Introduction
Nesprin-1 is a protein isoform of the nesprin
protein family that contains spectrin repeats similar to those in
mAKAP, which forms homodimers and specifically targets the nuclear
envelope through a KASH domain (1–5).
Nesprin-1α is also a candidate for a mAKAP nuclear envelope
receptor. It has been reported that mutations in the nesprin-1 gene
may be responsible for adult cerebella ataxia and mutations of
nesprin-1 which interact with lamin A/C may lead to at least 2
distinct human disease phenotypes, myopathic or neurologic, a
feature similar to that found in laminopathies. Puckelwartz et
al (6) reported Δ/Δ KASH mice
expresses nesprin-1 without its carboxyl-terminal KASH domain;
these Δ/Δ KASH mice have a normally assembled but dysfunctioning
nuclear membrane complex and provide a model for nesprin-1
mutations and developing cardiomyopathy with associated cardiac
conduction system disease.
Mesenchymal stem cells (MSCs) are bone
marrow-derived cells that retain the capability to differentiate
into various types of tissue cells and contribute to the
regeneration of a variety of tissues, including bone, cartilage,
muscle and adipose tissue (7–9).
MSCs, after being transplantated into the ischemic myocardial
tissue, secrete a variety of factors including vascular endothelial
growth factor (VEGF). The cardioprotective effects of MSCs are
known to be mediated not only by their differentiation into
cardiomyocyte-like cells, but also by their ability to supply large
amounts of angiogenic, anti-apoptotic and mitogenic factors
(10–12). These findings suggest the
therapeutic potential of MSCs for heart failure.
From the literature, we know that nesprin proteins
exist only in multiple tissues (skeletal, cardiac and vascular
smooth muscle) and not in stem cells (2). The study of nesprin-1 protein, which
is speculated to be localized to the nuclear membrane, may aid in
the understanding of the process through which MSCs differentiate
into cardiomyocyte-like cells.
Materials and methods
Animals
Clean Sprague-Dawley (SD) rats, weighing 250–300 g,
were obtained from the Experimental Animal Center of Shanghai
Jiaotong University Medical School, Shanghai, China (production
license: scxk (hu)2004-0001; use license no. syxk (hu)2003–2009).
The present study was reviewed and approved by the University
Institutional Animal Care and Use Committee.
Reagents
Except where otherwise specified, all reagents were
obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and
Gibco (Grand Island, NY, USA), including cell culture medium
[low-glucose Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS)]. The cardiac-specific antibodies (TNI,
α-sarcomeric actin, desmin), FITC-conjugated goat anti-rat
antibodies (CD45), PE-conjugated rabbit anti-rat antibodies (CD90),
allophycocyanin (APC)-conjugated rabbit anti-rat antibodies (CD29)
and FITC-conjugated rabbit anti-rat nesprin-1 antibodies, were
purchased from Abcam (Cambridge, UK). The BLOCK-iT™ POLIImiR RNAi
Expression Vector kit with EmGFP, pcDNA™ 6.2-GW/EmGFPmiR,
Escherichia coli (E. coli) DH5, Lipofectamine®
2000, Opti-MEM, TRIzol reagent and pLenti6.3/V5-DEST were purchased
from Invitrogen (Carlsbad, CA, USA). Restriction endonuclease, DNA
ligase and the Large Plasmid DNA Extraction kit were purchased from
Qiagen (Dusseldorf, Germany). The reagents and instruments for
immunohistochemistry, immunofluorescence and western blot analysis
were purchased from Gibco, Sigma-Aldrich Chemical Co. and
Invitrogen, respectively.
Cell culture
Eight-week-old SD rats (250–300 g) were prepared as
donors. The procedures were performed in accordance with the
guidelines for animal experimentation of Shanghai Jiaotong
University and approved by the institutional ethics committee.
Under general anesthesia with ether (approximately 100 μl) bone
marrow was aspirated from both the tibia and femur with a 20-gauge
needle attached to a 10-ml syringe containing 0.5 ml DMEM with 40
U/ml heparin.
The concentration of the cells in suspension was
adjusted to 5×105 mononuclear cells/ml culture medium at
37°C in a humidified atmosphere with 5% CO2; the cells
were then seeded on culture plates, without removal of the red
blood cells. Since bone marrow-derived MSCs (BMSCs) grow initially
in colonies and do not reach confluence over the entire culture
dish, the cells were passaged 7 days after seeding, when half the
colonies reached 70–80% confluence; the cells were then passaged
weekly when the cells reached confluence. For subcultures, adherent
BMSCs wer harvested using 0.125% trypsin and plated at a ratio of
1:3.
For flow cytometry, the cells were detached using
accutase instead of trypsin, in order to achieve a better
preservation of the cell surface molecules. 293T cells were
maintained in MEM supplemented with 5% FBS and 50 mg/ml gentamycin.
The cells were trypsinized by a 0.05% trypsin-0.5 mM EDTA
solution.
Flow cytometry
Flow cytometry was performed using a FACSAria flow
cytometer/cell sorter (BD Biosciences, San Jose, CA, USA).
Following accutase treatment, the cells were resuspended at a
density of 1×105 cells/200 μl phosphate-buffered saline
(PBS) and incubated with 2% FCS (PBS-FCS) on ice. The cells were
stained with antibody, and incubated with FITC-conjugated CD45
monoclonal antibody, PE-conjugated CD90 monoclonal antibody and
APC-conjugated CD29 monoclonal antibody (at concentrations
indicated by the manufacturer) for 30 min at 4°C in the dark, and
then washed in PBS-FCS. After washing, the cells were analyzed in
the cytometer. At least 5,000 events were analyzed for each sample.
Negative controls, used to detect the unspecific bindings, included
an irrelevant antibody or PBS-FCS alone. The acquired data were
analyzed using Summit software (Cytomation, Inc., Fort Collins, CO,
USA).
Immunofluorescence microscopy
BMSCs grown on glass coverslips were fixed by a
20-min incubation in 4% formaldehyde (freshly prepared from
paraformaldehyde), rinsed in PBS, and stored in 70% ethanol at
−20°C. The fixed cells were blocked for 30 min in blocking solution
(PBS supplemented with 2% goat serum, 1% BSA, 0.1% gelatin, 0.1%
Triton X-100 and 0.05% Tween-10), and incubated overnight with the
primary antibody (at the dilution indicated by the manufacturer) at
4°C. After washing, the cells were incubated with the secondary
antibody (FITC-conjugated anti-rat IgG for nesprin-1) for 30 min.
