Introduction
Experimental autoimmune encephalomyelitis (EAE) is a
widely used model of multiple sclerosis (MS). Both are
characterized by immune cell infiltration from small blood vessels
into the central nervous system (CNS) and subsequent
CD4+ T cell-mediated axonal demyelination (1). MS is a chronic CNS inflammatory
disease that exhibits an earlier onset and higher incidence rate in
females than males (2). MS
severity and relapse frequency decrease significantly during late
pregnancy (3). However, the
disease usually shows increased relapse frequency and severity
during the postpartum period (4),
and this is associated with reduced estrogen levels (5). Clinical studies have indicated that
oral estrogen administration exerts immunoregulatory effects and
reduces the number and size of gadolinium-enhancing lesions in
relapsing-remitting MS patients, suggesting that estrogen is an
effective drug for MS therapy (6,7).
Certain studies have reported that estriol exerts anti-inflammatory
effects, such as inhibiting Th1 and Th17 cell priming (8,9),
regulatory B cells (10) and T
cells (11), as well as
inhibiting matrix metalloproteinase-9 (MMP-9) activity (12). Other studies have demonstrated
that estriol decreases gray matter atrophy (13) and reduces inappropriate excitatory
synaptic transmission and other neuropathological hippocampal
changes during EAE (14). It has
been reported that chronic neuroinflammation and nuclear
factor-κB-dependent chemokine C-C-motif ligand (CCL)2 expression in
reactive astrocytes can be inhibited by estriol (15). Estrogen also plays a protective
role. A number of published studies have described in detail that
different types of estrogen receptor (ER) signaling ameliorate
EAE-induced damage (9–11,16,17). Collectively, these reports, as
well as others indicate that estrogen treatment exerts
neuroprotective effects against EAE on the CNS. In this study, we
expanded on this line of research and investigated the utility of
inducing the overexpression of ERα using a lentivirus.
The biological effects of estrogens are
differentially mediated through ERα and ERβ, and G protein-coupled
ER30 (GPR30). Some studies have investigated the impact of ERβ
signaling. Tiwari-Woodruff and Voskuhl found that the ERβ ligand
had no effect at EAE onset, but promoted recovery (18). Du et al reported that ERβ
ligand administration did not reduce overall CNS inflammation in
EAE, but it did decrease the percentage of dendritic cells (DCs)
(19). Other studies have
reported a protective effect of GPR30 activation in EAE. Blasko
et al suggested that during EAE, GPR30 indirectly mediates a
decrease in proinflammatory cytokines, including interleukin
(IL)-17 and interferon-γ (IFN-γ) in immune cells (20). GPR30 plays a key role in the
estrogen-dependent and vitamin D3-mediated protection in EAE, which
could enhance IL-10 and IL-6 secretion by myelin oligodendrocyte
glycoprotein (MOG) peptide-reactive splenocytes and promote CCL5,
chemokine receptor (CCR)1, and CCR3 expression in spleen tissue
(21). GPR30 membrane ER
treatment has been shown to alter cytokine profiles and enhance the
suppressive activity of CD4+Foxp3+ T cells
through a GPR30 and programmed death-1 (PD-1)-dependent mechanism
(16). ERα also plays an
important role in EAE. Firstly, estrogens exert protective effects
during EAE through ERα by inhibiting the recruitment of
blood-derived inflammatory cells into the CNS (22). Secondly, certain studies have
reported estrogen-mediated protection against EAE in regulatory B
cells and the mediation of MMP-9 activity through ERα (10,23). Thirdly, Lelu et al
demonstrated that ERα expression is critical in hematopoietic, but
not endothelial cells; it mediates the estrogen inhibitory effect
on Th1 and Th17 cell priming, which protects against EAE (8). Finally, reactive astrocytes are a
target of the inhibitory action of nuclear ERα on chemokine
expression; this observation suggests that targeting astrocytic
nuclear factor-κB may be a useful therapeutic goal (15,24). Although exclusively investigated,
the exact role of ERα in EAE requires further study.
Evidence indicates that therapeutic genes expressed
in lentiviral vectors are applicable for the treatment of many
types of neurological disorders, due to the characteristics of the
sustained expression of targeted genes and no obvious unwanted
side-effects of lentivirus vectors per se (25). Foster et al found that
restoring ERα expression via lentiviral hippocampal delivery
improved the hippocampal response to estrogen in ERα−/−
mice (26), suggesting that the
presence of ERα in the CNS is crucial for estrogen function and
that a lentiviral delivery system is a viable way of manipulating
CNS gene expression. In this study, we established an experimental
model to identify and characterize the effect and mechanism of
action of nuclear ER in EAE. We induced the overexpression of ERα
in the CNS by injecting recombinant lentivirus into the lateral
cerebral ventricle, to investigate the effect and mechanism of
action of ERα in the CNS in EAE mice; estradiol and the ERα
agonist, raloxifene, were used as the positive controls. Our
results demonstrate that the lentivirus-mediated delivery of an ERα
gene is a viable method of increasing CNS-targeted protein
expression and is effective in ameliorating the symptoms of EAE in
a mouse model of MS.
Materials and methods
Animals
We purchased 75 4-week-old female C57BL/6 mice
weighing 19–22 g from the Experimental Animal Center of Chongqing
Medical University [Certificate of Conformity: SCXK (Chongqing)
2007-000341]. All mice were handled in accordance with the
requirements of the Chongqing University Medical School Laboratory
Animal Ethics Committee.
Reagents
The plasmid pMD19-ERα, 293T (human embryonic kidney)
cells, and Escherichia coli DH5α were obtained from the
Central Laboratory of Guizhou People’s Hospital, Guizhou, China.
The lentiviral vector, LV-GFP-Flag [with green fluorescence protein
(GFP) and Flag tag] was purchased from Shanghai GeneChem Corp.
(Shanghai, China). The helper plasmids, pHelper 1.0 and pHelper
2.0, Lipofectamine 2000 and TRIzol were purchased from Invitrogen
(Carlsbad, CA, USA). Raloxifene hydrochloride tablets (easy Witte™)
were purchased from Eli Lilly (Indianapolis, IN, USA). Estradiol
valerate (Progynova™) was purchased from Schering AG
(Berlin-Wedding, Germany). MOG 35–55 peptide
(MEVGWYRSPFSRVVHLYRNGK, purity >97%) was purchased from the
Xi’an AP Peptide Synthesis Company (Xi’an, China). Pertussis toxin
was a gift from the Chengdu Institute of Biological Products in
China. Bacillus Calmette-Guerin (BCG) vaccine was purchased from
Chinese Pharmaceutical and Biological Products (Chengdu, China).
Trypsin was purchased from Ameresco (Solon, OH, USA). Dulbecco’s
modified Eagle’s medium (DMEM)/F12, D-Hank’s solution, and protein
assay kits were purchased from HyClone (Logan, UT, USA). Fetal
bovine serum and B-27 were purchased from Gibco (Carlsbad). Mouse
anti-ERα monoclonal antibody was purchased from Lifespan
Biosciences (Seattle, WA, USA). Mouse monoclonal antibody to
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and goat
anti-mouse IgG were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Rabbit anti-matrix MMP-9 polyclonal antibody and
fluorescein isothiocynate (FITC)-labeled goat anti-rabbit IgG were
purchased from Beijing Boao Sen Corp. (Beijing, China). Luxol fast
blue (LFB), cresyl fast violet and complete Freund’s adjuvant were
obtained from Sigma-Aldrich (St. Louis, MO, USA). The BioRT reverse
transcription-polymerase chain reaction (RT-PCR) kit was purchased
from Bio Flux Corp. (Tokyo, Japan). The SYBR Master Mixture kit was
obtained from Takara (Dalian, China). Mouse anti-myelin basic
protein (MBP) antibody was purchased from Abcam (Cambridge, UK).
Polyvinylidene fluoride (PVDF) membranes were obtained from Bio-Rad
(Hercules, CA, USA). The ACS 180E2 chemiluminescent immunoassay
reagent kit was obtained from Bayer Corp. (Pittsburgh, PA, USA).
Mouse TNF-α, IFN-γ, IL-4, IL-17, IL-23 and MMP-9 enzyme-linked
immunosorbent assay (ELISA) kits were purchased from R&D
Systems (Minneapolis, MN, USA). An enhanced chemiluminescence
(ECL)-Plus kit was obtained from Amersham (Piscataway, NJ, USA).
Primers were designed and synthesized by Sangon (Shanghai,
China).
Primary neuronal cell culture
Cell culture was conducted as previously described
(27). C57BL/6 mice were
sacrificed within 24 h of birth, and their cortical tissues were
dissected. A single cell suspension was obtained following
trypsinization, and these neuronal cells were seeded at a density
of 1×105 cells/well in 6-well plates and
1×107 cells/15 ml flask and cultured in an incubator
operating at 37°C under 5% CO2. After 24 h, the basal
medium was replaced with a serum-free medium (DMEM/F12 medium
supplemented with 1% growth factor B-27, 104 units/ml
penicillin and 10 mg/ml streptomycin). Half of the serum-free
medium was replaced with fresh medium every 3 days.
Determination of raloxifene
concentration
The blood drug concentration of raloxifene was
determined as follows: blood samples were collected from the EAE
mice. A precise volume of 1 ml of plasma sample was transferred to
centrifuge tubes. A total of 50 μl of internal reference solution
was then added and mixed evenly. Approximately 200 μl of NaOH
solution (1 mmol/l) was added into the mixed solution, rotated for
20 sec, followed by the addition of 5 ml of hexane-butanol (98:2,
v/v), rotation for 3 min and centrifugation at 4,000 rpm for 10
min. A precise volume of 4 ml of organic phase was collected and
transferred to another centrifuge tube, and then blow-dried with
nitrogen flow in a water bath at 40°C. The residue was dissolved in
200 μl of mobile phase and centrifuged at 16,000 rpm for 2 min. The
supernatant was collected and transferred to the small bottle in
the automated chemiluminescent immunoassay analyzer, and vertically
illuminated under an ultraviolet lamp at a wavelength of 254 nm for
10 min. A precise volume of 10 μl of the supernatant was collected
for determination.
