Introduction
Hepatocellular carcinoma is a serious disease which
develops rapidly and is difficult to detect at an early stage. Only
10–30% of patients can be treated by surgery (1,2)
and for those who cannot, targeted therapy is considered a good
choice which has always been a hot spot of research.
Transcatheter arterial chemoembolization (TACE),
percutaneous ethanol injection (PEI) and radiofrequency ablation
(RFA) play important roles in the treatment of both early and
advanced stage hepatocellular carcinoma. However, since many
advanced stage patients suffer from hepatic dysfunction and such
therapies may worsen the hepatic function, leading inclusively to
hepatic failure, such applications are avoided. Among these
techniques, PEI, a method of percutaneous intratumor injection, is
used to treat the tumor mainly by the injection of ethanol, which
causes protein denaturation, dehydration of the cancer cells and
coagulation of small vessels within the tumor. Nevertheless, some
patients are allergic to ethanol and the osmotic ability of ethanol
has been found to be limited; hence, larger doses and repeated
multiple-site injections are required in order to achieve complete
tumor necrosis, which inevitably increases the likelihood of a
variety of side-effects (3–5).
On the other hand, the intratumoral injection of chemotherapeutic
drugs for the treatment of hepatocellular carcinoma has been
extensively studied (6); however,
the drugs injected into the tumor do not diffuse evenly. Studies
have shown that the diffusion is even and the release is delayed
when poloxamer 407 (P407)-based thermosensitive gels are added to
the injected drugs (7,8). In recent years, the anticancer
effects of somatostatin (SST) and somatostatin analog (SSTA) have
gained increasing attention, with studies showing that the SSTA,
octreotide (OCT), can significantly inhibit the growth of tumor
cells and shrink the size of the tumor with prolonged intravenous
application (9–11). Compared with SST, OCT has a longer
half-life, stronger effects and fewer adverse effects. Targeted
intratumoral injection therapy can further improve the therapeutic
efficacy (12); however, for use
in the treatment of advanced hepatocellular carcinoma by
intratumoral injection, the half-life of OCT is still too short and
frequent applications will add to the economic, physical and mental
burden of patients.
In the present study, we hypothesized that P407 as a
carrier may be combined with OCT to obtain OCT-P407 as a
sustained-release preparation, which can make up for the
shortcomings of OCT. At the same time, we established a mouse model
of hepatocellular carcinoma by the subcutaneous transplantion of
Hca-F cells to confirm the effects of this new agent (administered
by intratumoral injection) on hepatocellular carcinoma. The
findings of the present study may provide patients with advanced
hepatocellular carcinoma with a novel, well-tolerated treatment
method administered by intratumoral injection.
Materials and methods
Animal care
Kunming mice (weighing 18–22 g) were obtained from
the Experimental Animal Center of Dalian Medical University,
Dalian, China. The present study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the Canadian Council on Animal Care.
The protocol was approved by the Committee on the Ethics of Animal
Experiments of Dalian Medical University (permit no. SCXK
2008-0002). All surgical procedures were performed under ether
anesthesia and all of the subcutaneous inoculations and tumor
injections were administered in a quiet and comfortable
environment.
Animal model
The mice were housed in a specific pathogen-free
(SPF) environment maintained at 24–26°C with a relative humidity of
60–65%. They were allowed free access to water and food throughout
the experimental period.
During the study, we tried our best to reduce the
pain and suffering of the mice. The animals ate and drank freely in
a comfortable and quiet environment while the mouse models of liver
cancer were established by the subcutaneous transplantion of cancer
cells. After the injection of the solutions to the 4 groups for 8
days, the mice were sacrificed. All the experiments were performed
under diethyl ether anesthesia and the in vivo experiments
conformed to the Medical Laboratory Animal Guidelines. This study
was performed in compliance with the Helsinki Declaration. The
surgical procedures were approved by the Animal Care and Use
Committee of Dalian Medical University.
Materials
Cell line
The mouse hepatocellular carcinoma ascites cell line
with a high lymphatic metastatic potential, Hca-F, was established
and preserved in the Morphology Laboratory of Dalian Medical
University.
Experimental animals
The SPF mice used in this study were provided by the
Experimental Animal Center of Dalian Medical University.
Reagents
OCT powder and OCT standard preparation (Xinlibang
Bio-Pharmaceutical Co., Chengdu, China; purity >99%); P407 and
OCT-P407 were prepared by the College of Pharmacy of Dalian Medical
University and the Dalian University of Technology, at a
concentration of 0.1 mg/ml; RPMI-1640 culture medium (HyClone Co.,
Logan, UT, USA); methyl thiazolyl tetrazolium (MTT) (Amresco LLC,
Solon, OH, USA); Annexin V-FITC kit (Jingmei BioTech Co., Shenzhen,
China); RT-PCR amplification kit (Thermo Fisher Scientific Inc.,
Waltham, MA, USA); rabbit anti-mouse VEGF antibody was acquired
from Thermo Fisher Scientific Inc.; rabbit anti-mouse caspase-3
antibody (Boster Biotechnology Inc., Wuhan, China); the
ready-to-use Immunohistochemistry kit and DAB coloration kit were
acquired from Beijing Zhongshan Golden Bridge Biotechnology Co.
(Beijing, China).
Methods
Preparation of thermosensitive gel and
OCT-P407
The P407-based thermosensitive gel consisted of
P407, cellogran and other excipients, such as PEG-PLA block
copolymer, ethylhydroxyethylcellulose and PEG-polymer-PLC block
copolymer. OCT solution of the required concentration was added to
the P407-based thermosensitive gel and incubated overnight at 4°C
after gentle stirring. A colorless and transparent viscous liquid
(OCT-P407) was thereby formed. OCT-P407 was placed in an ice water
bath before use.
In vitro experiments
Cell culture
Hca-F cell counting, passage, culture, recovery and
cryopreservation were performed as previously described (13,14). Cells expanded as a single-layer
suspension when observed under an inverted phase-contrast
microscope (Olympus Co., Tokyo, Japan).
Measurement of cell proliferation
Screening for the optimum concentration of cells was
performed as previously described (15). Groups were defined as follows
(n=5): the control group used for colorimetric zeroing, where no
cells were inoculated and only solution was added; the negative
control group, where the cells were inoculated, but no solution was
added; the OCT-P407 group, which was divided into 4 subgroups
according to the different OCT concentrations (0.1, 1, 10 and 100
μg/ml); the OCT group, defined as the positive control group,
divided into 4 subgroups containing the same OCT concentrations as
in the OCT-P407 group; the P407 group, which was the same as the
OCT-P407 group except that OCT-P407 was replaced by P407. There
were 4 parallel wells corresponding to each OCT concentration. The
calculation of the inhibition rate (IR) was carried out according
to the formula: IR = (1 - OD value of the experimental well/OD
value of the control well) ×100%. The 50% inhibitory concentration
(IC50) was calculated by the modified Karber method according to
the formula: LgIC50 = Xm - I [P - (3 - Pm - Pn)/4], where Xm
represents the l g of the maximum dose; I represents l g (maximum
dose/adjacent dose); P represents the sum of positive response
rates; Pm represents the maximum positive response rate; and Pn
represents the minimum positive response rate. After the optimum
concentration of cells and optimum inhibitory concentration were
calculated, cell suspension was inoculated into a 96-well plate.
OCT solution, OCT-P407 and P407 containing the same concentrations
of OCT (20 μg/ml) were added separately. Three 96-well plates were
placed in a 37°C incubator with 5% CO2 and saturated
humidity and taken out separately after 12, 24 and 48 h. After the
addition of 20 μl of 500 μg/ml MTT into each well, the cells were
cultured for an additional 4 h. The cells were then centrifuged at
1,000 rpm for 10 min, the supernatant was aspirated carefully and
150 μl of dimethyl sulfoxide (DMSO) were added to each well. The
plates were then shaken on a micro oscillator for 15 min, after
which the absorbance (OD) was detected using a microplate reader at
a wavelength of 492 nm. The OD detection was in duplicate and the
values averaged. The growth inhibition ratio of each group was
calculated by the IR formula.
Evaluation of apoptosis
Hca-F cells in the exponential phase were collected
and the cell concentration adjusted to 1×106 cells/ml.
The cells were divided into 4 groups as follows: negative control
group, only cells were inoculated and no solutions was added; the
OCT-P407, OCT and P407 groups, where the concentration of the
solution was the optimum inhibitory concentration detected by MTT
assay. After the cells were cultured with the different solutions
for 12, 24 and 48 h, apoptosis was detected by Annexin V-FITC/PI
staining and flow cytometry (on a Becton-Dickinson cytometer)
according to the manufacturer’s instructions as previously
described (16). Changes in cell
morphology were detected and photographed under an inverted optical
microscope after 48 h of culture.
