Introduction
Epithelial ovarian cancer (EOC) is described as a
silent killer as patients always present with local invasion or
distant metastasis at first diagnosis. In the developed world,
ovarian cancer is the leading cause of gynecological cancer-related
mortality among women (1).
Surgical cytoreduction and treatment with chemotherapeutic agents,
such as cisplatin and paclitaxel (Taxol), are commonly used to
treat this malignancy. However, the majority of ovarian cancer
survivors eventually suffer from recurrent disease that develops
resistance to multiple chemotherapeutic agents; thus, EOC is a
disease with a high mortality rate. Long-term survival is achieved
in less than a third of patients with advanced-stage ovarian cancer
(2).
The Janus kinase/signal transducer and activator of
transcription (JAK/STAT) signaling pathway is an important pathway
by which cytokines transfer information from the surface of the
cell into the nucleus. STAT3 is a crucial member of the JAK/STAT
signaling pathway. STAT3 has been described as a key regulator of
cell survival and proliferation (3). It is constitutively activated in a
variety of tumor cell lines and primary tumors, including prostate,
breast, head and neck cancer, multiple myeloma and glioma (4–7).
STAT3 is activated by various protein tyrosine kinases, JAK and the
proto-oncogene tyrosine-protein kinase (Src), as well as
membrane-bound growth factor receptor tyrosine kinases, such as the
epidermal growth factor receptor (EGFR) (8,9).
Thus, the functions of activated STAT3 proteins vary, and include
cell growth, differentiation, development, apoptosis and
angiogenesis (10,11). The constitutive activation of
STAT3 has been shown to contribute to tumorigenesis in ovarian
cancer (12). STAT3 has also been
reported to be a key factor for drug-resistance in ovarian cancer
(13). Therefore, STAT3 may be a
potential molecular target for the treatment of cancer (14).
The constitutive activation of STAT3 in tumor cells
points to STAT3 as a valuable target for attacking tumor cells.
Moreover, STAT3 is not essential for the functioning of mature
cells (15). Thus, it is a highly
valuable target for inducing tumor cell death; however, STAT3 lacks
more specific inhibitors. Several strategies have been investigated
to target the signaling pathway of STAT3, including RNA
interference (using siRNA), dominant-negative, antisense and
‘decoy’ oligodeoxynucleotides (ODN) technology (16–18). A recent study reported that a
STAT3 decoy oligonucleotide induced cell death in a human
colorectal carcinoma cell line by blocking nuclear transfer
(19). In our previous study, we
utilized the decoy ODN technology to examine the effects of a STAT3
decoy ODN on human ovarian cancer cells in vitro and found
that the STAT3 decoy ODN decreased the invasive capability of the
cancer cells and enhanced the sensitivity of ovarian cancer cells
to paclitaxel (20). However,
there is little information available as to the effects of STAT3
decoy ODNs on cancer in vivo, and little information as to
its toxicity in vivo.
In this study, we established subcutaneous
xenografts of SKOV3 human ovarian cancer cells in nude mice,
examined the antitumor effects of STAT3 decoy ODNs on xenografted
nude mice, investigated the potential mechanisms of the STAT3 decoy
ODNs, and examined the side-effects of the STAT3 decoy ODNs on the
vital organs of nude mice.
Materials and methods
Cell line and cell culture
The SKOV3 human ovarian epithelial cancer cell line
was provided by the Qilu Hospital Biotechnology Center, Shandong
University, Jinan, China. The cells were cultured in RPMI-1640
medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco-BRL) and incubated
under standardized conditions (37°C, 5% carbon dioxide).
STAT3 decoy and scrambled ODN
Phosphorothioate sense and antisense strands of
STAT3 decoy or scrambled control ODNs were synthesized using the
Expedite™ Nucleic Acid Synthesis System (Sangon Biotechnology,
Shanghai, China). The STAT3 decoy ODN sequence was 5′-CATTTCCCGTAA
ATC-3′ and 3′-GTAAAGGGCATTTAG-5′ and the scrambled ODN sequence was
5′-CATCTTGCCAATATC-3′ and 3′-GTAGAACGGTTA TAG-5′ (21,22). The sense and antisense strands
were then prepared by annealing complementary single-stranded ODNs
by heating to 95°C for 10 min followed by cooling to room
temperature slowly over a period of 2 h.
Subcutaneous xenografts in nude mice
Female nude mice (BALB/c, 4–5 weeks old) were
purchased from the Shanghai Experimental Animal Center (Chinese
Academy of Sciences, Shanghai, China). The mice were housed and
maintained under specific pathogen-free conditions according to the
experimental animal guidelines and approved by the Institutional
Animal Care and Use Committee of Shandong University. The tumor
model was established according to a previous study (23). SKOV3 cells were harvested and
resuspended in RPMI-1640 medium. A total of 200 μl SKOV3 cells
(2×107/ml) were injected into the right flanks of the
mice. Two weeks later, the mice were randomly assigned to 3 groups
with 5 mice in each group (STAT3 decoy ODN treatment group, STAT3
scrambled ODN treatment control group and PBS treatment control
group). ODN (50 μg in 50 μl PBS) was intratumorally injected every
other day for 30 days. Tumor sizes were measured by length (l) and
width (w) every 4 days, and the tumor volumes were calculated
according to the following formula: tumor volume =
lw2/2. The mental state, diet and stool of the mice were
observed daily. Twelve hours after the final injection, the mice
were sacrificed. The tumors, heart, liver and kidneys were removed,
parts of them were fixed in formalin and embedded in paraffin, and
parts of them were frozen at −80°C for western blot analysis.
