Introduction
Autologous myoblasts that have expanded from
satellite cells isolated from muscle biopsy samples constitute the
basis for cell replacement therapies in regenerative medicine for
the treatment of urinary incontinence, ischemia-damaged myocardium
or Duchenne muscular dystrophy. Clinical reports on autologous
muscle-derived cells (MDCs) used for the treatment of either
ischemia-damaged myocardium or urinary incontinence in humans have
demonstrated the dependency of the therapeutic effect on the number
of injected cells, with >1×108 myoblasts required to
obtain significant results or improved symptoms (1–3).
These observations indicate that this treatment strategy may
require a high number of competent cells to obtain a sufficient
therapeutic effect.
Satellite cells remain in a quiescent state in
uninjured tissue. Minor damage or injury to the tissue stimulates
the release of hepatocyte growth factor (HGF) and basic fibroblast
growth factor (bFGF) from injured myofibers (4,5).
HGF activates satellite cells and causes them to enter the cell
cycle, proliferate, differentiate and fuse to regenerate injured
muscle fibers (4). HGF has been
shown to be the only growth factor capable of activating satellite
cells in in vitro primary culture (6–8).
bFGF has been shown to enhance myoblast proliferation by increasing
cyclin-D1 mRNA expression between 4 and 8 h post-induction with a
return to initial levels by 32 h post-induction (9). Notably, bFGF has been reported to
enhance the HGF-stimulated proliferation of myoblasts (10) and to repress the terminal
differentiation of myoblasts (11). McGeachie and Grounds have shown
in vivo the presence of dividing myoblasts up to 120 h after
damage (12). However, this rate
of proliferation is not maximal and can be increased in
vitro by the addition of members of the fibroblast growth
factor family (13,14). Epidermal growth factor (EGF),
platelet-derived growth factor (PDGF) and tumor growth factor
(TGF)-β have also been reported to enhance myoblast proliferation
in vitro (15–17). When proliferating myoblasts must
withdraw from the cell cycle to differentiate, growth factors, such
as HGF and bFGF, which stimulate cell cycle progression, regulate
the activity of myogenic regulatory transcription factors, such as
MyoD, myogenic factor 5 (Myf5), myogenin and myogenic regulatory
factor (MRF)4, that have been shown to control the specification
and differentiation of the muscle lineage (18). During regeneration, activated
satellite cells reportedly initially express either Myf5, MyoD or
both (19,20). Myogenin is required for the
differentiation of myoblasts (21); MRF4 is thought to be involved in
the maturation of myotubes (22).
Myostatin, a growth factor and a TGF-β superfamily
member, is a specific negative regulator of skeletal muscle mass
(23). This growth factor has
been shown to play a role in regulating the activation, growth and
self-renewal of satellite cells (24) and to inhibit the growth of
myoblasts (25). Myostatin has
also been shown to negatively regulate myogenic differentiation by
inhibiting the mRNA and protein expression of MyoD, Myf5, myogenin
and myosin heavy chain 2A (MyHC-2A) (26,27). MyHC-2A is one of 3 fast-type
isoforms of a muscle contractile protein known as myosin heavy
chain (28). In low seeding
density cultures without supplemental growth factors, MyHC-2A mRNA
expression has been shown to increase in parallel with a decrease
in Myf5 and myogenin expression; this result indicates a
correlation with phenotypic differentiation (29).
Initial in vitro experiments with muscle cell
progenitor cultures have been performed in Ham’s F10 or Ham’s F-12
media (30,31) and have also been performed in
other media, such as Dulbecco’s modified Eagle’s medium (DMEM)
(32,33). However, the use of these media
results in a low number of cells. Published culture strategies
aimed at increasing the number of obtained myoblasts have
emphasized the importance of proteins used for flask covering,
supplementation with various growth factors and different cell
passaging strategies, as well as the effect of these variables on
the kinetics and the proliferation potential of myoblast expansion
(17,29,31,34–36). The effectiveness of EGF, FGF and
PDGF growth factors in enhancing expansion capacity has also been
reported (16,36). Thus, a higher number of myoblasts
can be obtained using skeletal muscle cell growth medium (SKGM)
(3,37) or DMEM with the addition of growth
factors. A high proportion of serum and non-confluent culture
conditions have been shown to prevent myogenic differentiation
(38). An automated culture
system indicated that the optimal seeding density and attainable
confluence was 1×103 cells/cm2 and 50%
confluence, respectively (39).
However, a recent study indicated that prolonged culture leads to
an increased percentage of senescent cells, a decreased ability of
myoblasts to form myotubes and alterations in glucose and lipid
metabolism (40).
