Introduction
Pericarpium zanthoxyli (PZ) is the dried
pericarp of the ripe fruit of Zanthoxylum schinifolium
(Sieb. and Zucc) or Zanthoxylum bungeanum (Maxim.), of the
Rutaceae family. Although it has been used to alleviate pain and
increase appetite in Asian medicine, the effects of PZ on adipocyte
differentiation and the underlying mechanisms have not been
elucidated.
Obesity is a worldwide epidemic, and there are
multiple obesity-associated health issues, including type 2
diabetes, hypertension and cardiovascular disease (1). Obesity is caused by adipocyte
hyperplasia, as well as hypertrophy. Adipocyte hypertrophy induces
the transformation of preadipocytes into adipocytes (2,3),
with preadipocytes initiating the expression of
differentiation-related transcription factors when the cells are
exposed to adipogenic inducers (4,5).
Adipocyte differentiation also requires a concerted cellular
program, including the growth arrest of confluent preadipocytes
[termed mitotic clonal expansion (MCE)] and the initiation of
transcriptional events during the early and late stages of
differentiation (4).
CCAAT/enhancer-binding protein (C/EBP)β and C/EBPδ are the first
transcription factors to be expressed during adipocyte
differentiation. The increased activities of C/EBPβ and C/EBPδ are
thought to mediate the expression of peroxisome
proliferator-activated receptor γ (PPARγ) and C/EBPα during
adipogenesis (5,6).
To investigate the mechanisms responsible for
adipocyte differentiation, glucose uptake by insulin and lipid
metabolism, the 3T3-L1 cell culture model has normally been used.
However, 3T3-L1 cells have significant limitations, including a
long interval between preadipocyte formation and adipocyte
maturation (7), and a limited
passage for differentiation. To overcome these limitations, we used
OP9 mouse stromal cells in this study, as first reported in the
study by Wolins et al (8)
as a useful new model of adipocyte differentiation. In their study,
OP9 cells differentiated into adipocytes after being confluent and
subsequent to many passages and long periods in culture, unlike
3T3-L1 cells. Furthermore, the OP9 cells initiated the same events,
including lipid metabolism, insulin signaling and glucose
transport, very similar to 3T3-L1 cells (8).
In the present study, the effects of PZ extract
(PZE) on the adipocytic differentiation of OP9 cells were
investigated by measuring lipid accumulation and evaluating the
expression levels of adipocyte marker genes and their target genes.
We also examined its mechanisms of action in adipocyte
differentiation by treating the cells with PZE during the early
(days 0–2) and late stages of differentiation (days 3–5).
Materials and methods
Reagents
The OP9 cells were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). Minimum essential
medium α (MEMα), fetal bovine serum (FBS), Alexa Fluor®
568 goat anti-rabbit IgG and BODIPY® 493/503 dye were
purchased from Invitrogen (Carlsbad, CA, USA). Insulin,
3-isobutyl-1-methylxanthine (IBMX), dexamethasone (DEXA) and Oil
Red O dye were purchased from Sigma Chemical Co. (St. Louis, MO,
USA). Antibodies against PPARγ, C/EBPα, C/EBPβ and β-actin were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Antibodies against extracellular signal-regulated kinases 1/2
(ERK1/2), phospho-ERK1/2, protein kinase B (Akt) and phospho-Akt
were obtained from Cell Signaling Technology (Beverly, MA, USA).
All the chemicals used were of analytical grade.
Preparation of PZE
The pericarp of Zanthoxylum piperitum D.C.
(Rutaceae) were purchased in May 2010 from the Wonkwang University
Oriental Herbal Drugstore, Iksan, Korea, and were identified by
Professor Youn-Chul Kim, College of Pharmacy, Wonkwang University.
A voucher specimen (no. WP10-05-1) was deposited at the Herbarium
of the College of Pharmacy, Wonkwang University. The dried and
pulverized pericarps of Zanthoxylum piperitum (50 g) were
extracted twice with hot 70% ethanol (1 liter) for 2 h at room
temperature and filtered with filter paper. The filtrate was
evaporated in vacuo to produce a 70% ethanol extract (10.64
g, 21.3 w/w %). The 70% ethanol extract was suspended in distilled
water (100 ml), followed by filtration. The residue derived from
the filtration was dissolved in hot ethanol and filtered again. The
filtrate was then evaporated in vacuo to obtain a
standardized fraction of Zanthoxylum piperitum (NNMBS142,
3.29 g, 6.58 w/w %). NNMBS142 was deposited at the Standardized
Material Bank for New Botanical Drugs, Wonkwang University.
Radix astragali extracts were also received from Professor
Youn-Chul Kim and used as a negative control. The extraction
methods for Radix astragali were the same as those used for
PZE.
Cell culture and induction of adipocyte
differentiation
The OP9 cells were cultured in MEMα containing 20%
FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C in a 5% CO2 incubator. To induce
differentiation, 1-day post-confluent preadipocytes were incubated
in differentiation medium containing 10% FBS, 0.5 mM IBMX, 0.25 μM
DEXA, 175 nM insulin, 2 mM L-glutamine, 100 U/ml penicillin and 100
μg/ml streptomycin for 2 days. The medium was then changed to MEMα
containing 10% FBS, 2 mM L-glutamine, and 175 nM insulin, and the
cells were cultured for 3 days. Control cells [no differentiation
(ND)] were cultured in MEMα containing 10% FBS, 2 mM L-glutamine,
100 U/ml penicillin, and 100 μg/ml streptomycin without IBMX, DEXA
and insulin for 5 days.
