Introduction
Atopic dermatitis (AD) is a common pruritic
inflammatory skin disorder caused by the excessive activation of
certain white blood cells and basophils due to IgE production in
response to environmental triggers (1,2).
AD is diagnosed based on eczematous skin lesions, which present as
skin erythematous, plaques, eruption and lichenification, as well
as give rise to asthma, allergic rhinitis, administration of food
allergies, and contact dermatitis (3–5).
Following pathological examination of AD patients, acute lesions
show hyperkeratiosis, spongiosis and parakeratosis, while chronic
lesions present as epidermal hyperplasia, acanthosis and
accumulation of lymphocytes and mast cells in skin tissue (5,6).
Traditional methods have been used to screen
allergens among substances in the surrounding environment, although
novel methods for screening of allergens have recently received
attention owing to their increased presence of environmental
allergens. The allergy skin prick test, a well-known traditional
method, is an inexpensive, rapid and accurate method measuring
allergen response through the use of a few drops of isolated
allergen gently pricked onto the surface of the forearm skin to
identify causative allergens (7).
The allergy-specific IgE antibody test screens allergens by
measuring the amounts of IgE antibody for suspected allergens in
blood samples (8,9). However, these methods are limited as
they do not accurately quantify the magnitude of the allergic
response. IL-4/Luc/CNS-1 transgenic (Tg) mice were recently
produced by microinjection with the luciferase gene under control
of the human IL-4 promoter and the IL-4 enhancer (CNS-1) to
overcome these limitations (10).
Three types of allergens were successfully quantified in
vivo in these mice, a respiratory sensitizer, vaccine
additives, and crude extracts of natural allergens (10). The therapeutic effects of aqueous
extract of Liriope platyphylla (AEtLP) on AD were also
successfully evaluated using IL-4/Luc/CNS-1 Tg mice (11). Therefore, evaluation of novel
substances for their ability to induce AD and the effects of
allergens can be conducted using IL-4/Luc/CNS-1 Tg mice.
Cheonggukjang (CKJ) is a fermented product
manufactured from soybean, usually by fermentation with Bacillus
subtilis (B. subtilis) (12). During fermentation of CKJ,
flavonoid glycosides are converted into aglycones by hydrolysis,
and many proteins are degraded into small peptides and amino acids
(13,14). CKJ contains many enzymes,
microorganisms, and bioactive compounds that are absent from
unfermented soybean (15).
Additionally, CKJ has various physiological activities caused by
antioxidant substances, fibrinolytic enzymes and many active
compounds including isoflavones, unsaturated fatty acids, dietary
fiber and oligosaccharides (16).
CKJ exhibits diverse biological and pharmacological
activities, including anti-mutagenic, anti-obesity and
anti-diabetic effects, as well as anti-inflammatory action,
fibrinolytic activity and thrombolytic effects on human chronic
diseases (17–20). However, few studies have reported
the potential efficacy of fermented soybean product or CKJ extract
on AD in humans. Using NC/Tnd mice as an AD model, the ferment soy
product, ImmuBalance, was found to reduce AD symptoms including
clinical skin severity score, scratching behavior, trans-epidermal
water loss (TEWL) value, and PGP9.5-positive neuronal fibers
(21). Moreover, the
administration of poly-γ-glutamic acid (γ-PGA), one of the
components of CKJ, significantly decreased the clinical skin
severity score, IgE and IgG1 concentration, epidermal thickness,
mast cell infiltration and CCR3+ cell number in NC/Nga
mice with BMAC-induced AD (22).
Furthermore, the anti-inflammatory activity of the ethanol extract
of CKJ was examined in an animal model with passive cutaneous
anaphylaxis and arachidonic acid-induced ear edema. Following oral
administration of this extract for 5 days, passive cutaneous
anaphylaxis was significantly reduced by 27.3% in a rat group
treated with 400 mg/kg/day (23).
However, no studies conducted thus far have used IL-4/Luc/CNS-1 Tg
mice with phthalic anhydride (PA)-induced AD as a model to quantify
the therapeutic effects of fermented soybean products.
Therefore, the present study was conducted to
investigate the use of IL-4/Luc/CNS-1 Tg mice with PA-induced AD to
investigate the effects of fermented soybean.
Materials and methods
Design of animal experiment
The animal protocol used in this study was reviewed
and approved based on the ethical and scientific care procedures of
the Pusan National University-Institutional Animal Care and Use
Committee (PNU-2013–0378). All the animals were handled in the
Pusan National University-Laboratory Animal Resources Center
accredited by AAALAC International in accordance with the USA NIH
guidelines (accredited unit no. 001525) and the Korean Food and
Drug Administration (FAD) in accordance with the Laboratory Animals
Act (accredited unit no. 00231). The mice were housed under
specific pathogen-free (SPF) conditions and a strict light cycle
(lights on at 08:00 and off at 20:00) at a temperature of 22±2°C
and 50±10% relative humidity and were provided with standard
irradiated chow diet (Purina Mills Inc., Seongnam, Korea) ad
libitum.
Nine-week-old IL-4/Luc/CNS-1 Tg mice (n=20) were
randomly divided into four groups, with five mice per group. The
first group of Tg mice [acetone-olive oil (AOO), n=5] had 100 ml of
AOO repeatedly spread on the dorsum of their ears three times a
week for 4 weeks. In the second group (PA, n=10), 100 ml of 15% PA
solution in AOO (4:1, v/v) was repeatedly spread on the dorsum of
the ears three times a week for 4 weeks. The second group was
further divided into the PA + Vehicle and PA + CKJ treatment
groups, which received a comparable volume of water daily via oral
administration and 50 mg/kg body weight of CKJ for 4 weeks,
respectively. Age-matched Tg mice (n=5) were used as a no treatment
group.
IL-4/Luc/CNS-1 Tg mice
IL-4/Luc/CNS-1 Tg mice used in this study were
obtained from the National Institute of Food and Drug Safety
Evaluation of the Korean FDA (Osong, Korea). Large numbers of
IL-4/Luc/CNS-1 Tg mice and non-Tg littermates were produced by
mating IL-4/Luc/CNS-1 Tg mice and HR1 mice. Founder mice, into
which the IL-4/Luc/CNS-1 transgene was inserted, were
identified by PCR analysis of tail-derived genomic DNA. For PCR, 10
pmol each of sense (5′-CTC GCA TGC CAG AGA TCC TA-3′) and antisense
(5′-CCA CAA CCT TCG CTT CAA AA-3′) primers were added into a
mixture containing genomic DNA template, and the reaction mixtures
were subjected to 35 cycles of amplification (1 min at 94°C; 1 min
at 56°C and 1 min at 72°C) using a Perkin-Elmer thermal cycler
(PerkinElmer, Waltham, MA, USA). The amplified PCR products were
separated by 1% agarose gel electrophoresis and band patterns were
detected using a Kodak Electrophoresis Documentation and Analysis
System 120 (Eastman Kodak, Rochester, NY, USA). IL-4/Luc/CNS-1 Tg
mice were screened from founder mice through the detection of PCR
products with 467 bp.
Preparation of CKJ extracts
CKJ extract was prepared as previously described
(24,25). The soybean (Daepung strain) used
to manufacture CKJ was kindly supplied by the National Institute of
Crop Science (Miryang, Korea) while B. subtilis MC31 was
obtained from the Food Microbiology Laboratory at Pusan National
University. To manufacture the CKJ extract, 15 g of soybeans were
washed and then soaked in three volumes of tap water at room
temperature for 24 h. The soybeans were then treated with hot steam
at 121°C for 50 min, after which they were allowed to cool to 45°C.
The steamed soybeans were inoculated with 2% (w/w) B.
subtilis MC31 and fermented for 72 h at 40°C. The fermented
soybeans were then powdered by freeze-drying, homogenization and
sifting. The final sample of CKJ extract was stored at −75°C until
use.
Analysis of GABA concentration
GABA concentration was measured in a
spectrophotometric assay containing GABase enzyme (Sigma-Aldrich,
St. Louis, MO, USA) using the method described by Zhang and Bown
(26). Briefly, the supernatant
of the CKJ extract was collected from the lyophilized powder of CKJ
(0.3 g) that had been soaked in 99% ethanol (1.2 ml) for 5 h. This
supernatant (0.1 ml) was then mixed with 0.4 ml of MeOH and
completely dried at 70–80°C for 30 min. Then, 70 mM
LaCl3 (1 ml) was added and the mixture was agitated for
10 min, centrifuged at 9,800 × g for 5 min. The supernatant (0.8
ml) was then mixed with 0.1 M KOH solution (0.16 ml) for 3–5 min,
after which it was purified by centrifugation and filtration. This
solution (0.55 ml) of CKJ was then dispensed into individual
cuvettes, each of which contained 0.2 ml of 0.5 mM
K4P2O7 buffer (pH 8.6), 0.15 ml of
4 mM NADP and 0.05 ml of GABase (2 U/ml). The initial absorbance
was then read at 340 nm using a spectrophotometer (Optizen POP;
Mecasys Co., Ltd., Daejeon, Korea), after which 0.05 ml of 20 mM
α-ketoglutarate was added and the samples were incubated for 60 min
at room temperature. The absorbance was read at the same
wavelength. The final concentration of GABA was then calculated by
comparing the differences of the two absorbances and by comparison
with a standard curve.
