Introduction
Pulmonary arterial hypertension (PAH) is a common
complication of congenital heart disease caused by a left-to-right
shunt. It is a condition characterized by an increase in pulmonary
vascular resistance and remodeling, structural remodeling of the
heart, right heart dysfunction and widespread loss of pulmonary
microvasculature, leading to right-heart failure and premature
decease (1–7). In recent years, despite significant
progress being made in the development of drugs for the tretment of
PAH, the effects of currently used drugs are not ideal, while the
mechanisms causing PAH formation have not yet been fully
elucidated. Vascular structural remodeling is considered the key
process in the pathology of hypoxic pulmonary hypertension
(8). Pulmonary vascular
structural remodeling is mainly caused by high pulmonary blood flow
(9), which leads to neointimal
formation and hyperplasia of the medial vascular wall, resulting
from an imbalance between proliferation and apoptosis in pulmonary
vascular smooth muscle cells favoring proliferation (VSMCs)
(10,11).
The cytoplasmic activity of the transcription factor
nuclear factor-κB (NF-κB) has been shown to be regulated through
its association with the IκB-α protein, itself regulated by the
ubiquitin (Ub)-proteasome system (UPS) (12,13). Previous studies have demonstrated
that the NF-κB pathway plays an important role in pulmonary
vascular remodeling (14,15). However, studies of the underlying
mechanisms of vascular remodeling via the NF-κB pathway remain are
limited.
The proteasome inhibitor, PS-341 (FDA approved drug:
Bortezomib/Velcade), has the potential to inhibit NF-κB activation
by reducing the degradation of IκB-α. In the present study, we
established a high blood flow-induced pulmonary hypertension model
to study vascular remodeling, using a surgical aorto-caval shunt
(ACS) method. We hypothesized that low doses of PS-341 may prevent
pulmonary vascular remodeling through the inhibition of the NF-κB
signaling pathway, by downregulating the UPS.
Materials and methods
Animals and reagents
Male Wistar rats, weighing 140–200 g, were purchased
from the Animal Experimental Center of Shandong University, Jinan,
China. All experimental procedures were approved by the
Institutional Animal Ethics Committee of Shandong University.
Six-week-old rats (n=45) were randomly divided into
3 groups (n=15 in each group): the sham-operated, shunt and PS-341
groups. PS-341 was kindly provided by Karolinska Institute,
Stockholm, Sweden and the dose used was in accordance with that
used in previous studies (13,16,17).
Animal model preparation
The animal model of ACS was established using a
previously described surgical method (18). Briefly, the animals were
anesthetized with an injection of 0.25% pentobarbital sodium (40
mg/kg). With the abdominal aorta and inferior vena cava fully
exposed, a bulldog vascular clamp was placed across the aorta
caudal to the left renal artery. The aorta was punctured using an
18-gauge disposable needle, which was subsequently withdrawn. A
silk thread was then used to stitch the puncture of the abdominal
wall. Swelling of the vena cava and mixing of arterial and venous
blood confirmed the implantation of the shunt. Three days
post-surgery, the rats that survived were randomly divided into 3
groups (n=15 in each group): the sham-operated, shunt and PS-341
groups. The sham-operated group was subjected to a sham operation
with the same procedure apart from the shunt implantation. The
PS-341 group received an intraperitoneal injection of PS-341 (50
μg/kg) for 8 weeks following shunt implantation, while the shunt
group received the same dose of saline instead of PS-341. Heparin
(0.5 mg/kg) was injected intra-abdominally 6 h post-surgery and
penicillin (20 mg/kg) was injected during the following 3 days.
Measurement of pulmonary arterial
pressure
Eight weeks following the establishment of the
model, the hemodynamic parameters of the animals were measured as
previously described (19).
Briefly, a 3F-microtip transducer catheter (Millar Instruments
Inc., Houston, TX, USA) connected to a data acquisition system
(Biopac Systems, Inc. Goleta, CA, USA) was inserted via the right
jugular vein into the right ventricle (RV) to obtain baseline
measurements of heart rate (HR), central venous pressure (CVP) and
right ventricular systolic pressure (RVSP).
Preparation of heart and lung
tissues
Following the measurement of hemodynamic parameters,
the rats were sacrificed, and the weights of the RV, the left
ventricle (LV) and the ventricular septum (SEP) were recorded. The
ratio of the right ventricular free wall weight to the sum of left
ventricular plus septal weight (RV/LV + S) was determined to
evaluate the extent of hypertrophy in the RV. Lung tissue samples
of a few animals were quickly harvested and embedded in optical
cutting temperature medium (OCT; Sigma-Aldrich, St. Louis, MO,
USA), frozen in liquid nitrogen and stored at −80°C. These samples
were subsequently used for western blot analysis. An additional
lung tissue sample was fixed in a 10% formaldehyde buffer solution
in situ using a tracheal cannula, and was embedded in
paraffin. The tissue sections were then cut into 5-μm-thick slices
and were stained with hematoxylin and eosin (H&E) and Masson’s
trichrome dye. The morphological alterations of the pulmonary
arterial wall were observed under an optical microscope as
previously described (20). The
percentages of medial wall thickness (WT%) and medial wall areas
(WA%) with an external diameter (ED) of 15–50 μm were calculated in
at least 10 arteries in each group to evaluate vascular remodeling.
