Introduction
Antioxidant compounds are reducing agents that
arrest the progression of chain reaction by scavenging the free
radicals and protect cells from undergoing degeneration (1). Nevertheless, a powerful antioxidant
enzyme network exists in all aerobic organisms that deals with
reactive oxygen species (ROS), including hydroxyl radicals (OH),
and superoxide anions (O2−). Antioxidant
enzymes such as Cu/Zn superoxide dismutase (SOD), Mn-SOD, catalase,
glutathione peroxidase (GPx) and glutathione (GSH) protect cells by
catalyzing the perilous ROS into less hazardous compounds (2). Apart from the endogenous antioxidant
system, antioxidants derived from plant sources also play a key
role in maintaining the equilibrium between cellular ROS production
and internal defense against oxidative stress (1,2).
Flavonoids are naturally occurring polyphenols found
ubiquitously in various fruits, leaves and seeds (3). Rutin, a bioflavonoid compound, is a
glycoside derivative of quercetin, a polyphenol well known for its
anticancer (4), anti-inflammatory
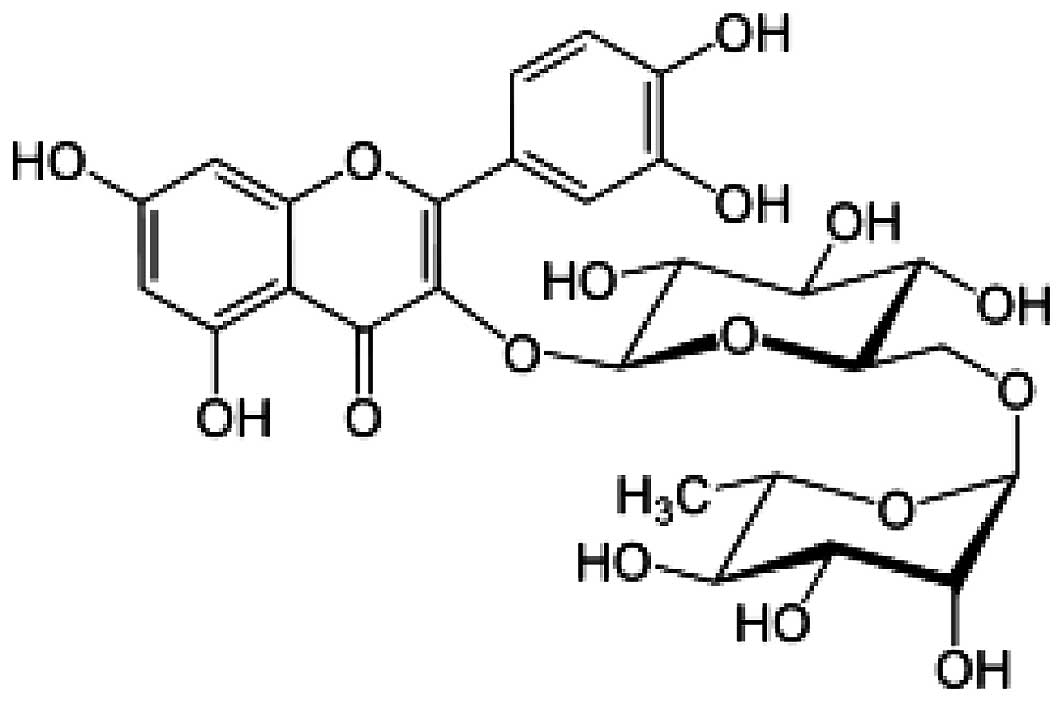
(5) anti-viral (6) and antioxidant (7) effects. The chemical structure of
rutin (Fig. 1) resembles that of
quercetin, with the exception that the hydrogen atom on the right
side in quercetin is replaced by the disaccharide rutinose
(rhamnose and glucose) molecule in rutin and is also known as
quercetin-3-O-rutinoside (8).
Rutin is widely found in citrus fruits and the rinds of grapes and
lime, in berries, including cranberries and mulberries (9), as well as buckwheat and asparagus
(8). Although many studies have
proven the neuroprotective role of quercetin, attention to rutin
has been lacking. Rutin has disaccharide sugar molecules as the
side chain (Fig. 1). Therefore,
rutin may exhibit improved antioxidant properties as well as
greater bioavailability potential as compared to quercetin
(10).
Although evidence suggests an association between
rutin and its neuroprotective activity, the antioxidant mechanism
of rutin is not well established. Thus, the objective of this study
was to establish the neuroprotective role of rutin as well as to
elucidate its antioxidant mechanism by specifically observing its
role in altering the natural antioxidant enzyme network in
6-hydroxydopamine (6-OHDA)-induced neurotoxicity in
pheochromocytoma (PC-12) neuronal cells. PC-12 cells are commonly
used in the investigation of neurotherapeutics study for
Parkinson’s disease (PD). PC-12 cells are known to secrete dopamine
neurotransmitters and contain high amounts of dopamine
transporters. In this experimental model, 6-OHDA (a hydroxylated
analogue of dopamine) was used to induce neurodegeneration.
6-OHDA-induced neuronal death provides the more comparable event of
PD as in human brains (11).
Materials and methods
Materials
PC-12 cells were purchased from ATCC (#CRL-1721.1
PC-12 ADH, Rattus norvegicus, Manassas, VA, USA). 6-OHDA,
rutin, poly-L-lysine, MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], and
dimethyl sulphoxide (DMSO) were purchased from Sigma Aldrich (St.
Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM),
pen-strep, horse serum, and fetal bovine serum (FBS) were purchased
from Gibco (Carlsbad, CA, USA). Antioxidant enzyme kits, GPx SOD,
catalase, thiobarbiturate, and GSH assay kits were purchased from
Cayman Chemicals.
Cell culture
PC-12 cells were grown in a humidified incubator
with 5% CO2 at a temperature of 37°C in DMEM medium
supplemented with 5% horse serum and 5% FBS and pen-strep (100
U/ml). The cells were cultured in poly-L-lysine-coated T-75 culture
flasks. The cells used in the experiments were taken between
passages 3 and 8, as cells become clumpy and difficult to
singularize after passage 10. When the cells were 60% confluent,
they were dislodged from the flask using cell scraper.
