Introduction
Intestinal ischemia/reperfusion (I/R) injury
represents a significant clinical problem in numerous situations,
such as small bowel transplantation, cardiopulmonary bypass, acute
mesenteric ischemia, abdominal aortic aneurysm surgery, intestinal
obstruction, as well as hemorrhagic, traumatic and septic shock
(1,2). Intestinal I/R injury triggers a
highly complex cascade of events resulting in decreased contractile
activity, increased microvascular permeability and the dysfunction
of the mucosal barrier (3–7).
Although the definitive pathogenesis regarding intestinal I/R
injury remains obscure, the hallmarks of this condition have been
well defined, including reactive oxygen species (ROS), recruitment
of activated leukocytes, cytokine release, cellular apoptosis and
mitochondrial dysfunction (8).
ROS, the most critical effector of intestinal I/R injury, can react
with all biological macromolecules (nucleic acids, proteins,
carbohydrates and lipids) in a destructive manner and initiate a
wide range of toxic oxidative reactions, which include the
initiation of lipid peroxidation, the direct inhibition of
mitochondrial respiratory chain enzymes and membrane
sodium/potassium adenosine 5′-triphosphate-ase activity,
inactivation of membrane sodium channels and other oxidative
modifications of proteins (9,10).
In addition, the overproduction of nitric oxide (NO) generated by
inducible NO synthase (iNOS) is also characterized in intestinal
I/R, which aggravates intestinal oxidative stress (11). The nuclear factor (NF)-κB pathway
has been reported to play an important role in intestinal I/R which
is responsible for ROS accumulation and cell apoptosis (12). Moreover, oxidative stress promotes
inflammatory cell infiltration and mitochondrial dysfunction. Thus,
the therapeutic strategy of removing free radicals and reducing NO
overproduction may be potentially effective for protection against
intestinal I/R injury.
However, to date, there is still lack of effective
therapeutic treatments for intestinal I/R injury. Osthole, a
natural derivative of coumarin, is an active constituent extracted
from some medicinal plants (13)
which has been shown to exert a variety of pharmacological and
therapeutic effects. Accumulating evidence has demonstrated that
osthole possesses a variety of pharmacological properties,
including antitumor (14),
anti-osteoporotic (15),
anti-diabetic (16),
anti-inflammatory (17) and
anti-allergic properties (18).
Recently, osthole has been demonstrated to exert therapeutic
effects against cerebral ischemic stroke (19,20) and cardiac hypertrophy in rats
(21). Thus, the effects of
osthole on intestinal I/R injury need to be further
investigated.
The aim of this study was to investigate the effect
of osthole in a mouse model of intestinal I/R and delineate the
underlying mechanisms. Using a mouse model, we first observed that
pre-treatment with osthole exerted protective effects on mice with
intestinal I/R injury in a dose-dependent manner. Pre-treatment
with osthole markedly ameliorated villus height and attenuated the
destruction of epithelial cells within the villi. The effect of
osthole against intestinal I/R injury was evaluated by measuring
the levels of myeloperoxidase (MPO), malondialdehyde (MDA),
superoxide dismutase (SOD) and glutathione (GSH). Pre-treatment
with osthole exerted protective effects, including the suppression
of oxidative stress, neutrophil infiltration and NO levels.
Increased IκBα phosphorylation and NF-κB nuclear translocation
induced by I/R injury were also significantly decreased following
pre-treatment with osthole. In this study, the effects of osthole
in the early phase following intestinal I/R injury in mice were
investigated to provide a mechanistic basis for the protective
effects of osthole against intestinal I/R injury.
Materials and methods
Animals and experimental groups
Adult male BALB/c mice were obtained from the
Laboratory Animal Center of the Fourth Military Medical University,
Xi’an, China and housed under standard conditions. They were kept
in cages at a fairly constant room temperature and were exposed to
a 12/12 h light/dark cycle. Each animal was fasted overnight prior
to surgery, but had access to water ad libitum. All animal
experiments were performed in accordance with the Prevention of
Cruelty to Animals Act 1986, under the guidelines of the National
Health and Medical Research Council for the Care and Use of Animals
for Experimental Purposes in China.
The mice were randomly divided into 3 groups (n=13
per group): i) Sham group: all the surgical steps were performed;
however, intestinal I/R was not induced. The animals were kept
under anesthesia for the duration of the intestinal I/R procedure.
ii) I/R group: intestinal I/R was induced and this group served as
the control for the osthole-treated group. iii) Osthole group: a
single dose of osthole (50 mg/kg daily for 3 days) was injected via
the tail vein. Osthole (>98% purity) was a gift from Dr W. Liu
(Department of Neurology, Xijing Hospital, Fourth Military Medical
University, Xi’an, China). The mice in each group were subdivided
into 3 subgroups. The first subgroup was used for histological
examination and immunofluorescence staining (n=4 per group); the
second subgroup was used for detecting MPO, GSH, SOD, MDA and NO
levels (n=6 per group); and the third subgroup was used for western
blot analysis (n=3 per group).
Model of intestinal I/R injury
Following an acclimation period of at least 3 days,
the mice were prepared for surgery under deep sodium pentobarbital
anesthesia. All procedures were performed with the animals
breathing spontaneously and body temperature was maintained at 37°C
using a water-circulating heating pad. The animals were subjected
to I/R as previously described (22). Briefly, a midline laparotomy was
performed. The superior mesenteric artery was identified and
isolated, and the blood supply to the intestine was interrupted for
1 h by occlusion of the superior mesenteric artery using a
microvascular clamp. Ischemia was confirmed by the loss of
pulsation of the mesenteric artery and its branches or a pale color
of the intestine. Following the ischemic phase, the clamp was
removed to allow the restoration of the blood flow (reperfusion)
for 6 h, which was confirmed by the return of the pulses,
re-establishment of the pink color and enhanced intestinal
peristalsis. Once reperfusion was secured, the wound was sutured
and the animal was allowed to awaken from the anesthesia. The mice
were administered an overdose of pentobarbital prior to
euthanasia.
Histology and tissue injury scoring
For isolation of the intestinal segments, tissue
from the entire intestine was cut into 6 equal segments, of which a
section of 1–1.5 cm at the middle of each segment was collected for
histological analysis. Formalin-fixed and paraffin-embedded tissue
sections were cut (4-μm-thick) and stained with hematoxylin
and eosin for histological examination. The above procedure was
performed by a physician.
Intestinal sections from each animal were scored for
mucosal damage as previously described (23). Briefly, a score of 0 was assigned
to a normal villus; villi with tip distortion were scored as 1;
villi lacking goblet cells and containing Guggenheims’ spaces were
scored as 2; villi with patchy disruption of the epithelial cells
were scored as 3; villi with exposed but intact lamina propria and
epithelial cell sloughing were assigned a score of 4; villi in
which the lamina propria was exuding were scored as 5; and finally,
villi displaying hemorrhage or denudation were scored as 6. All
histological analyses were performed by a pathologist in a blinded
manner. Five random high-power fields (×200) were examined per
sample.
MPO assay
The intestinal samples were weighed and homogenized
in a solution prepared from the assay kit (Jiancheng Institute of
Biotechnology, Nanjing, China), and homogenates were obtained and
used for MPO assay. The reaction was initiated by adding hydrogen
peroxide in the medium and 3,3,5,5-tetramethylbenzidine was used as
an oxidizable dye to produce yellow color compounds. The optical
density was recorded at 460 nm and the results were expressed as
U/g wet tissue.