High-performance liquid chromatography
(HPLC) analysis of CKJ
To determine the concentration of diadzein and
genistein in CKJ, the aqueous extract of CKJ was dissolved in 100
mg/ml of 50% MeOH and agitated at 200 rpm for 4 h. Following
incubation for 12 h at room temperature, the sample was centrifuged
at 3,000 rpm, after which the supernatant was harvested, diluted to
25 mg/ml in 50% MeOH and passed through a syringe filter (0.45
mm).
The CKJ was analyzed using an iLC 3000 HPLC system
(Interface Engineering Co., Ltd., Seoul, Korea) equipped with a
Corona® CAD® Detector (ESA Bioscience, Inc.,
Chelmsford, MA, USA). Chromatographic separation was performed
using a YMC-triart C18 column (4.6×250 mm, particle size 5 μm;
Shiseido Co., Ltd., Tokyo, Japan). The mobile phase consisted of
solvent A (0.1% formic acid in deionized water) and solvent B
(acetonitrile) using the following gradient elution program: 0–30
min, 20–40% of solvent B and 30–45 min, 40–70% of solvent B. A flow
rate of 1.0 ml/min was used for sample analysis and the nebulizer
gas was nitrogen. The gas flow rate and gas pressure were
maintained at 1.53 l/min and 35±2 psi, respectively. The output
signal of the detector was recorded using the Clarity™
Chromatography Software (DataApex, Prague, Czech Republic).
Measurement of body weight, organ weight
and ear thickness
Alterations of body weight during the experimental
procedure were measured using an electronic balance (Mettler
Toledo-International, Inc., Greifensee, Switzerland) three times a
week for 4 weeks. After final administration, the weights of the
spleen, thymus, auricular lymph node (ALN) collected from the
sacrificed mice were also measured by the same method. Ear
thickness was measured using a thickness gauge (Digimatic
Indicator; Matusutoyo Co., Tokyo, Japan) to determine the degree of
allergic skin inflammation induced by PA treatment.
Analysis of the bioluminescence
image
In vivo imaging was conducted using an IVIS
imaging system (Xenogen Corp., Alameda, CA, USA) as previously
described (11). Briefly,
IL-4/Luc/CNS-1 Tg mice were anesthetized with Zoletil and injected
i.p. with 150 mg/kg of D-luciferin (Sigma-Aldrich). Ten minutes
after the D-luciferin injection, images of mice were captured for 3
min using an IVIS imaging system. Photons emitted from specific
regions were then quantified using the Living Image software
(Xenogen Corp.). In vivo luciferase activity was expressed
in photons per second.
Enzyme-linked immunosorbent assay (ELISA)
for the detection of serum IgE concentration
The serum IgE concentration was measured using an
ELISA kit (Shibayagi, Co., Ltd., Gunma, Japan) according to the
manufacturer’s instructions. Briefly, capture antibodies were
plated in the Nunc C bottom immunoplate supplied in the kit. The
wells were then washed with washing solution (50 mM Tris, 0.14 M
NaCl, 0.05% Tween-20, pH 8.0) three times, after which serum
samples and standards diluted with buffer solution were added to
the wells, and the plate was incubated for 2 h. The wells were then
washed again with washing solution, after which 50 μl of
biotin-conjugated anti-IgE antibodies (1,000-fold dilution) were
added to each well and the samples were incubated for another 2 h
to bind with captured IgE. The wells were then washed again with
washing solution, after which horseradish peroxidase-conjugated
detection antibodies (2,000-fold dilution) were added to each well
and samples were incubated for 1 h. An enzyme reaction was then
initiated by adding tetramethylbenzidine (TMB) substrate solution
(100 mM sodium acetate buffer pH 6.0, 0.006%
H2O2) and the samples was incubated at room
temperature in the dark for 20 min. The reaction was terminated by
adding acidic solution (reaction stopper, 1 M
H2SO4), and the absorbance of yellow product
was measured spectrophotometrically at 450 nm. The final
concentration of IgE was calculated using a standard curve.
Histological analysis of ear tissue
Ear tissues were removed from mice, fixed with 10%
formalin, embedded in paraffin wax, routinely processed, and
sectioned into 4 μm slices. The ear tissue sections were then
stained with hematoxylin and eosin, after which they were examined
by light microscopy (Leica Microsystems, Heerbrugg, Switzerland)
for the presence of edema and inflammatory cell accumulation.
Thickness levels of the epidermis and dermis and the number of
crypts were also measured using the Leica Application Suite (Leica
Microsystems).
Mast cells were detected by staining with Toluidine
blue (Sigma-Aldrich) according to previously described methods
(27). Subsequent to
deparaffinization and dehydration, ear tissue sections were stained
with 0.25% Toluidine blue (Sigma-Aldrich) and examined by light
microscopy for the presence of mast cells. The number of cells per
specific area was measured with Leica Application Suite (Leica
Microsystems).
Western blot analysis
Ear tissues from a subset of the groups (n=5 per
group) were homogenized using a PRO-PREP™ Solution kit (Intron
Biotechnology, Seongnam, Korea) supplemented with 1/2 of a protein
inhibitor cocktail tablet (Roche Diagnostics GmbH, Penzberg,
Germany), followed by centrifugation at 13,000 rpm for 5 min. The
prepared proteins were then electrophoresed through a 10% SDS-PAGE
gel. The proteins were transferred onto a nitrocellulose membrane
(Amersham Biosciences, Corston, UK) for 2 h at 40 V in transfer
buffer (25 mM Trizma-base, 192 mM glycine and 20% methanol). The
efficiency of the transfer and equal protein loading were
determined by staining the gel with Coomassie Blue (Sigma-Aldrich).
Appropriate dilutions of primary antibodies, anti-IL-6 antibody
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-VEGF
antibody (Peprotech, Inc., Rocky Hill, NJ, USA), and anti-β-actin
(Sigma-Aldrich) were added to the membranes and allowed to
hybridize overnight at 4°C. After the antibodies were removed, the
membranes were washed three times in a solution composed of 10 mM
Trizma-base (pH 7.6), 150 mM NaCl, and 0.05% Tween-20 for 10 min.
This was followed by incubation with horseradish
peroxidase-conjugated anti-secondary antibody for 1 h at room
temperature. The membrane was washed again as described above and
developed using an enhanced chemiluminescence detection system
(Amersham Bioscience). The results were then quantified using the
Image Analyzer System (2000MM; Estman Kodak) and expressed as the
fold-increase over control values. Results were confirmed by two
independent investigators who performed the experiments at least
twice.
Statistical analysis
One-way ANOVA was used to identify significant
differences between the PA- and AOO-treated groups (SPSS for
Windows, Release 10.10, Standard Version; SPSS, Inc., Chicago, IL,
USA). Additionally, response differences between the Vehicle- and
CKJ-treated group in the PA-treated group were evaluated by a post
hoc test (SPSS for Windows, Release 10.10, Standard Version; SPSS,
Inc.) of the variance and significance levels. All the values are
expressed as the means ± standard deviation (SD). P<0.05 was
considered to indicate statistical significance.