Subsequently, the cells were dispersed by vigorous pipetting in and
out for several times. The dispersed cells were plated on a
poly-L-lysine-coated 96-well microplate at a density of
1×105 cells/ml and incubated overnight in order to
facilitate cell adhesion to the substrate. The cells were then
cultured for 4, 8 and 12 h in the presence of rutin at 10, 50 and
100 μM. Subsequently, the pretreated cells were induced
using 6-OHDA for 24 h and assayed for its antioxidant activities.
Control cells were not treated with rutin or 6-OHDA, while positive
control cells were treated with 6-OHDA only. Rutin was dissolved in
DMSO and then diluted with complete culture media. A concentration
of DMSO in the final culture media of <0.05% had no protective
or damaging effects on PC-12 cells.
Preparation of 6-OHDA stock solution
6-OHDA-HBr (1 mg) was dissolved in 2 ml of chilled
0.15% of ascorbic acid to produce up to 2 mM of stock solution. The
0.15% of chilled ascorbic acid arrested the oxidation of
6-OHDA-HBr. The tube used to dissolve 6-OHDA was covered with
aluminium foil to protect the stock solution from intense light
exposure. The stock solution was subsequently filtered using a 0.2
μm syringe filter and diluted to the desired concentration
using complete culture media.
Determination of cell viability
The cytotoxicity effect of rutin on 6-OHDA-induced
PC-12 was determined using MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay. MTT, a yellow tetrazole, was reduced to insoluble purple
formazan in the mitochondria of viable cells. The insoluble purple
formazan was dissolved using a solubilizing solvent and the colored
solution was measured at 570 nm using a microplate reader.
Following the incubation of PC-12 cells with different
concentrations of rutin at different time points, 10 μl of
MTT (5 mg/ml) was added to each well and incubated for 4 h. The
supernatant was removed, and 100 μl DMSO was added to
solubilize the insoluble purple formazan. The absorbance was read
at 570 nm using the Opsys microplate reader (DYNEX Technologies,
Chantilly, VA, USA). Data on cell viability were expressed as a
percentage of the surviving control cells in the study.
Biochemical analysis
Each sample of treated and untreated PC-12 cells was
collected using cold phosphate-buffered saline (PBS) by
centrifugation at 4°C at the specific centrifugation speed required
by each enzyme kit. The cell pellet was homogenized using cold
buffer and the clear supernatant was obtained for the measurements
of GSH, GPx, SOD, and catalase and malondialdehyde (MDA)
activities.
GSH assay
The sulfhydryl group of GSH in the sample reacted
with DTNB [5,5′-dithio-bis-2-(nitrobenzoic acid)] generating a
yellow-colored TNB (5-thio-2-nitrobenzoic acid). Glutathione
reductase reduced the disulphide mixture (GSH and TNB), producing
an increased amount of TNB. The rate of TNB production was directly
proportional to the amount of GSH present in the sample. GSH
concentration was determined by measuring the absorbance of TNB at
405–414 nm using a microplate reader.
GPx assay
GPx enzyme catalyzed the reduction of hydroperoxides
by using two molecules of reduced GSH to produce one molecule of
reduced hydroperoxides and oxidized glutathione (GSSG). This
reaction was coupled with a second reaction which recycled GSSG
into its reduced state by glutathione reductase and NADPH. The
oxidation of NADPH to NADP+ was followed by a decrease of
absorbance at 340 nm, which was measured every 1 min for 8 min and
was directly proportional to the GSH activity in the sample.
SOD assay
Total SOD activity (cytosolic and mitochondrial) in
the sample was assayed using a Cayman Chemical assay kit. SODs are
metalloenzymes that catalyze the dismutation of free radicals,
specifically superoxide anion, to less toxic compounds including
oxygen and hydrogen peroxide molecules. The superoxide anion
radicals in the reaction were generated by hypoxanthine and
xanthine oxidase. The dismutation reaction by SOD utilized the
tetrazolium salt resulting in color change and the quantity of SODs
was measured at 440 nm using a microplate reader.
Catalase assay
Catalase enzyme activity is usually estimated by
catalytic activity. However, for the Cayman Chemicals enzyme kit,
the catalase activity was measured using peroxidatic activity. In
the peroxidatic reaction, catalase mediated the reaction between
hydrogen peroxidase and low molecular alcohol, which served as
electron donors for the production of water and formaldehyde
molecules. The formaldehyde molecules reacted with the chromogen,
4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Purpald), resulting
in purple color change and were measured colorimetrically at 540 nm
using a microplate reader.
Lipid peroxidation assay
Lipid peroxidation was determined by the
thiobarbituric acid reactive (TBARS) method. The cell lysates were
mixed with thiobarbituric acid (TBA) and allowed to react at a
temperature of 95–100°C. The naturally occurring product of lipid
peroxidation, MDA reacted with TBA, forming the MDA-TBA adduct, an
acidic condition that was measured colorimetrically at 540 nm using
a microplate reader.
Statistical analysis
The experiments were repeated three times in
triplicate. Results were shown as the mean ± SEM. Data were
analyzed using one-way ANOVA and SPSS Inc. software (SPSS
Statistics Desktop, V20.0.0). P<0.05 was considered
statistically significant.