Detection of MDA, GSH and SOD in
intestinal tissue
The intestinal samples were homogenized in 0.05
mol/l phosphate buffer. The homogenates were centrifuged at 4,000
rpm for 20 min at 4°C and the MDA, GSH and SOD content in the
supernatant was measured using the corresponding kits (Jiancheng
Institute of Biotechnology).
SOD activity and MDA levels were measured using the
xanthine oxidase and thiobarbituric acid method, respectively. SOD
activity was measured at 500 nm. One SOD activity unit was defined
as the enzyme amount causing 50% inhibition in 1 ml reaction
solution/mg tissue protein and the result was expressed as U/mg
protein. MDA was determined at 532 nm and the results were
expressed as nmol/mg protein. GSH levels were measured through a
reaction using dithiobisnitrobenzoic acid. GSH activity was
determined at 420 nm and the results were expressed as mg/g
protein.
NO synthesis can contribute to nitrite accumulation.
The concentration of nitrite in the intestinal samples was measured
following a reaction with Griess reagent (sulfanilamide 1%,
naphthylethylene diamine 0.01%, H3PO4 5%) to
reflect the production of NO at 550 nm. The results were expressed
as μmol/g protein. Detailed procedures for the above
measurements were performed according to the instructions of kit.
The protein content was determined using a kit based on the
Bradford assay and bovine serum albumin was used as a standard.
Western blot analysis
Total protein lysates from frozen tissues were
prepared in ice-cold RIPA buffer (20 mM HEPES pH 7.5, 150 mM NaCl,
1 mM EDTA, 10% glycerol, 0.5% sodium deoxycholate, 1% Nonidet P-40,
0.1% SDS and protease inhibitor cocktails). Nuclear extracts were
prepared using a Nuclear Protein Extraction kit (Beyotime
Biotechnology, Shanghai, China). Protein samples were immunoblotted
with antibodies to IκB, p-IκB and NF-κB-p65 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), and the protein-antibody
immune complexes were detected with horseradish
peroxidase-conjugated secondary antibodies and enhanced
chemiluminescence reagents (Pierce Biotechnology, Rockford, IL,
USA). For detection, an ECL chemiluminescence system (GE
Healthcare, Piscataway, NJ, USA) was used. Signals were detected
and quantified using the LAS-3000 image analyzer (FujiFilm, Tokyo,
Japan).
Immunofluorescence staining
Staining was performed on slides of
4-μm-thick sections from intestinal tissue. Following
deparaffinization and dehydration, the slides were immersed in 10
mM sodium citrate buffer containing 0.05% Tween-20 (pH 6.0) for 5
min at 55°C for antigen retrieval. The slides were then
permeabilized with 10 μg/ml proteinase K in 10 mM Tris/HCl
(pH 7.6) for 15 min and incubated with 5% BSA in a humidified
chamber for 30 min to block non-specific binding. The slides were
incubated with antibody against NF-κB-p65 (1:200 in PBS containing
1% BSA; Santa Cruz Biotechnology, Inc.) in a humidified chamber
overnight at 4°C, and with fluorescent-fluorescein
isothiocyanate-conjugated donkey anti-rabbit immunoglobulin G
(1:500 in PBS) at room temperature for 1 h. The nuclei were
counterstained with Hoechst 33258 (1:5,000; 10 min). Fluorescence
imaging was assessed under a fluorescence microscope (Fluorescence
E-1000 microscopy, Nikon, Japan) and subjected to identical
exposure times. Instead of the primary antibody, PBS was used for
the negative controls.
Statistical analysis
Data were analyzed using SPSS v 11.5 software for
Windows (SPSS Inc, Chicago, IL, USA). All values are presented as
the means ± standard error of the mean (SEM). Differences between
groups were compared using analysis of variance with the Bonferroni
correction for multiple comparisons. Values of P<0.05 were
considered to indicate statistically significant differences.
Results
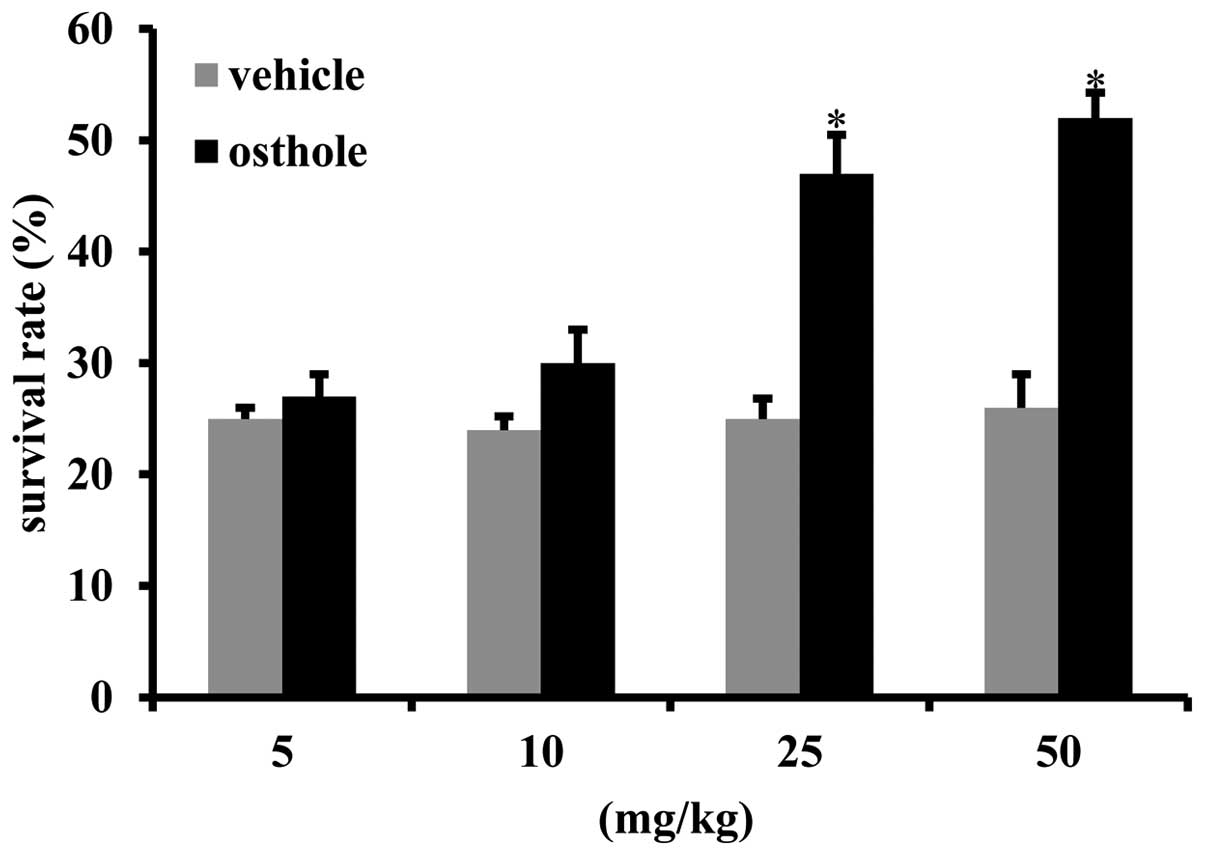
Osthole improves the survival rate of
mice subjected to intestinal I/R injury
In order to determine the effect of osthole on
intestinal I/R injury, the mice were administered various doses of
osthole (5, 10, 25, 50 mg/kg) for 3 days prior to the induction of
intestinal I/R injury. The results revealed that osthole
preconditioning (25 mg/kg and 50 mg/kg) prior to intestinal I/R
injury significantly improved the survival rate at 24 h following
reperfusion as compared to the vehicle-treated mice (Fig. 1).