ERα recombinant lentivirus construction
and transfection
Lentiviral transfection was performed as described
in a previous study (28).
Briefly, a PCR-amplified gene fragment encoding ERα was cut using
the restriction enzymes, AgeI/NheI, and cloned into
the LV-GFP-Flag vector. The plasmid sequences were confirmed by
sequencing. The ERα recombinant vector or the empty viral vector
was co-transfected with the pHelper 1.0 and pHelper 2.0 helper
plasmids into 293T cells using Lipofectamine 2000. The
lentivirus-containing supernatant was collected, centrifuged and
filtered. The titer of ERα recombinant lentivirus was determined
using real-time quantitative PCR. Neuronal cells cultured for 7
days were infected with ERα recombinant lentivirus at a
multiplicity of infection (MOI) of 5. GFP expression in the
infected cells was observed daily under an inverted fluorescence
microscope. Five days after infection, ERα recombinant
lentivirus-infected and -uninfected neuronal cells were collected,
and total RNA and protein were extracted. ERα expression was
examined by RT-PCR and western blot analysis. The primer sequences
are shown in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer name | Primer sequence
(5′→3′) |
|---|
| ERα coding
region | Forward |
GAGGATCCCCGGGTACCGGTCGCCACCATGACCATGACCCTTCACAC |
| Reverse |
TCATCCTTGTAGTCGCTAGCGATCGTGTTGGGGAAGC |
| ERα lentivirus
vector | Forward |
GATCCACCTGATGGCCAAAG |
| Reverse |
AGCGTAAAAGGAGCAACATAG |
| ERα | Forward |
AATTCCTGGTGTTGTCG |
| Reverse |
AAGGTCCGCTGGATTGAG |
| β-actin | Forward |
GTGGACATCCGCAAAGAC |
| Reverse |
AAAGGGTGTAACGCAACTA |
| MMP-9 | Forward |
GCCCTGGAACTCACACGACA |
| Reverse | TTGGAAA
CTCACACGCCAGAAG |
| TNF-α | Forward |
GCCACAAGCAGGAATGAGAAG |
| Reverse | GCCACAAG
CAGGAATGAGAAG |
| IFN-γ | Forward |
TTTGCAGCTCTTCCTCAT |
| Reverse |
TGCCAGTTCCTCCAGATA |
| IL-4 | Forward |
TCTCGAATGTACCAGGAGCCATATC |
| Reverse |
AGCACCTTGGAAGCCCTACAGA |
| IL-23 | Forward |
ACATGCACCAGCGGGACATA |
| Reverse |
CTTTGAAGATGTCAGAGTCAAGCAG |
| IL-17 | Forward |
ACGCGCAAACATGAGTCCAG |
| Reverse |
AGGCTCAGCAGCAGCAACAG |
Ovariectomy and EAE animal model
preparation
A total of 75 female C57BL/6 mice were anesthetized
and underwent bilateral tubal ligation and ovariectomy. Two weeks
later, an mouse model of MS was established. MOG 35–55 peptide was
diluted with 0.01 mol/l phosphate-buffered saline (PBS) to 300
μg/ml, combined with the same amount of complete Freund’s adjuvant
(final concentration of BCG of 10 mg/ml) and mixed with a glass
syringe to form an antigen emulsion. EAE was induced with a
subcutaneous injection of 0.2 ml antigen emulsion at the back,
neck, armpit and groin at 0 and 7 days post-immunization, and
intraperitoneal injections of 0.4 mg pertussis toxin were
administered at 0 and 2 days post-immunization. EAE mice were
divided into 5 groups (n=17 per group): the estrogen, ERα agonist,
ERα recombinant lentivirus, empty virus vector and normal saline
(NS) group. From days 7 to 30 following immunization, the estrogen
and ERα agonist groups were subcutaneously treated with a saline
suspension of estradiol valerate (0.04 mg/kg/day) (29) and a raloxifene saline suspension
(2 mg/kg/day) (30),
respectively. On the day of immunization, mice in the ERα, empty
virus and NS group were anesthetized with sodium pentobarbital (40
mg/kg) and injected with 20 μl ERα lentivirus, empty virus, or
saline, respectively. Injections were made into the lateral
cerebral ventricle located 2.0 mm posterior and 1.5 mm lateral to
the bregma at a depth of 2.5 mm using stereotaxic techniques.
Animals in each group were euthanized at 5, 10, 20 and 30 days
post-injection, and the spinal cords and brain tissue were
harvested for analysis. Hematoxylin and eosin (H&E) staining
was performed to assess the inflammatory response in the brain and
spinal cord tissues of normal mice following injection with ERα
recombinant lentivirus.
To examine the effects of raloxifene and estradiol
valerate on EAE induction and severity, mice received treatment or
placebo by subcutaneous injection from days 10 to 30 following
immunization.
Determination of estradiol
concentraion
Blood samples were collected from the EAE mice and
dissolved at room temperature, mixed evenly, and centrifuged at
4,000 rpm for 5 min. Approximately 250 μl of serum samples were
collected and transferred to tubes for subsequent determination.
The cleaning and filling procedures of the fully automated
chemiluminescent immunoassay analyzer were performed. The luminous
agent and solid-phase secondary antibody were added according to
the instructions provided with the ACS 180E2 chemiluminescent
immunoassay reagent kit, and the mixed procedure was executed for
20 min. The calibration solution, control solution and antibody
were added into the sample plates, and the calibration and control
programs were set up on a computer. The blood drug concentration of
estradiol was automatically determined using the immunoassay
analyzer.
EAE evaluation
Body weight and clinical scores were recorded daily.
We employed a standard scale used for EAE model evaluation
(31) that consists of: 1,
paresis of the tail; 2, paresis of a hind limb; 3, paralysis of a
hind limb; 4, paralysis of a forelimb; and 5, a moribund state or
death.
Pathological analysis
Five EAE mice in each group were sacrificed at 16
days post-immunization (dpi) (acute phase), 5 mice were sacrificed
at 23 dpi (remission phase), and 5 mice were sacrificed at 30 dpi
(chronic phase). The spinal cords of the acute phase mice were
fixed in 4% paraformaldehyde for 24 h, embedded in paraffin, and
cut into 5 μm-thick sections for H&E staining and LFB-H&E
staining as previously described (27). The scores of the inflammatory
responses were evaluated on a 5-point scale as described previously
(32): 0, no infiltration of
inflammatory cells; 1, infiltration only into the subarachnoid
space; 2, mild infiltration into the brain parenchyma; 3, moderate
infiltration into the brain parenchyma; and 4, severe and diffuse
inflammatory cell infiltration into the brain parenchyma. Nerve
demyelination scores were evaluated on a 4-point scale as follows:
0, no demyelination; 1, mild demyelination; 2, moderate
demyelination; and 3, severe demyelination.
MMP-9 immunofluorescence staining
Paraffin-embedded tissues were cut into 6 μm-thick
sections, dewaxed, rehydrated, subjected to antigen retrieval and
permeabilized with 0.1% Triton X-100. The sections were blocked in
pre-immune serum blocking solution (1:10) for 20 min, incubated
with an anti-MMP-9 antibody (1:100), followed by incubation with a
FITC-labeled secondary antibody (1:100). The sections were observed
using an Axiovert 200M fluorescence microscope (Zeiss, Jena,
Germany). Five random and non-overlapping fields were collected
from each section, and the integral optical density (IOD) values
were calculated and averaged with the microscope’s image analysis
system.
Real-time quantitative PCR
Total brain RNA was extracted with TRIzol and
reverse transcribed to cDNA using a BioRT reverse transcription
kit. Real-time quantitative PCR was performed using an SYBR Master
Mixture kit according to the manufacturer’s instructions. An RT
quality control and a PCR negative control were included, and
β-actin was used as an internal control. Relative gene expression
levels were calculated according to the cycle threshold (Ct) values
of target genes relative to the internal control gene. The primers
are shown in Table I.
ELISA
Brain tissues were washed with ice-cold PBS, dried
on filter paper, diluted in saline to form a 10% brain tissue
homogenate, and centrifuged at 2,800 × g for 10 min at 4°C. TNF-α,
IFN-γ, MMP-9, IL-4, IL-17 and IL-23 expression levels in the
supernatants were detected using a double antibody sandwich ELISA
kit according to the manufacturer’s instructions.
Western blot analysis
Cell lysates were equally loaded, and the proteins
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). The separated proteins were transferred
onto PVDF membranes at 4°C, 400 mA for 120 min. Membranes were
blocked in Tris-buffered saline buffer containing Tween-20 (TBST)
and 5% skimmed milk for 1 h and incubated with mouse anti-ERα
monoclonal antibody (1:500), mouse anti-MBP antibody (1:1,000), or
mouse anti-GAPDH monoclonal antibody (1:2,500) at 4°C overnight.
The following day, membranes were incubated with sheep anti-mouse
IgG (1:5,000) at room temperature for 1 h, and the blots were
visualized using an ECL-PLUS kit. GAPDH was used as an internal
control. Relative protein expression levels are described as the
band density of target proteins relative to GAPDH.
Statistical analysis
All experiments were performed at least in
triplicate. Statistical analyses included one-way analysis of
variance and Newman-Keuls multiple comparisons tests. The
summarized data are presented as the means ± standard deviation,
and a P-value <0.05 was considered to indicate a statistically
significant difference.