In the present study, we compared the commercial
medium, SkGM™-2 BulletKit™Medium (SKGM-2; current version of the no
longer available SKGM), with a medium designed by our group
[DMEM/F12 medium supplemented with EGF, bFGF, HGF, insulin and
dexamethasone (DFEFH)]. The aim of this study was to seek the
optimal large-scale expansion conditions of functional MDCs for
regenerative medicinal use by comparing the expansion efficacy and
the fusogenic potential of skeletal myoblasts in these 2 media.
We demonstrate culturing conditions that produce up
to 5×109 myoblasts, presenting fusogenic potential in 24
days. We also demonstrate that the number of myoblast colonies, the
doubling time, the duration of the logarithmic growth phase, the
number of expanded cells and the cell morphology strongly depend on
culture conditions. Our results demonstrated that the use of an
in-house medium resulted in an approximately 100-fold greater
number of cells of less differentiated morphology and higher fusion
potential during 3 passages compared with cells cultured in SKGM-2
medium. Additionally, we show that the expansion of myoblasts over
23 generations results in a rapid decrease in the proliferation
potential, and an increase in the doubling time, the appearance of
vacuoles and the loss of the fusion potential of these cells.
Materials and methods
Muscle biopsy and primary cell
culture
Deltoid muscle samples of approximately 0.2 g were
acquired from women with stress urinary incontinence (20% available
for the experiments), aged over 50 years, by a needle biopsy
performed under local anesthesia. Both institutional review board
approval and informed consent from all participants were obtained
prior to the biopsy procedure. All muscle biopsies were transported
in phosphate-buffered saline (PBS) with antibiotics (PAA
Laboratories GmbH, Goetzis, Austria) and stored at room temperature
until processing, which was generally performed 2 h after the
biopsy had been obtained. First, the muscle samples were rinsed in
PBS and cleaned by removing adherent fat and tendon tissues using a
scalpel. The muscle tissue was then cut into small sections and
digested with 2 mg/ml of collagenase 1A (Sigma-Aldrich, Seelze,
Germany) for 40 min at 37°C, as previously described (33). Individual fibers were liberated by
rigorous pipetting and were centrifuged at 300 × g for 5 min. The
pellet was resuspended in a small volume of PBS, was divided into 2
parts and was centrifuged again.
To compare the expansion efficacy of the SKGM-2
medium (Lonza, Walkersville, MD, USA) (formulated by combining
SKBM™-2 Basal Medium, CC-3246 with the SkGM™-2 SingleQuots™ kit,
CC-3244) with that of the in-house medium, one-tenth of the muscle
fibers was resuspended in SKGM-2 medium and another one-tenth was
resuspended in the medium designed by our group: DMEM/F-12 (PAA
Laboratories GmbH) supplemented with dexamethasone, insulin (both
from Sigma-Aldrich), 18% fetal bovine serum (FBS) (PAA Laboratories
GmbH) and the growth factors, EGF, FGF (2) and HGF, (DFEFH). The muscle fibers
were then transferred into 6-well plates coated with laminin
(Sigma-Aldrich) to enable the released satellite cells to adhere to
or migrate out of the muscle fibers. The plates were incubated for
48 h in a humidified atmosphere containing 5% CO2. After
48 h, the medium with the muscle fragments that had not adhered to
the plates was changed (P0) and the supernatant containing the
muscle fibers was transferred into new laminin-coated wells (P1).
After a further 48 h, the procedure was repeated and the medium in
all wells was changed every 3 days. At day 8 of culture, the number
of colonies, defined as a cluster of at least 10 cells, was counted
in wells P0 and P1 and summarized.
Myoblast culture
The first passage was performed on day 11; the cells
were passaged from a 6-well plate to a 25 cm2 flask,
with a seeding density of 1,000 cells/1 cm2. The cells
were cultured in SKGM-2 or DFEFH medium at 37°C, 5% CO2
and 95% humidity. The medium was changed twice a week. The cells
were harvested after 7 days and reseeded again at the same density
of 1×103 cells/1 cm2.
Manual cell counts to assess myoblast
number
Every 7 days, the cells were harvested for
reseeding; the cell number was counted using a hematocytometer and
a light microscope (Olympus, Tokyo, Japan).
RNA extraction and reverse
transcription
Total RNA was extracted using an RNeasy mini kit
(Qiagen, Valencia, CA, USA). The reverse polymerase transcription
was performed using reverse transcription reagents (Roche/Applied
Biosystems, Foster City, CA, USA) or M-MLV Reverse Transcriptase
(Promega, Madison, WI, USA) according to the manufacturer’s
instructions.