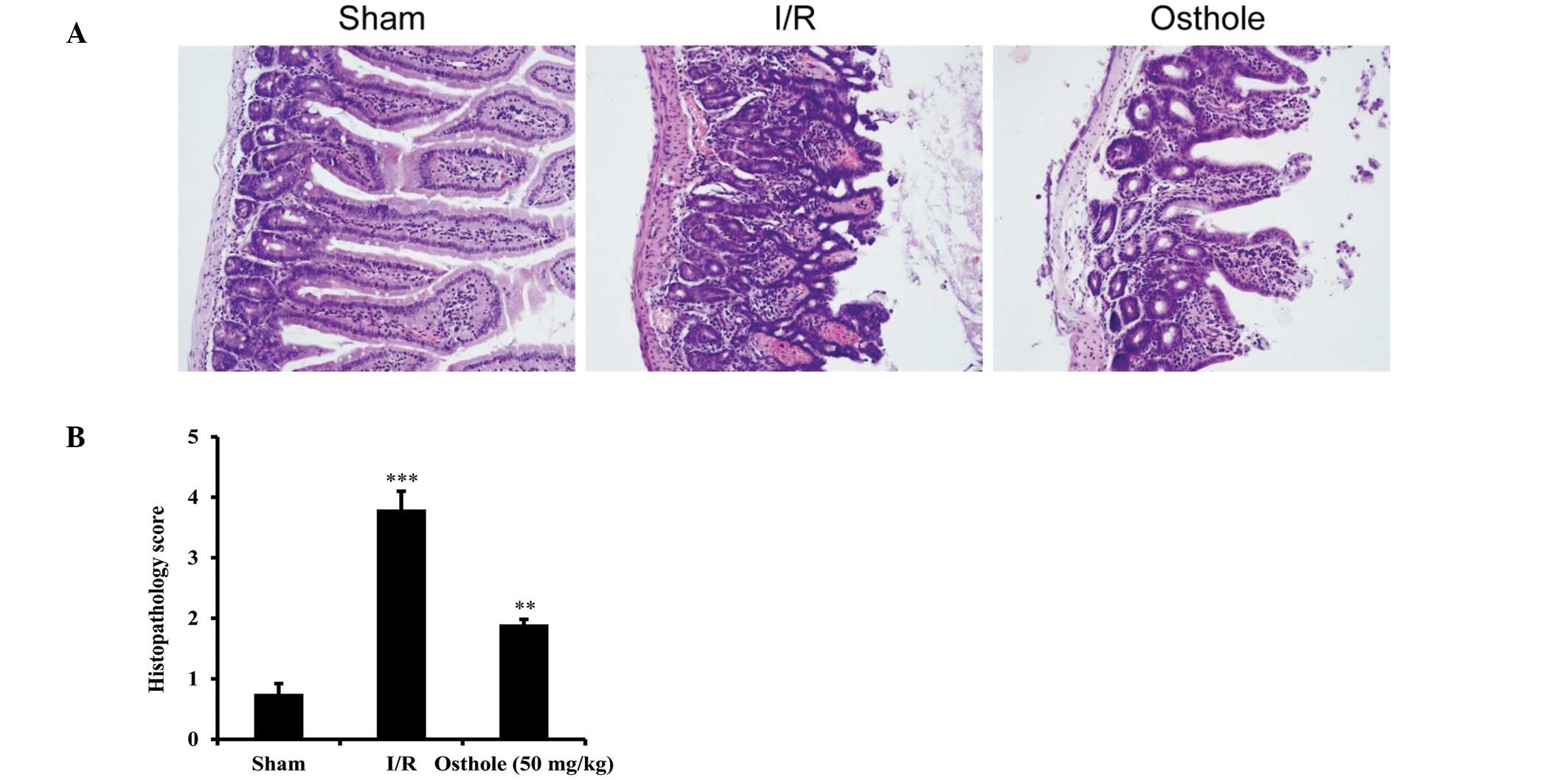
Osthole attenuates intestinal injury
induced by I/R
To further investigate the effect of osthole on
intestinal I/R injury, the morphology of the intestinal epithelium
and villi was examined by immunohistochemistry. In the sham group,
the results of histopathological examination of the intestinal
epithelium and villi were normal. Mucosal necrosis and bleeding
were observed in the intestinal mucosa and submucosa in the I/R
group which also showed a marked reduction in villus height and
destruction of epithelial cells within the villi. In the osthole
group, mice pre-treated with osthole (50 mg/kg) demonstrated
intestinal architecture with almost normal histological features.
Although the median intestinal injury score of the osthole group
was higher than the sham group (P<0.001), it was significantly
lower than that of the I/R group (P<0.01) (Fig. 2B). Taken together, these data
suggest that osthole exerts protective effects against intestinal
I/R injury.