These studies were performed by 2 examiners blinded to treatment
allocation.
Immunohistochemical analysis
The lung tissue sections were blocked with 2% goat
serum for 30 min and then washed with Public Broadcasting Service
(PBS) working fluid, cut into 5-μm-thick slices and incubated with
primary antibodies following fixation in acetone for 10 min at 4°C.
The protein content of the pulmonary arterial smooth muscle cells
(PASMCs) was semi-quantitatively assessed by immunohistochemistry,
carried out with monoclonal mouse anti-ubiquitin antibody (1:200)
and rabbit anti-NF-κB p65 antibody (1:100; Abcam, Cambridge, UK)
following the manufacturer’s recommendations. Subsequently, the
3,3′-diaminobenzidine (DAB) dye was added to visualize the
antibodies, and following washing of the tissue sections with
phosphate-buffered saline (PBS) solution, the sections were
observed and photographed under a microscope.
Western blot analysis
The lung tissue samples were homogenized in liquid
nitrogen and equal amounts of protein were denatured and separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE). Protein concentrations were semi-quantitatively
assessed using the BCA Protein Assay kit (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). Proteins (50 μg), loaded onto a 10%
SDS-polyacrylamide gel and electroblotted onto a nitrocellulose
membrane. The membrane was blocked for 16 h at 4°C in blocking
buffer containing 5% skim milk powder in TBST [20 mM Tris HCl (pH
7.4), 150 mM NaCl and 0.1% Tween-20] and incubated with
anti-ubiquitin (1:1,000), anti NF-κB p65 (1:2,000) and anti-IκB-α
(1:1,000) antibodies (all from Santa Cruz Biotechnology, Inc.) and
washed with Tris-buffered saline with TBST. The goat anti-rabbit
IgG (Boshide Inc., Shanghai, China) was incubated at 37°C for 1 h
as the secondary antibody. Immunoreactions were visualized using an
electrochemiluminescence (ECL) system according to the
manufacturer’s instructions (Thermo Fisher Scientific Co., Ltd.,
Shanghai, China).
Statistical analysis
The data are presented as the means ± SD. A one-way
ANOVA was performed for the statistical comparison of differences
between groups, using SPSS 16.0 software. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Mortality of the animals
No mortality was observed in the sham-operated
group, while 2 rats in the shunt group died from acute pulmonary
edema within the first week post-surgery and were replaced. In
total, 45 rats in each group were available for further
analyses.
Evaluation of hemodynamic parameters
There was no significant difference in the initial
HR, CVP and RVSP values among the 3 groups. Eight weeks after the
establishment of the ACS model, RVSP was significantly elevated in
the shunt group compared with the sham-operated group (P<0.05),
and was significantly lower compared with the PS-341 group
(P<0.05). The ratio of RV/(LV + S) was significantly higher in
the shunt compared with the sham-operated group (P<0.05), and
was significantly lower in the PS-341 group compared with the shunt
group (P<0.05). These results indicated that severe pulmonary
hypertension and right ventricular hypertrophy occurred; thus our
model of high blood flow-induced PAH was successfully
established.