Determination of cell viability
The effects of PZE on OP9 cell viability were
determined using an established MTT assay. Briefly, the cells were
seeded in a 96-well dish and incubated at 37°C for 24 h to allow
attachment. The attached cells were either untreated [control
(CON)] or treated with 10 or 20 μg/ml PZE for various periods of
time at 37°C. The cells were washed with phosphate-buffered saline
(PBS) prior to the addition of MTT (0.5 mg/ml PBS) and incubated at
37°C for 30 min. Formazan crystals were dissolved with dimethyl
sulfoxide (100 μl/well) and detected at OD570 with a
model Emax (Molecular Devices, Sunnyvale, CA, USA).
Oil Red O staining
After the induction of adipocyte differentiation,
the cells were washed with cold PBS, fixed at room temperature with
4% formalin for 1 h, and then rinsed with 60% isopropanol. The OP9
cells were stained with Oil Red O for 1 h at room temperature and
washed 4 times with distilled water. The retained Oil Red O dye in
the cells was quantified by elution into isopropanol, and the
OD500 was measured.
Automated image acquisition and
processing
Following adipocyte differentiation, the cells were
washed with a cold PBS, fixed at room temperature with 4%
paraformaldehyde for 30 min, washed 3 times with cold PBS, and then
added to a blocking buffer and incubated for 45 min at room
temperature to prevent non-specific antibody binding. PPARγ or
C/EBPβ antibodies were then added to the cells following by
overnight incubation; the cells were then washed, and washed again
3 times, and incubated with BODIPY 493/503 dye for lipid droplets,
DAPI for the nucleus and Alexa Fluor 568 goat anti-rabbit or
anti-mouse IgG for PPARγ and C/EBPβ, respectively, for 1 h. Images
were acquired on an ArrayScan™ VTi automated microscopy and image
analysis system (Cellomics Inc., Pittsburgh, PA, USA). Using the
system of an automated highly sensitive fluorescence imaging
microscope with a ×20 objective and suitable filter sets, the
stained cells were identified with DAPI in fluorescence channel 1,
BODIPY 493/503 in channel 2 and Alexa Fluor 568 in channel 3. The
arbitrary value for BODIPY, C/EBPβ and PPARγ calculated from the
standard deviation of the intensity of the pixels under the channel
measuring DAPI reflected the content of the intact DNA.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the cells using a
FastPure™ RNA kit (Takara, Shiga, Japan). The RNA concentration and
purity were determined by absorbance at 260/280 nm. cDNA was
synthesized from 1 μg of total RNA using a PrimeScript™ RT reagent
kit (Takara). Adipocyte differentiation-related gene mRNA
expressions were determined by real-time (quantitative) PCR using
the ABI PRISM® 7900 Sequence Detection System and
SYBR®-Green I (Applied Biosystems, Foster City, CA,
USA). The primer sequences are listed in Table I. All the results were normalized
to the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase
(GAPDH), to control for variation in mRNA concentrations. Relative
quantification was performed using the comparative ΔΔCt
method according to the manufacturer’s instructions (Applied
Biosystems).
 | Table IPrimers and probes for real-time
quantitative PCR. |
Table I
Primers and probes for real-time
quantitative PCR.
| Genes | Primer sequences | Accession no. |
|---|
| PPARγ |
5′-GAAAGACAACGGACAAATCACC-3′
5′-GGGGGTGATATGTTTGAACTTG-3′ | NM_011146 |
| C/EBPα |
5′-TTGTTTGGCTTTATCTCGGC-3′
5′-CCAAGAAGTCGGTGGACAAG-3′ | NM_007678 |
| FABP4 |
5′-AGCCTTTCTCACCTGGAAGA-3′
5′-TTGTGGCAAAGCCCACTC-3′ | NM_024406 |
| FAS |
5′-TGATGTGGAACACAGCAAGG-3′
5′-GGCTGTGGTGACTCTTAGTGATAA-3′ | NM_007988 |
| HSL |
5′-GGAGCACTACAAACGCAACGA-3′
5′-TCGGCCACCGGTAAAGAG-3′ | NM_010719 |
| LPL |
5′-GGACGGTAACGGGAATGTATGA-3′
5′-TGACATTGGAGTCAGGTTCTCTCT-3′ | NM_008509 |
| GAPDH |
5′-CGTCCCGTAGACAAAATGGT-3′
5′-TTGATGGCAACAATCTCCAC-3′ | NM_008084 |
Western blot analysis
The OP9 cells were pre-treated with 20 μg/ml PZE for
1 h and then differentiation was induced at 37°C. The cells were
lysed with ice-cold M-PER® Mammalian Protein Extraction
Reagent (Pierce Biotechnology, Rockford, IL, USA), and the protein
concentration in the lysate was determined using the Bradford
method (9). Samples (20 μg) were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis with 10% acrylamide, and transferred onto Hybond™-P
polyvinylidene fluoride membranes (GE Healthcare Life Sciences,
Buckinghamshire, UK) using a western blot apparatus. Each membrane
was blocked for 2 h with 2% bovine serum albumin or 5% skim milk
and then incubated overnight at 4°C with 1 μg/ml of a 1:2,000
dilution of the primary antibody. HRP-conjugated IgG (1:2,000
dilution) was used as the secondary antibody. Protein expression
levels were determined by signal analysis using an image analyzer
(Fuji-Film, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using analysis of
variance and Duncan’s test. Differences with P-values <0.05 were
considered statistically significant.