Results
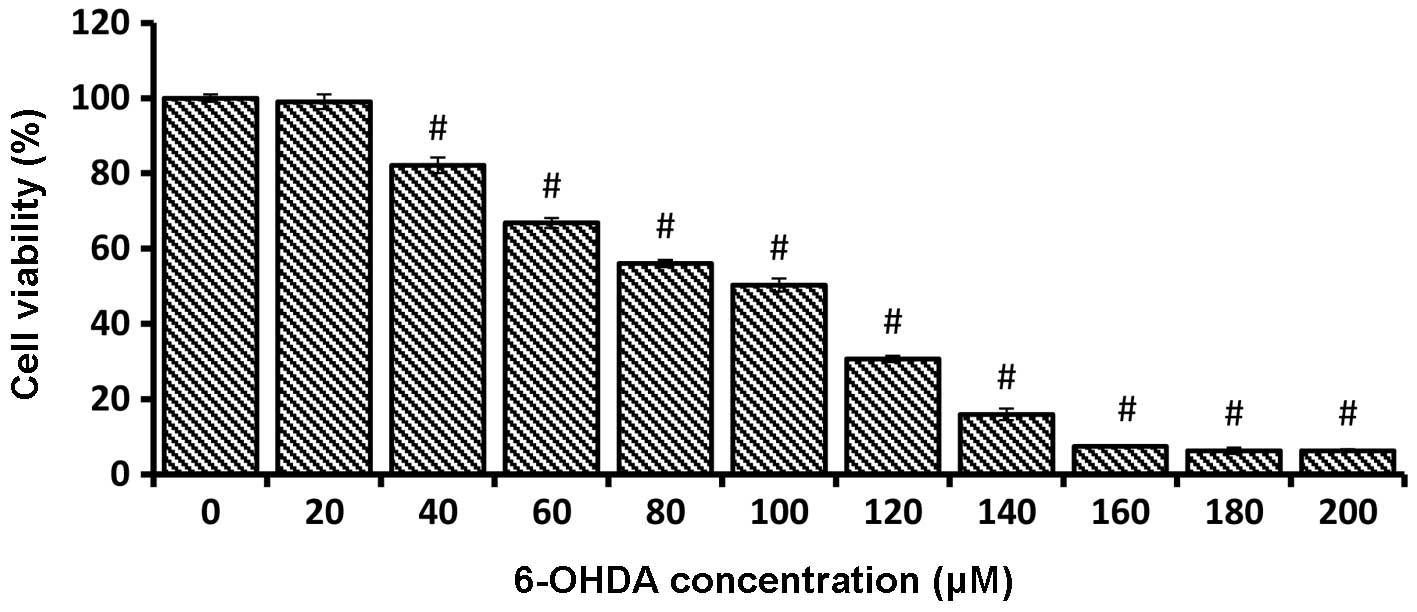
Dose response of 6-OHDA toxicity
Cell viability markedly decreased following a 24-h
incubation of PC-12 cells with an increasing concentration of
6-OHDA (0–200 μM). The dose response study was crucial to
determine the IC50 value of the 6-OHDA concentration.
The 6-OHDA concentration, which resulted in 50% PC-12 cell
inhibition, was 100 μM (50.33±1.72) on 1×104
cells/ml. The cell viability of each sample concentration was
compared with the mean percentage of the untreated control and
reported as the mean ± SEM (Fig.
2).
Effect of rutin in 6-OHDA-induced
oxidative stress in PC-12 cells
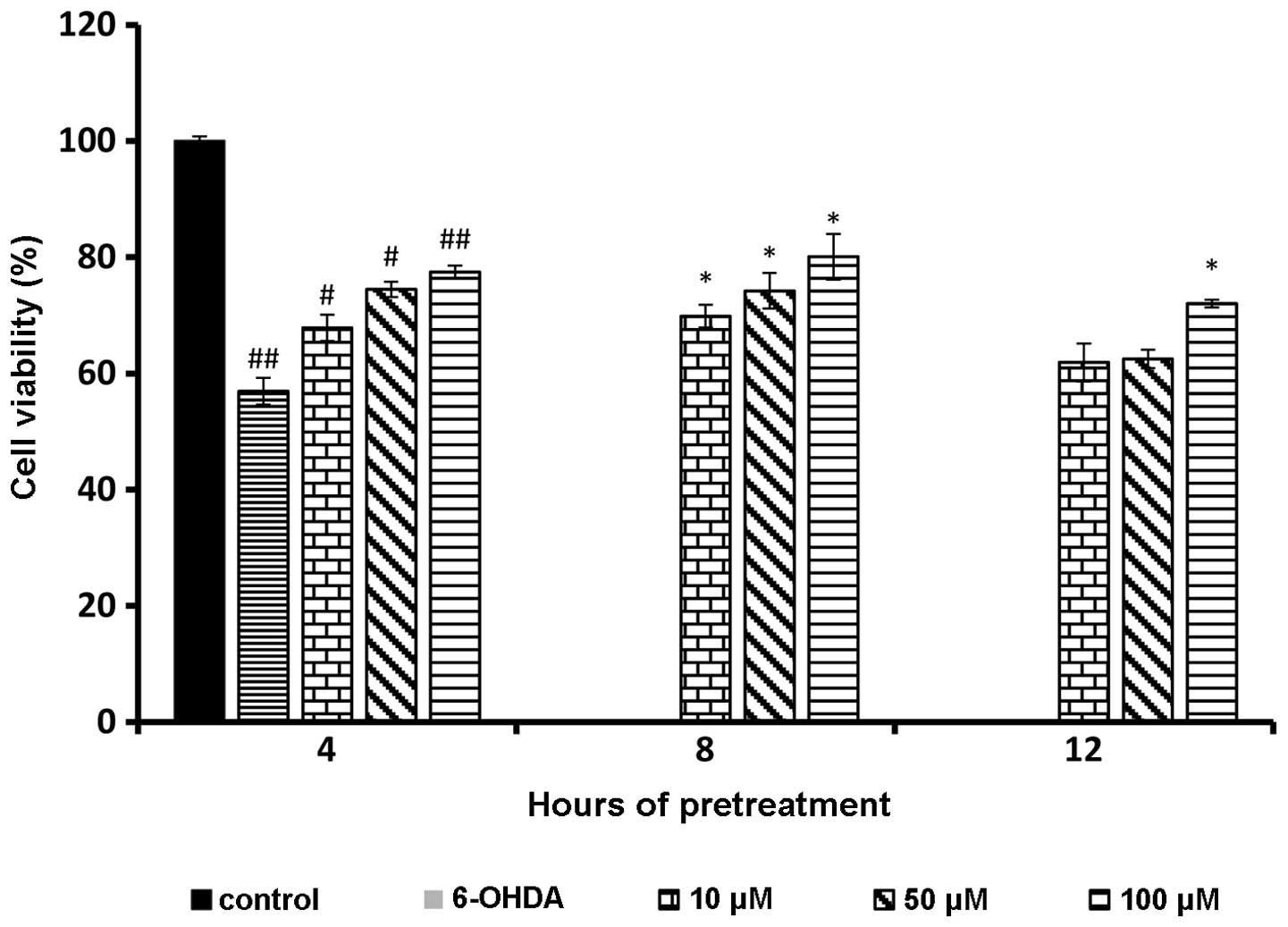
Results of MTT assay following rutin pretreatment
showed a significant increase in cell viability in a dose-dependent
manner. The 100 μM of 6-OHDA treatment caused 50% cell
inhibition (50.33%±1.72) on 1×104 cells/ml of PC-12
cells. Thus, 100 μM of 6-OHDA was used for the subsequent
experiments to assess the protective effect of rutin. The 100
μM of 6-OHDA resulted in cell viability of 56.92±2.31. The
cell viability was the highest at 100 μM of rutin at all
three time points. However, the optimum reading was recorded at 100
μM of rutin at 8 h of pretreatment (80.06±3.94). However,
the protective ability of rutin decreased after 12 h of incubation
(Fig. 3).
