adipogenic and osteogenic or (B)
chondrogenic medium. Magnification, (A) ×200; (B) left, ×200 and
right, ×400. ALP, alkaline phosphatase; bFGF, basic fibroblast
growth factor; EGF, epidermal growth factor; aFGF, acidic
fibroblast growth factor. |
Discussion
Our results are consistent with several previous
studies that have demonstrated the multipotency of rat or human
skin dermal fibroblasts (16–20). It has been established that the
bulge area of the hair follicle is a rich source of epidermal stem
cells (21–24). The present study further supports
these data by showing that HF-MSCs express CD44, CD73, CD90 and
CD105 which are the surface markers that characterize MSCs. HF-MSCs
have the potential to differentiate into multiple cell lineages,
particularly chondrocytes, adipocytes and osteocytes (2), suggesting that HF-MSCs may be highly
similar to MSCs derived from the bone marrow, adipose tissue, or
other organs. Mainly, the hair follicle may be a readily accessible
source of autologous human MSCs that can be used for tissue
engineering and regenerative medicine.
The present study demonstrated that the use of aFGF,
EGF and bFGF supplementation during the expansion culture of
HF-MSCs improves their proliferation rate yet preserves their
significant trilineage differentiation potentials. It has been
demonstrated that EGF can increase the expansion of BMSCs for in
vivo transplantation (14)
and, if added subsequent to induction, enhanced adipogenesis
(25). Moreover, EGF had notable
effects on the growth and development of human adipose-derived
stromal/stem cells at physiological concentrations (0.5–2 ng/ml)
(13). Our results support the
original studies which showed that EGF can increase the
proliferation of HF-MSCs. Moreover, when 5 ng/ml EGF was added into
the culture medium for 3 weeks, individual cells showed positive
Oil Red O staining without culturing in adipogenic medium. Several
studies have shown that bFGF increases proliferation and prevents
differentiation of BM-MSCs (hMSCs) (26–28) and embryonic stem cells (10). Furthermore, bFGF preconditioning
enhances the differentiation potential of the BMSCs (28,29). Our results showed that aFGF is
similar to bFGF in its ability to stimulate proliferation, but
compared with bFGF the necessary concentration of aFGF was higher
to achieve a similar proliferation rate.
It remains to be determined how EGF, aFGF and bFGF
increase HF-MSC proliferation. They may act as downstream elements
in its signal transduction pathway such as the FGF, the BMPs and
the classic Wnt signaling pathway (30–33). Thus, further studies will focus on
fully characterizing the in vitro and in vivo effects
of EGF and FGFs.
Acknowledgements
This study was supported by the State Key
Development Program of Basic Research of China (211CB606200), the
National Natural Science Foundation of China (30930026/C100101),
the Science and Technology Planning Project of Jilin Province,
China (200905180), the National Natural Science Foundation of China
(31040028). The authors thank Willam Orr for the valuable editorial
assistance.
References
|
1
|
Park IH, Zhao R, West JA, et al:
Reprogramming of human somatic cells to pluripotency with defined
factors. Nature. 451:141–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu JY, Peng HF, Gopinath S, Tian J and
Andreadis ST: Derivation of functional smooth muscle cells from
multipotent human hair follicle mesenchymal stem cells. Tissue Eng
Part A. 16:2553–2564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoogduijn MJ, Gorjup E and Genever PG:
Comparative characterization of hair follicle dermal stem cells and
bone marrow mesenchymal stem cells. Stem Cell Dev. 15:49–60. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jahoda CA, Whitehouse J, Reynolds AJ and
Hole N: Hair follicle dermal cells differentiate into adipogenic
and osteogenic lineages. Exp Dermatol. 12:849–859. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rufaut NW, Goldthorpe NT, Wildermoth JE
and Wallace OA: Myogenic differentiation of dermal papilla cells
from bovine skin. J Cell Physiol. 209:959–966. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jahoda CA, Reynolds AJ, Chaponnier C,
Forester JC and Gabbiani G: Smooth muscle alpha-actin is a maker
for hair follicle dermis in vivo and in vitro. J Cell Sci.
99:627–636. 1991.PubMed/NCBI
|
|
7
|
Lin X, Buff EM, Perrimon N and Michelson
AM: Heparan sulfate proteoglycans are essential for FGF receptor
signaling during Drosophila embryonic development.
Development. 126:3715–3723. 1999.PubMed/NCBI
|
|
8
|
Böhlen P, Esch F, Baird A and
Gospodarowicz D: Acidic fibroblast growth factor (FGF) from bovine
brain: amino-terminal sequence and comparison with basic FGF. EMBO
J. 4:1951–1956. 1985.PubMed/NCBI
|
|
9
|
Gospodarowicz D, Massoglia S, Cheng J and
Fujii DK: Effect of fibroblast growth factor and lipoproteins on
the proliferation of endothelial cells derived from bovine adrenal
cortex, brain cortex, and corpus luteum capillaries. J Cell
Physiol. 127:121–136. 1986. View Article : Google Scholar
|
|
10
|
Vallier L, Alexander M and Pedersen RA:
Activin/Nodal and FGF pathways cooperate to maintain pluripotency
of human embryonic stem cells. J Cell Sci. 118:4495–4509. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang G, Zhang H, Zhao Y, Li J, Cai J, Wang
P, Meng S, Feng J, Miao C, Ding M, Li D and Deng H: Noggin and bFGF
cooperate to maintain the pluripotency of humanembryonic stem cells
in the absence of feeder layers. Biochem Biophys Res Commun.