Results
ERα overexpression with recombinant
lentivirus in vitro and in vivo
We achieved ERα overexpression with recombinant
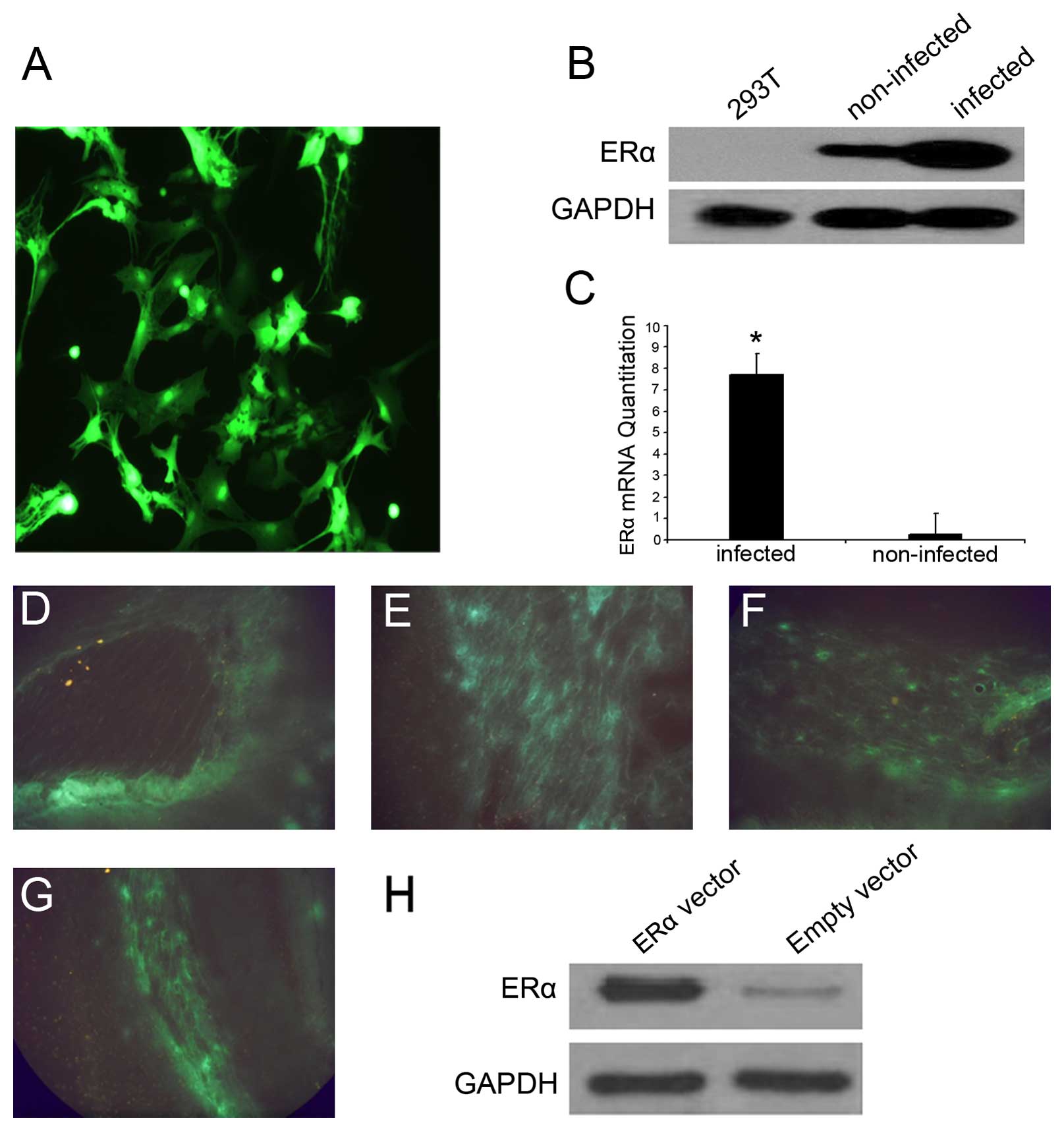
lentiviral transfection in vitro and in vivo. In the
cultured neuronal cells, a sustained GFP expression was observed
for at least 8 weeks (Fig. 1A)
following infection with ERα recombinant lentivirus (titer of
2×108 TU/ml; MOI of 5), suggesting that the lentiviral
infection of the neuronal cells was effective and stable. Moreover,
ERα mRNA and protein levels were significantly upregulated in the
lentiviral-transfected neuronal cells (Fig. 1B and C). We also induced the
overexpression of ERα in EAE mice by injecting recombinant
lentivirus into the lateral ventricle of the mouse brains. At 5,
10, 20 and 30 days post-injection, EAE mice receiving ERα
recombinant lentivirus and the empty vector controls were
sacrificed. We detected a strong GFP signal in the EAE mice that
received ERα recombinant lentivirus in several CNS regions,
including the hippocampus (Fig.
1D), the region around the lateral ventricle (Fig. 1E), brain parenchyma (Fig. 1F) and spinal cord (Fig. 1G), suggesting the successful
overexpression of ERα in vivo. By 5 days post-injection, ERα
protein expression in the animals receiving ERα recombinant
lentivirus was increased relative to the controls (Fig. 1H), and this was maintained
throughout the course of the experiment. H&E staining showed
that ERα recombinant lentivirus injection did not induce
inflammatory responses in the brain or spinal cord tissues of the
normal mice.
ERα overexpression improves clinical
signs of disease in EAE mice
The estrogen and ERα agonist group received a
subcutaneous injection containing saline suspensions of estradiol
valerate (0.04 mg/kg/day) and a raloxifene saline suspension (2
mg/kg/day), respectively. The doses were selected to produce
relevant serum therapeutic levels. Baseline measurements were made
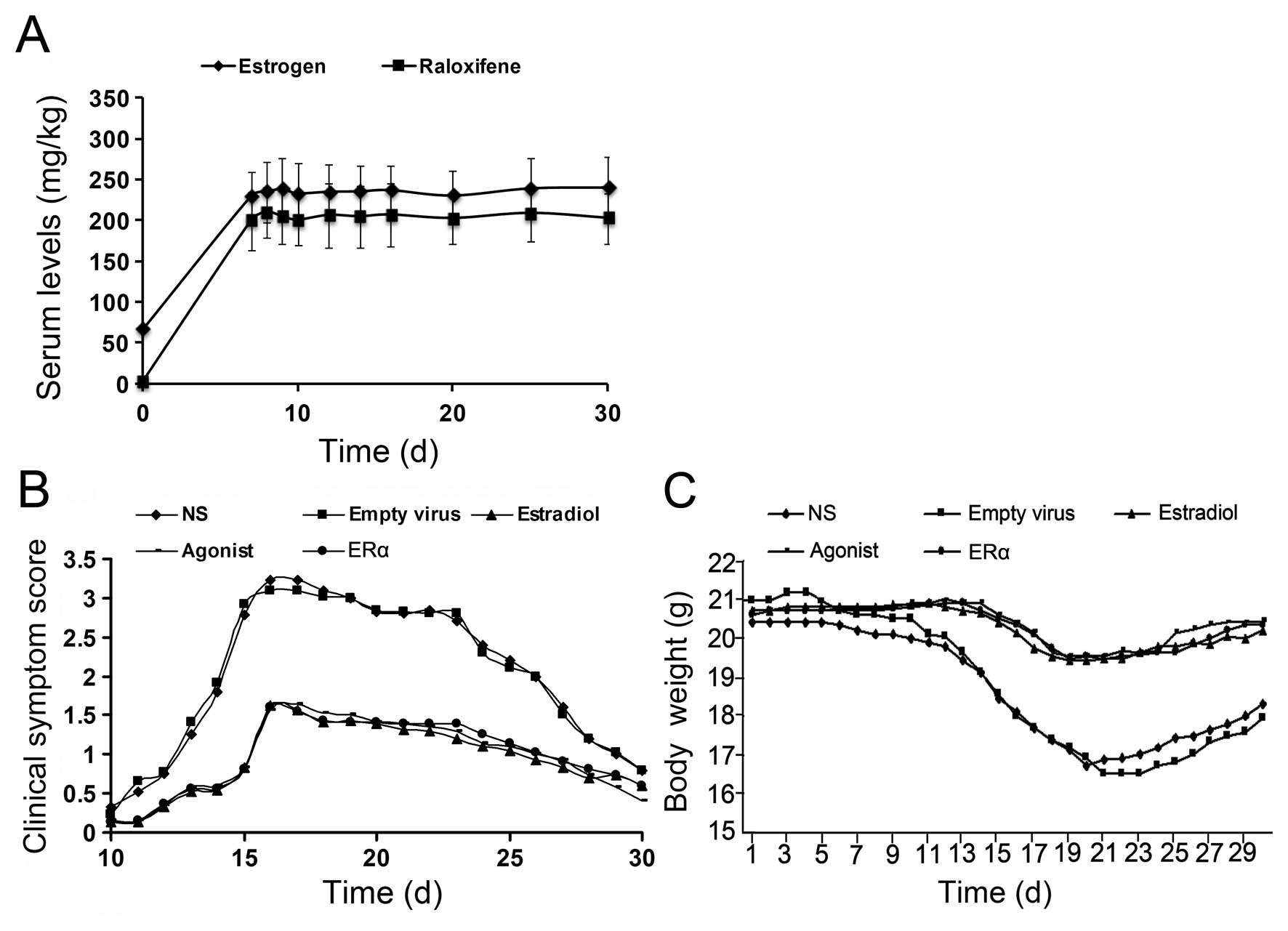
at day 0. The graph in Fig. 2A
shows that the treatment successfully increased the serum levels of
estrogen and raloxifene over a period of 30 days.
Mice that received recombinant ERα lentivirus were
used to assess the possible protective role of ERα against the
clinical manifestations of EAE (Fig.