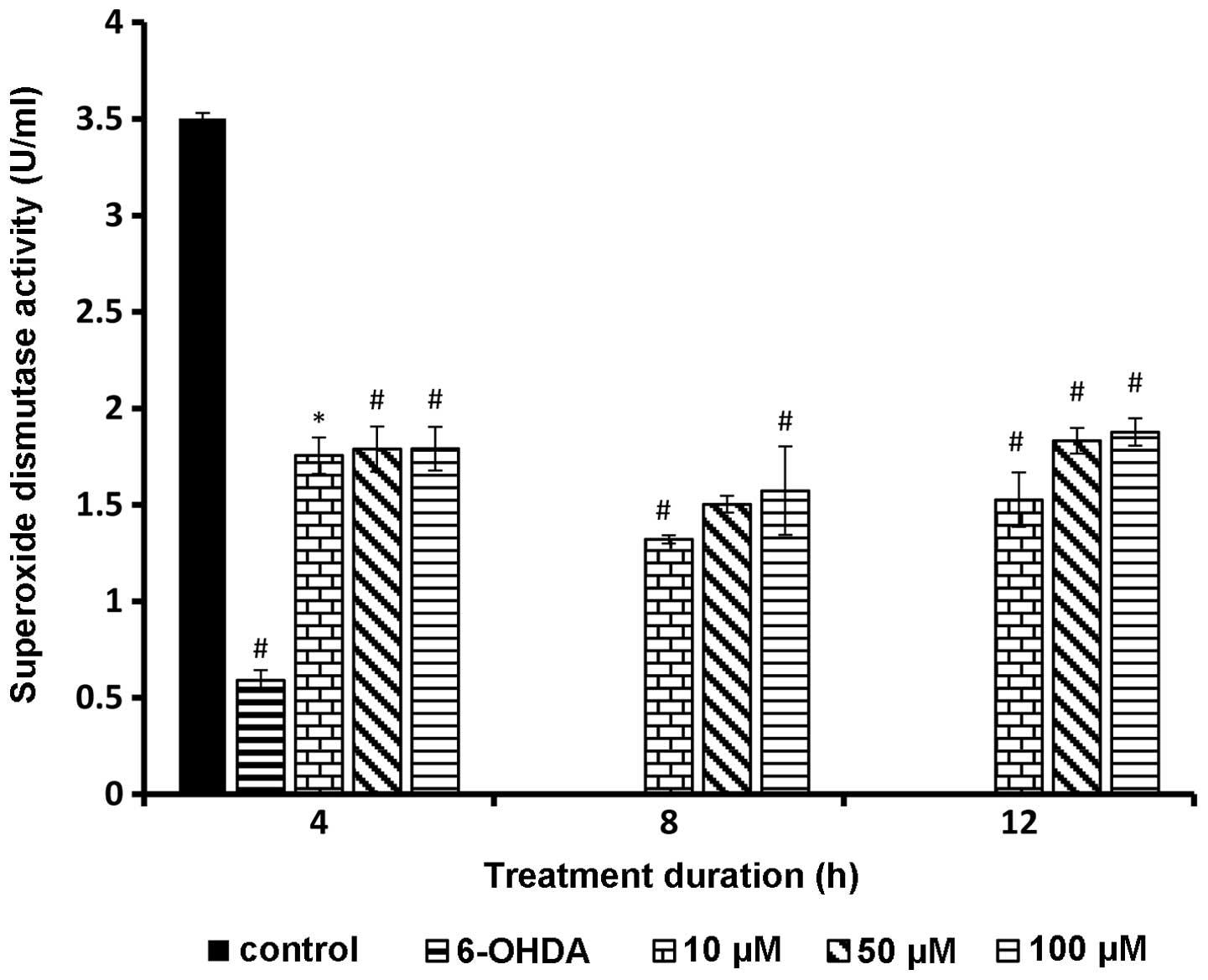
SOD activity following rutin
pretreatment
SOD level significantly increased in all the rutin
pretreated PC-12 cells. Test groups showed a statistically
significant difference compared to 6-OHDA alone (P<0.01). The
SOD activity showed a positive trend with increasing rutin
concentration (Fig. 4).
