Introduction
The breakdown of normal mucosal immunity causes the
development of inflammatory bowel disease (IBD), which can be
classified as Crohn’s disease (CD) and ulcerative colitis (UC)
(1). IBD is a chronically
relapsing and remitting condition of unknown origin that exhibits
various features of immunological inflammation and affects at least
1 in 1,000 people in western countries. IBD is characterized by
inflammation in the intestine, and is associated with diarrhea,
occult blood, abdominal pain, weight loss, anemia and leukocytosis.
IBD primarily affects young adults, and the disease initially
manifests in childhood in 15–25% of cases. Therefore, IBD patients
often develop severe symptoms that decrease their quality of life
(2). Consequently, there is a
need for innovative therapies for IBD.
Current treatments for IBD focus on suppressing
inflammation or modulating the immune response using
corticosteroids, mercaptopurines, 5-ASA, or monoclonal antibodies
against inflammatory cytokines, e.g., the anti-tumor necrosis
factor (TNF)-α antibody infliximab (3). However, despite the wide variety of
pharmacologic options for patients with IBD, consistent cures and
prolonged remissions have yet to be achieved.
Hepatocyte growth factor (HGF) was originally
identified (4–7) and cloned (8,9) as
a potent mitogen for hepatocytes, but has since been established as
a multifunctional cytokine that exhibits mitogenic, motogenic,
morphologic, angiogenic, antiapoptotic and organotrophic effects in
a variety of tissues (10). HGF
is upregulated in inflamed colonic mucosal tissue in patients with
CD or UC (11–13), and plasma HGF levels are elevated
in animal models of acute colitis (14). In vitro, HGF modulates
intestinal epithelial cell proliferation and migration (15), thereby enhancing epithelial cell
restitution, which is the initial step of gastrointestinal wound
healing. In addition, administration of recombinant human HGF
(hHGF) protein reduces the severity of colitis and accelerates
colonic mucosal repair in models of TNBS-induced and DSS-induced
colitis (16–19), as well as in HLA-B27 transgenic
rats with colitis (20). Mukoyama
et al (21) showed that
the intrarectal administration of an adenoviral (Ad) vector
carrying the HGF gene prevented TNBS-induced colitis. Additionally,
Hanawa et al (22)
demonstrated the attenuation of mouse DSS colitis by naked gene
transfer of rat HGF into the liver, and Kanbe et al
(23) reported the amelioration
of mucosal damage in DSS colitis by the intrarectal administration
of the naked HGF gene. In their study, Kanayama et al
(24) demonstrated the promotion
of colonic epithelial regeneration by HGF gene transfer through
electroporation. Findings by those authors suggest that HGF is
potentially an important new treatment modality for promoting the
repair of intestinal mucosa in patients with IBD.
In the majority of previous studies, HGF was
provided in the form of recombinant hHGF protein. However, due to
the rapid clearance of the HGF protein, large doses and frequent
administration of recombinant hHGF were required. Naked gene
transfer is a simple and easy method, but the efficiency of gene
transduction is extremely low, possibly leading to insufficient
clinical effectiveness in human patients. By contrast, the
intrarectal administration of an Ad carrying the HGF gene is
considered to be extremely stressful for patients. Therefore, in
this study we injected an Ad carrying the hHGF gene in single
rounds of injections into both hindlimbs of mice 1 day after
administration of DSS. We then investigated the therapeutic effects
and mechanisms of systemically circulating HGF protein, produced by
a gene introduced into the limbs, in the DSS-induced acute colitis
model.
Materials and methods
Recombinant Ad
The Ad expressing hHGF under the transcriptional
control of the cytomegalovirus immediate-early enhancer and a
modified chicken β-actin promoter (Ad.HGF) was generated as
described previously (25). The
Ad.HGF and the control Ad expressing the LacZ gene (Ad.LacZ) were
amplified in HEK-293 cells, purified twice on CsCl gradients, and
desalted as described previously (26–29).
Animal studies
Six- to 7-week-old female BALB/c mice weighing 17–20
g (Japan SLC, Inc., Hamamatsu, Japan) were housed in cages in a
temperature-controlled environment under a 12-h light-dark cycle
with free access to food and water. The animal studies were
performed in accordance with the National Institutes of Health
guidelines, as specified by the Animal Care Facility at Gifu
University School of Medicine.
To induce dextran sodium sulfate (DSS) colitis, the
mice were provided with distilled drinking water containing 5%
(w/v) DSS (MW, 36,000–50,000; ICN Biomedicals Inc., Aurora, OH,
USA) for 7 days. Subsequently, colitis was maintained by feeding
the mice 1% DSS (30–32) in the drinking water.
One day after the administration of DSS, Ad.HGF was
injected into both hindlimbs of each mouse for a total dose of
1×1011 particles/mouse (i.e., 5×1010
particles each into the left and right thigh muscles) (n=8).
Ad.LacZ was injected in a similar manner into control mice (n=8).
These groups were followed until day 15 (i.e., 8 days after the end
of the 7-day period of 5% DSS administration). To evaluate the
severity of colitis, body weight was examined on a daily basis. On
day 15, all the mice were sacrificed by inhaled anesthetics, and
colon samples were collected for examination. In other experiments,
on day 5 of 5% DSS administration, 5-bromo-2′-deoxyuridine (BrdU,
100 mg/kg) was administered intraperitoneally to mice (n=8)
infected with Ad.HGF or Ad.LacZ, and the animals were sacrificed by
inhaled anesthetics 2 h later. These samples were used for analyses
of HGF signal transduction, cell proliferation, apoptosis,
cytokines and lymphocyte surface markers. The concentration of
exogenous hHGF in serum was analyzed using the same dose (i.e.,
1×1011 particles/mouse) of Ad.LacZ or Ad.HGF in intact
mice (n=16).
Enzyme-linked immunosorbent assay
The plasma concentration of hHGF following
adenoviral intramuscular gene transduction (IMGT) was measured in
mice at each time point (n=4) using the Quantikine human HGF
Immunoassay kit (R&D Systems, Inc., Minneapolis, MN, USA).
TNF-α, interleukin (IL)-1β, IL-6, interferon (IFN)-γ, IL-2, IL-4
and IL-5 levels in the colons of colitic mice were measured using
commercially available enzyme-linked immunosorbent assay (ELISA)
kits (BioSource International, Inc., Camarillo, CA, USA) according
to the manufacturer’s instructions.
Immunoprecipitation and c-Met receptor
phosphorylation assay
The phosphorylation and activation of the c-Met
receptor in colon tissues were detected by immunoprecipitation, as
described previously (33,34).
In brief, 1 g of colon tissue was homogenized in 4 ml of lysis
buffer [1% Triton X-100, 150 mM NaCl, 50 mM Tris-HCl (pH 7.6), 10%
glycerol, 1 mM vanadate, and 1 mM phenylmethylsulfonyl fluoride]
with a protease-inhibitor cocktail (Sigma-Aldrich, Tokyo, Japan).
Following centrifugation, the supernatant was incubated with 0.5
μg/ml anti-mouse c-Met antibody (sc-162; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) for 4 h, and then sequentially incubated
with 5 μl of protein G-Sepharose beads for 3 h. After washing,
proteins bound to the beads were dissolved in sample buffer and
subjected to SDS-PAGE. Phosphorylated c-Met was immunoblotted using
the anti-phosphotyrosine antibody PY20 (Transduction Laboratories,
Lexington, KY, USA).
Histopathological analysis
After each mouse was sacrificed, the intestine was
dissected from the anus to the cecum and rinsed with physiological
saline. The colon length was measured, and the colon sample was
divided into three sections (cecum, proximal colon and distal
colon), with the cecum being separated first, and then the
remaining part of the colon being divided into two equal segments
(proximal colon and distal colon). The cecum, proximal colon and
distal colon were opened longitudinally, and the proximal and
distal colon were equally divided longitudinally and transversely.
Thus, the cecum was divided into two sections, and the proximal and
distal colon were divided into four sections. The colon tissues
were fixed in 10% formalin and embedded in paraffin, and 4-μm
sections were cut and stained with hematoxylin and eosin (H&E)
to determine the inflammation and crypt scores (35). Briefly, the sections were graded
on a scale of 0–3 to indicate the severity of inflammation: 0,
none; 1, mucosa; 2, mucosa and submucosa; and 3, transverse, and on
a scale of 0–4 to indicate the severity of crypt damage: 0, none;
1, basal 1/3 damage; 2, basal 2/3 damage; 3: loss of the entire
crypt with the surface epithelium remaining intact; and 4, loss of
the entire crypt and surface epithelium. The changes were also
scored with regard to the extent of tissue involvement, measured as
a percentage: i) 1–25%, ii) 26–50%, iii) 51–75%, and iv) 76–100%.