Results
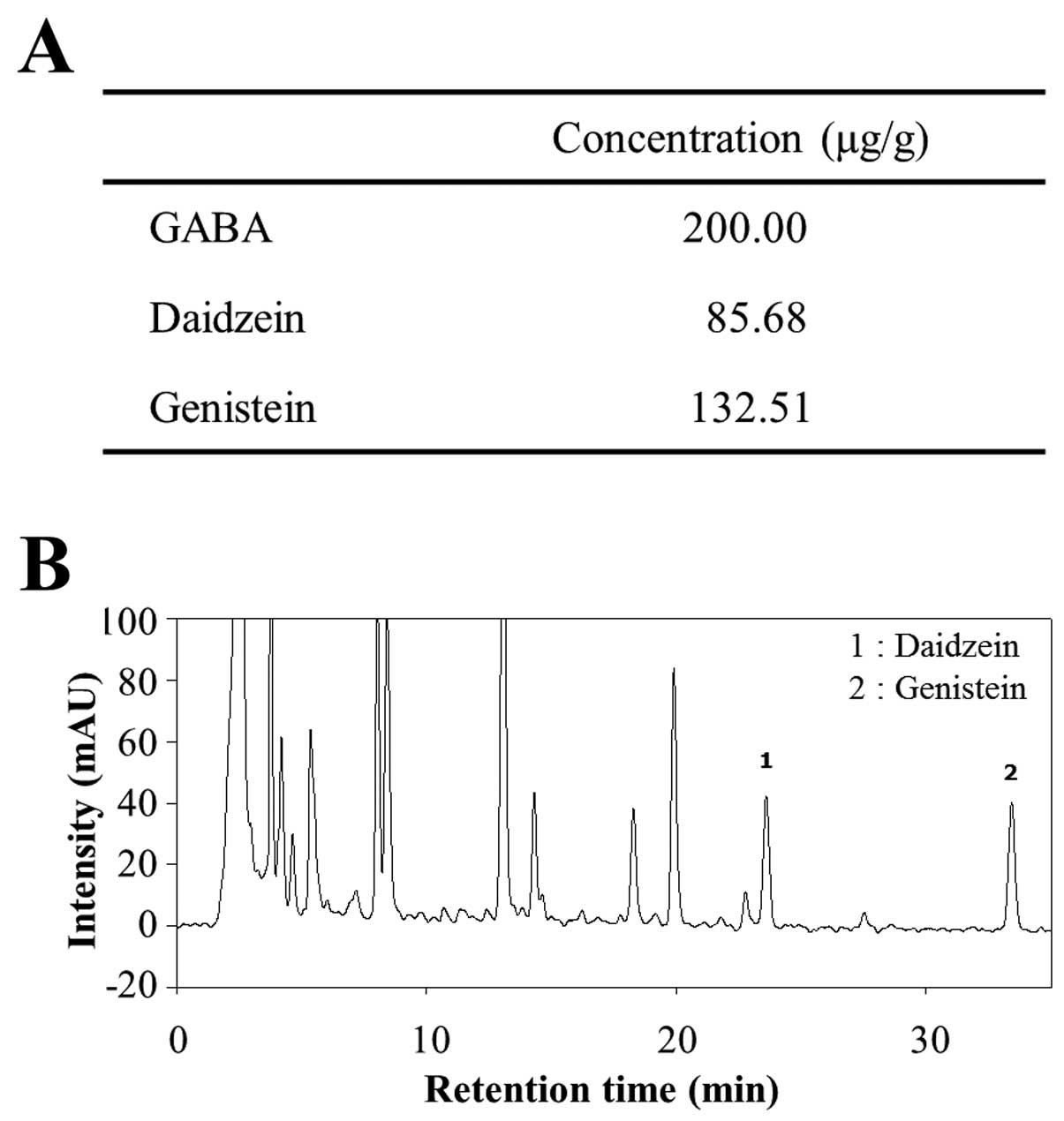
Distribution of key components in
CKJ
The distribution of three functional compounds in
CKJ was analyzed by enzyme assay and HPLC analysis. GABA was found
to be present at 200.00 μg/g, while the levels of the flavonoids
daidzein and genistein were 85.68 and 132.51 μg/g, respectively
(Fig. 1). Therefore, above
results indicated that CKJ extract contained high concentrations of
GABA although the concentration of flavonoids was slightly low.
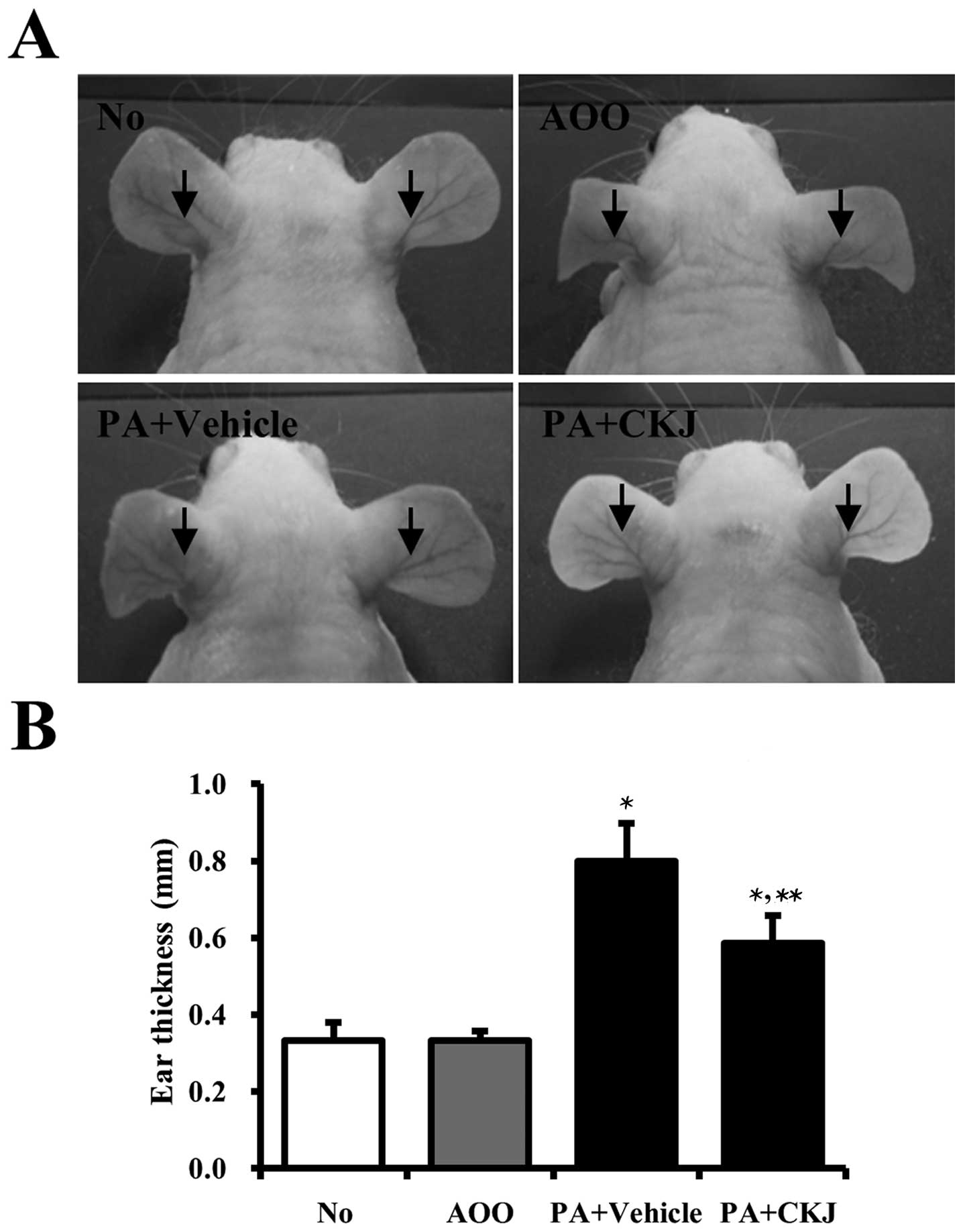
Effects of CKJ-treatment on ear
morphology and thickness
To determine whether CKJ treatment suppressed
changes in ear phenotype induced by PA treatment, ear morphology
and thickness were observed in IL-4/Luc/CNS-1 Tg mice after CKJ
treatment for 4 weeks. The outline of the ear vein became distinct
or thickened in the PA + Vehicle-treated group compared to the
AOO-treated group, and ear color changed from flesh tint to dark
brown. These alterations were slightly reversed in the PA + CKJ
co-treated group (Fig. 2B). In
addition, ear thickness rapidly increased in PA + Vehicle-treated
mice compared to non- or AOO-treated mice. However, these levels in
PA + CKJ-treated mice were significantly lower than those in the PA
+ Vehicle-treated group (Fig.
2C). Therefore, these findings demonstrated that CKJ treatment
successfully decreases ear and vein thickness induced by
PA-treatment.
Quantification of the therapeutic effects
of CKJ treatment in IL-4/Luc/CNS-1 Tg mice treated with PA
To quantify the therapeutic effects of CKJ on the
allergenic response to PA treatment using the luciferase reporter
system, luciferase signals from the whole body and eight organs
were measured using the Living Image software in IL-4/Luc/CNS-1 Tg
mice after individual PA + Vehicle or PA + CKJ treatment. In the
whole body image, the luciferase signal was highly detected in the
abdominal region of IL-4/Luc/CNS-1 Tg mice treated with PA +
Vehicle, whereas the no treatment or AOO-treated group showed no
luciferase signal. However, this signal was greatly reduced in
groups treated with PA + CKJ (Fig.