Paraffin sections were made from the tumor, heart, liver and kidney
tissues of the nude mice, and the tissues were stained with
hematoxylin and eosin (H&E).
TUNEL assay for the detection of
apoptosis induced by STAT3 decoy ODN in vivo
To detect the poptotic cells in the tumor tissues,
TUNEL assay, using a Fluorometric TUNEL System (KeyGen Biotech,
Nanjing, China) was performed according to the manufacturer's
instructions. The tissue sections were rinsed with PBS for 5 min
following incubation with proteinase K (18 μg/ml) for 20 min, and
then blocked with fetal bovine serum for 15 min at room
temperature. TdT reaction mix (50 μl) was added to the sections
followed by incubation in a humidified chamber for 60 min at 37°C.
The the sections were then rinsed with PBS and observed under a
fluorescence microscope. Cell nuclei with green fluorescent
staining were defined as TUNEL-positive nuclei. To quantify the
TUNEL-positive cells, the number of green fluorescence-positive
cells was counted in 5 random fields of view on each section at
×200 magnification.
Western blot analysis
The tumor tissues were lysed in lysis buffer. Sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was
performed as previously described (24). The whole cell extracts (30
μg/lane) were separated by SDS-PAGE and transferred onto
nitrocellulose membranes (Millipore, Bedford, MA, USA). The
membranes were blocked in Tris-buffered saline with 5% (w/v)
non-fat dry milk, and then incubated with a primary antibody
against β-actin, MMP-2, MMP-9, Bcl-2 and caspase-3 (1:1,000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C. After
washing with TBST 3 times, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibody.
Immunoreactive proteins were visualized using an enhanced
chemiluminescence (ECL) detection system (Pierce, Rockford, IL,
USA). The bands were examined using a densitometer analysis system
(Flurochem 9900-50; Alpha Innotech, San Leandro, CA, USA). Band
density was analyzed using BandScan software (Glyko, Novato, CA,
USA) and the results were expressed as a ratio of the protein of
interest/β-actin to correct for loading for each sample.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software. All data are expressed as the means ± SD of at least
3 independent experiments. The differences between groups were
analyzed using the Student's t-test; P-values <0.05 were
considered to indicate statistically significant differences in all
cases.
Results
STAT3 decoy ODN inhibits ovarian cancer
cell growth in vivo
The SKOV3 cells formed xenografts in the nude mice,
and the average time taken for the tumors to be formed was 10–12
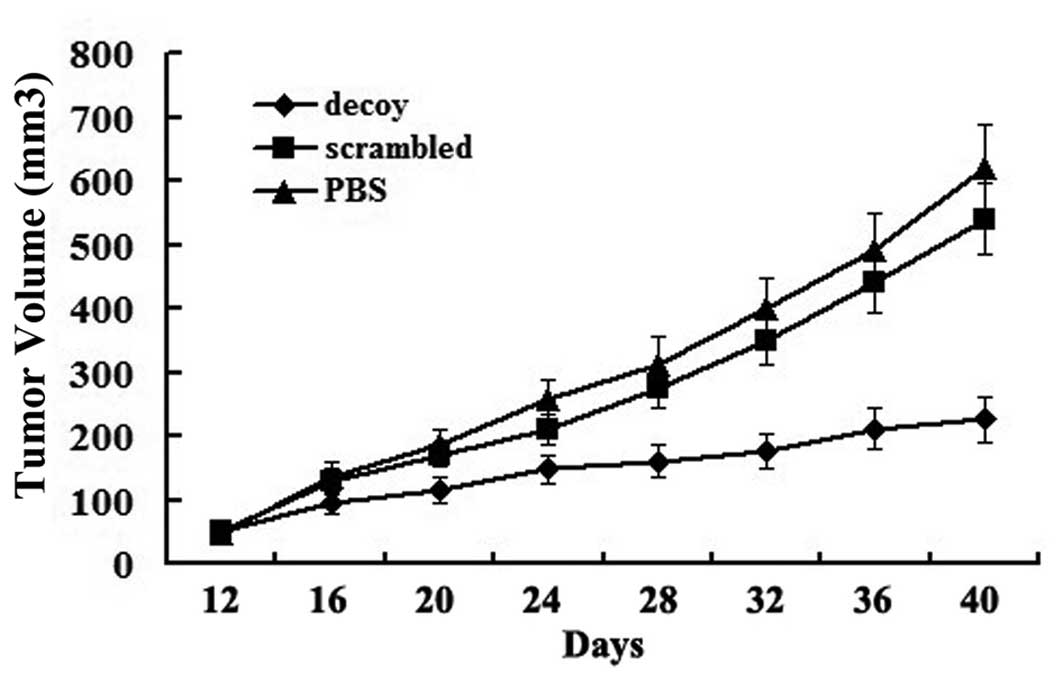
days. Images of the xenografted nude mice are shown in Fig. 1. Tumor growth curves showed that
the growth of the tumors treated with the STAT3 decoy ODN was
significantly inhibited compared with the other treatment groups
(Fig. 2). After the treatment was
terminated, the average volume of the xenografts was as follows:
620±68 mm3 in the PBS group, 540±55 mm3 in
the scrambled group and 226±35 mm3 in the decoy group.