Evaluation of changes in the pulmonary
artery wall structure
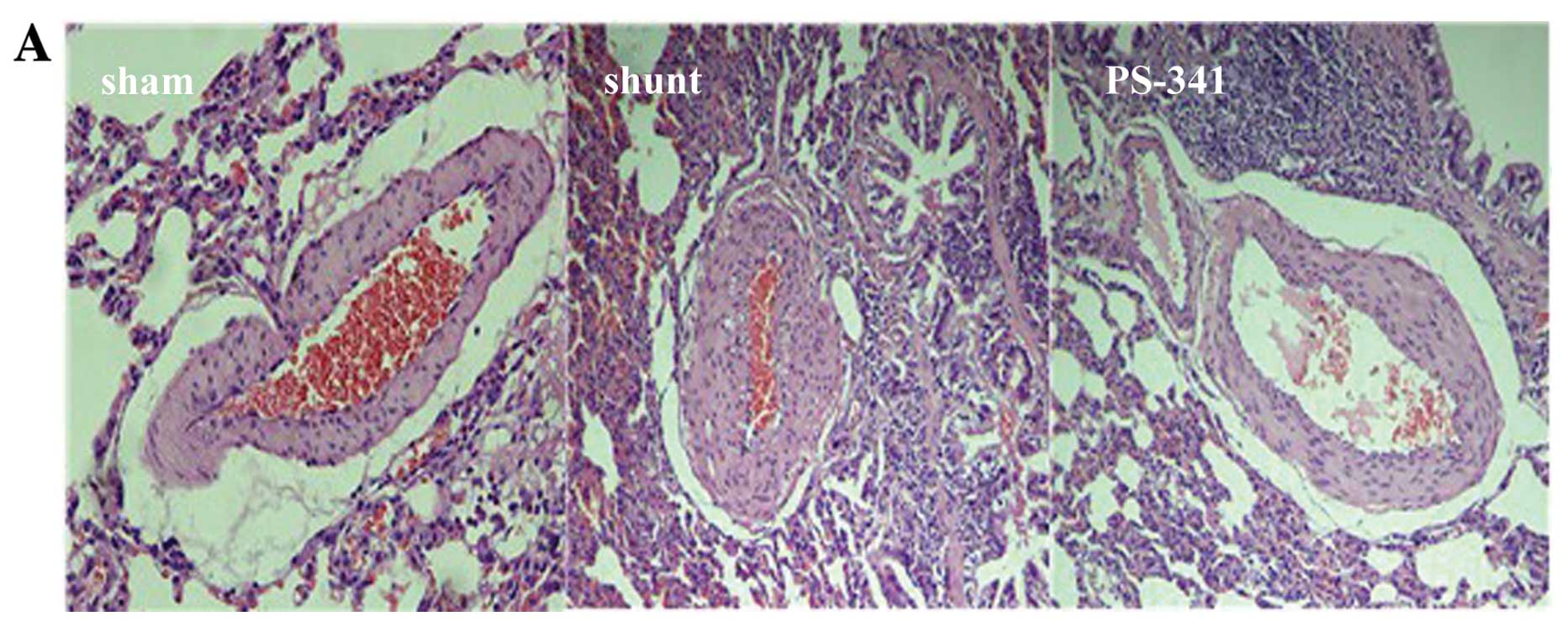
Eight weeks post-surgery, H&E staining
demonstrated a significant thickening of the intima and stenosis of
muscular arteries in the shunt compared with the sham-operated
group. The WT% and WA% of muscular arteries with an ED of 15–50 μm
was significantly decreased in the PS-341 group compared with the
shunt group (P<0.05) (Fig. 1).
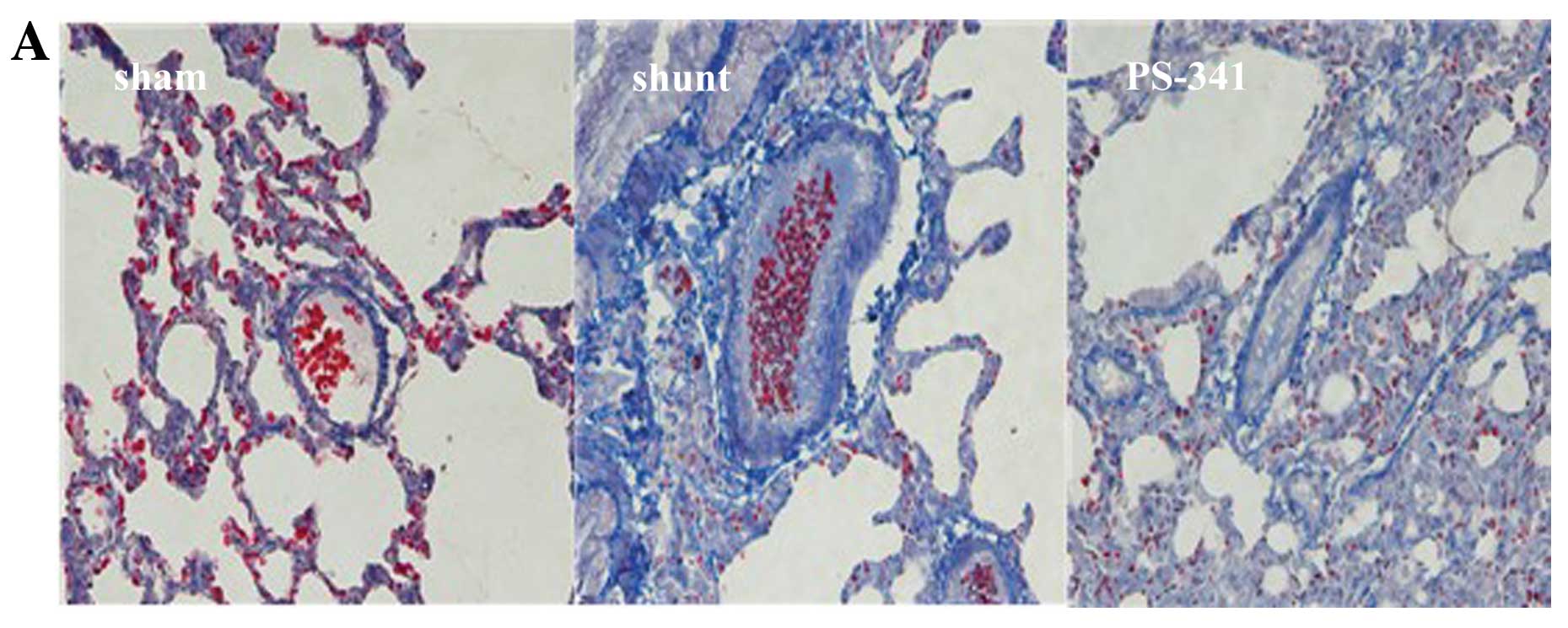
Masson’s trichrome staining (Fig.