3A). Following organ image analysis, a high luciferase signal
was detected in the thymus of IL-4/Luc/CNS-1 Tg mice treated with
PA + Vehicle, however, the signal was markedly reduced in the
thymus of IL-4/Luc/CNS-1 Tg mice treated with PA + CKJ (Fig. 3B). Overall, these results
indicated that the effects of CKJ on the allergic response induced
by PA treatment could be quantified using IL-4/Luc/CNS-1 Tg mice
without sacrificing the animals.
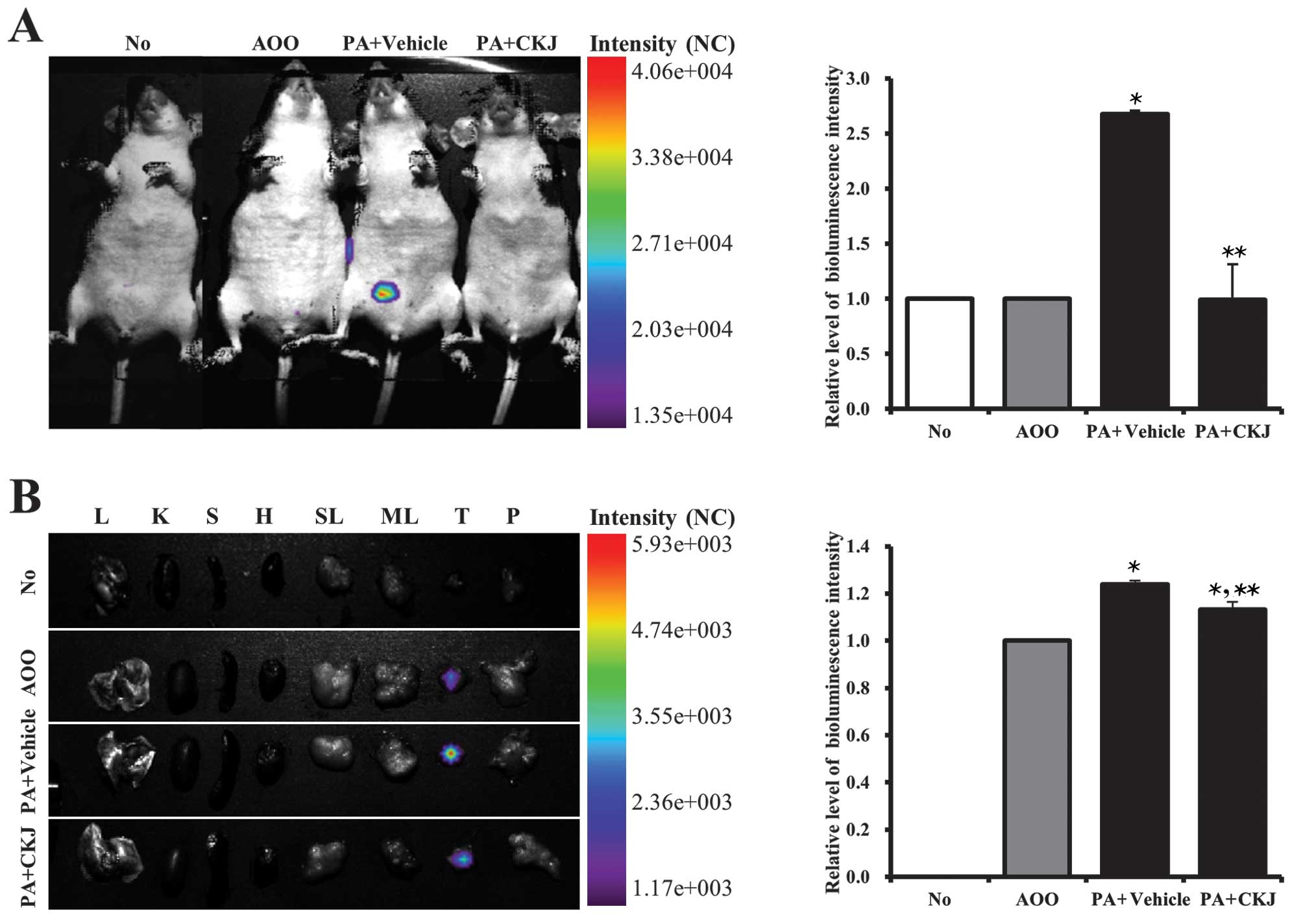 | Figure 3(A) Detection of luciferase signals
in the whole body and (B) each organ of IL-4/Luc/CNS-1 transgenic
(Tg) mice. Mice were treated with acetone-olive oil (AOO), phthalic
anhydride (PA) + Vehicle and PA + cheonggukjang (CKJ) for 4 weeks
and then imaged at 24 h after final treatment using the Living
Image software. Color overlay on the image represents the photons
per second emitted from the organs in accordance with the
pseudocolor scale shown next to the image. In this image, red
indicates the highest number of photons per second, while blue
indicates the lowest number of photons per second. L, lung; K,
kidney; S, spleen; H, heart; SL, submandibular lymph node; ML,
mesenteric lymph node; T, thymus; P, pancreas. Data shown are the
means ± standard deviation (SD) (n=5). *P<0.05
indicates a significant difference compared to the AOO-treated
group. **P<0.05 indicates a significant difference
compared to the PA + Vehicle-treated group. |
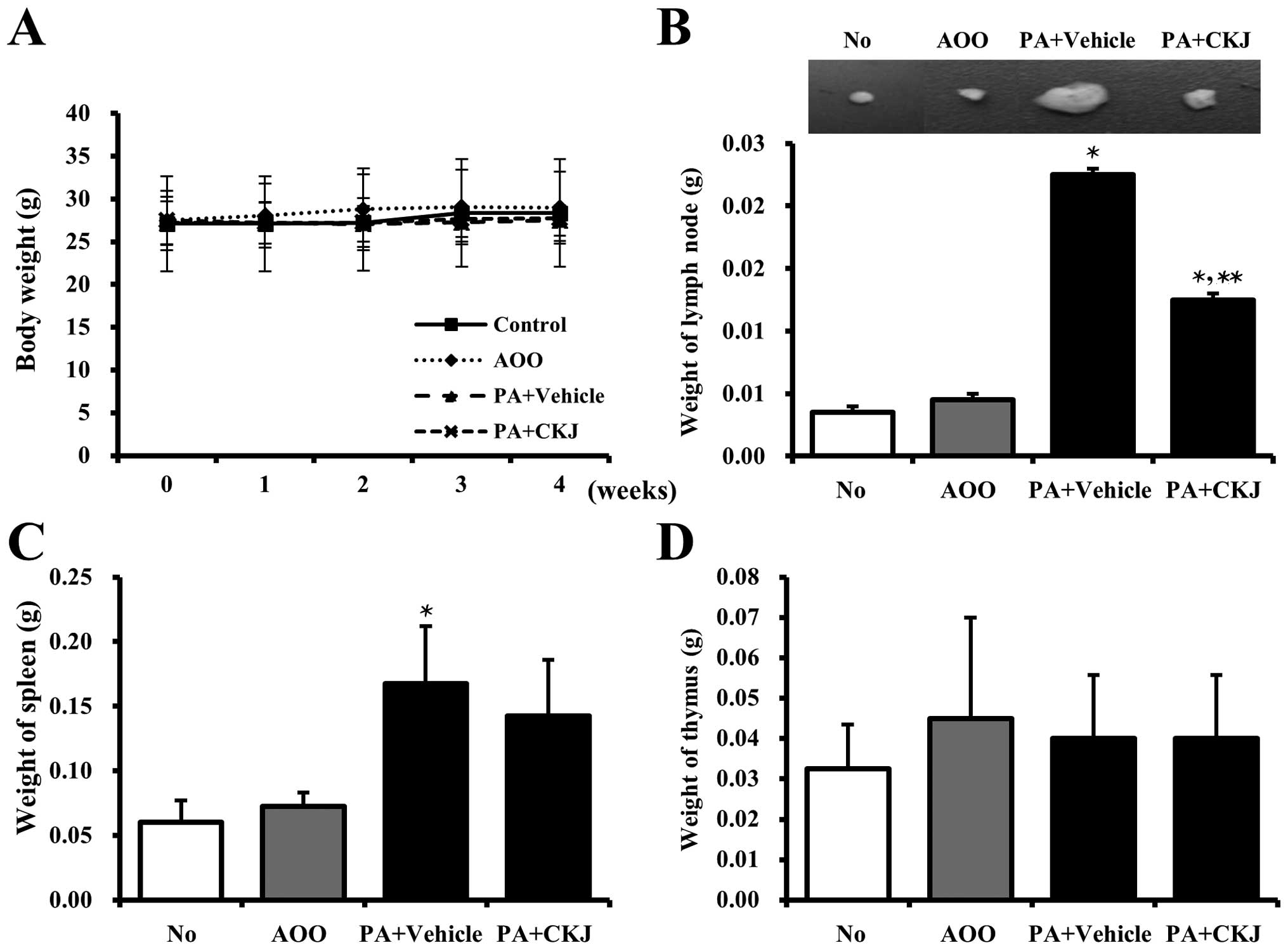
Alteration of body and organ weight
Alterations in body weight were measured during all
experimental periods to examine the effects of CKJ treatment on
whole body growth. However, no significant changes in body weight
were in observed in any of the groups (Fig. 4A).