Results
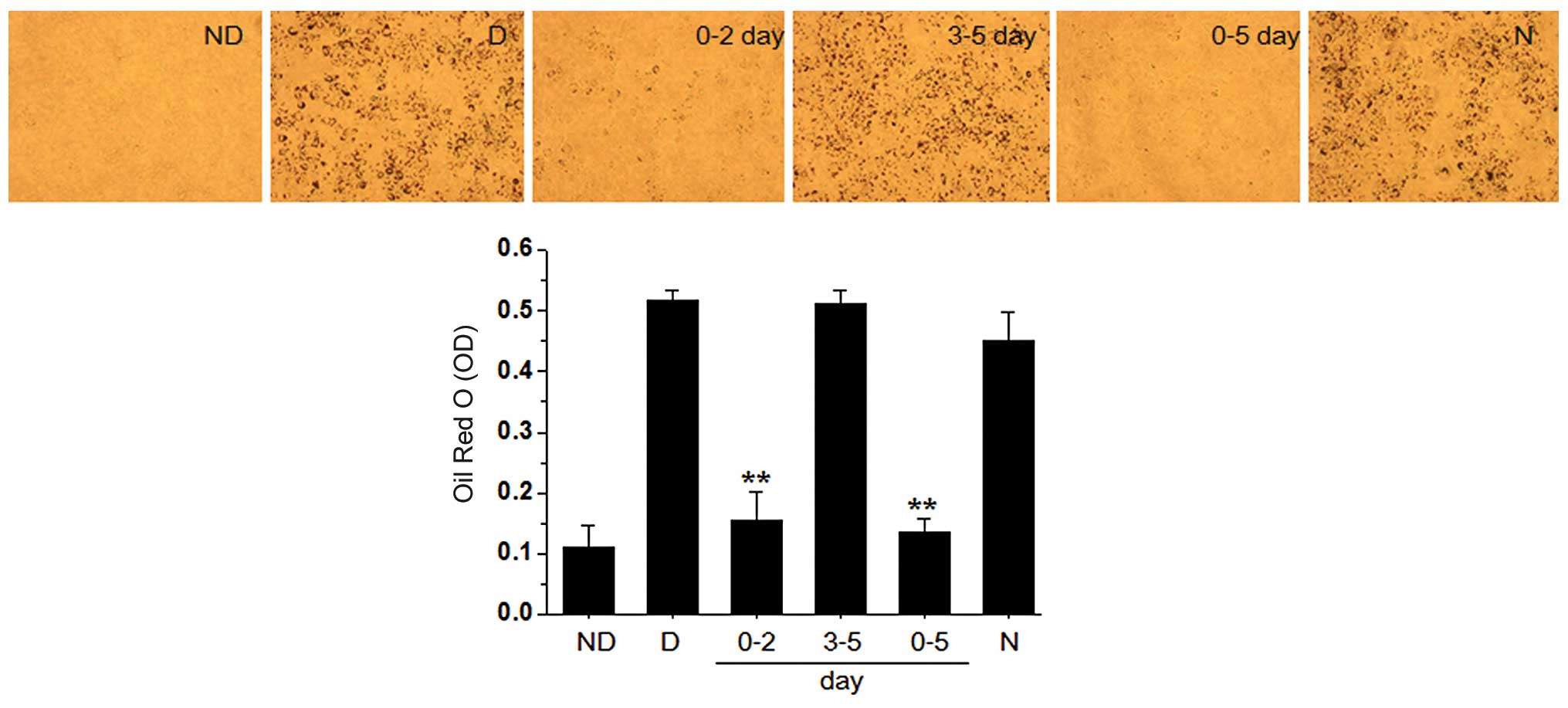
PZE inhibits adipocyte
differentiation
In our experiments, we investigated whether PZE
inhibits the differentiation of OP9 preadipocytes into mature
adipocytes. To understand the molecular basis underlying
PZE-inhibited adipogenesis, we first attempted to clarify the key
stage during adipocyte differentiation that are critical to the
anti-adipogenic effects of PZE, and we divided the adipogenesis
process into an early (days 0–2) and late (days 3–5) stage. The
formation of lipid droplets and the accumulation of triglycerides
in the adipocytes treated with 20 μg/ml PZE were completely blocked
during the early stage, as confirmed by Oil Red O staining in
Fig. 2. We further investigated
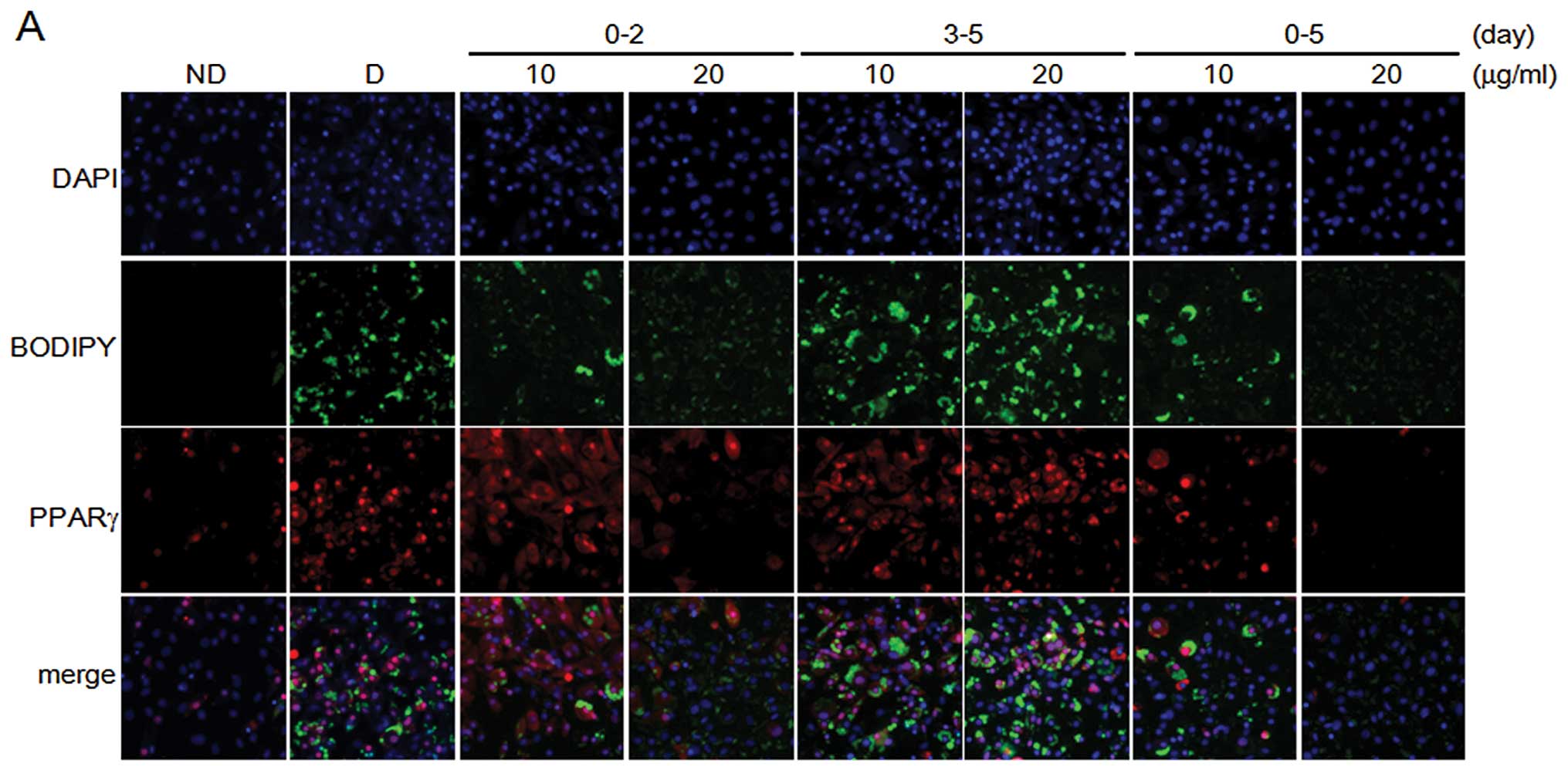
the inhibitory effects of PZE in adipocyte differentiation using
the automated image acquisition and processing method. Early-stage
