Introduction
Stroke affects over 780,000 individuals each year in
the United States (1) and results
in functional and structural brain impairment, as well as in poor
motor function (2). Major efforts
are underway to discover more effective methods of improving
outcomes in patients with stroke in the motor and cognitive arenas
(3). As a result, following
rehabilitation, the majority of patients have partially recovered
or are left with significant physical dysfunctions (4–6).
Post-stroke rehabilitation may improve recovery and reduce
long-term disability (7);
however, objective methods for evaluating the specific effects of
rehabilitation are required. While the findings of several studies
support the hypothesis that changes in brain function accompany
therapy-mediated improvements in motor skills (8–13),
the spatial specificity of current evaluation methods is inadequate
to allow the clear neuroanatomical localization of functional
changes. In biomedical imaging research, various mechanisms have
been explored based on plastic reorganization of the peri-infarct
and infarct areas on axonal sprouting (14,15) and on the migration of immature
neurons into the peri-infarct cortex (16). Diffusion tensor imaging
(DTI)-derived measures are valid biomarkers of neuroplasticity and
have been used successfully (17). Previous studies have shown that
neuroplasticity may play a role in motor recovery following stroke
in terms of the structural remodeling of white matter in the
ipsilesional and contralesional hemispheres (18), as well as in the functional
reorganization of activity in the sensorimotor cortices (19). Several studies have shown
structural plasticity in stroke survivors, demonstrating the
reorganization of the central nervous system, as well as
experimental evidence of ‘in vivo’ post-stroke plasticity
(20). Evidence shows that the
cerebral cortex undergoes significant structural plasticity for
several weeks to months following stroke (21). The reorganization taking place in
the central nervous system possibly includes both cellular and
anatomical phenomena, as well modifications of synaptic efficacy
within neuronal networks (22).
Additionally, plastic functional reorganization involves the
contralesional supplementary motor area (SMA) and premotor cortex
(23) and potentially the
ipsilesional primary motor cortex (24). Other clinical studies have shown
the benefits of using robot-assisted therapy in patients during
neurological recovery (25–36). The incremental improvements in
clinical scales following intensive robotic therapy, although
minimal, are statistically significant and certainly meaningful to
patients (32,37–39). It has been demonstrated that
neurological deficits may be better predicted and more precisely
characterized by incorporating brain maps of injury assessed using
magnetic resonance imaging (MRI) (40) and that neurorehabilitation with a
robotic devise is more beneficial than conventional paradigms
(41). Brain maps can provide
insight into which parts of a system are still functioning, thereby
potentially providing information not evident from clinical
observations (42). A recent
study provided additional support for the hypothsesis that
extensive time-dependent anatomical changes occur in residual
tissue and must be considered when evaluating plasticity-related
cortical changes associated with post-stroke recovery of function
(43).
The aim of the present study was to examine the
hypothesis that brain mapping using a novel magnetic resonance
(MR)-compatible hand device in conjunction with state-of-the-art
MRI can serve as a novel biomarker for brain plasticity induced by
rehabilitative motor training in patients with chronic stroke.
Thus, we explored brain plasticity after chronic stroke using
volumetric and diffusion imaging developed in the molecular
medicine era in conjunction with a novel MR-compatible hand-induced
robotic device (MR_CHIROD). We challenge the longstanding view that
neuroplasticity is not possible beyond 6 months post-stroke, which
has been a critical barrier to progress in the field of
rehabilitation in chronic stroke.
Materials and methods
Study design
We examined 15 healthy controls using DTI as part of
an overall patient MR session, which included 3D high-resolution
T1-weighted MRI, functional MRI (fMRI) and DTI; we also serially
examined 4 patients with chronic stroke. All experiments were
approved by the Institutional Review Board at Massachusetts General
Hospital and performed at the Athinoula A. Martinos Center for
Biomedical Imaging. The patients had first-ever left-sided ischemic
subcortical middle cerebral artery (MCA) stroke ≥6 months prior to
enrollment in this study, with no spasticity or joint stiffness.
Patients trained at home and underwent serial MR evaluation at
baseline (prior to training), during training and after 8 weeks of
training. Training at home consisted of squeezing a gel exercise
ball with the paretic hand at approximately 75% of maximum strength
for 1 h/day, 3 days/week. For each patient, reference (100%) was
own maximum force, defined as the force at which subjects could
completely squeeze the MR_CHIROD [group max force, 128 ± 13 N (n=5,
male)]. The appropriate hand exercise ball was selected after
measuring maximum hand-grip strength using a dynamometer. MRI
examinations were performed at baseline (prior to the commencement
of training); 4 weeks later, halfway through the exercise period;
another 4 weeks later; at the end of the training period; and again
4 weeks after completing the training period. All examinations were
performed on a Siemens Tim Trio 3-Tesla (3T) MRI scanner.