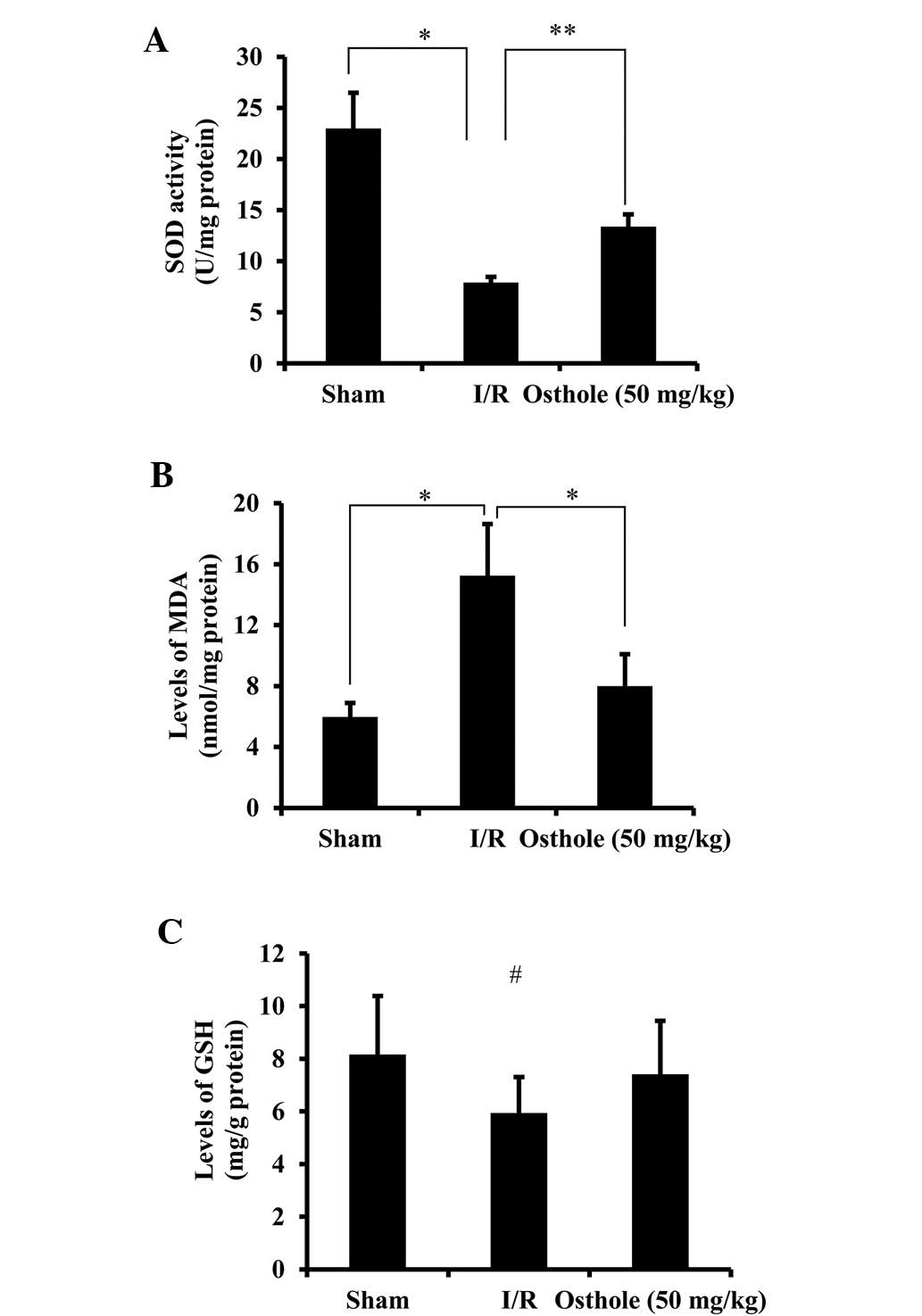
Osthole exerts antioxidant effects in
intestinal I/R injury
To elucidate the underlying mechanisms of action of
osthole in protecting against intestinal I/R injury, the
antioxidant and oxidant products were evaluated. The levels of SOD,
an enzyme occurring naturally in the body which protects cells by
cleaning up free radicals, were significantly reduced in the I/R
group compared with the sham group (P<0.05). Following
pre-treatment with osthole, SOD activity was significantly improved
compared with the I/R group (P<0.01) (Fig. 3A). The levels of MDA, an important
marker of lipoperoxidation associated with oxidative stress, were
decreased following pre-treatment with osthole (P<0.05 vs. I/R
group) (Fig. 3B). Following I/R
injury, the levels of GSH, a common antioxidant, were slightly
decreased; however, following pre-treatment with osthole, these
levels increased. However, the difference was not statistically
significant among the different groups (Fig. 3C). These results demonstrate that
osthole relieves oxidative stress through the upregulation of SOD
and the downregulation of MDA levels following intestinal I/R
injury.
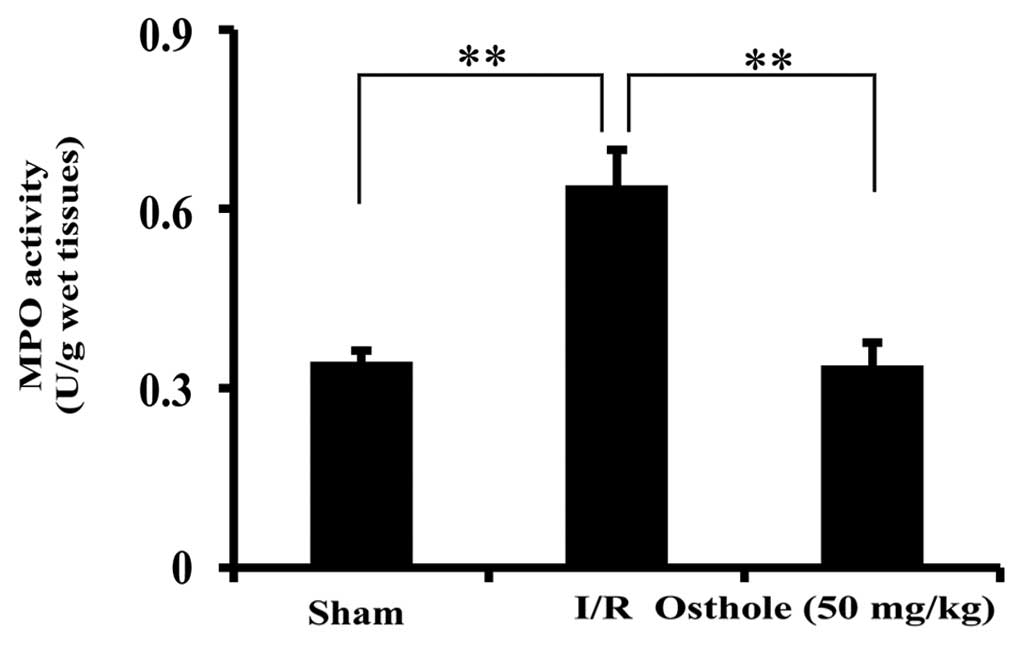
Osthole reduces neutrophil infiltration
following intestinal I/R injury
Tissue MPO activity is a hallmark of intestinal I/R
injury which reflects the infiltration of neutrophils. In order to
examine the effect of osthole on inflammation, the activity of MPO
was measured. Compared to the sham group, a significant increase in
MPO activity was observed in the I/R group, which was alleviated in
the osthole-treated group (P<0.01) (Fig. 4).