treatment with PZE in the adipocyte differentiation process
inhibited lipid droplet formation in a dose-dependent manner, as
shown by BODIPY staining (green), which is a specific fluorescence
dye for intracellular lipids (Fig.
3). In the same region, we examined PPARγ protein expression
levels. PPARγ expression was downregulated follwoing early-stage
treatment with PZE, but not after late-stage treatment. The effects
of PZE on the formation of lipid droplets and PPARγ protein
expression during the early stage were similar to those during the
entire period of adipocyte differentiation (days 0–5). When the
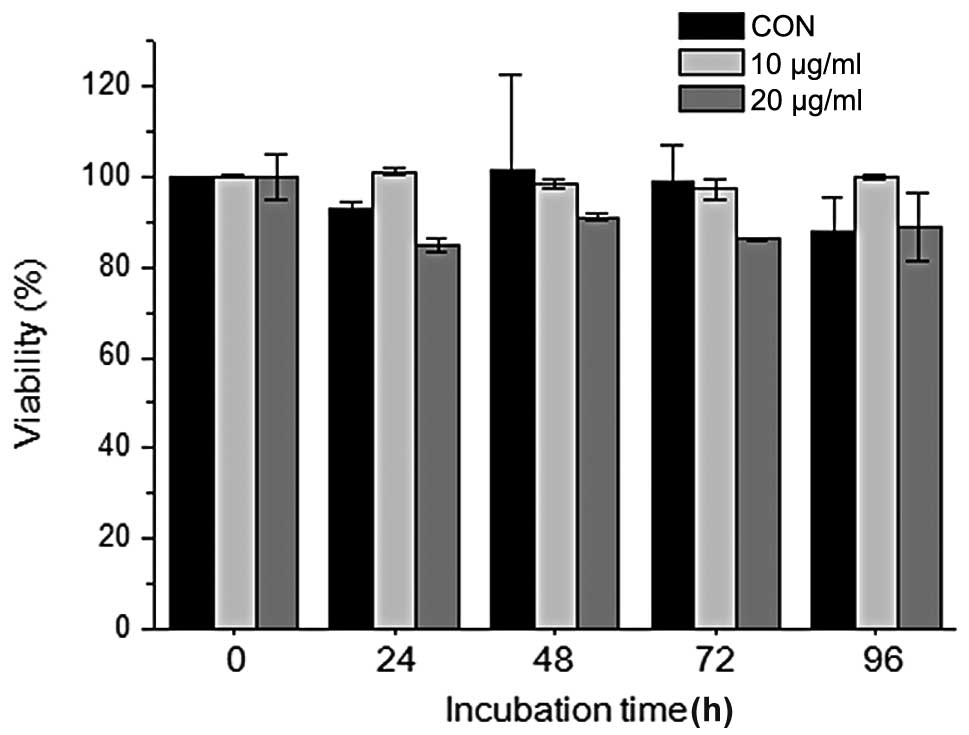
cells were treated with 10 or 20 μg/ml PZE during adipocyte
differentiation, cytotoxicity was not demonstrated at the various
time points compared to the control (untreated cells) cells
(Fig. 1).
