Introduction
Solar ultraviolet (UV) radiation is considered a
major source of environmental damage for human skin (1). Results of previoius studies have
shown that exposure of UV radiation in skin cells triggers various
cell changes, including generation of reactive oxidative stress,
cell cycle arrest, and altered expression of genes encoding
inflammation-related proteins (2–4).
Strong evidence suggests that UV induces cell membrane disruption
as well as nuclear DNA damage, resulting in skin cell loss and/or
apoptosis (5–8). It is assumed that keratinocytes are
the most numerous cells in human skin and likely the first cells to
be damaged by UV radiation (9).
However, the molecular and cellular mechanisms underlying
UV-induced apoptosis in keratinocytes remain to be adequately
determined.
Mounting evidence suggests that the induction of
keratinocyte apoptosis by UVB radiation is mediated by the action
of a variety of proteins and/or factors. For example, it has been
previously shown that activation of caspase-9, a member of the
caspase family, is important in initiating apoptosis in
keratinocytes exposed to UVB radiation (10). Moreover, it has been demonstrated
that several members of the B-cell lymphoma-2 (Bcl-2) family, such
as Bcl-2 and myeloid cell leukemia-1 (Mcl-1), are involved in
UV-induced apoptosis in keratinocytes (11–14). Involvement of the downregulation
of certain members of the inhibitor of apoptosis protein (IAP)
family, including human IAP-1 (HIAP-1), HIAP-2, X-linked IAP
(XIAP), and survivin, in the UVB-induced apoptosis of keratinocyte
has also been proposed (15–17). A number of studies have emphasized
that activities of multiple signaling proteins, such as protein
kinase B (PKB), mitogen-activated protein kinases (MAPKs), and
protein kinase Cs (PKCs), are necessary for UVB-induced apoptosis
of human keratinocytes and skin cell damage (18–21). It has also been shown that
forcible overexpression of C/EBP homologous protein (CHOP), a
transcription factor induced by endoplasmic reticulum (ER) stress,
causes apoptosis in keratinocytes in culture (22), suggesting that ER stress may play
a role in UVB-induced keratinocyte apoptosis (22). However, the role and/or
cross-regulation (crosstalk) among the abovementioned
anti-apoptotic and signaling proteins and ER stress pathways in
UVB-induced growth inhibition and/or apoptosis of keratinocytes
remain to be determined.
In this study, we investigated the effect of UVB on
survival and apoptosis of HaCaT human keratinocytes and determined
possible molecular, cellular and signaling mechanisms including
cross-regulation, which are responsible for the UVB’s anti-survival
and/or pro-apoptotic effects.
Materials and methods
Materials
DMEM/F12, penicillin-streptomycin, and fetal bovine
serum (FBS) were purchased from WelGENE (Daegu, Korea).
Enzyme-linked chemiluminescence (ECL) western detection reagents
were purchased from Thermo Scientific (Waltham, MA, USA). Bradford
reagent was purchased from Bio-Rad (Hercules, CA, USA). Antibodies
of procaspase-9 and -3 were purchased from Stressgen
Biotechnologies (Victoria, BC, Canada). Antibodies of Mcl-1, Bcl-2
and glucose-regulated protein 78 (GRP78) were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies of XIAP
and HIAP-1 were purchased from R&D Systems (Minneapolis, MN,
USA). An antibody of poly(ADP-ribose) polymerase (PARP) was
purchased from Roche Diagnostics GmbH (Mannheim, Germany).
Antibodies of phospho-PKB (p-PKB) and total PKB (T-PKB) were
purchased from Cell Signaling Technology (Danvers, MA, USA).
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk),
GF109203X, GO6983, and proteinase inhibitor cocktail (100X) were
purchased from Calbiochem (Madison, WI, USA). Plasticwares,
including 6- and 24-well plates, were purchased from SPL Life
Sciences Co., Ltd. (Gyeonggi-do, Korea). Other reagents, including
mouse monoclonal anti-human actin antibody, were purchased from
Sigma (St. Louis, MO, USA).
Cell culture
HaCaT human keratinocytes [Korean Cell Line Bank
(KCLB), Seoul, Korea] were grown at 37°C in a humidified condition
of 95% air and 5% CO2 in DMEM/F12 supplemented with 10%
heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml
streptomycin.
UVB radiation
UVB was supplied by a closely spaced array of seven
Westinghouse FS-40 sunlamps, which delivered uniform radiation at a
distance of 38 cm. The energy output of UVB (290–320 nm) at 38 cm
was measured with a UVB photometer (IL1350 photometer;
International Light, Newburyport, MA, USA). Briefly, for the
preparation of whole cell lysate mentioned below, HaCaT cells were
seeded in a 60-mm culture dish at a density of 1.6×106
cells in 4 ml volume the day prior to UVB radiation. To prevent
light absorption by cell culture medium, the medium was removed
from just prior to UVB radiation and replaced with a thin layer of
phosphate-buffered saline (PBS) to cover cells. Cells were then
exposed for 0, 50, 100, 200 and 400 sec of UVB, corresponding to
the dose of 0, 50, 100, 200 and 400 mJ/cm2,
respectively. PBS was then removed from cells after the specified
time of UVB radiation. Fresh culture medium was added to cells
exposed to UVB radiation for an additional 8 h.
Preparation of whole cell lysates
HaCaT cells (1.6×106/4 ml) plated in a
60-mm culture dish overnight were initially pretreated with or
without a broad-spectrum caspase inhibitor (z-VAD-fmk) or a pan-PKC
inhibitor (GF109203X or GO6983) for 1 h and then treated with or
without UVB at 400 mJ/cm2 for an additional 8 h. The
conditioned cells were then washed twice with PBS and exposed to a
lysis buffer [50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 0.1% sodium
dodecyl sulfate, 0.25% sodium deoxycholate, 1% Triton X-100, 1%
Nonidet P-40, 1 mM EDTA, 1 mM EGTA, proteinase inhibitor cocktail
(1X)]. Whole cell lysates were collected in a 1.5 ml tube and
centrifuged for 20 min at 4°C at 14,240 × g. The supernatant was
removed and its protein concentration was determined with Bradford
reagent.
Western blotting
Whole cell lysates (50 μg protein) were separated by
SDS-PAGE (10%) and transferred onto nitrocellulose membranes
(Millipore, Billerica, MA, USA). The membrane was washed with TBS
(10 mM Tris, 150 mM NaCl) supplemented with 0.05% (vol/vol)
Tween-20 (TBST) followed by blocking with TBST containing 5%
(wt/vol) non-fat dry milk. The membrane was incubated with
respective antibody specific for procaspase-9 (1:2,000),
procaspase-3 (1:2,000), PARP (1:2,000), PKB (1:2,000), HIAP-1
(1:2,000), XIAP (1:1,000), Mcl-1 (1:1,000), Bcl-2 (1:1,000), GRP78
(1:1,000) or actin (1:5,000) at 4°C overnight. The membrane was
then exposed to secondary antibodies coupled with horseradish
peroxidase for 2 h at room temperature. The membrane was washed
three times with TBST at room temperature. Immunoreactivities were
detected by ECL reagents. Equal protein loading was assessed by
expression levels of actin protein.
Cell count assay
HaCaT cells were seeded in 24-well plates at a
density of 2×105 cells in 500 μl volume overnight. The
cells were then exposed to UVB (400 mJ/cm2) for 2, 5 or
8 h. The number of surviving YD-8 cells that could not be stained
with trypan blue dye was counted under microscope. Approximately
<100 cells were counted for the analysis.