2B). In the empty virus and NS group, symptoms appeared on
approximately the 12th day post-immunization, including decreased
physical activity, loss of appetite and body weight and tail and
limb paralysis. The most severe symptoms manifested on the 14–18th
day post-immunization (peaking on day 16, acute stage) and
gradually improved from day 23 on (remission stage). After 30 days,
the mice had fully recovered, but mild neurological dysfunction was
detected in some animals (chronic phase). The total incidence rate
of EAE was >90% in the empty virus and NS group; the maximum
weight loss was 4.183±1.358 and 4.3±1.226 g, and the maximum
clinical scores were 3.23±0.831 and 3.09±0.834, respectively
(Fig. 2B and C). Compared to the
empty virus and NS group, ERα overexpression significantly improved
EAE symptoms throughout the entire observation period, including
delayed disease onset (symptoms appeared on the 14th day
post-immunization), low EAE incidence rate (14.29%) and evident
symptom relief (Fig. 2B), similar
to the estrogen and ERα agonist group. In the estrogen, ERα agonist
and ERα recombinant lentivirus group, the maximum weight loss was
1.244±0.554, 1.172±0.63 and 1.341±0.481 g, and the maximum clinical
scores were 1.62±1.082, 1.57±1.117 and 1.61±1.135, respectively,
which were all significantly lower than those in the empty virus or
NS group (P<0.05, Fig. 2B and
C). At day 30 post-immunization, observation of the ovarian and
uterine tissues of mice in each group revealed a single EAE mouse
with endometrial thickening in the estrogen group, but similar
findings were not observed in the other groups.
ERα overexpression inhibits inflammatory
cell infiltration and nerve fiber demyelination in EAE mice
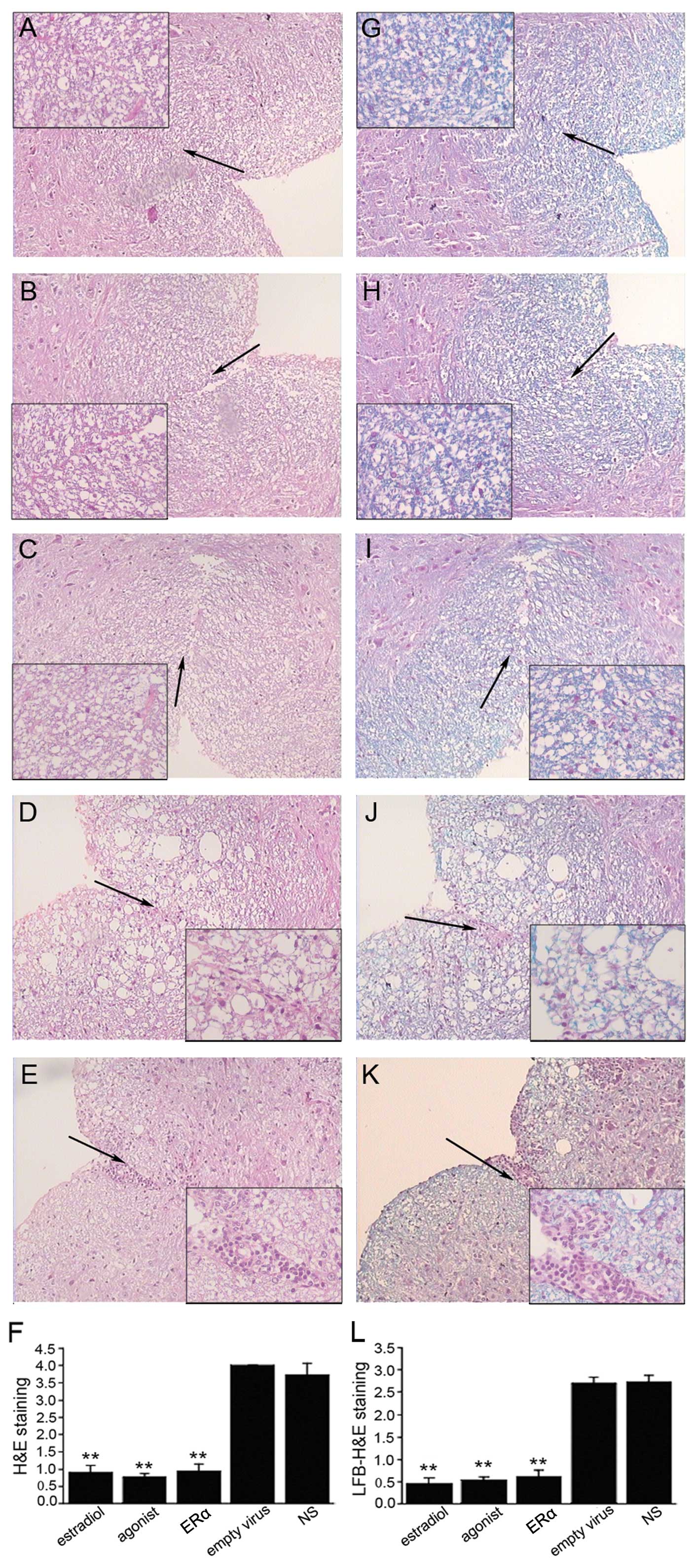
Since the most significant features of EAE are
inflammatory cell invasion and nerve fiber demyelination, we
investigated the effect of ERα overexpression on both these
parameters. We observed significant inflammatory cell infiltration,
mainly lymphocytes, into the spinal cord during acute EAE in the
empty virus and NS group (Fig. 3D and
E). This was dramatically attenuated by ERα overexpression and
the administration of estrogen or ERα agonist (Fig. 3A-C and F, P<0.001). Similarly,
significant demyelination was observed in the empty virus and NS
group, which also improved following treatment with ERα recombinant
lentivirus and estrogen or ERα agonist (Fig. 3G-L).
ERα overexpression increases MBP
expression
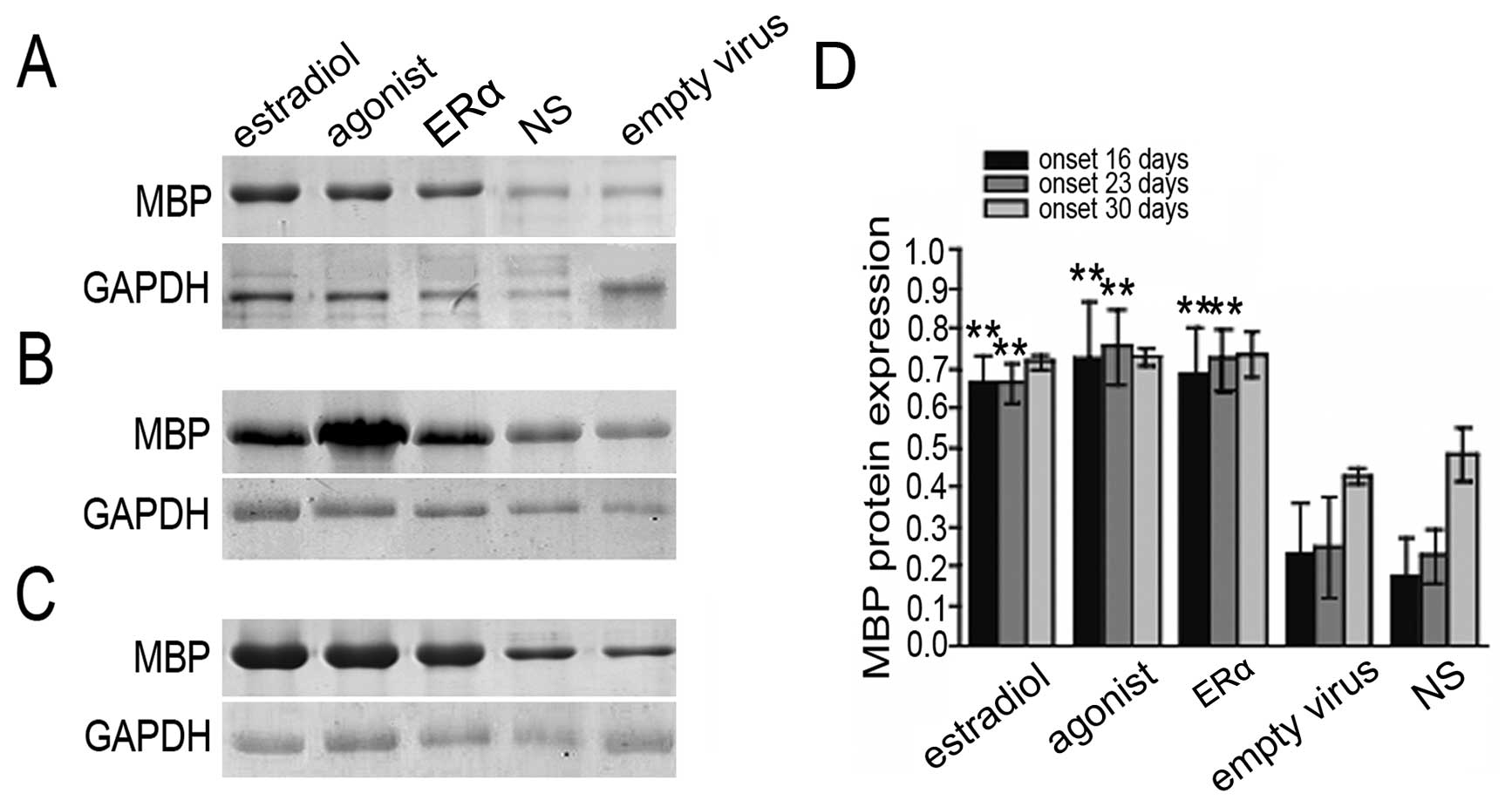
MBP is a membrane protein that is specifically
expressed in myelin-forming cells (33). Mice with a reduced MBP expression
exhibit serious nerve fiber myelination defects in the CNS
(33,34), suggesting that MBP plays an
important role in myelin formation. To further confirm that ERα
overexpression inhibits nerve fiber demyelination in EAE mice, MBP
expression was analyzed by immunohistochemistry. We found that MBP
protein levels were significantly increased in the brain in the
ERα, estradiol, and raloxifene groups (Fig. 4). These results indicate that ERα
overexpression has a similar effect to estrogen therapy on
demyelination in EAE mice.