Description of MR_CHIROD
The design and testing of the first generation
MR_CHIROD has been previously reported (44–47). A detailed description of the
second generation MR_CHIROD used in this study has been previously
published (48). Briefly, the
MR_CHIROD mainly consists of 3 major subsystems: a) an
electro-rheological fluid (ERF) based resistive element, b) handles
and c) 2 sensors, including an optical encoder to measure
patient-induced motion and a force sensor. Each subsystem includes
several components of varying complexity. All components are
optimally designed with strength and safety in mind for
MR-compatibility and for regular and high-stress testing. The
MR_CHIROD is configured to securely attach to the scanner table
close to the subject who thus feels no weight.
MRI examination protocol
All examinations were performed on a
state-of-the-art 3T MRI system for increased signal-to-noise ratio
(SNR). We used a 12-channel Siemens Tim coil and collected MR
images and the examinations were completed in approximately 45 min.
DTI images were acquired as part of an MR session for each patient,
which included 3D high-resolution T1-weighted MRI, fMRI and DTI. In
addition, a rapid, low resolution fully-sampled T1 magnetization
prepared rapid gradient echo (MP-RAGE) or a fast spin-density
weighted 3D fast low-angle shot (FLASH) gradient echo sequence was
acquired (typical acquisition time, 6 sec) in order to guide the
calculation of the generalized autocalibrating partially parallel
acquisitions (GRAPPA) reconstruction parameters. Imaging parameters
were as follows: sagittal orientation; 7° flip angle; echo time
(TE) = 4.73 msec; repetition time (TR) = 2,530 msec; inversion time
(TI) = 1,100 msec; 1-mm slice thickness; 352×352×192 matrix; GRAPPA
factor = 3–6 to achieve the shortest acquisition time. Each
volunteer performed the paradigm at 45%, 60% and 75% of their
maximum grip strength and could fully squeeze the device at all
levels. The percentage levels compensate for the performance
confounds. Care was taken to minimize elbow flexion and/or
reflexive motion and head motion (typically 0.1–0.4 mm). Typical
imaging parameters for DTI were: 2×2×2 mm voxel size, 64 slices, 2
diffusion weightings (b = 0 sec/mm2, b = 1,000
sec/mm2), TR/TE = 8,600 msec/100 msec, 12 diffusion
directions, 4 dummy scans, 10 T2 weighted images, 2 averages. The
imaging sequence employs the twice-refocused spin-echo method for
reduction of eddy current.
Data analysis
To assess the thickness of cortical gray matter, we
used the FreeSurfer automated tool (http://surfer.nmr.mgh.harvard.edu) and voxel-based
morphometry (VBM) conducted using SPM8 calculated deviations of the
brain volume of a patient and from 11 age- and gender-matched
controls. The total acquisition time for DTI was 10 min. DTI fiber
tract reconstruction was performed using the DTI Studio software
package. Deterministic tractography was performed using the fuzzy
art with add clustering technique (FACT) algorithm (49). All tracts were visualized and
subsequently visually inspected for directionality and location.
The regions of interest (balls of 3 mm diameter) were designated in
the ascending fibers of the pons to visualize the corticospinal
tract (CST). The purpose of this analysis was to probe alterations
in diffusion-based tractography, and consequently, to demonstrate
changes in structural plasticity in addition to the functional
changes we observed in the brains of the chronic stroke patients as
a result of hand training. We reconstructed the CST tract,
selecting as seeding areas the pons, the posterior limb of the
internal capsule and the motor cortex.