Measurement of DNA fragmentation
HaCaT cells (1.6×106/4 ml) were seeded in
a 60-mm culture dish the day prior to UVB radiation. The cells were
incubated with varying doses of UVB for 8 h, harvested, washed, and
lysed in a buffer [50 mM Tris (pH 8.0), 0.5% sarkosyl, 0.5 mg/ml
proteinase K, and 1 mM EDTA] at 55°C for 3 h. RNase A (0.5 μg/ml)
was then added and the cells were incubated at 55°C for 18 h. The
lysates were centrifuged at 10,000 × g for 20 min. Genomic DNA in
the supernatant was extracted with equal volume of neutral
phenol-chloroform-isoamyl alcohol mixture (25:24:1), and analyzed
by electrophoresis on 1.7% agarose gel. DNA was visualized and
photographed under UV illumination after staining with ethidium
bromide (0.1 μg/ml).
Statistical analysis
Cell count analysis was performed in triplicate and
repeated three times. Data were expressed as mean ± standard error
(SE). Significant differences were determined by one-way ANOVA.
P<0.05 was considered to indicate statistical significance.
Results
UVB radiation leads to reduction of cell
survival and induction of apoptosis of HaCaT cells
Initially, we investigated whether UVB radiation
induces apoptosis of HaCaT cells. In this study, apoptosis
induction by UVB was assessed by the ability of UVB to induce
genomic DNA fragmentation, an index of apoptosis in HaCaT cells.
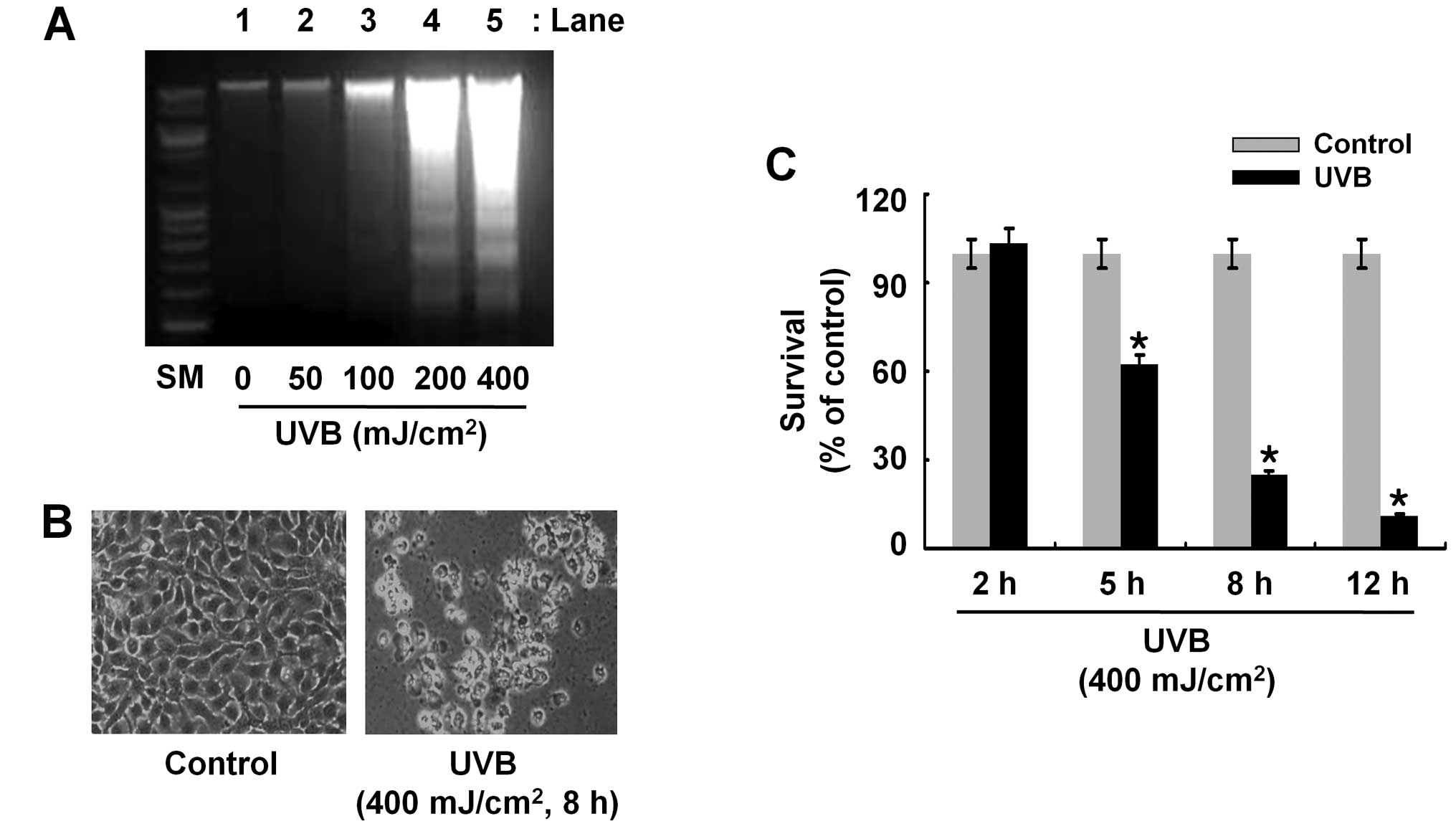
There was no DNA fragmentation in HaCaT cells exposed to UVB
radiation at 50 or 100 mJ/cm2 for 8 h (lanes 2 or 3)
(Fig. 1A). However, there was
strong induction of DNA fragmentation in HaCaT cells exposed to UVB
at 200 or 400 mJ/cm2 (lanes 4 or 5). Cells undergoing
apoptosis have distinct morphological changes, such as cell
shrinkage. Compared with the control, UVB (400 mJ/cm2, 8
h) radiation induced severe cell shrinkage of HaCaT cells,
supporting the apoptosis-inducing effects of UVB on HaCaT cells
(Fig. 1B). Using 400
mJ/cm2, time course experiments were conducted to
determine the effect of UVB (400 mJ/cm2) radiation on
survival of HaCaT cells over time. Data of cell count analysis
demonstrated ~47, 73 and 90% reduction of HaCaT cell survival by
UVB radiation at 5, 8 and 12 h, respectively (Fig. 1C). These results collectively
suggest that UVB radiation at 400 mJ/cm2 for 8 h has
strong anti-survival and pro-apoptotic effects on HaCaT cells.
