Introduction
Vaults are predominantly cytoplasmic
ribonucleoprotein particles identified in preparations of coated
vesicles (1). The vault complex
has a barrel-like structure with an invaginated waist and two
protruding caps. Mammalian vaults are composed of major vault
protein (MVP) and two minor vault proteins [vault
poly(ADP-ribose)polymerase (VPARP) and telomerase-associated
protein 1 (TEP1)] as well as small untranslated RNAs (2). Vault particles have been highly
conserved throughout evolution and are found in numerous eukaryotic
species (3). These data suggest
that vaults have an important cellular function. However, the
precise function of vault particles has not yet been completely
elucidated.
MVP is the main component of vault particles and
covers over 70% of the particle mass. Since MVP is identical to
lung resistance-related protein (LRP), which was first identified
in a non-P-gp multidrug-resistant cancer cell line and has been
shown to be overexpressed in several multidrug resistant cell lines
(4,5), it has been suggested that MVP is
involved in multidrug resistance.
On the other hand, a number of previous studies have
reported that MVP expression is induced by a variety of cellular
stresses such as DNA damaging agents, UV irradiation, hypoxia and
hyperthermia (6–8). Kowalski et al (9) reported that MVP is involved in host
resistance to infection with Pseudomonas aeruginosa. Ryu
et al (10) demonstrated
that MVP enhanced the resistance of cells to apoptosis induced by
H2O2 in senescent human diploid fibroblasts
(HDFs). These findings suggest that MVP plays an important role in
cellular responses to stress.
It has previously been reported that the human MVP
promoter lacks a TATA-box, as well as other core promoter elements,
but harbors putative transcriptional factor binding sites for p53,
STAT1, MyoD, specificity protein 1 (Sp1), GATA and YB-1 (11). Transcription factors are involved
in the regulation of MVP in different cell types (6–9,12,13). We previously reported that
hyperosmotic stress upregulated the expression of MVP (14). However, it is unclear which
transcription factor affects MVP expression under hyperosmotic
stress conditions and the underlying mechanism has not yet been
elucidated. Therefore, the aim of this study was to investigate the
mechanism behind the induction of MVP expression under hyperosmotic
stress conditions.
Materials and methods
Cell line, antibodies and chemicals
The SW620 human colon carcinoma cell line was
provided by Dr A.T. Fojo (National Cancer Institute, Bethesda, MD,
USA). HA-tagged ubiquitin for constructing the plasmid was provided
by Dr Dirk Bohmann (European Molecular Biology Laboratory,
Heidelberg, Germany). RPMI-1640 medium was purchased from Nissui
Seiyaku Co. (Tokyo, Japan). Fetal calf serum (FCS) was obtained
from JRH Biosciences (Lenexa, KS, USA). Sucrose was obtained from
Wako Pure Chemical Industries, Ltd. (Osaka, Japan). A rabbit
anti-ERK polyclonal antibody (C-16), mouse anti-Sp1 monoclonal
antibody (E-3), rabbit anti-Sp1 polyclonal antibody (PEP2), rabbit
anti-HA polyclonal antibody (Y-11) and mouse phosphothreonin
monoclonal antibody (H-2) were obtained from Santa Cruz
Biotechnology, Inc. A mouse anti-STAT1 monoclonal antibody (610185)
was obtained from BD Biosciences. A rabbit anti-MVP polyclonal
antibody was prepared using a glutathione S-transferase (GST)-MVP
(aa 694–794) fusion protein as an antigen (5).
Immunoblot analysis
Immunoblot analysis was carried out as described
previously (5,17).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from cultured cells was isolated using
TRIzol reagent (Invitrogen). RT-PCR was performed with the
SuperScript One-Step RT-PCR system and gene-specific primers
according to the manufacturer’s instructions (Invitrogen). Reaction
mixtures contained total RNA (500 ng of each), 0.2 mM dNTPs, 0.2 μM
of Sp1 primer (sense, 5′-TGGAAGCAGCTGAGGCAATGG-3′ and
antisense, 5′-ATCCAGCCTCAGCTAGTTCAA-3′) and GAPDH primer
(sense, 5′-AGAACATCATCCCTGCCTCTA CTGG-3′ and antisense,
5′-AAAGGTGGAGGAGTGGGTGT CGCTG-3′), enzyme mixture containing
SuperScript II RT, Platinum TaqDNA polymerase, and 1X buffer with
1.2 mM MgSO4. The reaction was performed at 50ºC for 20
min, 94ºC for 2 min, followed by 28 cycles of 94ºC for 15 sec, 55ºC
for 30 sec and 70ºC for 30 sec.
Dual-luciferase reporter assay
The generation of the MVP promoter-luciferase
construct (pMVP78) has been described in a previous study of ours
(6). The luciferase assay was
performed using the Dual-Luciferase Reporter Assay System according
to the manufacturer’s instructions (Promega). In order to perform
the luciferase assay, transfected SW620 cells were cultured in the
presence of sucrose. Cells were washed with PBS and lysed using 100
μl of passive lysis buffer per well. Luminescence assays were
performed using a luminometer (TD-20/20 Luminometer; Turner
Designs, Sunnyvale, CA, USA). All experiments were performed in
triplicate and the results were normalized to pRL-TK activity.