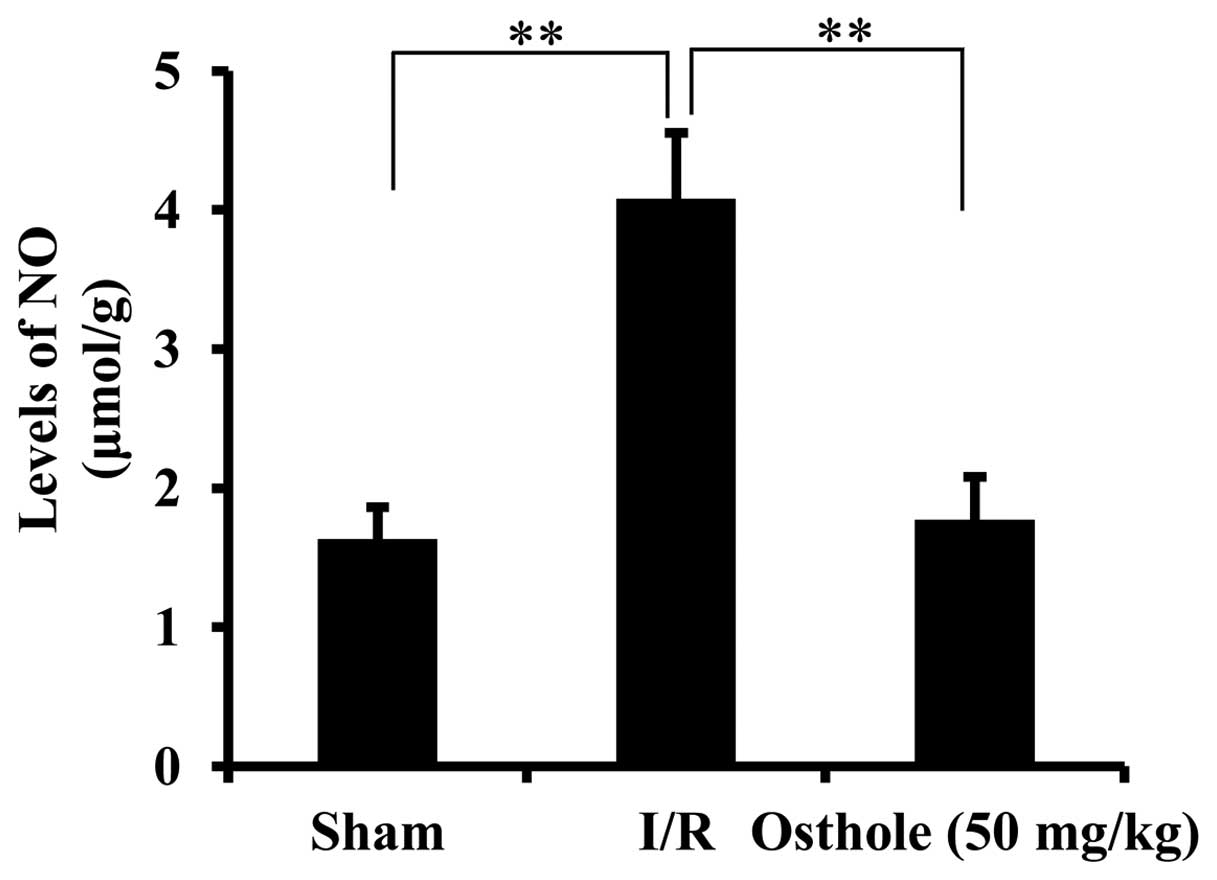
Osthole inhibits the elevation of NO
levels following intestinal I/R injury
The overproduction of NO following intestinal I/R
injury has been reported to be closely associated with oxidative
damage (11). NO synthesis can
contribute to nitrite accumulation. The concentration of nitrite in
the intestinal samples was measured to reflect the production of
NO. As shown in Fig. 5,
pre-treatment with osthole significantly inhibited the elevation of
NO levels in the I/R group (P<0.01).
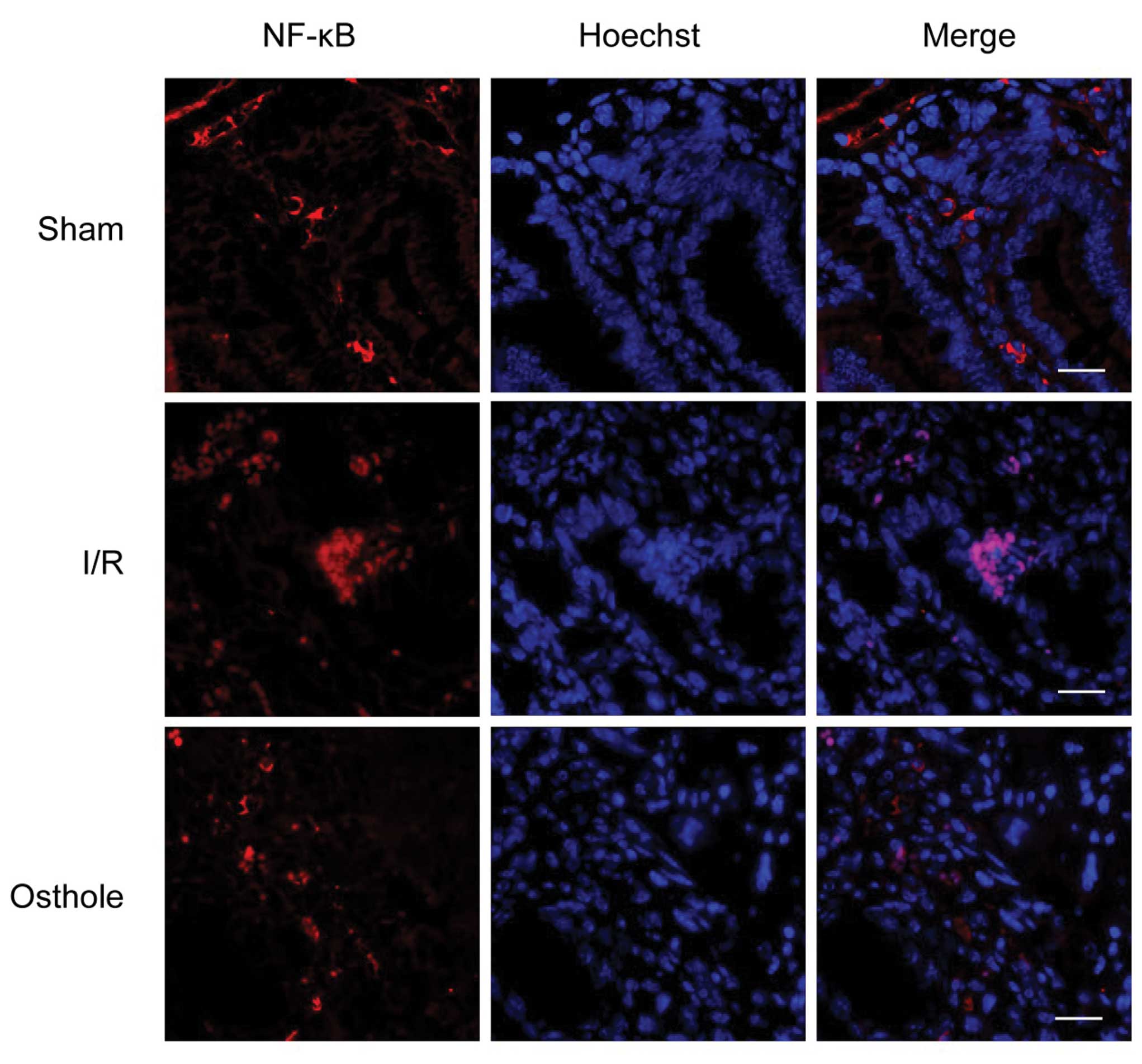
Osthole protects against intestinal I/R
injury by inhibitig the activation of the NF-κB signaling
pathway
It has been reported that NO produced by the iNOS
gene is induced during inflammation (24). NF-κB is an important nuclear
transcription factor that regulates iNOS gene expression by binding
the iNOS promoter region and plays a central role in inflammation
through its ability to induce the transcription of pro-inflammatory
genes (25). The data mentioned
above suggest that osthole reduces neutrophil infiltration and
inhibits the NO levels induced by intestinal I/R injury. Thus, we
hypothesized that osthole protects against intestinal I/R injury by
inhibiting the activation of the NF-κB signaling pathway. To
confirm our hypothesis, we examined the effects of osthole on NF-κB
nuclear translocation. In the sham group, NF-κB was localized in
the cytoplasm and translocated to the nucleus in the I/R group.