Finally, the coverslips were washed, mounted in glycerol and
examined under an epifluorescence microscope (Olympus, Tokyo,
Japan).
Western blot analysis
After washing with PBS, the BMSCs were scraped off
the culture dish and transferred to centrifuge tubes. Following
centrifugation at 700 × g for 10 min at 4°C, the pellets were lysed
in hot Laemmli loading buffer (62.5 mmol/l Tris-HCl, pH 6.8, 2%
SDS, 10% glycerol, 0.05% β-mercaptoethanol, 0.05% bromophenol
blue). Equal amounts of protein extracts (20 mg/lane) were
subjected to SDS-PAGE on a 5% stacking gel and a 10% separating
gel, followed by transfer of the proteins onto nitrocellulose
membranes (20 min at 10 V). After blocking in PBS containing 0.05%
Triton X-100 (TBS) and 5% FCS for 1 h, the blots were incubated
overnight with primary antibodies (rabbit anti-rat nesprin-1) at
4°C. After washing, the membranes were incubated with the secondary
antibody (HRP-conjugated goat anti rabbit IgG) for 1 h; the bound
antibody was detected by ECL. β-actin was used as an internal
control.
Design and cloning of small interfering
RNA (siRNA) cassettes
The nesprin-1 DNA and protein sequence was according
to the GenBank accession no. NM_001029909.1. The nesprin-1 gene
siRNA sequence and the corresponding miRNA oligonucleotide sequence
were then designed and synthesized using Ambion design software and
the sequences were verified using BLAST software: (5′-CGGGAGTTGTTG
ACTATGAAA-3′); the corresponding miRNA oligonucleotide sequence was
as follows: nesprin-1 forward,
TGCTGTTTCATAGTCAACAACTCCCGGTTTTGGCCACTGACTGACCGGGAGTTTGACTATGAAA;
and reverse,
CCTGTTTCATAGTCAAACTCCCGGTCAGTCAGTGGCCAAAACCGGGAGTTGTTGACTATGAAAC.
The Vector Cloning kit was then used for restructuring; the
double-stranded miRNA oligonucleotide was inserted into the miRNA
expression vector (Invitrogen), and the cells were transfected with
miRNA plasmids infected with E. coli DH5. The
oligonucleotides were annealed and cloned into the
BglII-HindIII site. The pDONR221 vector was processed
with BP recombination reaction, in order to obtain the entry vector
containing the siRNA. The sequence of the entry vector with siRNA
and the lentiviral expression vector pLenti6/V5-DEST were processed
with LR recombination reaction in order to obtain the lentiviral
expression vector expressing siRNA targeting nesprin-1
(LV-siNesprin-1).
Transfection
The 293T cells were co-transfected with a plasmid
expressing GFP together with a plasmid expressing siRNA specific
for GFP (siGFP) at a ratio of 1:5 using FuGENE®
transfection reagent (Roche, Indianapolis, IN, USA).
Lentiviral vector production
Recombinant lentiviruses were produced by the
transient transfection of 293T cells using the calcium-phosphate
method as previously described (13–15). Infectious lentiviruses were
harvested at 48 and 72 h post-transfection and filtered through
0.22-μm-pore cellulose acetate filters as previously described
(13–15). Recombinant lentiviruses were
concentrated by ultracentrifugation (2 h at 50,000 × g) and
subsequently purified on a sucrose 20% gradient (2 h at 46,000 × g)
as previously described (16).
Vector concentrations were analyzed using an immunocapture p24-gag
ELISA (Alliance; DuPont/NEN, Boston, MA, USA) as previously
described (16); the concentrated
suspension of the activity of the viral titer was measured
(1×106 TU/ml).
Protein levels were analyzed by western blot
analysis (concrete steps as the former) and immunoblotting was
carried out according to standard methods with rabbit moclonal
antibody against GFP (Abcam) or β-actin (Sigma-Aldrich Chemical
Co.). Fluorescence-activated cell-sorter analysis was carried out
as previously described (17–19).
MTT cell proliferation assay
The cells were plated on 24-well plates
(2×104 cells/well) in the growth medium for the assays.
The protocols for MTT assays were as previously described (20). In brief, growth medium containing
0.25 mg/ml MTT was added to each well and the cells were further
incubated at 37°C for 20 min, following which the medium was
replaced by 0.2 ml DMSO/well. MTT dye conversion was determined by
measuring the OD540 nm of the DMSO extracts using DMSO
as the blank control.
Detection of cell cycle of MSCs by low
cytometry
The cells (5×105) were centrifugated for
5 min at 800 rpm, the supernatant was collected, and they were then
washed with cold PBS twice and fixed with 700 ml/l cold ethanol at
4°C overnight. The ethanol was removed by centrifugation prior to
detection. The cells were washed with PBS twice, then stained with
1 ml PI (bromide tablets) at 4°C for 30 min in the dark.
Detection of MSC apoptosis by flow
cytometry
The cells (5×106) were centrifugated for
5 min at 1,000 rpm, then the culture medium was discarded. They
were then washed once with PBS, centrifuged and the supernatant was
removed; they were then fixed with 70% cold ethanol at 4°C for 1–2
h. The ethanol was then removed by centrifugation. The cells were
resuspended with 3 ml PBS for 5 min, filtered once and centrifuged
at 1,500 rpm for 5 min. The PBS was discarded prior to detection.
The cells were stained with 1 ml PI and FITC-Annexin V in 4°C for
30 min in the dark.
Morphology of the nucleus following
staining of MSCs with 4,6-diamidino-2-phenylindole (DAPI) for 72 h
and transfection with LV-siNesprin-1
The cells were passaged when they became nearly
confluent. Sterile DAPI solution was added to the culture medium.
The MSCs were rinsed 6 times in PBS solution to remove all excess
unbound DAPI. The MSCs were cultured in culture medium [DMEM,
supplemented with 20% FBS and penicillin (100 U/ml)/streptomycin
(100 μg/ml)] at 37°C in a humidified atmosphere with 5%
CO2 for 72 h; they were then examined under a
microscope.
Statistical analysis
Image programmer software was used to analyze the
images. Data are presented as the means ± standard deviation (SD).
Statistical analyses were performed using paired t-tests where
applicable. Statistical analysis was performed using SPSS and
GraphPad Prism 5 Demo software. A p-value <0.05, based on a
two-tailed test, was considered to indicate a statistically
significant difference.