It is well known that the weight of some immune
organs increase in response to the topical application of agents
that have allergenic or sensitizing potential (28,29). As shown in Fig. 4B and C, PA treatment induced an
increase in the weight of the spleen and lymph node in
IL-4/Luc/CNS-1 Tg mice compared to the AOO-treated group. However,
their weights were significantly reduced in the PA + CKJ-treated
group, although not to the level of the AOO-treated group. The
weight of the thymus was maintained in the PA + Vehicle and PA +
CKJ-treated group (Fig. 4D).
Taken together, these results suggested that CKJ treatment
contributes to the reduction of spleen and lymph node weight
observed in IL-4/Luc/CNS-1 Tg mice in response to PA treatment.
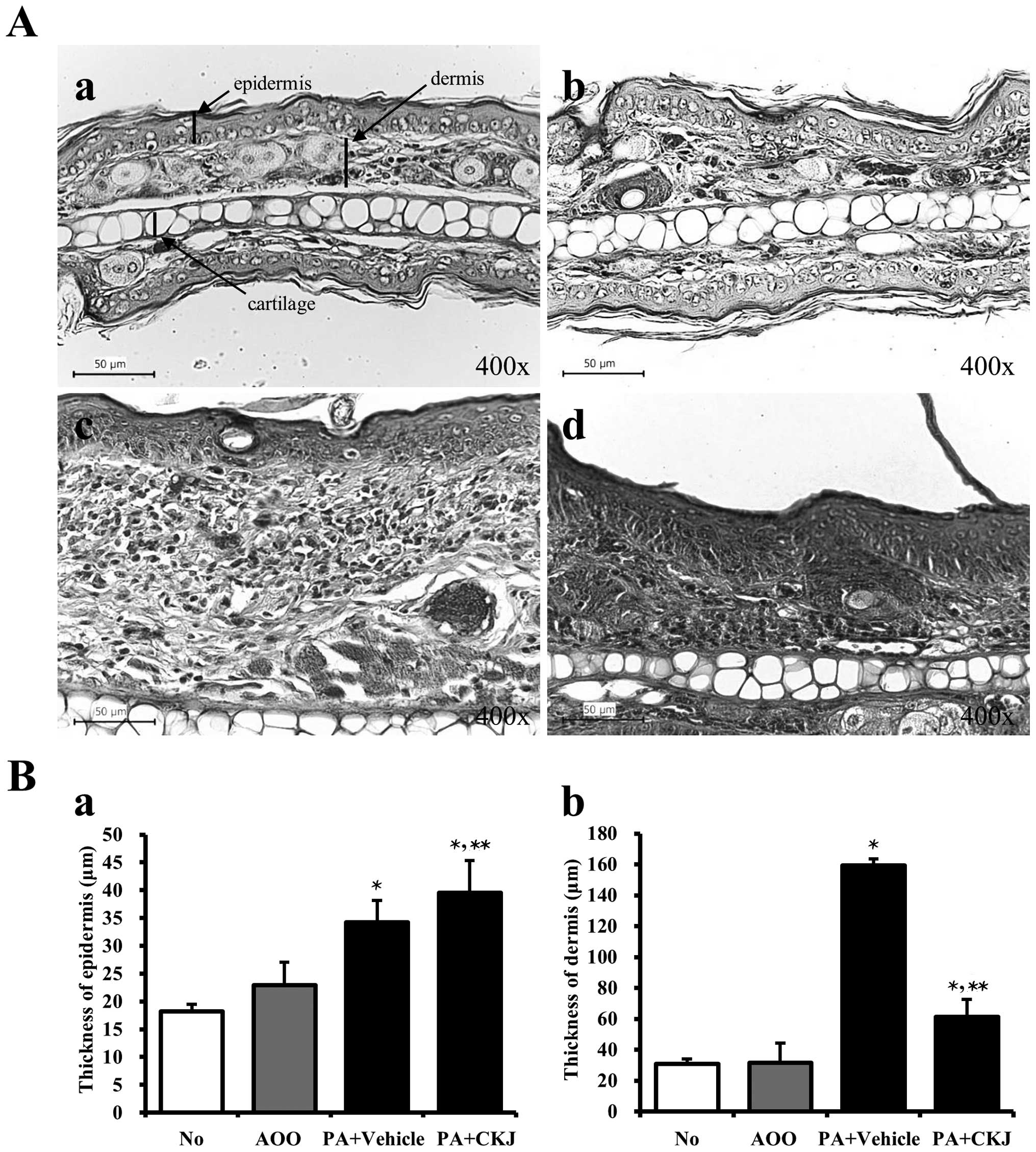
Effects of CKJ treatment on ear
histology
To verify the suppressive effects of CKJ treatment
on ear histology, the histological analysis of ear tissue from
IL-4/Luc/CNS-1 Tg mice was performed. The epidermis and dermis of
the ear tissue were thicker in the PA + Vehicle-treated group than
in the AOO-treated group. The thickness of the dermis greatly
decreased in the PA + CKJ-treated group, but was not completely
recovered to that of the AOO-treated group (Fig. 5A and Bb). The thickness of the
epidermis increased in the PA + CKJ-treated group compared to the
PA + Vehicle-treated group (Fig. 5A
and Ba). Taken together, these results showed that CKJ may
improve the dermis thickness among the AD responses induced by
PA-treatment.
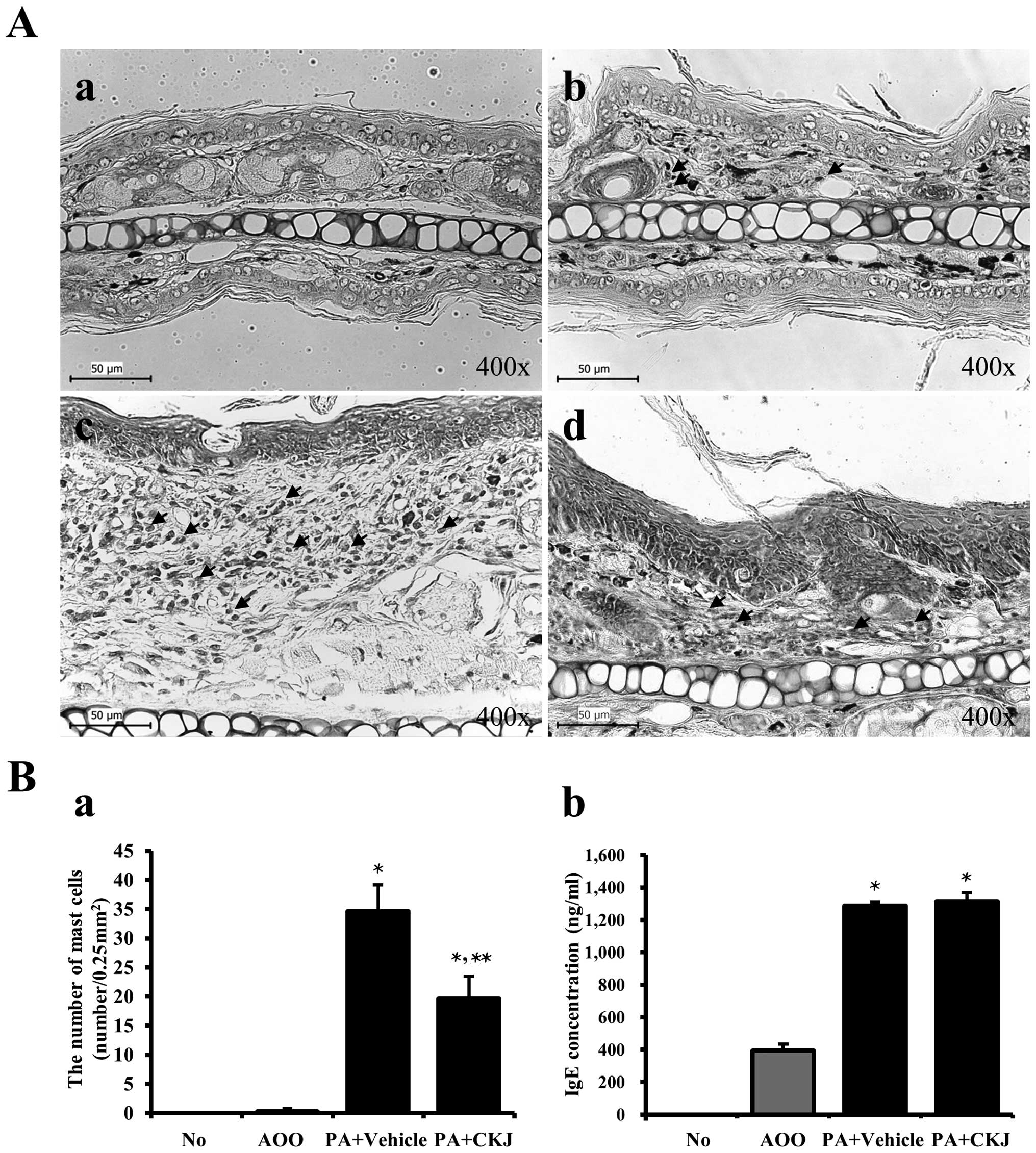
Effects of CKJ treatment on infiltration
of mast cells
Mast cells play important roles in asthma, eczema,
itch, allergic rhinitis, and allergic conjunctivitis (30). Therefore, ear tissue sections were
stained with Toluidine blue and observed under a microscope to
examine the effects of CKJ on the infiltration of mast cells. The
number of mast cells stained blue was significantly greater in the
PA + Vehicle-treated group than that in the AOO-treated group
(Fig. 6). However, their number
was significantly reduced following PA + CKJ-treatment, although
not to that of the AOO-treated group (Fig. 6A and Ba). These data suggest that
CKJ contributes to the suppression of mast cell infiltration in the
dermis of ear skin.