Results
Table I summarizes
the results from patients showing that the number of fibers and the
average tract length were significantly altered after hand training
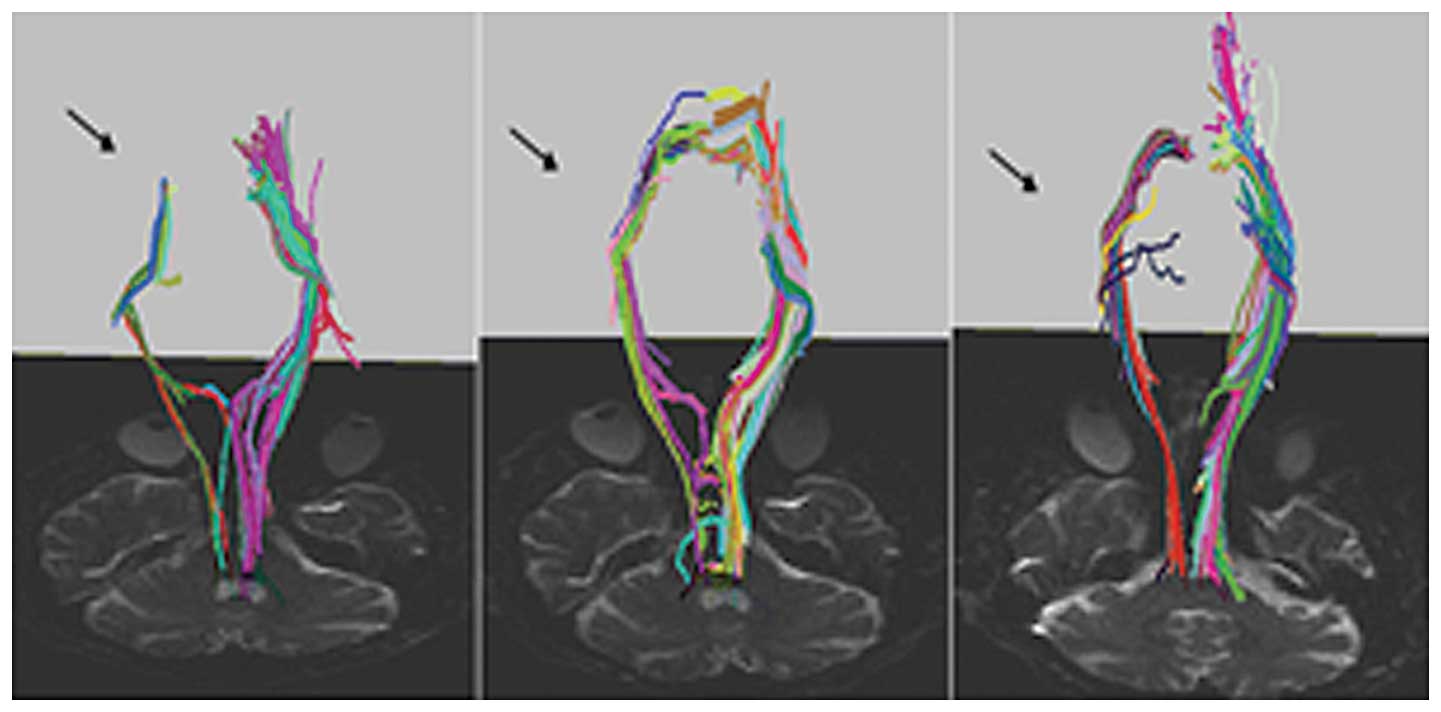
(p<0.001). Fig. 1 depicts DTI
images from a representative patient who suffered a single
left-sided ischemic subcortical MCA stroke ≥6 months prior to
enrollment in this study and did not have spasticity or joint
stiffness. New CST fibers (arrows) projecting progressively closer
to motor cortex appeared during training (Fig. 1). CST fiber (blue fibers, Fig. 1) density was altered during
training and SMA recruitment was indicated from a bundle of fibers
(Fig. 1).
 | Table IComparison of CST fibers of the
affected hemisphere before and after 2 months of training. |
Table I
Comparison of CST fibers of the
affected hemisphere before and after 2 months of training.
| Affected
fibers | Average no. ±
SD | Average length ± SD
(mm) |
|---|
| Before training
(baseline) | 46±8.1a | 43.6±3.6 |
| After training | 96.8±7.1 | 71.4±4.5 |
| Percentage change
from baseline | 110.4b,c | 63.7c |
| p-value | <0.001 | <0.001 |
Our data analysis using volumetric techniques showed
a decrease in cortical thickness, volume and neural density
extending far beyond the stroke infarct and included most of the
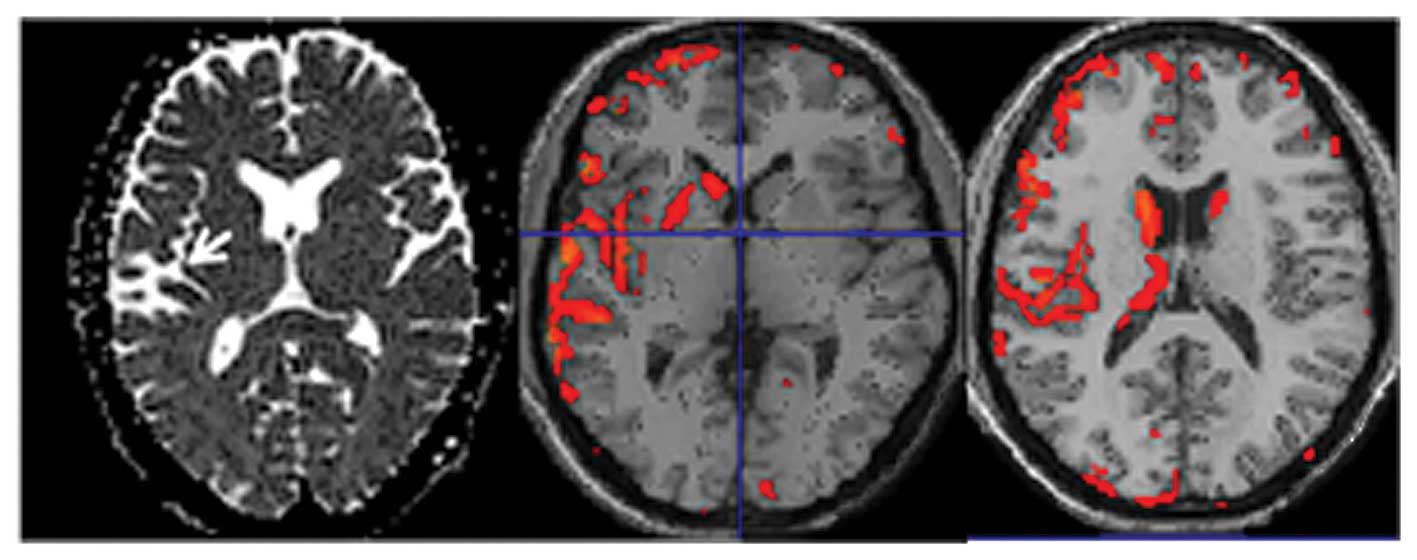
sensorimotor regions of the stroke and intact hemispheres (Fig. 2). We present a typical case with a
stroke at the left temporal lobe showing an intense signal on the
ADC map (Fig. 2, arrow). VBM was
conducted using SPM8 calculated deviations of the brain volume of a
patient and from 11 age- and gender-matched controls and showed
cortical atrophy mainly in the affected hemisphere and noticeably
even beyond the stroke region (middle and left images). Our data
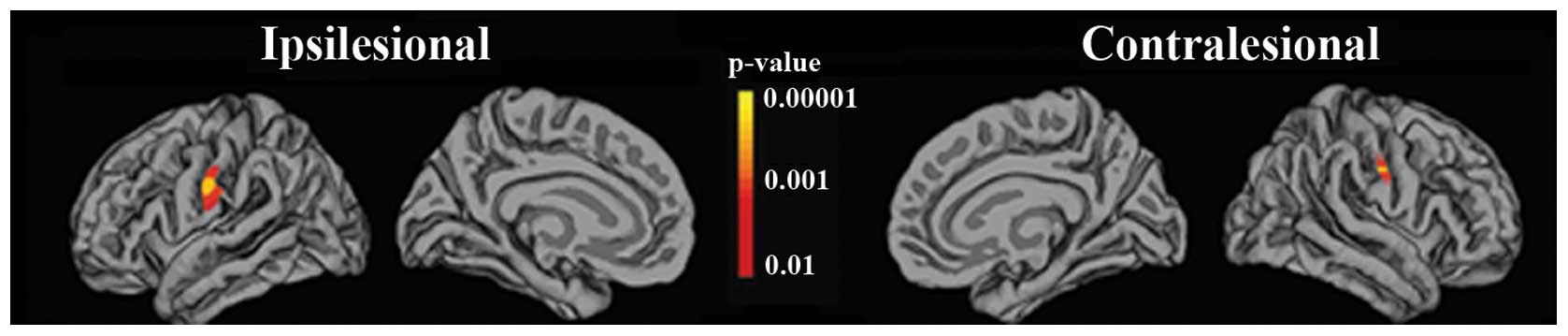
also showed a significant (p<0.05) increase in the cortical
thickness in the ventral postcentral gyrus areas of patients after
training relative to the cortical thickness before training. Our
volumetric data analysis showed a significant increase in the
cortical thickness of the ventral postcentral gyrus areas of
patients after training relative to pre-training cortical thickness