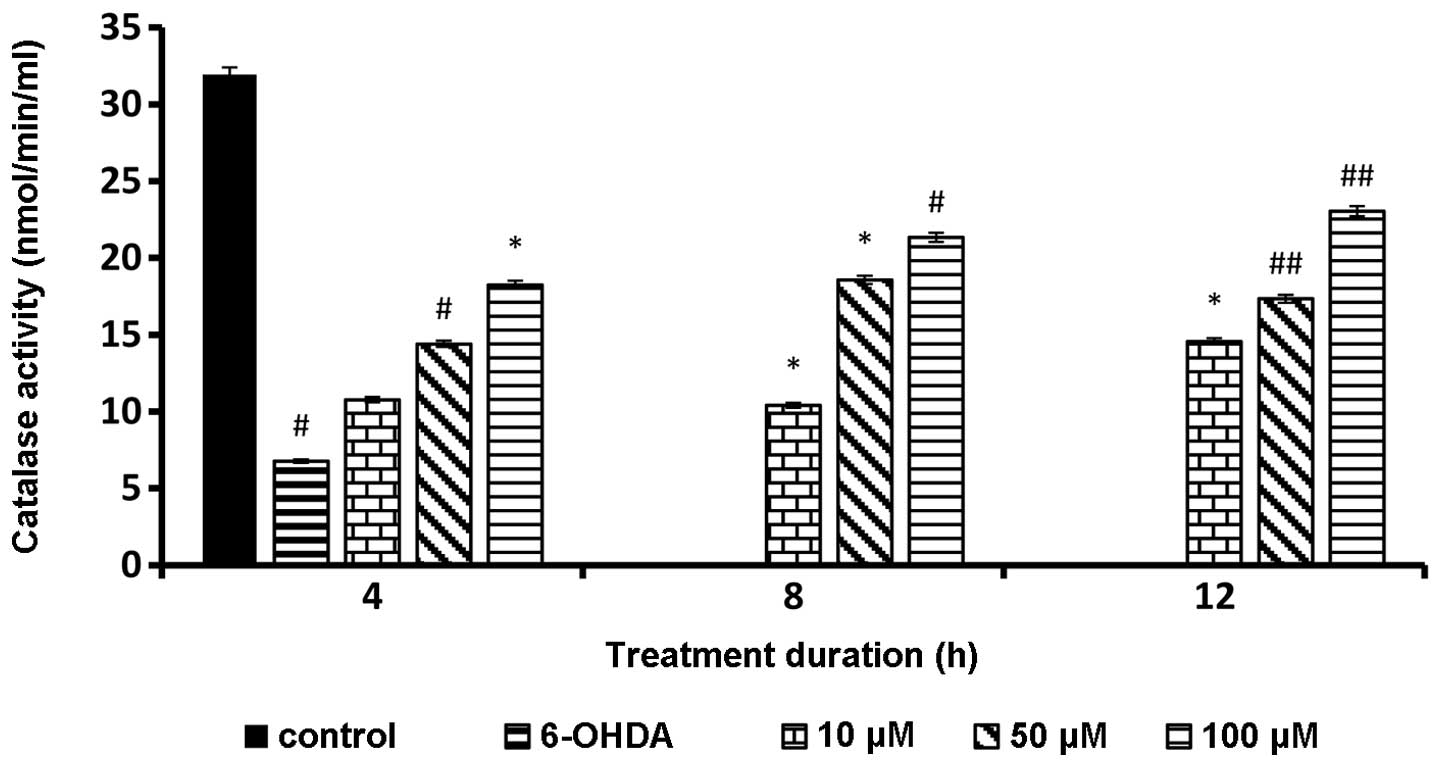
Catalase level following rutin
pretreatment
Catalase level was significantly increased in all
the rutin pretreated groups and the trend was directly proportional
to the rutin concentration. Almost all data showed a statistically
significant value in comparison with 6-OHDA alone (Fig. 5).
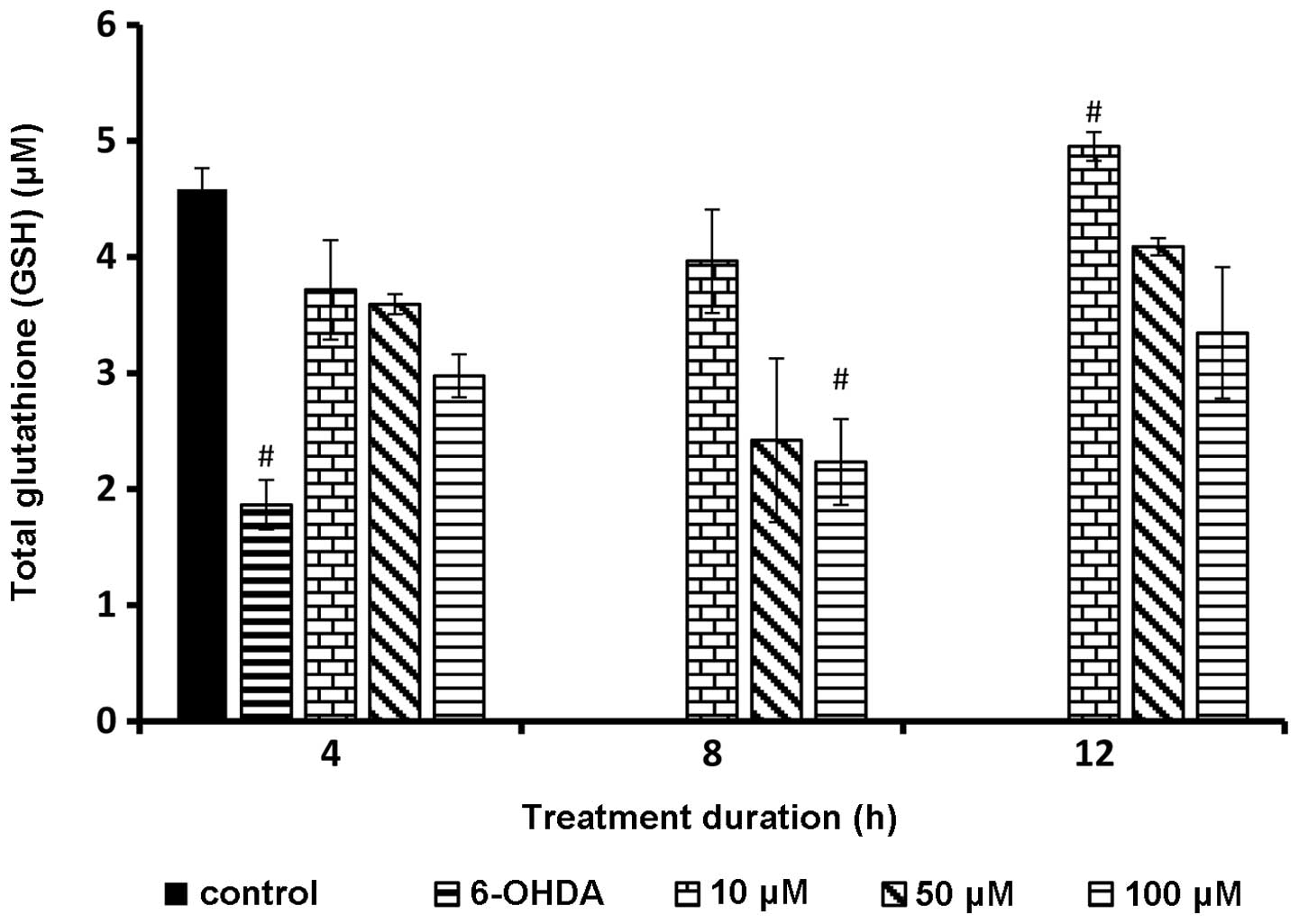
GSH level following rutin
pretreatment
The internal GSH in response to different
concentrations of rutin yielded significant (P<0.01) positive
results. The GSH level was significantly elevated in the untreated
sample and decreased in the sample incubated with 6-OHDA alone. Of
note, the samples incubated with rutin demonstrated significantly
higher amounts (P<0.01) of GSH concentrations. The GSH
concentration was indirectly proportional to the rutin
concentration. As the rutin concentration was increased the cells
produced a reduced amount of GSH (Fig. 6).