UVB radiation leads to activation of
caspase-9 and -3 and cleavage of PARP in HaCaT cells
Induction of apoptosis is influenced by the activity
of a number of proteins, including caspases. The effect of UVB
radiation on the activation of caspases in HaCaT cells was
determined using western blot analysis. Activation of caspases by
UVB was assessed by the ability of UVB to alter the cellular levels
of the inactive proform and active form (cleaved product) of
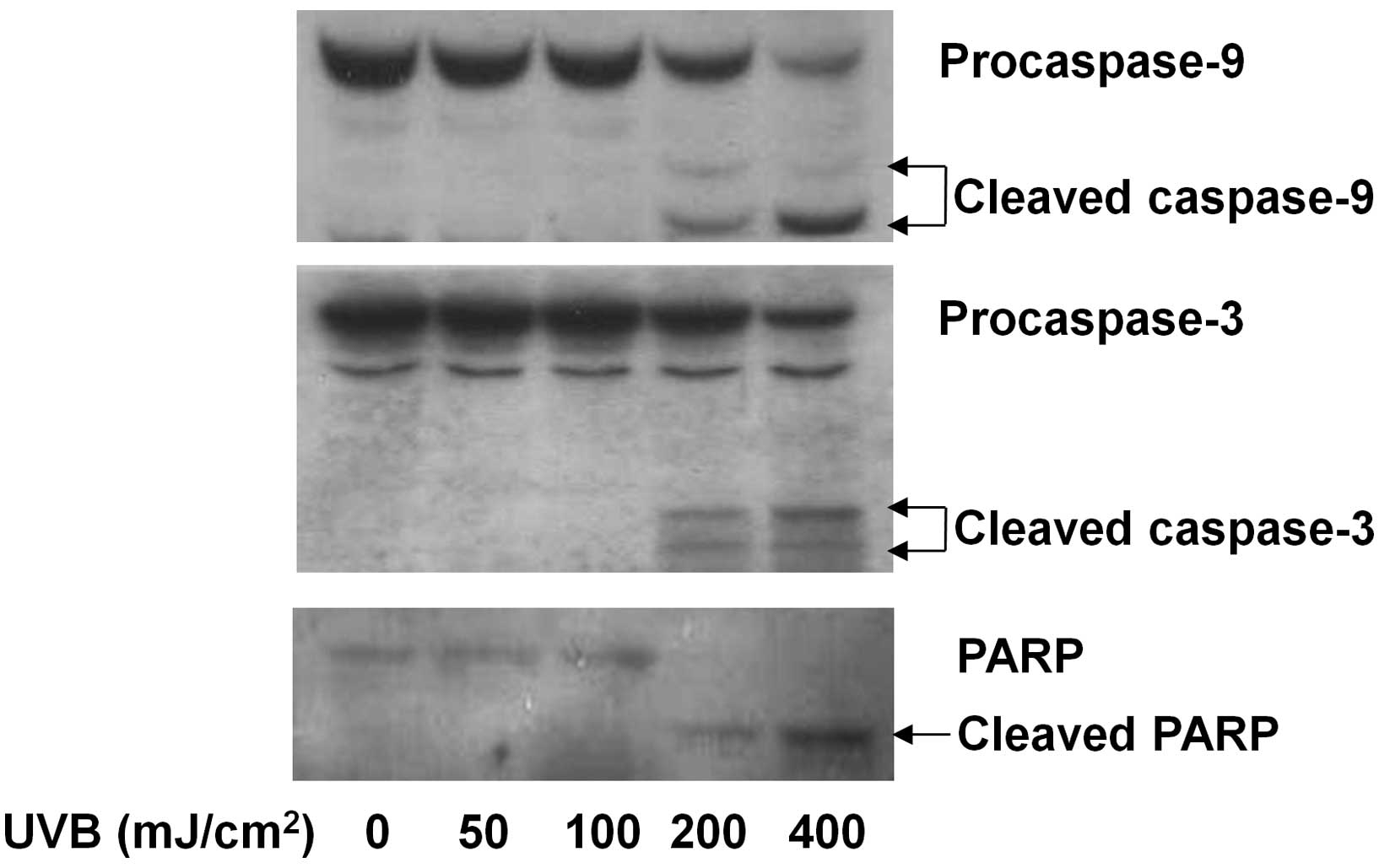
caspases in HaCaT cells. Compared with the control (lane 1), UVB
radiation at 50 or 100 mJ/cm2 for 8 h did not affect the
cellular levels of procaspase-9 and -3 in HaCaT cells (lanes 2 or
3) (Fig. 2). However, the same
time exposure of UVB at 200 or 400 mJ/cm2 decreased the
cellular levels of procaspase-9 and -3, while largely increasing
those of cleaved caspase-9 and -3. PARP is a downstream substrate
of caspase-9 and -3. There was a UVB radiation dose-dependent
increase in the cellular levels of cleaved PARP in HaCaT cells
(lanes 2–5) in which UVB at 400 mJ/cm2 induced the
highest cellular accumulation of cleaved PARP, confirming the
UVB-induced activation of caspase-9 and -3 in HaCaT cells.
UVB radiation leads to the altered
expression of Mcl-1, HIAP-1, XIAP and PKB in HaCaT cells
We investigated whether UVB radiation affects
expression of anti-apoptotic and signaling proteins involved in
cell survival and/or apoptosis, including the Bcl-2 or IAP family
and PKB, in HaCaT cells. Compared with the control (lane 1), there
was little effect on Mcl-1 protein expression by UVB at 50, 100 or
200 mJ/cm2 for 8 h (lanes 2–4) (Fig. 3). However, there was a strong
downregulation of Mcl-1 protein expression by UVB at 400
mJ/cm2. UVB radiation at the doses applied did not
affect Mcl-1 protein expression in HaCaT cells (lanes 2–5) compared
with the control (lane 1). Instead, a slight increase of Bcl-2
protein expression by UVB at 200 or 400 mJ/cm2 was
observed. UVB at 50 or 100 mJ/cm2 did not affect HIAP-1
and XIAP protein expression (lanes 2 or 3), but there was strong
repression of HIAP-1 and XIAP proteins by UVB at 200 or 400
mJ/cm2 (lanes 4 or 5). In the case of PKB, UVB at 50,
100 or 200 mJ/cm2 did not affect PKB protein
phosphorylation and expression (lanes 2–4) compared with the
control (lane 1). However, phosphorylated levels of PKB and total
expression levels of this protein by UVB at 400 mJ/cm2
(lane 5) were markedly reduced. Evidence suggests that ER stress
plays a role in the induction of apoptosis. Cells with ER stress
express high GRP78, a molecular chaperone protein that involves in
protein folding. Compared with the control (lane 1), no enhancement
of GRP78 protein expression in HaCaT cells exposed to UVB at 50 or
100 mJ/cm2 (lanes 2 or 3) was identified. However, a
marked increase in GRP78 protein expression by UVB at 200 or 400
mJ/cm2 (lanes 4 or 5) was noted. Actin protein
expression of the control was not altered by UVB at the doses
tested.
