Introduction
Nerve injury induced-neuropathic pain (NPP) causes
local functional and biochemical changes and it also leads to
pathology of the spinal cord and brain (1,2).
Currently, no effective treatment is available for NPP, and the
mechanisms underlying its occurrence and development are poorly
understood. In traditional animal models of chronic constriction
injury (CCI) of the sciatic nerve, which are widely used in
studying NPP, it is difficult to replicate the level of nerve
injury due to variables in surgery and severity of nerve ligation.
This often leads to variation among the individual models (such as
the percentage of autophagy), making the experiments unreplicable.
Therefore, the development of new animal models and understanding
the mechanisms underlying NPP are essential (3,4) in
order to meet clinical needs in pain management.
The complement system has a vital role in human
nonspecific immunity. In the normal physiological condition,
components of the complement system (i.e., complements) exist in
blood plasma in the form of enzyme precursors. Once they are
activated, a cascade chain of enzymatic reactions occurs, leading
to the formation of series of activated fragments of the complement
proteins and eventually membrane attack complexes (MACs) (5). The activation of complements plays a
significant physiological role in clearing apoptotic cells and
neuronal fragments. However, improper activation can also cause
pathological injury to the host (5,6).
There is increasing research on the association
between complement activation and neuropathic pain. Griffin et
al (7) employed a gene chip
technique to screen 216 genes that were detected in three NPP
models induced by peripheral nerve injury. Among them, 54 genes
were co-expressed in all three models and they are related with
immunity, including genes expressing complement proteins. These
findings indicate that activation of complements may play a role in
the development of NPP. It has also been shown that intrathecal
injection of dissolvable soluble complement receptor 1 (sCR1) could
inhibit activation of the complement reaction cascade and
attenuates pain intensity in diverse rat models of inflammatory,
constrictive, and viral infection injuries of the sciatic nerve
(8,9). Kleinschnitz et al (10) found a reduced pain response and a
decrease in the accumulation and activity of macrophages in the
injured nerves of rats with a complement deficiency after CCI. It
is well known that there are several significant changes in the
spinal dorsal horn in NPP models such as abnormal discharge of
neurons and cytokine release of glial cells which contribute to the
development of neuropathic pain. However, it is unknown whether the
complements in the spinal dorsal horn are abnormally activated and
whether complements are associated with development of neuropathic
pain.
Among all the components of the complement system in
serum, complement component 3 (C3) has the highest percentage
(5). It is also the meeting point
of the classic, alternative, and mannose-binding lectin (MBL)
pathways of complement activation, and has a key role in the
defense mechanism of the host (5). It has been demonstrated that neurons
and glial cells synthesize the components of the complement cascade
reaction (11,12). They can also express complement
regulatory proteins and their receptors such as complement
component 3a receptor 1 (C3ar1) which is upregulated following
inflammatory central nervous system disease (CNS) injuries
(11). Increased C3a was found to
provoke the production of inflammatory mediators, which in turn
further enhanced synthesis of the complement. Thus, a feedback loop
is formed between activation of inflammatory cytokines and the
complement system, leading to nerve-immune activation (13). Chinese cobra venom factor (CVF),
an acidic glycoprotein extracted from Chinese cobra venom, is the
main anti-complement component in the venom (14). In the present study, we
hypothesized that C3 is a key mediator in the NPP mechanism. We
used a modified CCI (mCCI) rat model to explore the association
between the abnormal activation of C3 in the spinal dorsal horn and
the occurrence and development of NPP. Moreover, CVF, as a
non-specific inhibitor of complement function, could be an
effective intervention reagent to identify the roles of abnormally
activated complements in NPP.
Materials and methods
Animals and experimental groupings
Healthy adult male Sprague-Dawley rats (250–300 g)
were employed in this study, and were provided by the Animal
Experiment Center of the Third Military Medical University, with
Animal Medical Certificate no. SCXK (Military) 2002008. Rats were
housed four to a cage. The cage floor was covered with sawdust at a
room temperature of 20±2°C. The animals were exposed to a strictly
alternating light-dark pattern of illumination, each for 12 h, and
they were provided with adequate water and food. After a one-week
adaptation period, the modified rat models were studied.
Thirty-six rats were divided into three groups
(n=12): sham-operated, traditional CCI, and mCCI. Traditional CCI
models were prepared as previously described by Bennett and Xie
(15), and the mCCI models were
treated with slight modifications as introduced later. Behavioral
responses consisting of changes in paw withdrawal thermal latency
(PWTL) and paw withdrawal threshold to mechanical stimuli (PWMT)
were observed at two weeks post-surgery. After these observations,
the mCCI model was adopted in subsequent experiments.
Animals were randomly divided into seven groups
(designated A to G), including 11 subgroups (n=12 in each group).
Group A was the normal controls, rats that did not undergo surgery
but that were otherwise treated identically, with the same survival
requirement as the other groups. Group B rats were sham-operated,
they underwent the same course of surgery as the model rats but
without ligation of the sciatic nerve. Subgroups B1, B3 and B7
corresponded to 1, 3 and 7 days post-surgery, respectively. Group C
was the mCCI group, rats that underwent the mCCI model operation.
Subgroups C1, C3 and C7 corresponded to 1, 3 and 7 days
post-surgery, respectively. Group D, or sham + saline, underwent a
sham operation with saline intravenous (IV) injection at the same
volume as Group F. Group E, or mCCI + saline, underwent mCCI
surgery and IV injection of normal saline each day pre- and
post-surgery. Group F, or mCCI + CVF, underwent mCCI surgery and
received IV CVF (50 μg/kg body weight pre-surgery, 20 μg/kg
post-surgery, each day). Group G, mCCI + saline + CVF, underwent
mCCI surgery and a single IV CVF (50 μg/kg body weight) 4 days
post-surgery, and saline injection as for Group E. All the
experiments were conducted in the daytime.
The Animal Care and Use Committee at the Third
Military Medical University in the P.R. China approved the
experimental protocols and all protocols were in accordance with
the guidelines for animal study set by the International
Association for the Study of Pain. Every effort was made to
minimize both animal suffering and the number of animals used.
Experiment preparations
Chinese CVF was produced by Beijie Biotechnology
Co., Ltd. (Kunming, China). A solution (0.5 mg/ml) was prepared by
dissolving 1 mg of CVF powder in 2 ml of 0.01 M phosphate-buffered
saline (PBS; pH 7.2–7.4). The solution was filtered and sterilized
through disposable aseptic filters, sub-packaged into aseptic
bottles, and stored at −20°C.
Goat anti-rat C3 antibody was produced by Cappel
(USA) (cat. no. 55713). The superoxide dismutase (SOD) test box
(article no. A00) and maleic dialdehyde (MDA) kit were provided by
Jiancheng Bioengineering Institute (Nanjing, China). The RNAprep
tissue/bacteria kit was manufactured by Tiangen Biotech (Beijing,
China) (cat. no. DP401). The cDNA synthesis kit was produced by
Toyobo, code no. FSK-100. The reverse transcription (RT)-PCR kit
was produced by Toyobo, code no. PCR-400. The C3 assay kit was
manufactured by Shanghai XiTang Biotechnology Co., Ltd. (Shanghai,
China; batch no. 0610225).