Pre-treatment with osthole inhibited the nuclear translocation of
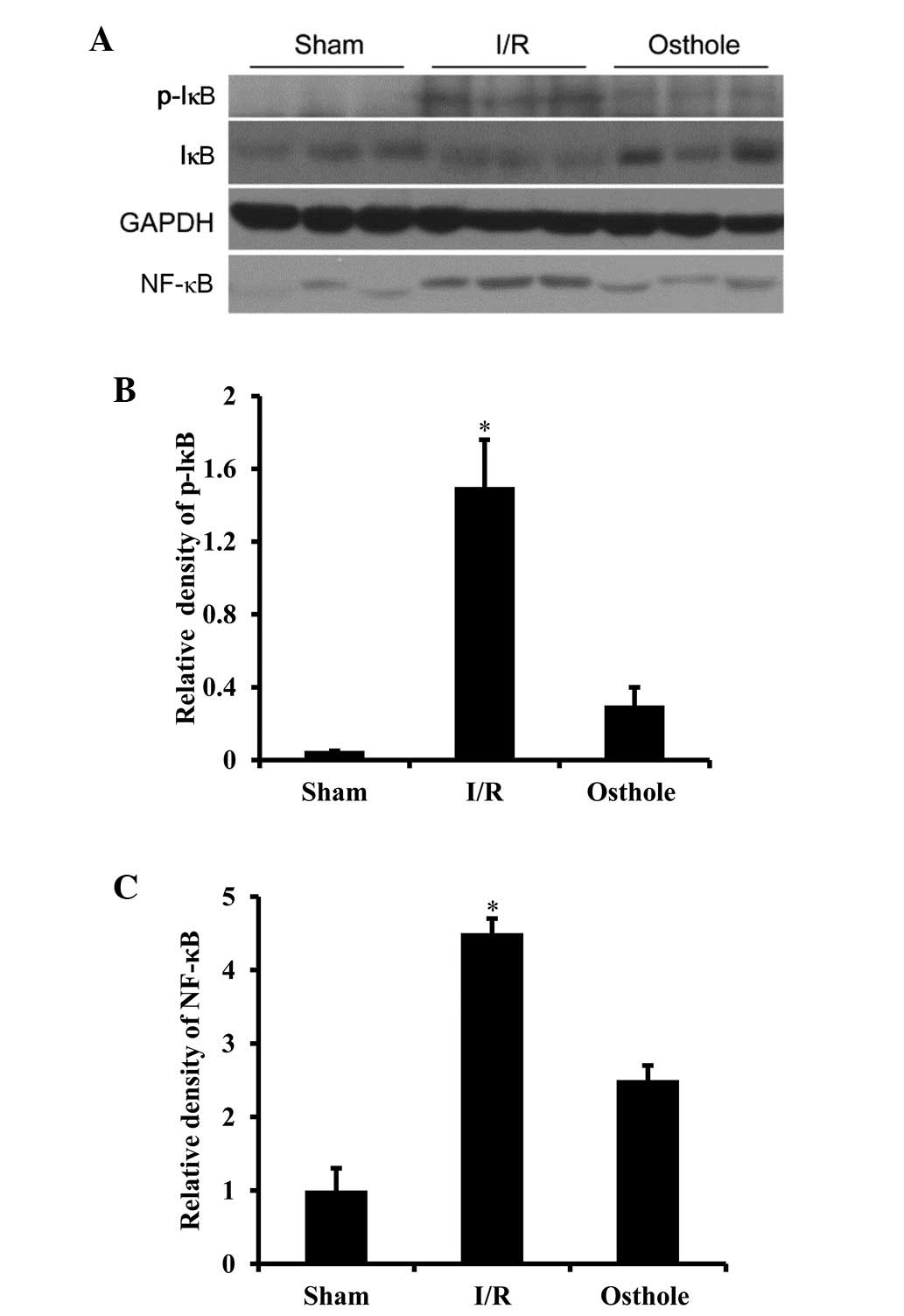
NF-κB compared with the I/R group (Fig. 6). In order to further confirm
these results, western blot analysis was performed to examine the
nuclear expression of NF-κB and the phosphorylation state of IκBα.
Consistent with the immunofluorescence staining results, we
observed that the nuclear expression of NF-κB was markedly
increased in the I/R group, while pre-treatment with osthole
inhibited the nuclear expression of NF-κB (P<0.01 vs. I/R
group). We also found that phosphorylated IκBα was increased in the
I/R group. In the osthole-treated group, phosphorylated IκBα was
significantly inhibited (P<0.01 vs. I/R group) (Fig. 7).
Discussion
Osthole, a Chinese herbal medicine possessing a
variety of pharmacological properties, has gained considerable
attention over the years and has been used for the treatment of a
number of diseases, including carcinoma (14), amnesia (26), metabolic syndromes (27,28), seizures (29) and osteoporosis (30). Recent studies have suggested that
osthole is also beneficial in preventing I/R injury. It has been
shown that osthole prevents motor impairment, decreases the levels
of NO, interleukin (IL)-1β and IL-8, inhibits iNOS and MPO
activity, and decreases infarct volume following middle cerebral
artery occlusion (31).
Similarly, it has been demonstrated that osthole significantly
inhibits neuronal cell death in the CA1 area in the hippocampus by
decreasing caspase-3 protein expression in male SD rats subjected
to transient global brain ischemia (32). However, no studies have been
conducted on the role and potential mechanisms of action of osthole
in intestinal I/R injury. In the present study, we demonstrated
that pre-treatment with osthole exerted protective effects against
intestinal I/R injury. Pre-treatment with osthole improved the
survival rates of mice subjected to I/R injury and protected
intestinal epithelial cells and villi against I/R injury. Osthole
also suppressed stress, neutrophil infiltration and NO
overproduction. Furthermore, we found that the underlying
mechanisms of action of osthole in preventing intestinal I/R injury
involved the inhibition of NF-κB.
The production of free radicals from oxygen
molecules derived from the electron transport chains of
mitochondrial cells, endothelial cells and activated neutrophils
elicits oxidative stress and enhances intestinal I/R injury
(33). Thus, the antioxidant,
SOD, is considered to play a crucial role in relieving oxidative
stress during I/R injury. In this study, we found that
pre-treatment with osthole upregulated SOD activity which was
suppressed in the I/R group. Osthole also downregulated MDA levels
following intestinal I/R injury, which are a marker of
lipoperoxidation associated with oxidative stress. These results
are in agreement with those from previous reports demonstrating
that osthole is effective in treating hyperlipidemic and alcoholic
fatty liver by the inhibition of ROS production, the enhancement of
antioxidative enzyme activity and the reduction of peroxidation
(34,35). In addition, it was found that the
neuroprotective effects of osthole against acute ischemic stroke
and 1-methyl-4-phenylpyridinium ion-induced cytotoxicity in PC12
cells is partly attributed to its antioxidative action (36). Neutrophil infiltration has been
proposed as a hallmark of intestinal I/R injury. The activity of
MPO which is mainly released by neutrophils can be evaluated as
neutrophil infiltration (11). In
our study, MPO activity was significantly augmented in the I/R
group and was decreased following pre-treatment with osthole.
Furthermore, the involvement of NO in intestinal I/R injury has
been widely suggested. iNOS can produce excessive amounts of NO
following intestinal I/R injury, which is considered responsible
for the cytotoxic potential of NO, resulting in oxidative stress
and tissue damage (11,37,38). A previous study demonstrated that
osthole decreased NO levels and inhibited NOS activity in a rat
model of carrageenan-induced hind paw edema (17). In the current study, we found that
osthole markedly inhibited the increased NO levels induced by
intestinal I/R injury. These results suggest that osthole exerts
therapeutic effects against intestinal I/R injury by attenuating
oxidative stress, reducing excessive neutrophil infiltration and
modulating NO levels.