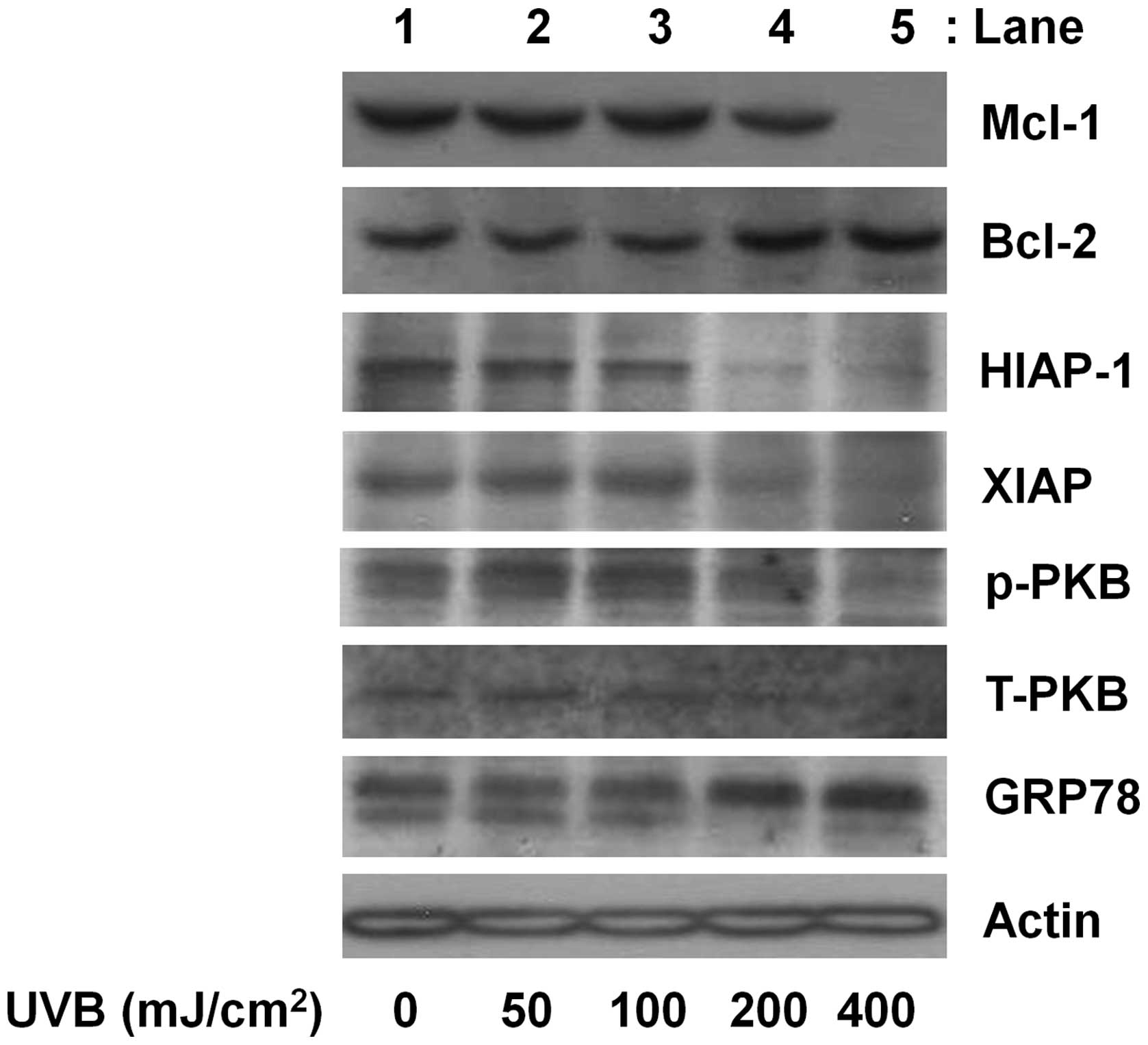 | Figure 3The effect of UVB radiation on the
expression and/or phosphorylation of myeloid cell leukemia-1
(Mcl-1), human inhibitor of apoptosis protein-1 (HIAP-1), B-cell
lymphoma-2 (Bcl-2), HIAP-1, X-linked IAP (XIAP), protein kinase B
(PKB) and glucose-regulated protein 78 (GRP78) in HaCaT cells.
HaCaT cells were treated with or without UVB at the indicated doses
for 8 h. Whole cell lysates were prepared, and analyzed by western
blotting using the specific antibodies of Mcl-1, HIAP-1, Bcl-2,
HIAP-1, XIAP, PKB and GRP78. p-PKB, phosphorylated PKB; T-PKB,
total PKB. Results are representative of three independent
experiments. |
Important roles of the caspase activation
in UVB-induced apoptosis and downregulation of Mcl-1, XIAP and PKB
(but not HIAP-1) in HaCaT cells
Involvement of caspases in the UVB-induced apoptosis
of HaCaT cells was determined using z-VAD-fmk, a broad-spectrum
caspase inhibitor. The UVB-induced apoptosis in HaCaT cells (lane
2) was strongly blunted by the caspase inhibitor (lane 3) (Fig. 4A). The UVB-induced morphological
changes (cell shrinkage) of HaCaT cells were also largely blocked
by z-VAD-fmk (Fig. 4B). Notably,
the UVB-induced activation of caspase-9 and production of cleaved
PARP in HaCaT cells (lane 2) was not shown in the presence of
z-VAD-fmk (lane 3), suggesting the drug efficacy to inhibit the
caspase pathway (Fig. 4C). Of
note, the UVB-induced downregulation of Mcl-1 and XIAP, but not
HIAP-1, in HaCaT cells (lane 2) was strongly blocked by z-VAD-fmk
(lane 3). Furthermore, the UVB-induced downregulation of
phosphorylated and total PKB proteins in HaCaT cells (lane 2) was
largely inhibited by z-VAD-fmk (lane 3). Actin protein expression
remained unchanged under these experimental conditions (Fig. 4C).
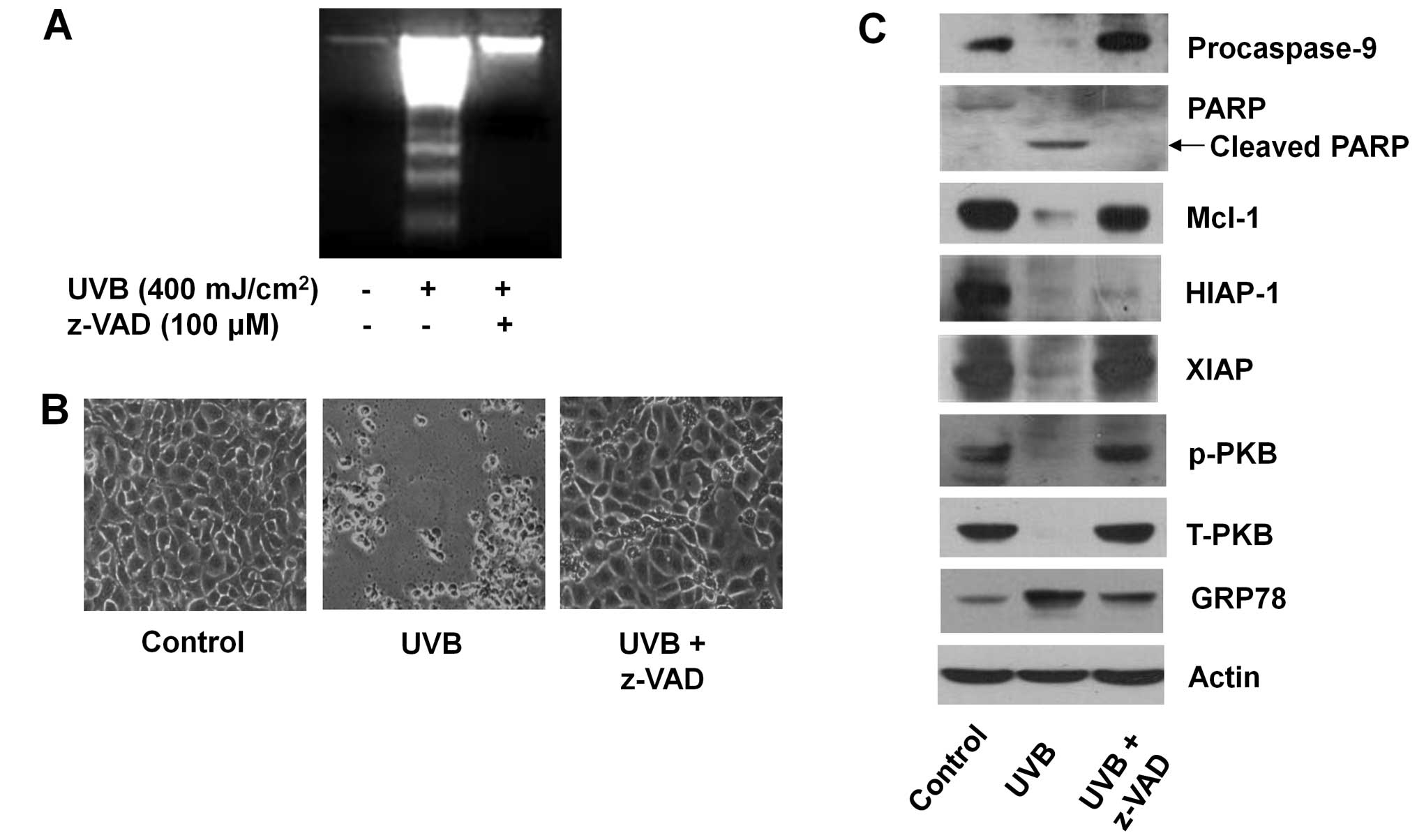 | Figure 4The effect of ultraviolet B (UVB)
radiation and/or z-VAD-fmk on the survival, apoptosis, and
expression of myeloid cell leukemia-1 (Mcl-1), human inhibitor of
apoptosis protein-1 (HIAP-1), B-cell lymphoma-2 (Bcl-2), HIAP-1,
X-linked IAP (XIAP), protein kinase B (PKB), and glucose-regulated
protein 78 (GRP78) in HaCaT cells. (A) HaCaT cells were pretreated
with or without z-VAD-fmk, a pan-caspase inhibitor for 1 h and then
treated with or without UVB in the absence or presence of the
caspase inhibitor for 8 h. Extra nuclear-fragmented DNA from the
conditioned cells was then extracted, and analyzed on a 1.7%