Establishment of the mCCI model and
administration of CVF
Modifications were made to the traditional
procedures for creating the CCI rat model, described in the
following steps (15). After an
intraperitoneal (IP) injection of 1% pentobarbitone (40 mg/kg) and
adequate anesthesia, the rat was put in the prone position, with
four limbs and incisor teeth fixed to the operating table. The
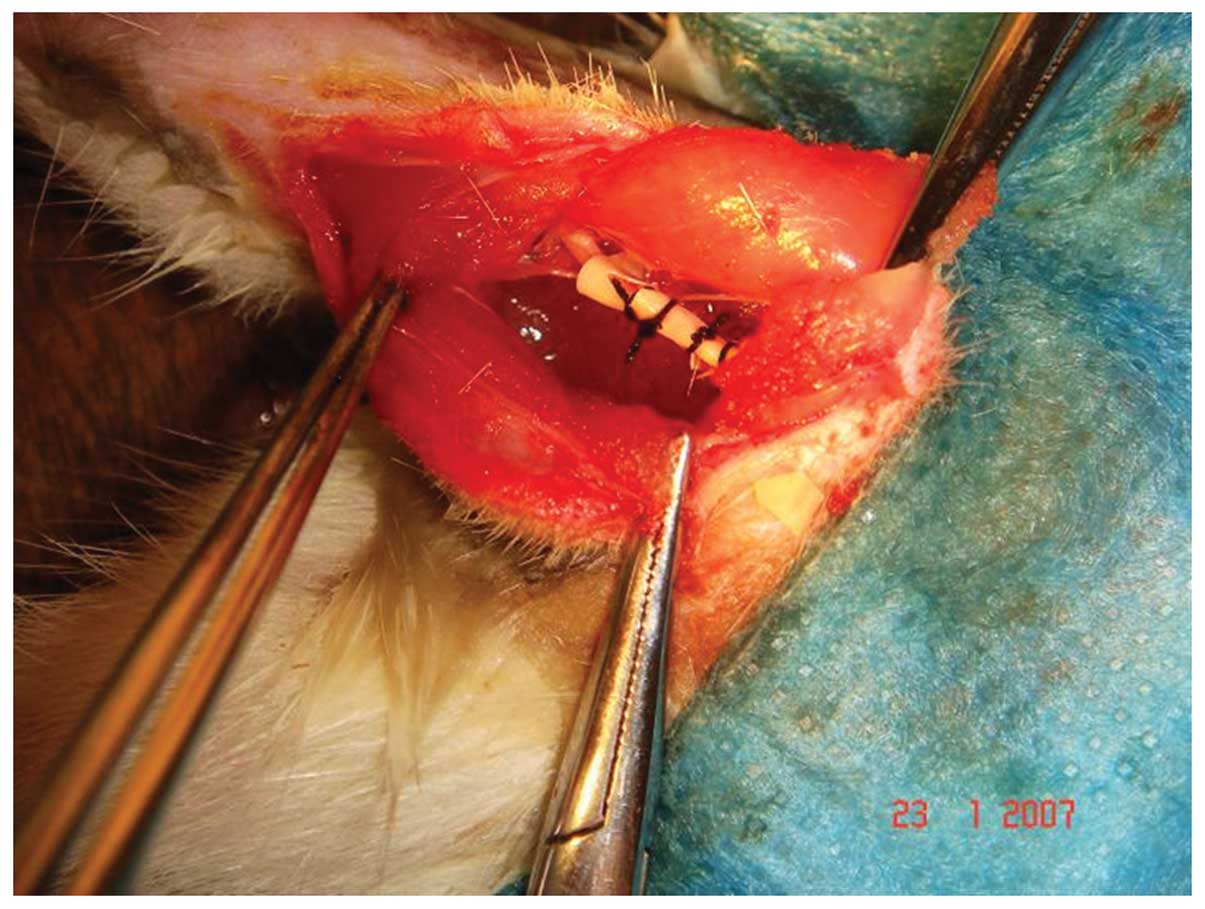
sciatic nerve was exposed with the routine method, and the
peripheral tissues were liberated. The sciatic nerve was enveloped
with porous films ∼5 mm from the proximal end of the nerve
branching. Four ligation bands were then made with 4/0 chromic
suture at an interval of 1 mm, to the degree that the epineurial
blood flow was not affected (Fig.
1). The main difference between the mCCI and traditional models
was that the sciatic nerve was enveloped with porous films in the
mCCI rats which makes the strength of ligation more symmetrical and
protects the nerve from acute crush and incised injuries. CVF was
administered via tail vein injection 10 min before surgery (50
μg/kg body weight) and each day after surgery (20 μg/kg) in rats in
Group F (mCCI + CVF). An equivalent volume of IV normal saline was
injected daily in rats in Group D (sham + saline) and Group E (mCCI
+ saline) before and after surgery. Rats in Group G (mCCI + saline
+ CVF) were injected with CVF (50 μg/kg) 4 days after surgery and
with normal saline at other time-points similar to Group F.
Observation of behavioral changes
Observations were made of the gait and posture of
the operated limb for spontaneous pain behaviors as well as for the
presence of autophagy, and were scored according to Attal et
al (16). Behaviors of the
operated hind limb were scored as: 0, the limb rests on the floor
normally; 1, the limb rests partially on the floor, with toes in
the ventral flexion; 2, only the medial part of foot rests on the
floor; 3, only the heel rests on the floor, with toes lifted up; 4,
the whole posterior foot is lifted up away from the floor; 5, the
animal licks the foot of the operated limb. Behaviors were observed
for 5 min each time at 10 a.m. every day for two weeks after
surgery. The score of the behavior with the longest duration was
used to represent the rat's level of spontaneous pain.
The PWMT was determined according to the method
introduced by Dixon (17). The
rat was placed onto an elevated metal mesh that was then covered
with a transparent plexiglass cover. After 20 min of adaptation,
von Frey filaments (range of measurement power 0.2–20 g) were used
to perpendicularly stimulate the skin of the sole of the foot
between the 2nd and 3rd toes, with the pressure increasing
gradually. The stimulation persisted for 6–8 sec. A positive
reaction was recorded if prompt paw withdrawal occurred during the
stimulation period, or upon the removal of the von Frey filaments.
Paw withdrawal resulting from body movement was not deemed a
positive reaction. Ten stimuli were applied around the threshold,
with an interval of at least 15 sec, waiting for the disappearance
of the response to the previous stimulus. The 50% withdrawal
threshold was calculated according to the median calculation method
and the pressure was recorded as the PWMT.
The PWTL was determined according to the method
described by Rokyta et al (18). An RYT-1 thermal stimulator (made
in the Fourth Military Medical University, Xian, China) for pain
threshold was used. The PWTL of each rat was tested in the morning.
Following adaptation for 5 min on the glass bedplate, the rat was
irradiated with a fixed intensity of light (the first level of 3)
for <30 sec. The test was repeated three times for each rat with
a resting interval between tests of 5 min. The mean time of paw
withdrawal for the three tests was recorded as the PWTL.
Biochemical measurements of C3, total SOD
(T-SOD), and MDA
Immunoturbidimetry was adopted to determine the
concentration of C3 in spinal cord homogenate. The spinal cord
(segments L4-6) was exposed at different time-points (3, 5 or 7
days) after surgery. The dorsal corner on the operated side was cut
rapidly and preserved in liquid nitrogen. After weighing, the
spinal cord samples were added into a blender, added to iced salt
water, one part tissue to nine parts liquid to prepare a 10% spinal
cord homogenate. The homogenate was then centrifuged for 10 min at
3,000 rpm at 4°C. The clear supernatant was diluted with normal
saline (1:11), and blended for 30 sec with a vortex mixer; the
mixture was added to anti-C3 serum (100 μl/ml). After adequate
mixing, this was incubated in a thermostatic water bath at 37°C for
15 min. A UV spectrophotometer was set at a wavelength of 340 nm,
and normal saline was used to determine the zero adjustment. The
absorbance of each tube was measured, and a standard preparation
was diluted in the proportion given by the kit's instructions and
measured as value A.
Based on the concentration of the standard
preparations given by the kit and the value A obtained in this
experiment, we drew a standard curve and then obtained the linear
equation Y = aX + b, where Y is value A, and X is the concentration
of C3 of the standard preparation at different dilution ratios, a
and b are coefficients obtained from the kit's information. Using
this equation, we calculated the concentration of C3 of each sample
(19).
A spectrophotometric method was employed to
determine T-SOD activity and the MDA content of the spinal cord
tissues. Xanthine oxidase was used to measure SOD activity and an
optical density (OD) value for the test tube was read at a
wavelength of 550 nm. The thiobarbituric acid (TBA) method was used
to assess the concentration of MDA, and the OD value of each tube
was read at a wavelength of 532 nm (20).
RT-PCR analysis of C3 mRNA
expression
Animals were euthanized with an overdose of
pentobarbital at different time-points. We adopted RT-PCR to
determine the level of C3 mRNA in the spinal cord segments
L4–6, on Days 1, 3 and 7 after surgery. The dorsal corner of the
ipsilateral spinal cord segments (L4–6) was dissected, and
preserved in liquid nitrogen. The methods of extraction,
identification, and RT of RNA were the same as previously described
(21). The two-step approach for
RT-PCR analysis was employed to evaluate the expression of
C3 mRNA in the spinal cord tissues. For the primer
sequences, we looked up the corresponding RNA sequence in GenBank,
and designed the primer with software WinStar 5.08 (WinStar Bio.
Co.). The primer sequences were: for Actb, forward,
5′-CGTAAAGACCTCTATGCCAACA-3′ and reverse,
5′-CGTAAAGACCTCTATGCCAACA-3′; for C3, forward,
5′-GCTGTGCCTTATGTCATTGTCC-3′ and reverse,
5′-ATTTCTCCCACTGTTCGGTCTG-3′. The results were analyzed with the
help of the software Quantity One E4.0.
Expression of C3 protein by
immunohistochemistry
The immunoreactivity of C3 in the spinal cord
segments L4–6 was tested. On post-surgical days 1, 3 and 7, 1%
pentobarbitone (IP, 40 mg/kg) was administered to the sham-operated
and mCCI rats. The chest was then opened and the heart was exposed.
After infusing normal saline and paraformaldehyde through the left
ventricle and aortic cannula, segments L4–6 of the spinal cord were
dissected, preserved and fixed for 24 h, followed by dehydration,
infiltration with supporting paraffin, and paraffin embedment.
Serial sections (thickness, 4 μm) were cut. Immunohistochemical
staining was performed. The primary antibody was goat anti-rat C3
(50 μl, 1:200) and the second antibody was biotin-labeled rabbit
anti-goat IgG (50 μl). Following incubation in enzyme-labeled
horseradish peroxidase anti-biotin fluid (50 μl) at 37°C for 20
min, diaminobenzidine coloration was performed, then after staining
with hematoxylin, sections were cleared and mounted, and observed
under an Olympus light microscope. With a clear background, we were
able to clearly visualize blue nucleus staining, and the brownish
yellow intra-cytoplasmic particles which were identified as cells
testing positive for C3 (22).