Quantitative PCR
Myostatin, Myf5, myogenin and MYH2 expression levels
were determined by quantitative (real-time) PCR on an ABI PRISM
7300 Sequence Detection System (Applied Biosystems) using a
commercially available TaqMan PCR master mix. Spanning an exon
junction, the TaqMan probes used for this analysis were as follows:
myostatin (Hs00976237_m1), Myf5 (Hs00271574_m1), myogenin
(Hs01032275_m1), and MYH2 (Hs00430042_m1), all from Applied
Biosystems. The 2−ΔΔCT method was used to quantify the
relative expression levels of the genes. The mRNA expression levels
for all samples were normalized to the housekeeping gene,
GAPDH.
Purity evaluation
The percentage of CD56+ cells was
assessed using monoclonal anti-CD56-APC antibodies (BD Biosciences,
San Jose, CA, USA) and cytofluorymetric evaluation on a FACSCalibur
with a CellQuest analysis software (BD Biosciences Immunocytometry
Systems).
Evaluation of the fusion potential of
MDCs
To evaluate fusion potential, the cells were seeded
in 96-well plates in duplicate; once the cells reached 80%
confluence, the culture medium was changed to DMEM with 2% FBS and
25 μmol/l insulin, as previously described (40). The medium was changed twice a
week. On day 11, the cells were fixed and stained with Wright’s
eosin methylene blue solution (Merck, Darmstadt, Germany). The
number of nuclei in the 6 largest myotubes was counted in each
well.
Statistical analysis
Statistical analysis was performed using a one- or
two-tailed paired Student’s t-test with Microsoft Excel; a value of
p<0.05 was considered to indicate a statistically significant
difference.
Results
Growth of primary myoblast colonies
To isolate the satellite cells, the digested muscle
tissue was seeded in culture medium on laminin-coated plates to
enhance the adherence of satellite cells, as previously described
(34). At the beginning of
myoblast expansion, the same volume of digested muscle tissue was
seeded in single wells of a laminin-coated 6-well plate in 2 types
of medium, SKGM-2 and DFEFH. On day 6, the number of growing
myoblast colonies was counted using an inverted microscope. As
shown in Fig. 1A, culture in the
DFEFH medium resulted in an average of 41 myoblast colonies
compared with 27 myoblast colonies obtained with culture in the
SKGM-2 medium. Thus, a statistically significant increase in the
colony number was observed.
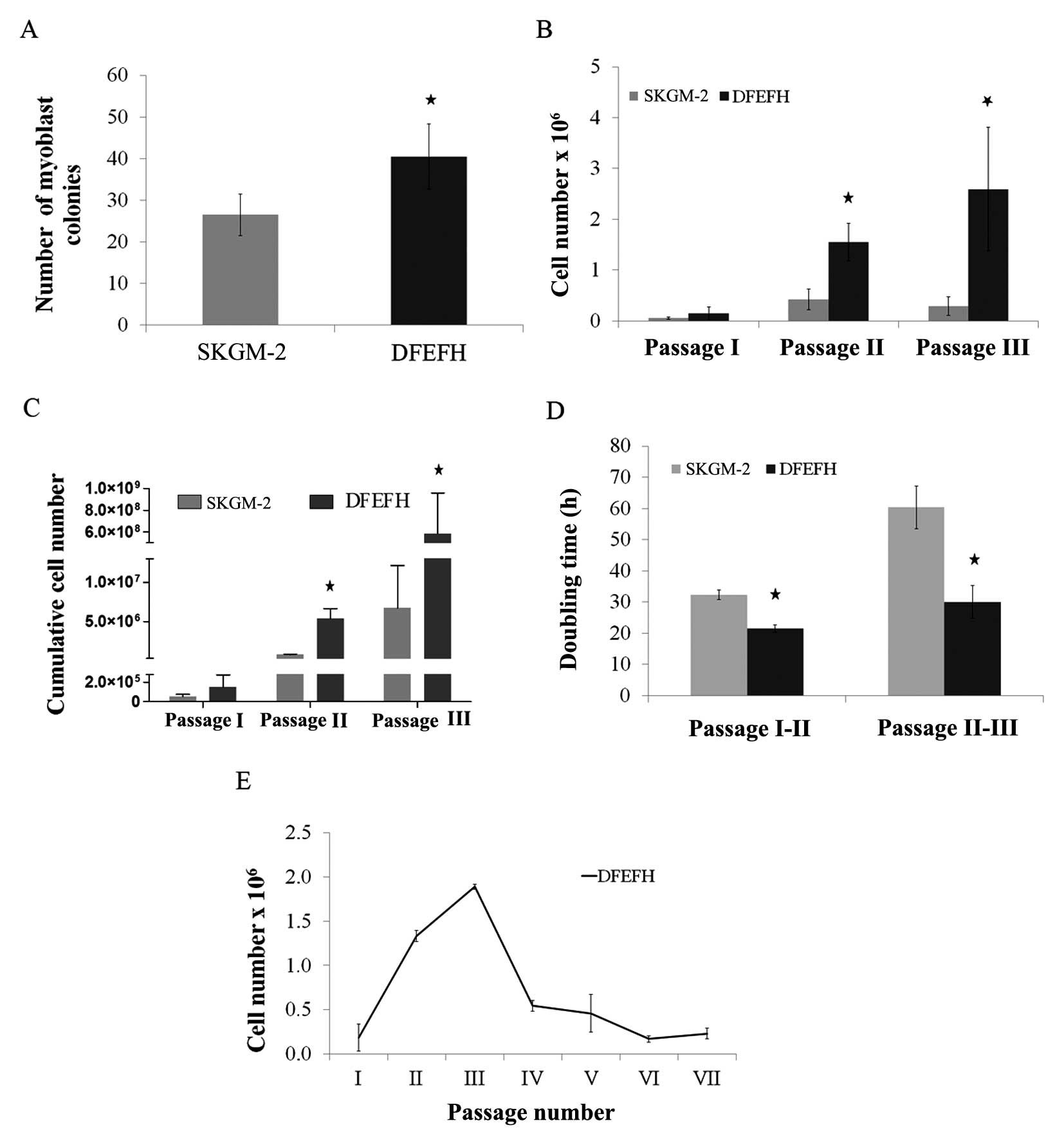 | Figure 1Effect of the culture medium on the
number of myoblast colonies, the doubling time and expansion
efficacy. Suspensions containing muscle fibers and single cells
after collagenase digestion of muscle tissue from approximately
0.02 g of biopsy fragments were seeded on laminin-coated wells of a
6-well plate in skeletal muscle cell growth medium-2 (SKGM-2) or
DFEFH medium (P0). After 48 h, supernatants were transferred into