RNA interference
STAT1 siRNA was obtained from Cell Signaling
Technology (no. 6544). siRNA duplexes were synthesized using the
Silencer™ siRNA construction kit (Ambion Inc., Austin, TX, USA).
The siRNA used in this study consisted of a 21-nucleotide sense
strand and a 21-nucleotide antisense strand with a two-nucleotide
overhang at the 3′-end. Sequences were as follows: GFP-siRNA
target sequence, 5′-AAGCGTTCAACTA GCAGACCA-3′; Sp1-siRNA
target sequence, 5′-AAGGAA CAGAGTGGCAGCAGT-3′. siRNA (100 nM) was
introduced into the SW620 cells using Lipofectamine™ 2000 according
to the manufacturer’s instructions (Invitrogen).
In vitro ubiquitination assays
COS-7 cells were transfected with expression vectors
encoding HA-tagged ubiquitin for 24 h after transfection, and were
exposed for 12 h to normal osmotic or hyperosmotic conditions in
the presence of the proteasome inhibitor, MG132 (5 μM). The cells
were then lysed in RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM
NaCl, 1% Nonidet P-40 (NP-40), 0.1% SDS, 0.5% sodium deoxycholate,
1 mM p-amidinophenyl methanesulfonyl fluoride hydrochloride (APMSF)
and 1 mg/ml aprotinin] and lysates (500 μg protein) were
immunoprecipitated with anti-Sp1 antibody followed by
immunoblotting with anti-HA antibody.
Immunoprecipitation
Cells were lysed with RIPA buffer and centrifuged at
7,500 rpm for 10 min at 4ºC. Subsequent to protein extraction, 500
μg of total cell lysates were incubated with anti-Sp1 monoclonal
antibody at 4ºC for 1 h. A total of 30 μl of a 50% slurry of
protein G-Sepharose 4B in RIPA buffer was then added to the
reaction mixtures and incubated for 12 h at 4ºC with rotation.
Following rapid centrifugation, the resulting pellets were washed
three times with RIPA buffer, and the immunoprecipitated proteins
were analyzed by immunoblotting using anti-phosphothreonin antibody
or anti-Sp1 polyclonal antibody.
Chromatin immunoprecipitation (ChIP)
assay
Cells were fixed with 1% formaldehyde for 10 min at
37ºC to cross-link protein to DNA. A chromatin immunoprecipitation
(ChIP) assay was carried out using a ChIP assay kit (Upstate
Biotechnology), according to the manufacturer’s instructions. The
soluble DNA fraction was mixed with an anti-Sp1 polyclonal antibody
or non-immunized rabbit IgG (Santa Cruz Biotechnology), and the
precipitated DNA was amplified with primers for the MVP promoter
[5′-GCCAGCTGGCTCCAAGGTAG-3′ (sense) and 5′-ATCACTTCCCGGCAGGGCAA-3′
(antisense)].
Results
Sp1 directly regulates the transcription
of the MVP gene under hyperosmotic stress conditions
We previously reported that hyperosmotic stress
upregulates MVP promoter activity. To identify important MVP
promoter elements that are involved in the induction of MVP
expression by hyperosmotic stress, we generated luciferase reporter
constructs containing various promoter elements. The pMVP78
construct contained three elements, Sp1, p53 and STAT, which were
reported as transcription factors involved in MVP promoter
activity (Fig. 1A). Following the
transfection of the plasmids into the SW620 cells, cells were
subjected to hyperosmotic stress and MVP promoter activity
was monitored by luciferase assay. Compared to basal luciferase
activity, luciferase activity was approximately 2-fold higher in
the pMVP78-transfected cells treated with sucrose (Fig. 1B). These results suggest that Sp1,
p53, or STAT contribute to the enhancement of MVP promoter
activity by hyperosmotic stress.