agarose gel. (B) HaCaT cells were pretreated with or without
z-VAD-fmk for 1 h and then treated with or without UVB in the
absence or presence of the caspase inhibitor for 8 h. Morphological
change was then observed via microscopy. (C) HaCaT cells were
pretreated with or without z-VAD-fmk for 1 h and then treated with
or without UVB in the absence or presence of the caspase inhibitor
for 8 h. Whole cell lysates were prepared, and analyzed by western
blotting using specific antibodies of procasapse-9,
poly(ADP-ribose) polymerase (PARP), Mcl-1, HIAP-1, XIAP, PKB and
GRP78. p-PKB, phosphorylated PKB; T-PKB, total PKB. Results are
representative of three independent experiments. |
Involvement of PKCs in the UVB-induced
reduction of cell survival and downregulation of HIAP-1, XIAP, and
PKB (but not Mcl-1) in HaCaT cells
PKCs are involved in cell survival and/or apoptosis
of keratinocytes exposed to UVB. Using GF109203X or GO6983, a
broad-spectrum of PKC inhibitor, we determined whether activities
of PKCs are linked to the UVB-induced anti-survival and/or
pro-apoptotic responses in HaCaT cells. The anti-survival effect of
UVB on HaCaT cells (column 2) was largely blocked in the presence
of GF109203X (column 3) or GO6983 (column 4) (Fig. 5A). Notably, the UVB effects on
activation of caspase-9, cleavage of PARP, downregulation of
HIAP-1, XIAP and PKB in HaCaT cells (lane 2) was partially or
largely blunted by GF109203X (lane 3) or GO6983 (lane 4) (Fig. 5B). The UVB-induced downregulation
of Mcl-1 (lane 2) was not changed by each PKC inhibitor (lanes 3 or
4). Actin protein expression remained constant by treatment with or
without UVB in the absence or presence of GF109203X or GO6983
(Fig. 5B).
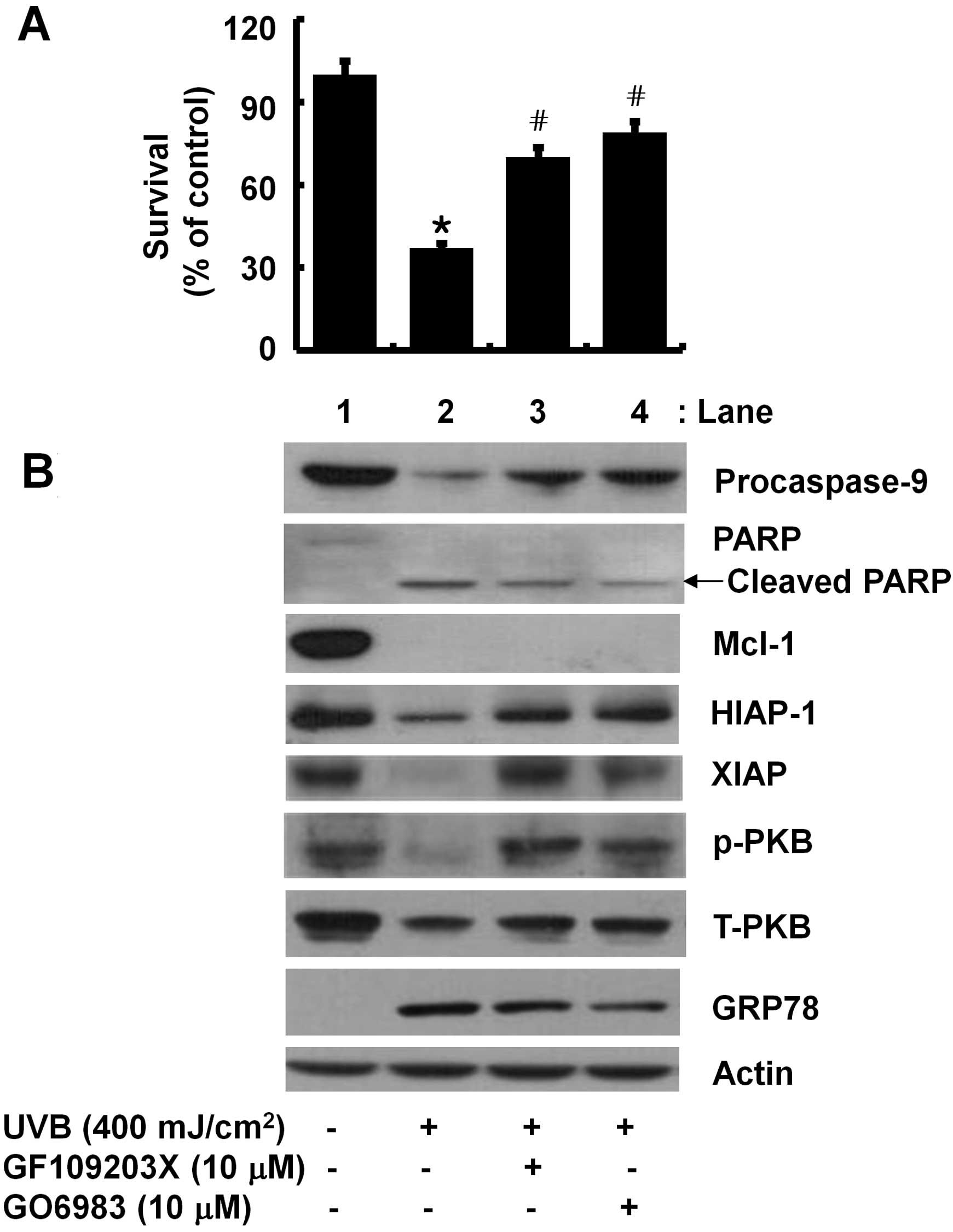 | Figure 5The effect of ultraviolet B (UVB)
radiation and/or GF109203X or GO6983 on the survival and expression
of myeloid cell leukemia-1 (Mcl-1), human inhibitor of apoptosis
protein-1 (HIAP-1), B-cell lymphoma-2 (Bcl-2), HIAP-1, X-linked IAP
(XIAP), protein kinase B (PKB), and glucose-regulated protein 78
(GRP78) in HaCaT cells. (A) HaCaT cells were pretreated with or
without GF109203X or GO6983, a broad spectrum PKC inhibitor for 1 h
and then treated with or without UVB in the absence or presence of
the PKC inhibitor for 8 h. Data are the mean ± SE of three
independent experiments. The number of surviving cells was
normalized as a percentage of UVB-free control.