Results
Characterization of MSCs
After discarding the non-adherent cells by the first
medium change and washing with PBS 3 times at 24 h of primary
culture, approximately 80% of the MSCs had adhered to the culture
dishes; the medium was then changed to remove the suspension of
hematopoietic stem cells. After 3 days in primary culture, the MSCs
adhered to the plastic surface, presenting a small population of
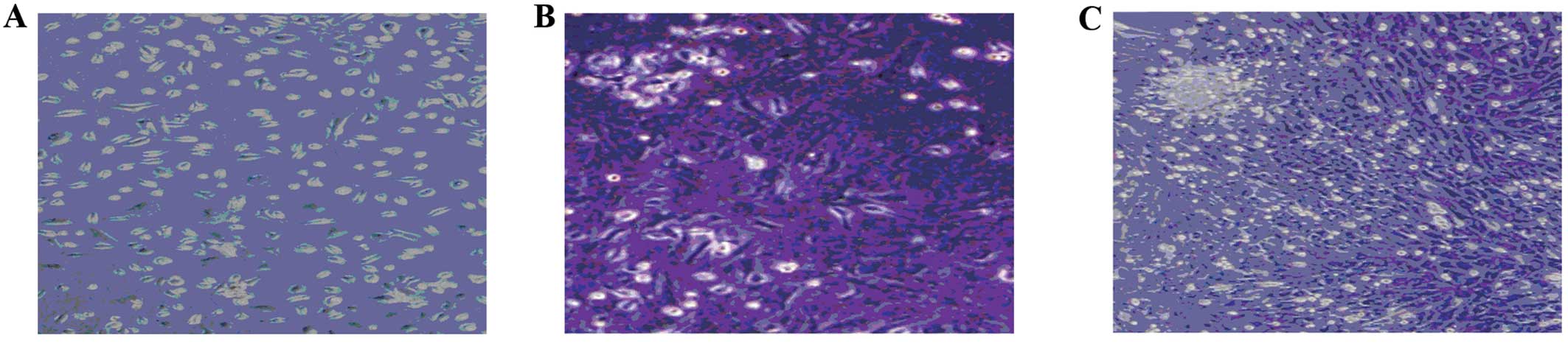
single cells. The cells were spindle-shaped with a single nucleus
(Fig. 1A). Seven to 10 days after
initial plating, the cells resembled long spindle-shaped fibroblast
cells and began to form colonies (Fig. 1B and C). After replating, almost
100% of the cells had adhered to the culture dishes, and were
polygonal or spindle-shaped, with long processes.
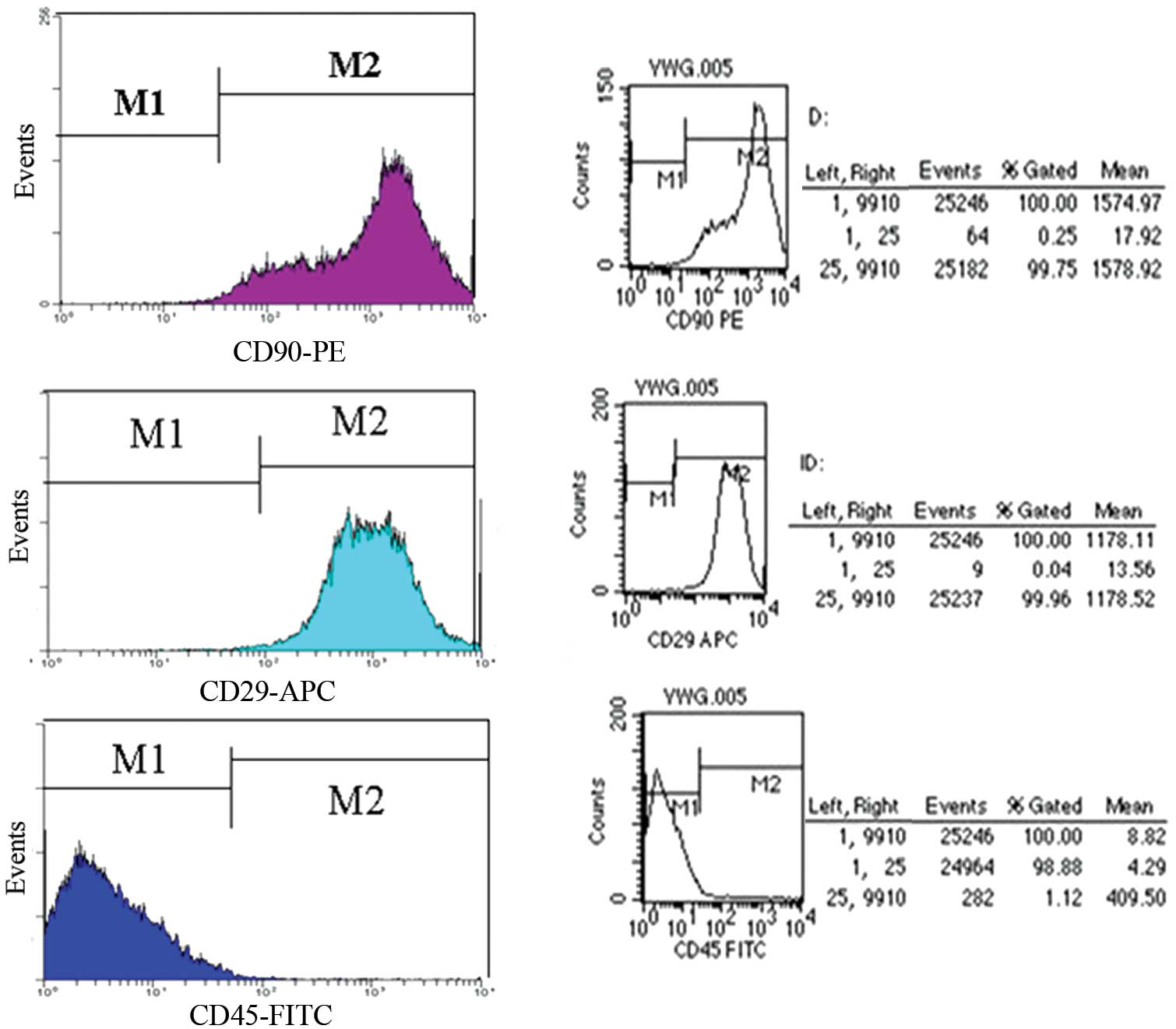
The rat MSC surface antigen profiles obtained by
flow cytometry (Fig. 2), were
positive for CD90, CD29 and negative for CD45. The percentage of
CD90 and CD29 was 99.96 and 99.75%, respectively; however, the
percentage of CD45 was 1.12%.