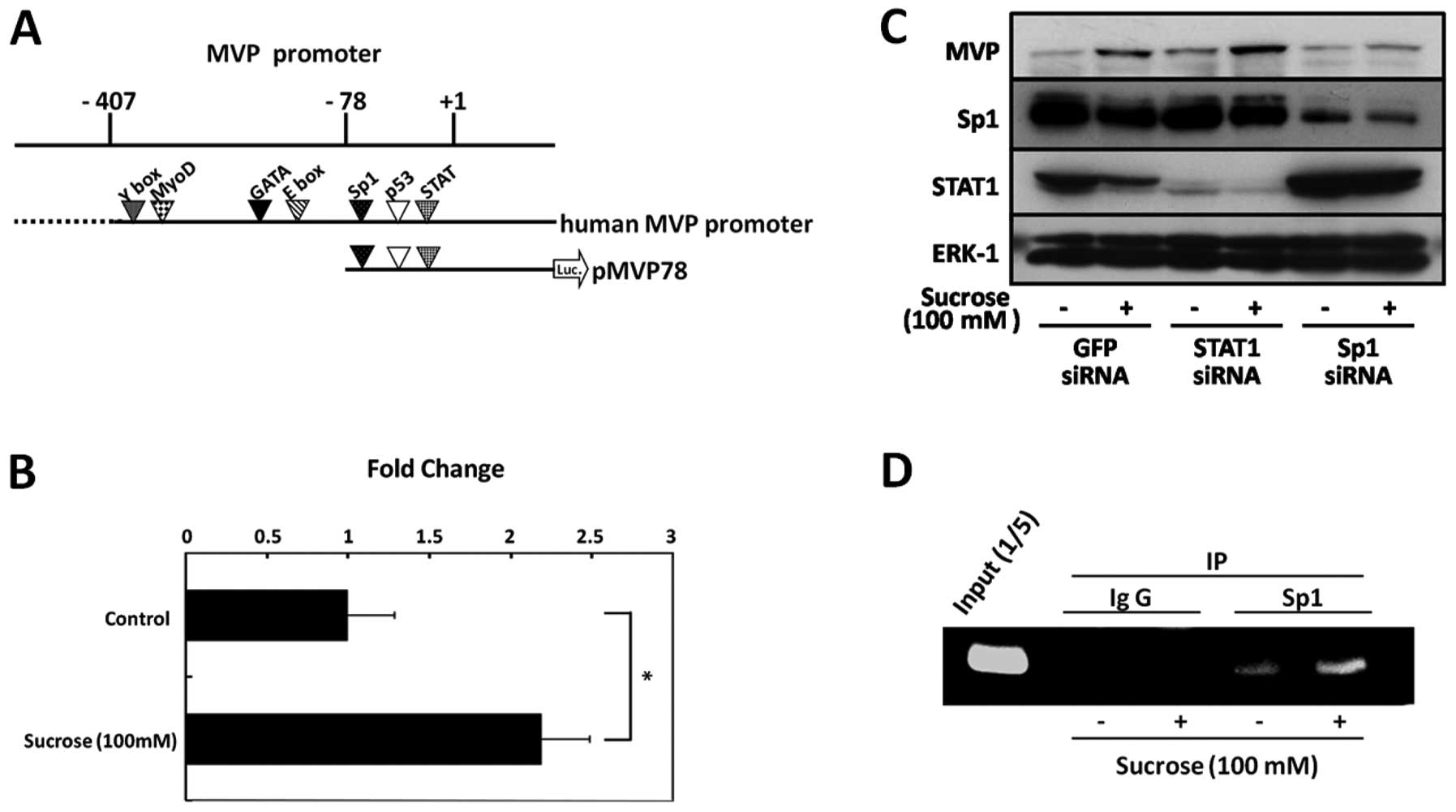 | Figure 1(A) Outline of the MVP-promoter
constructs used. The top line indicates the promoter region (−407)
upstream of the MVP transcription start site (+1). The pMVP78
construct harbors the Sp1, p53 and STAT binding sites. This
construct was linked to a luciferase reporter gene (luc). (B) SW620
cells were transiently transfected with pMVP78 (800 ng) and pRL-TK
(200 ng) reporter gene constructs using Lipofectamine 2000 reagent.
After transfection, the cells were incubated in the presence (+) or
absence (-) of 100 mM sucrose for 48 h and then assayed for
luciferase activity. Experiments were performed in triplicate and
the results were normalized to pRL-TK activity. Bars indicated the
means ± SD. *P<0.05. (C) SW620 cells were transfected
with siRNA targeting Sp1, STAT1, or GFP and cultured in 100 mM
sucrose for 48 h, and cell lysates were tehn prepared from these
cells. The expression of Sp1, STAT1, MVP and ERK-1 was detected by
immunoblot analsyis using anti-Sp1, anti-STAT1, anti-MVP and
anti-ERK-1 antibodies, respectively. (D) The SW620 cells were
treated with 100 mM sucrose for 72 h. Cells were fixed with
formaldehyde to form a DNA-protein complex and were subjected to
ChIP assay, as described in Materials and methods. A PCR primer for
the MVP promoter was used to detect the promoter fragments in the
immunoprecipitates. The input lane represents 20% of the total
chromatin used in the ChIP assay. |
SW620 cells have a mutation in the p53 gene (codon
273, Arg→His). This suggests that Sp1 or STAT1 is involved in the
induction of MVP expression by hyperosmotic stress. To determine
whether Sp1 or STAT1 are involved in the induction of MVP by
hyperosmotic stress, SW620 cells were transfected with siRNA
targeting Sp1 or STAT1. Sp1 knockdown inhibited the induction of
MVP expression by hyperosmotic stress in the SW620 cells (Fig. 1C). However, STAT1 knockdown did
not affect the induction of MVP expression. These results suggest
that Sp1 is involved in the induction of MVP expression by
hyperosmotic stress in SW620 cells.
To determine whether Sp1 can bind to the promoter
region of the MVP gene in SW620 cells under hyperosmotic
stress conditions, we performed a chromatin immunoprecipitation
(ChIP) assay using anti-Sp1 antibody. The presence of the
MVP promoter in chromatin immunoprecipitates was assessed by
PCR using a specific pair of primers spanning the Sp1-bind site in
the MVP gene promoter. Sp1 recruitment to the MVP
promoter was enhanced by hyperosmotic stress in SW620 cells
(Fig. 1D). These results indicate
that Sp1 directly regulates the transcription of the MVP
gene under hyperosmotic stress conditions.