*P<0.05 compared to the value of UVB-free control.
#P<0.05 compared to the value obtained at UVB
treatment without any drug. (B) HaCaT cells were pretreated without
or with GF109203X or GO6983 for 1 h and then treated with or
without UVB in the absence or presence of the PKC inhibitor for 8
h. Whole cell lysates were prepared, and analyzed by western
blotting using specific antibodies of procasapse-9,
poly(ADP-ribose) polymerase (PARP), Mcl-1, HIAP-1, XIAP, PKB and
GRP78. p-PKB, phosphorylated PKB; T-PKB, total PKB. Results are
representative of three independent experiments. |
Discussion
Solar UVB radiation induces various types of skin
damage, some of which may lead to loss of keratinocytes and/or
induction of keratinocyte apoptosis. In the present study, we have
demonstrated the ability of UVB radiation at 400 mJ/cm2
for 8 h to strongly reduce cell survival and induce apoptosis of
HaCaT human keratinocytes. Our data indicate that the UVB-induced
anti-survival and pro-apoptotic effects on HaCaT cells are mediated
through modulation of the expression and/or activity of caspases,
PKCs, PKB, XIAP, HIAP-1 and/or Mcl-1, along with induction of ER
stress.
Accumulating evidence suggests that UVB radiation,
depending on its dose and time, leads to a decrease in cell
survival and/or induction of apoptosis in various skin cells,
including normal keratinocytes, HaCaT cells, and JB6 murine
epidermal cells (21,26,27). Cells undergoing apoptosis have
distinct biochemical and morphological characteristics, such as
nuclear DNA fragmentation and cell shrinkage (28). Bearing this in mind and
considering the present findings that UVB radiation induces nuclear
DNA fragmentation and cell shrinkage in HaCaT cells (Fig. 1A and B), it appears that UVB
induces HaCaT cell death by apoptosis.
Induction of apoptosis is mainly linked to the
intrinsic (mitochondrial) and extrinsic (death receptor-mediated)
pathways. Caspases, a group of the essential proteases required for
the execution of cell death by apoptotic stimuli (29), are key to both apoptotic pathways.
Caspases are synthesized as zymogens (inactive precursors) in
resting cells, however, following exposure to apoptotic stimuli,
they become processed via partial proteolytic cleavage and are
activated in cells. Active caspases then cleave many cellular
proteins, including PARP and other vital proteins, leading to the
induction and/or execution of apoptosis (29–31). Among caspases, caspase-9 and -8 is
an initiator caspase of the extrinsic and intrinsic apoptotic
pathways, respectively (29,32). Findings of previous studies have
shown that UVB radiation triggers the activation of the intrinsic
and extrinsic pathways (9–11,33).
However, evidence suggests that the UVB-induced apoptosis of human
keratinocytes is primarily mediated via the intrinsic pathway
(15,34). Consistent with those findings, the
present study has also demonstrated the UVB-induced activation of
caspase-9 (Fig. 2), which is
crucial in the UVB-induced apoptosis of HaCaT cells (Fig. 4A).
Members of the Bcl-2 family, including Bcl-2 and
Mcl-1, are anti-apoptotic proteins and participate in apoptosis
initiation and/or caspase activation by regulating the
mitochondrial membrane integrity (23,24). Earlier studies have demonstrated
that Bcl-2 overexpression abrogates the UVB-induced apoptosis in
human keratinocytes in vitro and in vivo (6,11).
Additionally, Mcl-1 is a major epithermal survival protein and
alteration of Mcl-1 expression influences the UV-induced apoptosis
in normal keratinocytes (14),
suggesting involvement of Bcl-2 and Mcl-1 in the UV-induced
apoptosis of keratinocyes. In this study, however, we have observed
that UVB radiation strongly downregulates the expression of Mcl-1,
but does not affect that of Bcl-2 in HaCaT cells (Fig. 3). Thus, it is assumed that
downregulation of Mcl-1, but not Bcl-2, may contribute to the
UVB-induced apoptosis, reduction of cell survival, and/or
activation of caspase-9 in HaCaT cells. Differences in the
expression levels of Bcl-2 protein in HaCaT cells exposed to UVB in
this study and previous ones may be due to different experimental
conditions (UVB radiation dose and exposure time) applied.
The members of IAPs, including HIAP-1, HIAP-2, XIAP
and survivin, are other suppressors of apoptosis (25) and inhibitors of caspases (35,36). In particular, XIAP is shown to
directly inhibit caspase-9 and -3 (36). Studies have recently suggested
that UVB radiation decreases the cellular levels of HIAP-1, HIAP-2,
survivin, and livin in normal human keratinocytes (15) and of XIAP in HaCaT cells (16). In this study, we have also
demonstrated that UVB radiation leads to the downregulation of
HIAP-1 and XIAP in HaCaT cells (Fig.
3), which suggests that downregulation of HIAP-1 and XIAP may
facilitate the UVB-induced apoptosis and activation of caspase-9 in
HaCaT cells. At present, we have limited knowledge regarding the
mechanisms underlying the UVB-induced downregulation of Mcl-1,
HIAP-1 and XIAP in HaCaT cells. Notably, the present findings with
large blockage of the UVB-induced downregulation of Mcl-1 and XIAP,
but not HIAP-1, in HaCaT cells by z-VAD-fmk, a pan-caspase
inhibitor (Fig. 4C) indicate that
caspases are responsible for the UVB-induced Mcl-1 and XIAP
downregulation (proteolysis) in HaCaT cells.
PKB is a protein kinase involved in cell survival
and/or the apoptosis of a variety of cells. Notably, it has been
shown that UVB radiation induces the rapid activation of PKB
(within 15 min), which contributes to the UVB-activated cell
survival pathway in human skin in vivo (37). Moreover, a protective role of PKB
in the early-activated apoptotic pathway in keratinocytes exposed
to UVB radiation via BAD phosphorylation and translocation has been
suggested (38). In this study,
we have demonstrated that UVB radiation downregulates
phosphorylated and total PKB proteins in HaCaT cells (Fig. 3) and importantly the
downregulation is largely blocked by the pan-caspase inhibitor
z-VAD-fmk (Fig. 4C). These
results suggest that elimination of PKB further contributes to the
UVB-induced reduction of cell survival and/or apoptosis of HaCaT
cells and that caspases are also responsible for the UVB-induced
downregulation of PKB in the cells.