The average volume of the xenografts in the decoy group decreased
significantly compared with the PBS and scrambled group
(P<0.05). However, the difference between the PBS and scrambled
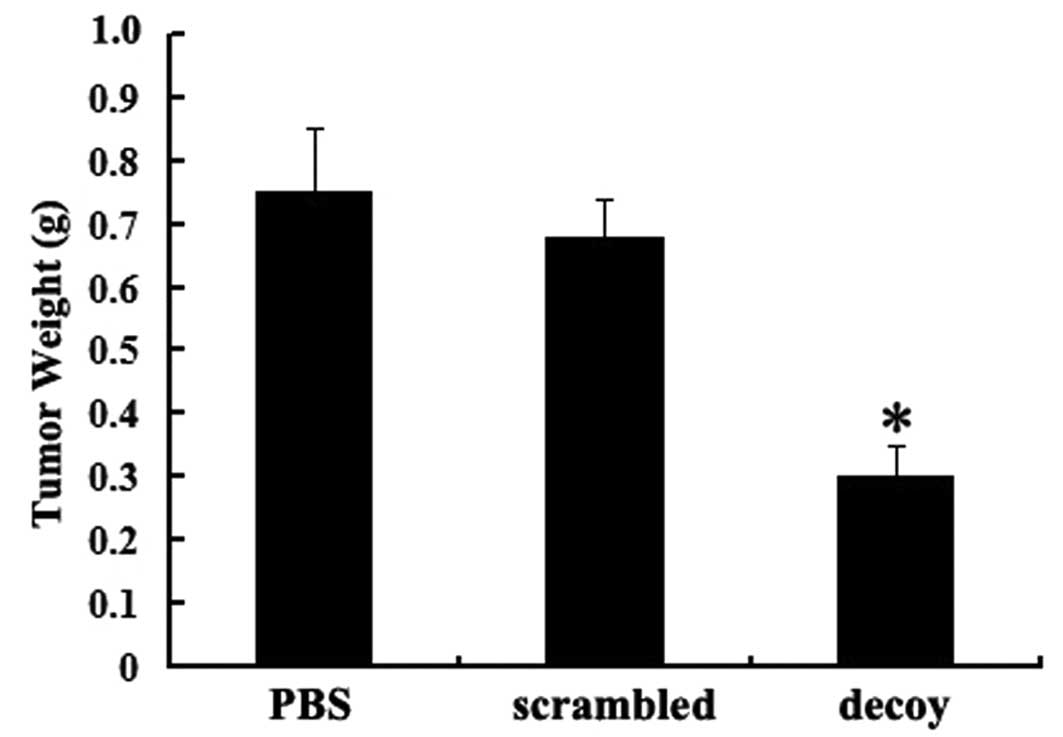
groups was not statistically significant. The average weight of the
tumor tissues in the decoy group was significantly lower than that
in the other control groups (P<0.05) (Fig. 3).
STAT3 decoy ODN promotes ovarian cancer
cell apoptosis in vivo
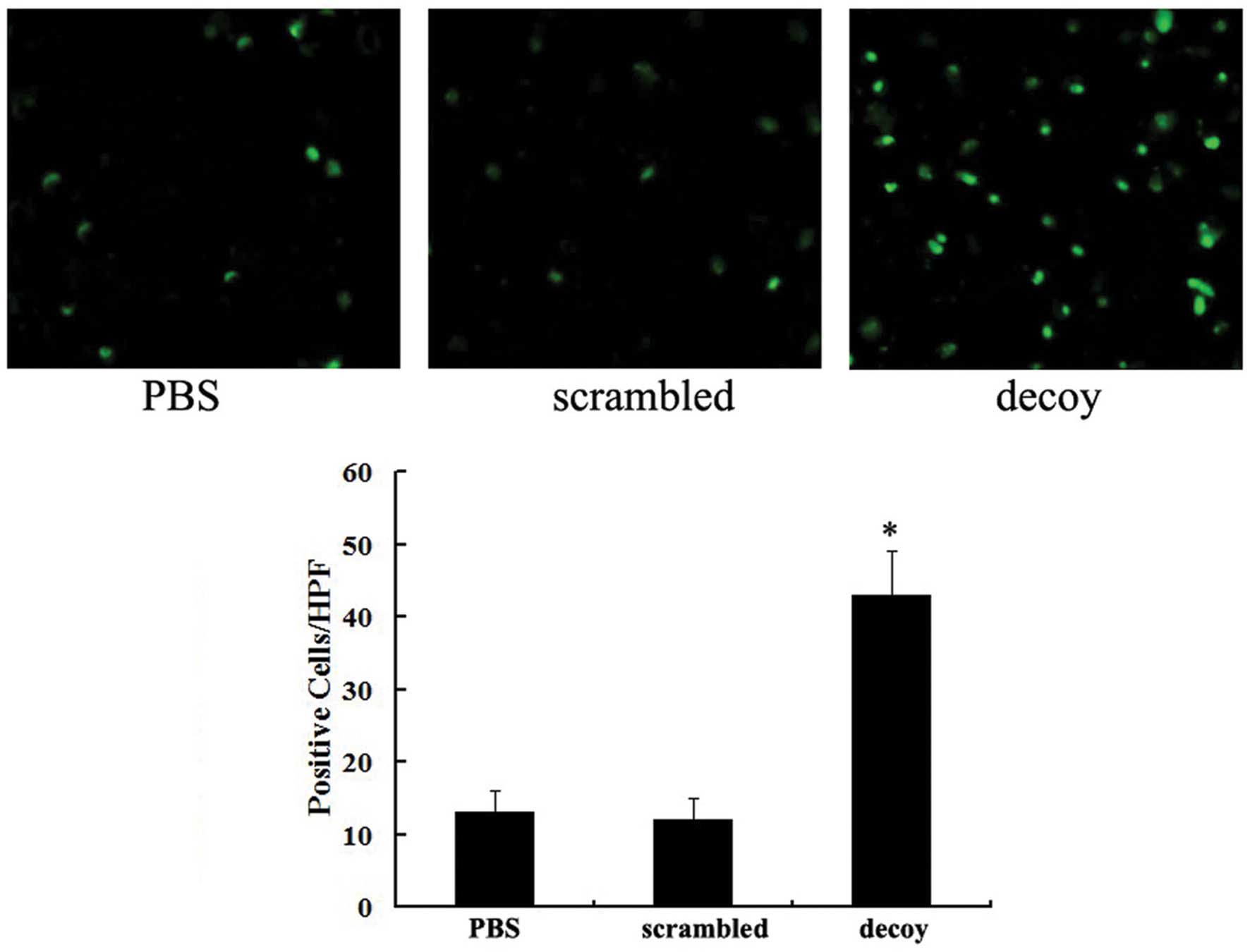
A fluorometric TUNEL assay was performed to detect
the apoptotic cells in the tumor tissues of the nude mice. Cell
nuclei with green fluorescent staining were defined as apoptotic
cells. TUNEL assay revealed that there were 43±7 apoptotic
cells/high power field (/HPF) in the group treated with the STAT3