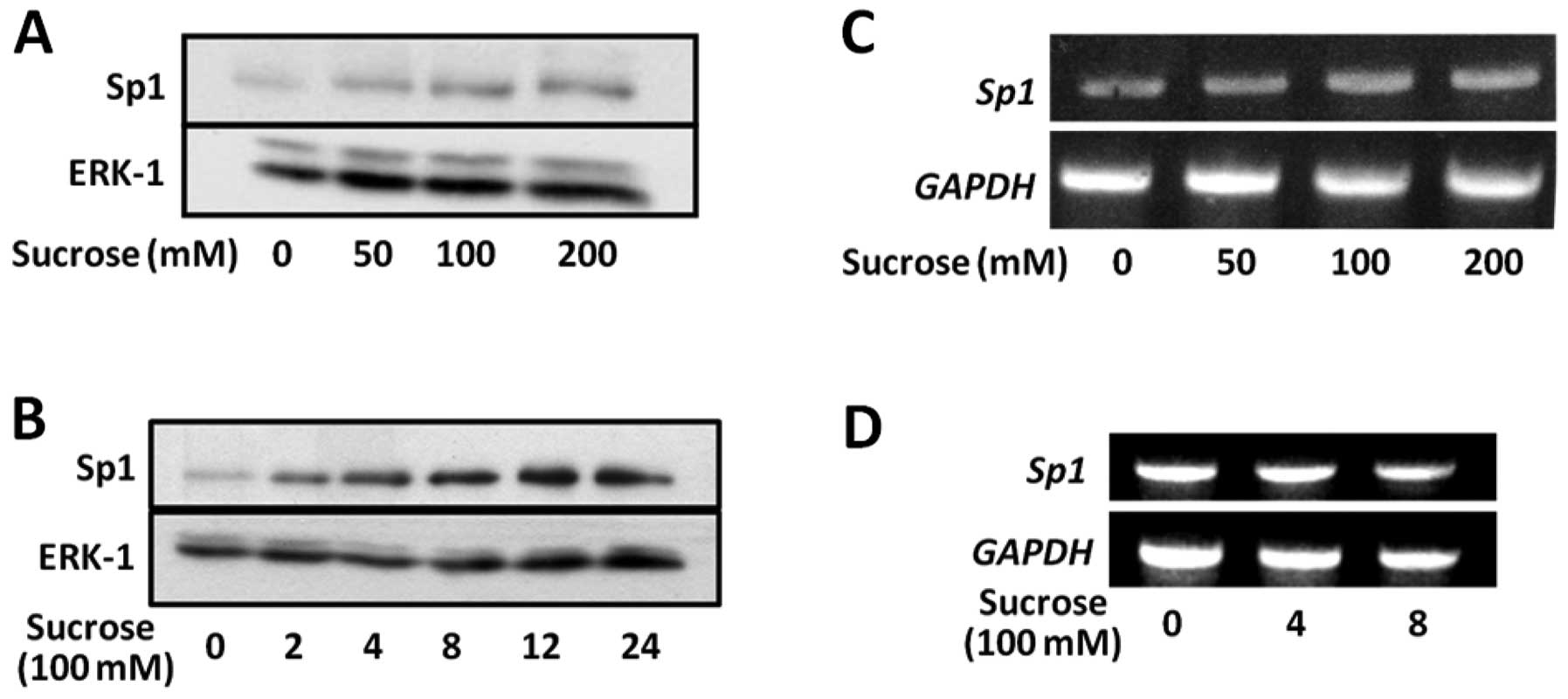
Hyperosmotic stress upregulates Sp1
protein levels
To determine whether hyperosmotic stress affects the
expression of Sp1, we performed immunoblot analysis using anti-Sp1
antibody. Fig. 2A shows that the
expression of Sp1 increased with increasing concentrations of
sucrose from 50 to 200 mM. Sp1 protein levels increased in a
time-dependent manner during treatment with sucrose at 100 mM, and
achieved a peak at 12 h (Fig.
2B).
We then examined the effect of hyperosmotic stress
on the expression levels of Sp1 mRNA in SW620 cells.
Hyperosmotic stress did not affect Sp1 mRNA levels (Fig. 2C and D). These results suggest
that hyperosmotic stress upregulates Sp1 protein levels by reducing
the turnover rate.