Each section was then separately scored for each feature by taking
the product of the severity score and the score for the extent of
tissue involvement. Thus, the inflammation score ranged from 0 to
12, and the crypt score ranged from 0 to 16. Apoptotic cells were
detected using a light microscope (Olympus, Tokyo, Japan) and the
terminal deoxynucleotidyltransferase-mediated deoxyuridine
triphosphate biotin nick end-labeling (TUNEL) assay (ApopTag kit;
Intergen Co., Purchase, NY, USA), as described previously (25,33,36). To detect proliferating cells, BrdU
incorporation was measured using a staining kit (Zymed
Laboratories, Inc., South San Francisco, CA, USA) according to the
manufacturer’s instructions.
Endothelial cells, CD4+ T lymphocytes,
and CD8+ T lymphocytes were detected in situ
using an anti-vWF antibody (Dako Cytomation Co., Ltd., Kyoto,
Japan), anti-CD4 antibody and anti-CD8 antibody (both from Zymed
Laboratories, Inc.), respectively, as described previously
(25,36).
Statistical analysis
Values provided are the means ± SEM values. The
significance of differences was evaluated using the Student’s
t-test.
Results
Intramuscular injection of Ad.HGF
produces circulating plasma hHGF, leading to c-Met activation in
the colonic mucosa
DSS-induced colitis was induced in 6- to 7-week-old
female BALB/c mice. One day after DSS administration, Ad.HGF was
administered in a single procedure involving injections into both
hindlimbs (total dose, 1×1011 particles/mouse; as
mentioned in Materials and methods). In the hHGF-overexpressing
mice, the plasma levels of hHGF were 1,140±101, 634±341 and
33.9±15.8 pg/ml at 2, 4 and 6 days after injection, respectively.
No hHGF was detected in the Ad.LacZ-treated mice at any time point,
demonstrating that this method accurately detected only hHGF
protein expressed from the hHGF transgene, without a cross-reaction
resulting in detection of the endogenous mouse HGF protein. These
results indicate that hHGF expression was effectively induced by
the intramuscular injection of Ad.HGF, leading to the presence of
hHGF in the plasma of the mice.
The biological effects of HGF are mediated by its
receptor c-Met, which is capable of activating multiple
intracellular transducers and signaling pathways. Therefore, we
examined c-Met tyrosine phosphorylation in the colonic mucosal
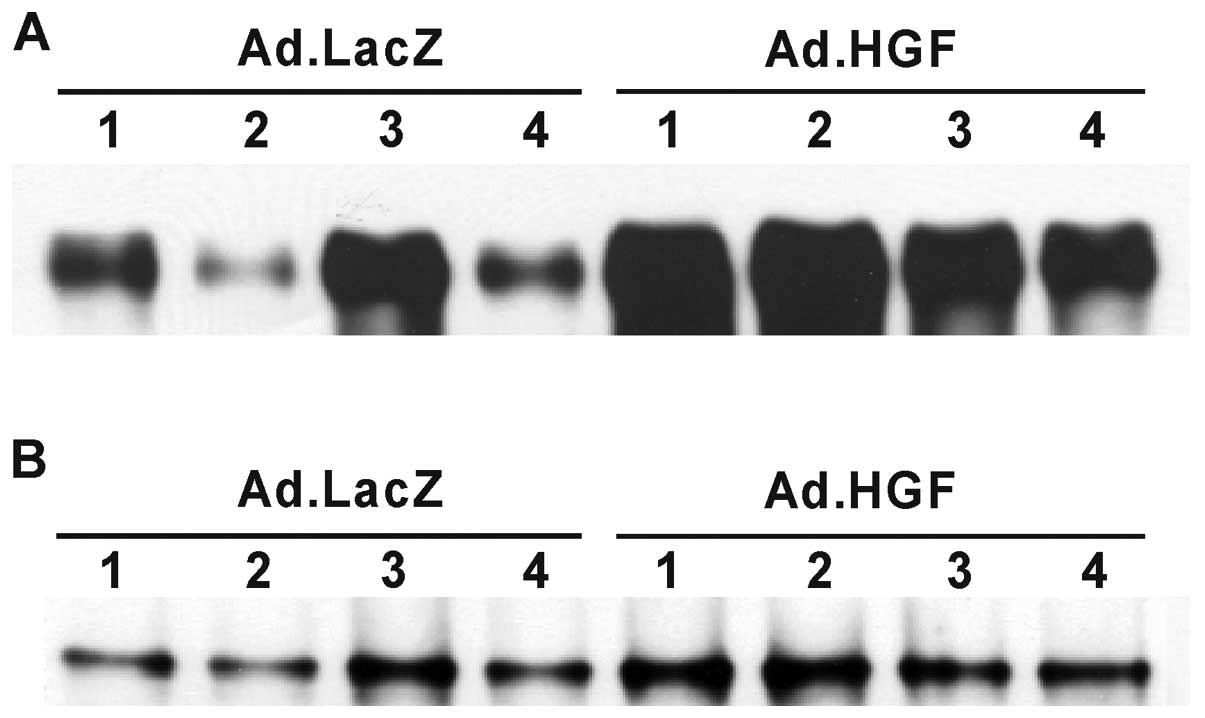
epithelium by western blotting (Fig.
1). Phosphorylated c-Met was detected at low or moderate levels
in the injured colonic mucosa of mice treated with Ad.LacZ,
presumably as a result of a DSS-induced increase in endogenous HGF
in response to colonic mucosal injury (14). By contrast, the injured colonic
mucosa of mice treated with Ad.HGF exhibited high levels of c-Met
tyrosine phosphorylation.
Adenoviral hHGF IMGT prevents weight loss
in DSS-induced colitis mice
DSS-induced colitis is characterized by bloody
stools and severe weight loss (30). In mice treated with Ad.LacZ, we
observed persistent liquid stool and waste with subsequent severe
weight loss. By contrast, colitic mice that received a single round
of injections of Ad.HGF exhibited significant reductions in liquid
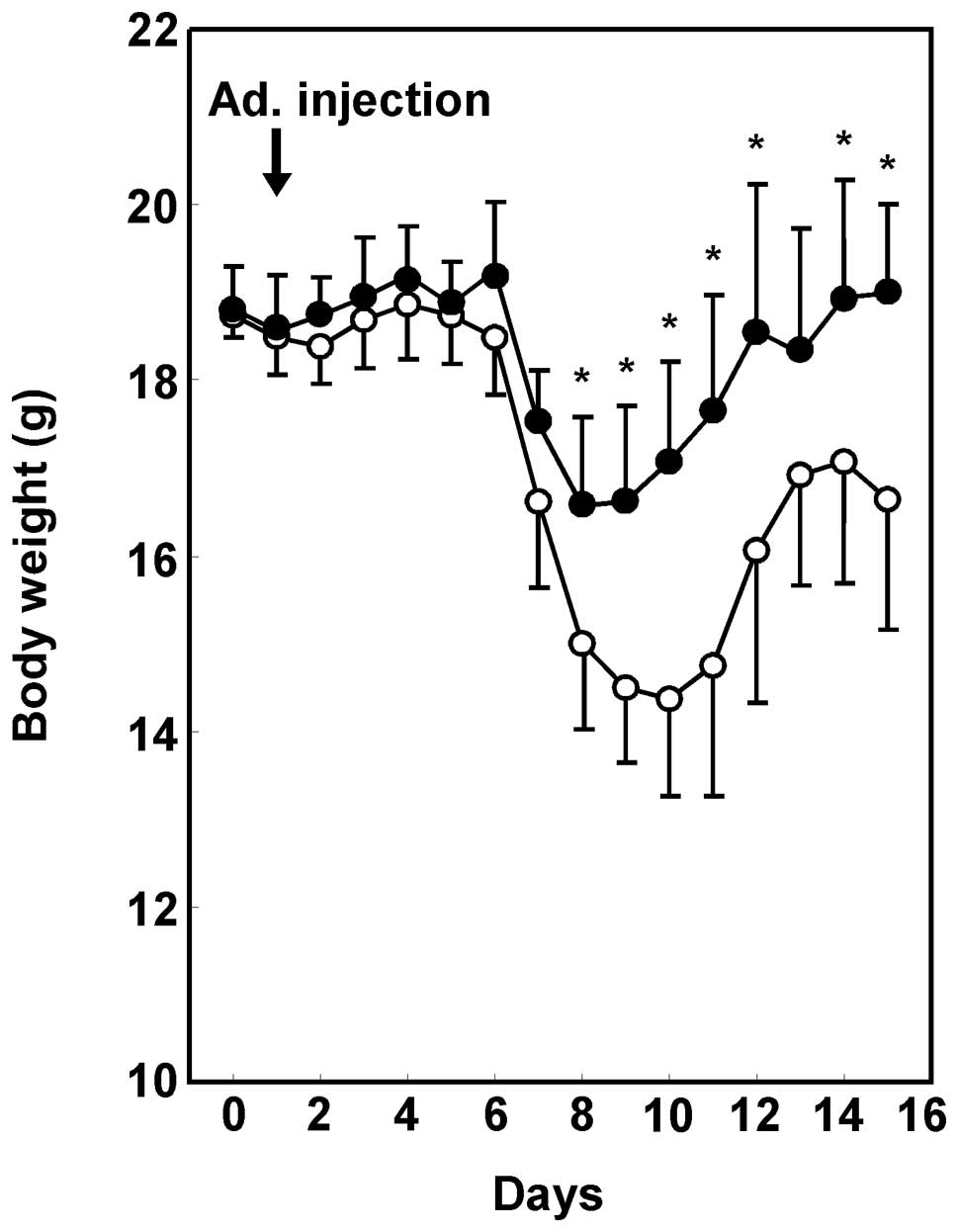
stool and gross bleeding from the rectum (data not shown). Fig. 2 shows the mean weight change, and
that the body weights of Ad.HGF-treated mice were significantly
higher than those of the Ad.LacZ-treated mice. In the
Ad.LacZ-treated control mice, weight loss occurred 6–7 days after
the initiation of DSS administration. Ad.HGF treatment
significantly prevented this weight loss.