decoy ODN, while there were 11±3 apoptotic cells in the scrambled
group and 13±4 apoptotic cells in the PBS group (Fig. 4). The difference between the decoy
group and the other 2 groups was statistically significant
(P<0.05). These results suggested that the STAT3 decoy ODN
promoted ovarian cancer cell apoptosis in vivo.
Side-effects of STAT3 decoy ODN on tumor
tissues and vital organs of nude mice
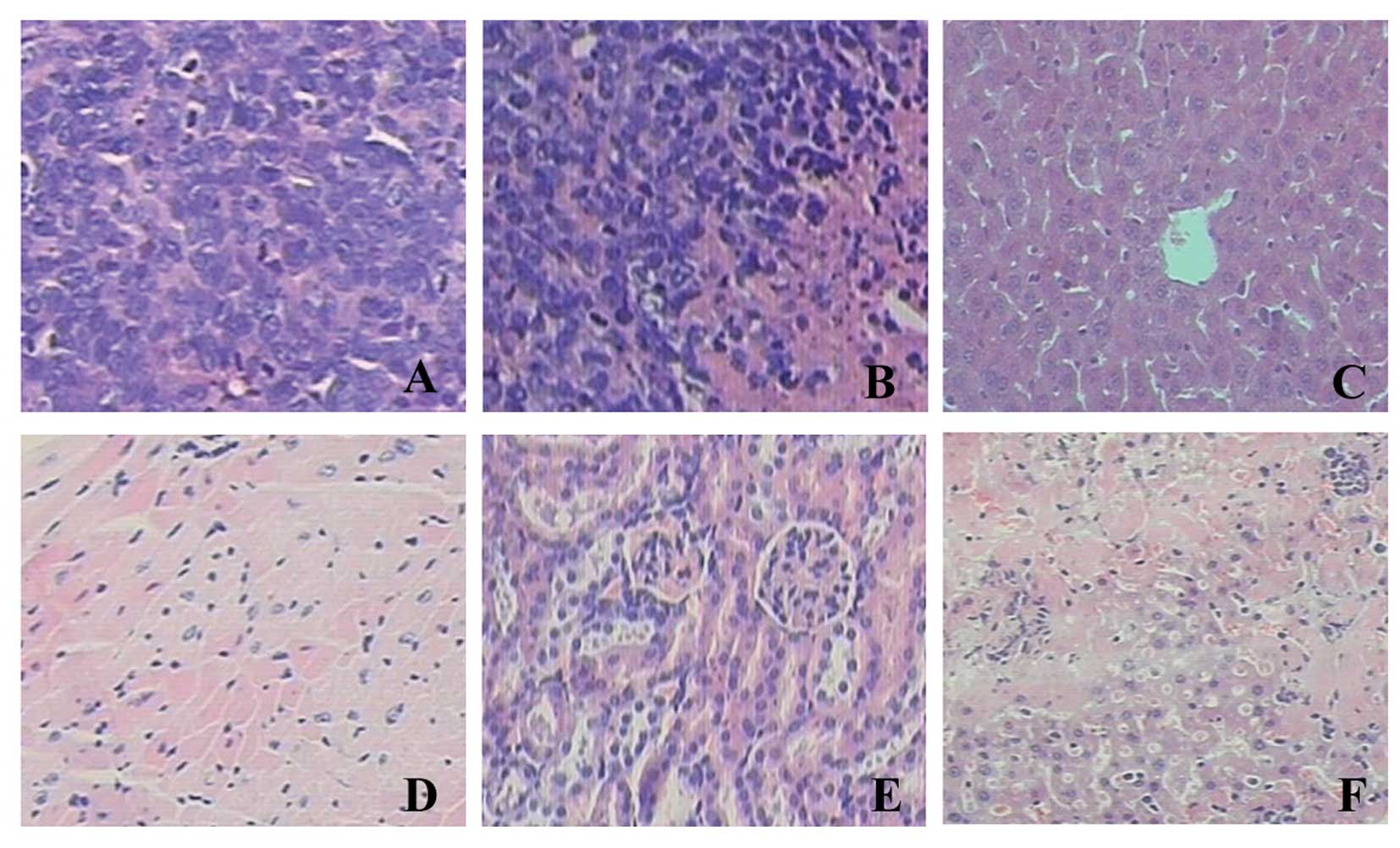
In the tumor tissue sections, H&E staining
revealed karyomegaly, anachromasis and karyokinesis in the cancer
cells (Fig. 5). Necrosis was
observed partly in the xenograft tissues. The sections of the heart
and kidney tissues showed no significant abnormalities. However, 1
in 5 nude mice treated with the STAT3 decoy ODN had slight
inflammation and necrosis in parts of the hepatic lobule.