new wells (P1), fresh medium was added to P0, and the procedure was
repeated after another 48 h. At day 8, the number of colonies,
defined as at least 10 cells, was counted in P0 and P1 and added.
At day 11, the first passage was performed and the cells were
counted. Cells were seeded at a density of 0.025×106/25
cm2 flask and passaged with a constant seeding density
every 6–7 days until the 3rd passage. The cell number was counted
and the doubling time was calculated at each passage. Cells
expanded in DFEFH medium were cultured until passage 7, starting
with the same seeding density of 0.025×106 cells/25
cm2 flask from passage 1 to the final passage. (A) The
number of myoblast colonies obtained in different media on day 6,
n=2 (2 different donors); *p<0.05. (B) The
proliferation rate of myoblast expansion during passages 1–3, n=3
(3 different donors); *p<0.05. (C) The cumulative
cells number, that could be obtained in SKGM-2 and DFEFH medium
after 3 passages, n=3 (3 different donors); *p<0.05.
(D) The doubling time of myoblasts cultured in different medias at
passage 2 and 3, n=3 (3 different donors); *p<0.05.
(E) The profile of the kinetics of myoblast proliferation in DFEFH
medium, n=2 (2 different donors). |
Proliferation rate and doubling time of
satellite cells
The cells were passaged for the first time after 11
days, when the colonies had begun to be more associated with each
other. As shown in Fig. 1B,
culture in DFEFH medium resulted in 0.15×106 cells, a
number which was approximately 3-fold higher than that of the cells
(0.05×106 ) cultured in SKGM-2 medium (Fig. 1B). The cells were seeded into 25
cm2 culture flasks with the same density, i.e.,
1×103 cells/cm2. The following passages were
performed once one of the cultures reached approximately 75%
confluence, after approximately 6–7 days. In 2 further passages,
the cells cultured in DFEFH medium required less time to obtain
passaging confluence; consequently, a higher number of cells was
obtained with this medium. After the 2nd passage, the difference in
the number of cells obtained was even higher, i.e.,
1.55×106 cells were obtained with DFEFH medium, a number
which was approximately 4-fold higher than that of cells
(0.4×106) obtained by culture in SKGM-2 medium. Finally,
after the 3rd passage, the greatest difference was observed, with
2.6×106 cells obtained from DFEFH medium cultures, that
is, a number which was approximately 9-fold higher than that of
cells (0.29×106) cultured in SKGM-2 medium. The
differences at passages 2 and 3 were statistically significant
(p<0.05). Cumulative cell numbers, which could be finally
achieved by seeding all the cells at each passage, were calculated.
Cell culture in DFEFH medium obtained 5.84×108
myoblasts, while culture in SKGM-2 obtaiend only 6.7×106
myoblasts (Fig. 1C).