In vivo experiments
Establishment of a mouse model of
liver cancer by subcutaneous transplantion of cancer cells
Second generation of ascites liver cancer cells
(with a survival rate >98%) was collected, washed twice with
phosphate-buffered saline (PBS; 0.2 ml) and the cells were adjusted
to 2.0×106/ml (17,18). One hundred mice were
subcutaneously injected with 0.2 ml of cell suspension
(~5×106 cells) in the left axillary fossa and the tumor
growth was monitored. Seven days after the injection, tumor
diameter was measured with a slide caliper rule. Mice whose tumor
diameter was 2 cm, were used for the detection of the P407
retention time in the tumor cavity, whereas 50 mice whose tumor
diameter was 1 cm were used for the detection of the therapeutic
effects of the intratumoral injection of the different
solutions.
Intratumoral injection
Fur around the tumor was shaved and the mice were
anaesthetized with ether and placed in a supine position. The
SP6-12 probe of Siemens Color Doppler ultrasound system (116834
type; Siemens Co., Munich, Germany) was used to scan the position
of the tumor. Following routine sterilization and draping, the
solutions were injected with a needle of a 1 ml syringe into the
center of the tumor under ultrasound guidance. The needle was
appropriately adjusted during the injection and the solution was
slowly injected from deep to shallow, in order to distribute it
homogeneously in the tumor cavity and the position of the needle
was appropriately adjusted during the injection. The whole process
was monitored in real-time and recorded by ultrasound.
Detection of P407 retention time in
the mouse tumor cavity
Methylene blue solution was mixed with P407 and
normal saline solution (NS) separately to obtain methylene
blue-P407 and methylene blue solution. Sixteen mice whose tumor
diameter was 2 cm were randomly divided into the methylene
blue-P407 and methylene blue solution groups, each group containing
8 mice. Under ultrasound guidance, the 2 reagents mentioned above
were slowly injected into the center of the tumor and the
pigmentation status of the skin around the tumor was observed.
Twenty-four hours after the injection, the mice were culled and the
tumors excised. The pigmentation status of the solid tumors
inoculated with the 2 reagents was compared. Forty-eight mice whose
tumor diameter was 2 cm were selected for receiving intratumoral
injections under ultrasound guidance. Mice were randomly divided
into the OCT-P407 and OCT groups, according to the solutions that
were injected (n=24) and at 12, 24 and 48 h after injection, 8 mice
(at each time point) were sacrificed by cervical dislocation.
Following the dissection of the tumors, the selection of the
wavelength of the high performance liquid chromatography (HPLC) and
the chromatographic condition, as well as the treatment of the
sample were performed as previously described (19).
Indicators and methods for tumor
detection
Fifty mice liver transplanted with cancer were
randomly divided into 5 groups: the OCT-P407, OCT, ethanol, P407
and NS groups (n=10), which were respectively injected with P407
plus 20 μg of OCT, 20 μg of OCT, 0.2 ml of ethanol, P407 and 0.2 ml
of NS.
Detection of tumor volume
On day 0, 2, 4, 6 and 8 after injection, 3
orthogonal radial lines of the tumor, the length, width and
thickness, were measured with a slide caliper rule; each radial
line was measured 3 times and the values were averaged. Calculation
of the tumor volume was carried out according to the formula: V =
π/6 × a × b × c (V, volume of the tumor; a, the longest radial
line; b, the shortest radial line; c, thickness).
Detection of tumor weight and IR
All mice were culled 8 days after injection. Tumors
were completely excised, weighed immediately and the tumor weight
IR was calculated according to the formula: tumor weight IR = (1 -
mean tumor weight of the drug administered group/mean tumor weight
of the negative control group) ×100%.
Mice from each group were sacrificed by cervical
dislocation and the tumor tissue was isolated. After the tumors
were weighed, part of them was immediately fixed in 10% neutral
formalin solution and then embedded in paraffin. The other part of
the tumor tissue was preserved in a liquid nitrogen container.
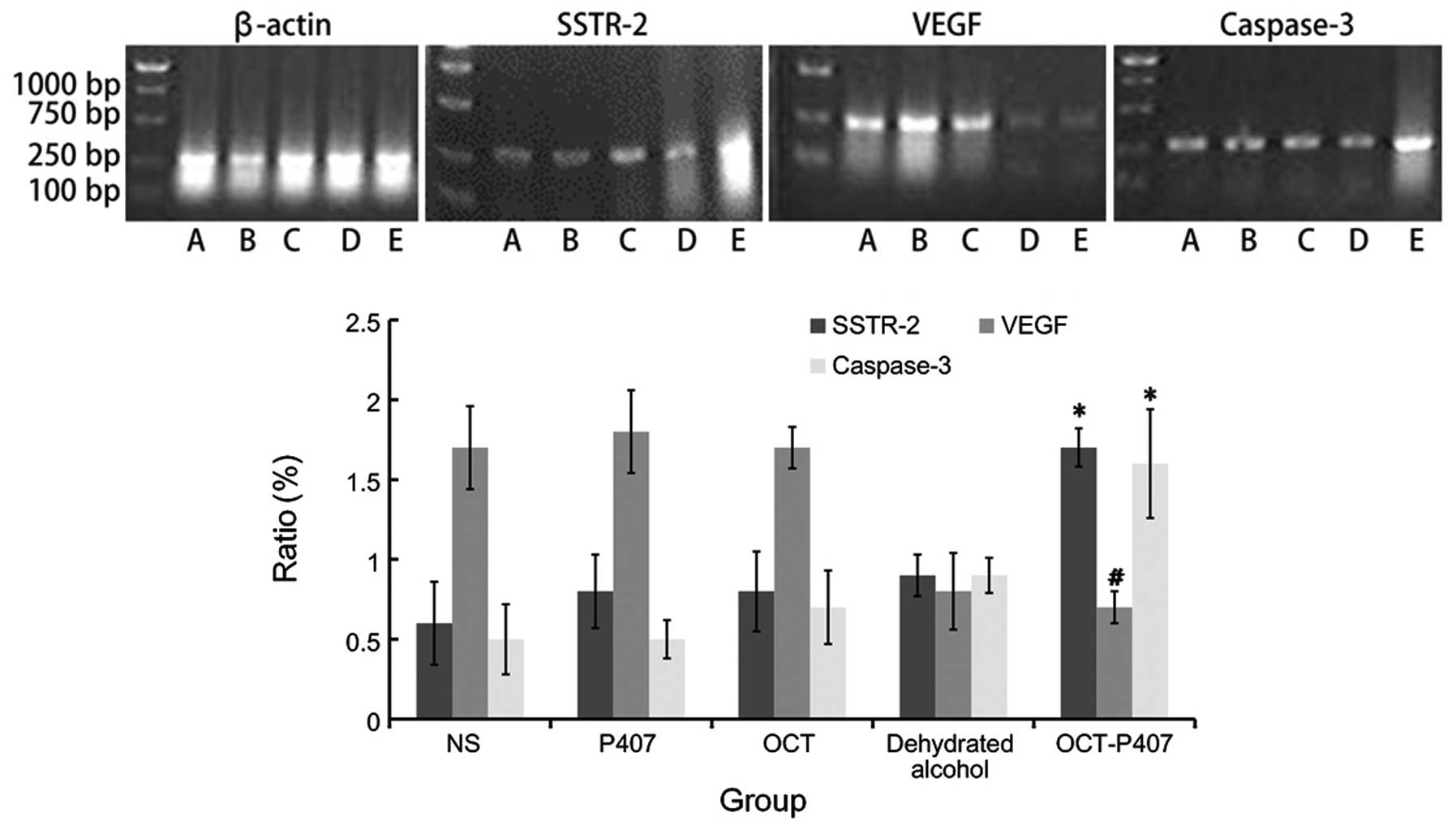
Detection of the expression of somatostatin receptor-2 (SSTR-2),
vascular endothelial growth factor (VEGF) and caspase-3 in the
tumor tissue was performed by immunohistochemistry according to
previous work in our laboratory (20).
Reverse transcription polymerase chain
reaction (RT-PCR)
Extraction of total RNA and evaluation of RNA
quality were carried out as described in our previous study
(20). SSTR-2, VEGF, caspase-3
and the control β-actin primers were designed by us in accordance
with GenBank data: SSTR-2 (154 bp) forward,
5′-GAGGCCTTTCCCCTAGAGTT-3′ and reverse, 5′-CACCGTAACGCTTGTCCTT-3′;
VEGF (228 bp) forward, 5′-ATGGCAGGAGCCCCGGGGTGTCCC-3′ and reverse,
5′-GGCTTGTCAATTTTTCTGGCTTTG-3′; caspase-3 (255 bp) forward,
5′-CAGACTCCGGCAGTAGTCGCC-3′ and reverse,
5-GTGGACGCAGCCAACCTCAGA-3′; β-actin (266 bp) forward,
5′-ACAGAGTACTTGCGCTCAGGAG-3′ and reverse,
5′-GTCACCCACACTGTGCCCATCT-3′. The one-step protocol was performed
according to the RT-PCR (Thermo Fisher Scientific Inc.)
manufacturer’s instructions followed by semi-quantitative
analysis.