(Fig. 3).
Discussion
In this study, we observed alterations in the number
of fibers, length, density and increased cortical thickness. In
addition, our volumetric data analysis showed a significant
increase in the cortical thickness of the ventral postcentral gyrus
areas of patients after training relative to pre-training cortical
thickness. These findings suggest structural neuroplasticity in
patients with chronic stroke, which may be concomitant with
connectivity alterations (7–9)
and are in agreement with data from previous reports of fiber tract
alterations (50–52). Movement of residual tissue towards
the infarct was observed, supporting the notion that extensive
time-dependent morphological changes that occur in residual tissue
must be considered when evaluating plasticity-related cortical
changes associated with post-stroke recovery of function, which was
the rationale for performing structural analysis in this study.
We were motivated to develop and use a hand device
as hand movements normally play a central role in the daily lives
of individuals; thus, we believe that more attention should be paid
to the study of rehabilitation of hand motor function following
stroke. Since a major issue in hand motor therapy is how to best
restore function, interventions emphasizing intense, active and
repetitive movement should be of high value. We believe that these
interventions should increase strength, accuracy and functional use
when applied to subjects with impairment due to stroke. For
patients with chronic stroke who are in the advanced stages of
recovery, rehabilitation should be aimed at returning an individual
to normal activities, and should thus incorporate resistance
exercises intended to support the renewed development of muscle
strength. Therefore, the rationale of our approach to providing
such a therapy using an MR-compatible hand robot was motivated
first by the limited efforts that have been made thus far
concerning robotic developments for the hand, and second by the
novel combination of features that render the use of our
MR-compatible hand robot promising for enhancing the effectiveness
of standard post-stroke therapy.
