Introduction
Acute lung injury (ALI) is a clinical syndrome
defined by acute hypoxemic respiratory failure and bilateral
pulmonary infiltrates consistent with edema, eventually leading to
lung fibrogenesis (1). ALI is
considered an immutable response to lung injury with transition
from alveolar capillary damage to a fibroproliferative phase,
independent of initial cause (2,3).
Currently, no specific strategies for the treatment of ALI are
available. It has been previously suggested that bone
marrow-derived mesenchymal stem cells (MSCs) incorporate into
various tissues and in some cases differentiate into a variety of
cell types of the tissue to which they have homed (4–8).
Conceivably, MSCs engrafted into injured lung tissue may assume
lung tissue cell type and contribute to lung repair. This fact
provides a strong rationale for exploring the potential use of MSCs
for the treatment of ALI.
Migration of circulating MSCs to the injured sites
is regulated by chemotactic signals. Stromal cell-derived factor
(SDF)-1 and its receptor CXC chemokine receptor (CXCR)4 have been
demonstrated to be the critical chemokines determining homing and
engraftment of MSCs associated with injury repair in many tissue
types (9–11). SDF-1 expression is upregulated in
the injured tissue sites (12).
CXCR4 is a critical chemokine receptor in stem cell homing, and the
differential expression of SDF-1 in injured tissue creates a
gradient essential for the migration of CXCR4-expressing cells. The
SDF-1/CXCR4 chemotactic axis is crucial for recruitment of
circulating or intravenously infused cells, suggesting that
modulation of these interactions may enhance stem cell engraftment
following injury (13–15). Therefore, the SDF-1/CXCR4
chemotactic axis was used to enhance homing and engraftment of MSCs
by transfecting with adenovirus combined with CXCR4 gene and the
therapeutic effect of MSCs for ALI was investigated.
MSCs localize to injured lungs and differentiate
into specific lung cell types, including alveolar epithelial cells
under appropriate conditions in order to promote lung remodeling.
However, it was shown that bone marrow-derived circulating
progenitor cells including bone marrow-derived MSCs accumulate in
the lung and differentiate into lung fibroblasts, lung
myofibroblasts and interstitial monocytes that contribute to a
fibrotic response (16–18). Xu et al (18) clarified that lung injury induces
the recruitment of CXCR4+ bone marrow-derived MSCs which
may assume a fibroblast phenotype and contribute to fibrogenesis in
the fibrogenic environment of the injured lung (18). The origin and nature of the
factors or signaling pathways that determine the differentiation of
MSCs remains to be determined, and the mechanisms of stem cell
involvement in tissue repair, regeneration and remodeling remain
unclear. It is therefore necessary to explore the possible
mechanisms that may regulate the differentiation of engraftment
MSCs in vivo. The types of cells differentiated from MSCs
are important for lung repair or pulmonary fibrosis.
Wnt/β-catenin signaling plays an important role in
tissue repair, wound closure, fibrosis and tissue remodeling
(19–21). Previously it was demonstrated that
aberrant activation of Wnt/β-catenin signaling has been connected
with pathogenesis of lung disease, such as asthma, pulmonary
fibrosis and lung cancer (22,23). Wnt/β-catenin signaling also plays
a vital role in fate determination and differentiation of MSCs
(24). The results of those
studies suggested that Wnt/β-catenin signaling may participate in
lung repair or pulmonary fibrosis via regulation of MSC
differentiation into lung tissue-specific cells.
In the present study, we investigated the effect of
intravenous transplantation of MSCs using the SDF-1/CXCR4
chemotactic axis to increase engraftment numbers in order to modify
the pathophysiology of HCl-induced lung injury. Additionally, the
role of the Wnt/β-catenin signaling pathway in the regulation of
MSC differentiation in vivo was examined.
Materials and methods
Ethics statement
The rats received care in compliance with the Guide
for the Care and Use of Experimental Animals formulated by the
National Society for Medical Research. the study was approved by
Nanjing University.
MSC isolation, culture and flow
cytometry
Adult Sprague-Dawley (SD) rats were purchased from
the Animal Feeding Center of Nanjing Medical University. MSC
isolation, culture and flow cytomery were performed as previously
described (24). In brief, MSCs
were obtained from the bone marrow of the femurs and tibias of rats
weighing 70–80 g. The marrow was flushed out using PBS under
aseptic conditions. The collected cells were filtered through a 70
μm cell strainer and centrifuged at 300 × g for 8 min. The cells
were subsequently cultured in low-glucose Dulbecco’s modified
Eagle’s medium (DMEM; HyClone, Thermo Scientific, San Jose, CA,
USA) with 10% FBS (Gibco-BRL, Invitrogen Life Technologies,
Paisley, UK; Invitrogen Life Technologies, Carlsbad, CA, USA), 1%
L-glutamine, and a 1% solution of penicillin and streptomycin
seeded at a density of 1×106 cells/ml into uncoated
flasks, and cultured in a humidified incubator at 37ºC in 5%
CO2. Non-adherent cells were removed after 72 h. When
MSCs reached 80% confluence, they were routinely passaged using
0.25% trypsin, with a dilution of 1:2 at each passage.
For flow cytometry, 1×105 passage-3 MSCs
were incubated with fluorescence-conjugated primary antibodies at
37ºC for 1 h in the dark following two washes with PBS and then
incubated with secondary antibody at 37ºC for 30 min. After washing
three times with PBS, the cells were analyzed using a FACSCalibur
flow cytometer and analyzed with Paint-A-Gate software
(Becton-Dickinson, San Jose, CA, USA). The antibodies employed
were: PE anti-rat CD29 from BioLegend, Inc. (San Diego, CA, USA),
R-PE anti-rat CD44 from Antigenix America, Inc. (Melville, NY,
USA), PE anti-rat CD90 from eBioscience, Inc. (San Diego, CA, USA),
mouse anti-rat CD73 from BD Pharmingen, Inc. (Franklin Lakes, NJ,
USA), mouse anti-rat CD34 from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA), rabbit anti-rat CD133 and FITC anti-rat CD79
from Abcam, Inc. (Cambridge, MA, USA), FITC anti-rat CD11B and FITC
anti-rat CD45 from Millipore Corporation (Billerica, MA, USA).
Transfection of MSCs and
immunofluorescent staining
The recombinant adenovirus vectors carrying the GFP
reporter gene (Ad-GFP) or CXCR4-GFP (Ad-CXCR4-GFP) were obtained
from Cyagen Biosciences, Inc. (Guangzhou, China). For transfection,
passage-3 MSCs were seeded at 1×104 cells/cm2
in a T-75 cm2 flask. The following day, MSCs were
transfected with Ad-GFP or Ad-CXCR4-GFP in FBS-free DMEM medium at
a multiplicity of infection (MOI) of 100 for 16 h. After
transfection, DMEM containing adenoviral particles was removed and
fresh MSC culture medium was added. Forty-eight hours after
transfection, MSCs were prepared for injection, and western blot
assay and immunofluorescence analysis were performed.
Immunofluorescent analysis of MSC-Ad-CXCR4/GFP cells
was performed as previously described (25). Briefly, MSCs transfected with
Ad-GFP or Ad-CXCR4-GFP for 48 h were first fixed with 4%
paraformaldehyde for 10 min. To block non-specific binding sites,
the cells were incubated with PBS containing 2% bovine serum
albumin (BSA) for 1 h at 37ºC. The primary antibody CXCR4 (Abcam,
Inc.) was diluted at a certain concentration (10 μg/ml) according
to the manufacturer’s instructions. Incubation was performed at 4ºC
for 16 h. After three washes with PBS, MSCs were incubated with a
secondary antibody (goat anti-rabbit Alexa Fluor 594; Invitrogen
Life Technologies, Gaithersburg, MD, USA) at a 1:400 dilution in 2%
BSA for 1 h at 37ºC in the dark. Non-transfection MSCs were used as
the control. The cells were stained with 5 μg/ml
4′,6′-diamidino-2-phenylindole (DAPI) (Biyuntian, Inc., Nantong,
Jiangsu, China) to identify cellular nuclei. Images were captured
using a confocal fluorescence microscope (Olympus, Tokyo,
Japan).
Hydrochloric acid-induced lung injury and
MSC administration
Male 8- to 10-week-old SD rats were anesthetized via
an intraperitoneal injection of ketamine (60 mg/kg) and diazepam
(60 mg/kg), then hydrochloric acid (HCl, pH 1.5, 1 ml/kg) was
administered intranasally. Normal controls were administered with
PBS (1 ml/kg) in the lung. MSC-Ad-GFP/CXCR4 cells (5×106
cells in 200 μl of PBS) were administered via tail vein injection
to rats 24 h after lung injury.