Statistical analysis
Statistical software SPSS v17.0 was used for the
analysis. Results are indicated as the means ± SD. Measurements
were performed in duplicate or triplicate and the t-test or one-way
or two-way ANOVA analysis was applied. P-values <0.05 were
considered to indicate statistically significant differences.
Results
Cell culture experiments
OCT-P407 effectively inhibits liver
cancer cell proliferation
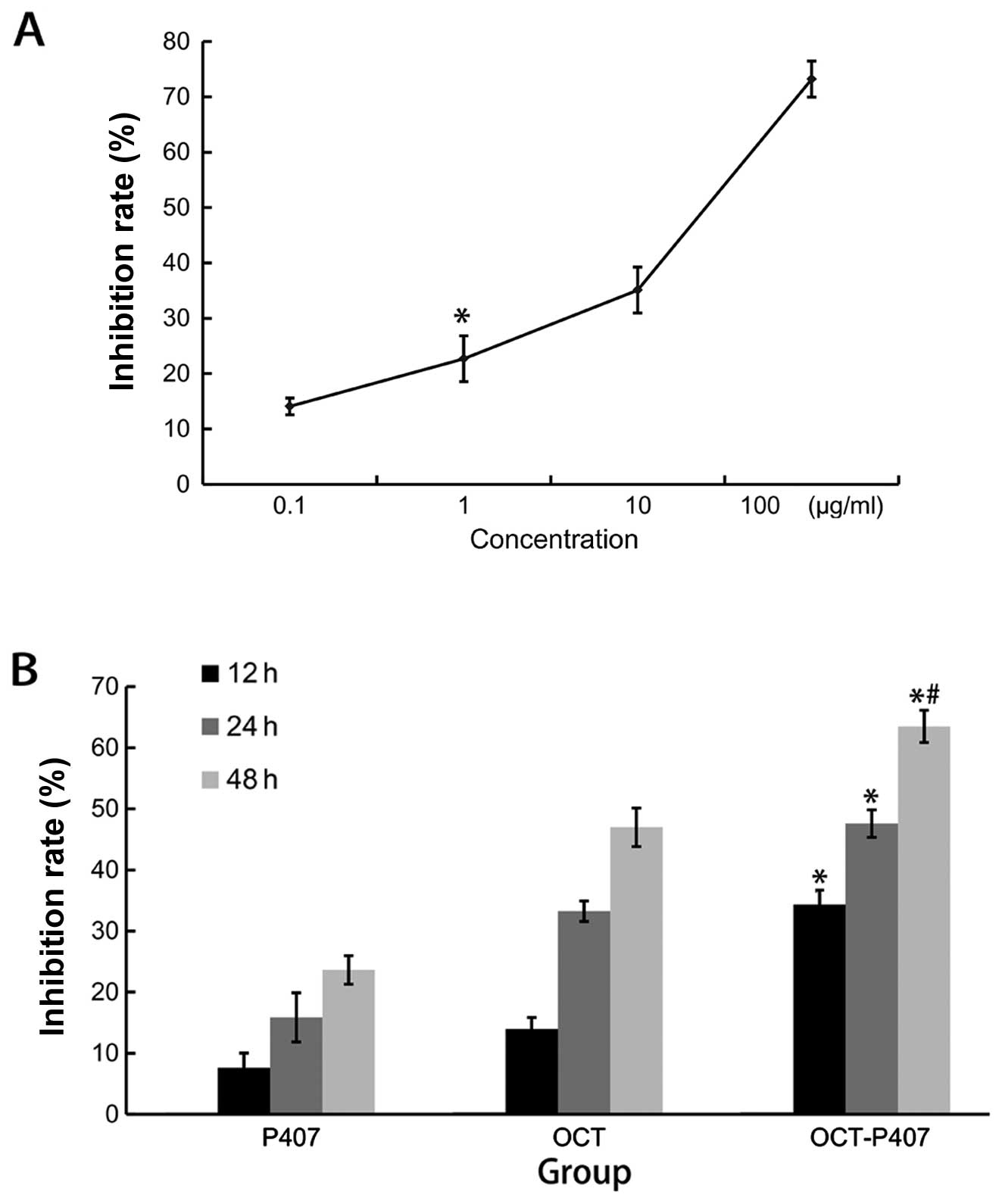
We first determined the optimum inhibitory
concentration of OCT in OCT-P407 by testing the effects of various
concentrations of OCT in OCT-P407 on liver cancer cell
proliferation after 24 h of treatment. We observed that OCT-P407
containing OCT at a concentration range of 1–100 μg/ml inhibited
the proliferation of Hca-F cells after 24 h in a
concentration-dependent manner, with an IC50 of 20 μg/ml (Fig. 1A). OCT (20 μg/ml) was therefore
defined as the optimum inhibitory concentration for the OCT-P407
and OCT groups and used in all subsequent experiments. We then
compared the IRs of OCT, OCT-P407 and P407 on liver cancer cell
proliferation over time. The IR of OCT-P407 was significantly
different (P<0.05) from the other groups and within the OCT-P407
group it was also significantly different (P<0.05) at 48 h when
compared with 12 and 24 h of treatment (Fig. 1B).
OCT-P407 effectively induces liver
cancer cell apoptosis
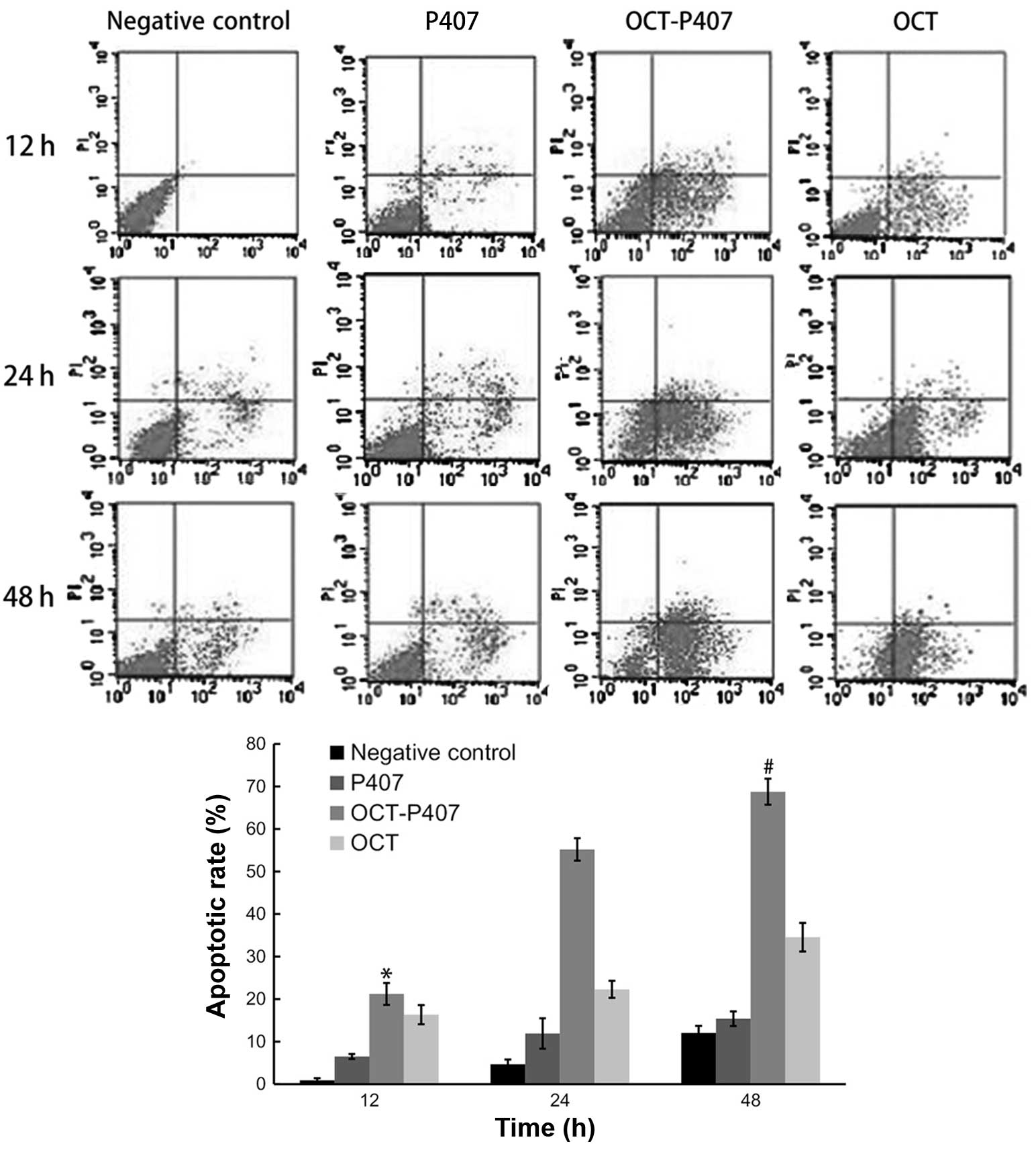
We compared the apoptotic rates of Hca-F cells in
the absence of the solution and in the presence of OCT, OCT-P407
and P407 over time. The apoptotic rate of OCT-P407 was
significantly different (P<0.05) compared with the other groups
and within the OCT-P407 group it was also significantly different
(P<0.05) at 48 h when compared with 12 and 24 h of treatment
(Fig. 2).