Pathomorphological observation
The structures of neurons in the dorsal part of the
spinal cord were observed under a transmission electron microscope
(TEM). The spinal cord samples were fixed by immersion in 2.5%
glutaraldehyde for 4 h, rinsed in PBS for 2 h, and fixed in osmic
acid for 2 h. Following dehydration through an alcohol series,
samples were stained through saturation with uranyl acetate for 30
min, and embedded in epoxide resin overnight at 37°C. The embedded
lump was made into the form of a pyramid with four smooth sides and
a trapezoid on the top, from which we obtained 60-nm sections using
an ultramicrotome. The sections were observed under a TEM (Tecnai
10; Philips, The Netherlands) after citric acid staining for 10
min, PBS rinsing three times, and air drying (23).
Statistical analysis
Statistical analysis was performed with SPSS 10.0
software. Repeated-measures ANOVA and the t-test were employed for
group comparisons, and the correlation between the complement
protein content and the pain threshold was analyzed using Pearson's
correlation analysis. All data are presented as mean ± standard
error (SE). P<0.05 was considered to indicate a statistically
significant difference.
Results
Rats with mCCI present no autophagy but
have greater thermal hyperalgesia than rats with traditional
CCI
The mCCI rats had no autophagy and maintained
thermal hyperalgesia for a longer period than did the traditional
CCI rats (3/12 rats with the traditional CCI showed signs of
autophagy of the posterior toes on the injured side). In regard to
spontaneous pain, the rats in the sham-operated group all scored 0
points in posture and walking, while scores in the traditional and
mCCI groups ranged from 2 to 4. The pain sensitivity of the mCCI
rats lasted >14 days, while the pain sensitivity in the
traditional group returned back to the level approximately similar
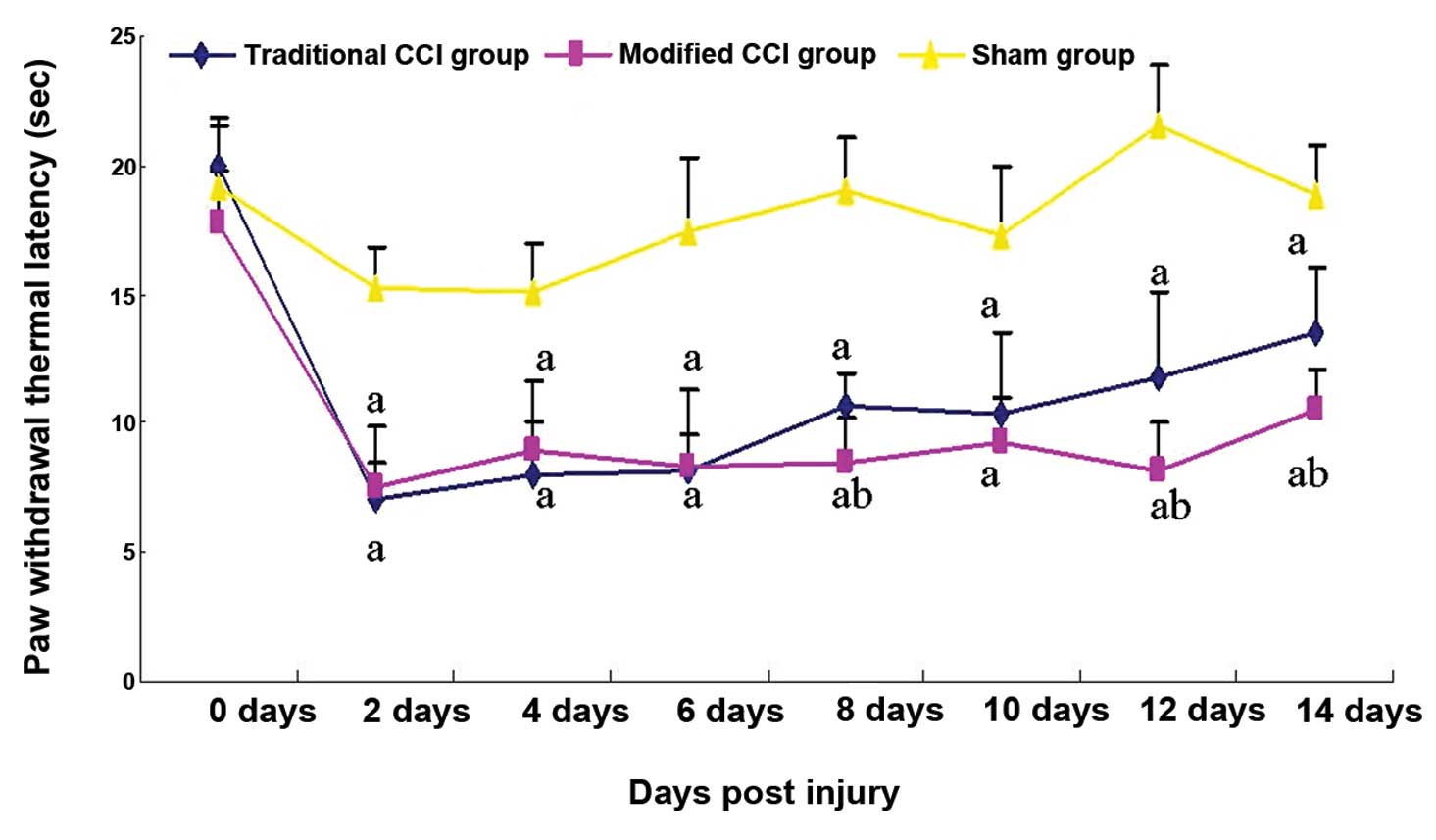
to that in the sham group on Day 14 after surgery (Fig. 2).
No statistically significant difference was found in
the preoperative thermalgesia threshold among the three groups. In
the sham-operated group, the thermalgesia threshold exhibited a
transitional and small decrease on post-surgical day 2; it then
returned to normal. In the traditional and mCCI groups, however,
hind PWMTs in response to thermal stimuli on the ligated side
significantly dropped by 53.6 and 51.0%, respectively, two days
after surgery, relative to the sham-operated rats. Six days after
surgery, the PWMTs dropped to the lowest level by 53.1 and 52.0%,
in the traditional and mCCI groups, respectively. Although the
PWMTs gradually increased afterwards until two weeks after surgery,
they were still lower than the preoperative level, 28.0 and 44.4%
lower than the sham-operated group, respectively (P<0.01)
(Fig. 2).
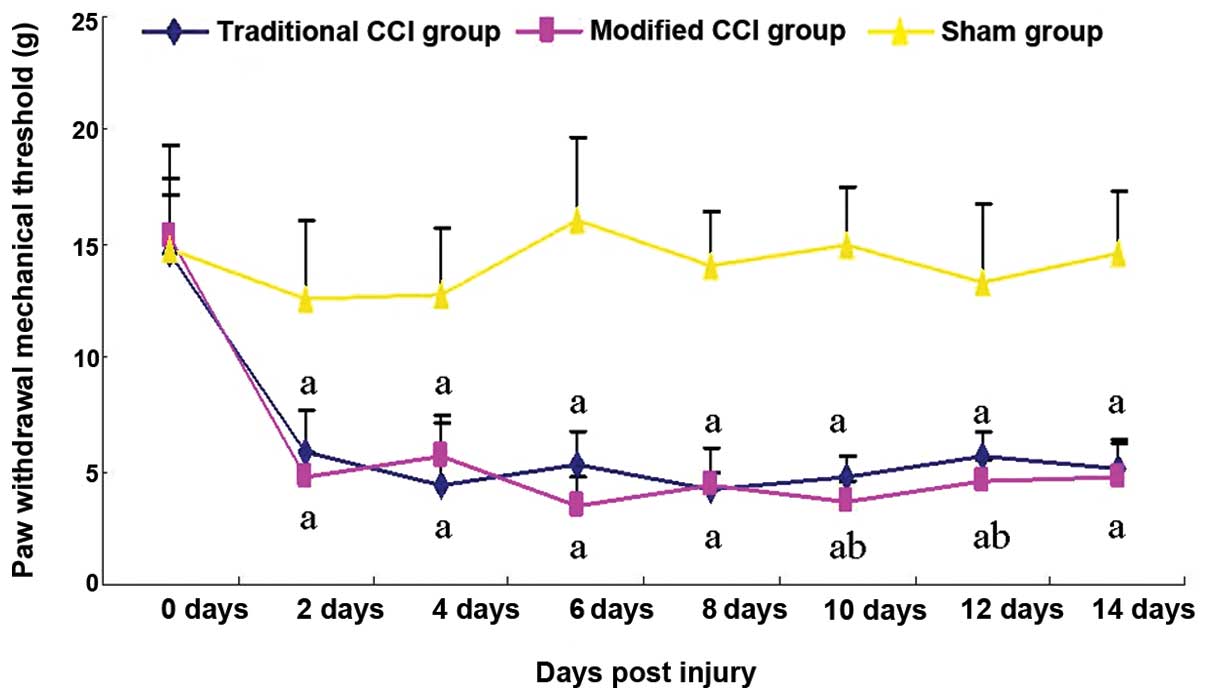
mCCI rats show greater mechanical
hyperalgesia than traditional CCI rats
To assess different pain responses to mechanical
stimuli in the rats with the mCCI and traditional CCI, the PWMTs in
the mCCI and traditional CCI rats were compared. The pain
sensitivity of mCCI rats remained unchanged over the 14 days
post-surgery, while that in the traditional group began to return
to the level similar to that in the sham group beginning on Day 8
(Fig. 3). Prior to surgery, there
were no significant differences in the PWMT among the three groups.