Animal groups and study design
SD rats were randomly divided into four groups (n=24
for each group): control: normal controls were injected with
MSC-Ad-CXCR4 cells. ALI: HCl instillation and injection of PBS;
ALI+MSC-GFP: ALI rats were injected with MSC-Ad-GFP cells; and
ALI+MSC-CXCR4: ALI rats were injected with MSC-Ad-CXCR4 cells.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Semiquantitative RT-PCR was performed as previously
described (26). CXCR4 mRNA was
obtained from MSC-Ad-GFP and MSC-Ad-CXCR4 cells. SDF-1 and
immunoreactive cytokine [tumor necrosis factor-α (TNF-α), IL-6,
IL-1β, IL-4, transforming growth factor-β1 (TGF-β1) and IL-10] mRNA
were obtained from lung tissue following HCl administration at 1,
3, 7 and 14 days. Total RNA was extracted using TRIzol (Invitrogen
Life Technologies, Gaithersburg, MD, USA) according to the
manufacturer’s instructions. cDNA was generated from 2 μg of total
RNA using random primers and EasyScript First-Strand cDNA Synthesis
SuperMix. RT-PCR was performed using primers specific for the
factors of interest. Quantification of the products was measured by
the amount of cDNA amplified and using amplification reactions of a
359-bp fragment from 18S cDNA as the control. The amount was
normalized using 18S as a standard. Primers used for detection are
shown in Table I.
 | Table IPrimer sequences for RT-PCR. |
Table I
Primer sequences for RT-PCR.
| Gene name | Forward
(5′–3′) | Reverse
(5′–3′) |
|---|
| TNF-α |
ACGCTCTTCTGTCTACTG |
GGATGAACACGCCAGTCG |
| IL-1β |
GAAGTCAAGACCAAAGTGG |
TGAAGTCAACTATGTCCCG |
| IL-6 |
GAAATGAGAAAAGAGTTGTGC |
GGAAGTTGGGGTAGGAAGGAC |
| IL-4 |
TCTCACGTCACTGACTGTA |
CTTTCAGTGTTGTGAGCGT |
| IL-10 |
CACTGCTATGTTGCCTGCTC |
TTCATGGCCTTGTAGACACC |
| TGF-β1 |
CTTCAGCTCCACAGAGAAGAACTGC |
CACGATCATGTTGGACAACTGCTCC |
| 18S |
TTTGGTCGCTCGCTCCTC |
GCTGCCTTCCTTGGATGTG |
Flow cytometry
Subsequent to injection of MSCs, the rats were
sacrificed at day 7 and 14. Lungs were removed and enzymatically
digested using dispase (BD Biosciences, San Jose, CA, USA) and
collagenases I (Sigma, St. Louis, MO, USA), yielding a mixed
population of lung cells. The cells were passaged through 70 μm
filters to obtain single-cell suspensions and analyzed by flow
cytometry using the FACSCalibur flow cytometer. GFP+
cells were gated in the green fluorescent channel, counted and
recorded as a percentage of nucleated cells. MSCs transfected with
adenovirus-GFP were used as a positive control, while non-injected
MSC lung cells were used as a negative control. Data analysis for
all flow cytometry experiments was performed using FloJo software
(Tree Star, Inc., Ashland, OR, USA).
Histologic examination
The left lung was inflated with 4% paraformaldehyde
through the trachea for 16 h followed by paraffin-embedding.
Sections (5 μm) were cut for hematoxylin and eosin (H&E) and
Masson’s trichrome staining. H&E staining was performed
according to the manufacturer’s instructions. The sections were
stained with H&E to determine histologic structure integrity.
The severity of pulmonary fibrosis in lung sections stained for
collagen with Masson’s trichrome stains was determined by the
histopathologist, who was blinded to the protocol design.
Detection of MSC differentiation in
vitro
To assess the regulation of Wnt/β-catenin signaling
on the differentiation of MSCs, 100 ng/ml Wnt3α and 20 ng/ml DKK1
(Peprotech, Inc., Rocky Hill, NJ, USA) were added into the cultured
MSCs. MSCs were treated with Wnt3 and DKK1 for 14 days, and then
MSCs were detected by immunofluorescence analysis as previously
described (25). The primary
antibodies were employed as follows: rabbit anti-β-catenin, rabbit
anti-α-SMA and mouse anti-vimentin (all antibodies purchased from
Abcam, Inc.).
Western blotting
Cell proteins were obtained from MSCs and tissue
proteins were obtained from the right lower lung in each group.
Western blot analysis of cellular lysates was performed as
previously described (25).
Briefly, cells or tissues were lysed in ice-cold extraction buffer
containing protease inhibitor cocktail (Roche Applied Science,
Indianapolis, IN, USA) for 30 min. The whole lysates were then
centrifuged at 12,000 × g for 30 min, and the protein concentration
in the supernatant was determined using BCA assays. Proteins were
separated using 12% SDS-polyacrylamide gel electrophoresis and were
electrophoretically transferred to polyvinylidene fluoride (PVDF)
membranes using standard procedures. The membranes were incubated
at 37ºC for 1 h in blocking buffer (PBS, 0.1% Tween-20, 1% BSA and
5% non-fat milk). The primary antibodies were added to the
membranes and incubated at 4ºC for 16 h. After three washes in PBS,
the membranes were incubated with the secondary antibody
(horseradish peroxidase-conjugated goat anti-rabbit/mouse IgG;
Boster Biological Technology Ltd., Wuhan, Hubei, China) at 37ºC for
1 h. Immunoreactive protein bands were detected using an Odyssey
Scanning System (LI-COR Biosciences, Lincoln, NE, USA). The primary
antibodies employed were: rabbit anti-CXCR4, rabbit anti-SDF-1,
rabbit anti-β-catenin, rabbit anti-MMP-2, rabbit anti-α-SMA, mouse
anti-vimentin and mouse anti-β-actin (Abcam, Inc.).
Immunofluorescence and
immunohistochemical staining
Immunofluorescence staining for the detection of
engraftment MSC differentiation and immunohistochemistry on
paraffin-embedded sections was performed as previously described
(27). Briefly, for
immunofluorescence staining, the lung tissue samples were fixed in
4% paraformaldehyde in PBS at 4ºC for 4 h followed by overnight
immersion at 4ºC in buffer containing 30% sucrose. The specimens
were then embedded in optimal cutting temperature (Sakura Finetek
USA Inc., Torrance, CA, USA), and stored at −70ºC until use. The
tissue was cut transversely at a thickness of 15 μm and the slides
were fixed in acetone at 4ºC for 15 min. The slides were then
washed twice in PBS for 5 min and blocked by incubation with 3% BSA
in PBS/0.3% Triton X-100 for 30 min at room temperature. After
draining this solution from the tissue section, the slides were
incubated overnight at 4ºC with primary antibodies [1:200; SDF-1,
α-SMA, vimentin, cytokeratin 18 (CK18), cytokeratin 19 (CK19) and
pro-surfactant protein C (SP-C)]. After three washes with PBS,
these slides were incubated with a secondary antibody (goat
anti-rabbit Alexa Fluor 594 or goat anti-mouse Alexa Fluor 594;
both from Invitrogen Life Technologies) at a 1:400 dilution in 2%
BSA for 1 h at 37ºC in the dark. The nuclei were stained with DAPI
(5 μg/ml). The images were captured using a laser scanning confocal
fluorescence microscope (Olympus). Negative control tissue sections
were similarly prepared from each rat except that no primary
antibody was added.
For immunohistochemical staining, tissue was fixed
in 4% paraformaldehyde in PBS overnight, and then stored in 70%
alcohol at 4ºC until processing for paraffin-embedded tissue
sectioning. Sections (5 μm) were mounted onto poly-L-lysine- coated
slides, dewaxed in xylene, and rehydrated by sequential rinses in
100, 95, 70 and 50% ethanol. Endogenous peroxidase activity was
inhibited using 3% H2O2 solution for 10 min.
After three washes in PBS, non-specific binding sites were blocked
with 3% BSA for 30 min at 37ºC, and then incubated overnight at 4ºC
with primary antibodies (1:200; α-SMA, vimentin, collagen I, CK18
and CK19). Negative control tissue sections were incubated with IgG
isotype replaced primary antibody. A secondary biotinylated
anti-mouse or anti-rabbit antibody (1:200; Boster Biological
Technology, Ltd.) was added and the slides were incubated for 30
min at 37ºC. After rinsing, the slides were incubated with
horseradish peroxidase-conjugated streptavidin and then washed with
deionized water. In the subsequent steps, the slides were incubated
with diaminobenzidine substrate solution for 10 min, and
counterstaining with hematoxylin. Images were captured on a Nikon
microscope. A brown reaction product was considered a positive
result.
SDF-1 enzyme-linked immunosorbent assay
(ELISA)
The protein concentrations of SDF-1 in the ALI rat
serum were measured using ELISA (R&D System, Minneapolis, MN,
USA), according to the manufacturer’s instructions. The serum was
obtained from the abdominal vein of ALI rats. Briefly, plates were
blocked and incubated at room temperature for 1 h, then samples
were added (100 μl/well) in duplicate for incubation at 37ºC for 90
min. Biotinylated antibodies were subsequently added (100 μl/well)
and incubated at 37ºC for 1 h. Incubation with
streptavidin-horseradish-peroxidase (at 37ºC for 30 min) was
followed by detection with tetramethylbenzidine (TMB) color
developing agent at 37ºC for 30 min. The reaction was stopped by
the addition of TMB stop solution. Plates were read on a microplate
reader (Alisei Quality System, Italy) using a wavelength of 450
nm.