In brief, our results indicated that culture in
DFEFH medium, starting with the same volume of digested tissue as
with culture in SKGM-2 medium, produced a greater number (100-fold)
of myoblasts compared with culture in SKGM-2 medium. The doubling
times for the cells cultured in DFEFH medium were 21.5 and 30 h
between passages 1 and 2 and between passages 2 and 3, respectively
(Fig. 1D). The cells cultured in
SKGM-2 medium had doubling times of 32.3 and 60.3 h between
passages 1 and 2 and between passages 2 and 3, respectively.
Only the cultures from the DFEFH medium were
submitted to further passages; after the 3rd passage, the
proliferation rate of the cultures considerably decreased and
remained low until the end of the expansion phase at the 7th
passage (Fig. 1E).
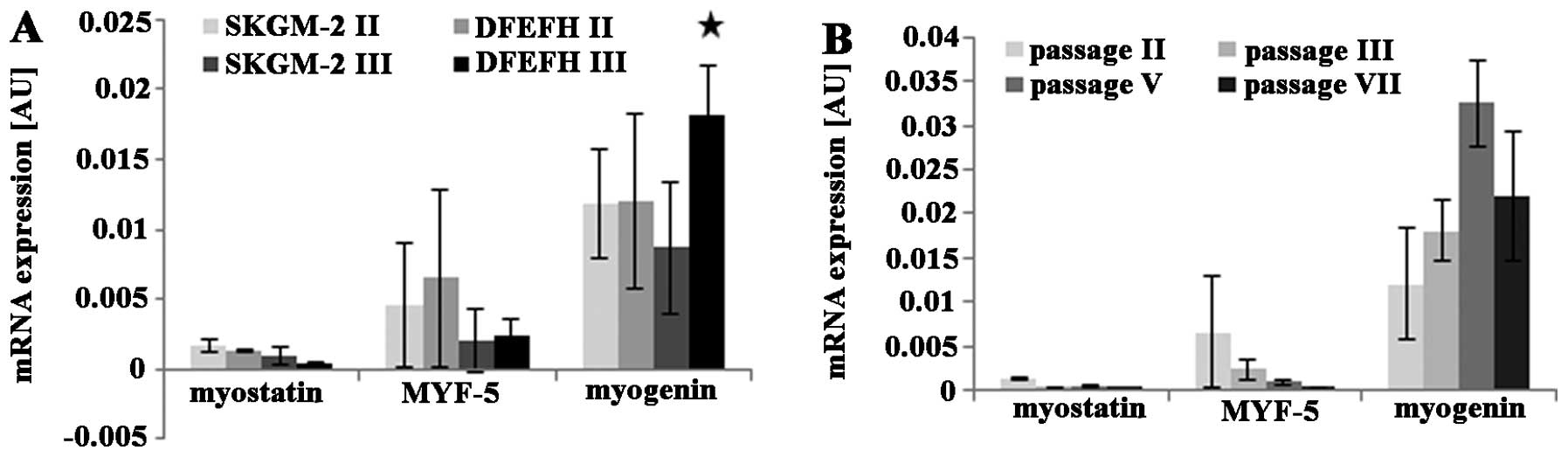
Expression of proliferation and
differentiation markers by expanded myoblasts
The mRNA expression levels of the selected genes
were investigated by quantitative PCR. The expression levels of the
myostatin, Myf5, and myogenin genes were slightly lower at passage
3 than at passage 2, when the myoblasts were cultured in SKGM-2
medium (Fig. 2A). Similar results
were observed for myoblasts cultured in DFEFH medium; the
expression of the myostatin and Myf5 genes in myoblasts cultured in
DFEFH medium was lower at passage 3 than at passage 2. By contrast,
the expression of myogenin was higher at passage 3 than at passage
2 for myoblasts cultured in DFEFH medium (Fig. 2A). Furthermore, a comparison of
the gene expression levels between myoblasts expanded in the
different media, but during the same passage indicated that
myostatin expression was 25% lower at passage 2 and approximately
3-fold lower in the cells cultured in DFEFH at passage 3. The
expression of Myf5 was similar at both passage 2 and passage 3. For
the cells cultured in DFEFH, myogenin expression was approximately
2-fold higher at passage 3 than at passage 2; this result was
statistically significant (p<0.05). An analysis of the mRNA
expression levels of myoblasts cultured in DFEFH medium from
passage 2 up to passage 7 indicated that the myostatin and Myf5
expression levels decreased from passages 2 to 5. The myogenin
expression levels increased by approximately 3-fold of those at the
initial phase during expansion and then decreased by approximately
30% at the end of the expansion phase (Fig. 2B).