The serum IgE concentration was measured in the four
groups of mice to determine whether CKJ suppressed the allergic
response induced by PA treatment. Repeated topical application of
PA solution induced a significant increase in serum IgE
concentration in IL-4/Luc/CNS-1 Tg mice. However, a significant
decrease of IgE concentration was not observed in the PA +
CKJ-treated group (Fig. 6Bb).
Overall, these results suggested that CKJ treatment did not
contribute to the reduction of IgE concentration in IL-4/Luc/CNS-1
Tg mice observed in response to PA treatment.
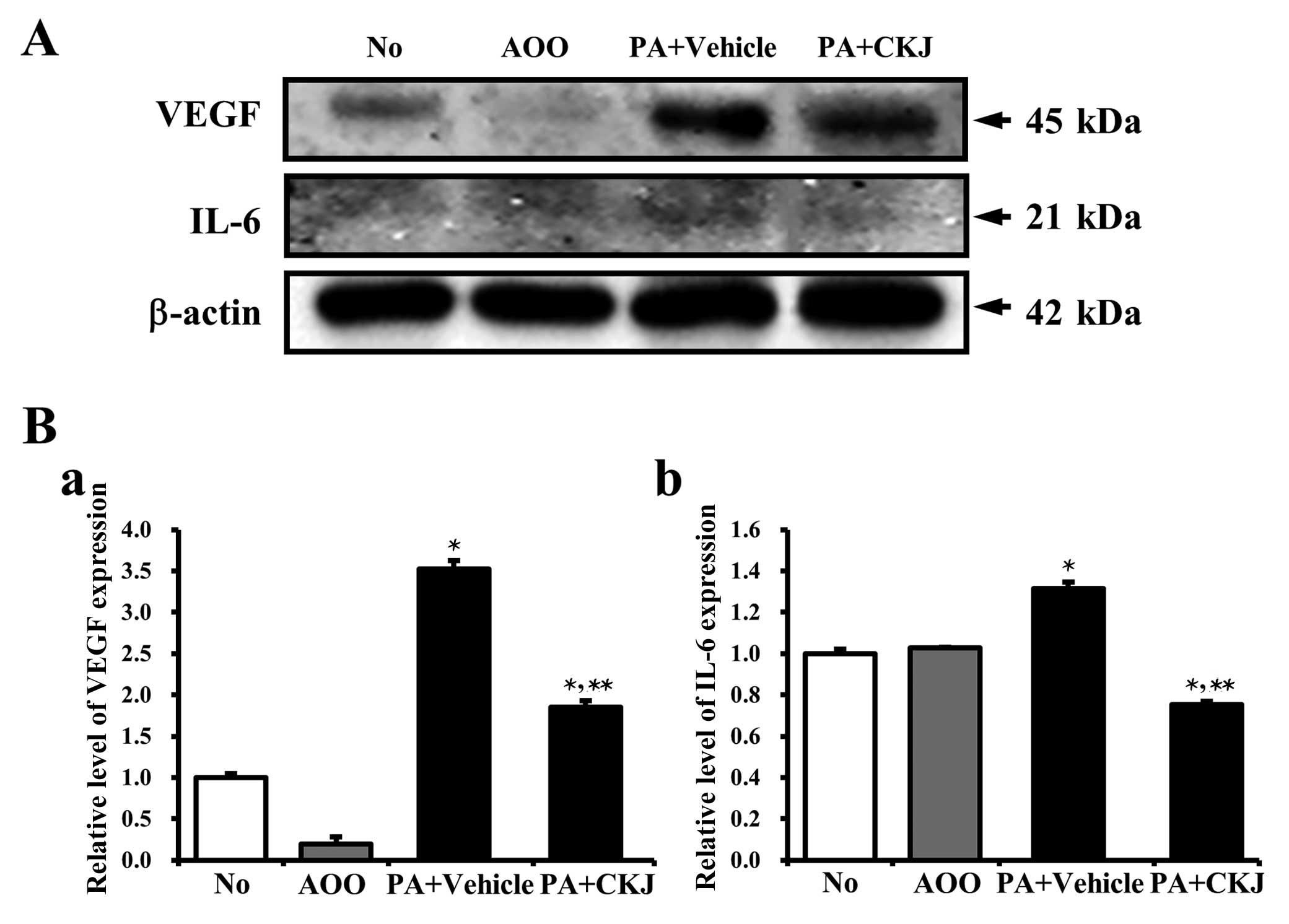
Effects of CKJ treatment on cytokine
expression
To determine whether CKJ induced the alteration of
atopic response-related cytokine expression, the expression levels
of VEGF and IL-6 were measured in ear tissues of IL-4/Luc/CNS-1 Tg
mice. A high expression of VEGF protein was observed in the PA +
Vehicle-treated group, whereas a low expression was detected in the
AOO-treated group and a reduction of VEGF protein expression was
observed in the PA + CKJ-treated group (Fig. 7A and Ba). Similar results were
observed following analysis of IL-6 expression. The increased IL-6
expression after PA treatment was reversed by CKJ treatment, but
not to levels of the AOO-treated group (Fig. 7A and Bb). The above results
suggested that CKJ treatment may relieve the allergic response
induced by PA treatment through the regulation of VEGF and IL-6
expression.
Discussion
Many Tg animal models characterizing allergic skin
inflammation have been developed and established to assess immunity
in vivo. These models can be classified into three groups:
i) models induced by epicutaneous (EC) application of sensitizers,
ii) Tg mice that either overexpress or defect selective proteins,
and iii) mice that spontaneously develop AD-like skin lesions
(5). In the first group, an
animal model of AD is induced by skin injury and EC sensitization
with allergens including ovalbumin (OVA) and PA. Following
sensitization, these models exhibit increased scratching behavior,
enhanced epidermal and dermal thickness, infiltration of
CD4+ T cells, and a high expression of Th2 cytokines
(31). Similar phenotypes can be
induced by EC application of house dust mite allergens, haptens
such as oxazolone and trinitrochlorobenzene treatment, and
superantigen treatment (32–34). In the second group, most animal
models of AD are produced by the overexpression of several related
genes [IL-4, IL-31, thymic stromal lymphopoietin (TSLP), caspase-1
and IL-18], as well as specific genes such as RelB and cathepsin.
These models also develop chronic dermatitis accompanied with
acanthosis, spongiosis, hyperkeratosis, dermal infiltration and
accumulation of mast cells, although their phenotypes do not remain
common in each mouse (35–40).