Furthermore, the rationale for using advanced MRI
methods in this study was that MRI takes advantage of anatomical,
as well as functional information provided by different imaging
techniques. In addition to fMRI, which depicts functional
plasticity, DTI has the advantage of addressing structural brain
plasticity directly by depicting alterations in the number of
fibers, length and density. Thus, our rationale for using an
MR-compatible hand robot in conjunction with DTI and volumetric MRI
is that while robotic therapy has been shown to improve arm motor
function following stroke (53,54), efforts to address brain structural
plasticity have not focused on the hand (36), although, as discussed above, hand
motor function is essential to everyday life. The available
literature on robotic studies demonstrates clear incremental
benefits in motor impairment, promoting a better outcome (36,38).
The findings of the present study suggest that
intensive rehabilitation training results in neuroplasticity, which
suggests that the brain is adaptable to rehabilitation even in
chronic stroke. Thus, we consequently suggest that for stroke
patients, rehabilitation is possible for a longer period of time
following stroke than originally thought, suggesting that motor
skill improvements are possible even after 6 months due to retained
brain plasticity. Indeed, intensive treatment protocols for
sensorimotor impairment have demonstrated benefits compared with
primary care in patients with chronic stroke (26). Robotic training has been shown to
enhance motor outcome in patients with stroke and the effects have
been maintained for over 3 years (29). More importantly, the region of the
stroke lesion may have a vital impact on the course of motor
recovery in cortical and subcortical sites. Patients with mixed
subcortical and cortical lesions have shown significantly greater
gains in motor coordination and strength during patient
rehabilitation than patients with lesions confined to the basal
ganglia (55).
From a neuroscience perspective, it has been
reported that stroke patients exhibit structural plasticity in the
same sensorimotor cortical areas that exhibit functional plasticity
(50,56,57). Our results on the cortical
thickness of the ventral postcentral gyrus are in agreement with
those of another study where there were regional differences in
cortical thickness across the ventral postcentral gyrus areas,
suggesting that the cortical thickness of the ventral postcentral
gyrus areas was greater in stroke patients compared with the
controls (50). Co-localized
structural and functional plasticity has also been previously
demonstrated in sensorimotor cortical areas of animals in response
to manipulations of sensorimotor experience (58,59) involved in motor recovery after
stroke (60,61). The results of a recent study on
rats are consistent with our findings and the notion that extensive
time-dependent anatomical changes that occur in residual tissue
must be considered when evaluating plasticity-related cortical
changes associated with post-stroke recovery of function (43).
We believe that our current results further extend
the knowledge on brain plasticity after training and encourage
further research on the specific role of structural
training-induced plasticity using robotic devices (62). To this end, our findings agree
with recent experimental data demonstrating changes revealed by DTI
parallel histological remodeling (1,2)
and recovery of function (37,63). Although it has been suggested that
diffusion MR imaging may enable the assessment of brain plasticity
(2,64,65), diffusion MR imaging needs to be
further explored and justified for its application and diagnostic
importance in humans. Other findings suggest that following stroke,
brain plasticity implicates synaptogenesis, changes in function in
pre-existing synapses, neurogenesis and cortical reorganization
(66). The cortex, contralateral
to the lesion, is active in post-stroke motor training, but the
pattern of cortical activation is then normalized (67). The recovery is better if some of
the relevant motor circuits are not damaged (67–69). Moreover, sensory stimulation may
also enhance motor recovery (67). Of note, it has been suggested that
acute and slow-growing lesions involve very different patterns of
reorganization (70). Our
findings support this notion in patients with chronic stroke.
Our study suggests that using advanced neuroimaging
in addition to novel robotic therapies induce neuroplasticity,
eventually leading to motor recovery. We believe that this approach
can influence stroke practice and policy in the future. We also
believe that our findings address the longstanding view that
neuroplasticity was not possible beyond 6 months post-stroke which
has been a critical barrier to progress in the field of
neurorehabilitation in patients with chronic stroke. Moreover, the
results from MR-compatible robotic devices can enhance accurate
monitoring and identify biomarkers of brain plasticity that can be
monitored during stroke patient rehabilitation. Therefore, our
results open new horizons for the design of novel robotic devices,
which would target other motor functions (i.e. arm, leg).
Therefore, the current study widens the horizons for future studies
focusing on verbal and memory impairments caused by stroke.
Finally, our study is an example of personalized medicine using
advanced neuroimaging methods in conjunction with robotics in the
molecular medicine era.
Acknowledgements
This study was supported in part by a grant from the
National Institute of Biomedical Imaging and Bioengineering of the
National Institutes of Health (Grant no. R21 EB004665-01A2) to A.
Aria Tzika. We thank Dr Mavroidis, the principal investigator of
the subcontract to Northeastern University, who built the
MR_CHIROD.