2A) revealed that the vessel wall was obscured, the lumen size
was reduced, and the level of collagen was increased, while a
significant increase in the number of interstitial fibrotic areas
was observed in the shunt group compared with the sham-operated
group (P<0.01) (Fig. 2B).
Following treatment with PS-341, the above changes were
significantly reversed. These results also indicated that the
vascular remodeling model was successful established.
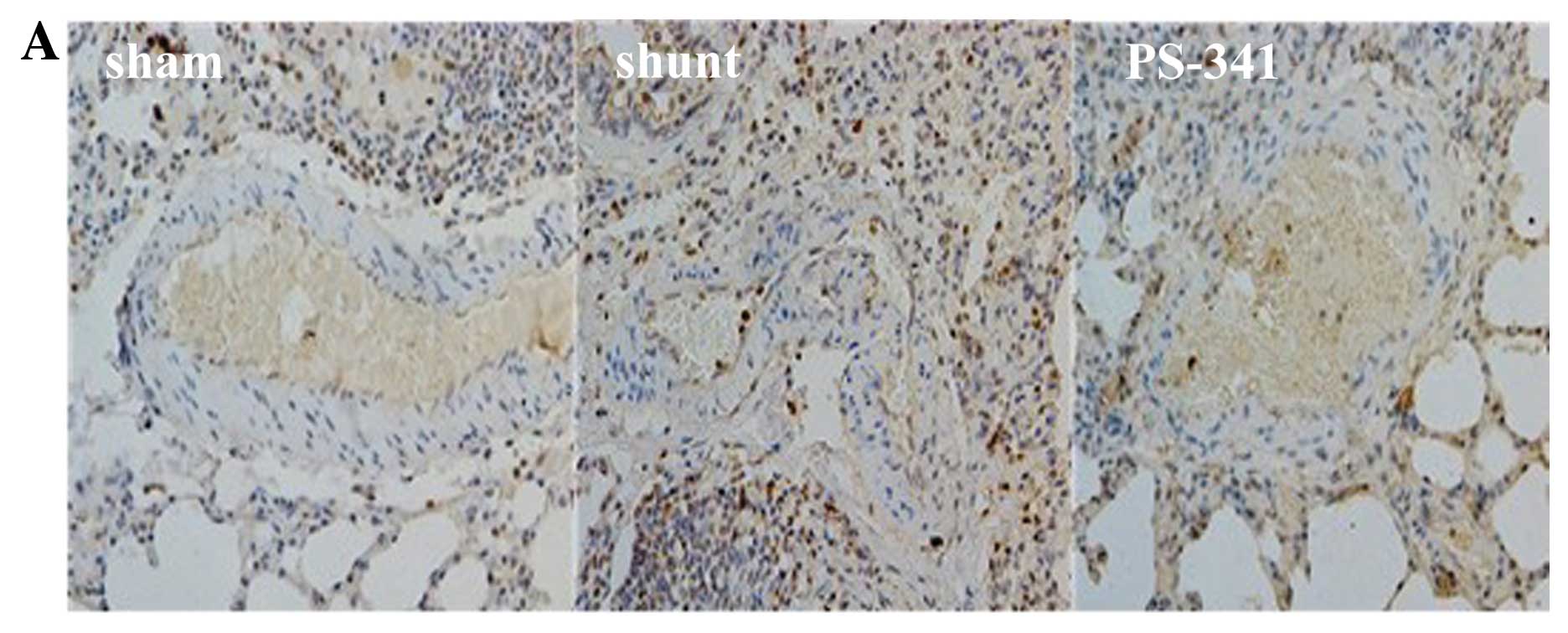
Ubiquitin and NF-κB p65 expression
detected by immunohistochemistry
There were significant differences observed in the
expression levels of ubiquitin and NF-κB p65 in the PASMCs in the 3
groups at 8 weeks post-surgery. Ubiquitin and NF-κB p65 were
detected in significantly higher levels in the shunt compared with
the sham-operated group (P<0.01), and in significantly lower
levels in the PS-341 group (P<0.05) (Fig. 3).