The sham-operated rats exhibited no evident change in response to
the von Frey hair stimulus, but relative to these the pain
thresholds of the traditional CCI and mCCI groups were reduced by
53.2 and 61.9%, respectively, 2 days after surgery. Six days after
the operation, the thresholds further declined by 67.0 and 78.1%,
respectively, their lowest value. Although the thresholds then
gradually increased until two weeks after surgery, they had
significantly decreased by 65.1 and 67.8%, respectively, compared
to the sham-operated group prior to surgery (P<0.01) (Fig. 3).
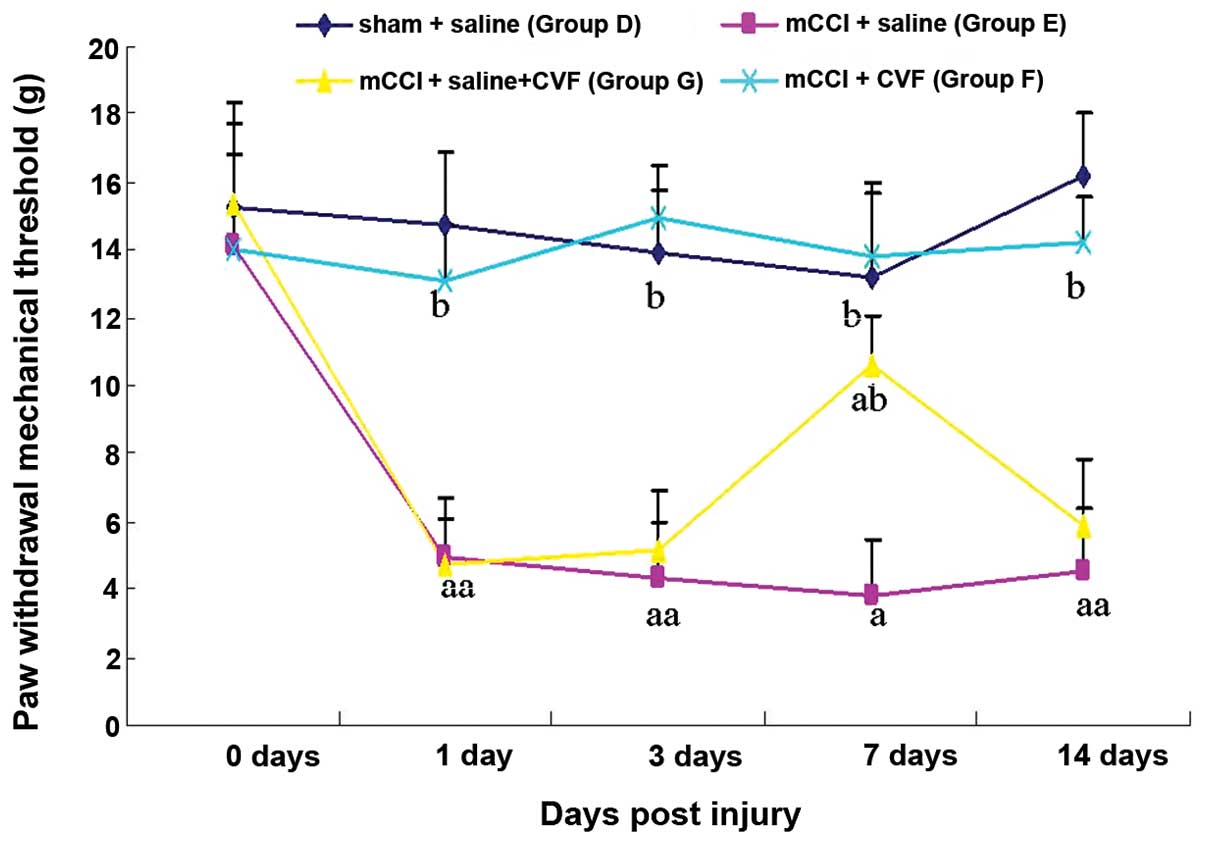
CVF reverses the mechanical hyperalgesia
induced by mCCI
To investigate if the complement cascade reaction
plays a significant role in the formation of NPP hyperalgesia, the
complement activity inhibitor CVF was administered by IV 50 μg/kg
prior to surgery and 20 μg/kg each day post-surgery, or by bolus IV
of 50 μg/kg on Day 4 after surgery. Continuous tail vein injection
of CVF completely reversed the mechanical hyperalgesia induced by
mCCI (Fig. 4); bolus IV CVF
showed such effects for about a week. Administration of normal
saline instead of CVF in mCCI rats did not produce significant
changes, when compared to the sham operation rats in mechanical
hyperalgesia (Fig. 4). These
results indicated that tail vein injection of CVF could reverse
mechanical hyperalgesia in rats with mCCI.
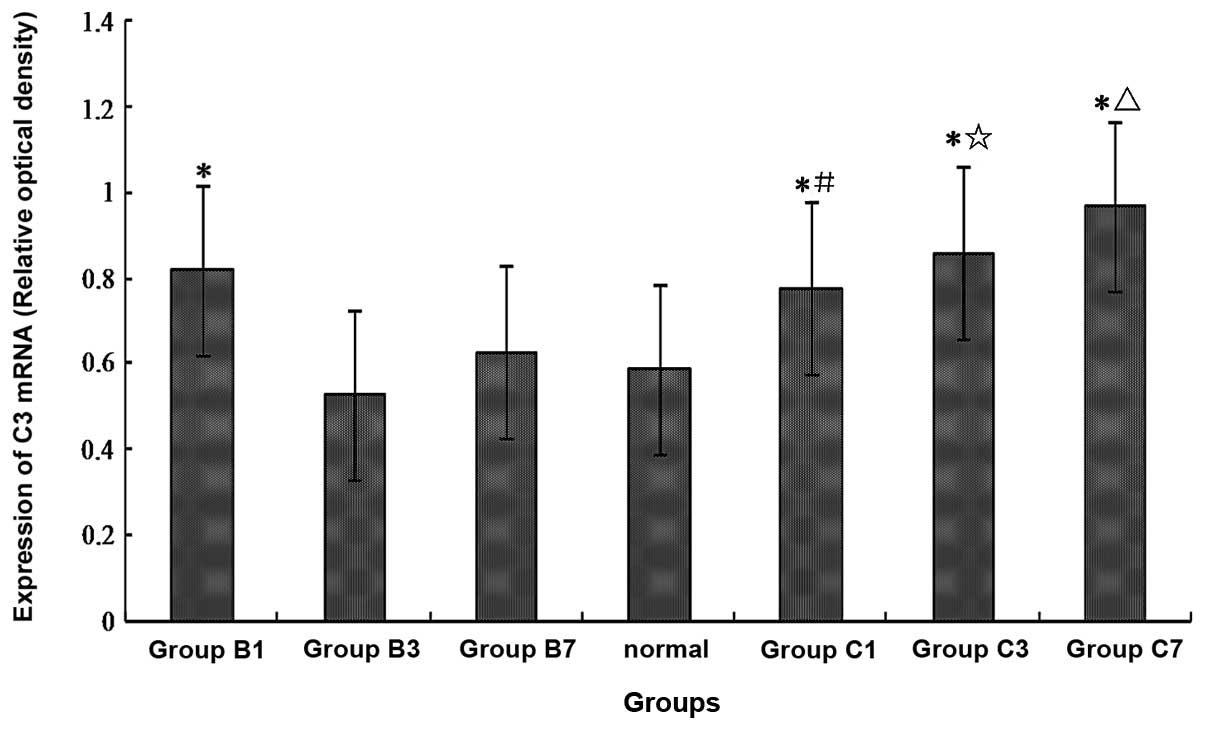
The expression of C3 mRNA increases in
the spinal cord of mCCI rats
One day after surgery, in the sham-operated and mCCI
groups, the expressions of spinal C3 mRNA were higher
(increased by almost 40%) than those in the normal control group
(Fig. 5) (P<0.01). Three and
seven days after surgery, there were no statistically significant
differences in the expression of spinal C3 mRNA in the
sham-operated rats when compared with the normal controls. However,
in the mCCI groups, the expressions of spinal C3 mRNA were
significantly higher 3 and 7 days post-surgery, compared to either
the normal controls or the corresponding 3- and 7-day sham-operated
rats (Fig. 5) (P<0.01).