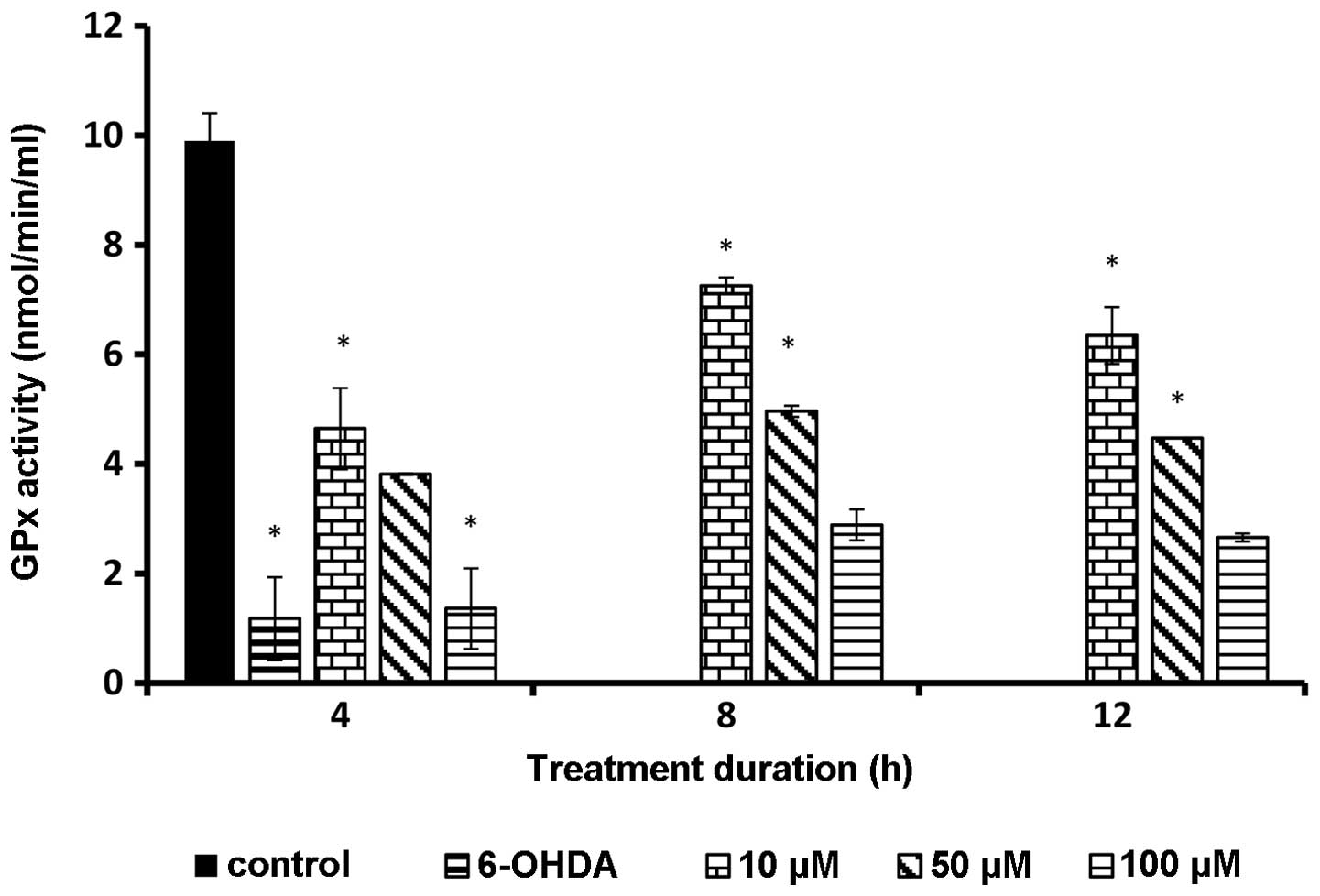
GPx level following rutin
pretreatment
GPx enzyme activity was determined colorimetrically.
The GPx enzyme was significantly (P<0.05) higher in rutin
pretreatment samples compared to the sample incubated with 6-OHDA
alone. The enzyme activity was elevated at a low concentration of
rutin and the data were statistically significant (P<0.01). This
result demonstrated that PC-12 cells produced a lower amount of GPx
in the presence of a high amount of external antioxidant (Fig. 7).