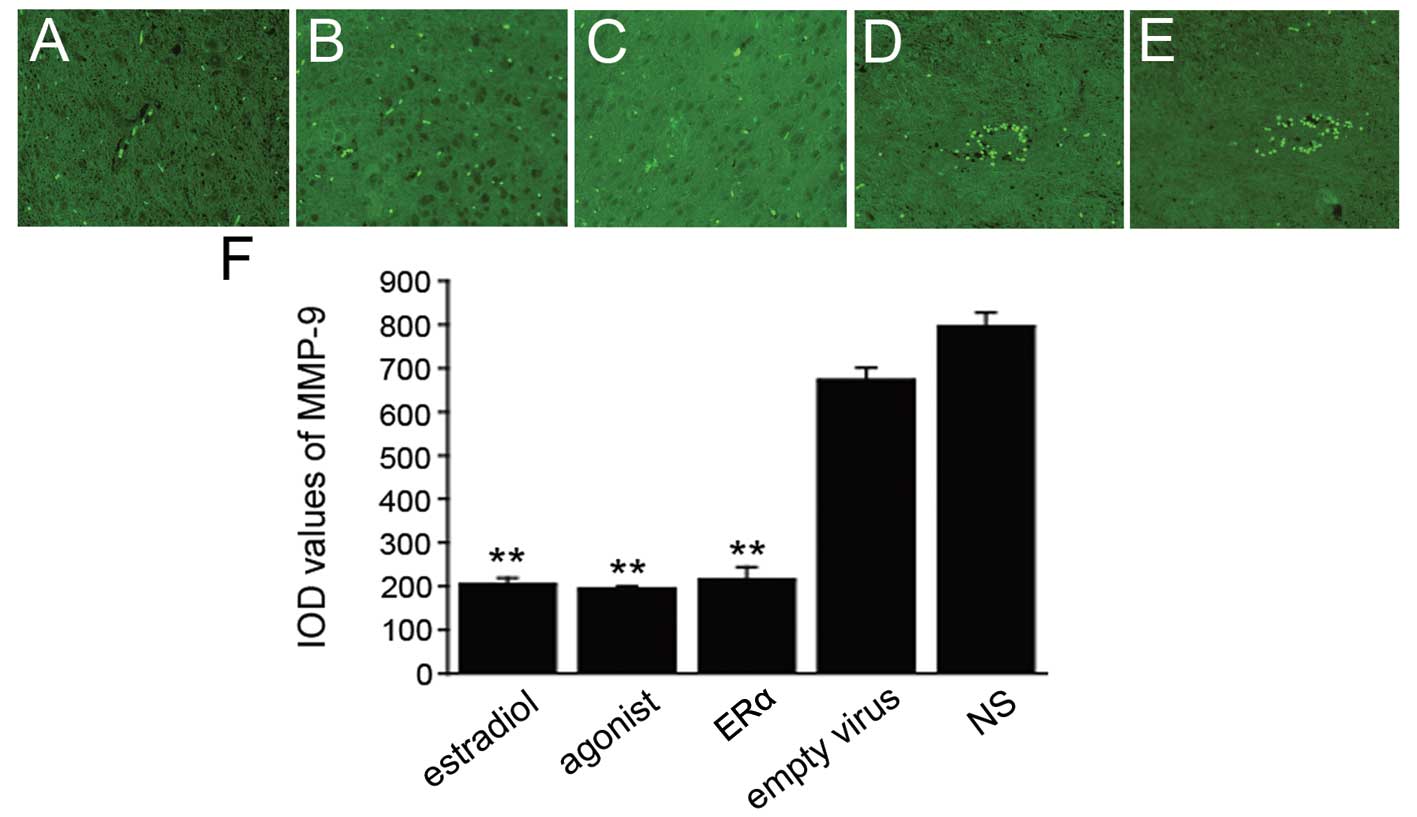
ERα overexpression inhibits MMP-9
expression
The expression of the proteolytic enzyme, MMP-9, is
increased in EAE models and results in blood-brain barrier damage
and a series of demyelinating events (35,36). We investigated MMP-9 expression
following the induction of ERα overexpression in an EAE mouse model
and investigated the mechanism behind the anti-inflammatory effects
exerted by ERα. We observed extensive infiltration of
MMP-9-positive cells into the spinal cord in the NS and empty virus
group. These cells, which typically present green cytoplasmic
fluorescence, formed infiltration lesions around small blood
vessels (Fig. 5D and E). Although
MMP-9-positive cell infiltration into the spinal cord was also
evident in the ERα, estrogen and ERα agonist group, the numbers of
MMP-9-positive cells were significantly decreased (Fig. 5A-C) compared to the empty virus
group, as evidenced by the significantly lower IOD values (Fig. 5F, P<0.001). These results
suggest that the inhibition of MMP-9 expression by ERα
overexpression may be one of the possible protective mechanisms
against EAE symptoms in mice.
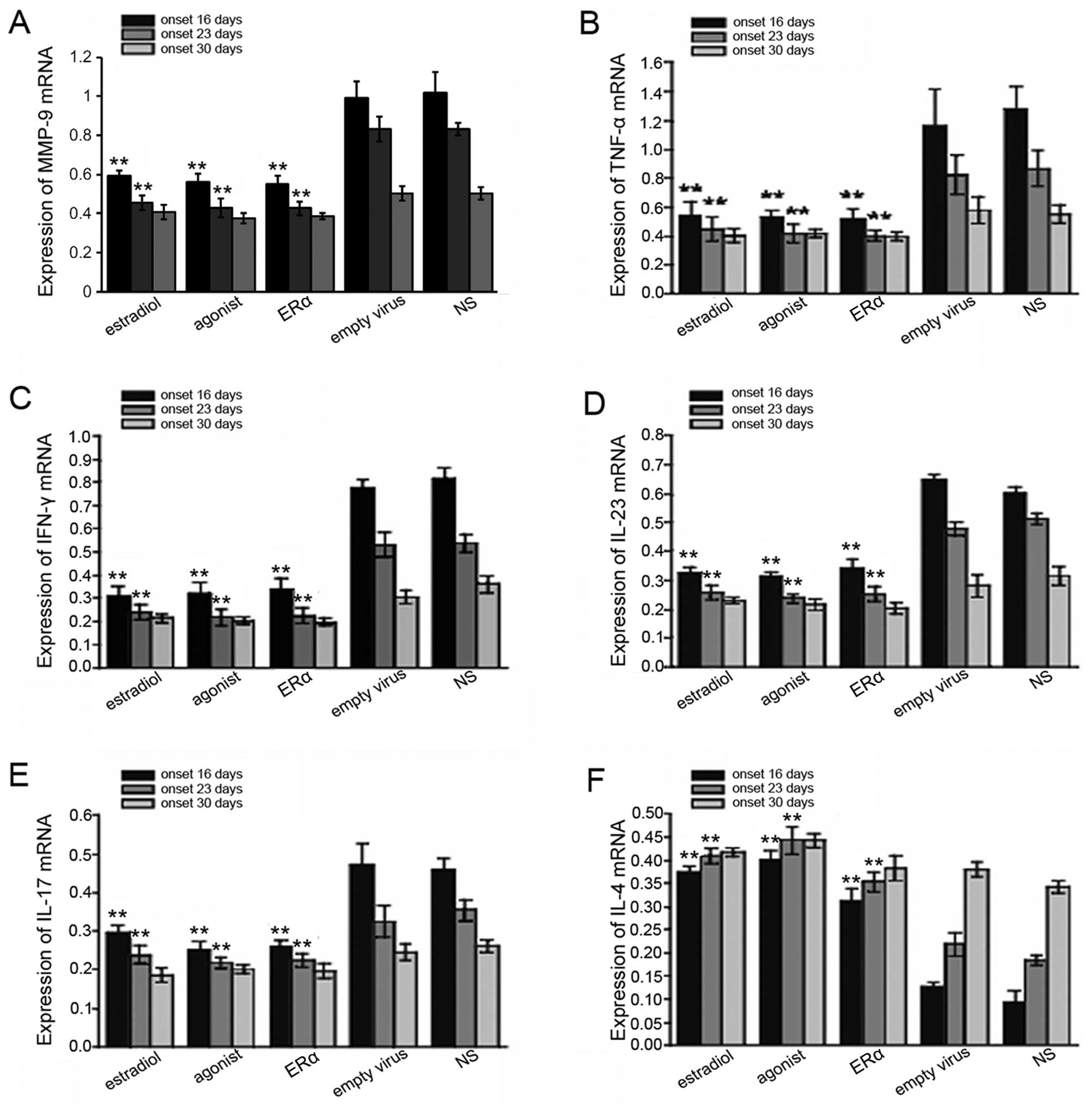
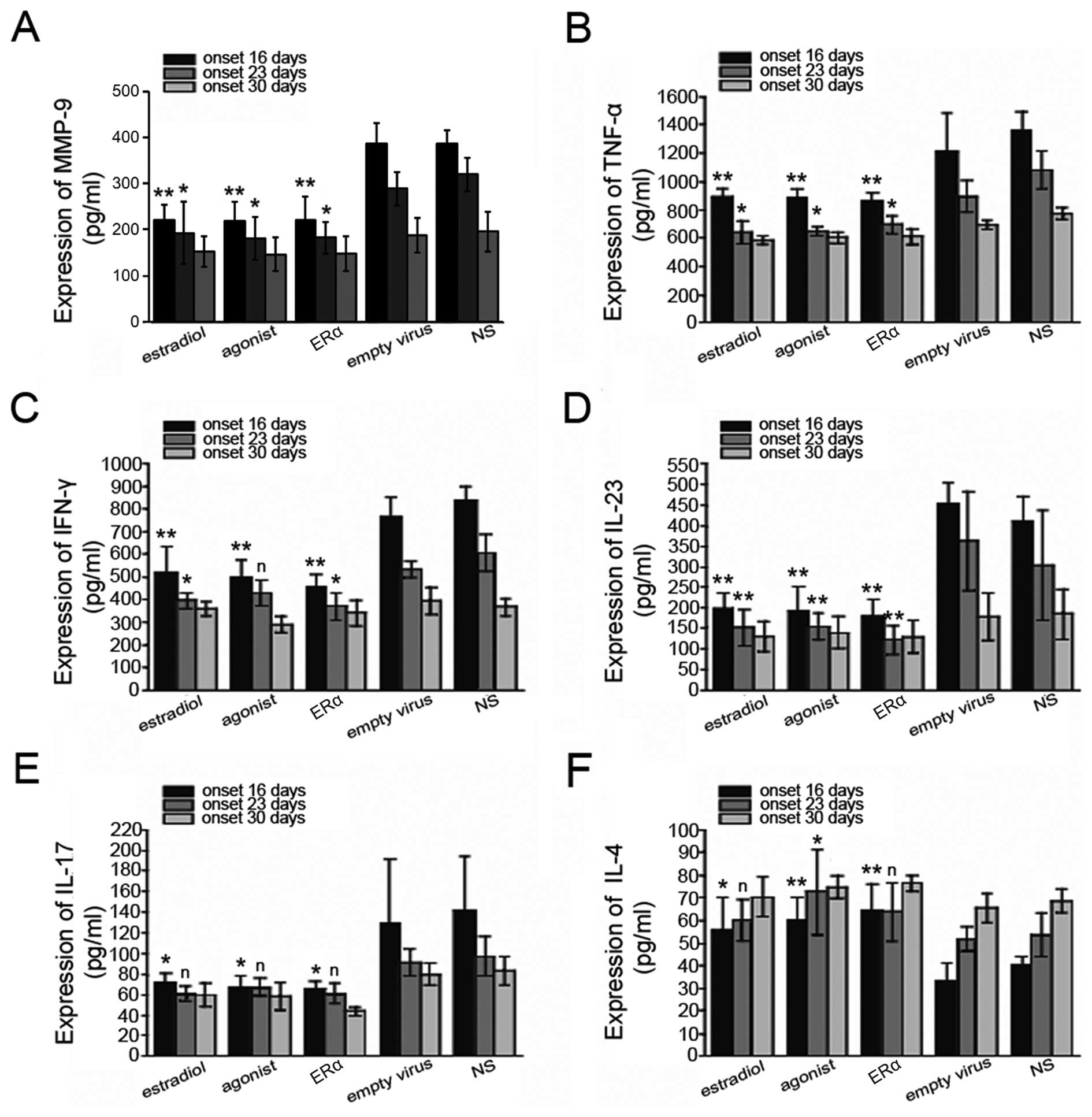
ERα overexpression decreases inflammatory
cytokine expression levels in EAE mice
It is known that the expression of inflammatory
cytokines, such as TNF-α, IFN-γ, IL-4, IL-17 and IL-23 is usually
increased in EAE. To further examine the correlation between
inflammation and protection against EAE by ERα overexpression, we
measured the levels of inflammatory cytokines, including TNF-α,
IFN-γ, IL-4, IL-17 and IL-23. We found that the levels of MMP-9,
TNF-α, IFN-γ, IL-17, IL-23 and IL-17 in the EAE mouse brains were
downregulated after the body weight recovery and disease symptoms
remittance, while IL-4 levels were increased. Compared to the empty
virus and NS group, ERα overexpression or treatment with estrogen
or ERα agonist significantly decreased the levels of inflammatory
cytokines in the EAE mice, but increased those of IL-4 (Figs. 6 and 7). Taken together, our results suggest
that the protective effects against EAE by lentiviral-mediated ERα
overexpression may occur through inflammatory response
inhibition.
Discussion
A number of studies have demonstrated that estrogen
or ERα agonists are beneficial for MS (5,12,20). The main aim of the present study
was to investigate the feasibility of lentivirus-mediated
overexpression of ERα in the CNS in EAE and to elucidate the
mechanisms underlying its effects. ERα overexpression resulted in
significant symptom improvement and successfully inhibited
inflammatory cytokines in EAE mice. We introduced the recombinant
lentivirus vectors via the administration of lateral cerebral
ventricle injections. Compared to adenovirus, lentivirus has high
transfection efficiency in non-dividing cells, a sustained
expression potency and minimal impact on normal cellular functions
(37–39). Previous studies have demonstrated
the feasibility of the lentivirus-mediated delivery of ERα
(26,40). These studies suggest that
lentivirus may have great potential for clinical gene therapy,
particularly for manipulating CNS gene expression (41). To date, to our knowledge, there
are no reports of the use of recombinant lentivirus overexpressing
ERα in EAE mouse models; the majority of studies have employed ERα
ligands or selective ER agonists. The results described in this
study illustrate that recombinant lentivirus increases ERα
expression at the gene and protein level and provide additional
evidence for the crucial role of ERα in EAE.
MS is an autoimmune disease characterized by
increased CNS inflammation and demyelination (42). Liu et al reported that
estrogen decreased TNF-α, IFN-γ and IL-12 production in mature DCs.
In addition, MBP-specific T cells co-cultured with
estrogen-pretreated mature DCs in the presence of antigen
demonstrated a shift towards the production of Th2 cytokines, IL-4
and IL-10, and a concomitant decrease in the production of Th1
cytokines, TNF-α and IFN-γ (43).
In the present study, clear demyelination and inflammatory cell
infiltration were observed in the CNS of EAE mice, and both were
attenuated by ERα overexpression. LFB-H&E staining showed that
demyelinated regions contained a high number of inflammatory cells,
suggesting that demyelination may be closely associated with the
activation of inflammation, and that ERα overexpression attenuates
the inflammatory response. Previous studies have suggested that
changes in oligodendrocyte markers can yield valuable information