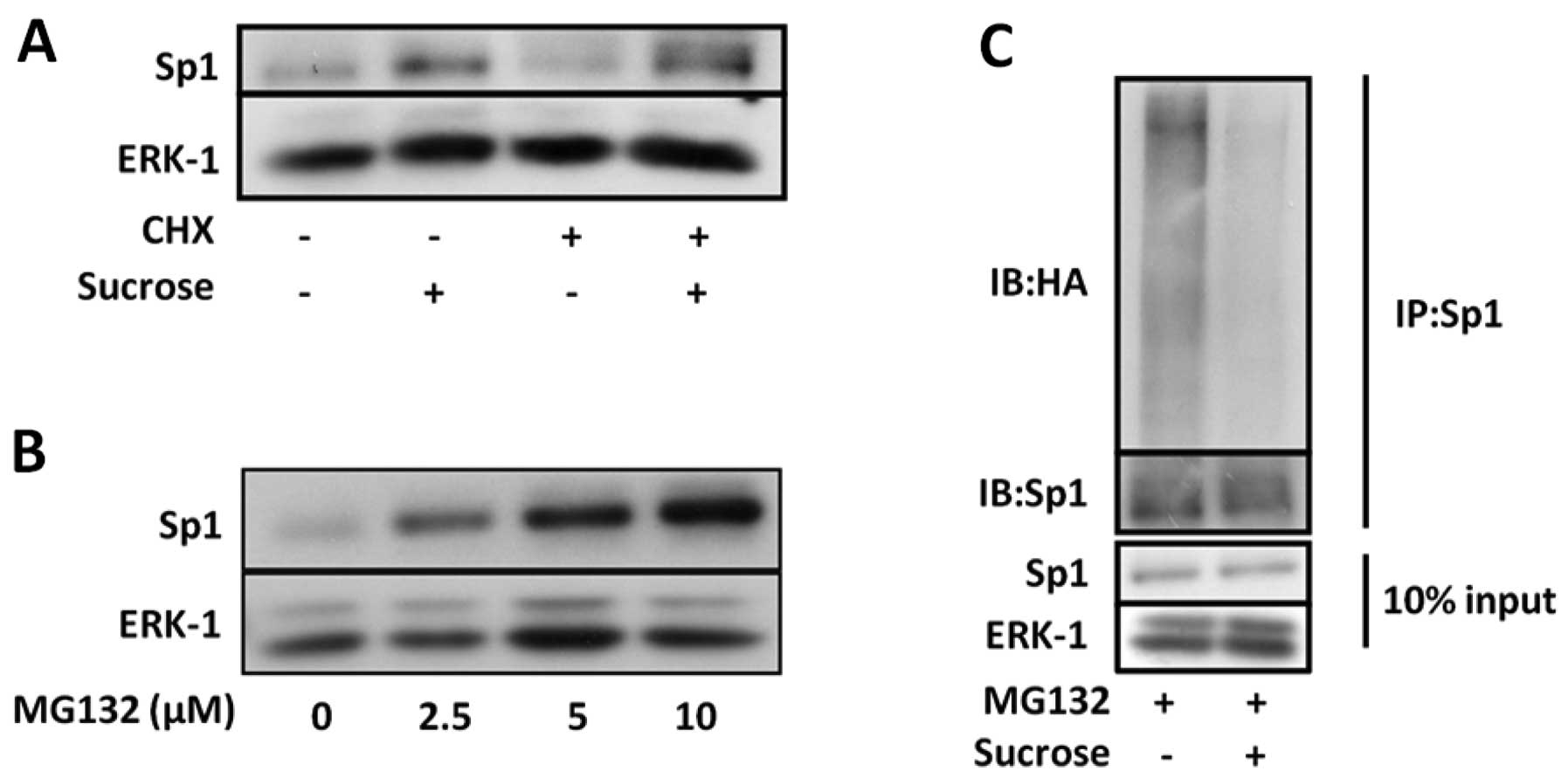
Hyperosmotic stress suppresses
proteasome-dependent Sp1 degradation by inhibiting
ubiquitination
To examine the possibility that the accumulation of
Sp1 protein reflects a reduction in its degradation rate, we
investigated whether the induction of Sp1 expression by
hyperosmotic stress occurs in SW620 cells exposed to
cycloheximide.
As shown in Fig.
3A, cycloheximide did not inhibit the induction of Sp1
expression under hyperosmotic conditions. This result suggests that
the degradation of Sp1 may be suppressed under hyperosmotic
conditions. We therefore investigated the effect of MG132, a
proteasome inhibitor, on Sp1 protein levels. Proteasome inhibition
in the SW620 cells by MG132 increased Sp1 protein levels (Fig. 3B). To determine whether
hyperosmotic stress affects the ubiquitination of Sp1, COS-7 cells
were transfected with HA-tagged ubiquitin. The transfected cells
were then incubated for 12 h under hyperosmotic conditions in the
presence or absence of MG132. Ubiquitinated Sp1 was detected by
immunoblot analsyis using anti-HA antibody following
immunoprecipitation with anti-Sp1 antibody. Furthermore, as shown
in Fig. 3C, hyperosmotic stress
suppressed the ubiquitination of Sp1. These results suggest that
hyperosmotic stress induces the accumulation of Sp1 by suppressing
its ubiquitination.