References
|
1
|
Towfighi A, Markovic D and Ovbiagele B:
Impact of a healthy lifestyle on all-cause and cardiovascular
mortality after stroke in the USA. J Neurol Neurosurg Psychiatry.
83:146–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cho HM, Choi BY, Chang CH, et al: The
clinical characteristics of motor function in chronic hemiparetic
stroke patients with complete corticospinal tract injury.
NeuroRehabilitation. 31:207–213. 2012.
|
|
3
|
Han C, Wang Q, Meng PP and Qi MZ: Effects
of intensity of arm training on hemiplegic upper extremity motor
recovery in stroke patients: a randomized controlled trial. Clin
Rehabil. 27:75–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Notturno F, Sepe R, Caulo M, Uncini A and
Committeri G: Pseudocortical and dissociate discriminative sensory
dysfunction in a thalamic stroke. Cortex. 49:336–339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pahlman U, Savborg M and Tarkowski E:
Cognitive dysfunction and physical activity after stroke: the
Gothenburg cognitive stroke study in the elderly. J Stroke
Cerebrovasc Dis. 21:652–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carter AR, Patel KR, Astafiev SV, et al:
Upstream dysfunction of somatomotor functional connectivity after
corticospinal damage in stroke. Neurorehabil Neural Repair.
26:7–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Indredavik B, Slordahl SA, Bakke F,
Rokseth R and Haheim LL: Stroke unit treatment. Long-term effects.
Stroke. 28:1861–1866. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liepert J, Miltner WH, Bauder H, et al:
Motor cortex plasticity during constraint-induced movement therapy
in stroke patients. Neurosci Lett. 250:5–8. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kopp B, Kunkel A, Muhlnickel W, Villringer
K, Taub E and Flor H: Plasticity in the motor system related to
therapy-induced improvement of movement after stroke. Neuroreport.
10:807–810. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liepert J, Bauder H, Wolfgang HR, Miltner
WH, Taub E and Weiller C: Treatment-induced cortical reorganization
after stroke in humans. Stroke. 31:1210–1216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nelles G, Jentzen W, Jueptner M, Muller S
and Diener HC: Arm training induced brain plasticity in stroke
studied with serial positron emission tomography. Neuroimage.
13:1146–1154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johansen-Berg H, Dawes H, Guy C, Smith SM,
Wade DT and Matthews PM: Correlation between motor improvements and
altered fMRI activity after rehabilitative therapy. Brain.
125:2731–2742. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaechter JD, Kraft E, Hilliard TS, et
al: Motor recovery and cortical reorganization after
constraint-induced movement therapy in stroke patients: a
preliminary study. Neurorehabil Neural Repair. 16:326–338. 2002.
View Article : Google Scholar
|
|
14
|
Dijkhuizen RM, Singhal AB, Mandeville JB,
et al: Correlation between brain reorganization, ischemic damage,
and neurologic status after transient focal cerebral ischemia in
rats: a functional magnetic resonance imaging study. J Neurosci.
23:510–517. 2003.
|
|
15
|
Mountz JM, Liu HG and Deutsch G:
Neuroimaging in cerebrovascular disorders: measurement of cerebral
physiology after stroke and assessment of stroke recovery. Semin
Nucl Med. 33:56–76. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carmichael ST: Cellular and molecular
mechanisms of neural repair after stroke: making waves. Ann Neurol.
59:735–742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lindenberg R, Renga V, Zhu LL, Betzler F,
Alsop D and Schlaug G: Structural integrity of corticospinal motor
fibers predicts motor impairment in chronic stroke. Neurology.
74:280–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JS, Han MK, Kim SH, Kwon OK and Kim
JH: Fiber tracking by diffusion tensor imaging in corticospinal
tract stroke: Topographical correlation with clinical symptoms.
Neuroimage. 26:771–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stinear CM, Barber PA, Smale PR, Coxon JP,
Fleming MK and Byblow WD: Functional potential in chronic stroke
patients depends on corticospinal tract integrity. Brain.
130:170–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rossini PM, Altamura C, Ferreri F, et al:
Neuroimaging experimental studies on brain plasticity in recovery
from stroke. Eura Medicophys. 43:241–254. 2007.PubMed/NCBI
|
|
21
|
Nudo RJ: Mechanisms for recovery of motor
function following cortical damage. Curr Opin Neurobiol.