Adenoviral hHGF IMGT reduces
colitis-induced intestinal shortening and pathological scores
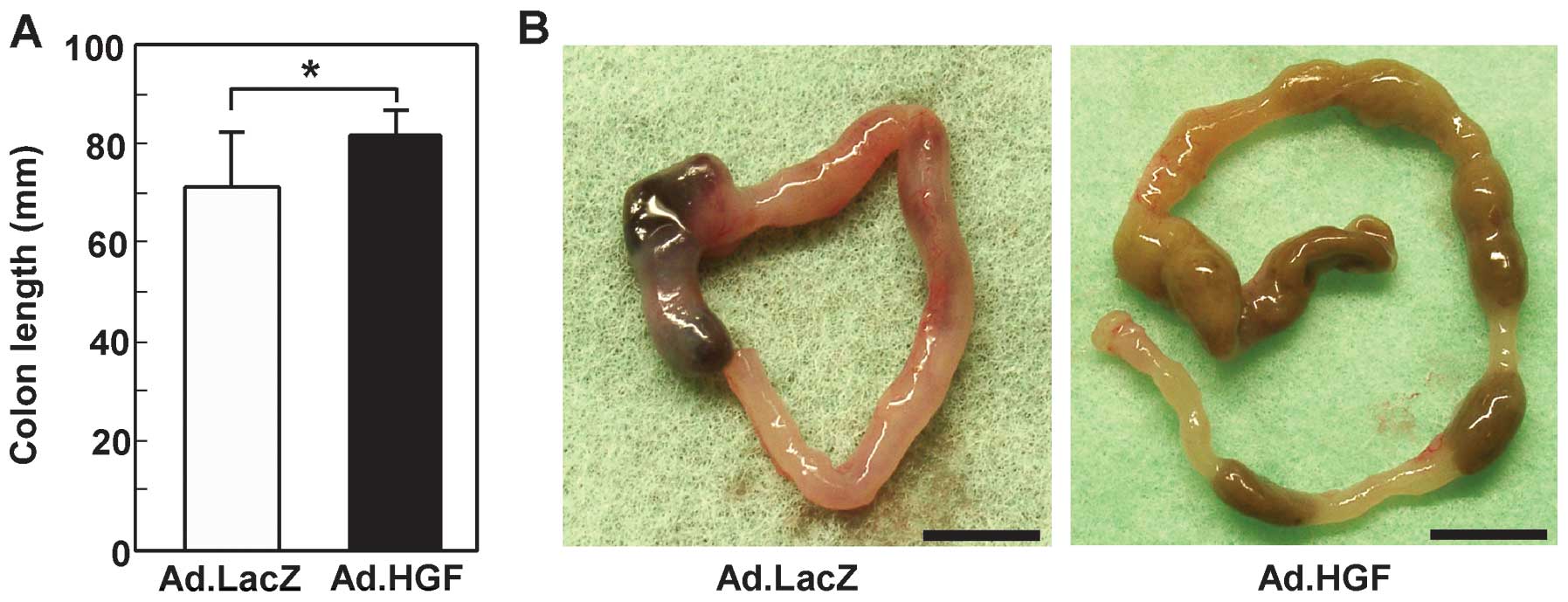
Shortening of the colon correlates well with
histologic changes, and colon length is therefore frequently used
as a morphologic parameter to indicate the degree of inflammation
(35). The colon lengths of mice
treated with Ad.LacZ and Ad.HGF were 72.0±10.6 and 82.0±4.7 mm,
respectively (Fig. 3A). In
contrast to the colons in the Ad.HGF-treated group, the colons in
the Ad.LacZ-treated group were short and severely inflamed, with
evident hemorrhages (Fig.
3B).
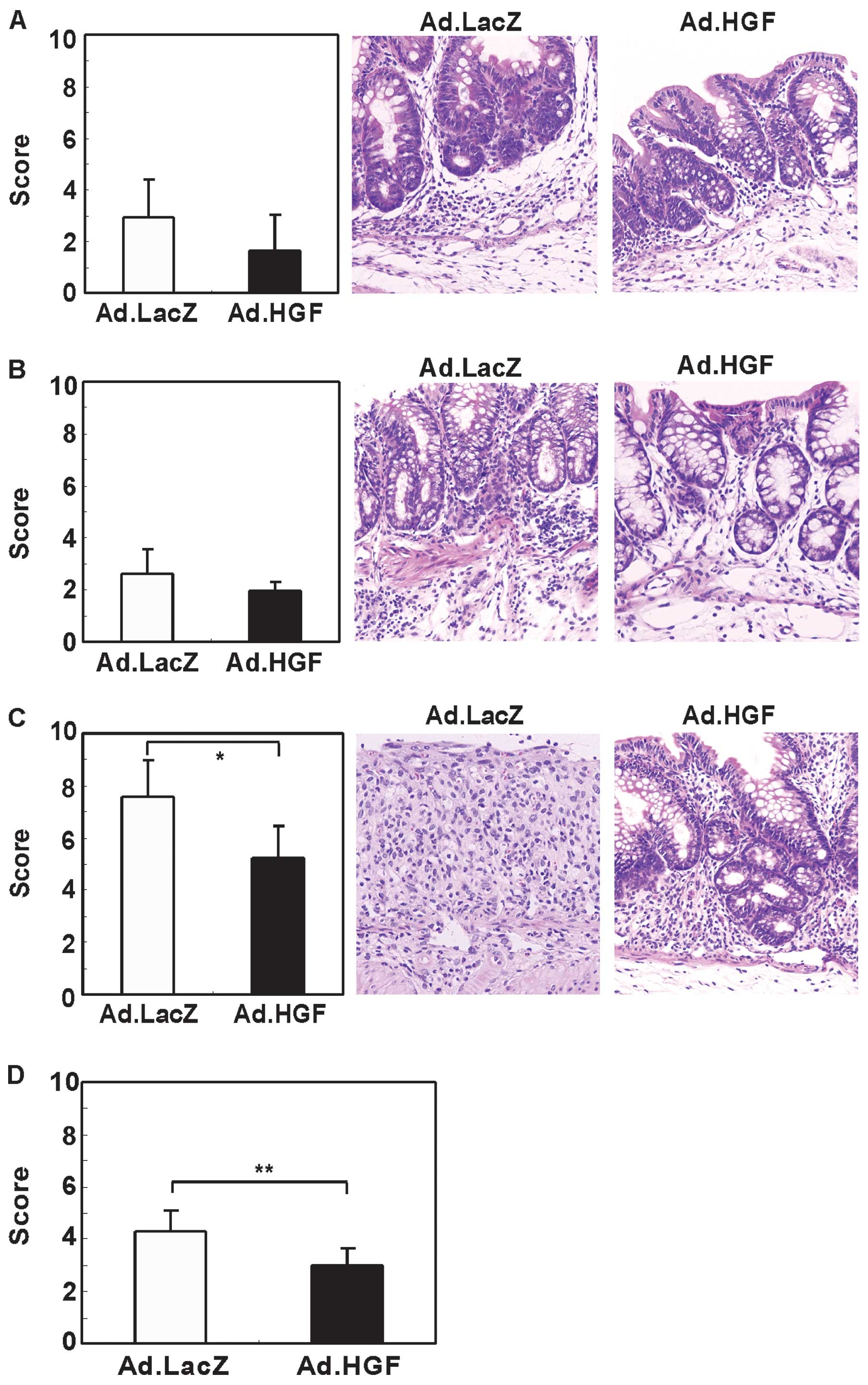
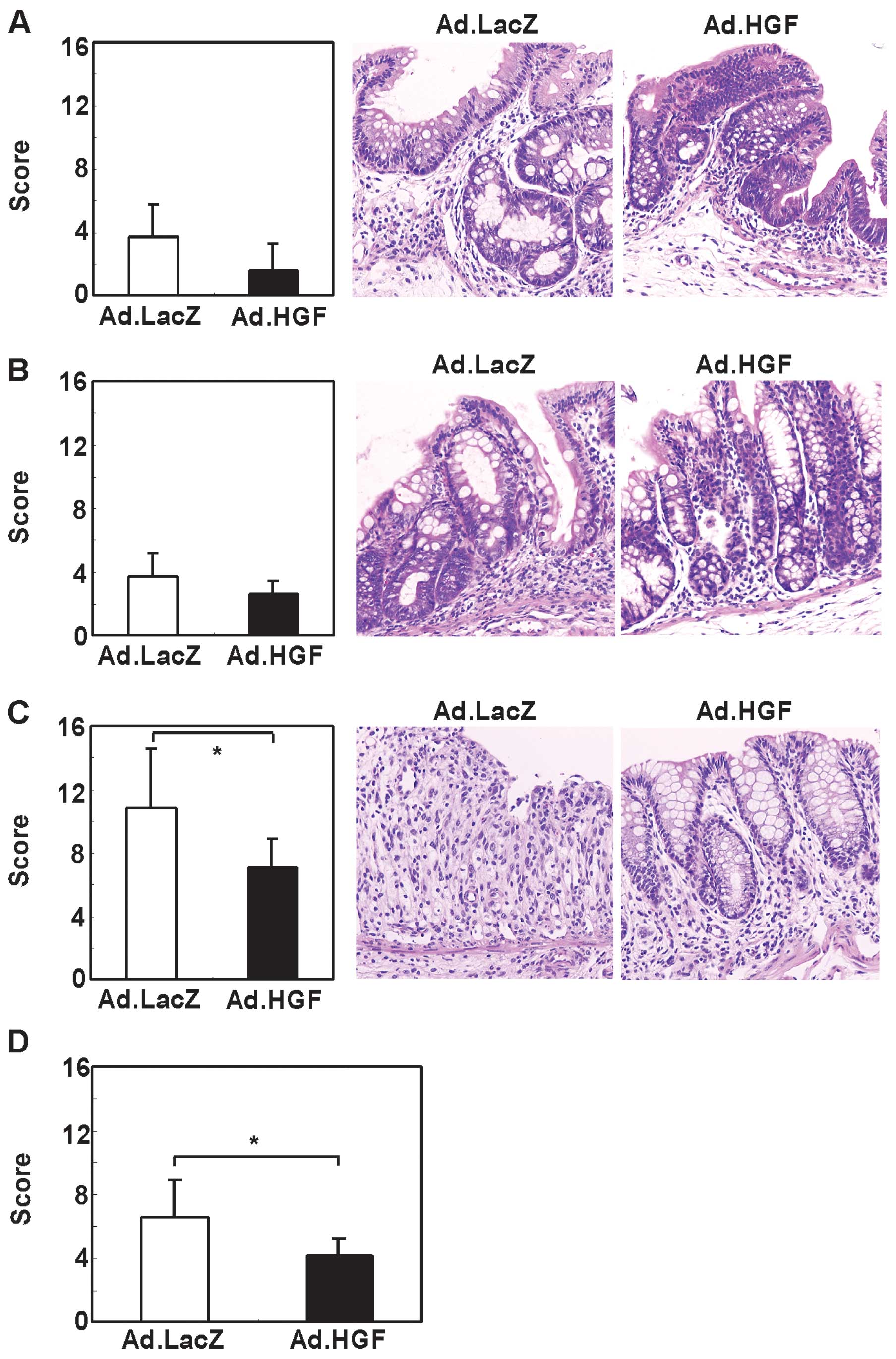
To validate this finding, we evaluated the effect of
Ad.HGF on DSS-induced colonic mucosal injury in mice by
histological analysis at day 15. In the cecum and proximal part of
the colon (i.e., towards the end of the cecum), the inflammation
and crypt scores appeared to be decreased by Ad.HGF administration