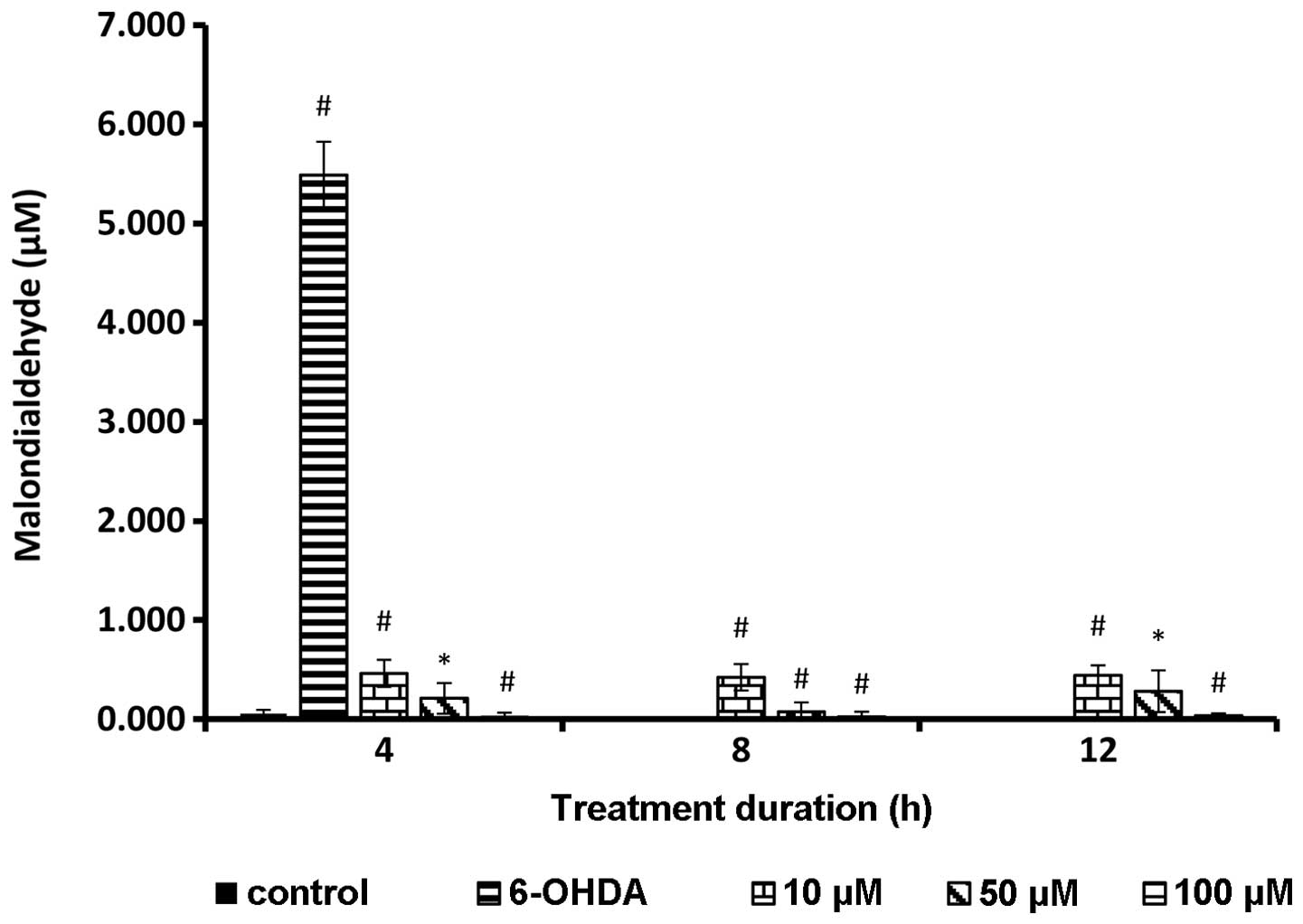
Lipid peroxidation following rutin
pretreatment
A significant reduction of the MDA level was
observed in cells treated with rutin (P<0.01). The cells treated
with 6-OHDA alone showed a markedly high level of MDA, which is an
indicator of lipid peroxidation or oxidative stress. Rutin
significantly reduced the lipid peroxidation in a dose-dependent
manner (P<0.01). The highest concentration of rutin showed the
greatest potential in suppressing lipid peroxidation in PC-12
cells. The test groups exhibited a statistically significant
decrease in lipid peroxidation compared to 6-OHDA treated cells
alone (Fig. 8).
Discussion
Reactive oxygen species (ROS) such as superoxide
anions (O2−) and hydrogen peroxide
(H2O2) have been proven to be the major
contributing factor in neurodegenerative diseases such as
Parkinson’s and Alzheimer’s diseases (12). Parkinson’s disease (PD) is caused
by selective loss of neuronal cells in substantia nigra pars
compacta, a region in the mid brain (13). Although various studies have been
conducted to identify a neurotherapeutic agent for PD, an
appropriate treatment regimen for PD remains elusive. Levodopa is
considered the cornerstone treatment for PD. However, the efficacy
of Levodopa tends to become depleted as the severity of disease
progresses (11). Therefore,
numerous attempts have been undertaken to identify a superior
neurotherapeutic agent for PD.
Rutin, a quercetin glycoside compound is widely
distributed in citrus fruits and buckwheat. The neuroprotective
potential of rutin has been studied and documented (7). However, the literature regarding the
neuroprotective effect of rutin is scanty. In this study, we have
explored the role of the bioflavonoid antioxidant rutin on
6-OHDA-induced neurotoxicity in PC-12 cells. PC-12 cells were used
as they resemble the neuronal cells at substantia nigra pars
compacta in human brain (14).
6-OHDA is a neurotoxin that is usually used to induce oxidative
stress in PC-12 cells. Subsequent to the pretreatment of PC-12
cells with rutin at 10, 50 and 100 μM for 4, 8 and 12 h, the
cells were incubated with 6-OHDA for 24 h to induce oxidative
stress. The cell viability assay and antioxidant enzyme assay were
performed on the treated cells and cell lysate, respectively.
Results of the cytoprotective study showed that
rutin markedly increased cell viability compared to cells with
6-OHDA alone. The cell viability increased significantly at 4 h of
treatment in a dose-dependent manner, reaching the highest
percentage at the 8th hour of pretreatment. The neuroprotective
effect was directly proportional to the rutin concentrations.
Aerobic cells are equipped with an extensive antioxidant enzyme
network that eliminates ROS which was formed during intracellular
signaling and defense against microorganisms (14). Imbalance in the ROS/antioxidant
enzyme homeostasis may result in disease state (15).
The endogenous antioxidant enzyme levels were
measured in all the samples that underwent rutin pretreatment and
oxidative stress by 6-OHDA. The enzymes examined in this study were
SOD, catalase, GSH, GPx and MDA concentrations. SOD enzymes are
metalloenzymes that occur as Cu/Zn SOD, Mn-SOD and EC-SOD in
aerobic cells. The enzymes are distributed in different
compartments of cells, thus, Cu/Zn-SOD has been identified in
cytosol, Mn-SOD in mitochondria and EC-SOD in the extracellular
compartment of aerobic cells (16). The SOD enzyme occurs in a
significantly high amount in brain, liver, heart, erythrocytes and
kidney cells. The function among the three SODs is similar and
involves catalyzing dismutation of the superoxide anion to oxygen
molecules and hydrogen peroxide, a less toxic molecule (17). In this study, the total SOD enzyme
was elevated in all the rutin pretreated cells in a dose-dependent
manner. Elevation of the SOD enzyme as the rutin concentration
increases proved that this antioxidant caused direct activation of
SOD to catalyze O2− produced by 6-OHDA.
Catalase, a tetrameric enzyme is a ubiquitous enzyme
identified in most aerobic cells that detoxifies hydrogen peroxide
which was generated by SOD (2,3).