16:638–644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vaina LM, Sikoglu EM, Soloviev S, et al:
Functional and anatomical profile of visual motion impairments in
stroke patients correlate with fMRI in normal subjects. J
Neuropsychol. 4:121–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krainik A, Duffau H, Capelle L, et al:
Role of the healthy hemisphere in recovery after resection of the
supplementary motor area. Neurology. 62:1323–1332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duffau H, Lopes M, Sichez JP, Bitar A and
Capelle L: A new device for electrical stimulation mapping of the
brainstem and spinal cord. Minim Invasive Neurosurg. 46:61–64.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aisen ML, Krebs HI, Hogan N, McDowell F
and Volpe BT: The effect of robot-assisted therapy and
rehabilitative training on motor recovery following stroke. Arch
Neurol. 54:443–446. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Volpe BT, Krebs HI, Hogan N, Edelstein OL,
Diels C and Aisen M: A novel approach to stroke rehabilitation:
robot-aided sensorimotor stimulation. Neurology. 54:1938–1944.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Volpe BT, Krebs HI, Hogan N, Edelsteinn L,
Diels CM and Aisen ML: Robot training enhanced motor outcome in
patients with stroke maintained over 3 years. Neurology.
53:1874–1876. 1999.PubMed/NCBI
|
|
28
|
Volpe BT, Krebs HI and Hogan N: Is
robot-aided sensorimotor training in stroke rehabilitation a
realistic option? Curr Opin Neurol. 14:745–752. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Volpe BT, Ferraro M, Lynch D, et al:
Robotics and other devices in the treatment of patients recovering
from stroke. Curr Neurol Neurosci Rep. 5:465–470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Volpe BT, Ferraro M, Krebs HI and Hogan N:
Robotics in the rehabilitation treatment of patients with stroke.
Curr Atheroscler Rep. 4:270–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferraro M, Palazzolo JJ, Krol J, Krebs HI,
Hogan N and Volpe BT: Robot-aided sensorimotor arm training
improves outcome in patients with chronic stroke. Neurology.
61:1604–1607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fasoli SE, Krebs HI, Stein J, Frontera WR,
Hughes R and Hogan N: Robotic therapy for chronic motor impairments
after stroke: follow-up results. Arch Phys Med Rehabil.
85:1106–1111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daly JJ, Hogan N, Perepezko EM, et al:
Response to upper-limb robotics and functional neuromuscular
stimulation following stroke. J Rehabil Res Dev. 42:723–736. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Macclellan LR, Bradham DD, Whitall J, et
al: Robotic upper-limb neurorehabilitation in chronic stroke
patients. J Rehabil Res Dev. 42:717–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Finley MA, Fasoli SE, Dipietro L, et al:
Short-duration robotic therapy in stroke patients with severe
upper-limb motor impairment. J Rehabil Res Dev. 42:683–692. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Prange GB, Jannink MJ, Groothuis-Oudshoorn
CG, Hermens HJ and Ijzerman MJ: Systematic review of the effect of
robot-aided therapy on recovery of the hemiparetic arm after
stroke. J Rehabil Res Dev. 43:171–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reinkensmeyer DJ: Robotic assistance for
upper extremity training after stroke. Stud Health Technol Inform.
145:25–39. 2009.PubMed/NCBI
|
|
38
|
Kwakkel G, Kollen BJ and Krebs HI: Effects
of robot-assisted therapy on upper limb recovery after stroke: a
systematic review. Neurorehabil Neural Repair. 22:111–121. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Astrakas LG, Naqvi SH, Kateb B and Tzika
AA: Functional MRI using robotic MRI compatible devices for
monitoring rehabilitation from chronic stroke in the molecular
medicine era (Review). Int J Mol Med. 29:963–973. 2012.PubMed/NCBI
|
|
40
|
Crafton KR, Mark AN and Cramer SC:
Improved understanding of cortical injury by incorporating measures
of functional anatomy. Brain. 126:1650–1659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang VS and Krakauer JW: Robotic
neurorehabilitation: a computational motor learning perspective. J
Neuroeng Rehabil. 6:52009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carey LM and Seitz RJ: Functional
neuroimaging in stroke recovery and neurorehabilitation: conceptual
issues and perspectives. Int J Stroke. 2:245–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Karl JM, Alaverdashvili M, Cross AR and
Whishaw IQ: Thinning, movement, and volume loss of residual
cortical tissue occurs after stroke in the adult rat as identified
by histological and magnetic resonance imaging analysis.
Neuroscience. 170:123–137. 2010.
|
|
44
|
Khanicheh A, Muto A, Triantafyllou C, et
al: fMRI-compatible rehabilitation hand device. J Neuroeng Rehabl.