Statistical analysis
Experimental results were expressed as mean ±
standard deviation (SD). Statistical analyses were performed using
one-way ANOVA techniques in Microsoft Excel 2003 using SPSS
software. This step was followed by a Student Newman-Keuls’
post-hoc test. P<0.05 was considered to be statistically
significant.
Results
Characterization of MSCs
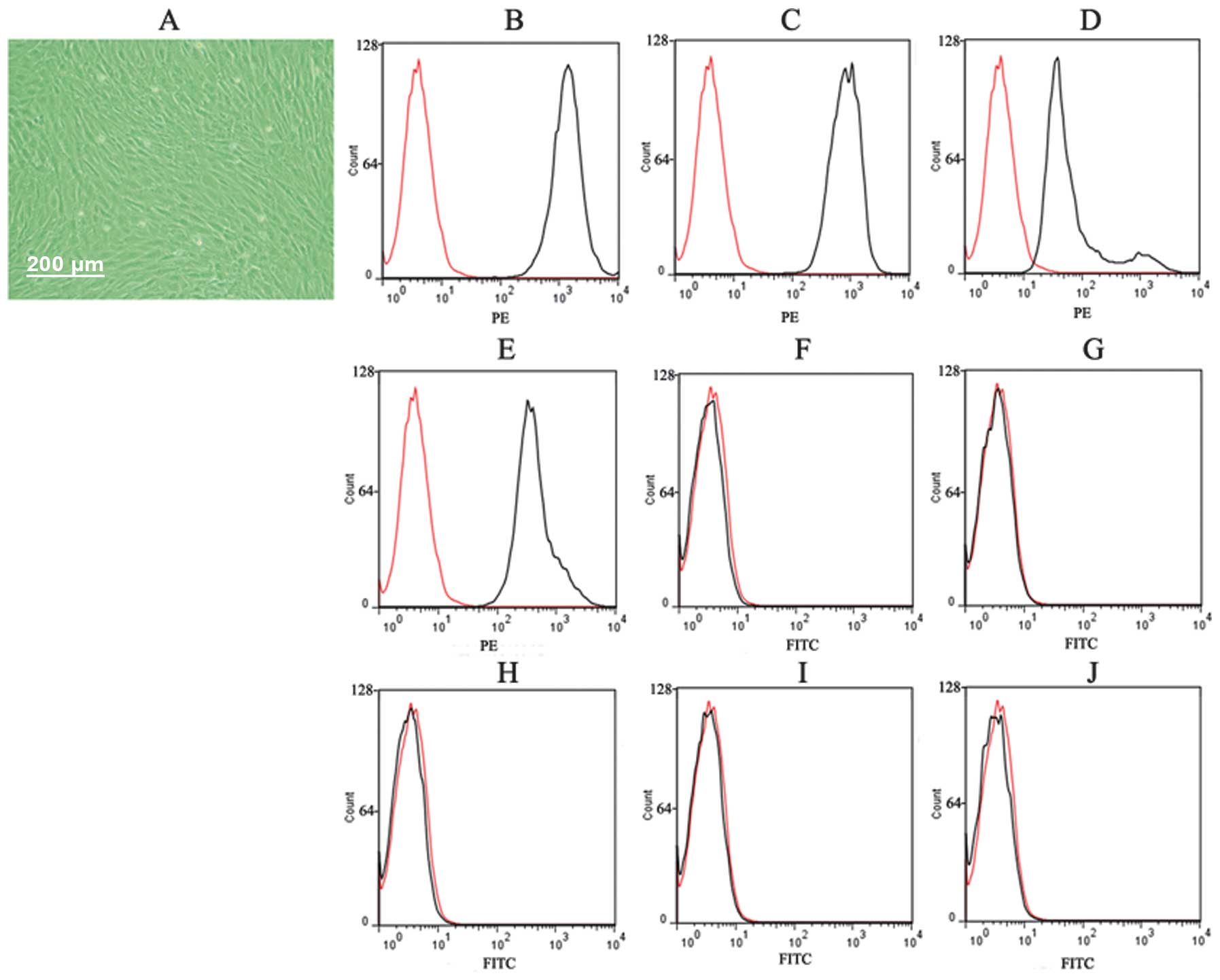
After three passages, the adherent cells showed a
typical fibroblast-like and spindle-shaped morphology (Fig. 1A). Results of the FACS analysis
demonstrated that cultured MSCs expressed CD29, CD44, CD73 and CD90
(Fig. 1B–E), but not CD11B, CD34,
CD45 and CD133 (Fig. 1F–J),
indicating that cultured adherent cells were MSCs with high purity.
The pure MSCs were used in subsequent experiments.
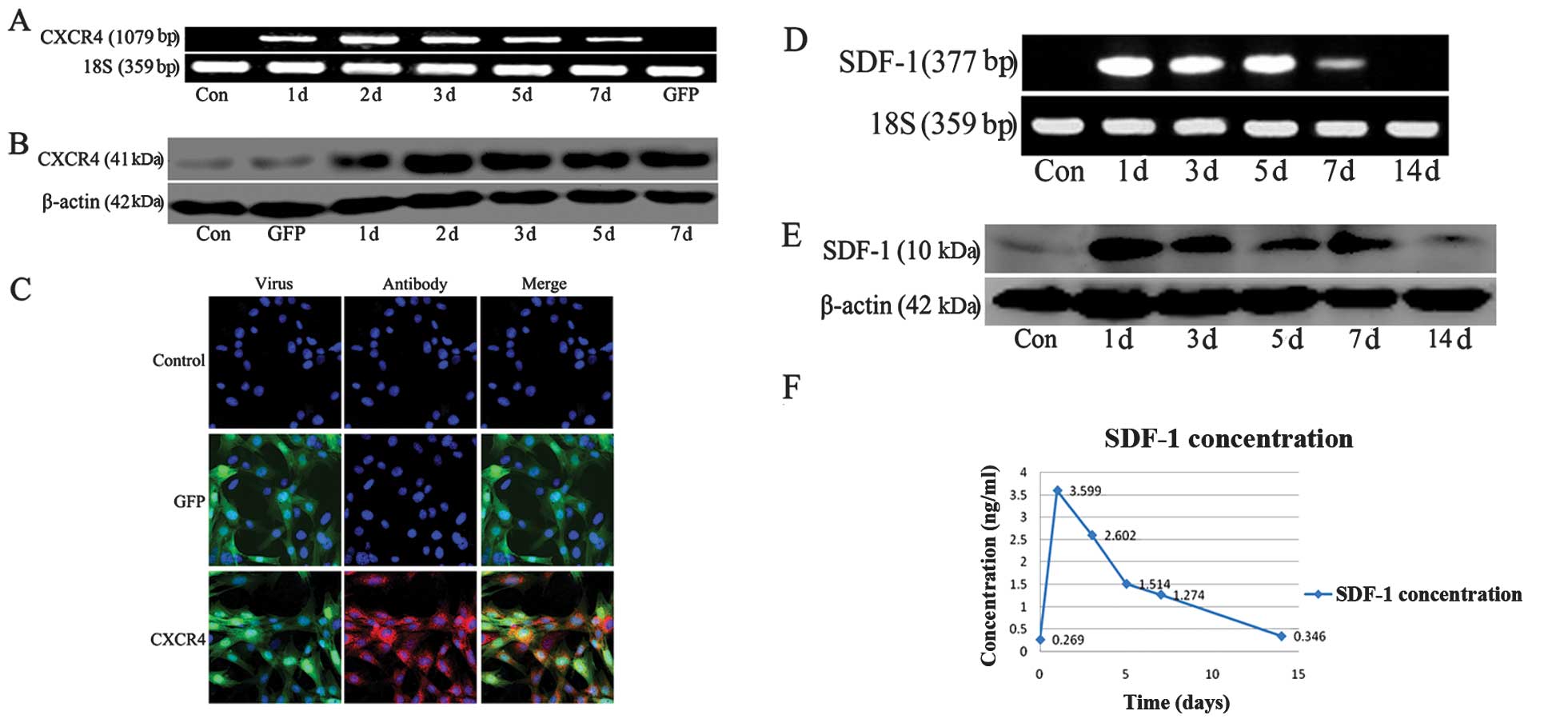
Detection of CXCR4 and SDF-1
expression
Results of previous studies have indicated that
primary MSCs expressed CXCR4 at a low level, whereas passaged MSCs
were not able to express CXCR4 following culture (28,29). To aid in cell tracking and explore
the role of SDF-1/CXCR4 axis, we transfected the adherent MSCs with
an adenovirus carrying the CXCR4 cDNA combined with enhanced green
fluorescent protein (eGFP) cDNA. Following transfection, mRNA and
the protein expression of CXCR4 in MSCs was increased, reaching a
peak at day 2 compared with the expression of MSC-Ad-GFP cells and
non-transfection MSCs (Fig. 2A and
B). Immunofluorescent analysis also indicated that the
transfection efficiency was ~90%, and that MSC-Ad-GFP cells and
non-transfection MSCs did not express CXCR4 (Fig. 2C).