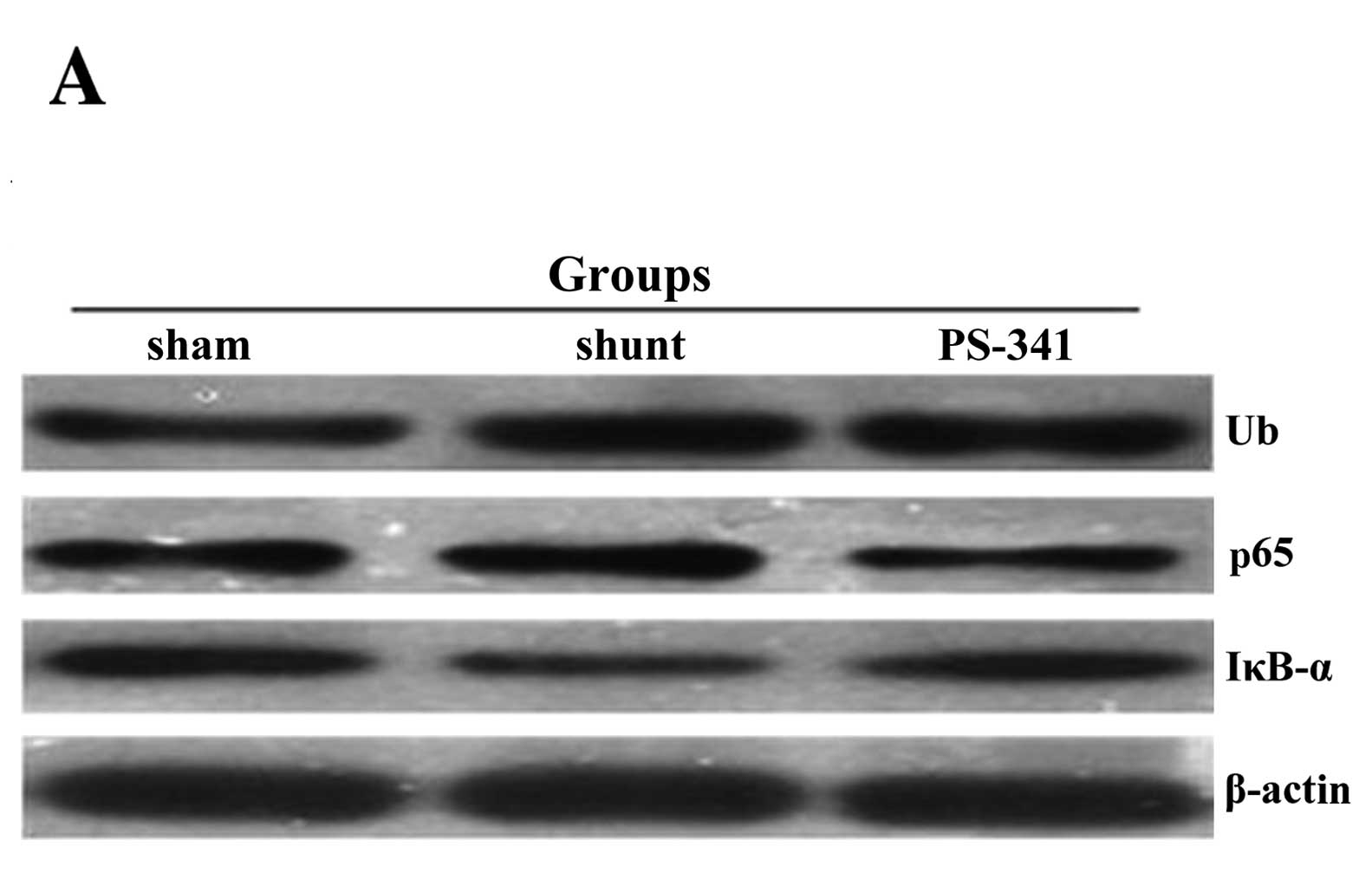
Ubiquitin, NF-κB p65 and IκB-α protein
quantities estimated by western blot analysis
The protein concentrations of ubiquitin and NF-κB
p65 in the lung tissue samples of the shunt group were
significantly increased, while the levels of IκB-α were
significantly reduced compared with the sham-operated group
(P<0.01) (Fig. 4). The protein
concentrations of ubiquitin and NF-κB p65 were significantly lower
(P<0.05) and those of IκB-α were significantly higher
(P<0.05) compared with the shunt group following treatment with
PS-341 for 8 weeks.