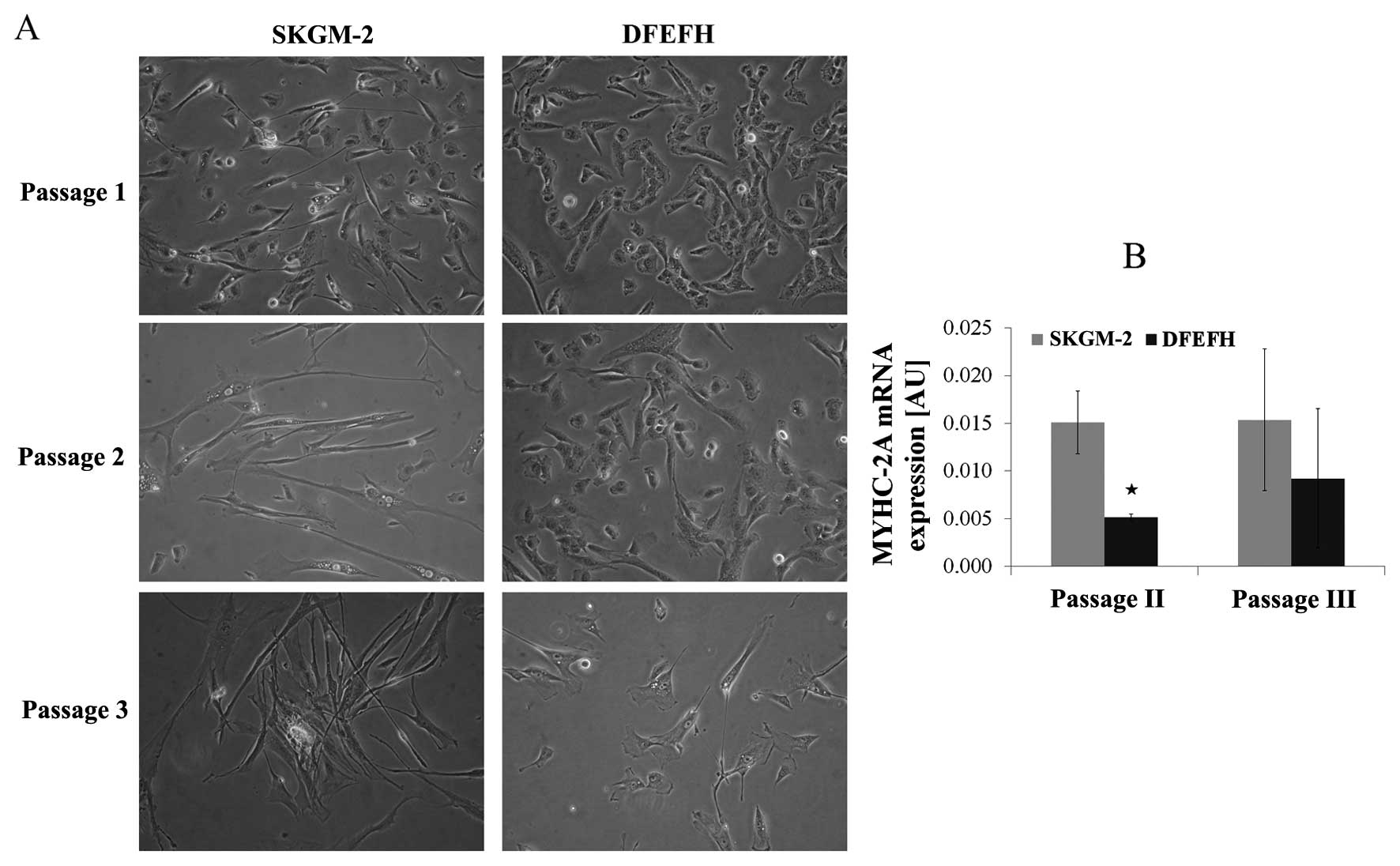
Morphological analysis of satellite cells
in culture and MyHC-2A expression
The morphological aspects of myoblasts cultured in 2
different culture media are shown in Fig. 3A. The morphology of the adherent
myoblasts differed between the 2 cultured media. At the beginning
of the culture, after passage 1, the difference was subtle. All
cells cultured in DFEFH medium were small and spindle-like, whereas
some elongated cells in between the spindle-like cells appeared
when the cells were cultured in SKGM-2 medium. After the 2nd
passage, the difference was considerable. Some elongated cells
appeared among the spindle-like cells cultured in DFEFH, but all
the cells cultured in SKGM-2 medium became elongated. This
considerable difference in the morphology of the cells was retained
after the 3rd passage.