although this difference was not statistically significant
(Figs. 4A and B, 5A and B). By contrast, treatment with
Ad.HGF significantly decreased the inflammation and crypt scores in
the distal part (i.e., towards the anus) and in the colon overall
(Figs. 4C and D, 5C and D).
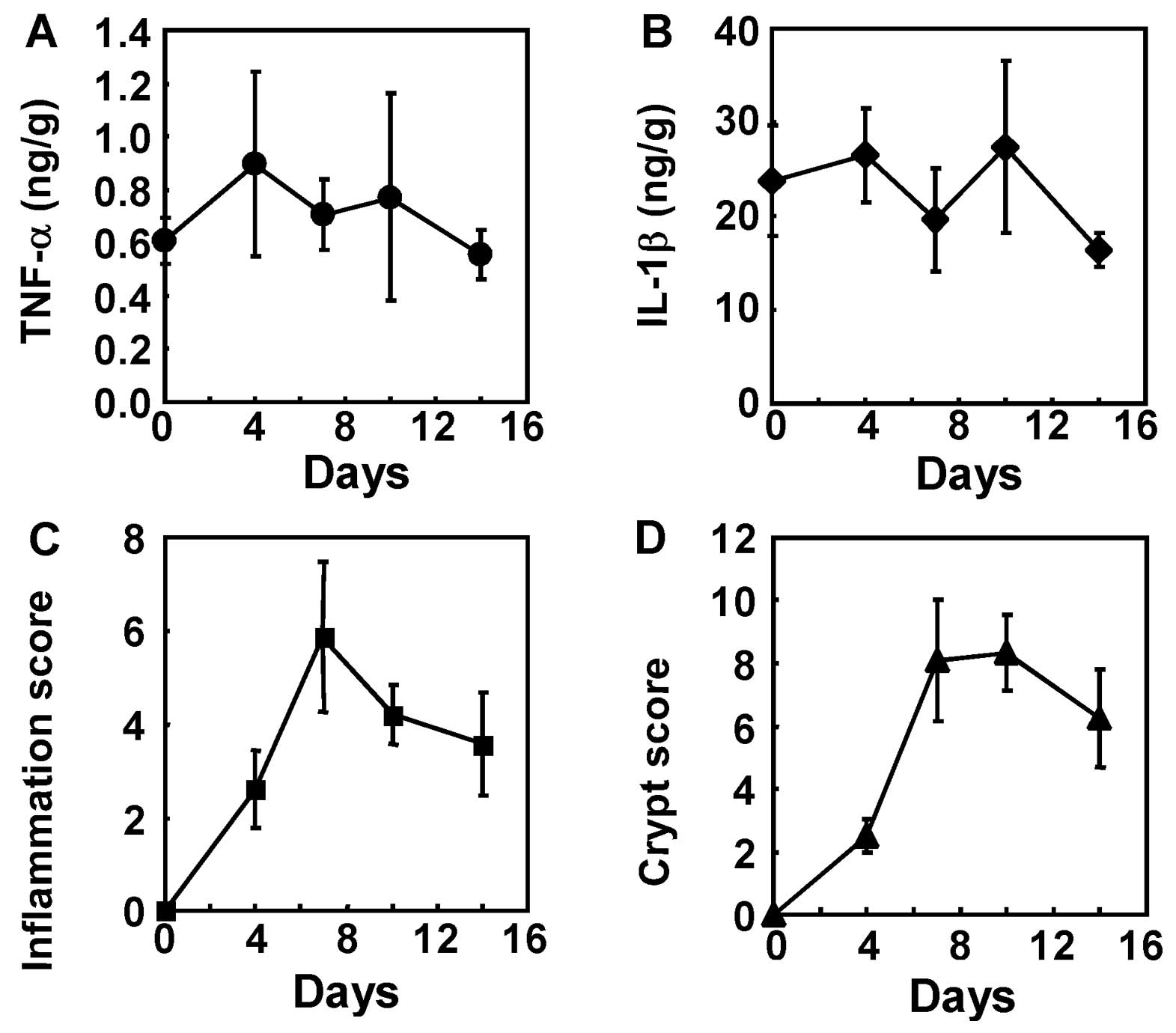
Kinetics of inflammation in colitic
mice
To elucidate the mechanism underlying the
therapeutic effect of hHGF, we studied the expression of TNF-α and
IL-1β in the colon and evaluated the inflammation and crypt scores
at days 4, 7, 10 and 14 of the experimental colitis model (Fig. 6). The expression of TNF-α and
IL-1β peaked as early as day 4 (Fig.
6A and B). The inflammation and crypt scores peaked as early as
day 7 (Fig. 6C and D). Given that
the plasma concentration of hHGF protein peaked on day 2 and
decreased thereafter, colon tissue were sampled and hHGF functions
were analyzed on day 5.