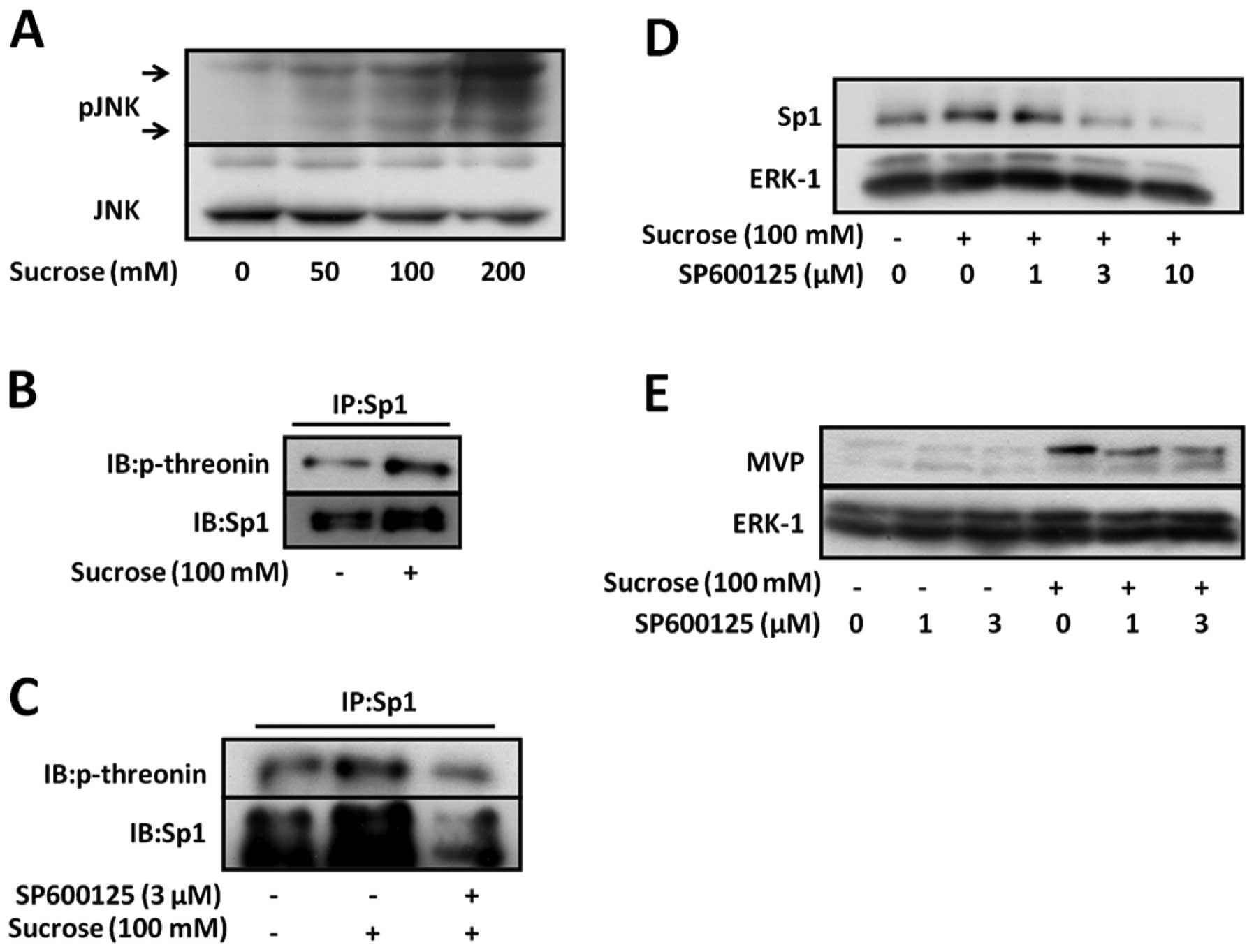
Induction of Sp1 is mediated by the
activation of c-Jun N-terminal kinase (JNK) activation under
hyperosmotic stress conditions
Hyperosmotic stress activates JNK, which plays an
important role in cellular stress responses. A recent study showed
that threonine phosphorylation by JNK-1 regulates the stability of
Sp1 (15). In order to
investigate whether JNK is activated in SW620 cells under
hyperosmotic stress conditions, we analyzed the phosphorylation of
JNK in SW620 cells under hyperosmotic stress conditions. The
phosphorylation of JNK increased in a manner dependent on the
sucrose concentration, which was increased from 50 to 200 mM
(Fig. 4A). These data suggest
that hyperosmotic stress activates JNK in SW620 cells.
Therefore, we investigated the threonine
phosphorylation of Sp1 under hyperosmotic stress conditions to
confirm that hyperosmotic stress affects the threonine
phosphorylation of Sp1 in SW620 cells. Hyperosmotic stress
increased the threonine phosphorylation of Sp1 (Fig. 4B). Moreover, as shown in Fig. 4C, SP600125, a specific JNK
inhibitor, inhibited the phosphorylation of Sp1 under hyperosmotic
conditions.
To investigate whether JNK regulates the induction
of Sp1 and MVP by hyperosmotic stress, SW620 cells were treated
with SP600125 in the presence or absence of hyperosmotic stress.
SP600125 inhibited the induction of Sp1 expression and consequently
that of MVP under hyperosmotic stress conditions (Fig. 4D and E). These data suggest that
JNK upregulates MVP expression by inhibiting Sp1 degradation under
hyperosmotic stress conditions. Therefore, the stability of Sp1 may
be mediated by the enhancement of Sp1 phosphorylation by JNK under
hyperosmotic stress conditions.
Discussion
One of the cellular stresses to which cells are
exposed to in vivo is hyperosmotic stress. It has previously
been reported that hyperosmotic stress induces apoptosis of cells
(16). We previously reported
that hyperosmotic stress modulates the PI3K/Akt pathway and induces
MVP, which exerts a cytoprotective effect against apoptosis,
thereby preventing cells from undergoing apoptosis by the
activation of Akt in SW620 colon cancer cells (14). However, the molecular basis for
the expression of MVP induced by hyperosmotic stress is unclear.