After lung injury, we detected the expression of
SDF-1 in lung tissue and serum. We found that the mRNA and protein
expression of SDF-1 in the lung was increased on day 1, 3, 5 and 7
following HCl-induced injury compared to the control group. The
SDF-1 expression reached a peak at day 1 after lung injury and
retained a high expression for one week in lung tissue (Fig. 2D and E). The expression of SDF-1
in the serum exhibited the same trend (Fig. 2F).
SDF-1/CXCR4 axis enhances MSC
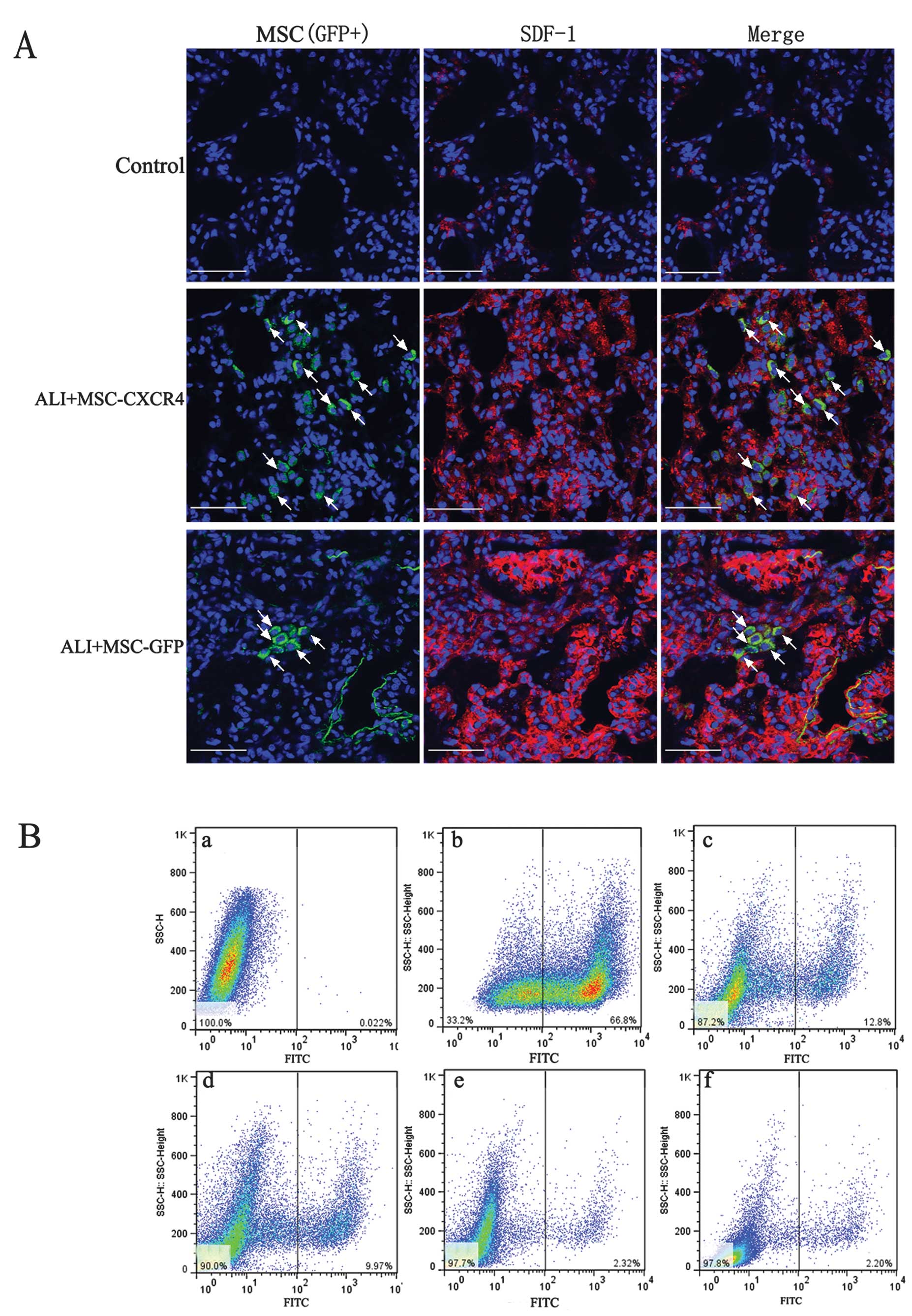
engraftment
To assess the engraftment ratio of MSCs at injury
sites, we measured the subpopulation of GFP+ cells in
lung tissue. Immunofluorescence staining was used to detect the
engraftment sites of MSCs (GFP+ cells) in lung tissue.
The results showed that MSCs engrafted the injury sites that
expressed SDF-1 at a high level (Fig.
3A). More GFP+ cells were identified in
SDF-1-expressed sites of the ALI+MSC-CXCR4 group compared to the
ALI+MSC-GFP group. Flow cytometry was used to analyze the
SDF-1-expressed sites to confirm the role of the SDF-1/CXCR4 axis
in the migration of MSCs. Following transplantation of the MSCs for
7 days, ~12% GFP+ cells in the cell population were
identified in the ALI+MSC-CXCR4 group; however, only ~2%
GFP+ cells were identified in the ALI+MSC-GFP group
(Fig. 3B). The results
demonstrated that a high expression of SDF-1 at the injury sites
and CXCR4 overexpression in MSCs improved the migration of MSCs
into injury sites. These results indicated that the SDF-1/CXCR4
chemotactic axis is crucial in regulating the migration of MSCs.
For long-term transplantation, GFP+ cells in the cell
population showed a similar time-dependent decrease. Following
transplantation of the MSCs for 14 days, ~10% GFP+ cells
were identified at the injury sites in the ALI+MSC-CXCR4 group.
Engraftment of MSCs in the lung marked effects on the fibrosis or
reparation of injured lung tissue.
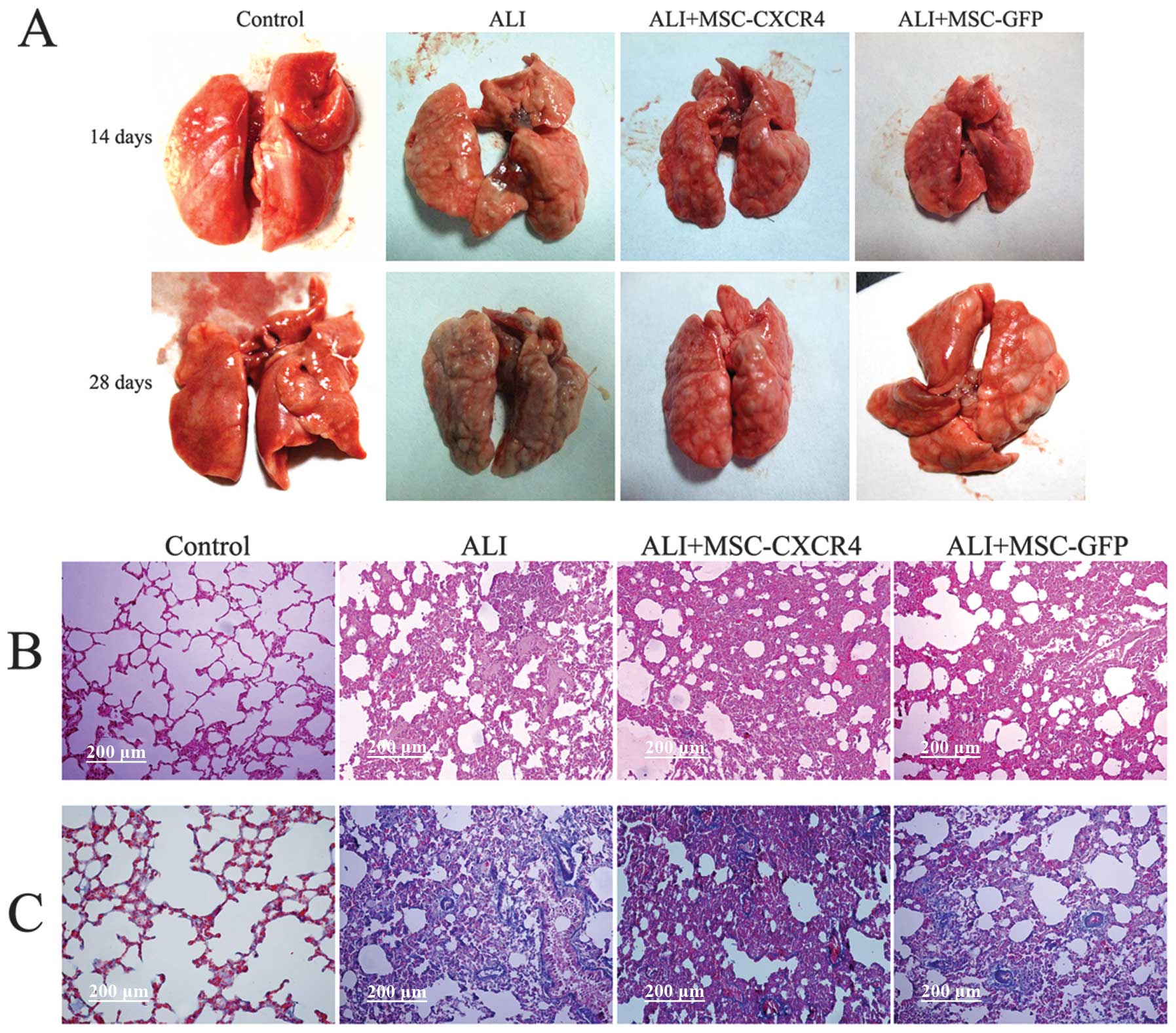
Effects of MSC transplantation in injured
lungs
After lung collection, the macroscopic appearance of
ALI lungs was intumescent and fibrotic (Fig. 4A). The lung samples were large,
yellowish and had several scars when compared with the saline
control lungs. However, ALI+MSC-GFP and ALI+MSC-CXCR4 lungs were
similar in appearance to that of the ALI lungs. MSC transplantation
did not attenuate lung injury and the fibrotic response.