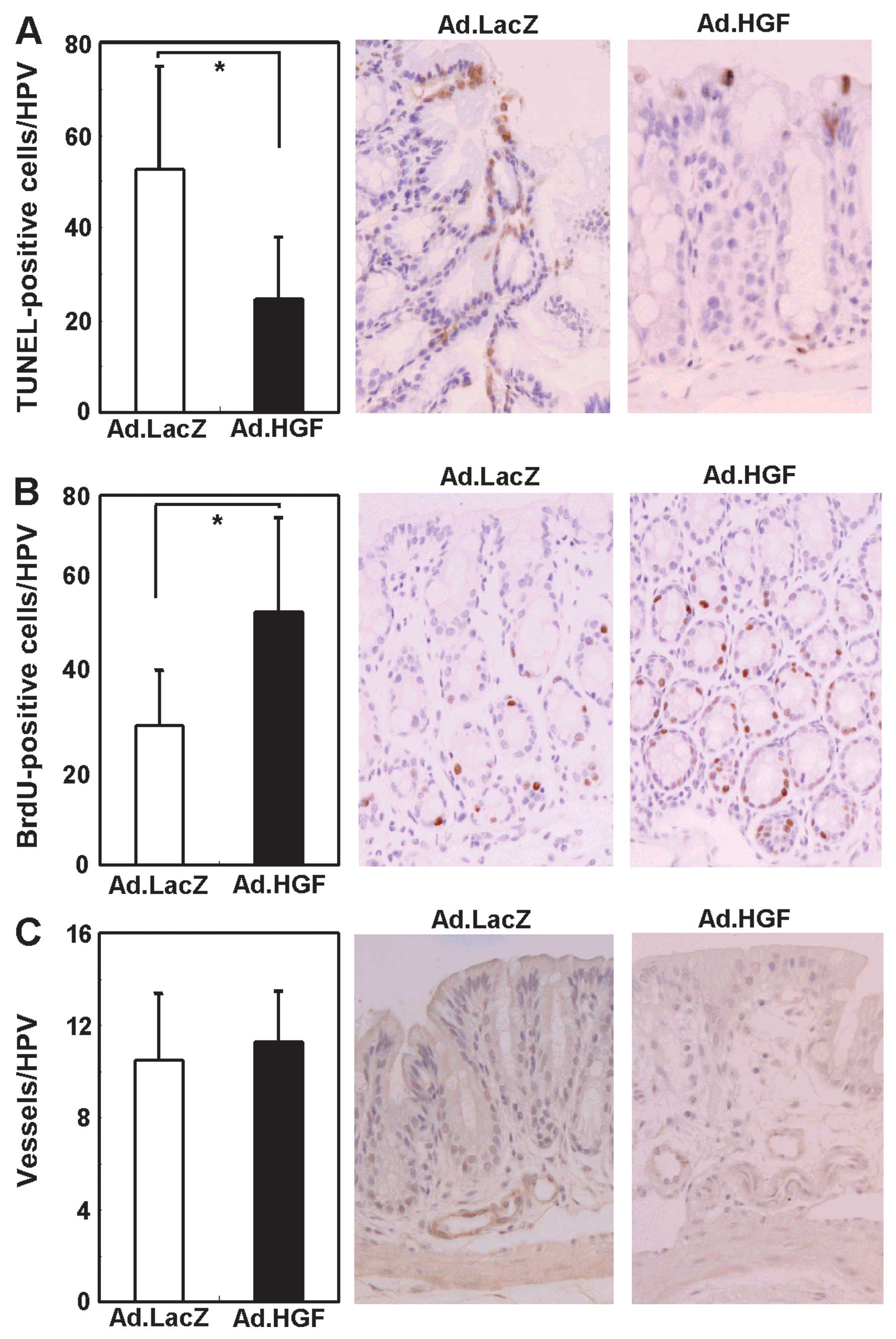
Adenoviral hHGF IMGT suppresses apoptosis
and enhances regeneration of the colonic epithelium
In DSS-induced colitis, loss of colonic mucosal
epithelial cells is closely associated with apoptosis (37,38). To evaluate the role of Ad.HGF in
preventing apoptosis in colonic epithelial cells, we performed the
TUNEL assay to detect apoptotic cells (Fig. 7A). Ad.HGF-treated colitic mice had
significantly (2.1-fold) fewer TUNEL-positive cells per high-power
field (HPF) than Ad.LacZ-treated colitic mice.
To determine whether Ad.HGF-injection stimulated the
proliferation of colonic epithelial cells, we measured the DNA
labeling index in the colonic mucosal epithelium. As shown in
Fig. 7B, the average number of
BrdU-positive cells in the colonic mucosal epithelium was
significantly (1.8-fold) higher in Ad.HGF-treated as compared to
Ad.LacZ-treated mice, suggesting that hHGF stimulates proliferation
in the colonic epithelial cells of colitic mice. These results
suggested that adenoviral hHGF IMGT promoted survival and
regeneration of the colonic mucosal epithelium in mice with
DSS-induced colitis. HGF is known to promote angiogenesis (10). Therefore, we hypothesized that the
angiogenic effect of HGF may contribute to the repair of the
damaged colonic epithelium. However, when we analyzed angiogenesis
in the distal part of the colon by anti-vWF immunohistochemistry,
the number of blood vessels in the colon did not differ
significantly between Ad.HGF-treated mice and controls, although a
few more vessels appeared to be present in Ad.HGF-treated animals
(Fig. 7C).
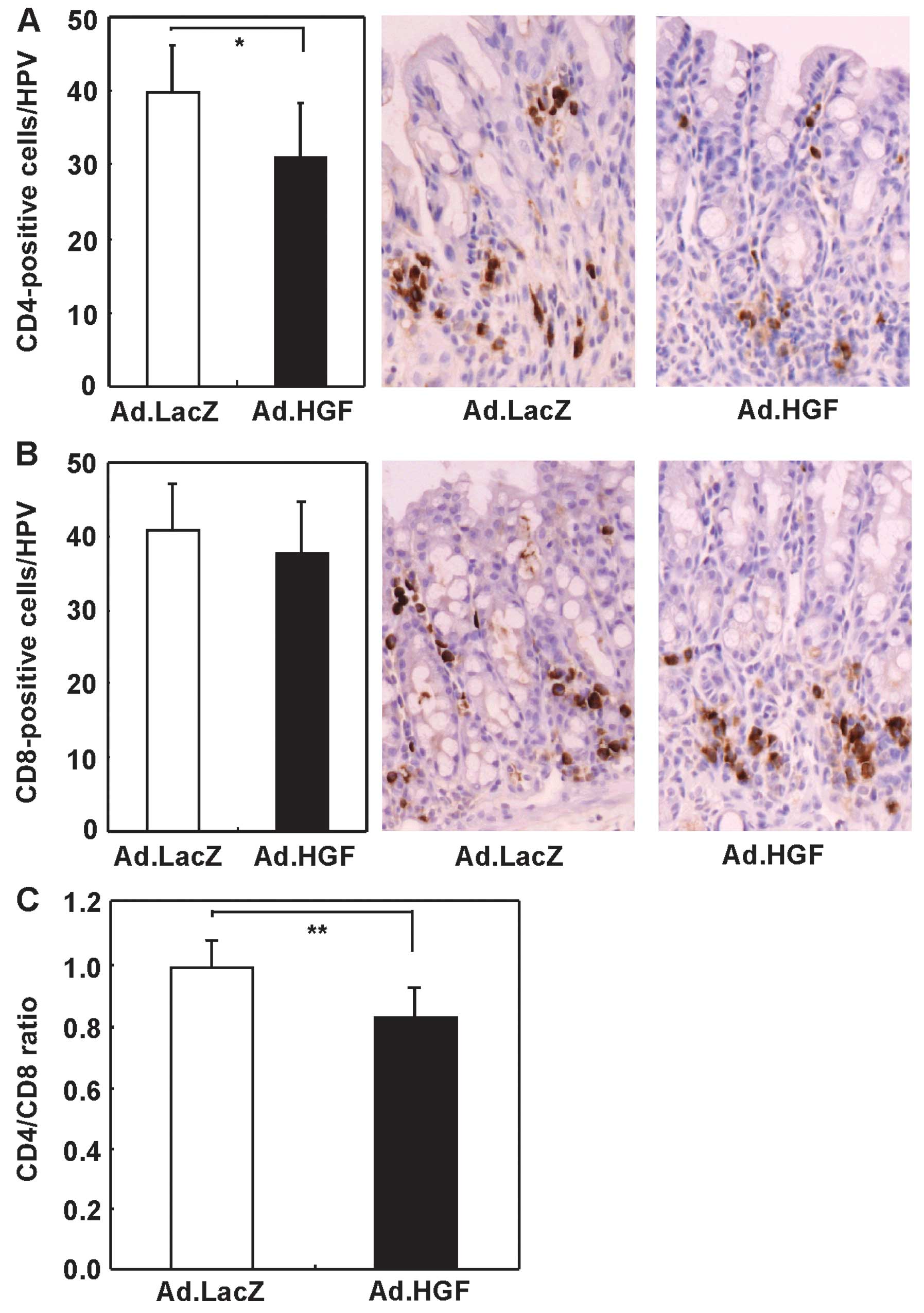
Effects of adenoviral hHGF IMGT on
immunoreactive cells and inflammatory cytokines in DSS-induced
colitis
To determine whether IMGT of hHGF affected the
immune system of DSS-treated mice, we directly detected immune
cells in the colon. Adenoviral hHGF IMGT decreased the number of
CD4+ T cells and the CD4/CD8 ratio, but not the number
of CD8+ T cells (Fig.