Therefore, in the current study, we elucidated that Sp1 and JNK
regulate the expression of MVP under hyperosmotic conditions.
Our results showed that hyperosmotic stress enhanced
the binding of Sp1 to the MVP promoter and upregulated the
transcription of the MVP gene. Sp1 is ubiquitous in
mammalian cells and is known as a housekeeping gene and a
transcription factor with a C2H2 zinc finger DNA binding domain,
and also modulates transcription of numerous genes (17). It has been reported that Sp1 is
activated by osmotic changes and regulates the transcription of a
number of genes (18,19). Our data are consistent with these
data from previous reports. Moreover, it has also been reported
that the knockdown of Sp1 enhances the
H2O2-induced apoptosis of U2OS osteosarcoma
cells (20). We previously
reported that MVP prevents cells from undergoing apoptosis
(14). Our findings, as well as
those from previous reports suggest that Sp1 augments the
expression of MVP and consequently protects cells from apoptosis
under hyperosmotic conditions.
JNK belongs to the family of mitogen-activated
protein kinases (MAPKs) and is activated by several types of
cellular stress, such as hypoxia and osmotic stress (21,22). This study demonstrates that the
inhibition of the ubiquitination and degradation of Sp1 by JNK
causes the stabilization and upregulation of Sp1 protein, which
participates in the induction of MVP expression under hyperosmotic
stress conditions. Chuang et al (15) reported that the phosphorylation by
JNK-1 regulates the stability of transcription factor Sp1 during
mitosis. We previously reported that the expression levels of MVP
in S1 small cell lung cancer cells increased in hypertonic culture
medium with sucrose (14). As Sp1
protein levels also increased in S1 cells (data not shown), JNK may
regulate the expression of MVP under hyperosmotic stress conditions
in other cell lines. Recent studies have shown that JNK suppresses
the interaction between Sp1 and ring finger protein 4 (RNF4), which
is an E3 ubiquitin ligase of Sp1 (15,23). These data suggest that JNK is
activated by hyperosmotic stress and may suppress the interaction
between Sp1 and RNF4 by phosphorylating the threonine residue of
Sp1. Consistent with these data, the results from our study showed
that hyperosmotic stress enhanced the threonine phosphorylation of
Sp1 and decreased the ubiquitination of Sp1 (Figs. 3C and 4B).
We previously reported that p38 MAPK, which belongs
to the family of MAPKs and is activated by cellular stress, is
partly involved in the expression of MVP induced by hyperosmolytes.
However, SB203580, a p38 MAPK-specific inhibitor, did not affect
the induction of Sp1 by hyperosmotic stress (data not shown). These
data suggest that p38 MAPK activates Sp1 without affecting the
expression of Sp1 under hyperosmotic conditions. In fact, certain
studies have shown that p38 MAPK activates Sp1 (24,25). Further studies are required to
define the role of p38 MAPK under hyperosmotic conditions.
In conclusion, the expression of MVP is upregulated
by several types of cellular stress. The data from our study
indicate that hyperosmotic stress upregulates Sp1 expression levels
by inhibiting ubiquitination through the activation of JNK, and the
induction of Sp1 expression directly enhances MVP
transcription.
Acknowledgements
We thank Dr Dirk Bohmann (European Molecular Biology
Laboratory, Heidelberg, Germany) for providing the HA-tagged
ubiquitin plasmid.
References
|
1
|
Kedersha NL and Rome LH: Isolation and
characterization of a novel ribonucleoprotein particle: large
structures contain a single species of small RNA. J Cell Biol.
103:699–709. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kickhoefer VA, Vasu SK and Rome LH: Vaults
are the answer, what is the question? Trends Cell Biol. 6:174–178.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kedersha NL, Miquel MC, Bittner D and Rome
LH: Vaults. II Ribonucleoprotein structures are highly conserved
among higher and lower eukaryotes. J Cell Biol. 110:895–901. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scheffer GL, Wijngaard PL, Flens MJ,
Izquierdo MA, Slovak ML, Pinedo HM, Meijer CJ, Clevers HC and
Scheper RJ: The drug resistance-related protein LRP is the human
major vault protein. Nat Med. 1:578–782. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitazono M, Sumizawa T, Takebayashi Y,
Chen ZS, Furukawa T, Nagayama S, Tani A, Takao S, Aikou T and
Akiyama S: Multidrug resistance and the lung resistance-related
protein in human colon carcinoma SW-620 cells. J Natl Cancer Inst.