on demyelination-associated diseases (44,45), and MBP appears to be the most
relevant. This abundant protein is believed to stabilize myelin
sheaths (46), and demyelinated
MS lesions and nearby areas have a reduced or absent MBP expression
(47,48). Levels of MBP (both mRNA and
protein) have been associated with de- and remyelination processes
in other neurological disorders, and it is considered a specific
and sensitive index for measuring oligodendrocyte injury and
demyelination. MBP loss is known to precede the onset of myelin
histological changes (49). In
this study, western blot analysis and RT-PCR revealed that MBP
expression was significantly reduced at the initial stage of EAE,
and myelin sheath damage was gradually alleviated during the
remission stage, indicating that myelin sheath damage is closely
associated with EAE development.
We observed significant increases in MBP expression
in the groups treated with ERα recombinant lentivirus and estrogen,
which also exhibited reduced myelin loss during EAE. Therefore, the
ERα-mediated attenuation of demyelination may at least partly be
due to MBP upregulation. Crawford et al reported that ERβ
ligand treatment exerted direct neuroprotective effects on
oligodendrocyte differentiation, myelination and axon conduction
following EAE (50). However,
these results are time-dependent; early treatment can reduce the
inflammatory response and myelin sheath damage, but late treatment
is ineffective (19). Therefore,
early intervention for EAE and MS is critical.
Although it is well established that a subcutaneous
injection of estrogen or ER agonist protects against
neurodegenerative and CNS immune diseases (51–53), long-term use may increase the risk
of breast cancer and cardiovascular disease (54). Conversely, the lentiviral
manipulation of CNS ERα expression can mimic the therapeutic effect
of estrogen, while avoiding unwanted side-effects. In this study,
we pathologically analyzed the ovarian and uterine tissues of EAE
mice in various groups during the experimental period and found a
single mouse with mild endometrial thickening in the estrogen
group, but similar changes were not observed in the other groups.
However, these animals were only treated acutely, and further
studies are required to validate the safety and clinical
feasibility of ERα recombinant lentivirus.
Th1/Th2 balance is crucial for immune defense and
surveillance. MS patients usually show an imbalance in Th1/Th2
cytokine expression (55–57), and addressing Th1/Th2 expression
levels has become a novel therapeutic strategy for MS patients
(58). Bebo et al found
that selective estrogen-receptor modulators (SERMs) caused a Th2
shift by decreasing IFN-γ- and TNF-α-producing CD4+ T
cells and increasing IL-4-producing CD4+ T cells. These
results suggest that SERMs may potentially be used to treat
inflammatory autoimmune disorders that affect the CNS (59). In this study, we found that TNF-α
and IFN-γ expression was significantly increased in the EAE mice,
particularly in the acute stage of the disease, and the level of
Th1 cytokines gradually decreased as the clinical symptoms
remitted. In addition, Th1 cytokine expression (TNF-α and IFN-γ)
was decreased following the induction of ERα overexpression and
treatment with estrogen or ER agonist. IFN-γ promotes the
differentiation of Th0 cells to Th1 cells and stimulates the
release of lymphotoxin, IL-1 and TNF-α, resulting in demyelination
and EAE development (60,61). In addition, EAE is also closely
related to the expression of the zinc calcium-dependent protease,
MMP-9, which is expressed in Th1 cells and increases their
migration (62), allowing immune
cells to access the CNS during the the early stages of MS. Abnormal
MMP-9 expression and increased activation in EAE mice can impair
the blood-brain barrier and allow myelin degradation (35,36). Gold et al reported that
estriol acts through ERα to reduce immune cell-mediated MMP-9
production, which is one of the potential mechanisms by which
estriol reduces MS and EAE inflammatory lesions (23). Increased MMP-9 serum levels have
also been observed in patients with clinically isolated syndrome
(CIS), and MMP-9 levels further increase in patients who go on to
develop MS compared to CIS patients who do not convert (63). We found that MMP-9 was
downregulated following the induction of ERα overexpression and
treatment with estrogen or ER agonist. We postulate that all 3
treatments decreased Th1 cytokine production and reduced the
differentiation of Th0 cells to Th1 cells. Conversely, the
expression of IL-4, which is released by Th2 cells, was increased.