PKCs are a group of serine/threonine kinases
involved in cell proliferation, survival and apoptosis (39,40). At present, 12 members of PKCs have
been identified, and can be classified into three groups based on
the co-factors required for activation, including the
Ca2+ and DAG-dependent classical PKCs (PKC-α, -β1, -β2
and -γ), the DAG-dependent, Ca2+-independent novel PKCs
(PKC-δ, -η, -ɛ and -θ), and the DAG- and
Ca2+-independent atypical PKCs (PKC-λ and -ζ) (41). Notably, results of previous
studies suggest a link between the activity of PKCs and
keratinocyte survival and/or apoptosis in response to UVB
radiation. For instance, UVB radiation causes the activation of
PKC-δ, ɛ, ζ, λ and η, leading to apoptosis or cell survival
(21,42). It has also been reported that
PKC-δ is activated in a caspase-dependent manner, and this
activation of PKC-δ contributes to the UVB-induced apoptosis in
human keratinocytes (18,20,43). However, PKC-ζ is suggested to be
rather anti-apoptotic in response to UV (44). Notably, results of the present
study, albeit activation of PKCs by UVB radiation in HaCaT cells is
not directly measured, demonstrate that GF109203X or GO6983,
pan-PKC inhibitors, effectively blocks the UVB-induced reduction of
cell survival (Fig. 5A),
activation of caspase-9, and downregulation of HIAP-1, XIAP and PKB
in HaCaT cells (Fig. 5B), which
strongly suggest a necessity for PKCs activities in such
UVB-induced growth inhibition and cellular changes in HaCaT cells.
Given that GF109203X or GO6983 is a pan-PKC inhibitor, it is likely
that multiple PKC members may be involved in UVB-induced growth
inhibition and cellular responses in HaCaT cells. Future studies,
using siRNA and/or cDNA transfection targeting each PKC, are
required to differentiate which isotype of PKCs is responsible for
the processes.
Evidence suggests that ER stress also mediates
induction of apoptosis. Cells undergoing ER stress have distinct
characteristics, including accumulation of unfolded and misfolded
proteins, upregulation of molecular chaperones (e.g., GRP78) and
transcription factors (e.g., CHOP), phosphorylation of eukaryotic
translation initiation factor 2α and inhibition of global
translation, and reduction of p90 activation of transcription
factor 6 (45–48). In a recent study, it was shown
that UVB radiation at low dose (10 mJ/cm2) for 4 h
induces ER stress, as assessed by the accumulation of unfolded
proteins in ER, CHOP upregulation, and downregulation of ATF6, in
HaCaT cells, without growth retardation (49). However, the same group has also
demonstrated that under this UVB radiation, unfolded protein
response and ER-associated degradation systems are activated in
order to protect cells from UVB-induced ER stress in HaCaT cells.
In the present study, we have demonstrated that UVB radiation (400
mJ/cm2, 8 h) enhances the expression of GRP78 in HaCaT
cells (Fig. 3), confirming
UVB-induced ER stress in HaCaT cells. Currently, the significance
or role of the induced ER stress in HaCaT cells exposed to UVB
radiation remains to be clarified. However, considering the present
findings that UVB radiation (400 mJ/cm2, 8 h) strongly
reduces cell survival and induces apoptosis of HaCaT cells, whether
the induced ER stress may facilitate and/or contribute to the
UVB-induced apoptotic responses in HaCaT cells remains to be
determined. Little is known about crosstalk between PKCs or
caspases and ER stress in cells, including keratinocytes, in
response to UVB radiation. Findings of the present study have shown
that the pan-caspase inhibitor (z-VAD-fmk) or pan-PKC inhibitor
(GO6983) substantially interferes with the UVB-induced enhancement
of GRP78 expression in HaCaT cells (Fig. 5A), suggesting a partial crosstalk
between caspases or PKCs and ER stress in the HaCaT cells exposed
to UVB radiation.
In summary, findings of this study have, to the best
of our knowledge, demonstrated for the first time that UVB
radiation has strong anti-survival and pro-apoptotic effects on
HaCaT cells and the effects are mediated through the activation of
caspase-9 and PKCs, which subsequently downregulates PKB, HIAP-1,
Mcl-1 and XIAP, and triggers ER stress.
Acknowledgements
The authors would like to thank Ms. Sun-Young Cho
for her excellent technical assistance.
References
|
1
|
Shea CR and Parrish JA: Non-ionizing
radiation and the skin. Physiology, Biochemistry and Molecular
Biology of the Skin. Goldsmith LA: 2. 2nd edition. Oxford
University Press; New York: pp. 910–927. 1991
|
|
2
|
Jin GH, Liu Y, Jin SZ, Liu XD and Liu SZ:
UVB induced oxidative stress in human keratinocytes and protective
effect of antioxidant agents. Radiat Environ Biophys. 46:61–68.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moniz LS and Stambolic V: Nek10 mediates
G2/M cell cycle arrest and MEK autoactivation in response to UV
irradiation. Mol Cell Biol. 31:30–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee KM, Lee KW, Jung SK, Lee EJ, Heo YS,
Bode AM, Lubet RA, Lee HJ and Dong Z: Kaempferol inhibits
UVB-induced COX-2 expression by suppressing Src kinase activity.
Biochem Pharmacol. 80:2042–2049. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheikh MS, Antinore MJ, Huang Y and
Fornace AJ Jr: Ultraviolet-irradiation-induced apoptosis is
mediated via ligand independent activation of tumor necrosis factor
receptor 1. Oncogene. 17:2555–2563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi H, Honma M, Ishida-Yamamoto A,
Namikawa K, Miwa A, Okado H, Kiyama H and Iizuka H: In vitro and in
vivo transfer of bcl-2 gene into keratinocytes suppresses
UVB-induced apoptosis. Photochem Photobiol. 74:579–586. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kulms D and Schwarz T: Independent
contribution of three different pathways to ultraviolet-B-induced
apoptosis. Biochem Pharmacol. 64:837–841. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang ES, Iwata K, Ikami K, Ham SA, Kim HJ,
Chang KC, Lee JH, Kim JH, Park SB, Kim JH, Yabe-Nishimura C and Seo
HG: Aldose reductase in keratinocytes attenuates cellular apoptosis
and senescence induced by UV radiation. Free Radic Biol Med.
50:680–688. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Larsson P, Andersson E, Johansson U,
Ollinger K and Rosdahl I: Ultraviolet A and B affect human
melanocytes and keratinocytes differently. A study of oxidative
alterations and apoptosis. Exp Dermatol. 14:117–123. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sitailo LA, Tibudan SS and Denning MF:
Activation of caspase-9 is required for UV-induced apoptosis of
human keratinocytes. J Biol Chem. 277:19346–19352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Assefa Z, Garmyn M, Vantieghem A, Declercq
W, Vandenabeele P, Vandenheede JR and Agostinis P: Ultraviolet B
radiation-induced apoptosis in human keratinocytes: cytosolic
activation of procaspase-8 and the role of Bcl-2. FEBS Lett.
540:125–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nijhawan D, Fang M, Traer E, Zhong Q, Gao
W, Du F and Wang X: Elimination of Mcl-1 is required for the
initiation of apoptosis following ultraviolet irradiation. Genes
Dev. 17:1475–1486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sitailo LA, Tibudan SS and Denning MF: The
protein kinase C delta catalytic fragment targets Mcl-1 for
degradation to trigger apoptosis. J Biol Chem. 281:29703–29710.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sitailo LA, Jerome-Morais A and Denning
MF: Mcl-1 functions as major epidermal survival protein required
for proper keratinocyte differentiation. J Invest Dermatol.