However, the mechanisms of action of osthole in
exerting its protective effects remain to be elucidated. The iNOS
promoter region contains binding sites for NF-κB, and its
expression during I/R injury requires de novo transcription,
which is under the control of NF-κB. NF-κB is usually present in
the cytoplasm in an inactive form bound to the inhibitory protein,
I-κB. NF-κB is activated by a variety of stimuli, including growth
factors, cytokines, oxidative stress and pro-inflammatory stimuli,
and the nuclear translocation of NF-κB subunits is induced
following intestinal I/R injury (39,40). It has been reported that iNOS is
capable of producing abundant NO under certain pathological
conditions, which presents beneficial effects but also contributes
to the overproduction of cytotoxic radicals (41). In a previous study, it was shown
that osthole, isolated from Clausena guillauminii, inhibited
the protein expression of iNOS induced by LPS in mouse macrophages
(42). Another study also
demonstrated that osthole reduced tumor necrosis factor (TNF)-α, NO
and cyclooxygenase (COX)-2 expression, inhibited NF-κB activation
and ROS release in LPS-stimulated macrophages (43). In this study, pre-treatment with
osthole inhibited the translocation of NF-κB to the nucleus and
increased I-κB phosphorylation induced by intestinal I/R injury.
These data suggest that osthole exerts protective effects against
intestinal I/R injury through the NF-κB-iNOS-NO pathway. Together
with the free radical scavenging and anti-inflammatory effects,
osthole may be a potential candidate for the development of
anti-ischemic drugs.
Certain limitations of this study should be noted.
Intestinal IR injury has been extensively investigated in animal
models and various approaches to diminish the damaging effects of
intestinal I/R injury have been investigated in animal models, as
well as in vitro (44,45). However, these models are not a
realistic model of the clinical situation and there are a number of
obvious differences between animal models and humans during
intestinal I/R injury, including antigens of specific proteins and
sensitivity to intestinal ischemia and inflammatory responses
(46–48). Moreover, advances in the clinical
treatment of I/R injury have been minimal. Pre-teatment with
osthole prior to ischemia in this study may not be a realistic
model of the clinical situation. The mice were pre-treated with
osthole prior to the induction of ischemia. Additionally, it was
confirmed that osthole preconditioning relieved cerebral ischemic
stroke (19). Therefore, although
a recent study confirmed that treatment with osthole subsequent to
ischemia significantly improved the cognitive deficits induced by
chronic cerebral hypoperfusion in rats (49), it can only be speculated whether
or not osthole postconditioning would also attenuate intestinal I/R
injury. To clarify this important point, treatment with osthole at
different starting points following the onset of intestinal
ischemia or reperfusion should be investigated.
Taken together, the data from our study demonstrate
that pre-treatment with osthole attenuates oxidative stress,
reduces neutrophil infiltration and decreases NO levels. The
mechanisms behind the protective effects of osthole involve the
inhibition of NF-κB nuclear translocation. Thus, the present study
suggests that osthole exerts protective and therapeutic effects
against intestinal I/R injury.
Acknowledgements
This study was supported by the
National Natural Science Foundation of Cultivating Projects of the
First Affiliated Hospital of Anhui Medical University (grant no.
2009KJ12) and the Anhui Province Natural Science Foundation (grant
no. 11040606Q21). The authors would like to thank Dr Xiao-Dong Cao
and Dr Wen-Bo Liu for their technical assistance.
References
|
1
|
Yasuhara H: Acute mesenteric ischemia: the
challenge of gastroenterology. Surg Today. 35:185–195. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berland T and Oldenburg WA: Acute
mesenteric ischemia. Curr Gastroenterol Rep. 10:341–346. 2008.
View Article : Google Scholar
|
|
3
|
Ozacmak VH, Sayan H, Arslan SO, Altaner S
and Aktas RG: Protective effect of melatonin on contractile
activity and oxidative injury induced by ischemia and reperfusion
of rat ileum. Life Sci. 76:1575–1588. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shibata C, Balsiger BM, Anding WJ, Duenes
JA, Miller VM and Sarr MG: Functional changes in nonadrenergic,
noncholinergic inhibitory neurons in ileal circular smooth muscle
after small bowel transplantation in rats. Dig Dis Sci.
43:2446–2454. 1998. View Article : Google Scholar
|
|
5
|
Blikslager AT, Moeser AJ, Gookin JL, Jones
SL and Odle J: Restoration of barrier function in injured
intestinal mucosa. Physiol Rev. 87:545–564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szabo A, Vollmar B, Boros M and Menger MD:
In vivo fluorescence microscopic imaging for dynamic quantitative
assessment of intestinal mucosa permeability in mice. J Surg Res.
145:179–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santora RJ, Lie ML, Grigoryev DN, Nasir O,
Moore FA and Hassoun HT: Therapeutic distant organ effects of
regional hypothermia during mesenteric ischemia-reperfusion injury.
J Vasc Surg. 52:1003–1014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vasileiou I, Kostopanagiotou G,
Katsargyris A, Klonaris C, Perrea D and Theocharis S: Toll-like
receptors: a novel target for therapeutic intervention in
intestinal and hepatic ischemiareperfusion injury? Expert Opin Ther
Targets. 14:839–853. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shah PC, Brolin RE, Amenta PS and Deshmukh
DR: Effect of aging on intestinal ischemia and reperfusion injury.
Mech Ageing Dev. 107:37–50. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Esposito E and Cuzzocrea S: Role of
nitroso radicals as drug targets in circulatory shock. Br J
Pharmacol. 157:494–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barocelli E, Ballabeni V, Ghizzardi P, et
al: The selective inhibition of inducible nitric oxide synthase
prevents intestinal ischemia-reperfusion injury in mice. Nitric
Oxide. 14:212–218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakano H, Nakajima A, Sakon-Komazawa S,
Piao JH, Xue X and Okumura K: Reactive oxygen species mediate
crosstalk between NF-kappaB and JNK. Cell Death Differ. 13:730–737.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoult JR and Paya M: Pharmacological and
biochemical actions of simple coumarins: natural products with
therapeutic potential. Gen Pharmacol. 27:713–722. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chou SY, Hsu CS, Wang KT, Wang MC and Wang
CC: Antitumor effects of osthole from Cnidium monnieri: an
in vitro and in vivo study. Phytother Res. 21:226–230. 2007.
View Article : Google Scholar
|
|
15
|
Zhang Q, Qin L, He W, et al: Coumarins
from Cnidium monnieri and their antiosteoporotic activity.
Planta Med. 73:13–19. 2007.PubMed/NCBI
|
|
16
|
Liang HJ, Suk FM, Wang CK, et al: Osthole,
a potential antidiabetic agent, alleviates hyperglycemia in db/db
mice. Chem Biol Interact. 181:309–315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Zhang W, Zhou L, Wang X and Lian Q:
Anti-inflammatory effect and mechanism of osthole in rats. Zhong
Yao Cai. 28:1002–1006. 2005.PubMed/NCBI
|
|
18
|
Chiu PR, Lee WT, Chu YT, Lee MS, Jong YJ
and Hung CH: Effect of the Chinese herb extract osthole on
IL-4-induced eotaxin expression in BEAS-2B cells. Pediatr Neonatol.