OCT-P407 induces evident liver cancer
cell morphological changes
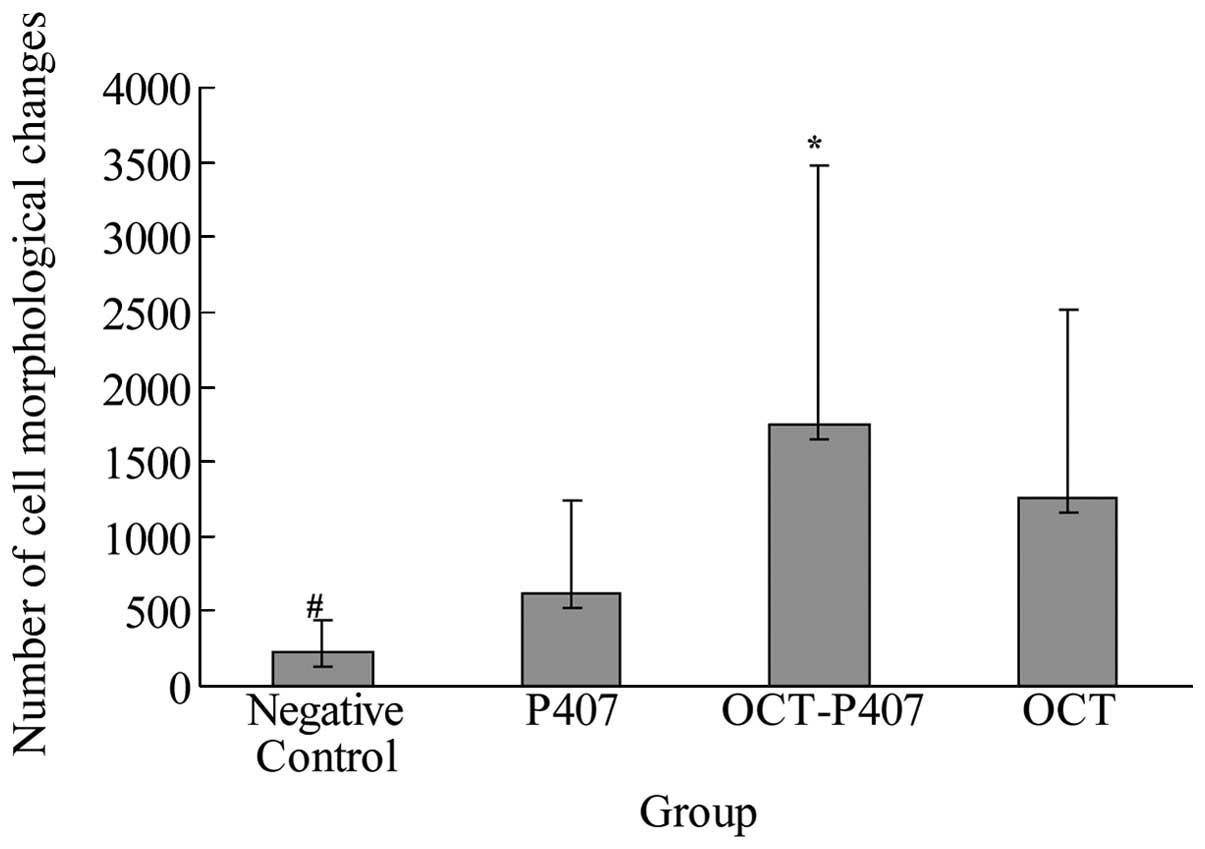
We analyzed the morphological changes of Hca-F cells
after 48 h of treatment with no solution, OCT, OCT-P407 and P407
under an inverted optical microscope. As was observed in the
negative control group, the cells were round-shaped, with a
transparent cytoplasm and they grew densely and were evenly
distributed; in the P407 group, some cells formed clusters; while
in the OCT-P407 group, they had an irregular shape, DNA
condensation and fragmentation and the majority aggregated into
clusters; in the OCT solution group, some of the cells showed
morphological changes as mentioned above. Thus, in the OCT-P407
group, the morphological changes of the Hca-F cells were greater
than those of cells in the OCT group and P407 group, as well as the
negative control group (P<0.05). In the OCT group and P407 group
the morphological changes of Hca-F cells were significantly greater
than those in the negative control group (P<0.05). In Fig. 3, we present a bar graph
illustrating the number of morphological changes observed in each
group.
Animal experiments
P407 has a long retention time in the
mouse tumor cavity
Methylene blue-P407 and methylene blue solution were
injected into the tumor cavities of the mice that were subcutaneous
transplanted with liver cancer cells. Our results revealed that
mice that were injected with methylene blue-P407 had less effluent
at the injection site and thereby a lighter blue-stained local
skin, while mice that were injected with methylene blue solution
had deeper blue-stained local skin due to a larger amount of
effluent. Similar results were observed at 3 min after the
injection, as well as 24 h later. When the solid tumor cavity was
opened, we found a stronger pigmentation of methylene blue-P407
than methylene blue solution, indicating that the retention time of
the former in the tumor cavity was longer than the latter (data not
shown).
OCT-P407 has a large residual
concentration in the mouse tumor cavity
At 12 h after the intratumoral injection of 100
μg/ml of OCT alone or in conjunction with P407, the peak areas (AU)
of the 2 groups were detected by HPLC and the residual
concentration of OCT (in μg/ml) was calculated by the external
reference method. Our results revealed that the residual
concentration of OCT in the OCT-P407 group was significantly higher
than the OCT group (P<0.05). Moreover, at 24 and 48 h after
injection, OCT could still be detected in the OCT-P407 group, but
no was longer detected in the OCT solution group.
OCT-P407 prevents the increase in
tumor volume and tumor weight
Before treatment there were no statistically
significant differences in the tumor volume between the groups
(P>0.05). As can be observed in Fig. 4B, the tumor volume in the
untreated (NS) group increased gradually from day 0 to 8 after
administration, growing to twice the original size on day 8.
Changes in tumor volume in the P407 group were similar to those in
the NS group, with these 2 groups presenting no significant
differences (P<0.05) at all time points (Fig. 4B). On the second day after
treatment, the tumor volume in the OCT group was not significantly
altered compared with the volume before administration, but was
significantly decreased when compared with the NS group
(P<0.05). However, after 4, 6 and 8 days of administration,
tumor volume increased and there was no statistically significant
difference compared with the NS group (P>0.05) (Fig. 4B). After 2, 4 and 6 days of
administration, tumor volumes in the OCT-P407 group were not
increased and on day 8, there was no statistically significant
difference compared with the volumes before administration
(P>0.05) (Fig. 4B). Although
on the second day after administration there was no significant
difference between the tumor volumes in the OCT-P407 and OCT
solution groups, from day 4 onwards, the tumor volume in the OCT
group increased and the differences between these 2 groups became
significant (P<0.05). Tumor volumes in the ethanol group did not
increase over time and had no statistically significant differences
when compared with the volumes before treatment (P>0.05) and
also when compared with the tumor volumes in the OCT-P407 group at
different time points (P>0.05) (Fig. 4B).