PZE decreases the expression of adipocyte
differentiation-related genes during early-stage treatment
Adipocyte differentiation is accompanied by the
increased expression of various transcription factors and
adipocyte-specific genes; PPARγ and C/EBPα are essential for
terminal adipocyte differentiation (6,7).
PPARγ and C/EBPα mRNA expression were markedly decreased following
treatment with 20 μg/ml PZE during the early stages, but not during
the late stages (Fig. 4). We
further investigated whether the PZE-induced reduction in PPARγ and
C/EBPα levels regulated the expression of their target genes,
including adipocyte protein 2 (aP2), fatty acid synthase (FAS),
hormone-sensitive lipase (HSL) and lipoprotein lipase (LPL).
Treatment with 20 μg/ml PZE during the early stages of
differentiation and during the entire differentiation period
markedly decreased the expression levels of aP2, FAS, HSL and
LPL.
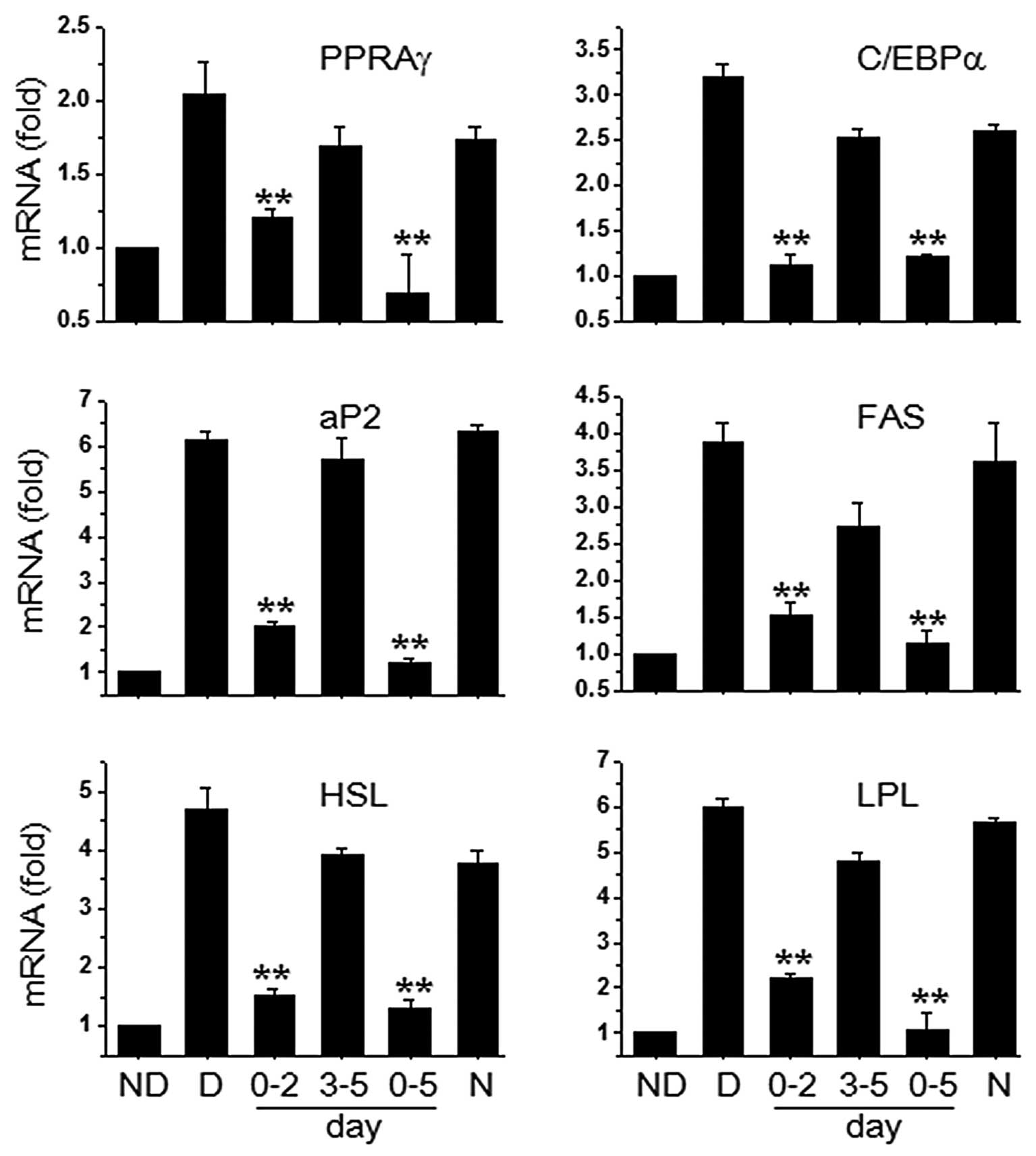 | Figure 4Effects of Pericarpium
zanthoxyli extract (PZE) on the expression of peroxisome
proliferator-activated receptor γ (PPARγ) and PPARγ-targeted genes.
OP9 cells were induced with MDI to induce differentiation into
adipocytes. Subsequently, 20 μg/ml PZE were added to the cells at
the early (0–2 days) and late stages of differentiation (3–5 days),
or the entire period (0–5 days). After 5 days of differentiation,
real-time PCR was carried out by using specific primers for PPARγ,
CCAAT/enhancer-binding protein α (C/EBPα), adipocyte protein 2
(aP2), fatty acid synthase (FAS), hormone-sensitive lipase (HSL)
and lipoprotein lipase (LPL). Data are the means ± standard
deviation (SD) values of at least 3 independent experiments.