330:934–942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Butterwith SC, Peddie CD and Goddard C:
Regulation of adipocyte precursor DNA synthesis by acidic and basic
fibroblast growth factors: interaction with heparin and other
growth factors. J Endocrinol. 137:369–374. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hauner H, Röhrig K and Petruschke T:
Effects of epidermal growth factor (EGF), platelet-derived growth
factor (PDGF) and fibroblast growth factor (FGF) on human adipocyte
development and function. Eur J Clin Invest. 25:90–96. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamama K, Fan VH, Griffith LG, Blair HC
and Wells A: Epidermal growth factor as a candidate for ex vivo
expansion of bone marrow-derived mesenchymal stem cells. Stem
Cells. 24:686–695. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zaragosi LE, Ailhaud G and Dani C:
Autocrine fibroblast growth factor 2 signaling is critical for
self-renewal of human multipotent adipose-derived stem cells. Stem
Cells. 24:2412–2419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toma JG, Akhavan M, Fernandes KJ,
Barnabé-Heider F, Sadikot A, Kaplan DR and Miller FD: Isolation of
multipotent adult stem cells from the dermis of mammalian skin. Nat
Cell Biol. 3:778–784. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernandes KJ, McKenzie IA, Mill P, et al:
A dermal niche for multipotent adult skin-derived precursor cells.
Nat Cell Biol. 6:1082–1093. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toma JG, McKenzie IA, Bagli D and Miller
FD: Isolation and characterization of multipotent skin-derived
precursors from human skin. Stem Cells. 23:727–737. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen FG, Zhang WJ, Bi D, Liu W, Wei X,
Chen FF, Zhu L, Cui L and Cao Y: Clonal analysis of nestin(−)
vimentin(+) multipotent fibroblasts isolated from human dermis. J
Cell Sci. 120:2875–2883. 2007.
|
|
20
|
Lorenz K, Sicker M, Schmelzer E, Rupf T,
Salvetter J, Schulz-Siegmund M and Bader A: Multilineage
differentiation potential of human dermal skin-derived fibroblasts.
Exp Dermatol. 17:925–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cotsarelis G, Sun TT and Lavker RM:
Label-retaining cells reside in the bulge area of pilosebaceous
unit: implications for follicular stem cells, hair cycle, and skin
carcinogenesis. Cell. 61:1329–1337. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morris RJ and Potten CS: Highly persistent
label-retaining cells in the hair follicles of mice and their fate
following induction of anagen. J Invest Dermatol. 112:470–475.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tumbar T, Guasch G, Greco V, Blanpain C,
Lowry WE, Rendl M and Fuchs E: Defining the epithelial stem cell
niche in skin. Science. 303:359–363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morris RJ, Liu Y, Marles L, Yang Z,
Trempus C, Li S, Lin JS, Sawicki JA and Cotsarelis G: Capturing and
profiling adult hair follicle stem cells. Nat Biotechnol.
22:411–417. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adachi H, Kurachi H, Homma H, et al:
Epidermal growth factor promotes adipogenesis of 3T3-L1 cell in
vitro. Endocrinology. 135:1824–1830. 1994.PubMed/NCBI
|
|
26
|
Tsutsumi S, Shimazu A, Miyazaki K, Pan H,
Koike C, Yoshida E, Takagishi K and Kato Y: Retention of
multilineage differentiation potential of mesenchymal cells during
proliferation in response to FGF. Biochem Biophys Res Commun.
288:413–419. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Solchaga LA, Penick K, Porter JD, Goldberg
VM, Caplan AI and Welter JF: FGF-2 enhances the mitotic and
chondrogenic potentials of human adult bone marrow-derived
mesenchymal stem cells. J Cell Physiol. 203:398–409. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin I, Muraglia A, Campanile G,
Cancedda R and Quarto R: Fibroblast growth factor-2 supports ex
vivo expansion and maintenance of osteogenic precursors from human
bone marrow. Endocrinology. 138:4456–4462. 1997.PubMed/NCBI
|
|
29
|
Stewart AA, Byron CR, Pondenis H and
Stewart MC: Effect of fibroblast growth factor-2 on equine
mesenchymal stem cell monolayer expansion and chondrogenesis. Am J
Vet Res. 68:941–945. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Itoh N and Ornitz DM: Evolution of the Fgf
and Fgfr gene families. Trends Genet. 20:563–569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Itoh N and Ornitz DM: Functional
evolutionary history of the mouse Fgf gene family. Dev Dyn.
237:18–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thisse B and Thisse C: Functions and
regulations of fibroblast growth factor signaling during embryonic
development. Dev Biol. 287:390–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mikels AJ and Nusse R: Wnts as ligands:
processing, secretion and reception. Oncogene. 25:7461–7468. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Santiago-Mora R, Casado-Díaz A, De Castro
MD and Quesada-Gómez JM: Oleuropein enhances osteoblastogenesis and
inhibits adipogenesis: the effect on differentiation in stem cells
derived from bone marrow. Osteoporos Int. 22:675–684. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi KH, Choi BH, Park SR, Kim BJ and Min
BH: The chondrogenic differentiation of mesenchymal stem cells on
an extracellular matrix scaffold derived from porcine chondrocytes.
Biomaterials. 31:5355–5365. 2010. View Article : Google Scholar : PubMed/NCBI
|