Detection of protein expression of
nesprin-1 in MSCs by immunofluorescence and western blot
analysis
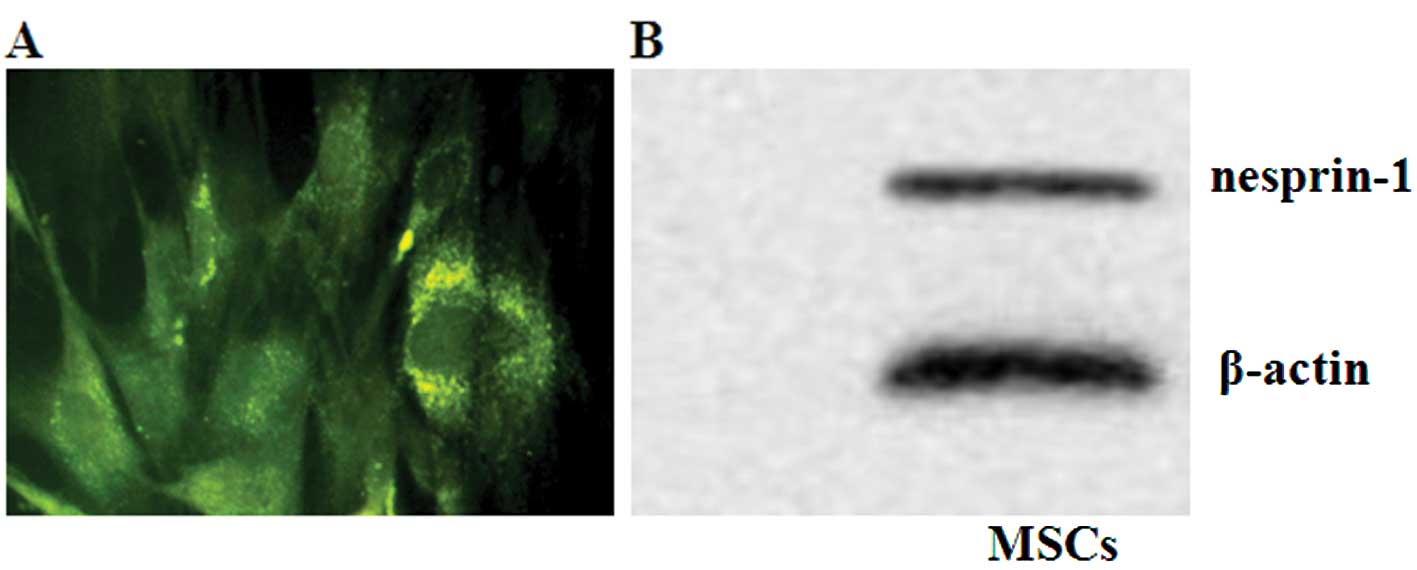
Immunofluorescent staining for nesprin-1 protein
verified the presence of rat MSCs (Fig. 3A), with granular green
fluorescence distributed around the nuclear membrane of the MSCs.
The protein expression of nesprin-1 was detected by western blot
analysis (Fig. 3B).
Transfection of MSCs with LV-siNesprin-1,
LV-GFP and the detection of protein expression of nesprin-1 by
western blot analysis
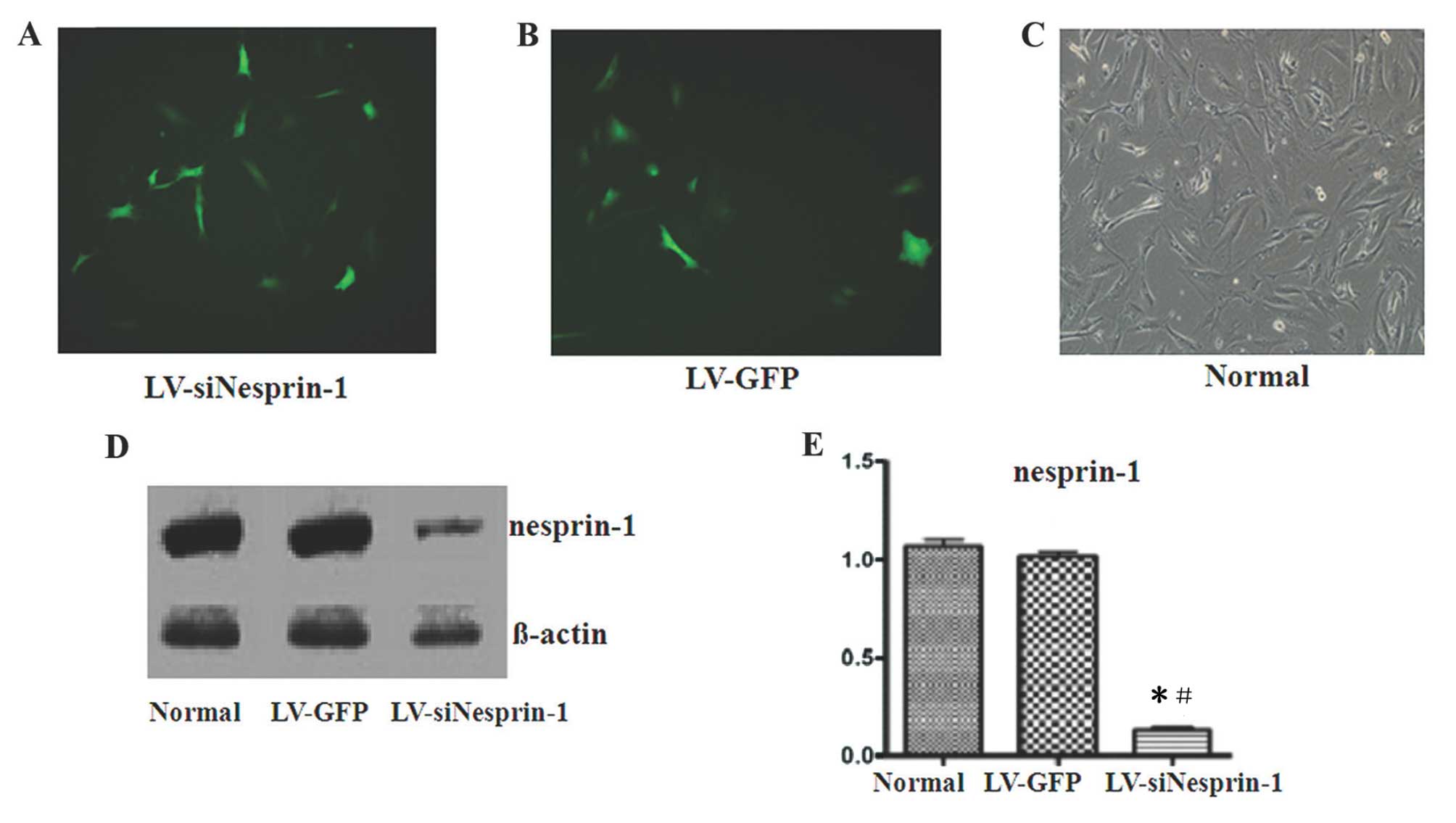
The MSCs were successfully transfected with
LV-siNesprin-1 or LV-GFP, as shown by green fluorescence (Fig. 4A–C). After the MSCs were
transfected with LV-siNesprin-1, the protein expression of
nesprin-1 in the LV-siNesprin-1 group was lower than that in the
LV-GFP and normal group (Fig.