To examine the effect of transplantation of MSCs in
ALI rats, serial lung sections obtained from rats following lung
injury over 28 days were stained with H&E or Masson’s trichrome
staining and examined by light microscopy. Lungs from rats in the
ALI group demonstrated marked alteration in lung architecture, with
extensive cellular thickening and fibrosis (Fig. 4B). At 28 days after MSC
transplantation, H&E staining indicated that the alveolar walls
were thickened and pulmonary interstitial fibrosis occurred.
Additionally, the extent of fibrosis was as severe as that observed
in the ALI group (Fig. 4B).
Masson’s trichrome staining demonstrated that collagen deposition
(blue staining) in the ALI and MSC transplantation groups was
increased compared with the control group (Fig. 4C). Histologic examination
indicated that MSC transplantation did not attenuate lung injury
and pulmonary fibrosis. However, we hypothesized that the
engraftment of MSCs contributes to pulmonary fibrosis. In the
fibrogenic environment of the injured lung, MSCs may differentiate
into fibroblast phenotype cells and contribute to fibrogenesis.
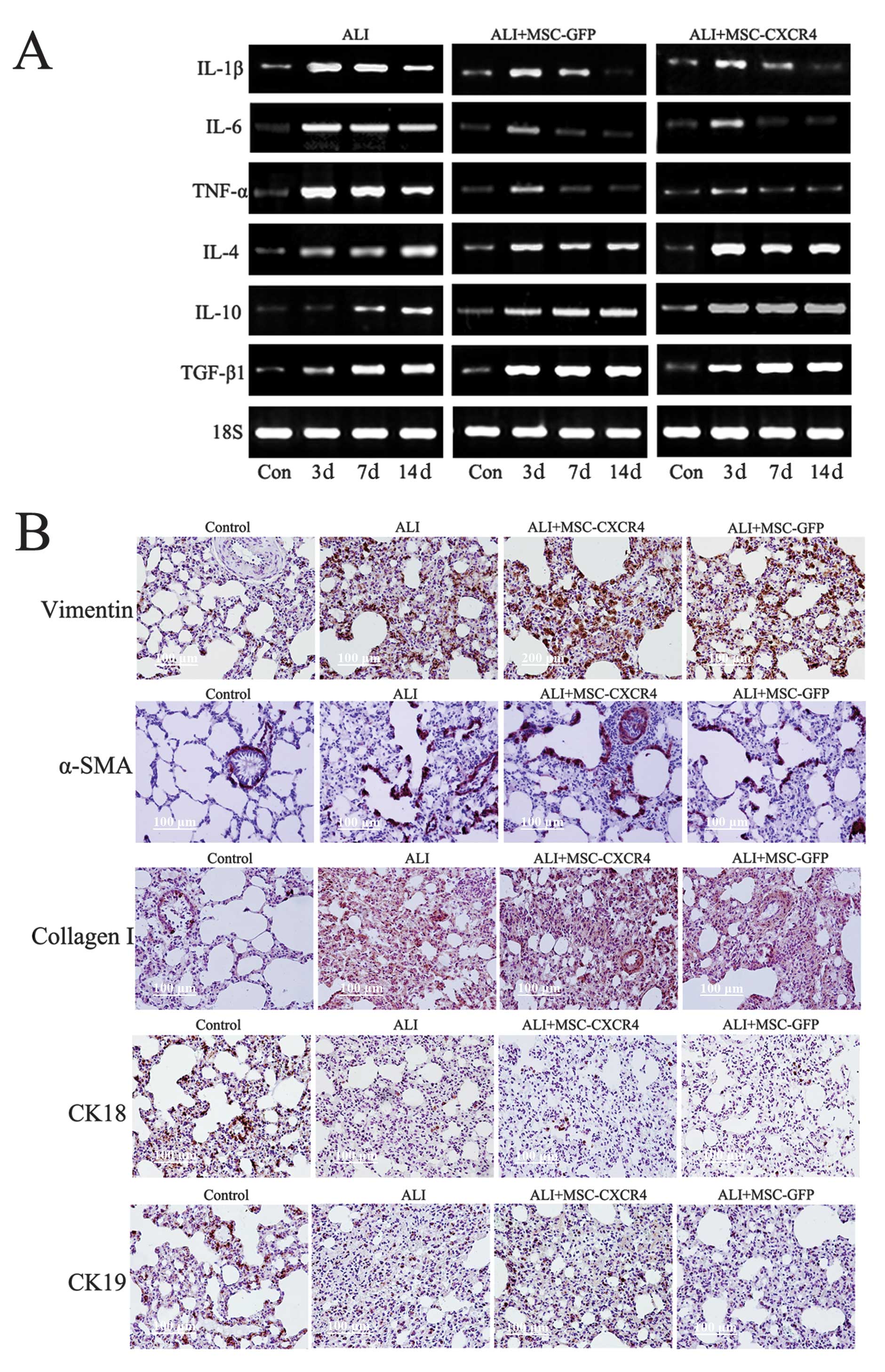
MSC transplantation reduced the
production of inflammatory cytokines
To determine the effects of transplantation of MSC
on the local inflammatory milieu in the lungs, we determined the
expression of several immune system-related cytokines in lung
tissue by RT-PCR (Fig. 5A). HCl
instillation resulted in increased production of the
pro-inflammatory cytokines TNF-α, IL-6 and IL-1β. By contrast, the
transplantation of MSCs (ALI+MSC-CXCR4 and ALI+MSC-GFP groups)
significantly decreased HCl-induced elevations of TNF-α, IL-6 and
IL-1β on 3, 7 and 14 days after ALI, but significantly increased
the expression of anti-inflammatory cytokines IL4, IL10 and TGF-β1.
The data indicated that MSCs was able to moderate the HCl-induced
lung inflammatory response.
MSC transplantation did not decrease the
expression of fibroblast markers
Based on immunohistochemical staining, we detected
the expression of fibroblast and epithelial markers in lung tissue
(Fig. 5B). After lung injury for
28 days, the expression of α-SMA, vimentin and collagen I was
clearly increased, whereas the expression of epithelial markers
CK18 and CK19 was decreased in the ALI group compared with the
control group. Four weeks after MSC transplantation (ALI+MSC-CXCR4
and ALI+MSC-GFP groups), no beneficial effects on lung injury and
pulmonary fibrosis were observed. Additionally, the content of
α-SMA, vimentin and collagen I in lung tissue was increased
compared with the controls. However, the expression of CK18 and
CK19 in lung tissue did not increase after MSC transplantation. The
immunohistochemical staining results indicated that MSC
transplantation did not reduce pulmonary fibrosis and attenuate
lung epithelium injury, but engraftment of MSCs was induced to
participate in pulmonary fibrogenesis by some cytokines or
signaling pathway at the injury sites.