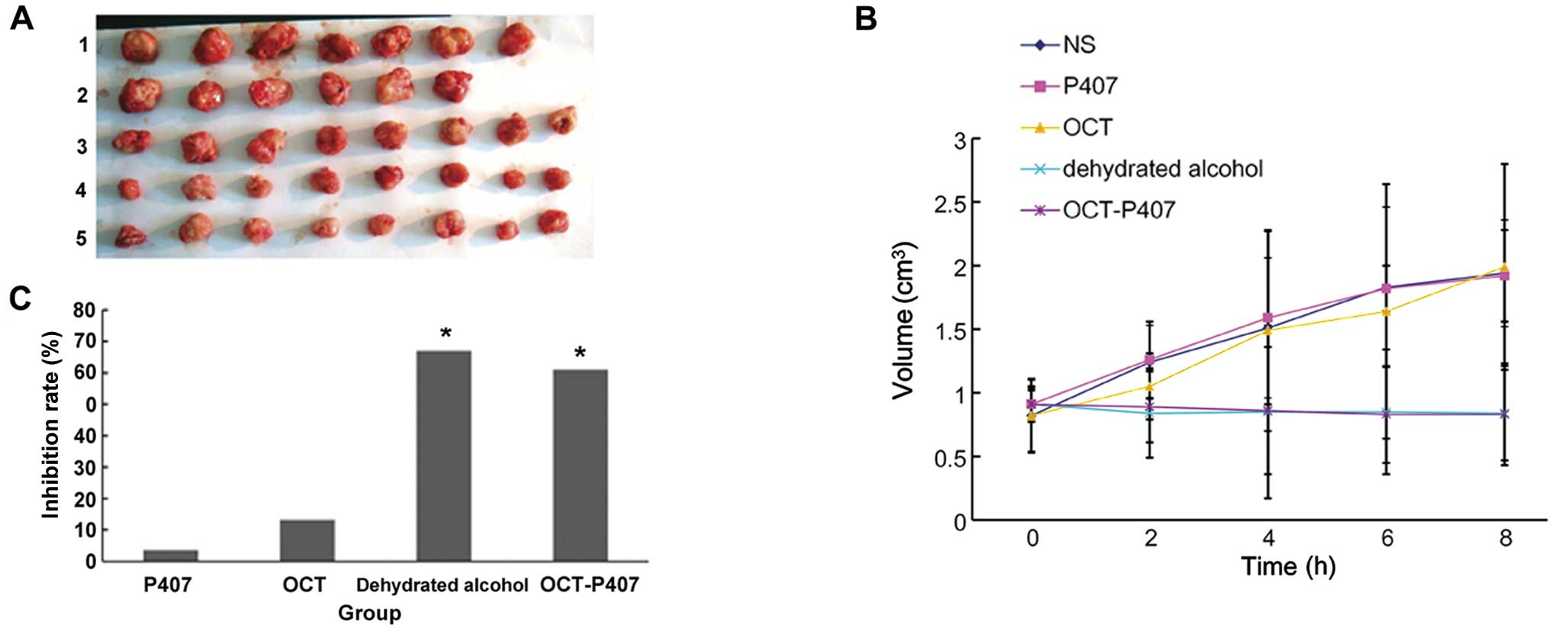 | Figure 4Changes in tumor volume and tumor
weight upon treatment with octreotide (OCT)-poloxamer 407 (P407).
(A) On day 8 of treatment with normal saline (NS), P407, OCT,
ethanol and OCT-P407, the mice were culled and the tumors excised.
Lane 1, NS group; lane 2, P407 group; lane 3, OCT group; lane 4,
dehydrated alcohol group; lane 5, OCT-P407 group. (B) Tumor volume
was measured at different time points, showing that the tumors in
the OCT-P407 and ethanol groups were significantly smaller than
those in the other groups (P<0.05). (C) On day 8 of treatment,
tumor weight was measured and the tumor inhibition rate was
calculated, showing that the tumors in the OCT-P407 and ethanol
groups were significantly lighter than those in the other groups
(*P<0.05). |
After 8 days of treatment, the tumors were excised
and we observed by the naked eye. The tumor volumes in the NS and
P407 groups were larger, followed by the OCT solution group. The
tumor volumes in the ethanol and OCT-407 groups were the lowest
(Fig. 4A). Tumor weight was then
measured and the tumor IR calculated. In accordance with the tumor
volumes, tumors in the OCT-P407 and particularly those in the
ethanol group were significantly lighter in weight (P<0.05) than
those in the OCT, NS and P407 groups (Fig. 4C).
OCT-P407 induces protein expression of
SSTR-2 and caspase-3 and decreases that of VEGF
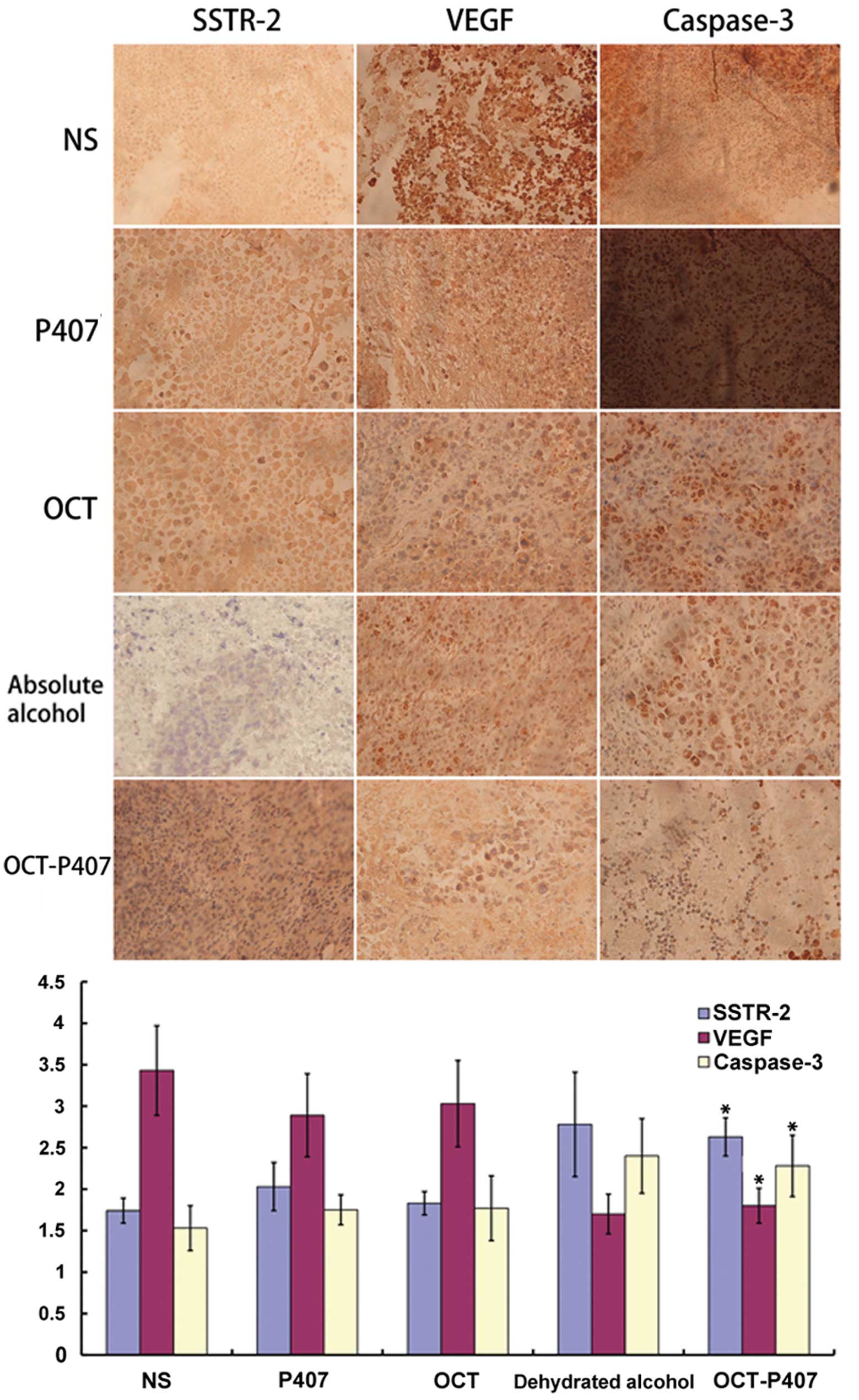
As detected by immunohistochemistry of the mouse
tumor sections, the number of positive cells, as well as the
staining intensity of SSTR-2 and the number of cytoplasm yellow
granules in the OCT-P407 group were significantly higher
(P<0.05) than those in the OCT, P407 and NS groups and, even
though there was no statistically significant difference
(P>0.05), they were also higher than those in the ethanol group
(Fig. 5). On the contrary, the
number of positive cells, as well as the staining intensity of VEGF
and the number of cytoplasm yellow granules in the OCT-P407 group
were significantly lower (P<0.05) than those in the OCT, P407
and NS groups and, although there was no statistically significant
difference (P>0.05), they were also lower than those of the
ethanol group (Fig. 5). Similar
to SSTR-2, the number of positive cells, as well as the staining
intensity of caspase-3 and the number of cytoplasm yellow granules
in the OCT-P407 group, were significantly higher (P<0.05) than
those in the OCT, P407 and NS groups; however, they were lower,
although not significantly (P>0.05), than those in the ethanol
group (Fig. 5).
OCT-P407 induces mRNA expression of
SSTR-2 and caspase-3 and decreases that of VEGF
As detected by RT-PCR in the mouse tumor sections,
the mRNA expression of SSTR-2 in the OCT-P407 group was
significantly higher (P<0.05) than that in the OCT, P407, NS and
even the ethanol group (Fig. 6).
On the contrary, the mRNA expression of VEGF in the OCT-P407 group
was significantly lower (P<0.05) than that in the OCT, P407 and
NS groups and, but was not statistically significant compared with
the ethanol group (P>0.05) (Fig.
6). Similar to SSTR-2, the mRNA expression of caspase-3 in the
OCT-P407 group was significantly higher (P<0.05) than that in
the OCT, P407, NS and even the ethanol group (Fig. 6).