4D). The protein expression of nesprin-1 in the LV-GFP group
was the same as that in the normal group, but was significanlty
different from that in the LV-siNesprin-1 group (P=0.03 and
P=0.028, respectively; P<0.05) (Fig. 4E). The expression of β-actin did
not differ between the 3 groups (P=0.10 and P=0.12, respectively;
P>0.05).
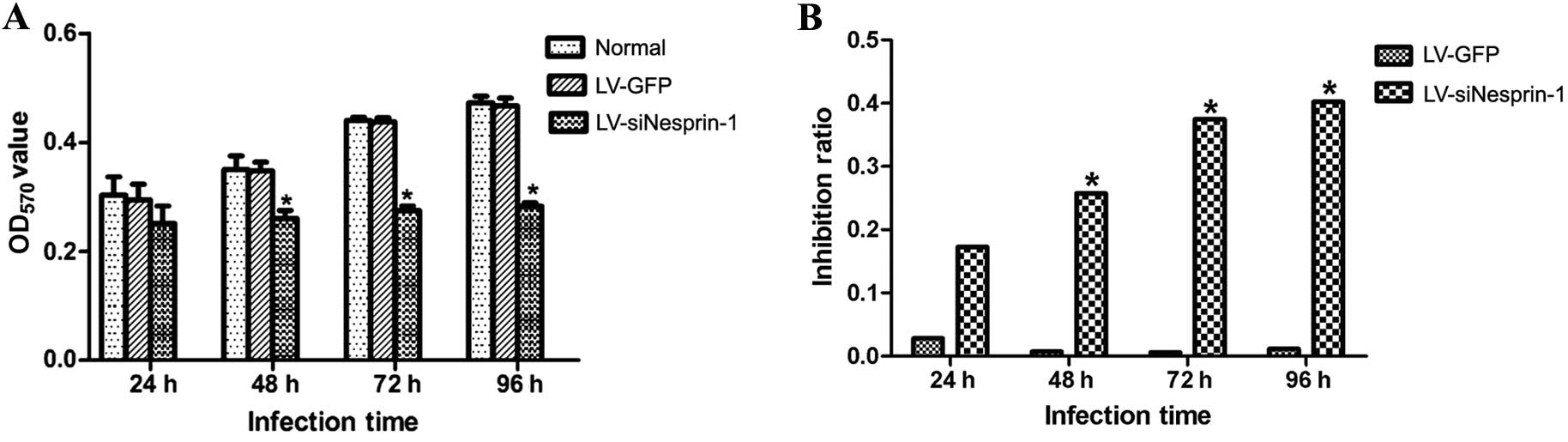
Detection of cell proliferation by MTT
assay
The 3 groups of cells (LV-siNesprin-1, LV-GFP and
normal group) were cultured for 24, 48, 72 and 96 h under the same
conditions. The proliferation of the cells was then detected by MTT
assay at 24, 48, 72 and 96 h. As shown in Fig. 5, the knockdown of nesprin-1
expression led to a decrease in the proliferation of MSCs; however,
the proliferation rate of the cells in the LV-GFP group was not
altered and was similar to the normal group.
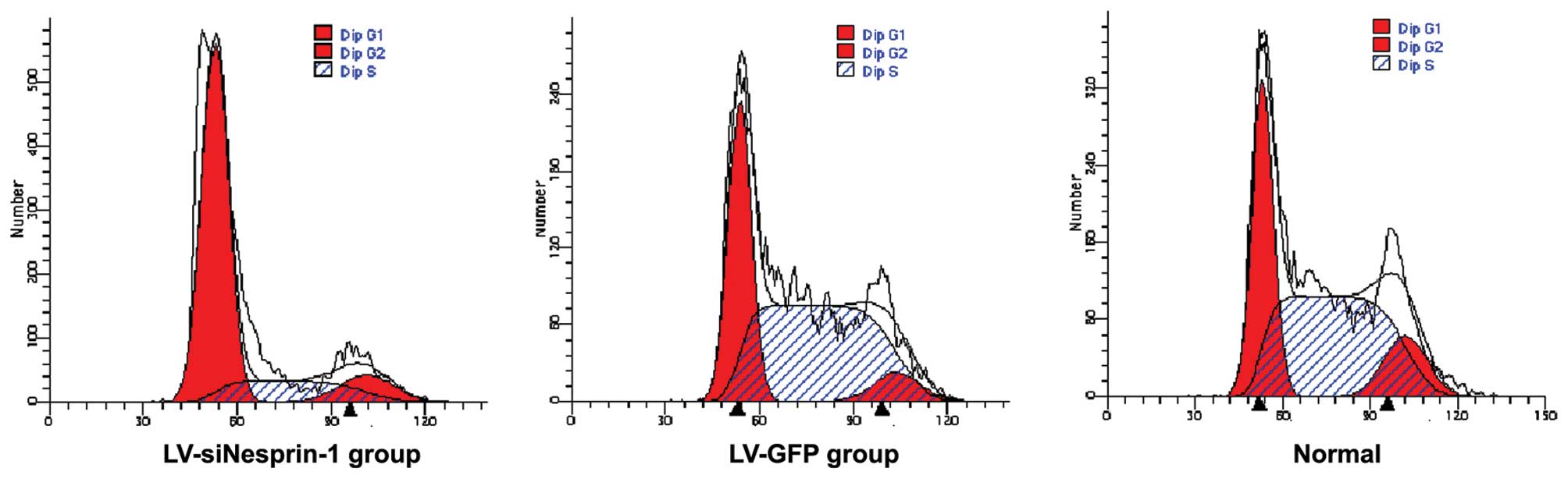
Cell cycle of MSCs in the 3 groups
(LV-siNesprin-1, LV-GFP and normal group)
The 3 groups of cells (LV-siNesprin-1, LV-GFP and
normal group) were cultured for 72 h under the same conditions. The
cell cycle was detected by flow cytometry. In the LV-siNesprin-1
group, the cells were mainly in the G0/G1 phase of the cell cycle
(Fig. 6).