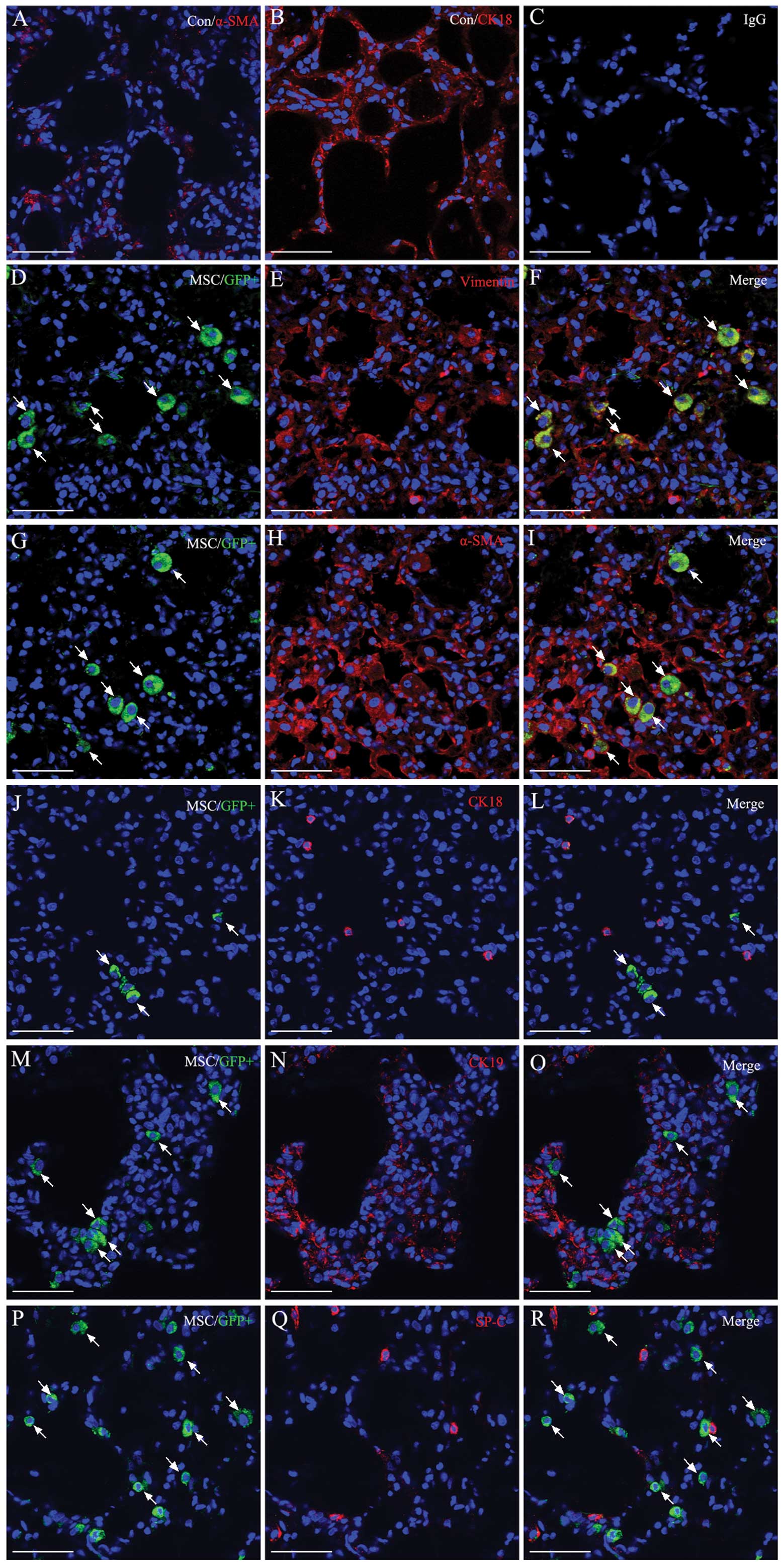
Detection of the MSC differentiation in
vivo
Although MSC transplantation decreased inflammatory
cytokine production, the beneficial effects of MSCs on lung injury
were not observed. We hypothesized that MSC differentiation is
important in lung repair or remodeling thereof. To investigate the
differentiation of engraftment MSCs in vivo, we used
immunofluorescent staining to detect the epithelial and fibroblast
marker expression of MSCs 14 days after MSCs transplantation in
ALI+MSC-CXCR4 group. We found that engraftment of MSCs
(GFP+ cells show in green) expressed myofibroblast
marker α-smooth muscle actin (α-SMA) and fibroblast marker vimentin
(Fig. 6A), but rarely expressed
epithelial markers CK18, CK19 and SP-C (Fig. 6B). These data suggested that MSCs
almost differentiated into lung fibroblasts or myofibroblasts, but
did not differentiate into lung epithelial cells. These fibroblasts
differentiated from exogenous MSCs may contribute to pulmonary
fibrogenesis. Some molecules or signaling pathway may be involved
in the regulation of MSCs differentiation at the injury sites.
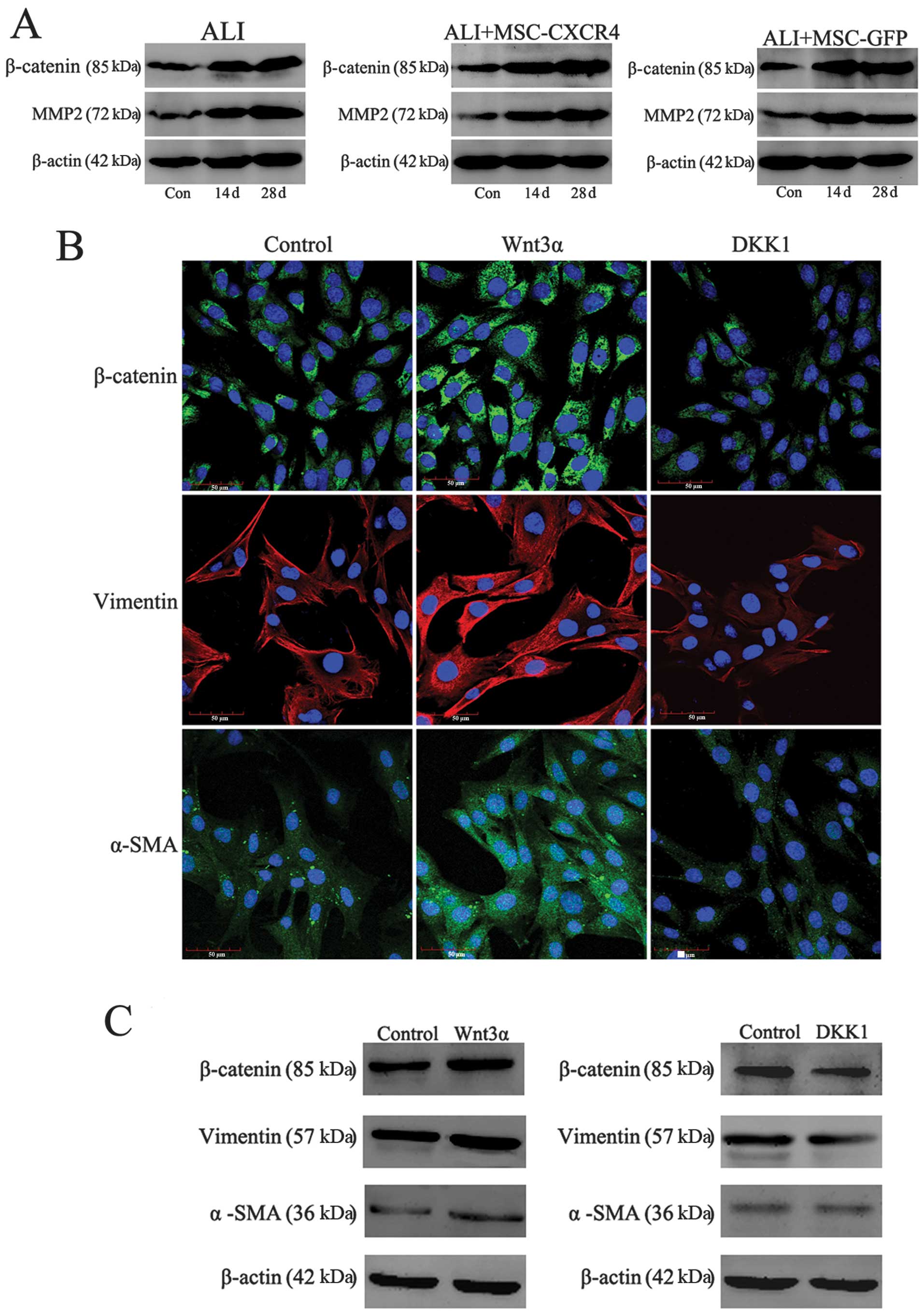
Wnt/β-catenin signaling regulates
myofibroblast differentiation of MSCs
Following transplantation, MSCs did not attenuate
lung injury and pulmonary fibrosis as we expected, however, MSCs
were involved in pulmonary fibrogenesis. We suspect that certain
factors or signaling pathway affected the differentiation process
of MSCs. It is necessary to explore the regulation mechanisms of
MSC differentiation in vivo. In a previous study, we
demonstrated that Wnt/β-catenin signaling regulates the MSC
differentiation in vitro (24). In a co-culture system, we
demonstrated that activation of Wnt signaling prevented the
epithelial differentiation of MSCs, but inhibition of Wnt signaling
promoted MSCs to differentiate into epithelial-like cells when MSCs
were co-cultured with epithelial cells. Wnt/β-catenin signaling is
also involved in regulating lung tissue remodeling, fibrosis or
destruction and lung diseases. Therefore, Wnt/β-catenin signaling
may regulate the differentiation of MSCs in lung tissue. In this
study, we detected the expression of β-catenin and MMP-2, which are
the essential components of canonical Wnt signaling. Western blot
analysis revealed that the protein expression of β-catenin and
MMP-2 was increased significantly in the ALI and ALI+MSC-CXCR4/GFP
groups compared with the control group, which demonstrated that Wnt
signaling is highly activated (Fig.