Discussion
The incidence and mortality rates of primary
hepatocellular carcinoma rank 7th and 4th, respectively worldwide
among tumors (21). Medication
cannot improve the condition of the patients, whereas surgical
procedures, such as hepatectomy or liver transplantation may be
more efficient. However, even if the tumor is small and localized,
the 5-year survival rate only reaches up to 37.9–50% (22,23). Studies have shown that the
survival rate can be significantly improved when TACE is combined
with physical or medical methodologies when treating solid tumors,
such as PEI, liquid nitrogen freezing, high frequency laser,
microwave and RFA among others. Progress has been made in TACE,
positron emission tomography (PET) and RFA (24,25) thus far, and studies have also
begun to focus on percutaneous intratumoral injection chemotherapy
(26–28). However, for those patients with
advanced liver cancer with liver dysfunction and a poor physical
condition, such treatments often have to be discontinued.
In recent years, OCT has been reported to have
antitumor effects (12,29–32). Compared with traditional
chemotherapeutics, OCT has a better specificity, fewer side-effects
and is harder to acquire resistance. Its action is mild and it has
hepatoprotective effects (33).
With these advantages, it is possible to conduct antitumor therapy
in such patients mentioned above. In order to overcome the
disadvantage of the short half-life of OCT, in the present study,
we tried to prolong the action time of OCT and improve its
treatment effectiveness by changing the pharmaceutical dosage
form.
As a type of polymer, P407-based thermosensitive
gels in situ have been extensively studied in recent years
(34–36). It uses the difference in
temperature between the environment and the human body to its
advantage. It can be administered by injection and forms a gel at
physiological temperatures, prolonging the action time of drugs,
improving their therapeutic effects (28). In vivo and in vitro
experiments have indicated that P407 has a stable release effect
(37–41) and compared with other similar
materials, it is more stable and can carry more drugs, in addition
to the fact that its toxicity is low, its surface is smooth and
soft and requires little stimulation. It will not be sensed as a
foreign body on the injection site. Furthermore, it has a good
biocompatibility and is easy to produce. It has been widely used in
delayed and controlled release gel systems and it is an ideal and
safe adjuvant used for the delayed release of drugs. P407 has been
reported to be combined with adriacin, chitosan and other polymers
to form depot preparations for intratumoral injection therapy
(42–46). In the present study, considering
that OCT has disadvantages, such as instability and a short
half-life and that P407 may increase drug stability, we tried to
combine these 2 materials to form the OCT-P407 depot preparation to
enhance the effects of intratumoral injection for the treatment of
hepatocellular carcinoma.
We conducted cell culture and in vivo
experiments to investigate the antitumor effects of OCT-P407.
First, MTT assay was used to detect the effects of OCT and OCT-P407
on the proliferation of Hca-F hepatocellular carcinoma cells. Our
results indicated that the proliferation of liver cancer cells in
the OCT solution group was inhibited. In our study, compared with
the control group, 24 h after treatment of the Hca-F cells with
OCT-P407 containing various concentrations of OCT, cell
proliferation was markedly suppressed. With the increasing drug
concentrations, the IR increased and once the best inhibitory
concentration was defined, the inhibitory effects increased over
time (P<0.05). The IRs of the OCT-P407 group upon 12, 24 and 48
h of treatment were significantly higher (P<0.05) than those of
the OCT solution group. This may be due to the cumulative effects
of drug delayed release that enhanced its effects.
Cell apoptosis is closely associated with the
occurrence, development and malignant transformation of tumors.
Studies have proven that OCT can not only inhibit the proliferation
of tumor cells, but can also induce tumor cell apoptosis (47,48). To assess this, we performed
quantitative analysis of tumor cell apoptosis using the Annexin
V-FITC/PI double staining method combined with flow cytometry. Our
results indicated that the effects of OCT-P407 on the induction of
apoptosis were significantly higher than those of OCT alone at
different time points (P<0.05) and that these effects increased
over time. We additionally observed the morphological changes in
the liver cancer cells 48 h after the addition of OCT-P407 and OCT
under an inverted light microscope and found that changes induced
by OCT-P407 were more obvious.
Mouse livers are relatively small and therefore are
not easy to operate on and observe and the Hca-F liver cancer cells
used in this study grow better in lymphatic tissue. Moreover,
establishing a mouse liver cancer implantation tumor model is
easier, faster and reliable; therefore we used it as our in
vivo experimental model.
It has been reported that the leaching method with
no membrane is commonly used to investigate the release time of
drugs in gels (49); however,
this method is time consuming and can only be conducted in
vitro. In this study, after establishing the mouse model of
liver cancer by the subcutaneous transplantation of cancer cells,
methylene blue and methylene blue thermosensitive gel were injected
into the tumor cavity to investigate their retention at different
time points. We observed a high amount of methylene blue in the
methylene blue P407 group 24 h later compared with the methylene
blue solution group. This experiment proved that P407 itself had an
outstanding adhesive attraction that slowed down the flow speed of
methylene blue in the solid tumor and prolonged its retention time
in the solid tumor cavity, providing direct evidence for its
delayed release effect in the tumor cavity. In this study, we
injected prepared OCT-P407 or OCT solution into the tumor cavities
of mice and observed the effects 12 h after injection. The OCT
content in the mouse tumor cavities was higher in the OCT-P407
group than in the OCT solution group. In contrast to the OCT
solution group, OCT could still be detected in the OCT-P407 group
24 and 48 h later. We concluded that the retention time of OCT-P407
was higher than that of OCT and this indicated that P407 has a
delayed release effect.
Since our in vitro data have confirmed that
OCT-P407 can inhibit liver cancer cell proliferation and induce
tumor cell apoptosis, we proceeded to investigate its antitumor
effects through in vivo experiments. We therefore compared
OCT-P407 with ethanol, a well known therapeutic drug administered
by intratumoral injection for the treatment of liver cancer, as
well as with a conventional OCT preparation, NS and P407. These
reagents were separately injected into mice subcutaneously
transplanted with Hca-F hepatocellular carcinoma cells. As a
result, the proliferation of tumor cells in the OCT-P407, ethanol
and OCT groups was inhibited, with the tumor volumes at different
time points after injection being smaller than those in the NS and
P407 groups (P<0.05). Two days after the injection, tumor
volumes in the OCT-P407 and ethanol groups were very similar to
those in the OCT solution group (P<0.05); however, 4, 6 and 8
days after injection, their volumes were significantly lower than
those in the OCT solution group (P<0.05). This indicated that
the action time of OCT-P407 was significantly longer than that of
OCT. Even though the tumor volume in the ethanol group tended to be
lower than that in the OCT-P407 group, there was no statistically
significant difference between them (P>0.05). In the end, the
tumor weights in the OCT-P407 and ethanol groups were lower than
those in the OCT, NS and P407 groups, although the tumor weight in
the ethanol group was lower than that in the OCT-P407 group
(P<0.05). Their IRs were 67 and 61%, respectively, with the
difference between them being statistically significant
(P<0.05). Our results confirmed that OCT-P407 had a greater
inhibitory effect on tumor cell proliferation than OCT and that its
effects were similar to those of ethanol as regards tumor volume,
although its effects were not as prominent as those of the latter
as regards tumor weight and IR. This may be due to the central
tumor necrosis caused by ethanol.
Previous studies have suggested that the antitumor
mechanism of OCT may be related to the following factors: i) The
direct inhibition of tumor cell proliferation, which is related to
the expression of the SSTR on the tumor cell surface (50). In 1998, Kouroumalis et al
(51) found that there were SSTRs
in human liver cancer cells contrary to normal liver tissue. The
combination of OCT and SSTR produces vasoconstrictive effects and
induces a dysfunction of the blood circulation in tumor tissue. It
has been described that there are one or more types of SSTRs in the
majority of tumor cells, which can be inhibited by OCT and SSTR-2
is the most common. OCT has a great affinity for SSTR-2 and it is
its selective receptor antagonist. The combination of OCT and SSTR
can exert cytotoxic effects, thereby inducing a lethal effect on
target cells (51,52). ii) The inhibition of angiogenesis,
which inhibits tumor cell proliferation by reducing tumor blood
supply. It has been reported that OCT can also reduce angiogenesis
by inhibiting VEGF (53). VEGF is
the strongest vascular growth factor with the highest specificity.
It acts on vascular endothelial cells specifically, participates in
the angiogenesis of solid tumors and it is closely associated with
the biological characteristics of solid tumors, such as invasion
and metastasis (54,55). iii) The induction of tumor cell
apoptosis. It has been described that one of the antitumor
mechanisms of OCT may be the induction of the apoptosis of
malignant cells (56,57). The core component of the apoptotic
cascade is the cysteinyl aspartate specific proteinase (caspase)
family (58), with caspase-3
being the crucial effector molecule in this process (59,60).
In this study, we investigated the mRNA and protein
expression of SSTR-2, VEGF and caspase-3 and found that, compared
with the ethanol group, the OCT-P407 group tended to have increased
mRNA and protein expression levels of SSTR-2 and caspase-3;
however, there was no statistically significant difference
(P>0.05). The difference was however, significant when compared
with the OCT solution, P407 and NS groups (P<0.05). The
expression of VEGF protein in the OCT-P407 group tended to be lower
than that in the ethanol group, despite the absence of significance
(P>0.05). VEGF mRNA and protein expression in the OCT-P407 group
was lower than that in the OCT solution, P407 and NS groups
(P<0.05), but higher than that in the ethanol group as regards
mRNA expression (P<0.05).