**P<0.01 vs. D group. ND, no differentiation; D,
differentiation; N, negative control (20 μg/ml Radix
astragali extract). |
PZE-inhibits the expression of C/EBPβ
during the early stages of adipogenesis
C/EBPβ is a specific transcription factor expressed
during the early stages of adipogenesis. C/EBPβ expression in OP9
adipocytes treated with 10 or 20 μg/ml PZE during the early stages
markedly decreased in a dose-dependent manner (Fig. 5A and B). When growth-arrested
preadipocytes were treated with adipogenic inducers, the number of
adipocytes increased by approximately 2-fold during the early
stages. PZE markedly inhibited adipocyte proliferation during the
early stages of differentiation, and the number of PZE-treated OP9
adipocytes was similar to that of the control group (Fig. 5C). To determine the signaling
pathway through which PZE inhibited clonal expansion during the
early stages of adipogenesis, the expression of ERK and Akt was
examined. Adipogenic inducers increased the phosphorylation of
ERK1/2 and Akt. When the OP9 adipocytes were treated with 20 μg/ml
PZE for 10 min or 3 h, ERK1/2 phosphorylation was slightly
decreased, but Akt phosphorylation was not decreased by treatment
with PZE.
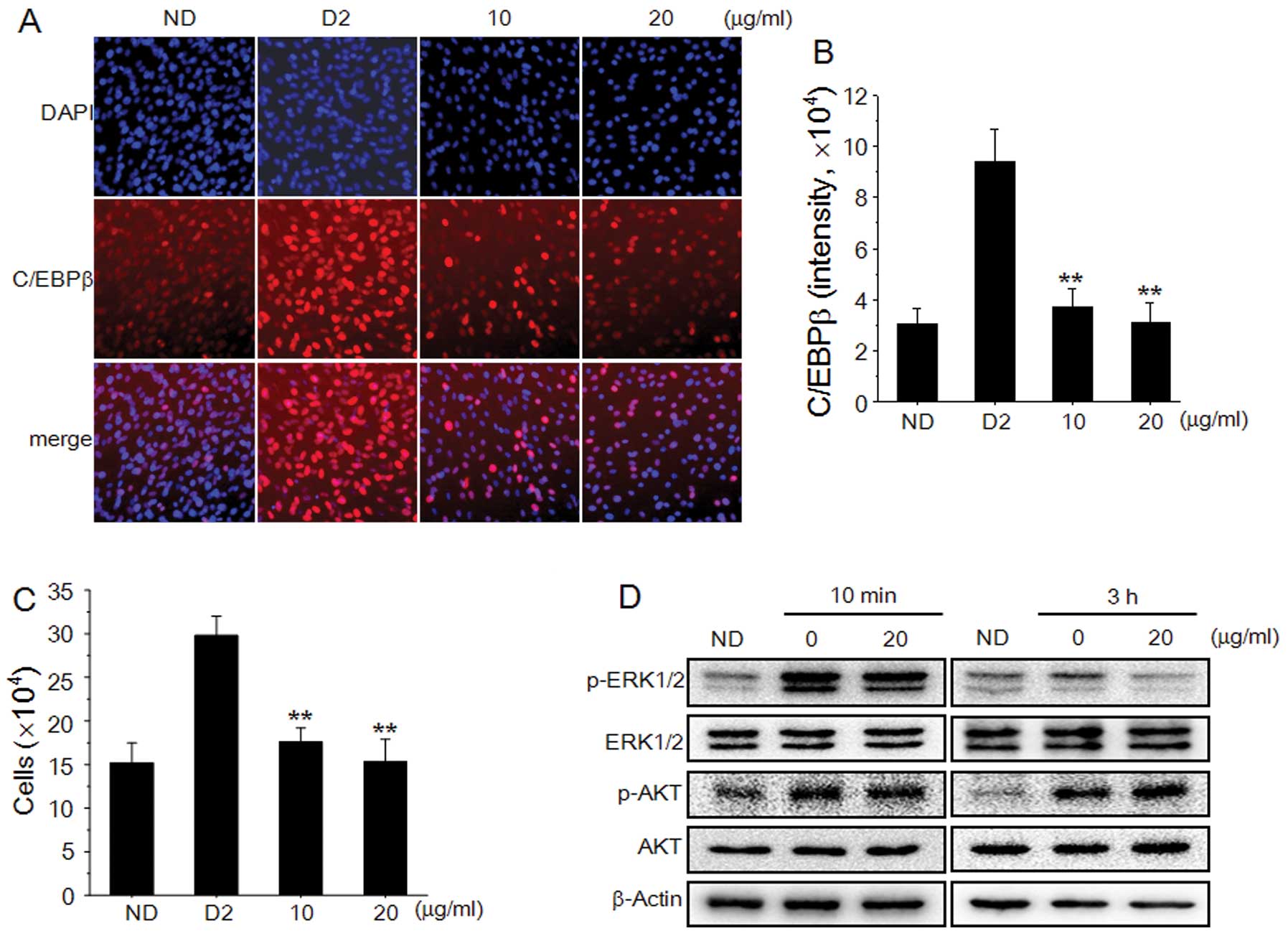 | Figure 5Effects of Pericarpium
zanthoxyli extract (PZE) on CCAAT/enhancer-binding protein β
(C/EBPβ) expression, cell proliferation and extracellular
signal-regulated kinases 1/2 (ERK1/2) phosphorylation in OP9 cells.