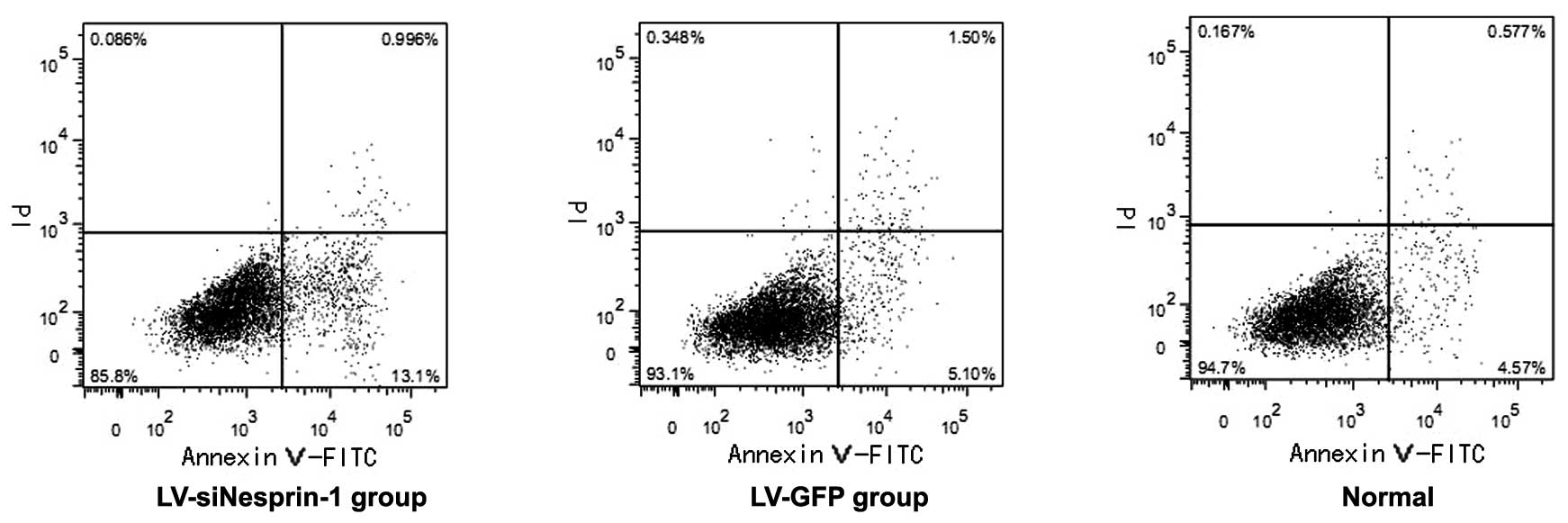
Apoptosis of MSCs in the 3 groups
(LV-siNesprin-1, LV-GFP and normal group)
The 3 groups of cells (LV-siNesprin-1, LV-GFP and
normal group) were cultured for 72 h under the same conditions. The
apoptosis of the cells was detected by flow cytometry. In the
LV-siNesprin-1 group, the apoptotic rate of the cells was higher
than that of the cells in the LV-GFP and normal group (Fig. 7).
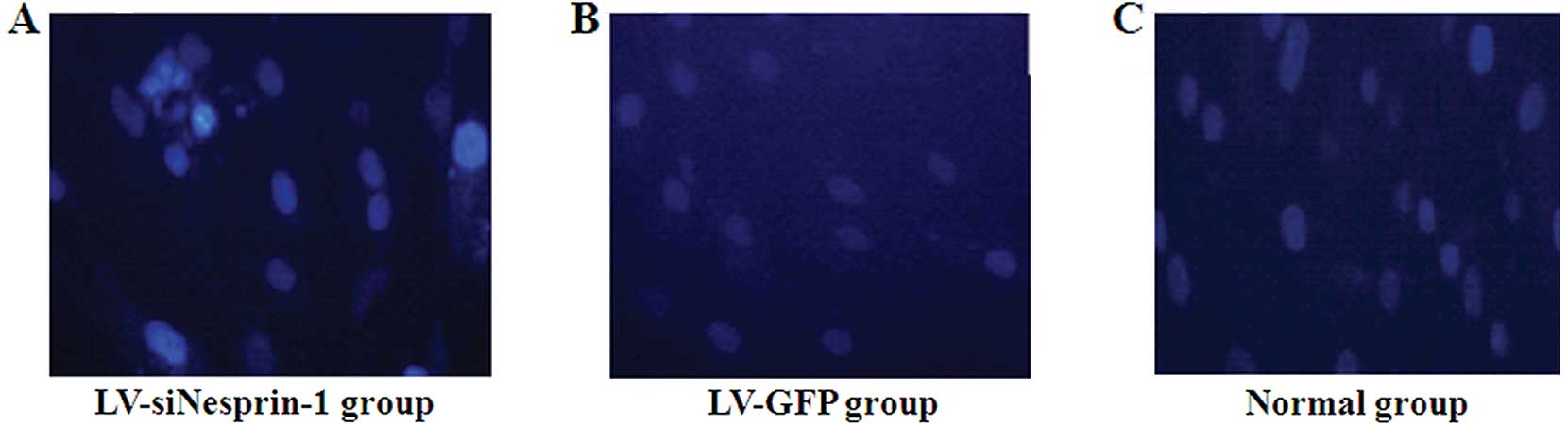
Changes in the morphology of the nucleus
following transfection of MSCs with LV-siNesprin-1
As shown in Fig.