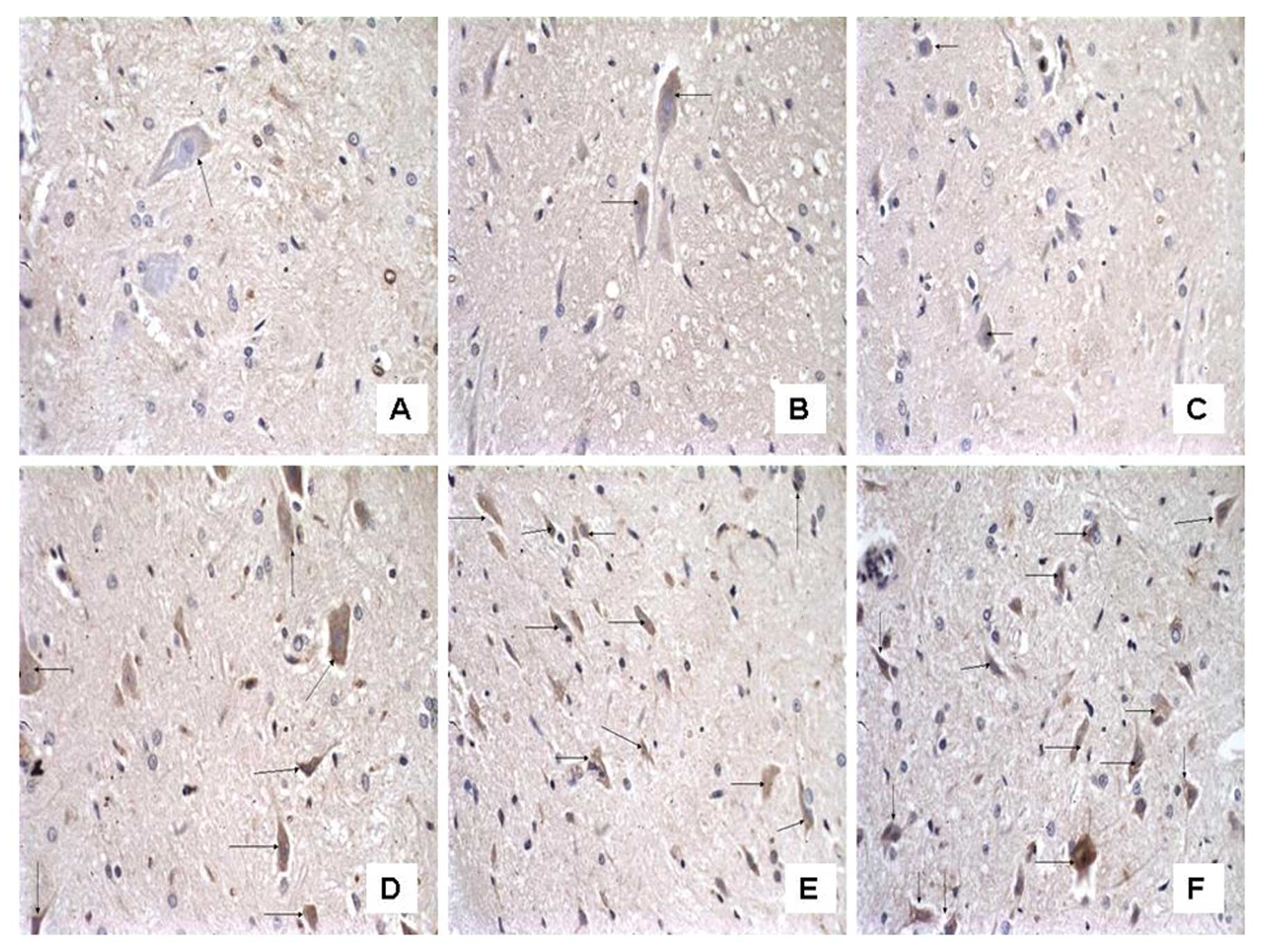
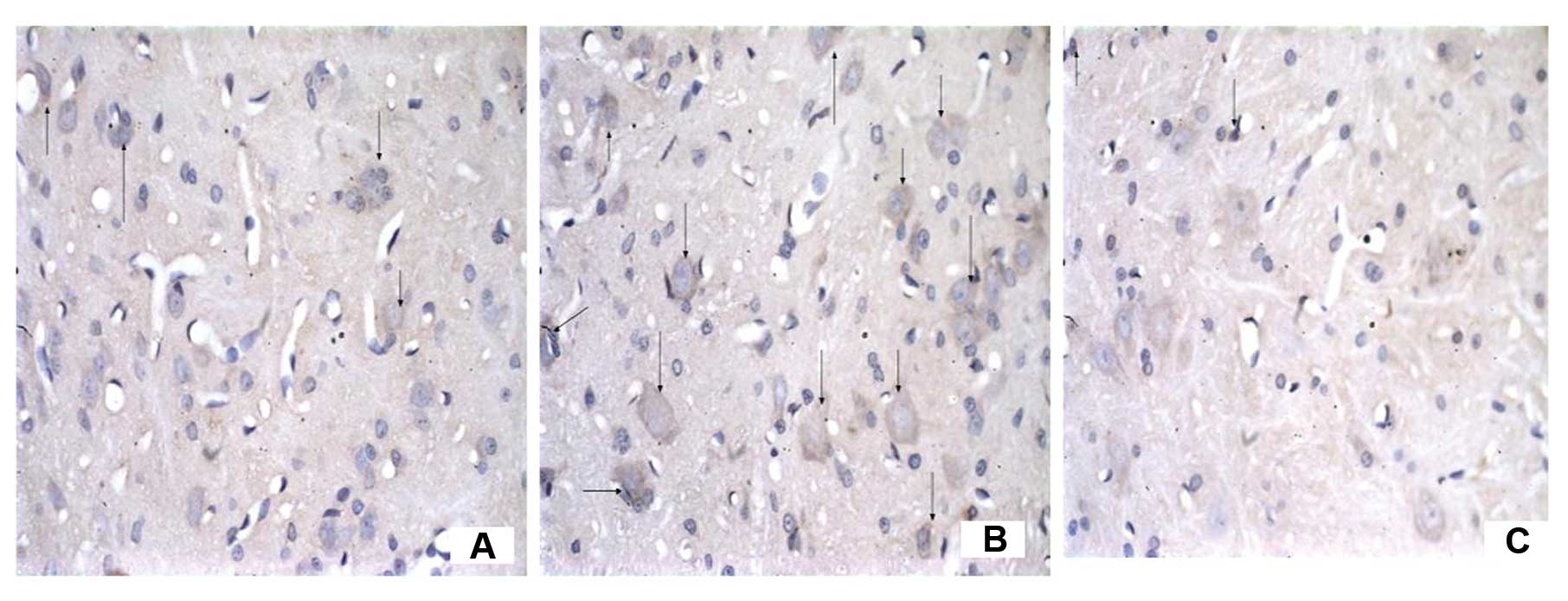
The number of C3-immunoreactive cells
increases on the ipsilateral spinal cord in mCCI rats and the
increase is reversed by CVF
To examine the effect of CVF on the expression of C3
after mCCI, we observed the morphology and number of C3-positive
cells under the microscope following immunohistochemistry. In the
dorsal horn of the spinal cord, segments L4–6, of the sham-operated
rats there were only a few C3-positive cells, appearing as brownish
yellow particles in the cytoplasm and a clear blue nuclear stain
(Figs. 6 and 7). However, their numbers were almost
equivalent on both the ipsilateral and contralateral sides on Days
1, 3 and 7 (Fig. 6A, B and C,
respectively). By contrast, the ipsilateral side of mCCI rats had
more than a 3-fold increase in C3-positive cells compared to the
contralateral one day after surgery. Furthermore, in the mCCI rats,
a greater number of C3-positive cells (1.5- to 2-fold) was found in
the 3- and 7-day groups than in the 1-day group (Fig. 6D, E and F for Group C at 1, 3 and
7 days, respectively). However, the number of C3-positive cells in
the spinal dorsal horn markedly decreased by 20% at 14 days in the
rats with mCCI + CVF (Group F) (Fig.
7C), compared with those without CVF administration (Fig. 7B), and with the sham-operated rats
(Fig. 7A).
We noted that C3 immunoreactivity was also observed
in the cells which were similar in morphology to neurons, and we
considered that C3 could be expressed by glial cells as well as by
neurons.