Discussion
Although a number of studies have addressed the role
of the UPS in the primary VSMC phenotype and survival signaling and
in ventricular remodeling (14,15), its involvement in pulmonary
vascular remodeling induced by high blood flow-induced PAH remains
unclear and the underlying mechanisms have not yet been fully
elucidated. To our knowledge, the present study is the first to
demonstrate that 8 weeks following the intraperitoneal injection of
PS-341, pulmonary vascular remodeling was considerably attenuated
in a model of high blood flow-induced PAH. Following treatment with
PS-341, we observed the following results: i) hemodynamic
parameters were significantly improved; ii) the WT% and WA% (ED,
50–150 μm), the fibrotic area and the RV/(LV + S) weight ratio were
significantly decreased; iii) ubiquitin and NF-κB p65 were detected
at lower levels in the PASMCs; and iv) the protein concentrations
of ubiquitin and NF-κB p65 were significantly decreased; the levels
of IκB-α (an NF-κB inhibitor) were significantly increased. These
results suggested that the activation of the NF-κB pathway was
significantly inhibited following treatment with PS-341.
UPS is an ATP-dependent multi-enzymatic process
(21). It consists of an
ubiquitin-conjugating system and the proteasome, and it is the main
intracellular protein degradation pathway in eukaryotic cells
(22,23). Ubiquitin is an 8 kDa protein of 76
amino-acids, expressed in all eukaryotic cells. The process of
protein ubiquitination involves the covalent attachment of
ubiquitin to a target protein, which serves as a signal for protein
degradation by the proteasome (24). The degradation of the targeted
protein is performed by the 26S proteasome, which consists of a
barrel-shaped proteolytic core complex (the 20S proteasome) and a
19S cap complex (11,25). A previous study (26) demonstrated that the inhibition of
cell cycle-controlling ubiquitin ligases or the proteasome can
reduce VSMC proliferation and prevent the modulation of their
synthetic phenotype. UPS can promote protein degradation underlying
the transition from a contractile to a proliferative VSMC
phenotype; thus, proteasome inhibition leads to a shift from a
synthetic and proliferative phenotype to a contractile one
(27,28). All the above studies have
demonstrate that UPS has a critical effect on the phenotypic
changes of VSMCs and underlies the transition towards the synthetic
phenotype. NF-κB plays a key role in the process of vascular
remodeling in a variety of physiological and pathophysiological
states (13). The activity of
NF-κB is regulated in the cytoplasm through its association with
the IκB-α protein, which is regulated by the UPS. The
proteasome-mediated degradation of the inhibitor, IκB-α, is
required for NF-κB activation, with the phosphorylation of IκB-α
promoting its ubiquitination and subsequent proteasomal degradation
(29).
PAH occurs when most of the very small arteries in
the lungs narrow in diameter (30), leading to increased pulmonary
vascular resistance, functional and structural changes in the
pulmonary vasculature, lung vascular remodeling, loss of the distal
pulmonary vasculature (31), and
RV dysfunction (32). A number of
therapies has been proven useful for decreasing pulmonary arterial
pressure and improving tolerance to exercise and life quality;
however, an effective and long-lasting treatment for this disorder
is still lacking (33,34). The main pathological features of
PAH are: remodeling of the pulmonary arteries, resulting from
endothelial dysfunction and proliferation, smooth muscle
hyperplasia and hypertrophy and expansion of the adventitial
matrix. Studies have shown that endothelial dysfunction is a key
feature and an early event in the pathogenesis of PAH (33,35).
In the present study, we established a model of high
blood flow-induced PAH by a surgical method that implanted a
left-to-right shunt. Eight weeks post-surgery, RVSP and the ratio
of RV/(LV + S) were significantly higher; H&E and Masson’s
staining illustrated that the intima thickened, the vessel wall was
obscured, the lumen size was reduced, and the fibrotic areas were
increased; the WT% and WA% of muscular arteries with an ED of 15–50
μm were significantly increased (Figs. 1 and 2). Following treatment with PS-341, the
hemodynamic and morphometric parameters were improved. These
results indicate that the PAH model was successfully established
and that PS-341 can prevent PHA and can attenuate pulmonary
vascular remodeling in high blood flow-induced PAH.
The proteasome inhibitor, PS-341, is currently the
only FDA approved drug (Bortezomib/Velcade) with well characterized
inhibitory effects on the activity of NF-κB (36,37). In order to further examine the
beneficial effects of PS-341 on vascular remodeling in PAH, we
performed immunohistochemical staining and western blot analysis to
detect the levels of UPS-related proteins, such as ubiquitin, NF-κB
p65 and IκB-α. Our results revealed that the expression levels of
ubiquitin and NF-κB p65 were significantly reduced; the levels of
IκB-α were significantly increased 8 weeks post-treatment with
PS-341, as compared with the shunt group. These results indicated
that the proteasome inhibitor, PS-341, inhibited UPS by
downregulating the expression of NF-κB p65 and preventing the
degradation of IκB-α. Other studies (12,38,39) have demonstrated that a basal NF-κB
activity may serve to sustain the proliferation and survival of
smooth muscle cell; treatment with the proteasome inhibitors,
PS-341 or MG-132, has been shown to effectively and
dose-dependently reduce neointimal formation in balloon-injured rat
carotid arteries, which was associated with anti-proliferative,
anti-inflammatory and pro-apoptotic effects mediated by the
inhibition of tumor necrosis factor-α-induced NF-κB activation. Our
study demonstrates that treatment with PS-341 clearly suppresses
pulmonary vascular remodeling through the inhibition of the NF-κB
signaling pathway.