Effects of STAT3 decoy ODN on the protein
expression of MMP-2, MMP-9, Bcl-2 and caspase-3 in vivo
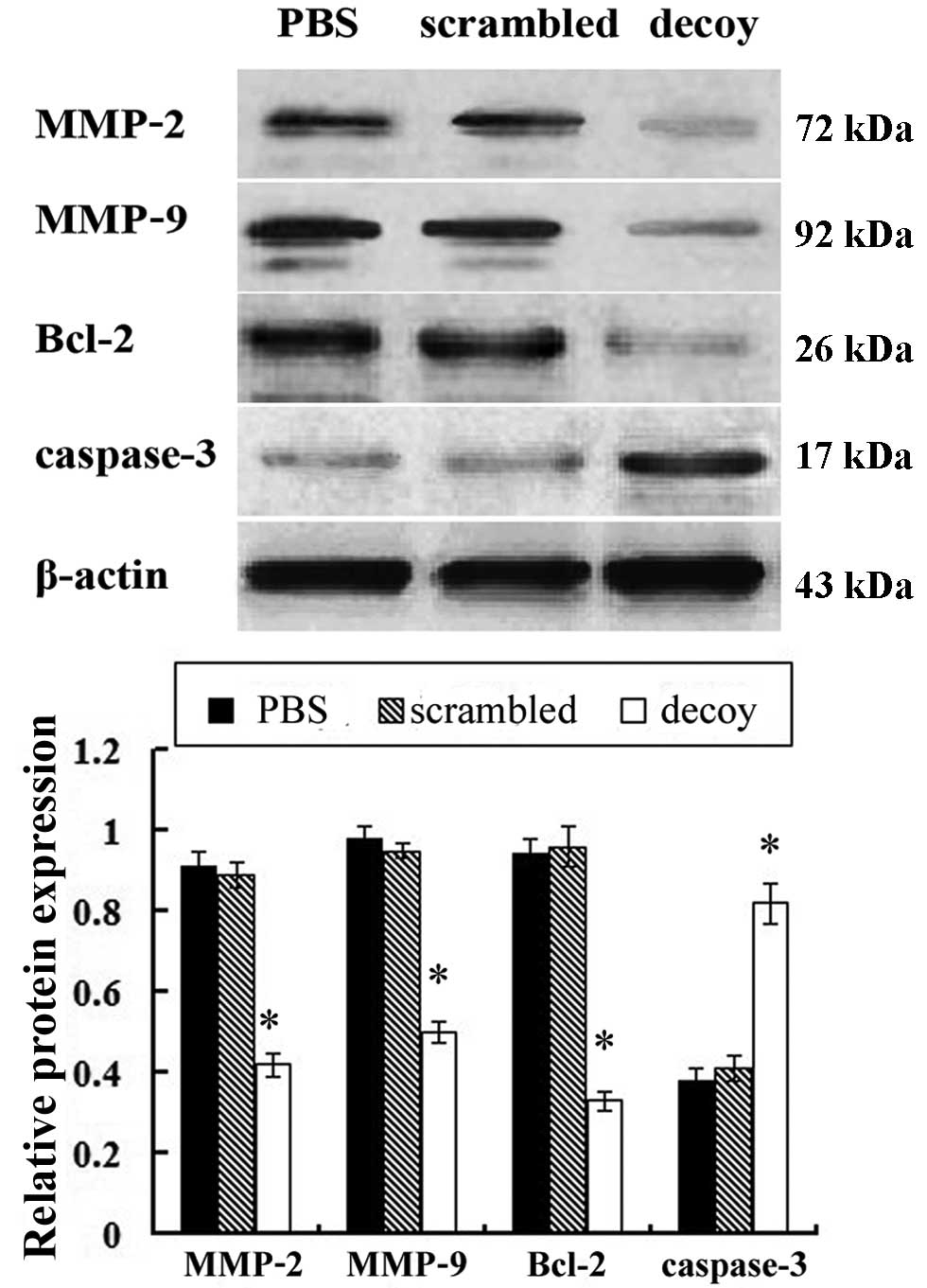
Western blot analysis was used to examine the
protein expression of MMP-2, MMP-9, Bcl-2 and caspase-3 in the
xenograft tissues of the nude mice. Compared with the PBS and
scrambled treatment group, the protein expression level of MMP-2,
MMP-9, Bcl-2 in the STAT3 decoy ODN treatment group was
significantly downregulated, while the protein expression level of
caspase-3 was significantly upregulated (Fig. 6).