(A) OP9 cells were pre-treated with 10 or 20 μg/ml PZE for 1 h, and
then cultured with multiple daily insulin (MDI) for 2 days. After 2
days of differentiation, immunohistochemical staining of OP9 cells
was carried out by using a specific antibody to visualize C/EBPβ
(red) and DAPI to visualize nuclei (blue). (B) C/EBPβ
concentrations were obtained by averaging intensities of antibody
staining from the nuclei of 5,000 individual cells. (C) The number
of cells treated with 10 and 20 μg/ml PZE was determined using a
hemocytometer. (D) OP9 cells treated with 20 μg/ml PZE for 10 min
or 3 h were harvested, and the lysates were subjected to western
blot analysis for ERK1/2, phospho-ERK1/2 (p-ERK1/2), protein kinase
B (Akt), and phospho-Akt (p-Akt). Data are representative of
triplicate experiments, and are the means ± standard deviation (SD)
values of at least 3 independent experiments.
**P<0.01 vs. D2 group. ND, no differentiation; D2,
differentiation day 2. |
Discussion
In the present study, we investigated the
anti-obesity effects of PZE in OP9 cells by measuring lipid
accumulation, and by analyzing changes in adipocyte
differentiation, which modulates adipocyte-specific gene
expression. Preadipocytes can differentiate into adipocytes, which
possess a spherical shape and accumulate lipid droplets (5,6,10).
In this study, treatment with PZE inhibited lipid accumulation and
the differentiation of OP9 preadipocytes into adipocytes in a
dose-dependent manner. Treatment with PZE also decreased the
expression of key adipocyte differentiation regulators, including
C/EBPβ and PPARγ, and downregulated ERK phosphorylation.
At the molecular level, adipocyte differentiation is
regulated by a complex transcriptional cascade that involves the
sequential activation of C/EBPs and PPARγ (11). C/EBPβ and C/EBPδ are rapidly and
transiently expressed after hormonal induction of a differentiation
cocktail, and C/EBPβ is required for MCE in the immediate early
stages of adipocyte differentiation (12). These temporally expressed
transcription factors are induced and activated by cAMP and
glucocorticoids, and act synergistically to induce the expression
of C/EBPα and PPARγ, the master adipogenic transcription regulators
(13). The expression of C/EBPα
and PPARγ cross-regulate each other through a positive feedback
loop and transactivate downstream target genes (aP2, LPL, FAS and
HSL) that are adipocyte-specific and are involved in maintaining
the adipocyte phenotype.
The OP9 adipocyte differentiation system was
originally established by Wolins et al (8), and has often been used for
adipocyte-related research (14–16). In our study, as shown in Figs. 2 and 3 confluent OP9 cells differentiated into
adipocytes upon exposure to IBMX, DEXA and multiple daily insulin
(MDI), which then activated a cascade of the adipogenic program.
Treatment with PZE inhibited early-stage (days 0–2) adipocyte
differentiation through the inhibition of C/EBPβ (Fig. 5A and B).
Adipogenesis is divided into the preadipocyte, early
and late stages. OP9 cells undergo MCE through the upregulation of
C/EBPβ during the early stages of adipocyte differentiation. This
is followed by the activation of the downstream signaling
transcription factors, PPARγ and C/EBPα (17). In this study, PZE inhibited the
formation of lipid droplets and triglyceride accumulation, and
suppressed C/EBPβ expression during the early stages of
differentiation, as confirmed by Oil Red O staining (Fig. 2) and BODIPY staining (Fig. 3).
Clonal expansion occurs during the early stages of
adipocyte differentiation, at which time the cell population is
increased by 2-fold (18). In
this study, PZE inhibited adipocyte differentiation through the
suppression of OP9 cell proliferation (Fig. 5C). Taken together, these results
indicate that the major target of PZE for the inhibition of
adipocyte differentiation in OP9 cells may be clonal expansion by
targeting C/EBPβ expression during the early stages of
differentiation.
The ERK pathway is necessary for the initiation of
the early stages of adipogenesis, and acts as a mitogenic signaling
molecule in adipocyte differentiation (19,20). Adipogenic inducers stimulate the
MAPK/ERK pathway, which is followed by the enhanced activity of
C/EBPβ and the induction of adipocyte differentiation (20,21). The activation of the Akt pathway
in 3T3-L1 preadipocytes can also induce adipogenesis (4,22,23). In this study, adipogenic inducers
stimulated the phosphorylation of ERK1/2 and Akt following
treatment with PZE for 10 min and 3 h, but ERK1/2 phosphorylation
was only decreased by treatment with PZE for 3 h (Fig. 5D). Akt phosphorylation and cyclin
D1 (data not shown) expression were not affected by treatment with
PZE. Muise-Helmericks et al (24) reported that the PI3K/Akt pathway
affects cell cycle progression through the regulation of cyclin D
and p27 expression (Fig. 5D).
This suggests that the inhibition of C/EBPβ expression by PZE is
the result of the decrease in ERK phosphorylation, not Akt
phosphorylation.
In conclusion, this study indicates a new role for
PZE in adipocyte differentiation through targeting the early
cellular events of adipogenesis, such as MCE and the expression of
early adipogenic transcription factors. These results identify a
possible mechanism of action of PZE, suggesting that the
PZE-induced inhibition of ERK phosphorylation suppresses
adipogenesis by inhibiting other signaling cascades that include
C/EBPs and PPARγ during the process of OP9 adipocyte
differentiation. Taken together, our findings provide important
insight into the mechanisms underlying the anti-obesity activity of
PZE.