In conclusion, our study demonstrates that the
intraperitoneal injection of PS-341 for 8 weeks prevents pulmonary
vascular remodeling in a model of high blood flow-induced PAH
through the inhibition of the NF-κB pathway.
Acknowledgements
This study was supported by the Key Technologies
R&D Program of Shandong Province (2010GSF10234) and the
Shandong Provincial Natural Science Foundation (ZR2011HM078).
References
|
1
|
Li B, Yang L, Shen J, Wang C and Jiang Z:
The antiproliferative effect of sildenafil on pulmonary artery
smooth muscle cells is mediated via upregulation of
mitogen-activated protein kinase phosphatase-1 and degradation of
extracellular signal-regulated kinase 1/2 phosphorylation. Anesth
Analg. 105:1034–1041. 2007. View Article : Google Scholar
|
|
2
|
Ohata Y, Ogata S, Nakanishi K, et al:
Expression of P2X4R mRNA and protein in rats with hypobaric
hypoxia-induced pulmonary hypertension. Circ J. 75:945–954. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yildiz P: Molecular mechanisms of
pulmonary hypertension. Clin Chim Acta. 403:9–16. 2009. View Article : Google Scholar
|
|
4
|
Can MM, Tanboğa IH, Demircan HC, et al:
Enhanced hemostatic indices in patients with pulmonary arterial
hypertension: an observational study. Thromb Res. 126:280–282.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Junbao D, Hui Y, Bing W, Jian L, Jianguang
Q and Chaoshu T: Effect of L-arginine on collagen of high
flow-induced pulmonary arterial remodeling. Circ J. 69:603–608.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baber SR, Deng W, Master RG, et al:
Intratracheal mesenchymal stem cell administration attenuates
monocrotaline-induced pulmonary hypertension and endothelial
dysfunction. Am J Physiol Heart Circ Physiol. 292:H1120–H1128.
2007. View Article : Google Scholar
|
|
7
|
Zhao YD, Courtman DW, Ng DS, et al:
Microvascular regeneration in established pulmonary hypertension by
angiogenic gene transfer. Am J Respir Cell Mol Biol. 35:182–189.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Y, Tian Y, Prabha M, et al: Effects of
sulfur dioxide on hypoxic pulmonary vascular structural remodeling.
Lab Invest. 90:68–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cuifen Z, Lijuan W, Li G, Wei X, Zhiyu W
and Fuhai L: Changes and distributions of peptides derived from
proadrenomedullin in left-to-right shunt pulmonary hypertension of
rats. Circ J. 72:476–481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McMurtry MS, Bonnet S, Wu X, et al:
Dichloroacetate prevents and reverses pulmonary hypertension by
inducing pulmonary artery smooth muscle cell apoptosis. Circ Res.
95:830–840. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li M, Dong X, Liu Y, Sun X, Li Z and He J:
Inhibition of ubiquitin proteasome function suppresses
proliferation of pulmonary artery smooth muscle cells. Naunyn
Schmiedebergs Arch Pharmacol. 384:517–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takami Y, Nakagami H, Morishita R, et al:
Ubiquitin carboxyl-terminal hydrolase L1, a novel deubiquitinating
enzyme in the vasculature, attenuates NF-kappaB activation.
Arterioscler Thromb Vasc Biol. 27:2184–2190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Y, Chen B, Liu D, et al: MG132
treatment attenuates cardiac remodeling and dysfunction following
aortic banding in rats via the NF-κB/TGFβ1 pathway. Biochem
Pharmacol. 81:1228–1236. 2011.PubMed/NCBI
|
|
14
|
Jin UH, Suh SJ, Chang HW, et al:
Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human
aortic smooth muscle cell migration and MMP-9 activity through AKT
signaling pathway. J Cell Biochem. 104:15–26. 2008.
|
|
15
|
Djordjevic T, Hess J, Herkert O, Görlach A
and BelAiba RS: Rac regulates thrombin-induced tissue factor
expression in pulmonary artery smooth muscle cells involving the
nuclear factor-kappaB pathway. Antioxid Redox Signal. 6:713–720.
2004. View Article : Google Scholar
|
|
16
|
Meiners S, Dreger H, Fechner M, et al:
Suppression of cardiomyocyte hypertrophy by inhibition of the
ubiquitin-proteasome system. Hypertension. 51:302–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wrana JL, Attisano L, Wieser R, Ventura F
and Massagué J: Mechanism of activation of the TGF-beta receptor.