All cells expressed MyHC-2A at both the 2nd and 3rd
passage. The myoblasts cultured in SKGM-2 medium had a constant,
higher expression of MyHC-2A at both passages than those cells
cultured in DFEFH (Fig. 3B).
Only the cells cultured in DFEFH medium were
submitted to further passage. However, during subsequent passages,
most of the cells cultured in DFEFH medium also became elongated
and vacuolated (data not shown).
Purity of myoblast cultures
The average expression of the CD56 antigen in
myoblasts cultured in DFEFH medium was 70% (60–80%), while in
myoblasts cultured in SKGM-2, it was 55% (20–90%) (data not
shown).
Fusogenic capacity
The formation of myotubes depends entirely on the
capacity of the cells to fuse with each other (29). In this study, after the 3rd
passage, myoblasts cultured in both types of culture medium had the
potential to fuse (Fig. 4A). The
average number of nuclei in the 12 randomly selected myotubes was
considerably higher for the myoblasts cultured in DFEFH medium
(Fig. 4B). For the cells cultured
in DFEFH medium, the effect of culture time on fusion potential was
also examined. We observed that the fusion potential of the cells
markedly decreased with the increasing number of passages, and
after passage 5, the fusion potential was very low (Fig. 4C). Moreover, the average number of
nuclei in the 12 randomly selected myotubes was considerably lower
at passages 4 and 5, in comparison with passage 3 (Fig. 4D).
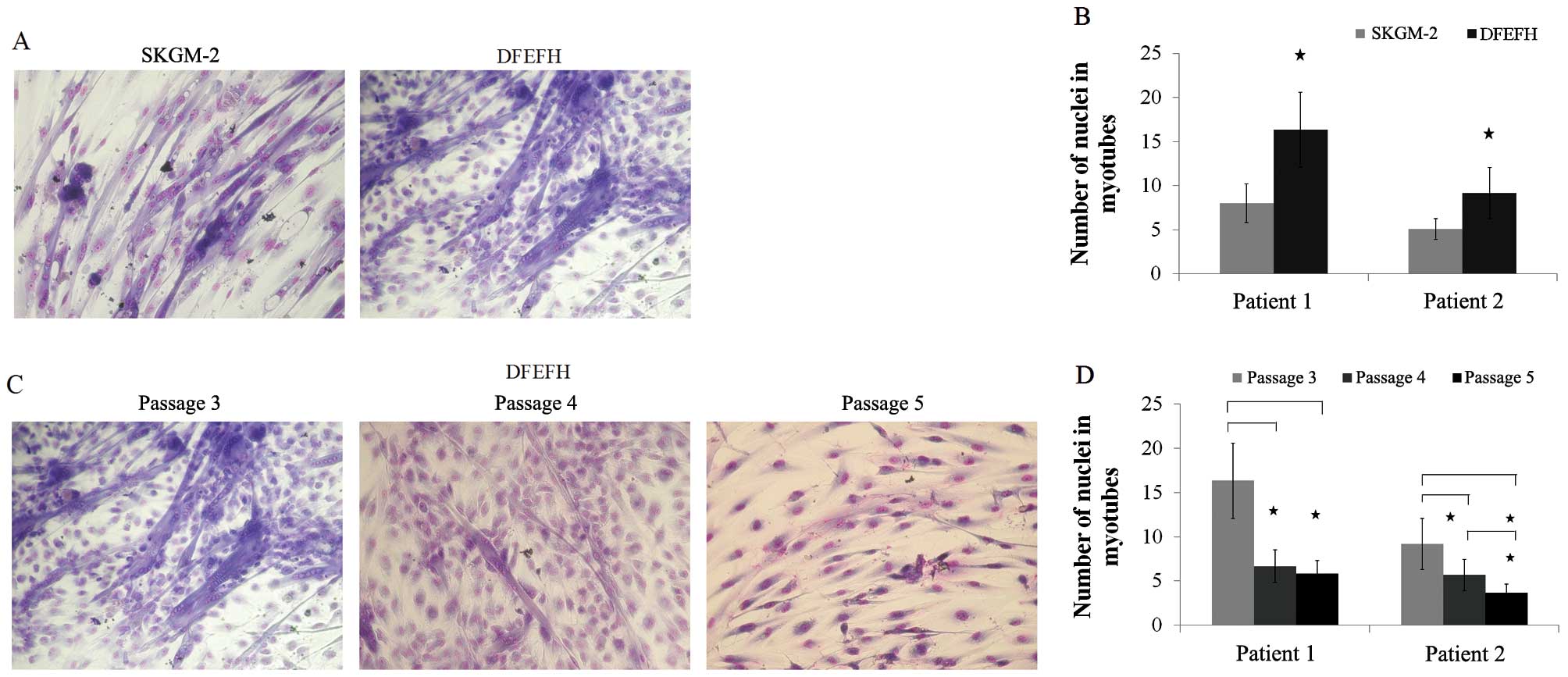 | Figure 4Fusion potential of cultured
myoblasts in different media. After passage 3, cells cultured in 2
different media were seeded in duplicate into 96-well plates, and
when 80% confluence was obtained, the culture medium was switched
to fusion medium [Dulbecco’s modified Eagle’s medium (DMEM), 2%
fetal bovine serum (FBS), 25 μmol/l insulin] and changed
every 3 days. After 11 days, cells were fixed and stained with
Wright’s eosin methylene blue solution. The number of nuclei in the
6 largest myotubes was counted in each well. Additionally, cells
cultured in DFEFH medium were subjected to higher passages and
their fusogenic potential was assessed in a similar manner. (A)
Comparison of the fusogenic potential of myoblasts cultured in
skeletal muscle cell growth medium-2 (SKGM-2) and DFEFH medium at
passage 3. Representative results of 2 independent experiments are
shown. (B) The average number of nuclei in the largest myotubes
created from myoblasts expanded in SKGM-2 and DFEFH medium at
passage 3, n=2 (2 different donors); *p<0.05. (C) The
fusion potential of myoblasts in prolonged cultured in DFEFH
medium. Representative results of 2 independent experiments are
shown. (D) The average number of nuclei in the largest myotubes
created from myoblasts expanded in DFEFH medium at passages 3, 4
and 5, n=2 (2 different donors); *p<0.05. |
Discussion
Myoblasts cultured from small muscle biopsies
constitute the basis for evolving cell replacement therapies.
Clinical reports on the use of myoblasts for cardiac indications