7A). It indicated that the abnormal activation of Wnt/β-catenin
signaling may induce engraftment of MSCs to differentiate into
myofibroblasts or fibroblasts and prevent the epithelial
differentiation of MSCs, resulting in tissue repair failure and
severe pulmonary fibrosis. The functional role of canonical Wnt
signaling in MSC differentiation in vitro was investigated.
Immunofluorescent staining and western blotting results revealed
that treatment of MSCs for 14 days with Wnt3α (100 ng/ml) resulted
in an increase in the protein expression of β-catenin, fibroblast
marker vimentin and myofibroblast marker α-SMA (Fig. 7B and C). Conversely, DKK1
decreased the expression levels of β-catenin, vimentin and α-SMA in
MSCs. The activation of Wnt/β-catenin signaling therefore induced
by Wnt3α ameliorated the possibility of MSCs to differentiate into
myofibroblasts. By contrast, the inhibition of Wnt/β-catenin
signaling caused by DKK1 prevented myofibroblast differentiation of
MSCs. It indicated that inhibition of the Wnt/β-catenin signaling
pathway following MSC transplantation suggests a positive unique
therapeutic approach for lung injury or pulmonary fibrosis.
Discussion
ALI is characterized by acute hypoxemic respiratory
failure, neutrophil accumulation in the lungs, interstitial edema,
disruption of epithelial and endothelial integrity and eventually
pulmonary fibrosis. Although most lung injury may be repaired by
locally derived progenitor cells, findings of previous studies
suggest that cells derived from bone marrow, especially MSCs, may
also repopulate the lung and repair the injured lung tissue.
Mesenchymal stem cell-based therapy is currently a promising and
novel treatment for lung injury (28,29). The ability of MSCs to engraft in
organs remotely from bone marrow suggests that exogenously
administered MSCs contribute to the repair of the injured alveolar
epithelium after lung injury. This finding is potentially of
significant clinical benefit for the regeneration of injured lung
tissue. However, key questions remain to be clarified, including
the best route of administration, the most favorable timing of cell
infusion, the types of cells differentiated from MSCs, whether
engraftment or differentiation requires enhancement of recruitment,
and the possible mechanism regulating the differentiation of MSCs.
The aim of the current study was to investigate the possible
involvement mechanism of MSCs in the treatment for ALI.
SDF-1 and its receptor CXCR4 are important mediators
of stem cell recruitment following tissue injury (30–32). Due to the therapeutic potential of
MSCs, the SDF-1/CXCR4 axis was used to improve MSC transplantation
levels at the injury sites. The results of the present study show
that SDF-1 levels in lung tissue and circulating blood increased
after HCl-induced lung injury, a finding that is consistent with
previous studies (18,33). After transfected MSC
administration, using flow cytometric and immunofluorescent
analyses, we confirmed that transplantation of MSCs overexpressing
CXCR4 specifically traffic to the injured lung which was induced by
the gradient SDF-1. The number of engraftment MSCs in the
ALI+MSC-CXCR4 group was more than that in the ALI+MSC-GFP and
control groups. Therefore, a high expression of SDF-1 at injury
sites and overexpressed CXCR4 on MSCs promoted more MSCs to engraft
into injured lung tissue sites. The SDF-1/CXCR4 axis promotes
exogenous MSCs to migrate and engraft into injured lung tissue.
Thus MSCs are crucial in lung remodeling. Based on this, we were
able to study clearly the mechanisms of injured lung epithelium
reparation following MSC transplantation.
Although the SDF-1/CXCR4 axis increased the number
of engraftment of MSCs, we found that engraftment of MSCs did not
ameliorate lung injury or pulmonary fibrosis. The amount of
collagen deposition in the MSC transplantation group was not
reduced by the administration of MSCs, but was as severe as in the
ALI group. The results of immunohistochemical staining indicated
that MSC transplantation did not ameliorate the epithelium injury
and repair epithelium integrity. However, the results demonstrated
that the hallmark of pulmonary fibrosis increased. Compared with
the control group, the expression of α-SMA, vimentin and collagen I
was increased in the lung tissue after lung injury and MSC
transplantation, suggesting pulmonary fibrogenesis. Circulating
exogenous MSCs may act as a significant source of lung fibroblasts
in response to lung injury. We hypothesized that in the fibrogenic
environment of the injured lung, the engraftment of MSCs did not
differentiate into lung epithelial cells, but may assume a
fibroblast phenotype and contribute to fibrogenesis.
Immunofluorescence results demonstrated that the engraftment of
MSCs rarely differentiated into lung epithelial cells, but almost
differentiated into myofibroblasts which contribute to pulmonary
fibrogenesis. This result may explain the reason for engraftment of
MSCs not ameliorating lung injury and fibrosis.
Recent studies have also shown that bone
marrow-derived circulating progenitor cells, including MSCs,
accumulate in the lung and contribute to pulmonary fibrosis after
lung injury (18,34–36). In other studies, however, a
protective rather than a pro-fibrotic effect of bone marrow-derived
MSCs has been reported (17,37–39). These contradictory data indicate
that the role of MSCs in the reparation or pathogenesis of
pulmonary fibrosis needs to be clarified. The differentiation
process of MSCs may be regulated by some cytokines and specific
signaling pathways at the injury sites. Although we found that MSC
transplantation decreased the pulmonary inflammatory process, the
pulmonary fibrosis was not completely prevented. This may be
because some of the fibrotic response signaling is due to
activation of fibroblasts already present in the lungs. Some
possible signaling pathways in injured lung may regulate the
differentiation of the exogenous MSCs, however, this should be
further investigated.
The Wnt/β-catenin signaling has been shown to be a
crucial mechanism in regulating embryonic development, cell
proliferation and motility, and cell fate determination (40,41). Wnt signaling is involved in the
regulation of the differentiation process of MSCs, including
adipogenesis, osteogenesis and myogenesis. Previously, we
demonstrated that Wnt signaling affected the epithelial
differentiation process of MSCs in a co-culture system (24). Wnt signaling pathway is also
involved in the regulation of tissue homeostasis, tissue damage and
remodeling, injury termination, tissue repair or destruction and
tissue diseases (42,43). Mounting evidence has suggested
that aberrant activation of Wnt signaling linked to the
pathogenesis of fibrotic lung disease, chronic obstructive
pulmonary disease, bleomycin-induced idiopathic pulmonary fibrosis
and dysregulated wound-healing response (20,44). Findings of those studies suggested
that Wnt/β-catenin signaling is relevant to the pathogenesis of
pulmonary fibrosis. In the present study, we detected the
expression of Wnt signaling in each group. We found that the
expression of β-catenin and MMP-2 in the ALI and MSC
transplantation groups was upregulated compared with the control
group. It indicated that Wnt signaling was highly activated in lung
tissue following lung injury. In addition, we have previously
demonstrated that the activation of Wnt/β-catenin prevented the
epithelial differentiation of MSCs co-cultured with epithelial
cells in vitro. However, the downregulation of Wnt/β-catenin
expression via Wnt antagonists DKK1 promoted MSCs to differentiate
into epithelial cells. In the present in vitro study, we
found that the activation of the Wnt/β-catenin signaling induced
the expression of vimentin and α-SMA in MSCs, suggesting that
activated Wnt signaling promoted MSCs to differentiate into
myofibroblasts. Therefore, Wnt signaling plays a critical role in
regulating engraftment of MSC differentiation following
transplantation in an HCl-induced lung injury model. The abnormal
activated Wnt signaling may promote myofibroblast differentiation
of MSCs to aggravate pulmonary fibrosis, however, it prevented
epithelial differentiation of MSCs, resulting in lung repair
failure. This study suggested that Wnt/β-catenin signaling may act
as a control point in the treatment for lung diseases, including
ALI and pulmonary fibrosis. Our in vitro study has
demonstrated that pharmacologic inhibition of the Wnt/β-catenin
signaling by DKK1 was able to prevent the myofibroblast
differentiation of MSCs. Additionally, aberrant activated
Wnt/β-catenin signaling is relevant to the pathogenesis of
pulmonary fibrosis via regulation of MSC differentiation after lung
injury.