8, the morphology of the nucleus in the LV-siNesprin-1 group
was altered; morphological changes such as fusion and fragmentation
were observed.
Discussion
Nesprins have been reported to bind to the nuclear
membrane. Through interaction with both emerin and lamin A/C,
nesprin is localized to the nuclear membrane (2,4,5,21).
The structure of the various nesprin isoforms suggests that they
also form a protein scaffold that links the nuclear membrane with
the nucleus, cytoplasmic organelles and the cell membrane via the
actin cytoskeleton. Nesprin (C. elegans ANC-1) mutations may
disrupt the positioning of the mitochondria in mononuclear cells
(22). These studies suggest a
structural role for nesprins in nuclear migration and the
positioning of major organelles (23). Nesprin (Drosophila MSP-300)
has been shown to be localized to regions of the cell margin
involved in exoskeletal attachment and to the Z-lines in
sarcomeres. These studies suggest its role in the structural
organization of the sarcomere and in signalling from the
extracellular environment to the nucleus. Gough et al
(24) concluded that the
nesprin-1 gene is expressed in a variety of forms that are
multifunctional and are capable of functioning at both the Golgi
and the nuclear envelope, with the linking of the 2 organelles
taking place during muscle cell differentiation.
Nesprins are widely expressed in a variety of
tissues; the high expression of both nesprin-1 and -2 has been
observed in skeletal, cardiac and vascular smooth muscls. Zhang
et al (2) suggested that
nesprins have a specific function in muscle cell differentiation.
However, the high expression of nesprin-1 has also been found in
peripheral blood leukocytes and spleen, and nesprin-2 in the
pancreas and testes. Nesprins are highly expressed in muscles with
both nesprin-1 and -2 muscle-specific isoforms (2,21,25). In vitro, during the
differentiation of C2C12 myoblasts into myotubes, nesprin changes
its localization from the nuclear membrane to the
cytoplasm/sarcomere, indicating its specific roles during muscle
differentiation (2,4). In the sarcomere of skeletal muscle
cells, different nesprin-1 and -2 epitopes are associated with the
Z-line, the A/I junction, the sarcoplasmic reticulum and the
mitochondrial membrane, indicating that nesprins may play the role
of maintaining sarcomeric structure (4,25).
In addition, sarcomeric proteins have been identified as potential
interacting partners for nesprins, including the ryanodine receptor
and mAKAP (25,26). mAKAP is targeted to the nuclear
membrane by nesprin-1 and they interact through their closely
related spectrin repeats (26).
Potentially, nesprins may be involved in maintaining and/or
targeting protein complexes common to both the nuclear membrane and
the sarcoplasmic reticulum (25).
Cardiomyocyte nuclei have been found to be elongated in Δ/Δ KASH
mouse hearts. These findings reflect what has been reported on
lamin A/C gene mutations and reinforce the importance of an intact
nuclear membrane complex for normal heart function (6). It has been shown that Δ/Δ KASH mice
(lacking the carboxy-terminus of nesprin-1) develop cardiomyopathy
with associated cardiac conduction system disease. Older mutant
animals have been shown to have elongated P wave duration, elevated
atrial and ventricular effective refractory periods, indicating
conduction system defects in the myocardium. It has been found that
cardiomyocyte nuclei are elongated with reduced heterochromatin in
Δ/Δ KASH mouse hearts (6).
The abovementioned data indicate that nesprin seems
to play a key role in adult cell mitosis, RNA transport and the
stability of the nuclear membrane. Yet, little is known about the
role of the nesprin-1 protein, particularly nesprin-1 in stem cells
(MSCs). In the present study, we aimed to elucidate the function of
nesprin-1 protein in BMSCs by designing a nesprin-1 siRNA
lentiviral vector. Following the transfection of the MSCs with
LV-siNesprin-1, we found that the protein expression of nesprin-1
in the LV-siNesprin-1 group was lower than that in the LV-GFP and
normal group; the proliferation of the MSCs in the LV-siNesprin-1
group was also reduced. However, the apoptotic rate was increased
in the LV-siNesprin-1 group compared with the LV-GFP and normal
group. As shown by morphological analysis using a microscope, in
the LV-siNesprin-1 group, morphological changes were observed in
the nucleus, such as fusion and fragmentation. Thus, nesprin-1
mediates cell differentiation and regulates the proliferation and
apoptosis of MSCs.
MSCs were first described in 1968 by Friedenstein
et al (27). These cells
can be expanded and induced, either in vitro or in
vivo, and terminally differentiate into osteoblasts,
chondrocytes, adipocytes, tenocytes, myotubes, neuronal cells and
hematopoietic cells with strong self-renewal ability and genetic
stability in vitro. Several research groups have reported
that MSCs are able to proliferate and potentially differentiate
in vitro (28–30). However, the ratio of MSCs in bone
marrow cells is very low, approximately 0.001–0.01%; hence, the
separation and amplification of MSCs is of vital significance.
Wakitani et al (31)
described a method of isolating MSCs from rat bone marrow with
Ficoll density gradient separation and adherent culture. Currently,
the International Society for Cellular Therapy proposed 3 minimal
criteria for defining MSCs (32):
i) MSCs must be plastic-adherent if maintained in standard culture
conditions; ii) MSCs must express CD105, CD73 and CD90, but must
not express haematopoietic markers, such as CD45, CD34, CD14 or
CD11b; and iii) MSCs must be capable of differentiating into
fibroblasts, osteoblasts, adipocytes and chondroblasts under the
corresponding lineage, particularly in in vitro conditions.
In this study, we detected the MSC surface antigens, CD90 and CD29,
by flow cytometry, with the percentage of CD90 and CD29 as high as
98%, and the percentage of CD45 approximately 1%.
Xu et al (33) reported that the ability of human
MSCs to proliferate remained strong between passages 2 and 6, then
the apoptosis of MSCs began to accelerate. In the process of MSC
proliferation and differentiation, the upregulation of the protein
expression of nesprin-1 may maintain the stability of the MSC
nuclear membrane and reduce the apoptosis of the MSCs; this may
provide a theoretical basis and gain more seed cells for the
improvement of therapeutic modalities for heart disease.
Acknowledgements
This study was supported by a grant from the
Municipal Committee of Science and Technology of Shanghai, China
(no. 064119636).