3:242006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Khanicheh A, Muto A, Triantafyllou C,
Astrakas LG, Mavroidis C and Tzika A: MR compatible erf-based
robotic device for hand rehabilitation after stroke. Proc Intl Soc
Mag Reson Med. 13:11102005.
|
|
46
|
Tzika A, Khanicheh A, Muto A,
Triantafyllou C, Astrakas LG and Mavroidis C: Novel rehabilitation
hand robots and fMRI in Stroke [Abstract]. Eur Radiol. 16(Suppl 1):
1832006.
|
|
47
|
Khanicheh A, Mintzopoulos D, Weinberg B,
Tzika AA and Mavroidis C: MR_CHIROD v.2: A fMRI Compatible
Mechatronic Hand Rehabilitation device. In: Proceedings of the 2007
IEEE 10th International Conference on Rehabilitation Robotics;
Noodwijk, The Netherlands. pp. 883–889. 2007
|
|
48
|
Khanicheh A, Mintzopoulos D, Weinberg B,
Tzika AA and Mavroidis C: MR_CHIROD v.2: magnetic resonance
compatible smart hand rehabilitation device for brain imaging. IEEE
Trans Neural Syst Rehabil Eng. 16:91–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lazar M, Weinstein DM, Tsuruda JS, et al:
White matter tractography using diffusion tensor deflection. Hum
Brain Mapp. 18:306–321. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schaechter JD, Moore CI, Connell BD, Rosen
BR and Dijkhuizen RM: Structural and functional plasticity in the
somatosensory cortex of chronic stroke patients. Brain.
129:2722–2733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Koganemaru S, Mima T, Thabit MN, et al:
Recovery of upper-limb function due to enhanced use-dependent
plasticity in chronic stroke patients. Brain. 133:3373–3384. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cauraugh JH and Summers JJ: Neural
plasticity and bilateral movements: a rehabilitation approach for
chronic stroke. Prog Neurobiol. 75:309–320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jack D, Boian R, Merians A, et al: Virtual
reality-enhanced stroke rehabilitation. IEEE Trans Neural Syst
Rehabil Eng. 9:308–318. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hesse S, Schulte-Tigges G, Konrad M,
Bardeleben A and Werner C: Robot-assisted arm trainer for the
passive and active practice of bilateral forearm and wrist
movements in hemiparetic subjects. Arch Phys Med Rehabil.
84:915–920. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shelton FN and Reding MJ: Effect of lesion
location on upper limb motor recovery after stroke. Stroke.
32:107–112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Smania N, Picelli A, Gandolfi M, Fiaschi A
and Tinazzi M: Rehabilitation of sensorimotor integration deficits
in balance impairment of patients with stroke hemiparesis: a
before/after pilot study. Neurol Sci. 29:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xerri C, Merzenich MM, Peterson BE and
Jenkins W: Plasticity of primary somatosensory cortex paralleling
sensorimotor skill recovery from stroke in adult monkeys. J
Neurophysiol. 79:2119–2148. 1998.PubMed/NCBI
|
|
58
|
Kleim JA, Barbay S, Cooper NR, et al:
Motor learning-dependent synaptogenesis is localized to
functionally reorganized motor cortex. Neurobiol Learn Mem.
77:63–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hickmott PW and Steen PA: Large-scale
changes in dendritic structure during reorganization of adult
somatosensory cortex. Nat Neurosci. 8:140–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Carmichael ST, Archibeque I, Luke L, Nolan
T, Momiy J and Li S: Growth-associated gene expression after
stroke: evidence for a growth-promoting region in peri-infarct
cortex. Exp Neurol. 193:291–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dijkhuizen RM, Ren J, Mandeville JB, et
al: Functional magnetic resonance imaging of reorganization in rat
brain after stroke. Proc Natl Acad Sci USA. 98:12766–12771. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Heller SL, Heier LA, Watts R, et al:
Evidence of cerebral reorganization following perinatal stroke
demonstrated with fMRI and DTI tractography. Clin Imaging.
29:283–287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lum P, Reinkensmeyer D, Mahoney R, Rymer
WZ and Burgar C: Robotic devices for movement therapy after stroke:
current status and challenges to clinical acceptance. Top Stroke
Rehabil. 8:40–53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Granziera C, Schmahmann JD, Hadjikhani N,
et al: Diffusion spectrum imaging shows the structural basis of
functional cerebellar circuits in the human cerebellum in vivo.
PLoS One. 4:e51012009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Thuen M, Olsen O, Berry M, et al:
Combination of Mn(2+)-enhanced and diffusion tensor MR imaging
gives complementary information about injury and regeneration in
the adult rat optic nerve. J Magn Reson Imaging. 29:39–51.
2009.
|
|
66
|
Carmichael ST: Plasticity of cortical
projections after stroke. Neuroscientist. 9:64–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dietrichs E: Brain plasticity after
stroke-implications for post-stroke rehabilitation. Tidsskr Nor
Laegeforen. 127:1228–1231. 2007.(In Norwegian).
|
|
68
|
O’Dell MW, Lin CC and Harrison V: Stroke
rehabilitation: strategies to enhance motor recovery. Annu Rev Med.
60:55–68. 2009.
|
|
69
|
Ward NS: Mechanisms underlying recovery of
motor function after stroke. Postgrad Med J. 81:510–514. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Desmurget M, Bonnetblanc F and Duffau H:
Contrasting acute and slow-growing lesions: a new door to brain
plasticity. Brain. 130:898–914. 2007. View Article : Google Scholar : PubMed/NCBI
|