Catalase converts two molecules of hydrogen peroxide to form two
molecules of water and one molecule of oxygen. This catalytic
reaction is a one-step process. In the present study, the catalase
activity was elevated as the concentration of rutin increased from
10 to 100 μM. Therefore, rutin likely has a synergistic
effect with catalase as it causes a direct activation of catalase
by eliminating the ROS molecules from the system.
GPx, a selenocysteine antioxidant enzyme identified
in the cytosol compartment of eukaryotic cells, is a vital
antioxidant defense mechanism. GPx catalyzes the reduction of
hydroperoxides at the expense of reduced GSH, a tripeptide to
produce GSSG and reduced hydroperoxide. The GSSG is recycled to GSH
by glutathione reductase and NADPH. In this study, the GPx and GSH
levels were significantly increased in all the rutin-treated cells
compared to 6-OHDA-treated cells alone. Findings showed that rutin
interacted with GPx and GSH to exhibit its antioxidant mechanism.
However, the trend seems to be inversely proportional to the rutin
concentration as a large amount of hydroperoxides was already
catalyzed by the enzyme catalase at high rutin concentrations,
leading to a decreased amount of GPx, while GSH were activated to
detoxify the hydroperoxides.
MDA is a naturally occurring end product of lipid
peroxidation. Lipid peroxidation is a largely accepted concept of
cell damage leading to disease onset. The antioxidant molecules
protect the cells by reversing or suppressing the progression of
lipid peroxidation in aerobic cells. Lipid peroxidation end
products measured as thiobarbituric acid reactive substances were
increased in the 6-OHDA-treated group. Rutin with antioxidant
properties may provide endogenous defense systems and reduce both
the initiation and propagation of reactive oxygen species. Rutin at
different doses effectively reduced the increased levels of
thiobarbituric acid reactive substances in treated cells.
The present findings demonstrated that rutin
protects PC-12 neuronal cells against 6-OHDA-induced neurotoxicity.
The drug may be considered as a potent therapeutic agent for
neurodegeneration associated with free radical generation in the
central nervous system. Additional experiments are required to
clarify the mechanisms of this bioflavonoid. Studies are currently
in progress to determine the molecular mechanisms of rutin-induced
neuroprotection in PC-12 cells.
References
|
1.
|
Uslu C, Taysi S and Bakan N: Lipid
peroxidation and antioxidant enzyme activities in experimental
maxillary sinusitis. Ann Clin Lab Sci. 33:18–22. 2003.PubMed/NCBI
|
|
2.
|
Nagata H, Takekoshi S, Takagi T, Honma T
and Watanabe K: Antioxidative action of flavonoids, quercetin and
catechin, mediated by the activation of glutathione peroxidase.
Tokai J Exp Clin Med. 24:1–11. 1999.PubMed/NCBI
|
|
3.
|
Agati G, Azzarello E, Pollastri S and
Tattini M: Flavonoids as antioxidants in plants: location and
functional significance. Plant Sci. 196:67–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nöthlings U, Murphy SP, Wilkens LR,
Henderson BE and Kolonel LN: Flavonols and pancreatic cancer risk:
the multi-ethnic cohort study. Am J Epidemiol. 166:924–931.
2007.
|
|
5.
|
Stewart LK, Soileau JL, Ribnicky D, Wang
ZQ, Raskin I, Poulev A, Majewski M, Cefalu WT and Gettys TW:
Quercetin transiently increases energy expenditure but persistently
decreases circulating markers of inflammation in C57BL/6J mice fed
a high-fat diet. Metabolism. 57(7 Suppl 1): S39–S46. 2008.
View Article : Google Scholar
|
|
6.
|
Wu LL, Yang XB, Huang ZM, Liu HZ and Wu
GX: In vivo and in vitro antiviral activity of hyperoside extracted
from Abelmoschus manihot (L) medik. Acta Pharmacol Sin.
28:404–409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Khan MM, Ahmad A, Ishrat T, Khuwaja G,
Srivastawa P, Khan MB, Raza SS, Javed H, Vaibhav K, Khan A and
Islam F: Rutin protects the neural damage induced by transient
focal ischemia in rats. Brain Res. 1292:123–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Suzuki T, Honda Y and Mukasa Y: Effects of
UV-B radiation, cold and desiccation stress on rutin concentration
and rutin glucosidase activity in tartary buckwheat (Fagopyrum
tataricum) leaves. Plant Sci. 168:1303–1307. 2005. View Article : Google Scholar
|
|
9.
|
Ramassamy C: Emerging role of polyphenolic
compounds in the treatment of neurodegenerative diseases: a review
of their intracellular targets. Eur J Pharmacol. 545:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hollman PC and Katan MB: Bioavailability
and health effects of dietary flavonols in man. Arch Toxicol Suppl.
20:237–248. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Blum D, Torch S, Lambeng N, Nissou M,
Benabid AL, Sadoul R and Verna JM: Molecular pathways involved in
the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the
apoptotic theory in Parkinson’s disease. Prog Neurobiol.
65:135–172. 2001.PubMed/NCBI
|
|
12.
|
Nikam S, Nikam P, Ahaley SK and Sontakke
AV: Oxidative stress in Parkinson’s disease. Indian J Clin Biochem.
24:98–101. 2009.
|
|
13.
|
Double KL, Reyes S, Werry EL and Halliday
GM: Selective cell death in neurodegeneration: why are some neurons
spared in vulnerable regions? Prog Neurobiol. 92:316–329. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Walkinshaw G and Waters CM:
Neurotoxin-induced cell death in neuronal PC12 cells is mediated by
induction of apoptosis. Neuroscience. 63:975–987. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yu DH, Bao YM, An LJ and Yang M:
Protection of PC12 cells against superoxide-induced damage by
isoflavonoids from Astragalus mongholicus. Biomed Environ
Sci. 22:50–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Qu CP, Xu ZR, Liu GJ, Liu C, Li Y, Wei ZG
and Liu GF: Differential expression of copper-zinc superoxide
dismutase gene of Polygonum sibiricum leaves, stems and
underground stems, subjected to high-salt stress. Int J Mol Sci.
11:5234–5245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hollman PC and Katan MB: Dietary
flavonoids: intake, health effects and bioavailability. Food Chem
Toxicol. 37:937–942. 1999. View Article : Google Scholar : PubMed/NCBI
|