Taken together, our results indicate that the
delayed release effect of OCT-P407 leads to a stronger antitumor
effect than OCT alone and that the mechanisms involved may be: i)
Upregulation of the expression of SSTR-2, inhibiting the growth of
liver cancer cells and inducing apoptosis. It has been shown that
with the application of an osmotic micropump containing OCT, the
long-term sustaining release of OCT can lead to the upregulation of
SSTR-2 and in tumor models, this upregulation of SSTR-2 depends on
the continuous contact with OCT, demonstrating that the sustained
release of small doses of OCT can lead to the upregulation of
SSTR-2 in tumor cells (61). ii)
OCT-P407 can enhance the inhibitory effects on angiogenesis by
enhancing the inhibitory effects of OCT on the gene and protein
expression of VEGF. The occurrence and development of liver cancer
are closely related to the expression levels of VEGF (57,62). The delayed release effect of
OCT-P407 can inhibit the occurrence and development hepatocellular
carcinoma. iii) OCT-P407 can induce apoptosis through the signal
transduction pathway mediated by caspase-3. OCT-P407 is similar to
ethanol in its antitumor mechanism. The only difference between
them was that the mRNA expression level of VEGF was lower in the
ethanol group than the OCT-P407 group, indicating that ethanol may
inhibit angiogenesis faster and more prominently by causing tissue
necrosis.
As stated above, in the present study, firstly, we
proved by in vitro cell culture experiments that OCT-P407
inhibits the proliferation and induces the apoptosis of liver
cancer cells. We then showed that the retention time of OCT-P407 in
the solid tumor cavity is longer than that of OCT, which indicates
that OCT-P407 has a delayed release effect. We also observed that
even though the antitumor effects of the intratumoral injection of
OCT-P407 were not as effective as those of ethanol in general, in
some aspects they had similar results. This suggests that OCT-P407
may prove to be effective as an antitumor drug. There were some
drawbacks in this experiment, such as the ratio of the
concentration of the thermosensitive gel and cell culture medium
which was difficult to handle in the in vitro cell culture
experiments and thus 3 days after the gels were added, the medium
became muddy and difficult to observe. Also, in the in vivo
experiments, the livers of the mice were too small to obtain tumor
tissue transplants and we were thus unable to establish the liver
solid tumor model. Moreover, in the design of the experiment, a
chemotherapeutics group was meant to be included in the control
groups; however, due to the variety of drugs already described, we
decided not to include such a group. To date, only a few cell
culture experiments using gels have been performed by us and others
and we hope that the experimental methodology can be improved in
the future. Our laboratory will select a more appropriate model of
liver cancer solid tumors for further experiments and we are
looking forward to providing patients with a new advanced liver
cancer treatment with the advantages of delayed release, low
toxicity and better compliance.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 30970886) and the
Science and Technology Project of Dalian (no. 2010E15SF179).
References
|
1
|
Han KH and Park JY: Systemic treatment in
advanced/metastatic hepatocellular carcinoma in the era of targeted
therapy. J Gastroenterol Hepatol. 25:1023–1025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang ZM, Guo JX, Zhang ZC, Jiang N, Zhang
ZY and Pan LJ: Therapeutic options for intermediate-advanced
hepatocellular carcinoma. World J Gastroenterol. 17:1685–1689.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bouza C, López-Cuadrado T, Alcázar R,
Saz-Parkinson Z and Amate JM: Meta-analysis of percutaneous
radiofrequency ablation versus ethanol injection in hepatocellular
carcinoma. BMC Gastroenterol. 9:312009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burton KR, O’Dwyer H and Scudamore C:
Percutaneous ethanol ablation of hepatocellular carcinoma:
periprocedural onset alcohol toxicity and pancreatitis following
conventional percutaneous ethanol ablation treatment. Can J
Gastroenterol. 23:554–556. 2009.
|
|
5
|
Danila M, Sporea I, Sirli R and Popescu A:
Percutaneous ethanol injection therapy in the treatment of
hepatocarcinoma-results obtained from a series of 88 cases. J
Gastrointestin Liver Dis. 18:317–322. 2009.PubMed/NCBI
|
|
6
|
Weijian F, Zan L, Suhong H, et al:
Destructive effect of percutaneous hydrochloric acid injection
therapy for liver cancer - a preliminary experimental and clinical
study. Gan To Kagaku Ryoho. 33:1852–1856. 2006.PubMed/NCBI
|
|
7
|
Castro DJ, Sridhar KS, Garewal HS, et al:
Intratumoral cisplatin/epinephrine gel in advanced head and neck
cancer: a multicenter, randomized, double-blind, phase III study in
North America. Head Neck. 25:717–731. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwak MK, Hur K, Yu JE, et al: Suppression
of in vivo tumor growth by using a biodegradable thermosensitive
hydrogel polymer containing chemotherapeutic agent. Invest New
Drugs. 28:284–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bornschein J, Drozdov I and Malfertheiner
P: Octreotide LAR: safety and tolerability issues. Expert Opin Drug
Saf. 8:755–768. 2009.PubMed/NCBI
|
|
10
|
Guo TK, Hao XY, Ma B, et al: Octreotide
for advanced hepatocellular carcinoma: a meta-analysis of
randomized controlled trials. J Cancer Res Clin Oncol.
135:1685–1692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Jin W, Wang X, Wang J, Zhang X
and Zhang Q: A novel octreotide modified lipid vesicle improved the
anticancer efficacy of doxorubicin in somatostatin receptor 2
positive tumor models. Mol Pharm. 7:1159–1168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu LQ, Lu Y, Lu HJ, Zhao ZG and Yang M:
Efficacy of intra-tumor injection of Kang-Lai-Te in treating
transplanted hepatoma in rats. Hepatobiliary Pancreat Dis Int.
3:580–584. 2004.PubMed/NCBI
|
|
13
|
Liu S, Sun MZ, Tang JW, Wang Z, Sun C and
Greenaway FT: High-performance liquid
chromatography/nano-electrospray ionization tandem mass
spectrometry, two-dimensional difference in-gel electrophoresis and
gene microarray identification of lymphatic metastasis-associated
biomarkers. Rapid Commun Mass Spectrom. 22:3172–3178. 2008.
View Article : Google Scholar
|
|
14
|
Yu SJ, Kang XH, Zhang JN, et al: Effects
of small interfering RNA targeting heparanase-1 combined with
heparin on invasiveness of mouse hepatocellular carcinoma cell
lines. Chin J Cancer. 29:816–823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasaki K, Tsuno NH, Sunami E, et al:
Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on
colon cancer cells. BMC Cancer. 10:3702010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aresvik DM, Pettersen RD, Abrahamsen TG
and Wright MS: 5-fluorouracil-induced death of Jurkat T-cells-a
role for caspases and MCL-1. Anticancer Res. 30:3879–3887.
2010.PubMed/NCBI
|
|
17
|
Song B, Tang JW, Wang B, Cui XN, Zhou CH
and Hou L: Screening for lymphatic metastasis-associated genes in
mouse hepatocarcinoma cell lines Hca-F and Hca-P using gene chip.
Ai Zheng. 24:774–780. 2005.(In Chinese).
|
|
18
|
Cui XN, Tang JW, Song B, Wang B, Chen SY
and Hou L: High expression of osteoglycin decreases gelatinase
activity of murine hepatocarcinoma Hca-F cells. World J
Gastroenterol. 15:6117–6122. 2009. View Article : Google Scholar
|
|
19
|
Ballal N, Kundabala M, Bhat K, et al:
Susceptibility of Candida albicans and Enterococcus
faecalis to Chitosan, Chlorhexidine gluconate and their
combination in vitro. Aust Endod J. 35:29–33. 2009.
|
|
20
|
Guo SB, Duan ZJ, Li Q and Sun XY: Effect
of heme oxygenase-1 on renal function in rats with liver cirrhosis.
World J Gastroenterol. 17:322–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
International Agency for Research on
Cancer. World Hearth Organization: WHO IARC Report.
|
|
22
|
Alkofer B, Lepennec V and Chiche L:
Hepatocellular cancer in the non-cirrhotic liver. J Visc Surg.