49:135–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chao X, Zhou J, Chen T, et al:
Neuroprotective effect of osthole against acute ischemic stroke on
middle cerebral ischemia occlusion in rats. Brain Res.
1363:206–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li F, Gong Q, Wang L and Shi J: Osthole
attenuates focal inflammatory reaction following permanent middle
cerebral artery occlusion in rats. Biol Pharm Bull. 35:1686–1690.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou F, Zhong W, Xue J, Gu ZL and Xie ML:
Reduction of rat cardiac hypertrophy by osthole is related to
regulation of cardiac oxidative stress and lipid metabolism.
Lipids. 47:987–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Souza DG, Soares AC, Pinho V, et al:
Increased mortality and inflammation in tumor necrosis
factor-stimulated gene-14 transgenic mice after ischemia and
reperfusion injury. Am J Pathol. 160:1755–1765
|
|
23
|
Fleming SD, Monestier M and Tsokos GC:
Accelerated ischemia/reperfusion-induced injury in
autoimmunity-prone mice. J Immunol. 173:4230–4235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nathan C: Nitric oxide as a secretory
product of mammalian cells. FASEB J. 6:3051–3064. 1992.PubMed/NCBI
|
|
25
|
Tak PP and Firestein GS: NF-kappaB: a key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsieh MT, Hsieh CL, Wang WH, Chen CS, Lin
CJ and Wu CR: Osthole improves aspects of spatial performance in
ovariectomized rats. Am J Chin Med. 32:11–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Xie ML, Zhu LJ and Gu ZL:
Therapeutic effect of osthole on hyperlipidemic fatty liver in
rats. Acta Pharmacol Sin. 28:398–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Xie ML, Xue J and Gu ZL: Osthole
regulates enzyme protein expression of CYP7A1 and DGAT2 via
activation of PPARalpha/gamma in fat milk-induced fatty liver rats.
J Asian Nat Prod Res. 10:807–812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luszczki JJ, Andres-Mach M, Cisowski W,
Mazol I, Glowniak K and Czuczwar SJ: Osthole suppresses seizures in
the mouse maximal electroshock seizure model. Eur J Pharmacol.
607:107–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li XX, Hara I and Matsumiya T: Effects of
osthole on postmenopausal osteoporosis using ovariectomized rats;
comparison to the effects of estradiol. Biol Pharm Bull.
25:738–742. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He W, Liu JX, Zhou YM, et al: Protective
effects of osthole on cerebral ischemia-reperfusion injury in rats
and its mechanism. Chin Pharmacol Bull. 24:1528–1530. 2008.
|
|
32
|
Liu WB, Huo JL, Fei Z, Men XL and Li J:
Neuroprotection of osthole against transient global brain ischemia
in rats. Chin J Neurosurg Dis Res. 8:118–121. 2009.
|
|
33
|
Flessas II, Papalois AE, Toutouzas K,
Zagouri F and Zografos GC: Effects of lazaroids on intestinal
ischemia and reperfusion injury in experimental models. J Surg Res.
166:265–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song F, Xie ML, Zhu LJ, Zhang KP, Xue J
and Gu ZL: Experimental study of osthole on treatment of
hyperlipidemic and alcoholic fatty liver in animals. World J
Gastroenterol. 12:4359–4363. 2006.PubMed/NCBI
|
|
35
|
Sun F, Xie ML, Xue J and Wang HB: Osthole
regulates hepatic PPAR alpha-mediated lipogenic gene expression in
alcoholic fatty liver murine. Phytomedicine. 17:669–673. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu WB, Zhou J, Qu Y, et al:
Neuroprotective effect of osthole on MPP+-induced
cytotoxicity in PC12 cells via inhibition of mitochondrial
dysfunction and ROS production. Neurochem Int. 57:206–215.
2010.PubMed/NCBI
|
|
37
|
Naito Y, Takagi T, Ichikawa H, et al: A
novel potent inhibitor of inducible nitric oxide inhibitor,
ONO-1714, reduces intestinal ischemia-reperfusion injury in rats.
Nitric Oxide. 10:170–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suzuki Y, Deitch EA, Mishima S, Lu Q and
Xu D: Inducible nitric oxide synthase gene knockout mice have
increased resistance to gut injury and bacterial translocation
after an intestinal ischemia-reperfusion injury. Crit Care Med.
28:3692–3696. 2000. View Article : Google Scholar
|
|
39
|
Zou L, Attuwaybi B and Kone BC: Effects of
NF-kappa B inhibition on mesenteric ischemia-reperfusion injury. Am
J Physiol Gastrointest Liver Physiol. 284:G713–G721. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Souza DG, Vieira AT, Pinho V, et al:
NF-kappaB plays a major role during the systemic and local acute
inflammatory response following intestinal reperfusion injury. Br J
Pharmacol. 145:246–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Altavilla D, Squadrito F, Campo GM, et al:
The lazaroid, U-74389G, inhibits inducible nitric oxide synthase
activity, reverses vascular failure and protects against endotoxin
shock. Eur J Pharmacol. 369:49–55. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nakamura T, Kodama N, Arai Y, et al:
Inhibitory effect of oxycoumarins isolated from the Thai medicinal
plant Clausena guillauminii on the inflammation mediators,
iNOS, TNF-alpha, and COX-2 expression in mouse macrophage RAW
264.7. J Nat Med. 63:21–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liao PC, Chien SC, Ho CL, et al: Osthole
regulates inflammatory mediator expression through modulating
NF-kappaB, mitogen-activated protein kinases, protein kinase C, and
reactive oxygen species. J Agric Food Chem. 58:10445–10451. 2010.
View Article : Google Scholar
|
|
44
|
Duggan M, Engelberts D, Jankov RP, et al:
Hypocapnia attenuates mesenteric ischemia-reperfusion injury in a
rat model. Can J Anaesth. 52:262–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Spanos CP, Papaconstantinou P, Spanos P,
Karamouzis M, Lekkas G and Papaconstantinou C: The effect of
L-arginine and aprotinin on intestinal ischemia-reperfusion injury.
J Gastrointest Surg. 11:247–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bjorck M, Bergqvist D, Rasmussen I, Piehl
E and Haglund U: An experimental porcine model of partial ischaemia
of the distal colon. Eur J Surg. 163:843–850. 1997.PubMed/NCBI
|
|
47
|
Grootjans J, Lenaerts K, Derikx JP, et al:
Human intestinal ischemia-reperfusion-induced inflammation
characterized: experiences from a new translational model. Am J
Pathol. 176:2283–2291. 2010. View Article : Google Scholar
|
|
48
|
Robinson JW, Mirkovitch V, Winistorfer B
and Saegesser F: Response of the intestinal mucosa to ischaemia.
Gut. 22:512–527. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ji HJ, Hu JF, Wang YH, Chen XY, Zhou R and
Chen NH: Osthole improves chronic cerebral hypoperfusion induced
cognitive deficits and neuronal damage in hippocampus. Eur J
Pharmacol. 636:96–101. 2010. View Article : Google Scholar : PubMed/NCBI
|