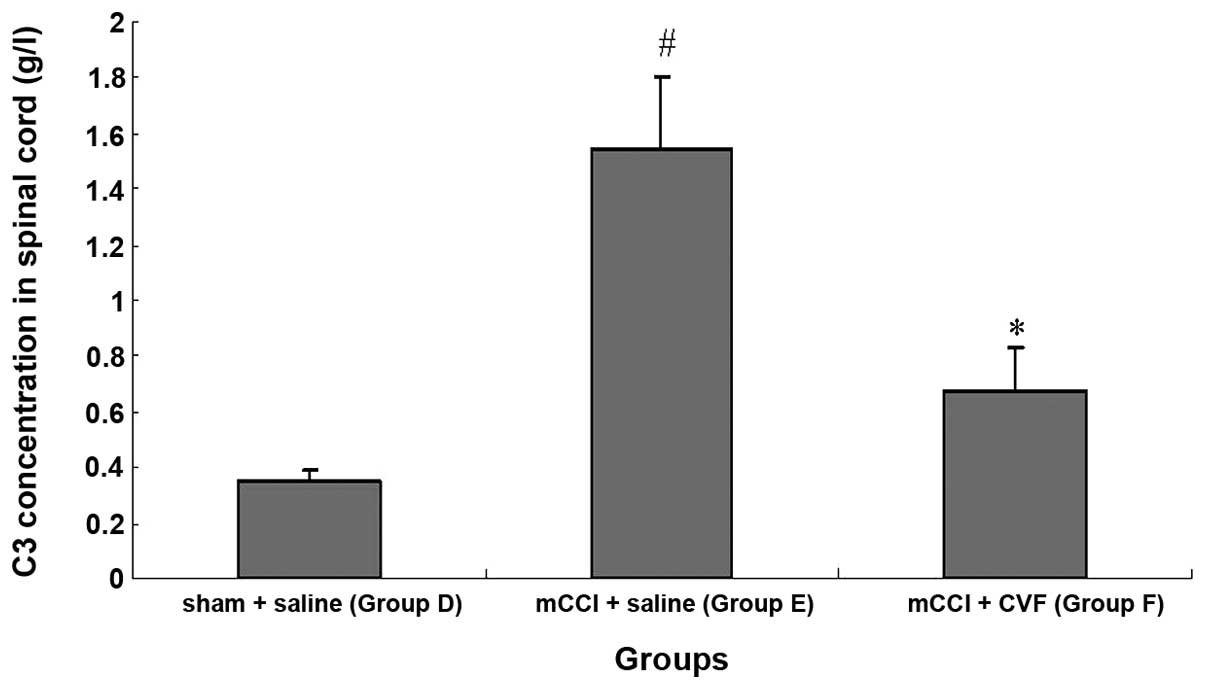
C3 concentration increases significantly
in the spinal cord homogenate of mCCI rats, and CVF inhibits the
increase
Immunoturbidimetry measurement of the concentration
of C3 in spinal cord homogenate showed that the content of C3 was
significantly elevated in spinal cord homogenate prepared from mCCI
rats. When compared with the normal controls, the C3 content of the
sham-operated rats was greater on Day 1 post-surgery, but
comparable on Days 3 and 7. However, for the 1-, 3- and 7-day mCCI
groups, the C3 content was ∼2.5- to 3.5-fold higher than that of
the normal control or sham-operated groups of the corresponding
period (Fig. 8) (P<0.01).
Furthermore, the increase in C3 in the spinal cord induced by mCCI
was markedly inhibited 14 days after daily tail vein injections of
CVF administered prior to and following surgery (Fig. 9) (P<0.01).
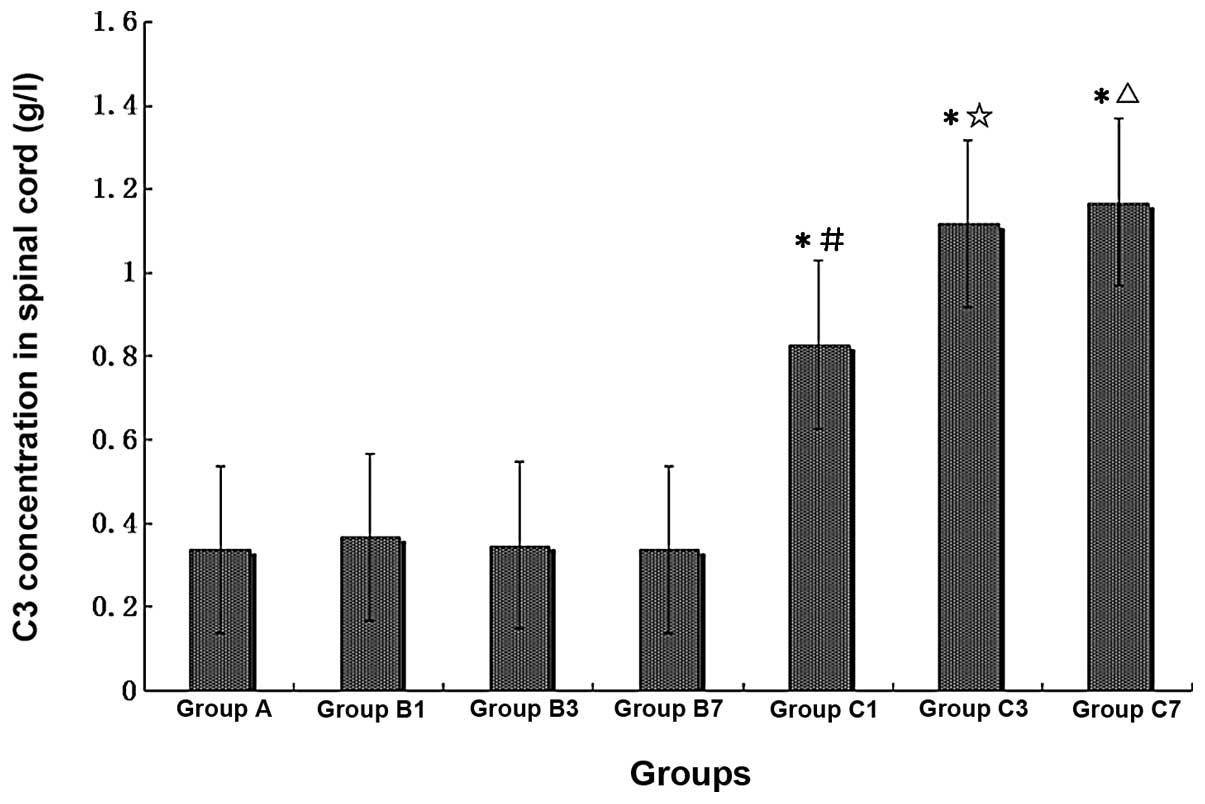 | Figure 8Comparison of C3 concentrations in
rat spinal cords of different treatment groups (n=6, each group).
A, normal, non-operated; B1, B3 and B7, sham-operated, 1, 3 and 7
days post-surgery, respectively; C1, C3 and C7, mCCI, 1, 3 and 7
days post-surgery, respectively. *P<0.01, compared
with Group A; #P<0.05, compared with Group B1;
☆P<0.01, compared with Group B3;
△P<0.01, compared with Group B7. |
The activity of SOD decreases and the
content of MDA increases in the spinal cord of mCCI rats, and CVF
reverses these changes
The activity of SOD in the spinal cord decreased
significantly by ∼45%, while the MDA content increased 5.5-fold in
the 14-day mCCI rats (Group E), when compared with the sham +
saline group (Group D, P<0.01) (Table I). However, the decreased SOD
activity and increased MDA level were significantly inhibited by
∼50% after the mCCI rats were treated with CVF (Group F, P<0.01)
(Table I).
 | Table IComparison of SOD and MDA in
different groups (mean ± SE). |
Table I
Comparison of SOD and MDA in
different groups (mean ± SE).
| Groups | Sham + saline
(Group D) | mCCI + saline
(Group E) | mCCI + CVF (Group
F) |
|---|
| SOD (U/mgprot) | 150.84±10.17 | 109.58±8.10a |
121.89±9.37a,b |
| MDA
(nmol/mgprot) | 1.64±0.27 | 9.19±0.67a |
4.71±0.52a,b |
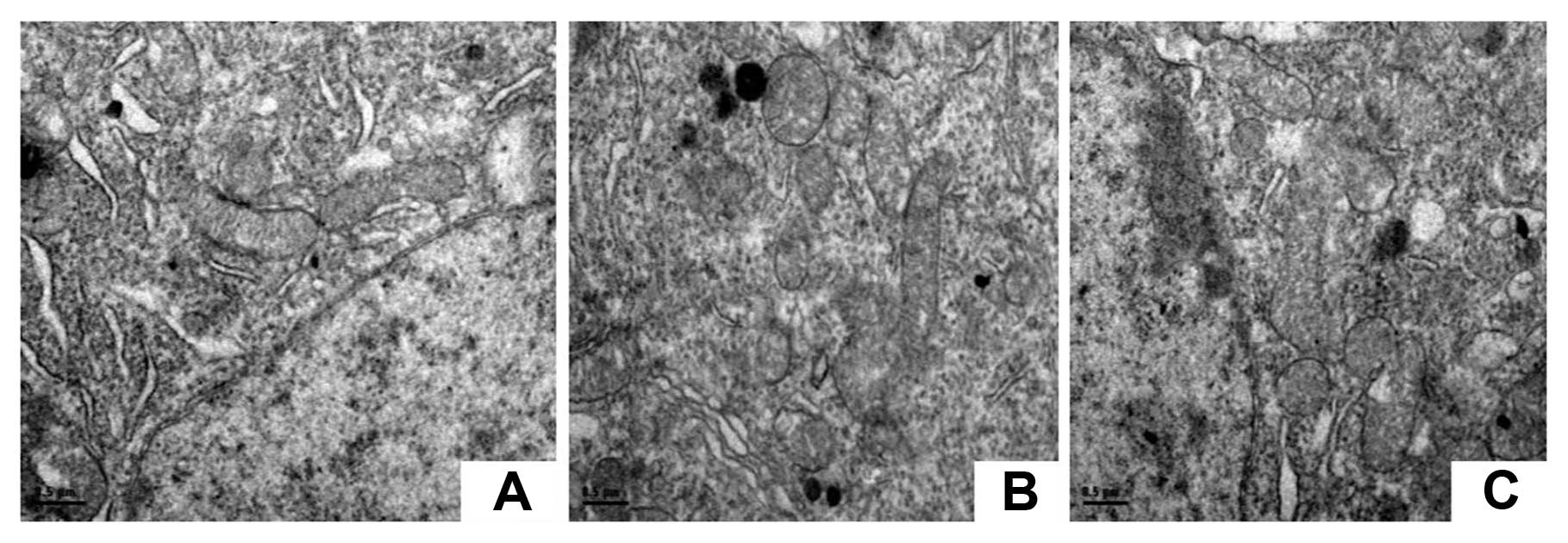
The neuronal mitochondria are damaged in
the spinal dorsal horn in the mCCI rats, and CVF attenuates the
injuries
Electron micrographs showed that neuronal
mitochondria and other subcellular structures in the spinal dorsal
horn were intact in sham-operated rats (Group D). However, in the
mCCI rats 14 days post-surgery, the neuronal mitochondria had
become swollen, the cell membrane was damaged and the cristae
fragmentized (Group E). Fourteen days after administration of CVF,
the swelling of the neuronal mitochondria had attenuated in mCCI
rats (Group F), as illustrated in Fig. 10.