As a result, the regulation of pro- or anti-inflammatory cytokines
by ERα overexpression suggests that the therapeutic effect of ERα
on EAE may be due to restoring Th1/Th2 balance. This is similar to
what has been reported previously; however, our experimental design
was more rigorous and included multiple time points. Our results
indicate that ERα recombinant lentivirus can control the Th1/Th2
cytokine imbalance at the acute stage of EAE in mice. This novel
finding demonstrates the effectiveness and possibility of using ERα
recombinant lentivirus for the treatment of EAE and MS.
We also demonstrated the effects of ERα
overexpression, estradiol and raloxifene on the IL-23/IL-17 axis
throughout EAE progression. The IL-23/IL-17 axis has been suggested
to play an important role in MS pathogenesis (64). IL-23 induces the production of
inflammatory cytokines, such as IL-16, IL-17, IL-17F and TNF-α
(65). IL-17 stimulates various
inflammatory cytokines, including IL-1, IL-6, TNF-α, nitric oxide
synthase, MMPs and chemical factors (66,67). EAE severity is reportedly
associated with serum IL-17 levels (68), and an injection of anti-IL-17
antibody has been shown to reduce the incidence of autoimmune
diseases and inflammation severity (69). Estrogen-induced EAE protection is
mediated by upregulated PD-1 expression within the Treg-cell
compartment, which reduces peripheral IL-17 production (9). Lelu et al demonstrated that
hematopoietic cell ERα expression is critical for mediating E2s
inhibitory effect on Th1 and Th17 cell priming, which results in
EAE protection (8). However, to
our knowledge, there have been no publications regarding the effect
of ERα on IL-23 in EAE. In our study, we observed that both IL-23
and IL-17 were downregulated following the induction of ERα
overexpression, as well as following the oral supplementation of
estrogen or ER agonist in EAE mice, suggesting that modulating the
IL-23/IL-17 axis by overexpressing ERα may contribute to its
protective effect against EAE.
In conclusion, the present study indicates that CNS
ERα overexpression with lentivirus attenuates EAE symptoms via
multiple mechanisms, including the suppression of inflammation and
reduced demyelination. Additionally, appropriate balances of
inflammatory cytokines in the Th1/Th2 and IL-23/IL-17 axes may also
contribute to the observed therapeutic effects of ERα
overexpression. These results provide evidence for the utility of
manipulating ERα expression in the CNS with lentivirus delivery.
Further studies are required to elucidate the effects of ERα
overexpression in EAE and MS.
Acknowledgements
This study was supported by a grant from the
Research Project of Science and Technology, Department of Guizhou
Province, No. sy[2009]3054 and the Special Fund of the Governor of
Guizhou Province for Excellent Scientific, Technological and
Educational Talents, No. Qian Sheng Zhuan He Zi (2010) 86. We would
like to thank the Central Laboratory and Molecular Biochemical
Laboratory of Guizhou Provincial People’s Hospital for the
excellent experimental environment and Anzhi Wen from the
Pathological Laboratory of Guizhou Provincial People’s Hospital for
providing technical support.
References
|
1
|
Ouallet J, Baumann N, Marie Y and
Villarroya H: Fas system up-regulation in experimental autoimmune
encephalomyelitis. J Neurol Sci. 170:96–104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barac-Latas V, Muhvic D and
Radosevic-Stabic B: The influence of pregnancy on development and
course of chronic relapsing experimental autoimmune
encephalomyelitis in rats: implications for multiple sclerosis.
Coll Antropol. 34(Suppl 1): 267–271. 2010.
|
|
3
|
Runmarker B and Andersen O: Pregnancy is
associated with a lower risk of onset and a better prognosis in
multiple sclerosis. Brain. 118:253–261. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Confavreux C, Hutchinson M, Hours MM,
Cortinovis-Tourniaire P and Moreau T: Rate of pregnancy-related
relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis
Group. N Engl J Med. 339:285–291. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niino M, Hirotani M, Fukazawa T, Kikuchi S
and Sasaki H: Estrogens as potential therapeutic agents in multiple
sclerosis. Cent Nerv Syst Agents Med Chem. 9:87–94. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sicotte NL, Liva SM, Klutch R, et al:
Treatment of multiple sclerosis with the pregnancy hormone estriol.
Ann Neurol. 52:421–428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soldan SS, Alvarez Retuerto AI, Sicotte NL
and Voskuhl RR: Immune modulation in multiple sclerosis patients
treated with the pregnancy hormone estriol. J Immunol.
171:6267–6274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lelu K, Laffont S, Delpy L, et al:
Estrogen receptor alpha signaling in T lymphocytes is required for
estradiol-mediated inhibition of Th1 and Th17 cell differentiation
and protection against experimental autoimmune encephalomyelitis. J
Immunol. 187:2386–2393. 2011. View Article : Google Scholar
|
|
9
|
Wang C, Dehghani B, Li Y, et al: Membrane
estrogen receptor regulates experimental autoimmune
encephalomyelitis through up-regulation of programmed death 1. J
Immunol. 182:3294–3303. 2009. View Article : Google Scholar
|
|
10
|
Bodhankar S, Wang C, Vandenbark AA and
Offner H: Estrogen- induced protection against experimental
autoimmune encephalomyelitis is abrogated in the absence of B
cells. Eur J Immunol. 41:1165–1175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Subramanian S, Yates M, Vandenbark AA and
Offner H: Oestrogen-mediated protection of experimental autoimmune
encephalomyelitis in the absence of Foxp3+ regulatory T
cells implicates compensatory pathways including regulatory B
cells. Immunology. 132:340–347. 2011.PubMed/NCBI
|
|
12
|
Gold SM and Voskuhl RR: Estrogen treatment
in multiple sclerosis. J Neurol Sci. 286:99–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
MacKenzie-Graham AJ, Rinek GA, Avedisian
A, et al: Estrogen treatment prevents gray matter atrophy in
experimental autoimmune encephalomyelitis. J Neurosci Res.
90:1310–1323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ziehn MO, Avedisian AA, Dervin SM, O’Dell
TJ and Voskuhl RR: Estriol preserves synaptic transmission in the
hippocampus during autoimmune demyelinating disease. Lab Invest.
92:1234–1245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giraud SN, Caron CM, Pham-Dinh D, Kitabgi
P and Nicot AB: Estradiol inhibits ongoing autoimmune
neuroinflammation and NFkappaB-dependent CCL2 expression in
reactive astrocytes. Proc Natl Acad Sci USA. 107:8416–8421. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bodhankar S and Offner H: Gpr30 forms an
integral part of E2-protective pathway in experimental autoimmune
encephalomyelitis. Immunol Endocr Metab Agents Med Chem.
11:262–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matejuk A, Bakke AC, Hopke C, Dwyer J,
Vandenbark AA and Offner H: Estrogen treatment induces a novel
population of regulatory cells, which suppresses experimental
autoimmune encephalomyelitis. J Neurosci Res. 77:119–126. 2004.
View Article : Google Scholar
|
|
18
|
Tiwari-Woodruff S and Voskuhl RR:
Neuroprotective and anti-inflammatory effects of estrogen receptor
ligand treatment in mice. J Neurol Sci. 286:81–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du S, Sandoval F, Trinh P, Umeda E and
Voskuhl R: Estrogen receptor-beta ligand treatment modulates
dendritic cells in the target organ during autoimmune demyelinating
disease. Eur J Immunol. 41:140–150. 2011. View Article : Google Scholar
|
|
20
|
Blasko E, Haskell CA, Leung S, et al:
Beneficial role of the GPR30 agonist G-1 in an animal model of
multiple sclerosis. J Neuroimmunol. 214:67–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Subramanian S, Miller LM, Grafe MR,
Vandenbark AA and Offner H: Contribution of GPR30 for 1,25
dihydroxyvitamin D(3) protection in EAE. Metab Brain Dis. 27:29–35.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lelu K, Delpy L, Robert V, et al:
Endogenous estrogens, through estrogen receptor alpha, constrain
autoimmune inflammation in female mice by limiting CD4+
T-cell homing into the CNS. Eur J Immunol. 40:3489–3498. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gold SM, Sasidhar MV, Morales LB, et al:
Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in
autoimmune demyelinating disease through estrogen receptor alpha
(ERalpha). Lab Invest. 89:1076–1083. 2009. View Article : Google Scholar
|
|
24
|
Spence RD, Hamby ME, Umeda E, et al:
Neuroprotection mediated through estrogen receptor-alpha in
astrocytes. Proc Natl Acad Sci USA. 108:8867–8872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong LF, Goodhead L, Prat C, Mitrophanous
KA, Kingsman SM and Mazarakis ND: Lentivirus-mediated gene transfer
to the central nervous system: therapeutic and research
applications. Hum Gene Ther. 17:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foster TC, Rani A, Kumar A, Cui L and
Semple-Rowland SL: Viral vector-mediated delivery of estrogen
receptor-alpha to the hippocampus improves spatial learning in
estrogen receptor-alpha knockout mice. Mol Ther. 16:1587–1593.