In conclusion, we utilized a rat model of
HCl-induced acute lung injury to evaluate the positive effects of
MSCs in lung. The results showed that engraftment of MSCs may act
as a source of new fibroblasts to promote pulmonary fibrogenesis
under lung tissue fibrotic conditions. Our data provides evidence
that Wnt/β-catenin signaling plays a critical role in regulating
the differentiation of MSCs in vivo. Aberrant activated
Wnt/β-catenin signaling after lung injury induced the engraftment
of MSCs to differentiate into myofibroblasts to contribute to
pulmonary fibrogenesis. MSCs have the ability for self-renewal,
unlimited proliferation and multi-potential differentiation, making
them attractive candidates for tissue repair or malignant change.
The possibility of utilizing MSCs as a type of cellular therapy for
conditions such as ALI is crucial. Therefore the manner in which
in vivo environments affect MSCs remains to be determined.
Our findings suggest that Wnt signaling is an essential mechanism
of regulating MSC differentiation in vivo. This study has
shown that aberrant activated Wnt/β-catenin signaling is linked to
the pathogenesis of pulmonary fibrosis via induction of
myofibroblast differentiation of MSCs following lung injury.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81170054 and 31200401), Natural
Science Foundation of Jiangsu Province of China (BK2011570 and
BK2012307), the National Basic Research Program of China (973
program 2010CB945103), and Open Research Fund of State Key
Laboratory of Bioelectronics, Southeast University.
References
|
1
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matute-Bello G, Frevert CW and Martin TR:
Animal models of acute lung injury. Am J Physiol Lung Cell Mol
Physiol. 295:L379–L399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meduri GU: The role of the host defence
response in the progression and outcome of ARDS: pathophysiological
correlations and response to glucocorticoid treatment. Eur Respir
J. 9:2650–2670. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prockop DJ: Marrow stromal cells as stem
cells for continual renewal of nonhematopoietic tissues and as
potential vectors for gene therapy. J Cell Biochem Suppl.
30–31:284–285. 1998.PubMed/NCBI
|
|
5
|
Okamoto R, Yajima T, Yamazaki M, et al:
Damaged epithelia regenerated by bone marrow-derived cells in the
human gastrointestinal tract. Nat Med. 8:1011–1017. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumoto T, Okamoto R, Yajima T, et al:
Increase of bone marrow-derived secretory lineage epithelial cells
during regeneration in the human intestine. Gastroenterology.
128:1851–1867. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krause DS: Plasticity of marrow-derived
stem cells. Gene Ther. 9:754–758. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lapidot T, Dar A and Kollet O: How do stem
cells find their way home? Blood. 106:1901–1910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Son BR, Marquez-Curtis LA, Kucia M, et al:
Migration of bone marrow and cord blood mesenchymal stem cells in
vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte
growth factor-c-met axes and involves matrix metalloproteinases.
Stem Cells. 24:1254–1264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liebler JM, Lutzko C, Banfalvi A, et al:
Retention of human bone marrow-derived cells in murine lungs
following bleomycin-induced lung injury. Am J Physiol Lung Cell Mol
Physiol. 295:L285–L292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lapidot T: Mechanism of human stem cell
migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice.
The role of SDF-1/CXCR4 interactions. Ann N Y Acad Sci. 938:83–95.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Askari AT, Unzek S, Popovic ZB, et al:
Effect of stromal-cell-derived factor 1 on stem-cell homing and
tissue regeneration in ischaemic cardiomyopathy. Lancet.
362:697–703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji JF, He BP, Dheen ST and Tay SS:
Interactions of chemokines and chemokine receptors mediate the
migration of mesenchymal stem cells to the impaired site in the
brain after hypoglossal nerve injury. Stem Cells. 22:415–427. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kollet O, Shivtiel S, Chen YQ, et al: HGF,
SDF-1, and MMP-9 are involved in stress-induced human
CD34+stem cell recruitment to the liver. J Clin Invest.
112:160–169. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hashimoto N, Jin H, Liu T, Chensue SW and
Phan SH: Bone marrow-derived progenitor cells in pulmonary
fibrosis. J Clin Invest. 113:243–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rojas M, Xu J, Woods CR, et al: Bone
marrow-derived mesenchymal stem cells in repair of the injured
lung. Am J Respir Cell Mol Biol. 33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu J, Mora A, Shim H, Stecenko A, Brigham
KL and Rojas M: Role of the SDF-1/CXCR4 axis in the pathogenesis of
lung injury and fibrosis. Am J Respir Cell Mol Biol. 37:291–299.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheon SS, Nadesan P, Poon R and Alman BA:
Growth factors regulate β-catenin-mediated TCF-dependent
transcriptional activation in fibroblasts during the proliferative
phase of wound healing. Exp Cell Res. 293:267–274. 2004.
|
|
20
|
Chilosi M, Poletti V, Zamò A, et al:
Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary
fibrosis. Am J Pathol. 162:1495–1502. 2003.
|
|
21
|
Pongracz JE and Stockley RA: Wnt
signalling in lung development and diseases. Respir Res. 7:152006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Königshoff M, Balsara N, Pfaff EM, et al:
Functional Wnt signaling is increased in idiopathic pulmonary
fibrosis. PLoS One. 3:e21422008.PubMed/NCBI
|
|
23
|
Uematsu K, He B, You L, Xu Z, McCormick F
and Jablons DM: Activation of the Wnt pathway in non small cell
lung cancer: evidence of dishevelled overexpression. Oncogene.
22:7218–7221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Sun Z, Qiu X, Li Y, Qin J and Han
X: Roles of Wnt/β-catenin signaling in epithelial differentiation
of mesenchymal stem cells. Biochem Biophys Res Commun.
390:1309–1314. 2009.
|
|
25
|
Sun Z, Wang Y, Gong X, Su H and Han X:
Secretion of rat tracheal epithelial cells induces mesenchymal stem
cells to differentiate into epithelial cells. Cell Biol Int.
36:169–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
White ES, Atrasz RG, Hu B, et al: Negative
regulation of myofibroblast differentiation by PTEN (Phosphatase
and Tensin Homolog Deleted on chromosome 10). Am J Respir Crit Care
Med. 173:112–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiu X, Lin H, Wang Y, et al:
Intracavernous transplantation of bone marrow-derived mesenchymal
stem cells restores erectile function of streptozocin-induced
diabetic rats. J Sex Med. 8:427–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cargnoni A, Gibelli L, Tosini A, et al:
Transplantation of allogeneic and xenogeneic placenta-derived cells
reduces bleomycin-induced lung fibrosis. Cell Transplant.
18:405–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang YS, Oh W, Choi SJ, et al: Human
umbilical cord blood-derived mesenchymal stem cells attenuate
hyperoxia-induced lung injury in neonatal rats. Cell Transplant.
18:869–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galvez BG, Sampaolesi M, Brunelli S, et
al: Complete repair of dystrophic skeletal muscle by
mesoangioblasts with enhanced migration ability. J Cell Biol.
174:231–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Romagnani P, Lasagni L, Mazzinghi B,
Lazzeri E and Romagnani S: Pharmacological modulation of stem cell
function. Curr Med Chem. 14:1129–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ratajczak MZ, Zuba-Surma E, Kucia M, Reca
R, Wojakowski W and Ratajczak J: The pleiotropic effects of the
SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis.
Leukemia. 20:1915–1924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song JS, Kang CM, Kang HH, Yoon HK, Kim
YK, Kim KH, Moon HS and Park SH: Inhibitory effect of CXC chemokine
receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary
fibrosis. Exp Mol Med. 42:465–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mehrad B, Burdick MD and Strieter RM:
Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary
fibrosis. Int J Biochem Cell Biol. 41:1708–1718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Phillips RJ, Burdick MD, Hong K, et al:
Circulating fibrocytes traffic to the lungs in response to CXCL12
and mediate fibrosis. J Clin Invest. 114:438–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Antoniou KM, Papadaki HA, Soufla G, et al:
Investigation of bone marrow mesenchymal stem cells (BM MSCs)
involvement in Idiopathic Pulmonary Fibrosis (IPF). Respir Med.
104:1535–1542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ortiz LA, Gambelli F, McBride C, et al:
Mesenchymal stem cell engraftment in lung is enhanced in response
to bleomycin exposure and ameliorates its fibrotic effects. Proc
Natl Acad Sci USA. 100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fine A: Marrow cells as progenitors of
lung tissue. Blood Cells Mol Dis. 32:95–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kotton DN, Summer R and Fine A: Lung stem
cells: new paradigms. Exp Hematol. 32:340–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ling L, Nurcombe V and Cool SM: Wnt
signaling controls the fate of mesenchymal stem cells. Gene.
433:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pinto D and Clevers H: Wnt control of stem
cells and differentiation in the intestinal epithelium. Exp Cell
Res. 306:357–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Van Amerongen R and Berns A: Knockout
mouse models to study Wnt signal transduction. Trends Genet.
22:678–689. 2006.PubMed/NCBI
|
|
44
|
Scotton CJ and Chambers RC: Molecular
targets in pulmonary fibrosis: the myofibroblast in focus. Chest.
132:1311–1321. 2007. View Article : Google Scholar : PubMed/NCBI
|