PWMT is negatively correlated with C3
content in the spinal cord
The correlation between the PWMT and C3 content in
the spinal cord was explored through Pearson's product moment
correlation analysis. The result showed that PWMT and C3 content
were well correlated, with a coefficient correlation of r=−0.899
and P<0.0001, suggesting that the pain threshold was negatively
correlated with C3 content in the spinal cord, i.e., the higher the
content of spinal cord C3, the greater the pain sensitivity.
Discussion
Using a model of CCI is the standard method for
studying NPP (24,25). In the traditional models, it is
difficult to control the level of nerve injury due to surgical
variables and differences in severity of nerve ligation. These
often lead to marked differences among individuals and
unreliability (26,27). Additionally, autophagy is often
observed in experimental animals, which not only contributes to
infection in local tissues, but also augments character differences
between the model and chronic NPP patients in the clinic. In the
present study, we modified the construction of the CCI models by
using porous films to envelop the sciatic nerve, thereby avoiding
acute nerve injuries, including acute crush and incised injuries.
The porosity of the film ensures material exchange between the
ligated nerve and the peripheral tissue space, and thus the ligated
nerves are provided with enough nutrition. The results of the
present study demonstrate that the mCCI model is an ideal
peripheral nerve injury-induced NPP model, as it is more stable,
longer-lasting, with less autophagy and longer pain sensitivity,
and it is more suitable for long-term animal observation and
research.
Research on the mechanisms underlying NPP has
demonstrated that it may result from the synergistic effects of
central and peripheral nerve sensitization (28–30). The complement system is an
essential component in human nonspecific immunity, and its
activation plays a significant physiological role in clearing
apoptotic cells and neuronal fragments. However, abnormal
activation of complements could also cause pathological injuries to
the host, and is involved in the formation and aggravation of
several CNS diseases. Bonifati and Kishore (31) reported that the activation of the
complement system is an important cofactor in the mechanisms of
numerous CNS diseases including Alzheimer's and Parkinson's disease
(31). Nevertheless, there have
been few reports on the association between the complement system
and the occurrence of NPP. C3 is at the crossing point of the
classic, alternative, and MBL pathways of complement activation,
and among the humoral components of the complement system it has
the highest percentage (5). Its
activating correlative fragments such as C3a, C5a and their protein
complexes such as C4b2a3b and C3bBb enable its multifunctional
characteristics including important roles in immunoprotection and
immunoregulation (11,13). For this reason, in this study, C3
content was considered an indicator for activation of the
complement system.
In the present study, mCCI-induced abnormal
activation of the complements in the spinal cord dorsal horn was
detected in the NPP model rats one day after surgery, and was
accentuated on Days 3 and 7. The results of RT-PCR analysis also
revealed that the levels of C3 mRNA in the spinal cord
dorsal horn of mCCI rats progressively increased 1, 3 and 7 days
after surgery. These results were consistent with the induction and
development of hyperalgesia in the mCCI rats. Moreover, Pearson's
correlation analysis also proved that the expression of C3 in the
spinal dorsal horn was negatively correlated with the pain
threshold in these rats.
The present study also demonstrated that a bolus
tail vein injection of CVF could temporarily reverse the thresholds
of thermalgesia and mechanical hyperalgesia, perhaps by intervening
in the expression of C3 in the spinal dorsal horn. Continuous tail
vein injection of CVF was clearly able to continually inhibit
hyperalgesia in mCCI rats; however, when CVF was administered only
on post-surgical day 4 the effect was maintained for no more than a
few days. It is possible that its inhibitory effect on the pain
threshold is gradually reduced and, consequently, hyperalgesia
returns when the effect of CVF administration is diminished.
Collectively, the results of the current study
indicate that the complement cascade reaction takes place in the
spinal cord dorsal horn after CCI of the sciatic nerve, and that
complement activation plays a significant role in the formation of
NPP hyperalgesia, which is consistent with previous studies.
Kleinschnitz et al (10)
developed a model of sciatic nerve constriction injury using
experimental animals with complement depletion, and found a
reduction of pain and a decrease in the accumulation and activity
of macrophages. Moreover, Twining et al (9) showed that intrathecal injection of
sCR1 in nerve injury rat models could inhibit activation of the
complement cascade reaction, consequently attenuating pain
intensity.
Currently, little is known regarding initiating
factors for abnormal activation of the complements in the spinal
cord and blood of NPP animal models. In physiological conditions,
the concentration of C3 in serum is 300-fold higher than that in
the cerebrospinal fluid (CSF). If inflammatory factors and
activated complement components are able to slightly change the
integrity of the blood-brain/spinal cord barrier, blood-borne
complements may penetrate into the CSF and markedly elevate its
concentration of complements (32). In the NPP model with ligated
spinal nerve, the content of glial fibrillary acidic protein, an
activated astrocyte marker, is increased in the rat spinal cord;
additionally, a microgliocyte marker, OX-42, is accentuated when
pain occurs (33). This indicates
that astrocytes and microglial cells are activated in the pain
model. These activated glial cells could synthesize and release
complements, and the released inflammatory mediators can also
activate the complement cascade reaction from the cells in the
spinal cord (33,34). We hypothesize that an increase in
complements could provoke generation of inflammatory mediators,
while the inflammatory mediators in return further activate or
upregulate the synthesis of complements. Thus, a positive feedback
loop is formed between inflammatory mediator release and complement
activation leading to inflammatory factor accumulation, which may
contribute to immuno-neuropathic injury (15).
Our study showed that there was a positive
correlation between a significant increase in C3 mRNA
expression in the spinal dorsal horn induced by mCCI and the
occurrence of hyperalgesia. This correlation was confirmed by the
inhibition of hyperalgesia affected by administration of CVF. These
results suggest that C3 in the spinal dorsal horn could play an
important role in the cascade reaction of complements, which may be
involved in the formation of NPP.
However, it remains unclear how complement
activation affects neuronal functions. We speculate that certain
mechanisms related to C3 could affect cellular functions. Once
complement is activated, cascade reaction occurs rapidly leading to
producing end products of terminal complement C5b-9 complex, i.e.
C5b-8 complex and MACs, making a pinhole in target cell membrane
(35). With continual extension
of the hole by multitude of C9 fragments enter the target cells,
the cell membrane ruptures, and consequently, the cells are
dissolved (36). It has been
shown that neurons are generally not damaged by the MAC (37), therefore, we assume that there are
two possible outcomes for complements released by the activated
glial cells: one is that complements act on neurons in which glial
cells regulate the transmission of peripheral nociceptive signals
released by the neurons; the other one is to act on the neighboring
glial cells and to increase their activations. Being activated by
the complements, the neurons are further activated and release ATP,
glutamatic acid, fractalkine, and other substances which in turn
act on the glial cells and induce them to release more inflammatory
factors (e.g., IL-1, IL-6 and TNF) and complements (38–40). The interaction between glial cells
and neurons further boosts the activation of glial cells and the
excitability of the neurons, and eventually the nociceptive signals
are amplified, leading to hyperalgesia, or even allodynia.
In this study, spinal SOD activity was significantly
lower but MDA levels were markedly higher in the mCCI rats,
compared to those in the sham-operated rat spinal cord. Moreover,
TEM revealed mitochondrial swelling, cell membrane damage, and
cristae fragmentation in the neurons of the spinal dorsal horn 14
days following mCCI surgery. These results suggest that
inflammatory cytokines play important roles in the abnormal
activation of complements of neurons and glial cells. We also
showed that tail vein injection of CVF enhanced the activity of
spinal SOD but decreased the quantity of MDA, and relieved the
swelling of mitochondria in the neurons of the spinal dorsal horn
in mCCI rats. The results suggest that CVF inhibits or even stops
the effects of the complement cascade reaction on neurons,
maintains the integrity of neuronal structure and protects neuronal
function, so as to regulate the sensation of pain.