129:1351–1360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chirico F, Fumelli C, Marconi A, Tinari A,
Straface E, Malorni W, Pellicciari R and Pincelli C:
Carboxyfullerenes localize within mitochondria and prevent the
UVB-induced intrinsic apoptotic pathway. Exp Dermatol. 16:429–436.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takasawa R and Tanuma S: Sustained release
of Smac/DIABLO from mitochondria commits to undergo UVB-induced
apoptosis. Apoptosis. 8:291–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dallaglio K, Marconi A and Pincelli C:
Survivin: a dual player in healthy and diseased skin. J Invest
Dermatol. 132:18–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim W, Yang HJ, Youn H, Yun YJ, Seong KM
and Youn B: Myricetin inhibits Akt survival signaling and induces
Bad-mediated apoptosis in a low dose ultraviolet (UV)-B-irradiated
HaCaT human immortalized keratinocytes. J Radiat Res. 51:285–296.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren SW, Li J, Wang W and Guan HS:
Protective effects of kappa-ca3000+CP against ultraviolet-induced
damage in HaCaT and MEF cells. J Photochem Photobiol B. 101:22–30.
2010.PubMed/NCBI
|
|
20
|
Denning MF, Wang Y, Tibudan S, Alkan S,
Nickoloff BJ and Qin JZ: Caspase activation and disruption of
mitochondrial membrane potential during UV radiation-induced
apoptosis of human keratinocytes requires activation of protein
kinase C. Cell Death Differ. 9:40–52. 2002. View Article : Google Scholar
|
|
21
|
Chen N, Ma Wy, Huang C and Dong Z:
Translocation of protein kinase C-epsilon and protein kinase
C-delta to membrane is required for ultraviolet B-induced
activation of mitogen-activated protein kinases and apoptosis. J
Biol Chem. 274:15389–15394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anand S, Chakrabarti E, Kawamura H, Taylor
CR and Maytin EV: Ultraviolet light (UVB and UVA) induces the
damage-responsive transcription factor CHOP/gadd153 in murine and
human epidermis: evidence for a mechanism specific to intact skin.
J Invest Dermatol. 125:323–333. 2005.PubMed/NCBI
|
|
23
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borner C: The Bcl-2 protein family: sensor
and checkpoints for life-or-death decisions. Mol Immunol.
39:615–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Svobodová A, Zdarilová A and Vostálová J:
Lonicera caerulea and Vaccinium myrtillus fruit
polyphenols protect HaCaT keratinocytes against UVB-induced
phototoxic stress and DNA damage. J Dermatol Sci. 56:196–204.
2009.
|
|
27
|
Won YK, Ong CN and Shen HM: Parthenolide
sensitizes ultraviolet (UV)-B-induced apoptosis via protein kinase
C-dependent pathways. Carcinogenesis. 26:2149–2156. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Allen RT, Hunter WJ III and Agrawal DK:
Morphological and biochemical characterization and analysis of
apoptosis. J Pharmacol Toxicol Methods. 37:215–228. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326:1–16. 1997.
|
|
30
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Casciola-Rosen L, Nicholson DW, Chong T,
Rowan KR, Thornberry NA, Miller DK and Rosen A: Apopain/CPP32
cleaves proteins that are essential for cellular repair: a
fundamental principle of apoptotic death. J Exp Med. 183:1957–1964.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI
|
|
33
|
Aragane Y, Kulms D, Metze D, Wilkes G,
Poppelmann B, Luger TA and Schwarz T: Ultraviolet light induces
apoptosis via direct activation of CD95 (Fas/APO-1) independently
of its ligand CD95L. J Cell Biol. 140:171–182. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daher A, Simbulan-Rosenthal CM and
Rosenthal DS: Apoptosis induced by ultraviolet B in
HPV-immortalized human keratinocytes requires caspase-9 and is
death receptor independent. Exp Dermatol. 15:23–34. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deveraux QL, Takahashi R, Salvesen GS and
Reed JC: X-linked IAP is a direct inhibitor of cell-death
proteases. Nature. 388:300–304. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ,
Li P, Srinivasula SM, Alnemri ES, Fairman R and Shi Y: Mechanism of
XIAP-mediated inhibition of caspase-9. Mol Cell. 11:519–527. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wan YS, Wang ZQ, Shao Y, Voorhees JJ and
Fisher GJ: Ultraviolet irradiation activates PI3-kinase/AKT
survival pathway via EGF receptors in human skin in vivo.
Int J Oncol. 18:461–466. 2001.PubMed/NCBI
|
|
38
|
Claerhout S, Van Laethem A, Agostinis P
and Garmyn M: Pathways involved in sunburn cell formation:
deregulation in skin cancer. Photochem Photobiol Sci. 5:199–207.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deacon EM, Pongracz J, Griffiths G and
Lord JM: Isoenzymes of protein kinase C: differential involvement
in apoptosis and pathogenesis. Mol Pathol. 50:124–131. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brodie C and Blumberg PM: Regulation of
cell apoptosis by protein kinase C delta. Apoptosis. 8:19–27. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang C and Kazanietz MG: Divergence and
complexities in DAG signaling: looking beyond PKC. Trends Pharmacol
Sci. 24:602–608. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Berra E, Municio MM, Sanz L, Frutos S,
Diaz-Meco MT and Moscat J: Positioning atypical protein kinase C
isoforms in the UV-induced apoptotic signaling cascade. Mol Cell
Biol. 17:4346–4354. 1997.PubMed/NCBI
|
|
43
|
Denning MF, Wang Y, Nickoloff BJ and
Wrone-Smith T: Protein kinase C-delta is activated by
caspase-dependent proteolysis during ultraviolet radiation-induced
apoptosis of human keratinocytes. J Biol Chem. 273:29995–30002.
1998. View Article : Google Scholar
|
|
44
|
Frutos S, Moscat J and Diaz-Meco MT:
Cleavage of zetaPKC but not lambda/iotaPKC by caspase-3 during
UV-induced apoptosis. J Biol Chem. 274:10765–10770. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee AS: The glucose-regulated proteins:
stress induction and clinical applications. Trends Biochem Sci.
26:504–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ma Y, Brewer JW, Diehl JA and Hendershot
LM: Two distinct stress signaling pathways converge upon the CHOP
promoter during the mammalian unfolded protein response. J Mol
Biol. 318:1351–1365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
De Haro C, Méndez R and Santoyo J: The
eIF-2 alpha kinases and the control of protein synthesis. FASEB J.
10:1378–1387. 1996.PubMed/NCBI
|
|
48
|
Haze K, Yoshida H, Yanagi H, Yura T and
Mori K: Mammalian transcription factor ATF6 is synthesized as a
transmembrane protein and activated by proteolysis in response to
endoplasmic reticulum stress. Mol Biol Cell. 10:3787–3799. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mera K, Kawahara K, Tada K, Kawai K,
Hashiguchi T, Maruyama I and Kanekura T: ER signaling is activated
to protect human HaCaT keratinocytes from ER stress induced by
environmental doses of UVB. Biochem Biophys Res Commun.
397:350–354. 2010. View Article : Google Scholar : PubMed/NCBI
|