Discussion
STAT3 has been recognized as an oncogene, due to its
oncogenic role (25).
Constitutive STAT3 activation is frequently involved in
uncontrolled tumor cell proliferation and therefore constitutes a
valuable target for antitumor therapy (26). Studies have shown that elevated
levels of total and/or tyrosine phosphorylated STAT3 (pSTAT3) in
the tumor are associated with decreased survival rates in cancer
patients, and this suggests that STAT3 may serve as a therapeutic
target (27). A previous study
reported that STAT3 is associated with the aggressive biological
behavior of ovarian cancer cells (28). However, the specific mechanisms
involved are unclear. Decoy ODNs have been shown to efficiently
induce cell death in a variety of cellular systems (29) and to have potential for the
specific targeting of tumor cells. The decoy ODN technology is a
novel tool, which has advantages of low cost, specificity,
simplicity and effectiveness. Moreover, the sequence of decoy ODNs
is relatively stable. This technology has been successfully used to
inhibit STAT3 pathway activation in squamous cell carcinoma of the
head and neck (SCCHN), pancreatic cancer and human lung cancer.
However, studies on the effects of STAT3 decoy ODNs on ovarian
cancer are limited, and the biological effects of STAT3 decoy ODNs
in vivo have not yet been fully elucidated.
In this study, to elucidate the antitumor effects of
STAT3 on ovarian cancer in vivo, we used the novel
technology, decoy ODNs, to regulate the STAT3 pathway in
xenografted nude mice. The major findings of the present study were
as follows: i) STAT3 decoy ODNs inhibited ovarian cancer cell
growth and promoted ovarian cancer cell apoptosis in vivo;
ii) STAT3 decoy ODNs downregulated the protein expression levels of
MMP-2, MMP-9, Bcl-2 and upregulated the protein expression level of
caspase-3 in vivo; iii) STAT3 decoy ODNs may have
side-effects on the livers of nude mice.
We monitored tumor growth during the treatment
period, and used tumor growth curves to reflect the inhibitory
effects of 3 different treatments. The growth curves revealed that
the growth of the tumors treated with STAT3 decoy ODN was
significantly inhibited compared with the other treatment groups
(Fig. 2). After the treatment was
terminated, we removed the tumor tissues from the nude mice. The
average volume and average weight of the tumor tissues in the decoy
group were significantly lower than those in the other control
groups (P<0.05). These findings indicated that the STAT3 decoy
ODN inhibited ovarian cancer growth in the nude mice. Studies have
shown that STAT3 decoy ODNs induce cancer cell apoptosis in
vitro (20,21). In this study, in order to detect
the apoptotic cells in the tumor tissues from the nude mice,
fluorometric TUNEL assay was performed. The results revealed that
the number of apoptotic cells/HPF in the STAT3 decoy ODN treatment
group was higher than that in the PBS and scrambled groups. These
results suggested that the STAT3 decoy ODN promoted ovarian cancer
cell apoptosis in vivo. These results are consistent with
those of other studies, showing that blocking the activation of
STAT3 suppresses tumor growth and induces tumor cell apoptosis
in vivo (22,30).
In this study, we used western blot analysis to
detect the expression levels of apoptosis-related proteins. The
protein expression levels of MMP-2, MMP-9, Bcl-2 in the STAT3 decoy
ODN treatment group significantly decreased, while the protein
expression level of caspase-3 significantly increased, compared
with the PBS and scrambled treatment group. MMPs are zinc-dependent
endopeptidases able to degrade components of the basement membrane
and the extracellular matrix (ECM) (31). MMP-2 and MMP-9 are believed to be
vital in the invasion of malignant tumors and angiogenesis. A
previous study found that MMP-2 and MMP-9 induced the release of
vascular endothelial growth factor (VEGF) in ovarian carcinoma
cells, and promoted ascite formation (32). Our results demonstrated that the
intratumoral injection of STAT3 decoy ODN decreased the expression
of MMP-2 and MMP-9 in the tumor tissues of nude mice. This finding
suggests that the downregulation of MMP-2 and MMP-9 may be one of
the mechanisms involved in the STAT3 decoy ODN inhibition of cancer
cell invasion and metastasis. Bcl-2 is a type of anti-apoptotic
protein, the tumorigenic potential of which has been demonstrated
in animal models and in certain human tumors (33). The anti-apoptotic protein, Bcl-2,
is associated with the inhibition of apoptosis, and its expression
correlates with chemoresistance in cancer patients. Several studies
have demonstrated that the inhibition of STAT3 by flavonoids and
synthetic compounds results in tumor cell apoptosis in vitro
(34,35). In our study, we found that cancer
cell apoptosis was promoted and that the expression of Bcl-2 was
decreased following the treatment of mouse xenografts with STAT3
decoy ODN. This result indicates that STAT3 decoy ODNs promote
cancer cell apoptosis by inhibiting the anti-apoptotic protein,
Bcl-2, in vivo. Caspase-3 is a central effector caspase in a
number of cell types, leading to DNase activation followed by DNA
fragmentation (36). Activated
caspase-3 has been shown to translocate to the nucleus during
apoptosis and to play an important role in the nuclear changes in
apoptotic cells (37). Moreover,
our study showed that the expression of caspase-3 was downregulated
following treatment with STAT3 decoy ODNs.