Additionally, strains of mice such as Naruto Research Institute
Otsuka Atrichia (NOA), NC/Nga and DS-Ng have been proposed as AD
models as they spontaneously develop AD-like phenotypes during the
breeding period (41,42). Our study focused on quantification
of therapeutic effects of a treatment for AD using the luciferase
system in IL-4/Luc/CNS-1 Tg mice. Since most previous studies have
been biased by the therapeutic substance screened and investigation
of their mechanism using the above model for AD, the present study
is important to understand the therapeutic effects of CKJ extract
that help improve AD in humans. However, it should be noted that
the present study was limited in that it only provided information
regarding the effects of CKJ during 4 weeks. Accordingly, further
long-term investigations and human clinical trials are needed to
clearly verify the therapeutic effects of CKJ and apply the
findings presented in this study to humans.
In most animal models, adverse effects on skin have
been detected and improved by treatment with several therapeutic
substances (11,27,43). Some fermented soybean products
have been shown to alleviate adverse skin conditions in the AD
model. For example, treatment with ImmuBalance for two weeks
gradually reduced the clinical skin severity scores in NC/Tnd mice
(21). Additionally, a
significant decrease in the clinical skin severity score was
detected in mice treated with low-molecular γ-PGA (PGA-LM) for
three weeks (22). The results of
the present study are in agreement with the abovementioned previous
reports, although the rate of decrease varied. This difference may
have occurred because the current study used the aqueous extract of
CKJ as the therapeutic substance, whereas the above studies
utilized a specific compound purified from fermented soybean
products.
Histological alterations of the epidermis and dermis
thickness, mast cell infiltration and immune cell accumulation are
considered key effects in IgE-mediated immediate hypersensitivity
and allergic disorders, as well as in immune responses that protect
organisms from parasites and bacteria (44,45). NC/Nga mice treated with PGA-LM
were found to have lower epidermis thickness, mast cell
infiltration and CCR3+ cell concentrations than a
control group (22). Similarly,
the number of PGP9.5-positive neuronal fibers was decreased in the
lesion skin of NC/Tnd mice after ImmuBalance treatment (21), while passive cutaneous anaphylaxis
was reduced by the ethanol extract of CKJ (23). To the best of our knowledge, the
results of the present study are the first to demonstrate the
therapeutic effects of CKJ aqueous extract on alteration of AD
phenotypes in IL-4/Luc/CNS-1 Tg mice. The thickness of dermis and
infiltrating mast cells decreased in the PA + CKJ-treated group
when compared with the PA + Vehicle-treated group, although the
epidermis thickness was maintained at a constant level, which is
partially in agreement with results of a previous study.
Hyperproduction of IgE is one of the key markers of
allergic hypersensitivity (46),
as well as an indicator of the magnitude of allergic immune
response, including AD (47).
Administration of PGA-LM induced a significant decrease in IgE and
IgG1 concentration compared to a control group in previous studies
(21,22). In the present study, IgE
concentration was not affected by CKJ treatment. This difference
was likely due to the main components of CKJ, the sample
preparation method and bacteria strain used for fermentation in the
various studies.
To the best of our knowledge, this is the first
study to employ IL-4/Luc/CNS-1 Tg mice to quantify the therapeutic
effects of AEtLP on the allergenic response to PA treatment. The
luciferase signal was highly detected in the abdominal region of
IL-4/Luc/CNS-1 Tg mice treated with PA + Vehicle alone, whereas the
AOO-treated group showed no luciferase signal. However, this signal
was greatly reduced in groups treated with PA + AEtLP25 and PA +
AEtLP50. Moreover, the highest luciferase signals were detected in
the thymus, followed by the pancreas and SL of IL-4/Luc/CNS-1 Tg
mice treated with PA + Vehicle, whereas these signals were markedly
reduced in these organs in IL-4/Luc/CNS-1 Tg mice treated with PA +
AEtLP25 or PA + AEtLP50 (11). In
the present study, CKJ treatment induced a marked decrease of
luciferase signal in the abdominal region of IL-4/Luc/CNS-1 Tg
mice, as shown in Fig. 3.
Moreover, this signal decreased in the thymus of IL-4/Luc/CNS-1 Tg
mice treated with PA + CKJ. These results were consistent with
previous results published by our group (11), although the pancreas and SL showed
different levels.
Taken together, the results of this study have
demonstrated two novel findings regarding the therapeutic effects
of CKJ using IL-4/Luc/CNS-1 Tg mice. Specifically, IL-4/Luc/CNS-1
Tg mice may be successfully applied to screen for the therapeutic
effects of CKJ-related products. Additionally, the aqueous extract
of CKJ can effectively relieve AD induced by PA treatment.
Furthermore, the findings presented herein indicate that CKJ may be
a beneficial food for the improvement and prevention of AD.
Acknowledgements
We thank Jin Hyang Hwang for directing the animal
management and care at the Laboratory Animal Resources Center. This
study was supported by grants to Dae Youn Hwang from the Korea
Institute of Planning Evaluation for Technology of Food,
Agriculture, Forestry and Fisheries (111030-3).
References
|
1
|
Hanifin JM and Rajka G: Diagnostic
features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh).
92:44–47. 1980.
|
|
2
|
Leung DY and Bieber T: Atopic dermatitis.
Lancet. 361:151–160. 2003. View Article : Google Scholar
|
|
3
|
Kay AB: Overview of ‘allergy and allergic
diseases: with a view to the future’. Br Med Bull. 56:843–864.
2000.
|
|
4
|
Kay AB: Allergy and allergic diseases.
First of two parts. N Engl J Med. 344:30–37. 2001. View Article : Google Scholar
|
|
5
|
Jin H, He R, Oyoshi M and Geha RS: Animal
models of atopic dermatitis. J Invest Dermatol. 129:31–40. 2009.
View Article : Google Scholar
|
|
6
|
Spergel JM and Paller AS: Atopic
dermatitis and the atopic march. J Allergy Clin Immunol. 112(Suppl
6): S118–S127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turner KJ, Stewart GA, Sharp AH and Czarny
D: Standardization of allergen extracts by inhibition of RAST, skin
test, and chemical composition. Clin Allergy. 10:441–450. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johansson SG: ImmunoCAP specific IgE test:
an objective tool for research and routine allergy diagnosis.
Expert Rev Mol Diagn. 4:273–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petersen AB, Gudmann P, Milvang-Grønager
P, Mørkeberg R, Bøgestrand S, Linneberg A and Johansen N:
Performance evaluation of a specific IgE assay developed for the
ADVIA centaur immunoassay system. Clin Biochem. 37:882–892. 2004.
View Article : Google Scholar
|
|
10
|
Bae CJ, Lee JW, Bae HS, Shim SB, Jee SW,
Lee SH, Lee CK, Hong JT and Hwang DY: Detection of allergenic
compounds using an IL-4/luciferase/CNS-1 transgenic mice model.
Toxicol Sci. 120:349–359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwak MH, Kim JE, Hwang IS, Lee YJ, An BS,
Hong JT, Lee SH and Hwang DY: Quantitative evaluation of
therapeutic effect of Liriope platyphylla on phthalic
anhydride-induced atopic dermatitis in IL-4/Luc/CNS-1 Tg mice. J
Ethnopharmacol. 148:880–889. 2013.PubMed/NCBI
|
|
12
|
Lee JJ, Lee DS and Kim HB: Fermentation
patterns of chungkookjang and Kanjang by Bacillus
licheniformis B1. Korean J Microbiol. 35:296–301. 1999.
|
|
13
|
Nakajima N, Nozaki N, Ishihara K, Ishikawa
A and Tsuji H: Analysis of isoflavone content in tempeh, a
fermented soybean, and preparation of a new isoflavone-enriched
tempeh. J Biosci Bioeng. 100:685–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwon DY, Jang JS, Lee JE, Kim YS, Shin DH
and Park S: The isoflavonoid aglycone-rich fractions of
Chungkookjang, fermented unsalted soybean, enhance insulin
signaling and peroxisome proliferator-activated receptor-gamma
activity in vitro. Biofactors. 26:245–258. 2006. View Article : Google Scholar
|
|
15
|
Su CL, Wu CJ, Chen FN, Wang BJ, Shen SR
and Won SJ: Supernatant of bacterial fermented soybean induces
apoptosis of human hepatocellular carcinoma Hep 3B cells via
activation of caspase-8 and mitochondria. Food Chem Toxicol.
45:303–314. 2007. View Article : Google Scholar
|
|
16
|
Kim MH, Kim SY, Ko JM, Jeong DY and Kim
YS: Biological activities of cheonggukjang prepared with several
soybean cultivars. Food Sci Biotechnol. 21:475–483. 2012.
View Article : Google Scholar
|
|
17
|
Kwon EY, Jung KO, Moon SH and Park KY:
Studies on enhancing chemopreventive effect of
chungkookjangs-antimutagenic activity of chungkookjangs prepared
with the different varieties of soybean and starter. J Korean Assoc
Cancer Prev. 7:200–209. 2002.
|
|
18
|
Choi J, Kwon SH, Park KY, Yu BP, Kim ND,
Jung JH and Chung HY: The anti-inflammatory action of fermented
soybean products in kidney of high-fat-fed rats. J Med Food.