2008. View Article : Google Scholar
|
|
27
|
Brewer GJ, Torricelli JR, Evege EK and
Price PJ: Optimized survival of hippocampal neurons in
B27-supplemented Neurobasal, a new serum-free medium combination. J
Neurol Sci. 35:567–576. 1993.PubMed/NCBI
|
|
28
|
Hu X, Lei L, Yuan J, Xing W, WJY and Qin
X: Construction of recombinant lentivirus carrying mouse estrogen
receptor α and identification in infected neurons. Acad J Sec Mil
Med Univ. 32:160–166. 2011.
|
|
29
|
Tiwari-Woodruff S, Morales LB, Lee R and
Voskuhl RR: Differential neuroprotective and antiinflammatory
effects of estrogen receptor (ER)alpha and ERbeta ligand treatment.
Proc Natl Acad Sci USA. 104:14813–14818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tapia-Gonzalez S, Carrero P, Pernia O,
Garcia-Segura LM and Diz-Chaves Y: Selective Er modulators reduce
microglia reactivity in vivo after peripheral inflammation:
potential role of microglial ERs. J Endocrinol. 198:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Legge KL, Min B, Bell JJ, et al: Coupling
of peripheral tolerance to endogenous interleukin 10 promotes
effective modulation of myelin-activated T cells and ameliorates
experimental allergic encephalomyelitis. J Exp Med. 191:2039–2052.
2000. View Article : Google Scholar
|
|
32
|
Murphy AC, Lalor SJ, Lynch MA and Mills
KH: Infiltration of Th1 and Th17 cells and activation of microglia
in the CNS during the course of experimental autoimmune
encephalomyelitis. Brain Behav Immun. 24:641–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jacobs EC: Genetic alterations in the
mouse myelin basic proteins result in a range of dysmyelinating
disorders. J Neurol Sci. 228:195–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Molineaux SM, Engh H, De Ferra F, Hudson L
and Lazzarini RA: Recombination within the myelin basic protein
gene created the dysmyelinating shiverer mouse mutation. Proc Natl
Acad Sci USA. 83:7542–7546. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fainardi E, Castellazzi M, Bellini T, et
al: Cerebrospinal fluid and serum levels and intrathecal production
of active MMP-9 as markers of disease activity in patients with
multiple sclerosis. Mult Scler. 12:294–301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kurzepa J, Bartosik-Psujek H,
Suchozebrska-Jesionek D, Rejdak K, Stryjecka-Zimmer M and
Stelmasiak Z: Role of matrix metalloproteinases in the pathogenesis
of multiple sclerosis. Neurol Neurochir Pol. 39:63–67. 2005.(In
Polish).
|
|
37
|
Rubinson DA, Dillon CP, Kwiatkowski AV, et
al: A lentivirus-based system to functionally silence genes in
primary mammalian cells, stem cells and transgenic mice by RNA
interference. Nat Genet. 33:401–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Trobridge G and Russell DW: Cell cycle
requirements for transduction by foamy virus vectors compared to
those of oncovirus and lentivirus vectors. J Virol. 78:2327–2335.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yip PK, Wong LF, Pattinson D, et al:
Lentiviral vector expressing retinoic acid receptor beta2 promotes
recovery of function after corticospinal tract injury in the adult
rat spinal cord. Hum Mol Genet. 15:3107–3118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ambrosino C, Tarallo R, Bamundo A, et al:
Identification of a hormone-regulated dynamic nuclear actin network
associated with estrogen receptor alpha in human breast cancer cell
nuclei. Mol Cell Proteomics. 9:1352–1367. 2010. View Article : Google Scholar
|
|
41
|
Vigna E and Naldini L: Lentiviral vectors:
excellent tools for experimental gene transfer and promising
candidates for gene therapy. J Gene Med. 2:308–316. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bruck W: The pathology of multiple
sclerosis is the result of focal inflammatory demyelination with
axonal damage. J Neurol. 252(Suppl 5): v3–v9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu HY, Buenafe AC, Matejuk A, et al:
Estrogen inhibition of EAE involves effects on dendritic cell
function. J Neurol Sci. 70:238–248. 2002.PubMed/NCBI
|
|
44
|
Baumann N and Pham-Dinh D: Biology of
oligodendrocyte and myelin in the mammalian central nervous system.
Physiol Rev. 81:871–927. 2001.PubMed/NCBI
|
|
45
|
Daigle JL, Hong JH, Chiang CS and McBride
WH: The role of tumor necrosis factor signaling pathways in the
response of murine brain to irradiation. Cancer Res. 61:8859–8865.
2001.PubMed/NCBI
|
|
46
|
Griffiths I, Klugmann M, Anderson T, et
al: Axonal swellings and degeneration in mice lacking the major
proteolipid of myelin. Science. 280:1610–1613. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
De Rosbo NK and Bernard CC: Multiple
sclerosis brain immunoglobulins stimulate myelin basic protein
degradation in human myelin: a new cause of demyelination. J
Neurochem. 53:513–518. 1989.PubMed/NCBI
|
|
48
|
Einstein ER, Csejtey J, Dalal KB, Adams
CW, Bayliss OB and Hallpike JF: Proteolytic activity and basic
protein loss in and around multiple sclerosis plaques: combined
biochemical and histochemical observations. J Neurochem.
19:653–662. 1972. View Article : Google Scholar
|
|
49
|
Harauz G, Ishiyama N, Hill CM, Bates IR,
Libich DS and Fares C: Myelin basic protein-diverse conformational
states of an intrinsically unstructured protein and its roles in
myelin assembly and multiple sclerosis. Micron. 35:503–542. 2004.
View Article : Google Scholar
|
|
50
|
Crawford DK, Mangiardi M, Song B, et al:
Oestrogen receptor beta ligand: a novel treatment to enhance
endogenous functional remyelination. Brain. 133:2999–3016. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Garay L, Gonzalez Deniselle MC, Gierman L,
et al: Steroid protection in the experimental autoimmune
encephalomyelitis model of multiple sclerosis.
Neuroimmunomodulation. 15:76–83. 2008.PubMed/NCBI
|
|
52
|
Sarkaki A, Amani R, Badavi M, et al:
Pre-treatment effect of different doses of soy isoflavones on
spatial learning and memory in an ovariectomized animal model of
Alzheimer’s disease. Pak J Biol Sci. 11:1114–1119. 2008.PubMed/NCBI
|
|
53
|
Sheldahl LC, Marriott LK, Bryant DM,
Shapiro RA and Dorsa DM: Neuroprotective effects of estrogen and
selective estrogen receptor modulators begin at the plasma
membrane. Minerva Endocrinol. 32:87–94. 2007.PubMed/NCBI
|
|
54
|
Riggs BL and Hartmann LC: Selective
estrogen-receptor modulators - mechanisms of action and application
to clinical practice. N Engl J Med. 348:618–629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chitnis T and Khoury SJ: Cytokine shifts
and tolerance in experimental autoimmune encephalomyelitis. Immunol
Res. 28:223–239. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
McGeachy MJ and Anderton SM: Cytokines in
the induction and resolution of experimental autoimmune
encephalomyelitis. Cytokine. 32:81–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Suryani S and Sutton I: An
interferon-gamma-producing Th1 subset is the major source of IL-17
in experimental autoimmune encephalitis. J Neuroimmunol.
183:96–103. 2007.PubMed/NCBI
|
|
58
|
Butti E, Bergami A, Recchia A, et al: IL4
gene delivery to the CNS recruits regulatory T cells and induces
clinical recovery in mouse models of multiple sclerosis. Gene Ther.
15:504–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bebo BF Jr, Dehghani B, Foster S,
Kurniawan A, Lopez FJ and Sherman LS: Treatment with selective
estrogen receptor modulators regulates myelin specific T-cells and
suppresses experimental autoimmune encephalomyelitis. Glia.
57:777–790. 2009. View Article : Google Scholar
|
|
60
|
Juedes AE, Hjelmstrom P, Bergman CM, Neild
AL and Ruddle NH: Kinetics and cellular origin of cytokines in the
central nervous system: insight into mechanisms of myelin
oligodendrocyte glycoprotein-induced experimental autoimmune
encephalomyelitis. J Immunol. 164:419–426. 2000. View Article : Google Scholar
|
|
61
|
Monteiro de Castro G, Eduarda Zanin M,
Ventura-Oliveira D, Aparecida Vilella C, Ashimine R and De Lima
Zollner R: Th1 and Th2 cytokine immunomodulation by gangliosides in
experimental autoimmune encephalomyelitis. Cytokine. 26:155–163.
2004.PubMed/NCBI
|
|
62
|
Abraham M, Shapiro S, Karni A, Weiner HL
and Miller A: Gelatinases (MMP-2 and MMP-9) are preferentially
expressed by Th1 vs. Th2 cells J Neuroimmunol. 163:157–164. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Correale J and Bassani Molinas Mde L:
Temporal variations of adhesion molecules and matrix
metalloproteinases in the course of MS. J Neuroimmunol.
140:198–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Illes Z, Safrany E, Peterfalvi A, et al:
3′UTR C2370A allele of the IL-23 receptor gene is associated with
relapsing-remitting multiple sclerosis. Neurosci Lett. 431:36–38.
2008.
|
|
65
|
Harrington LE, Hatton RD, Mangan PR, et
al: Interleukin 17- producing CD4+ effector T cells
develop via a lineage distinct from the T helper type 1 and 2
lineages. Nat Immunol. 6:1123–1132. 2005.PubMed/NCBI
|
|
66
|
Kawanokuchi J, Shimizu K, Nitta A, et al:
Production and functions of IL-17 in microglia. J Neuroimmunol.
194:54–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kolls JK and Linden A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tian AY, Zhang RW, Shi XG and Yu HM:
Alteration of T helper cell subsets in the optic nerve of
experimental autoimmune encephalomyelitis. Int J Mol Med.
25:869–874. 2010.PubMed/NCBI
|
|
69
|
Uyttenhove C, Sommereyns C, Theate I,
Michiels T and van Snick J: Anti-IL-17A autovaccination prevents
clinical and histological manifestations of experimental autoimmune
encephalomyelitis. Ann NY Acad Sci. 1110:330–336. 2007. View Article : Google Scholar
|