Studies on the toxicity of STAT3 decoy ODNs are
limited. In this study, we detected the biological effects of STAT3
decoy ODNs on heart, liver and kidney tissues from xenografted nude
mice. In the sections of xenograft tissues (stained with H&E),
we observed karyomegaly, anachromasis and karyokinesis in the
cancer cells. Necrosis was observed partly in the xenograft
tissues. The sections of the heart and liver tissues showed no
significant abnormalities. However, 1 in 5 nude mice treated with
STAT3 decoy ODN showed some abnormalities in the organs, such as
slight inflammation and necrosis in parts of the hepatic lobule.
These findings suggest that STAT3 decoy ODNs may have side-effects
on the livers of nude mice. The biological effects of STAT3 decoy
ODNs on tissues of the heart, liver and kidneys in vivo
require further investigation.
In conclusion, our study provides evidence that
STAT3 decoy ODNs suppress growth and induce the apoptosis of
ovarian cancer cells in vivo. The molecular mechanisms
behind the inhibitory effects of STAT3 decoy ODNs may involve the
downregulation of the protein expression of MMP-2, MMP-9 and Bcl-2
and the upregulation of the protein expression of caspase-3 in
vivo. Moreover, we examined the side-effects of STAT3 decoy
ODNs on the vital organs of nude mice. We found that there were no
significant abnormalities in the vital organs of the nude mice
apart from slight inflammation and necrosis in parts of the hepatic
lobule. Taken together, the data presented in this study
demonstrate that the blockade of aberrantly activated STAT3 with
decoy ODNs may be an efficient strategy for the treatment of
ovarian cancer in vivo.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics. CA Cancer J Clin.
56:106–130. 2006.
|
|
2
|
Edwards BK, Brown ML, Wingo PA, Howe HL,
Ward E, Ries LA, et al: Annual report to the nation on the status
of cancer, 1975–2002, featuring population-based trends in cancer
treatment. J Natl Cancer Inst. 97:1407–1427. 2005.
|
|
3
|
Bromberg JF: Activation of STAT proteins
and growth control. Bioessays. 23:161–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buettner R, Mora LB and Jove R: Activated
STAT signaling in human tumors provides novel molecular targets for
therapeutic intervention. Clin Cancer Res. 8:945–954.
2002.PubMed/NCBI
|
|
5
|
Catlett-Falcone R, Landowski TH, Oshiro
MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L,
Fernandez-Luna JL, Nunez G, Dalton WS and Jove R: Constitutive
activation of Stat3 signaling confers resistance to apoptosis in
human U266 myeloma cells. Immunity. 10:105–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao B, Shen X, Kunos G, Meng Q, Goldberg
ID, Rosen EM and Fan S: Constitutive activation of JAK-STAT3
signaling by BRCA1 in human prostate cancer cells. FEBS Lett.
488:179–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al Zaid Siddiquee K and Turkson J: STAT3
as a target for inducing apoptosis in solid and hematological
tumors. Cell Res. 18:254–267. 2008.PubMed/NCBI
|
|
8
|
Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia
W, Wei Y, Bartholomeusz G, Shih JY and Hung MC: Nuclear interaction
of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer
Cell. 7:575–589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park OK, Schaefer TS and Nathans D: In
vitro activation of Stat3 by epidermal growth factor receptor
kinase. Proc Natl Acad Sci USA. 93:13704–13708. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turkson J and Jove R: STAT proteins: novel
molecular targets for cancer drug discovery. Oncogene.
19:6613–6626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2648–2673. 2000. View Article : Google Scholar
|
|
12
|
Landen CN Jr, Lin YG, Armaiz Pena GN, et
al: Neuroendocrine modulation of signal transducer and activator of
transcription 3 in ovarian cancer. Cancer Res. 67:10389–10396.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duan Z, Foster R, Bell DA, Mahoney J,
Wolak K, Vaidya A, Hampel C, Lee H and Seiden MV: Signal
transducers and activators of transcription 3 pathway activation in
drug-resistant ovarian cancer. Clin Cancer Res. 12:5055–5063. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nefedova Y and Gabrilovich DI: Targeting
of Jak/STAT pathway in antigen presenting cells in cancer. Curr
Cancer Drug Targets. 7:71–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schlessinger K and Levy DE: Malignant
transformation but not normal cell growth depends on signal
transducer and activator of transcription 3. Cancer Res.