Acknowledgements
This study was supported by a National Research
Foundation of Korea (NRF) grant, funded by the Korean Government
(MEST) (no. 2011-0030130), Republic of Korea, and by a Basic
Science Research Program grant from the National Research
Foundation of Korea (NRF), funded by the Ministry of Education,
Science, and Technology (NRF-2012R1A1A4A0 1011520).
References
|
1
|
Schuster DP: Obesity and the development
of type 2 diabetes: the effects of fatty tissue inflammation.
Diabetes Metab Syndr Obes. 3:253–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caro JF, Dohm LG, Pories WJ and Sinha MK:
Cellular alterations in liver, skeletal muscle, and adipose tissue
responsible for insulin resistance in obesity and type II diabetes.
Diabetes Metab Rev. 5:665–689. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin RJ, Ramsay T and Hausman GJ:
Adipocyte development. Pediatr Ann. 13:448–453. 1984.
|
|
4
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998.PubMed/NCBI
|
|
5
|
Tong Q and Hotamisligil GS: Molecular
mechanisms of adipocyte differentiation. Rev Endocr Metab Disord.
2:349–355. 2001. View Article : Google Scholar
|
|
6
|
Ntambi JM and Young-Cheul K: Adipocyte
differentiation and gene expression. J Nutr. 130:3122S–3126S.
2000.PubMed/NCBI
|
|
7
|
Student AK, Hsu RY and Lane MD: Induction
of fatty acid synthetase synthesis in differentiating 3T3-L1
preadipocytes. J Biol Chem. 255:4745–4750. 1980.PubMed/NCBI
|
|
8
|
Wolins NE, Quaynor BK, Skinner JR, et al:
OP9 mouse stromal cells rapidly differentiate into adipocytes:
characterization of a useful new model of adipogenesis. J Lipid
Res. 47:450–460. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Otto TC and Lane MD: Adipose development:
from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 40:229–242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alessi MC, Lijnen HR, Bastelica D and
Juhan-Vague I: Adipose tissue and atherothrombosis. Pathophysiol
Haemost Thromb. 33:290–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang QQ, Otto TC and Lane MD:
CCAAT/enhancer-binding protein beta is required for mitotic clonal
expansion during adipogenesis. Proc Natl Acad Sci USA. 100:850–855.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farmer SR: Transcriptional control of
adipocyte formation. Cell Metab. 4:263–273. 2006. View Article : Google Scholar
|
|
14
|
Kotake D and Hirasawa N: Activation of a
retinoic acid receptor pathway by thiazolidinediones induces
production of vascular endothelial growth factor/vascular
permeability factor in OP9 adipocytes. Eur J Pharmacol. 707:95–103.
2013. View Article : Google Scholar
|
|
15
|
Saitoh Y, Mizuno H, Xiao L, Hyoudou S,
Kokubo K and Miwa N: Polyhydroxylated fullerene
C60(OH)44 suppresses intracellular lipid
accumulation together with repression of intracellular superoxide
anion radicals and subsequent PPARγ2 expression during spontaneous
differentiation of OP9 preadipocytes into adipocytes. Mol Cell
Biochem. 366:191–200. 2012.
|
|
16
|
Saitoh Y, Xiao L, Mizuno H, et al: Novel
polyhydroxylated fullerene suppresses intracellular oxidative
stress together with repression of intracellular lipid accumulation
during the differentiation of OP9 preadipocytes into adipocytes.
Free Radic Res. 44:1072–1081. 2010. View Article : Google Scholar
|
|
17
|
Park BO, Ahrends R and Teruel MN:
Consecutive positive feedback loops create a bistable switch that
controls preadipocyte-to-adipocyte conversion. Cell Rep. 2:976–990.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernlohr DA, Bolanowski MA, Kelly TJ Jr
and Lane MD: Evidence for an increase in transcription of specific
mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem.
260:5563–5567. 1985.PubMed/NCBI
|
|
19
|
Roberts EC, Shapiro PS, Nahreini TS, Pages
G, Pouyssegur J and Ahn NG: Distinct cell cycle timing requirements
for extracellular signal-regulated kinase and phosphoinositide
3-kinase signaling pathways in somatic cell mitosis. Mol Cell Biol.
22:7226–7241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang QQ, Otto TC and Lane MD: Mitotic
clonal expansion: a synchronous process required for adipogenesis.
Proc Natl Acad Sci USA. 100:44–49. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prusty D, Park BH, Davis KE and Farmer SR:
Activation of MEK/ERK signaling promotes adipogenesis by enhancing
peroxisome proliferator-activated receptor gamma (PPARgamma) and
C/EBPalpha gene expression during the differentiation of 3T3-L1
preadipocytes. J Biol Chem. 277:46226–46232. 2002. View Article : Google Scholar
|
|
22
|
Kohn AD, Summers SA, Birnbaum MJ and Roth
RA: Expression of a constitutively active Akt Ser/Thr kinase in
3T3-L1 adipocytes stimulates glucose uptake and glucose transporter
4 translocation. J Biol Chem. 271:31372–31378. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Magun R, Burgering BM, Coffer PJ, et al:
Expression of a constitutively activated form of protein kinase B
(c-Akt) in 3T3-L1 preadipose cells causes spontaneous
differentiation. Endocrinology. 137:3590–3593. 1996.PubMed/NCBI
|
|
24
|
Muise-Helmericks RC, Grimes HL, Bellacosa
A, Malstrom SE, Tsichlis PN and Rosen N: Cyclin D expression is
controlled post-transcriptionally via a phosphatidylinositol
3-kinase/Akt-dependent pathway. J Biol Chem. 273:29864–29872. 1998.
View Article : Google Scholar : PubMed/NCBI
|