References
|
1
|
Lammerding J, Schulze PC, Takahashi T, et
al: Lamin A/C deficiency causes defective nuclear mechanics and
mechanotransduction. J Clin Invest. 113:370–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Q, Skepper JN, Yang F, et al:
Nesprins: a novel family of spectrin-repeat-containing proteins
that localize to the nuclear membrane in multiple tissues. J Cell
Sci. 114:4485–4498. 2001.PubMed/NCBI
|
|
3
|
Apel ED, Lewis RM, Grady RM and Sanes JR:
Syne-1, a dystrophin- and Klarsicht-related protein associated with
synaptic nuclei at the neuromuscular junction. J Biol Chem.
275:31986–31995. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Q, Ragnauth CD, Skepper JN, et al:
Nesprin-2 is a multi-isomeric protein that binds lamin and emerin
at the nuclear envelope and forms a subcellular network in skeletal
muscle. J Cell Sci. 118:673–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mislow JM, Kim MS, Davis DB and McNally
EM: Myne-1, a spectrin repeat transmembrane protein of the myocyte
inner nuclear membrane, interacts with lamin A/C. J Cell Sci.
115:61–70. 2002.PubMed/NCBI
|
|
6
|
Puckelwartz MJ, Kessler EJ, Kim G, et al:
Nesprin-1 mutations in human and murine cardiomyopathy. J Mol Cell
Cardiol. 48:600–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Minguell JJ, Erices A and Conget P:
Mesenchymal stem cells. Exp Biol Med (Maywood). 226:507–520.
2001.PubMed/NCBI
|
|
9
|
Devine SM: Mesenchymal stem cells: will
they have a role in the clinic? J Cell Biochem Suppl. 38:73–79.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagaya N, Fujii T, Iwase T, et al:
Intravenous administration of mesenchymal stem cells improves
cardiac function in rats with acute myocardial infarction through
angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol.
287:H2670–H2676. 2004. View Article : Google Scholar
|
|
11
|
Miyahara Y, Nagaya N, Kataoka M, et al:
Monolayered mesenchymal stem cells repair scarred myocardium after
myocardial infarction. Nat Med. 12:459–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagaya N, Kangawa K, Itoh T, et al:
Transplantation of mesenchymal stem cells improves cardiac function
in a rat model of dilated cardiomyopathy. Circulation.
112:1128–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naldini L, Blömer U, Gallay P, et al: In
vivo gene delivery and stable transduction of nondividing cells by
a lentiviral vector. Science. 272:263–267. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dull T, Zufferey R, Kelly M, et al: A
third-generation lentivirus vector with a conditional packaging
system. J Virol. 72:8463–8471. 1998.PubMed/NCBI
|
|
15
|
Guenechea G, Gan OI, Inamitsu T, et al:
Transduction of human CD34+ CD38− bone marrow
and cord blood-derived SCID-repopulating cells with
third-generation lentiviral vectors. Mol Ther. 1:566–573. 2000.
|
|
16
|
Naldini L, Blömer U, Gage FH, Trono D and
Verma IM: Efficient transfer, integration, and sustained long-term
expression of the transgene in adult rat brains injected with a
lentiviral vector. Proc Natl Acad Sci USA. 93:11382–11388. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kafri T, Blömer U, Peterson DA, Gage FH
and Verma IM: Sustained expression of genes delivered directly into
liver and muscle by lentiviral vectors. Nat Genet. 17:314–317.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar
|
|
19
|
Koopman G, Reutelingsperger CP, Kuijten
GA, Keehnen RM, Pals ST and van Oers MH: Annexin V for flow
cytometric detection of phosphatidylserine expression on B cells
undergoing apoptosis. Blood. 84:1415–1420. 1994.PubMed/NCBI
|
|
20
|
Park C, Moon DO, Choi IW, et al: Curcumin
induces apoptosis and inhibits prostaglandin E2
production in synovial fibroblasts of patients with rheumatoid
arthritis. Int J Mol Med. 20:365–372. 2007.PubMed/NCBI
|
|
21
|
Mislow JM, Holaska JM, Kim MS, et al:
Nesprin-1alpha self-associates and binds directly to emerin and
lamin A in vitro. FEBS Lett. 525:135–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Starr DA and Han M: Role of ANC-1 in
tethering nuclei to the actin cytoskeleton. Science. 298:406–409.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Starr DA and Han M: ANChors away: an actin
based mechanism of nuclear positioning. J Cell Sci. 116:211–216.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gough LL, Fan J, Chu S, Winnick S and Beck
KA: Golgi localization of Syne-1. Mol Biol Cell. 14:2410–2424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Q, Ragnauth C, Greener MJ, Shanahan
CM and Roberts RG: The nesprins are giant actin-binding proteins,
orthologous to Drosophila melanogaster muscle protein
MSP-300. Genomics. 80:473–481. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pare GC, Easlick JL, Mislow JM, McNally EM
and Kapiloff MS: Nesprin-1alpha contributes to the targeting of
mAKAP to the cardiac myocyte nuclear envelope. Exp Cell Res.
303:388–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friedenstein AJ, Petrakova KV, Kurolesova
AI and Frolova GP: Heterotopic of bone marrow. Analysis of
precursor cells for osteogenic and hematopoietic tissues.
Transplantation. 6:230–247. 1968.PubMed/NCBI
|
|
28
|
Dennis JE and Charbord P: Origin and
differentiation of human and murine stroma. Stem Cells. 20:205–214.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Campagnoli C, Roberts IA, Kumar S, Bennett
PR, Bellantuono I and Fisk NM: Identification of mesenchymal
stem/progenitor cells in human first-trimester fetal blood, liver,
and bone marrow. Blood. 98:2396–2402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martin DR, Cox NR, Hathcock TL, Niemeyer
GP and Baker HJ: Isolation and characterization of multipotential
mesenchymal stem cells from feline bone marrow. Exp Hematol.
30:879–886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wakitani S, Saito T and Caplan AI:
Myogenic cells derived from rat bone marrow mesenchymal stem cells
exposed to 5-azacytidine. Muscle Nerve. 18:1417–1426. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal
cells. The International Society for Cellular Therapy position
statement. Cytotherapy. 8:315–317. 2006. View Article : Google Scholar
|
|
33
|
Xu W, Zhang X, Qian H, et al: Mesenchymal
stem cells from adult human bone marrow differentiate into a
cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood).
229:623–631. 2004.PubMed/NCBI
|