14:232–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jalin AMA, Lee CK, Lee CY, Kang AR, Park
CM, Cha J, Kim JH, Lee SW, Song YS, Lee JT and Kang SG:
Thrombolytic activity of cheonggukjang kinase in recovery from
brain damage in a rat cerebral embolic stroke model. Neural Regen
Res. 5:1875–1882. 2010.
|
|
20
|
Ko JA, Koo SY and Park HJ: Effects of
alginate microencapsulation on the fibrinolytic activity of
fermented soybean paste (cheonggukjang) extract. Food Chem.
111:921–924. 2008. View Article : Google Scholar
|
|
21
|
Matsuda A, Tanaka A, Pan W, Okamoto N,
Oida K, Kingyo N, Amagai Y, Xia Y, Jang H, Nishikawa S, Kajiwara N,
Ahn G, Ohmori K and Matsuda H: Supplementation of the fermented soy
product ImmuBalance™ effectively reduces itching behavior of atopic
NC/Tnd mice. J Dermatol Sci. 67:130–139. 2012.
|
|
22
|
Jang SN, Kim KL, Yun MY and Kang SM: The
effect of γ-PGA on NC/Nga mice, a mouse model for mite
antigen-induced atopic dermatitis. Korean J Microbiol Biotechnol.
38:53–63. 2010.(In Korean).
|
|
23
|
Choi YH, Lim H, Heo MY, Kwon DY and Kim
HP: Anti-inflammatory activity of the ethanol extract of
chungkukjang, Korean fermented bean: 5-lipoxygenase inhibition. J
Med Food. 11:539–543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang IS, Kim JE, Lee YJ, Kwak MH, Lee HG,
Kim HS, Lee HS and Hwang DY: Growth sensitivity in the epiphyseal
growth plate, liver and muscle of SD rats is significantly enhanced
by treatment with a fermented soybean product (cheonggukjang)
through stimulation of growth hormone secretion. Mol Med Rep.
9:166–172. 2014.
|
|
25
|
Lee YJ, Kim JE, Kwak MH, Go J, Son HJ, Kim
DS and Hwang DY: In vitro and in vivo study of effects of fermented
soybean product (chungkookjang) on NGF secretion ability and NGF
receptor signaling pathway. Lab Anim Res. 29:113–126. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang G and Brown AW: The rapid
determination of gamma-aminobutyric acid. Phytochemistry.
44:1007–1009. 1997. View Article : Google Scholar
|
|
27
|
Kim JE, Lee YK, Nam SH, Choi SI, Goo JS,
Jang MJ, Lee HS, Son HJ, Lee CY and Hwang DY: The symptoms of
atopic dermatitis in NC/Nga mice were significantly relieved by the
water extract of Liriope platyphylla. Lab Anim Res.
26:377–384. 2010. View Article : Google Scholar
|
|
28
|
Stahlmann R, Wegner M, Riecke K, Kruse M
and Platzek T: Sensitising potential of four textile dyes and some
of their metabolites in a modified local lymph node assay.
Toxicology. 219:113–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bae CJ, Shim SB, Jee SW, Lee SH, Kim MR,
Lee JW, Lee CK and Hwang DY: IL-6, VEGF, KC and RANTES are a major
cause of a high irritant dermatitis to phthalic anhydride in
C57BL/6 inbred mice. Allergol Int. 59:389–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prussin C and Metcalfe DD: 4. IgE, mast
cells, basophils, and eosinophils. J Allergy Clin Immunol.
111(Suppl 2): S486–S494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spergel JM, Mizoguchi E, Brewer JP, Martin
TR, Bhan AK and Geha RS: Epicutaneous sensitization with protein
antigen induces localized allergic dermatitis and
hyperresponsiveness to methacholine after single exposure to
aerosolized antigen in mice. J Clin Invest. 101:1614–1622. 1998.
View Article : Google Scholar
|
|
32
|
Kimura M, Tsuruta S and Yoshida T:
Correlation of house dust mite-specific lymphocyte proliferation
with IL-5 production, eosinophilia, and the severity of symptoms in
infants with atopic dermatitis. J Allergy Clin Immunol. 101:84–89.
1998. View Article : Google Scholar
|
|
33
|
Man MQ, Hatano Y, Lee SH, Man M, Chang S,
Feingold KR, Leung DY, Holleran W, Uchida Y and Elias PM:
Characterization of a hapten-induced, murine model with multiple
features of atopic dermatitis: structural, immunologic, and
biochemical changes following single versus multiple oxazolone
challenges. J Invest Dermatol. 128:79–86. 2008. View Article : Google Scholar
|
|
34
|
Laouini D, Kawamoto S, Yalcindag A, Bryce
P, Mizoguchi E, Oettgen H and Geha RS: Epicutaneous sensitization
with superantigen induces allergic skin inflammation. J Allergy
Clin Immunol. 112:981–987. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chan LS, Robinson N and Xu L: Expression
of interleukin-4 in the epidermis of transgenic mice results in a
pruritic inflammatory skin disease: an experimental animal model to
study atopic dermatitis. J Invest Dermatol. 117:977–983. 2001.
View Article : Google Scholar
|
|
36
|
Dillon SR, Sprecher C, Hammond A, et al:
Interleukin 31, a cytokine produced by activated T cells, induces
dermatitis in mice. Nat Immunol. 5:752–760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Soumelis V, Reche PA, Kanzler H, et al:
Human epithelial cells trigger dendritic cell mediated allergic
inflammation by producing TSLP. Nat Immunol. 3:673–680.
2002.PubMed/NCBI
|
|
38
|
Kim E, Lee JE, Namkung JH, Park JH, Kim S,
Shin ES, Cho EY and Yang JM: Association of the single-nucleotide
polymorphism and haplotype of the interleukin 18 gene with atopic
dermatitis in Koreans. Clin Exp Allergy. 37:865–871. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weih F, Warr G, Yang H and Bravo R:
Multifocal defects in immune responses in RelB-deficient mice. J
Immunol. 158:5211–5218. 1997.PubMed/NCBI
|
|
40
|
Tsukuba T, Okamoto K, Okamoto Y, Yanagawa
M, Kohmura K, Yasuda Y, Uchi H, Nakahara T, Furue M, Nakayama K,
Kadowaki T, Yamamoto K and Nakayama KI: Association of cathepsin E
deficiency with development of atopic dermatitis. J Biochem.
134:893–902. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Watanabe O, Natori K, Tamari M, Shiomoto
Y, Kubo S and Nakamura Y: Significantly elevated expression of PF4
(platelet factor 4) and eotaxin in the NOA mouse, a model for
atopic dermatitis. J Hum Genet. 44:173–176. 1999. View Article : Google Scholar
|
|
42
|
Hikita I, Yoshioka T, Mizoguchi T,
Tsukahara K, Tsuru K, Nagai H, Hirasawa T, Tsuruta Y, Suzuki R,
Ichihashi M and Horikawa T: Characterization of dermatitis arising
spontaneously in DS-Nh mice maintained under conventional
conditions: another possible model for atopic dermatitis. J
Dermatol Sci. 30:142–153. 2002. View Article : Google Scholar
|
|
43
|
Kim JE, Hwang IS, Goo JS, Nam SH, Choi SI,
Lee HR, Lee YJ, Kim YH, Park SJ, Kim NS, Choi YH and Hwang DY:
LP9M80-H isolated from Liriope platyphylla could help
alleviate diabetic symptoms via the regulation of glucose and lipid
concentration. J Life Sci. 22:634–641. 2012.
|
|
44
|
Kawakami T, Ando T, Kimura M, Wilson BS
and Kawakami Y: Mast cells in atopic dermatitis. Curr Opin Immunol.
21:666–678. 2009. View Article : Google Scholar
|
|
45
|
Galli SJ: Mast cells and basophils. Curr
Opin Hematol. 7:32–39. 2000. View Article : Google Scholar
|
|
46
|
Gao XK, Nakamura N, Fuseda K, Tanaka H,
Inagaki N and Nagai H: Establishment of allergic dermatitis in
NC/Nga mice as a model for severe atopic dermatitis. Biol Pharm
Bull. 27:1376–1381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dearman RJ, Skinner RA, Humphreys NE and
Kimber I: Methods for the identification of chemical respiratory
allergens in rodents: comparisons of cytokine profiling with
induced changes in serum IgE. J Appl Toxicol. 23:199–207. 2003.
View Article : Google Scholar
|