Nature. 370:341–347. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Jin HF, Liu D, et al: Hydrogen
sulfide induces apoptosis of pulmonary artery smooth muscle cell in
rats with pulmonary hypertension induced by high pulmonary blood
flow. Chin Med J (Engl). 122:3032–3038. 2009.PubMed/NCBI
|
|
19
|
Luan Y, Zhang ZH, Wei DE, et al:
Implantation of mesenchymal stem cells improves right ventricular
impairments caused by experimental pulmonary hypertension. Am J Med
Sci. 343:402–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YY, Luan Y, Zhang X, et al:
Proteasome inhibitor PS-341 attenuates flow-induced pulmonary
arterial hypertension. Clin Exp Med. June.16–2013.(Epub ahead of
print).
|
|
21
|
Depre C, Wang Q, Yan L, et al: Activation
of the cardiac proteasome during pressure overload promotes
ventricular hypertrophy. Circulation. 114:1821–1818. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naujokat C and Hoffmann S: Role and
function of the 26S proteasome in proliferation and apoptosis. Lab
Invest. 82:965–980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doll D, Sarikas A, Krajcik R and Zolk O:
Proteomic expression analysis of cardiomyocytes subjected to
proteasome inhibition. Biochem Biophys Res Commun. 353:436–442.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Voutsadakis IA: Ubiquitination and the
Ubiquitin-Proteasome System as regulators of transcription and
transcription factorsin epithelial mesenchymal transition of
cancer. Tumour Biol. 33:897–910. 2012. View Article : Google Scholar
|
|
25
|
Moore BS, Eustáquio AS and McGlinchey RP:
Advances in and applications of proteasome inhibitors. Curr Opin
Chem Biol. 12:434–440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Razeghi P, Baskin KK, Sharma S, et al:
Atrophy, hypertrophy, and hypoxemia induce transcriptional
regulators of the ubiquitin proteasome system in the rat heart.
Biochem Biophys Res Commun. 342:361–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meiners S, Laule M, Rother W, et al:
Ubiquitin-proteasome pathway as a new target for the prevention of
restenosis. Circulation. 105:483–489. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barringhaus KG and Matsumura ME: The
proteasome inhibitor lactacystin attenuates growth and migration of
vascular smooth muscle cells and limits the response to arterial
injury. Exp Clin Cardiol. 12:119–124. 2007.PubMed/NCBI
|
|
29
|
Sanchez-Ponce D, Tapia M, Muñoz A and
Garrido JJ: New role of IKK alpha/beta phosphorylated I kappa B
alpha in axon outgrowth and axon initial segment development. Mol
Cell Neurosci. 37:832–844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farber HW and Loscalzo J: Pulmonary
arterial hypertension. N Engl J Med. 351:1655–1665. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kee K and Naughton MT: Heart failure and
the lung. Circ J. 74:2507–2516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iyer SS, Co C and Rojas M: Mesenchymal
stem cells and inflammatory lung diseases. Panminerva Med. 51:5–16.
2009.PubMed/NCBI
|
|
33
|
Baber SR, Deng W, Master RG, et al:
Intratracheal mesenchymal stem cell administration attenuates
monocrotaline-induced pulmonary hypertension and endothelial
dysfunction. Am J Physiol Heart Circ Physiol. 292:H1120–H1128.
2007. View Article : Google Scholar
|
|
34
|
Patel KM, Crisostomo P, Lahm T, et al:
Mesenchymal stem cells attenuate hypoxic pulmonary vasoconstriction
by a paracrine mechanism. J Surg Res. 143:281–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Diller GP, van Eijl S, Okonko DO, et al:
Circulating endothelial progenitor cells in patients with
Eisenmenger syndrome and idiopathic pulmonary arterial
hypertension. Circulation. 117:3020–3030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Daroczi B, Kari G, Ren Q, Dicker AP and
Rodeck U: Nuclear factor kappa B inhibitors alleviate and the
proteasome inhibitor PS-341 exacerbates radiation toxicity in
zebrafish embryos. Mol Cancer Ther. 8:2625–2634. 2009. View Article : Google Scholar
|
|
37
|
Zavrski I, Kleeberg L, Kaiser M, et al:
Proteasome as an emerging therapeutic target in cancer. Curr Pharm
Des. 13:471–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Erl W, Hansson GK, de Martin R, Draude G,
Weber KS and Weber C: Nuclear factor-kappa B regulates induction of
apoptosis and inhibitor of apoptosis protein-1 expression in
vascular smooth muscle cells. Circ Res. 84:668–677. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grassia G, Maddaluno M, Musilli C, et al:
The I{kappa}B kinase inhibitor nuclear factor-{kappa}B essential
modulator-binding domain peptide for inhibition of injury-induced
neointimal formation. Arterioscler Thromb Vasc Biol. 30:2458–2466.
2010.
|