As indicated by Mika (41), clinically safe and reliable
intervention agents which target glial cells and the proteins they
produce should be the direction of research and development in the
treatment of NPP. Based on previous studies and our findings, we
strongly believe that NPP can be managed by means of blocking the
reaction chain of the complement activation or by improving
resistance of the neuronal cell membrane to activation of the
complement.
In conclusion, the mCCI animal model is more
suitable for the long-term observation and research of peripheral
nerve injury-induced NPP. In this study, mCCI induced a significant
increase in expression of C3 mRNA in the spinal dorsal horn,
and was consistent with the occurrence of hyperalgesia.
Administration of CVF reversed the hyperalgesia induced by mCCI.
These results demonstrated that abnormal complement activation
occurs in the dorsal horn of the spinal cord in rats with NPP, and
that C3 in the spinal dorsal horn could play an important role in
the cascade reaction of complements which is involved in the
formation of hyperalgesia.
Acknowledgements
The authors extend their gratitude to
Professor Jiangkai Lin in the Department of Neurosurgery, Southwest
Hospital, who made several suggestions for improving the technical
details of the experiment. This study was supported by grants from
the National Natural Science Foundation of China
(NSFC-30772077).
References
|
1
|
Adler JE, Nico L, VandeVord P and Skoff
AM: Modulation of neuropathic pain by a glial-derived factor. Pain
Med. 10:1229–1236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howorth PW, Thornton SR, O'Brien V, et al:
Retrograde viral vector-mediated inhibition of pontospinal
noradrenergic neurons causes hyperalgesia in rats. J Neurosci.
29:12855–12864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dib-Hajj SD, Black JA and Waxman SG:
Voltage-gated sodium channels: therapeutic targets for pain. Pain
Med. 10:1260–1269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Connor AB and Dworkin RH: Treatment of
neuropathic pain: an overview of recent guidelines. Am J Med.
122(Suppl 10): S22–S32. 2009.
|
|
5
|
Walport MJ: Complement. First of two
parts. N Engl J Med. 344:1058–1066. 2001.PubMed/NCBI
|
|
6
|
Carroll MC: The complement system in
regulation of adaptive immunity. Nat Immunol. 5:981–986. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Griffin RS, Costigan M, Brenner GJ, et al:
Complement induction in spinal cord microglia results in
anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci.
27:8699–8708. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Milligan E, Zapata V, Schoeniger D, et al:
An initial investigation of spinal mechanisms underlying pain
enhancement induced by fractalkine, a neuronally released
chemokine. Eur J Neurosci. 22:2775–2782. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Twining CM, Sloane EM, Schoeniger DK, et
al: Activation of the spinal cord complement cascade might
contribute to mechanical allodynia induced by three animal models
of spinal sensitization. J Pain. 6:174–183. 2005. View Article : Google Scholar
|
|
10
|
Kleinschnitz C, Hofstetter HH, Meuth SG,
Braeuninger S, Sommer C and Stoll G: T cell infiltration after
chronic constriction injury of mouse sciatic nerve is associated
with interleukin-17 expression. Exp Neurol. 200:480–485. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chow WN, Lee YL, Wong PC, Chung MK, Lee KF
and Yeung WS: Complement 3 deficiency impairs early pregnancy in
mice. Mol Reprod Dev. 76:647–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holmskov U, Thiel S and Jensenius JC:
Collections and ficolins: humoral lectins of the innate immune
defense. Annu Rev Immunol. 21:547–578. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davoust N, Jones J, Stahel PF, Ames RS and
Barnum SR: Receptor for the C3a anaphylatoxin is expressed by
neurons and glial cells. Glia. 26:201–211. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen Z, Teng X, Qian X, et al:
Immunoregulation effect by over-expression of heme oxygenase-1 on
cardiac xenotransplantation. Transplant Proc. 43:1994–1997. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar
|
|
16
|
Attal N, Fermanian C, Fermanian J,
Lanteri-Minet M, Alchaar H and Bouhassira D: Neuropathic pain: are
there distinct subtypes depending on the aetiology or anatomical
lesion? Pain. 138:343–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dixon WJ: Efficient analysis of
experimental observations. Annu Rev Pharmacol Toxicol. 20:441–462.
1980. View Article : Google Scholar
|
|
18
|
Rokyta R, Stopka P, Kafunkova E, Krizova
J, Fricova J and Holecek V: The evaluation of nociceptive intensity
by using free radicals direct measurement by EPR method in the tail
of anaesthetized rats. Neuro Endocrinol Lett. 29:1007–1014.
2008.PubMed/NCBI
|
|
19
|
Denham E, Mohn B, Tucker L, Lun A, Cleave
P and Boswell DR: Evaluation of immunoturbidimetric specific
protein methods using the Architect ci8200: comparison with
immunonephelometry. Ann Clin Biochem. 44:529–536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong Y, Hu HY, Xie X, Sakoda A, Sagehashi
M and Li FM: Gramine-induced growth inhibition, oxidative damage
and antioxidant responses in freshwater cyanobacterium
Microcystis aeruginosa. Aquat Toxicol. 91:262–269. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lange S, Bambir SH, Dodds AW, et al:
Complement component C3 transcription in Atlantic halibut
(Hippoglossus hippoglossus L.) larvae. Fish Shellfish
Immunol. 20:285–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morita H, Suzuki K, Mori N and Yasuhara O:
Occurrence of complement protein C3 in dying pyramidal neurons in
rat hippo-campus after systemic administration of kainic acid.
Neurosci Lett. 409:35–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kovacs B, Bukovics P and Gallyas F:
Morphological effects of transcardially perfused SDS on the rat
brain. Biol Cell. 99:425–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Decosterd I and Woolf CJ: Spared nerve
injury: an animal model of persistent peripheral neuropathic pain.
Pain. 87:149–158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watkins LR and Maier SF: Beyond neurons:
evidence that immune and glial cells contribute to pathological
pain states. Physiol Rev. 82:981–1011. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gazda LS, Milligan ED, Hansen MK, et al:
Sciatic inflammatory neuritis (SIN): behavioral allodynia is
paralleled by peri-sciatic proinflammatory cytokine and superoxide
production. J Peripher Nerv Syst. 6:111–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kiguchi N, Maeda T, Kobayashi Y, Fukazawa
Y and Kishioka S: Activation of extracellular signal-regulated
kinase in sciatic nerve contributes to neuropathic pain after
partial sciatic nerve ligation in mice. Anesth Analg.
109:1305–1311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bujalska M and Makulska-Nowak H:
Bradykinin receptor antagonists and cyclooxygenase inhibitors in
vincristine- and streptozotocin-induced hyperalgesia. Pharmacol
Rep. 61:631–640. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marchand F, Perretti M and McMahon SB:
Role of the immune system in chronic pain. Nat Rev Neurosci.
6:521–532. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watkins LR, Milligan ED and Maier SF:
Glial activation: a driving force for pathological pain. Trends
Neurosci. 24:450–455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bonifati DM and Kishore U: Role of
complement in neurode-generation and neuroinflammation. Mol
Immunol. 44:999–1010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reichert F and Rotshenker S:
Complement-receptor-3 and scavenger-receptor-AI/II mediated myelin
phagocytosis in microglia and macrophages. Neurobiol Dis. 12:65–72.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li JY, Xie W, Strong JA, Guo QL and Zhang
JM: Mechanical hypersensitivity, sympathetic sprouting, and glial
activation are attenuated by local injection of corticosteroid near
the lumbar ganglion in a rat model of neuropathic pain. Reg Anesth
Pain Med. 36:56–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suter MR, Berta T, Gao YJ, Decosterd I and
Ji RR: Large A-fiber activity is required for microglial
proliferation and p38 MAPK activation in the spinal cord: different
effects of resiniferatoxin and bupivacaine on spinal microglial
changes after spared nerve injury. Mol Pain. 5:532009. View Article : Google Scholar
|
|
35
|
Guo RF and Ward PA: Role of C5a in
inflammatory responses. Annu Rev Immunol. 23:821–852. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nguyen HX, Galvan MD and Anderson AJ:
Characterization of early and terminal complement proteins
associated with polymorphonuclear leukocytes in vitro and in vivo
after spinal cord injury. J Neuroinflammation. 5:262008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Jonge RR, van Schaik IN, Vreijling JP,
Troost D and Baas F: Expression of complement components in the
peripheral nervous system. Hum Mol Genet. 13:295–302. 2004.
|
|
38
|
Levin ME, Jin JG, Ji RR, et al: Complement
activation in the peripheral nervous system following the spinal
nerve ligation model of neuropathic pain. Pain. 137:182–201. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pekny M, Wilhelmsson U, Bogestal YR and
Pekna M: The role of astrocytes and complement system in neural
plasticity. Int Rev Neurobiol. 82:95–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiong ZQ and McNamara JO: Fleeting
activation of ionotropic glutamate receptors sensitizes cortical
neurons to complement attack. Neuron. 36:363–374. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mika J: Modulation of microglia can
attenuate neuropathic pain symptoms and enhance morphine
effectiveness. Pharmacol Rep. 60:297–307. 2008.PubMed/NCBI
|