8).
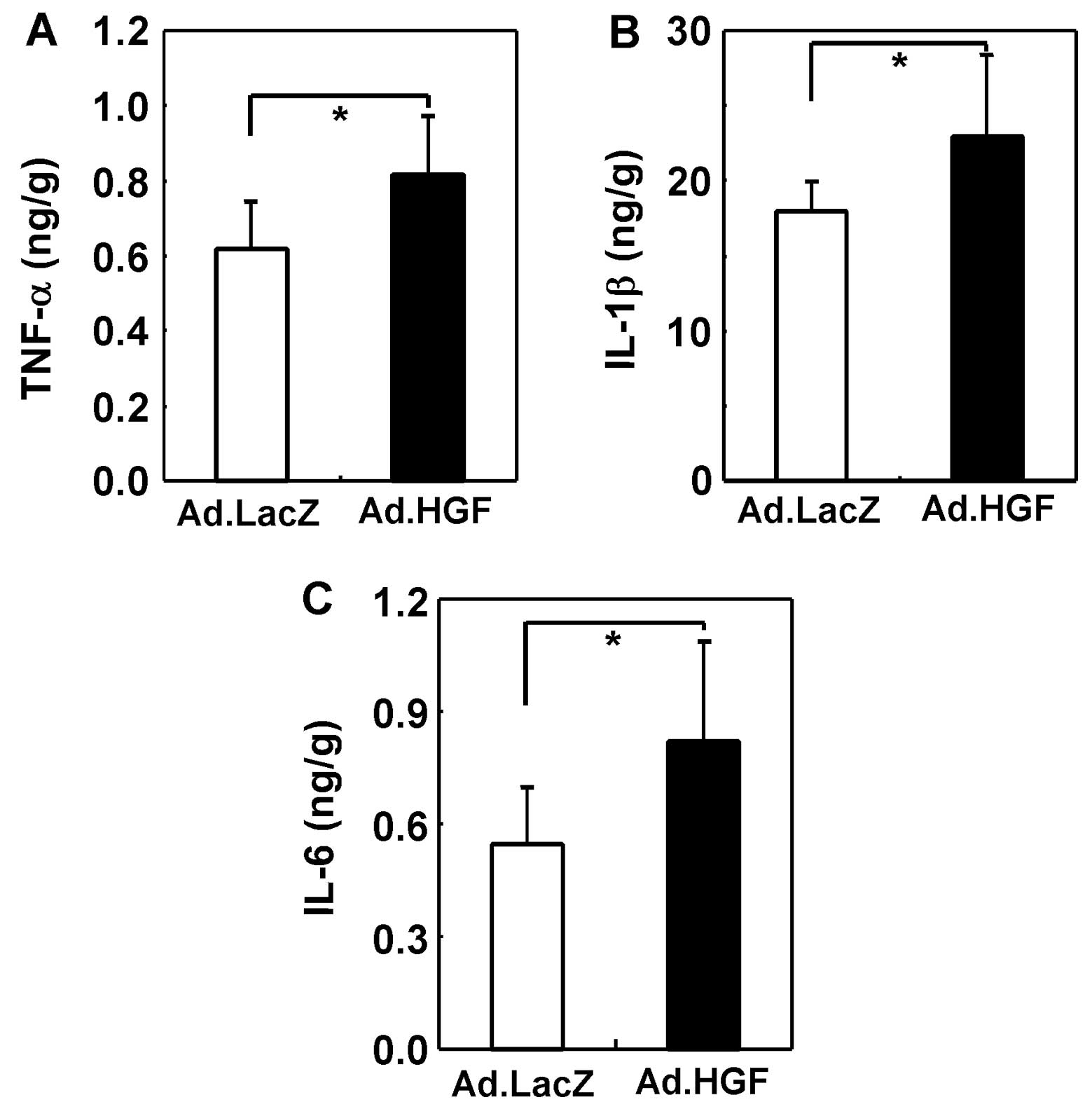
The inflammatory cytokine cascade plays an important
role in the pathogenesis of DSS-induced colitis. Therefore, we
analyzed the cytokine profile of the entire colon by ELISA. In
general, we observed upregulation of pro-inflammatory cytokines
(TNF-α, IL-1β and IL-6) in the colitic mice (39,40). The expression levels of TNF-α,
IL-1β and IL-6 were further increased by hHGF IMGT (Fig. 9).
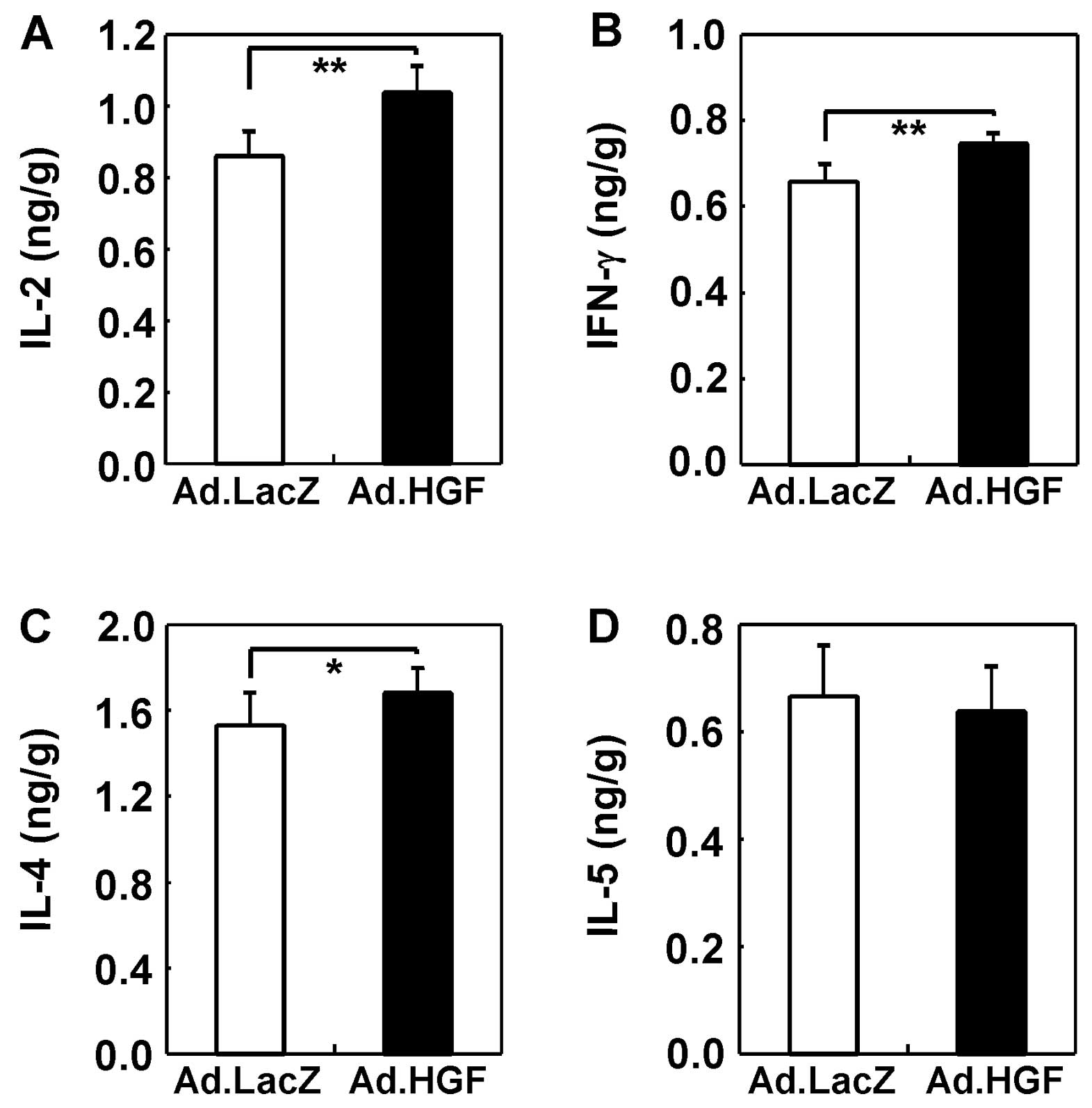
We also examined the effect of hHGF IMGT on Th1
(IFN-γ and IL-2) and Th2 (IL-4 and IL-5) cytokine expression in the
colons of colitic mice. IFN-γ, IL-2 and IL-4 were upregulated by
hHGF treatment (Fig. 10).