65:5828–5834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai L, Zhang G, Tong X, You Q, An Y, Wang
Y, Guo L, Wang T, Zhu D and Zheng J: Growth inhibition of human
ovarian cancer cells by blocking STAT3 activation with small
interfering RNA. Eur J Obstet Gynecol Reprod Biol. 148:73–80. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang L, Ma X, Xiao L, Tang M, Weng X, Sun
L and Cao Y: Uniquely modified RNA oligonucleotides targeting STAT3
suppress melanoma growth both in vitro and in vivo. Cancer Biol
Ther. 8:2065–2072. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu J, Li G, Sun T, Su Y, Zhang X, Shen J,
Tian Z and Zhang J: Blockage of the STAT3 signaling pathway with a
decoy oligonucleotide suppresses growth of human malignant glioma
cells. J Neurooncol. 89:9–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Souissi I, Najjar I, Ah-Koon L, et al: A
STAT3-decoy oligonucleotide induces cell death in a human
colorectal carcinoma cell line by blocking nuclear transfer of
STAT3 and STAT3-bound NF-κB. BMC Cell Biology. 12:142011.PubMed/NCBI
|
|
20
|
Zhang X, Liu P, Zhang B, Wang A and Yang
M: Role of STAT3 decoy oligodeoxynucleotides on cell invasion and
chemosensitivity in human epithelial ovarian cancer cells. Cancer
Genet Cytogenet. 197:46–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leong PL, Andrews GA, Johnson DE, Dyer KF,
Xi S, Mai JC, Robbins PD, Gadiparthi S, Burke NA, Watkins SF and
Grandis JR: Targeted inhibition of Stat3 with a decoy
oligonucleotide abrogates head and neck cancer cell growth. Proc
Natl Acad Sci USA. 100:4138–4143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Zhang L, Wang L, Wei H and Tian
Z: Therapeutic effects of STAT3-decoy oligodeoxynucleotide on human
lung cancer in xenograft mice. BMC Cancer. 7:1492007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Devalapally H, Duan Z, Seiden MV and Amiji
MM: Modulation of drug resistance in ovarian adenocarcinoma by
enhancing intracellular ceramide using tamoxifen-loaded
biodegradable polymeric nanoparticles. Clin Cancer Res.
14:3193–3203. 2008. View Article : Google Scholar
|
|
24
|
Mao HL, Liu PS, Zheng JF, Zhang PH, Zhou
LG, Xin G and Liu C: Transfection of Smac/DIABLO sensitizes
drug-resistant tumor cells to TRAIL or paclitaxel-induced apoptosis
in vitro. Pharmacol Res. 56:483–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bromberg J: Stat proteins and oncogenesis.
J Clin Invest. 109:1139–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fletcher S, Turkson J and Gunning PT:
Molecular approaches towards the inhibition of the signal
transducer and activator of transcription 3 (Stat3) protein.
ChemMedChem. 3:1159–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leeman RJ, Lui VW and Grandis JR: STAT3 as
a therapeutic target in head and neck cancer. Expert Opin Biol
Ther. 6:231–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Colomiere M, Findlay J, Ackland L and
Ahmed N: Epidermal growth factor-induced ovarian carcinoma cell
migration is associated with JAK2/STAT3 signals and changes in the
abundance and localization of alpha6beta1 integrin. Int J Biochem
Cell Biol. 41:1034–1045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jing N and Tweardy DJ: Targeting Stat3 in
cancer therapy. Anticancer Drugs. 16:601–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen J, Li R and Li G: Inhibitory effects
of decoy-ODN targeting activated STAT3 on human glioma growth in
vivo. In Vivo. 23:237–243. 2009.PubMed/NCBI
|
|
31
|
Liotta LA and Stetler-Stevenson WG: Tumor
invasion and metastasis: an imbalance of positive and negative
regulation. Cancer Res. 51(Suppl 18): 5054S–5059S. 1991.PubMed/NCBI
|
|
32
|
Belotti D, Paganoni P, Manenti L, Garofalo
A, Marchini S, Taraboletti G and Giavazzi R: Matrix
metalloproteinases (MMP9 and MMP2) induce the release of vascular
endothelial growth factor (VEGF) by ovarian carcinoma cells:
implications for ascites formation. Cancer Res. 63:5224–5229.
2003.
|
|
33
|
dos Santos LG, Lopes-Costa PV, dos Santos
AR, Facina G and da Silva BB: Bcl-2 oncogene expression in estrogen
receptor-positive and negative breast carcinoma. Eur J Gynaecol
Oncol. 29:459–461. 2008.
|
|
34
|
Alas S and Bonavida B: Inhibition of
constitutive STAT3 activity sensitizes resistant non-Hodgkin's
lymphoma and multiple myeloma to chemotherapeutic drug-mediated
apoptosis. Clin Cancer Res. 9:316–326. 2003.
|
|
35
|
Selvendiran K, Koga H, Ueno T, et al:
Luteolin promotes degradation in signal transducer and activator of
transcription 3 in human hepatoma cells: an implication for the
antitumor potential of flavonoids. Cancer Res. 66:4826–4834. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Enari M, Sakahira H, Yokoyama H, Okawa K,
Iwamatsu A and Nagata S: A caspase-activated DNase that degrades
DNA during apoptosis and its inhibitor ICAD. Nature. 391:43–50.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kamada S, Kikkawa U, Tsujimoto Y and
Hunter T: Nuclear translocation of caspase-3 is dependent on its
proteolytic activation and recognition of a substrate-like
protein(s). J Biol Chem. 280:857–860. 2005. View Article : Google Scholar : PubMed/NCBI
|