148:3–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi GH, Park JY, Hwang HK, et al:
Predictive factors for long-term survival in patients with
clinically significant portal hypertension following resection of
hepatocellular carcinoma. Liver Int. 31:485–493. 2011. View Article : Google Scholar
|
|
24
|
Johnson PJ: Non-surgical treatment of
hepatocellular carcinoma. HPB (Oxford). 7:50–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kurokohchi K, Hosomi N, Yoshitake A, et
al: Successful treatment of large-size advanced hepatocellular
carcinoma by transarterial chemoembolization followed by the
combination therapy of percutaneous ethanol-lipiodol injection and
radiofrequency ablation. Oncol Rep. 16:1067–1070. 2006.
|
|
26
|
Jang JW, Park YM, Bae SH, et al:
Therapeutic efficacy of multimodal combination therapy using
transcatheter arterial infusion of epirubicin and cisplatin,
systemic infusion of 5-fluorouracil, and additional percutaneous
ethanol injection for unresectable hepatocellular carcinoma. Cancer
Chemother Pharmacol. 54:415–420. 2004. View Article : Google Scholar
|
|
27
|
Oh YJ, Park YM, Kim BH, et al: A case of
hepatocellular carcinoma with pulmonary metastases treated
successfully with a combination of repeated hepatic arterial
infusion epirubicin and Cisplatin chemotherapy and systemic
low-dose infusion of 5-Fluorouracil. Gut Liver. 3:343–348. 2009.
View Article : Google Scholar
|
|
28
|
Yang L, Wang B, Qiao W and Liu P: A novel
combination chemotherapy integrating with intratumoral
chemotherapy. Medical hypotheses. 73:334–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen S, Xie Y, Wang CH and Tang CW:
Effects of octreotide on necrosis of hepatocellular carcinoma
xenografts in nude mice. Ai Zheng. 28:673–678. 2009.(In
Chinese).
|
|
30
|
Varas-Lorenzo MJ: Long-standing malignant
pancreatic carcinoid treated with octreotide. Rev Esp Enferm Dig.
102:662–666. 2010.PubMed/NCBI
|
|
31
|
Pettit L and El-Modir A: The role of
somatostatin analogues in the treatment of advanced malignant
thymomas: case report and review of the literature. Br J Radiol.
84:e7–e10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeuchi K, Fujiwara K, Tsujino T and
Morita H: Successful medical treatment with octreotide for
chyloperitoneum following paraaortic lymphadenectomy in the
treatment of gynecologic malignancies: a report of 2 cases. J
Reprod Med. 56:75–77. 2011.
|
|
33
|
Tracy TF Jr, Tector AJ, Goerke ME, Kitchen
S and Lagunoff D: Somatostatin analogue (octreotide) inhibits bile
duct epithelial cell proliferation and fibrosis after extrahepatic
biliary obstruction. Am J Pathol. 143:1574–1578. 1993.PubMed/NCBI
|
|
34
|
Cao Y, Zhang C, Shen W, Cheng Z, Yu LL and
Ping Q: Poly(N-isopropylacrylamide)-chitosan as thermosensitive in
situ gel-forming system for ocular drug delivery. J Control
Release. 120:186–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Batrakova EV and Kabanov AV: Pluronic
block copolymers: evolution of drug delivery concept from inert
nanocarriers to biological response modifiers. J Control Release.
130:98–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Zhu YY, Wei G and Lu WY: Effect of
carrageenan on poloxamer-based in situ gel for vaginal use:
improved in vitro and in vivo sustained-release properties. Eur J
Pharm Sci. 37:306–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Y, Li L, Liu H, Peng B and Jin R:
Preparation and release in vitro of injectable thermosensitive in
situ gel of Glabrous Sarcandra herb extract. Zhongguo Zhong
Yao Za Zhi. 34:2586–2589. 2009.(In Chinese).
|
|
38
|
Ammar HO, Salama HA, Ghorab M and Mahmoud
AA: Development of dorzolamide hydrochloride in situ gel
nanoemulsion for ocular delivery. Drug Dev Ind Pharm. 36:1330–1339.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baloğlu E, Karavana SY, Hyusein IY and
Köse T: Design and formulation of mebeverine HCl semisolid
formulations for intraorally administration. AAPS PharmSciTech.
11:181–188. 2010.PubMed/NCBI
|
|
40
|
Monti D, Burgalassi S, Rossato MS, et al:
Poloxamer 407 microspheres for orotransmucosal drug delivery. Part
II: In vitro/in vivo evaluation. Int J Pharm. 400:32–36. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kojarunchitt T, Hook S, Rizwan S, Rades T
and Baldursdottir S: Development and characterisation of modified
poloxamer 407 thermoresponsive depot systems containing cubosomes.
Int J Pharm. 408:20–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ishihara M, Obara K, Nakamura S, et al:
Chitosan hydrogel as a drug delivery carrier to control
angiogenesis. J Artif Organs. 9:8–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo DD, Moon HS, Arote R, et al: Enhanced
anticancer effect of conjugated linoleic acid by conjugation with
Pluronic F127 on MCF-7 breast cancer cells. Cancer Lett.
254:244–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sastre RL, Olmo R, Teijón C, Muñíz E,
Teijón JM and Blanco MD: 5-Fluorouracil plasma levels and
biodegradation of subcutaneously injected drug-loaded microspheres
prepared by spray-drying poly(D,L-lactide) and
poly(D,L-lactide-co-glycolide) polymers. Int J Pharm. 338:180–190.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Al-Abd AM, Hong KY, Song SC and Kuh HJ:
Pharmacokinetics of doxorubicin after intratumoral injection using
a thermosensitive hydrogel in tumor-bearing mice. J Control
Release. 142:101–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bae WK, Lee JH, Lee SJ, et al: Enhanced
anti-cancer effect of 5-fluorouracil loaded into thermo-responsive
conjugated linoleic acid-incorporated poloxamer hydrogel on
metastatic colon cancer models. J Nanosci Nanotechnol.
11:1425–1428. 2011. View Article : Google Scholar
|
|
47
|
Liu HL, Huo L and Wang L: Octreotide
inhibits proliferation and induces apoptosis of hepatocellular
carcinoma cells. Acta Pharmacol Sin. 25:1380–1386. 2004.PubMed/NCBI
|
|
48
|
Hua YP, Yin XY, Peng BG, et al: Mechanisms
and influence of octreotide-induced regulation of somatostatin
receptor 2 on hepatocellular carcinoma. Chemotherapy. 55:312–320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Moore T, Croy S, Mallapragada S and Pandit
N: Experimental investigation and mathematical modeling of Pluronic
F127 gel dissolution: drug release in stirred systems. J Control
Release. 67:191–202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Susini C and Buscail L: Rationale for the
use of somatostatin analogs as antitumor agents. Ann Oncol.
17:1733–1742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kouroumalis E, Skordilis P, Thermos K,
Vasilaki A, Moschandrea J and Manousos ON: Treatment of
hepatocellular carcinoma with octreotide: a randomised controlled
study. Gut. 42:442–447. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Patel YC: Somatostatin and its receptor
family. Front Neuroendocrinol. 20:157–198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jia WD, Xu GL, Xu RN, et al: Octreotide
acts as an antitumor angiogenesis compound and suppresses tumor
growth in nude mice bearing human hepatocellular carcinoma
xenografts. J Cancer Res Clin Oncol. 129:327–334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang ZL, Liu ZS and Sun Q: Expression of
angiopoietins, Tie2 and vascular endothelial growth factor in
angiogenesis and progression of hepatocellular carcinoma. World J
Gastroenterol. 12:4241–4245. 2006.PubMed/NCBI
|
|
55
|
Kandalaft LE, Motz GT, Busch J and Coukos
G: Angiogenesis and the tumor vasculature as antitumor immune
modulators: the role of vascular endothelial growth factor and
endothelin. Curr Top Microbiol Immunol. 344:129–148.
2011.PubMed/NCBI
|
|
56
|
Ferrante E, Pellegrini C, Bondioni S, et
al: Octreotide promotes apoptosis in human somatotroph tumor cells
by activating somatostatin receptor type 2. Endocr Relat Cancer.
13:955–962. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kaseb AO, Hanbali A, Cotant M, Hassan MM,
Wollner I and Philip PA: Vascular endothelial growth factor in the
management of hepatocellular carcinoma: a review of literature.
Cancer. 115:4895–4906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
McNeish IA, Bell S, McKay T, Tenev T,
Marani M and Lemoine NR: Expression of Smac/DIABLO in ovarian
carcinoma cells induces apoptosis via a caspase-9-mediated pathway.
Exp Cell Res. 286:186–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Krajewska M, Wang HG, Krajewski S, et al:
Immunohistochemical analysis of in vivo patterns of expression of
CPP32 (Caspase-3), a cell death protease. Cancer Res. 57:1605–1613.
1997.PubMed/NCBI
|
|
60
|
Tsagarakis NJ, Drygiannakis I, Batistakis
AG, Kolios G and Kouroumalis EA: Octreotide induces caspase
activation and apoptosis in human hepatoma HepG2 cells. World J
Gastroenterol. 17:313–321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Plate KH, Breier G, Weich HA, Mennel HD
and Risau W: Vascular endothelial growth factor and glioma
angiogenesis: coordinate induction of VEGF receptors, distribution
of VEGF protein and possible in vivo regulatory mechanisms. Int J
Cancer. 59:520–529. 1994. View Article : Google Scholar
|
|
62
|
Tammela T, Zarkada G, Wallgard E, et al:
Blocking VEGFR-3 suppresses angiogenic sprouting and vascular
network formation. Nature. 454:656–660. 2008. View Article : Google Scholar : PubMed/NCBI
|