Discussion
This study evaluated the therapeutic potential of
the intramuscular injection of HGF-expressing Ad for treating IBD,
using a mouse model of DSS-induced colitis. The therapeutic
strategy of adenoviral HGF IMGT, in which hHGF protein was produced
at distal sites (hindlimbs) and systemically delivered to the
target organ (the injured colon epithelium), functioned well.
Epithelial cell injury in DSS-induced colitis was potently
prevented by this method, which is clinically feasible, less
invasive, and does not suffer from the drawbacks associated with
the direct treatment of colitic tissues. Although previous studies
(16–18) have shown that HGF exerts
protective effects in bowel disease, the regimens tested involved
high levels of recombinant HGF protein (>100 μg/kg) and repeated
injections.
Recent advances in molecular techniques have
provided several strategies for in vivo gene delivery,
including naked plasmid DNA, liposomes encapsulating DNA, and viral
vectors (41,42). For instance, Hanawa et al
(22) reported that
administration of the naked HGF gene into the liver attenuated
acute colitis in mice, and Kanbe et al (23) showed that intrarectal
administration of a plasmid carrying the HGF gene ameliorated
DSS-induced colitis in mice. Kanayama et al (24) found that colonic epithelial
regeneration is promoted by HGF gene transfer via electroporation.
Oh et al (43) reported
that HVJ liposomes encapsulating the hHGF gene ameliorated
TNBS-induced colitis in mice, and that intrarectal administration
of an Ad carrying the HGF gene improved colonic damage in
TNBS-induced colitis (21).
However, each type of gene therapy system used thus far has some
associated limitations and concerns, particularly from the
viewpoints of clinical applicability, feasibility and safety
(41,42).
In this study, we assessed for the first time the
therapeutic potential of a unique method of adenoviral hHGF IMGT
for treating IBDs. In accordance with the results obtained in our
previous studies of a mouse model of myocardial infarction
(25,36), we successfully detected
circulating hHGF in the plasma of colitic mice after adenoviral
hHGF IMGT. In the colons of colitic mice that received adenoviral
hHGF IMGT, the c-Met/HGF receptor was highly phosphorylated on
tyrosine, demonstrating the functional efficacy of the adenoviral
hHGF IMGT system. Furthermore, hHGF IMGT stimulated proliferation
and inhibited apoptosis in the disrupted intestinal epithelial
barrier. These results indicate that our hHGF IMGT system induces
protection and regeneration in the colon, suggesting that it would
be useful in clinical treatments for bowel diseases.
The effects of HGF on carcinogenesis remain unclear.
Some studies suggest that HGF may promote the growth and metastasis
of some cancer types, probably via the stimulation of cancer cell
growth and angiogenesis (44,45). By contrast, carcinogenesis or
malignant phenotypes in other cancer types are potently inhibited
by overexpressed HGF (33). The
effects of HGF on IBDs are also unclear. In general, tumor
development may be caused by long-term exposure of cells to an
abnormally overexpressed growth factor. In our therapeutic system,
the duration of hHGF secretion after single rounds of intramuscular
injection was relatively short; therefore, we consider the risk of
cancer occurrence to be very low. In addition, a previous study
demonstrated the efficacy of repeated administration of Ad into
muscles, suggesting that this approach may yield sustained and
elevated therapeutic efficiency: neutralizing antibodies against
adenovirus should hinder only Ad circulating in the bloodstream,
but not Ad administered into the muscle (46). These findings are encouraging with
regard to the potential safety and clinical applicability of this
approach.
With regard to the therapeutic mechanism, previous
studies have reported that administration of recombinant HGF
protein (16) and vector encoding
HGF gene (43) ameliorate
TNBS-induced colitis and reduced inflammation, decreasing the
levels of inflammatory cytokines such as TNF-α. In particular, Oh
et al (43) showed that
administration of a plasmid carrying the HGF gene reduced the
invasion of CD4+ cells and neutrophils and suppressed
the expression of Th1 cytokines such as IL-12, IL-1β and IFN-γ in a
TNBS-induced colitis model. Hanawa et al (22) showed that administration of an HGF
gene-containing plasmid in the liver by intravenous injection
suppressed the mRNA levels of IFN-γ, IL-18 and TNF-α, and increased
the mRNA levels of anti-inflammatory cytokines such as IL-10.
Jeschke et al (47) found
that recombinant HGF reduced burn-related damage to the small
intestine. The serum levels of TNF-α, IL-1β and IL-6 were higher in
the HGF-treated group than in the control group. However, Jeschke
et al (47) did not
explain why the levels of these cytokines were increased by HGF.
Our data indicate that the number of CD4+ cells
decreased, but the levels of TNF-α, IL-1β and IL-6, as well as
those of Th1 and Th2 cytokines such as IL-2, IFN-γ and IL-4, were
elevated in the Ad.HGF-treated group. We hypothesize that the
reasons for the differences between our findings and those of
previous studies may involve differences among mouse strains, our
use of intramuscular gene administration mediated by an Ad, and our
selection of the early phase of DSS colitis for analysis of
inflammation and cytokine expression.
Futamatsu et al (48) reported that HGF suppressed T-cell
proliferation and IFN-γ production and increased IL-4 and IL-10
secretion from CD4+ T cells in vitro, and also
reduced the severity of experimental autoimmune myocarditis in
vivo by inducing Th2 cytokines and suppressing apoptosis of
cardiomyocytes. Kuroiwa et al (49) demonstrated that HGF gene delivery
inhibited Th2 immune responses and ameliorated lupus nephritis,
autoimmune sialadenitis, and cholangitis in chronic GVHD mice.
Another study indicated that treatment with HGF potently suppressed
dendritic cell functions such as antigen-presenting capacity, both
in vitro and in vivo, thus downregulating
antigen-induced Th1 and Th2 immune responses in a mouse model of
allergic airway inflammation (50). HGF has been suggested to suppress
airway hyper-responsiveness, inflammation, remodeling, and
eosinophil function in asthma (51). Okunishi et al (52) reported that HGF suppresses
antigen-induced T-cell priming by regulating the functions of
dendritic cells through IL-10 downregulation in the
antigen-sensitization phase. By contrast, they found that repeated
treatment with HGF induced Th2 immune responses with the
upregulation of IL-10 by DCs in the chronic inflammation phase of a
mouse model of collagen-induced arthritis. Thus, it is clear that
HGF induces various immune responses in different disease models.
However, further analysis is required to clarify the effects of HGF
on the immune system.
In conclusion, we have shown that a single round of
intramuscular injections of adenoviral hHGF is sufficient to
inhibit apoptosis and reconstitute the epithelium in a mouse model
of DSS-induced colitis. Based on these results, this approach shows
promise for clinical application in IBD.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Scientific Research (C) from the Ministry of Education,
Culture, Sports, Science and Technology of Japan. We would like to
thank Hatsue Oshika for her technical assistance.
References
|
1
|
Hisamatsu T, Kanai T, Mikami Y, Yoneno K,
Matsuoka K and Hibi T: Immune aspects of the pathogenesis of
inflammatory bowel disease. Pharmacol Ther. 137:283–297. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernklev T, Jahnsen J, Aadland E, et al:
Health-related quality of life in patients with inflammatory bowel
disease five years after the initial diagnosis. Scand J
Gastroenterol. 39:365–373. 2004.PubMed/NCBI
|
|
3
|
Burger D and Travis S: Conventional
medical management of inflammatory bowel disease. Gastroenterology.
140:1827–1837. e18222011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura T, Nawa K and Ichihara A: Partial
purification and characterization of hepatocyte growth factor from
serum of hepatectomized rats. Biochem Biophys Res Commun.
122:1450–1459. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Russell WE, McGowan JA and Bucher NL:
Partial characterization of a hepatocyte growth factor from rat
platelets. J Cell Physiol. 119:183–192. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thaler FJ and Michalopoulos GK:
Hepatopoietin A: partial characterization and trypsin activation of
a hepatocyte growth factor. Cancer Res. 45:2545–2549.
1985.PubMed/NCBI
|
|
7
|
Gohda E, Tsubouchi H, Nakayama H, et al:
Human hepatocyte growth factor in plasma from patients with
fulminant hepatic failure. Exp Cell Res. 166:139–150. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura T, Nishizawa T, Hagiya M, et al:
Molecular cloning and expression of human hepatocyte growth factor.