91:1647–1653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimamoto Y, Sumizawa T, Haraguchi M,
Gotanda T, Jueng HC, Furukawa T, Sakata R and Akiyama S: Direct
activation of the human major vault protein gene by DNA-damaging
agents. Oncol Rep. 15:645–652. 2006.PubMed/NCBI
|
|
7
|
Stein U, Jürchott K, Schläfke M and
Hohenberger P: Expression of multidrug resistance genes MVP, MDR1,
and MRP1 determined sequentially before, during, and after
hyperthermic isolated limb perfusion of soft tissue sarcoma and
melanoma patients. J Clin Oncol. 20:3282–3292. 2002. View Article : Google Scholar
|
|
8
|
Iwashita K, Ikeda R, Takeda Y, Sumizawa T,
Furukawa T, Yamaguchi T, Akiyama S and Yamada K: Major vault
protein forms complexes with hypoxia-inducible factor (HIF)-1alpha
and reduces HIF-1alpha level in ACHN human renal adenocarcinoma
cells. Cancer Sci. 101:920–926. 2010. View Article : Google Scholar
|
|
9
|
Kowalski MP, Dubouix-Bourandy A, Bajmoczi
M, Golan DE, Zaidi T, Coutinho-Sledge YS, Gygi MP, Gygi SP, Wiemer
EA and Pier GB: Host resistance to lung infection mediated by major
vault protein in epithelial cells. Science. 317:130–132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryu SJ, An HJ, Oh YS, Choi HR, Ha MK and
Park SC: On the role of major vault protein in the resistance of
senescent human diploid fibroblasts to apoptosis. Cell Death
Differ. 15:1673–1680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lange C, Walther W, Schwabe H and Stein U:
Cloning and initial analysis of the human multidrug
resistance-related MVP/LRP gene promoter. Biochem Biophys Res
Commun. 278:125–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stein U, Bergmann S, Scheffer GL, Scheper
RJ, Royer HD, Schlag PM and Walther W: YB-1 facilitates basal and
5-fluorouracil-inducible expression of the human major vault
protein (MVP) gene. Oncogene. 24:3606–3618. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steiner E, Holzmann K, Pirker C, Elbling
L, Micksche M and Berger W: SP-transcription factors are involved
in basal MVP promoter activity and its stimulation by HDAC
inhibitors. Biochem Biophys Res Commun. 317:235–243. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ikeda R, Iwashita K, Sumizawa T, Beppu S,
Tabata S, Tajitsu Y, Shimamoto Y, Yoshida K, Furukawa T, Che XF,
Yamaguchi T, Ushiyama M, Miyawaki A, Takeda Y, Yamamoto M, Zhao HY,
Shibayama Y, Yamada K and Akiyama S: Hyperosmotic stress
up-regulates the expression of major vault protein in SW620 human
colon cancer cells. Exp Cell Res. 314:3017–3026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chuang JY, Wang YT, Yeh SH, Liu YW, Chang
WC and Hung JJ: Phosphorylation by c-Jun NH2-terminal kinase 1
regulates the stability of transcription factor Sp1 during mitosis.
Mol Biol Cell. 19:1139–1151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mak SK and Kültz D: Gadd45 proteins induce
G2/M arrest and modulate apoptosis in kidney cells exposed to
hyperosmotic stress. J Biol Chem. 279:39075–39084. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kadonaga JT, Carner KR, Masiarz FR and
Tjian R: Isolation of cDNA encoding transcription factor Sp1 and
functional analysis of the DNA binding domain. Cell. 51:1079–1090.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ramos A, Ho WC, Forte S, Dickson K,
Boutilier J, Favell K and Barker PA: Hypo-osmolar stress induces
p75NTR expression by activating Sp1-dependent transcription. J
Neurosci. 27:1498–1506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bell LM, Leong ML, Kim B, Wang E, Park J,
Hemmings BA and Firestone GL: Hyperosmotic stress stimulates
promoter activity and regulates cellular utilization of the serum-
and glucocorticoid-inducible protein kinase (Sgk) by a p38
MAPK-dependent pathway. J Biol Chem. 275:25262–25272. 2000.
View Article : Google Scholar
|
|
20
|
Olofsson BA, Kelly CM, Kim J, Hornsby SM
and Azizkhan-Clifford J: Phosphorylation of Sp1 in response to DNA
damage by ataxia telangiectasia-mutated kinase. Mol Cancer
Res. 5:1319–1330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huangfu WC, Omori E, Akira S, Matsumoto K
and Ninomiya-Tsuji J: Osmotic stress activates the TAK1-JNK pathway
while blocking TAK1-mediated NF-kappaB activation TAO2 regulates
TAK1 pathways. J Biol Chem. 281:28802–28810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ho CY and Li HY: DNA damage during mitosis
invokes a JNK-mediated stress response that leads to cell death. J
Cell Biochem. 110:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang YT, Yang WB, Chang WC and Hung JJ:
Interplay of posttranslational modifications in Sp1 mediates Sp1
stability during cell cycle progression. J Mol Biol. 414:1–14.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D’Addario M, Arora PD, Ellen RP and
McCulloch CA: Interaction of p38 and Sp1 in a mechanical
force-induced, beta 1 integrin-mediated transcriptional circuit
that regulates the actin-binding protein filamin-A. J Biol Chem.
277:47541–47550. 2002.
|
|
25
|
Lin HH, Lai SC and Chau LY: Heme
oxygenase-1/carbon monoxide induces vascular endothelial growth
factor expression via p38 kinase-dependent activation of Sp1. J
Biol Chem. 286:3829–3838. 2011. View Article : Google Scholar : PubMed/NCBI
|