Nature. 342:440–443. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyazawa K, Tsubouchi H, Naka D, et al:
Molecular cloning and sequence analysis of cDNA for human
hepatocyte growth factor. Biochem Biophys Res Commun. 163:967–973.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsumoto K and Nakamura T: Hepatocyte
growth factor (HGF) as a tissue organizer for organogenesis and
regeneration. Biochem Biophys Res Commun. 239:639–644. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuno M, Shiota G, Umeki K, Kawasaki H,
Kojo H and Miura K: Clinical evaluation of hepatocyte growth factor
in patients with gastrointestinal and pancreatic diseases with
special reference to inflammatory bowel disease. Res Commun Mol
Pathol Pharmacol. 97:25–37. 1997.PubMed/NCBI
|
|
12
|
Kitamura S, Kondo S, Shinomura Y, et al:
Expression of hepatocyte growth factor and c-met in ulcerative
colitis. Inflamm Res. 49:320–324. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Srivastava M, Zurakowski D, Cheifetz P,
Leichtner A and Bousvaros A: Elevated serum hepatocyte growth
factor in children and young adults with inflammatory bowel
disease. J Pediatr Gastroenterol Nutr. 33:548–553. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ortega-Cava CF, Ishihara S, Kawashima K,
et al: Hepatocyte growth factor expression in dextran sodium
sulfate-induced colitis in rats. Dig Dis Sci. 47:2275–2285. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dignass AU, Lynch-Devaney K and Podolsky
DK: Hepatocyte growth factor/scatter factor modulates intestinal
epithelial cell proliferation and migration. Biochem Biophys Res
Commun. 202:701–709. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Numata M, Ido A, Moriuchi A, et al:
Hepatocyte growth factor facilitates the repair of large colonic
ulcers in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in
rats. Inflamm Bowel Dis. 11:551–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tahara Y, Ido A, Yamamoto S, et al:
Hepatocyte growth factor facilitates colonic mucosal repair in
experimental ulcerative colitis in rats. J Pharmacol Exp Ther.
307:146–151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohda Y, Hori K, Tomita T, et al: Effects
of hepatocyte growth factor on rat inflammatory bowel disease
models. Dig Dis Sci. 50:914–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Setoyama H, Ido A, Numata M, et al:
Repeated enemas with hepatocyte growth factor selectively stimulate
epithelial cell proliferation of injured mucosa in rats with
experimental colitis. Life Sci. 89:269–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arthur LG, Schwartz MZ, Kuenzler KA and
Birbe R: Hepatocyte growth factor treatment ameliorates diarrhea
and bowel inflammation in a rat model of inflammatory bowel
disease. J Pediatr Surg. 39:139–143. 2004. View Article : Google Scholar
|
|
21
|
Mukoyama T, Kanbe T, Murai R, et al:
Therapeutic effect of adenoviral-mediated hepatocyte growth factor
gene administration on TNBS-induced colitis in mice. Biochem
Biophys Res Commun. 329:1217–1224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanawa T, Suzuki K, Kawauchi Y, et al:
Attenuation of mouse acute colitis by naked hepatocyte growth
factor gene transfer into the liver. J Gene Med. 8:623–635. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanbe T, Murai R, Mukoyama T, et al: Naked
gene therapy of hepatocyte growth factor for dextran sulfate
sodium-induced colitis in mice. Biochem Biophys Res Commun.
345:1517–1525. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanayama M, Takahara T, Yata Y, et al:
Hepatocyte growth factor promotes colonic epithelial regeneration
via Akt signaling. Am J Physiol Gastrointest Liver Physiol.
293:G230–G239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Takemura G, Kosai K, et al:
Postinfarction treatment with an adenoviral vector expressing
hepatocyte growth factor relieves chronic left ventricular
remodeling and dysfunction in mice. Circulation. 107:2499–2506.
2003. View Article : Google Scholar
|
|
26
|
Chen SH, Chen XH, Wang Y, et al:
Combination gene therapy for liver metastasis of colon carcinoma in
vivo. Proc Natl Acad Sci USA. 92:2577–2581. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takahashi T, Kawai T, Ushikoshi H, et al:
Identification and isolation of embryonic stem cell-derived target
cells by adenoviral conditional targeting. Mol Ther. 14:673–683.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okabe Y, Kusaga A, Takahashi T, et al:
Neural development of methyl-CpG-binding protein 2 null embryonic
stem cells: a system for studying Rett syndrome. Brain Res.
1360:17–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horikawa Y, Wang Y, Nagano S, et al:
Assessment of an altered E1B promoter on the specificity and
potency of triple-regulated conditionally replicating adenoviruses:
implications for the generation of ideal m-CRAs. Cancer Gene Ther.
18:724–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990.PubMed/NCBI
|
|
31
|
Tomoyose M, Mitsuyama K, Ishida H,
Toyonaga A and Tanikawa K: Role of interleukin-10 in a murine model
of dextran sulfate sodium-induced colitis. Scand J Gastroenterol.
33:435–440. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanauchi O, Nakamura T, Agata K, Mitsuyama
K and Iwanaga T: Effects of germinated barley foodstuff on dextran
sulfate sodium-induced colitis in rats. J Gastroenterol.
33:179–188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuge K, Takahashi T, Nagano S, et al:
Adenoviral gene transduction of hepatocyte growth factor elicits
inhibitory effects for hepatoma. Int J Oncol. 27:77–85.
2005.PubMed/NCBI
|
|
34
|
Kamisasanuki T, Tokushige S, Terasaki H,
et al: Targeting CD9 produces stimulus-independent antiangiogenic
effects predominantly in activated endothelial cells during
angiogenesis: a novel antiangiogenic therapy. Biochem Biophys Res
Commun. 413:128–135. 2011. View Article : Google Scholar
|
|
35
|
Murthy SN, Cooper HS, Shim H, Shah RS,
Ibrahim SA and Sedergran DJ: Treatment of dextran sulfate
sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis
Sci. 38:1722–1734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Takemura G, Kosai K, et al: Critical
roles for the Fas/Fas ligand system in postinfarction ventricular
remodeling and heart failure. Circ Res. 95:627–636. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iwamoto M, Koji T, Makiyama K, Kobayashi N
and Nakane PK: Apoptosis of crypt epithelial cells in ulcerative
colitis. J Pathol. 180:152–159. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sträter J, Wellisch I, Riedl S, et al:
CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a
possible role in ulcerative colitis. Gastroenterology. 113:160–167.
1997.PubMed/NCBI
|
|
39
|
Rogler G and Andus T: Cytokines in
inflammatory bowel disease. World J Surg. 22:382–389. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Egger B, Bajaj-Elliott M, MacDonald TT,
Inglin R, Eysselein VE and Büchler MW: Characterisation of acute
murine dextran sodium sulphate colitis: cytokine profile and dose
dependency. Digestion. 62:240–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tomanin R and Scarpa M: Why do we need new
gene therapy viral vectors? Characteristics, limitations and future
perspectives of viral vector transduction. Curr Gene Ther.
4:357–372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boulaiz H, Marchal JA, Prados J, Melguizo
C and Aránega A: Non-viral and viral vectors for gene therapy. Cell
Mol Biol (Noisy-le-grand). 51:3–22. 2005.
|
|
43
|
Oh K, Iimuro Y, Takeuchi M, et al:
Ameliorating effect of hepatocyte growth factor on inflammatory
bowel disease in a murine model. Am J Physiol Gastrointest Liver
Physiol. 288:G729–G735. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shiota G, Kawasaki H, Nakamura T and
Schmidt EV: Characterization of double transgenic mice expressing
hepatocye growth factor and transforming growth factor alpha. Res
Commun Mol Pathol Pharmacol. 90:17–24. 1995.PubMed/NCBI
|
|
45
|
Sakata H, Takayama H, Sharp R, Rubin JS,
Merlino G and LaRochelle WJ: Hepatocyte growth factor/scatter
factor overexpression induces growth, abnormal development, and
tumor formation in transgenic mouse livers. Cell Growth Differ.
7:1513–1523. 1996.
|
|
46
|
Chen P, Kovesdi I and Bruder JT: Effective
repeat administration with adenovirus vectors to the muscle. Gene
Ther. 7:587–595. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jeschke MG, Bolder U, Finnerty CC, et al:
The effect of hepatocyte growth factor on gut mucosal apoptosis and
proliferation, and cellular mediators after severe trauma. Surgery.
138:482–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Futamatsu H, Suzuki J, Mizuno S, et al:
Hepatocyte growth factor ameliorates the progression of
experimental autoimmune myocarditis: a potential role for induction
of T helper 2 cytokines. Circ Res. 96:823–830. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kuroiwa T, Iwasaki T, Imado T, Sekiguchi
M, Fujimoto J and Sano H: Hepatocyte growth factor prevents lupus
nephritis in a murine lupus model of chronic graft-versus-host
disease. Arthritis Res Ther. 8:R1232006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Okunishi K, Dohi M, Nakagome K, et al: A
novel role of hepatocyte growth factor as an immune regulator
through suppressing dendritic cell function. J Immunol.
175:4745–4753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ito W, Takeda M, Tanabe M, et al:
Anti-allergic inflammatory effects of hepatocyte growth factor. Int
Arch Allergy Immunol. 146(Suppl 1): S82–S87. 2008. View Article : Google Scholar
|
|
52
|
Okunishi K, Dohi M, Fujio K, et al:
Hepatocyte growth factor significantly suppresses collagen-induced
arthritis in mice. J Immunol. 179:5504–5513. 2007. View Article : Google Scholar : PubMed/NCBI
|