(2,3) and urinary incontinence (1,41)
indicate that a high number of cells (≥1×108) is
required to obtain therapeutic effects or to improve patient
symptoms. Thus, the in vitro culture of myoblasts for
clinical use aims at obtaining the number of myoblasts with
differentiation and fusion potentials that will be sufficient and
will be able to regenerate damaged muscle. Culturing myoblasts is
challenging as differentiation and myotube formation reduce their
ability to grow.
A low-density passaging strategy
(1×103cells/cm2) has been demonstrated to be
the most appropriate for preferable myoblast culture achievement
(39). In this study, The
commercially available SKGM-2 medium, containing EGF, was compared
with DFEFH medium designed by our group, containing EGF, HGF and
FGF growth factors.
Our resuls revealed that a) a higher number of
satellite cells became activated in the in-house medium, b) their
daughter myoblasts had a shorter doubling time and c) their
logarithmic growth phase lasted longer than those cells cultured in
the commercial medium, SKGM-2. The higher number of myoblast
colonies observed in DFEFH medium (Fig. 1A) may indicate that some of the
satellite cells present in the muscle biopsy sample may remain
inactivated and may be lost when cultured in SKGM-2 medium. DFEFH
medium, in contrast to SKGM-2 medium, contained an addition of HGF,
shown to be the only factor able to activate these cells in
vivo and in vitro (4,6,7,42).
During in vitro isolation, mechanical disruption precedes
enzymatic digestion. The HGF released during this step may not be
sufficient for the activation of all satellite cells; thus, the
addition of this activating growth factor may be beneficial for the
activation of a higher number of satellite cells.
There was an almost 3-fold higher proliferation rate
at the first passage in the DFEFH medium than in the SKGM-2 medium
(Fig. 1B), resulting from a
greater number (1.6-fold) of colonies, indicating the more rapid
growth of the cells in DFEFH medium. The more rapid growth of cells
in the DFEFH medium may be a result of stronger proliferation
stimulation and/or differentiation prevention. Thus, the
proliferation rate of myoblasts obtained by the stimulation of EGF
alone in SKGM-2 medium can be enhanced by the stimulation of EGF
combined with bFGF and HGF growth factors present in DFEFH medium.
We hypothesized that the mechanism responsible for the higher
proliferation rate in DFEFH medium may be the result of two
additional growth factors, HGF and bFGF. Both HGF and bFGF, present
in DFEFH medium, have been shown to increase the in vitro
proliferation of satellite cells. HGF influences the cells by
accelerating cyclin-D1 expression and causing an earlier entry of
the cells into the cell cycle (6), while bFGF influences the cells
through the stimulation of cyclin-D1 expression (9). Additionally, FGF has been shown to
prevent the terminal differentiation of expanded myoblasts through
the suppression of MyHC-2A expression (8,11).
The addition of insulin to the DFEFH medium, which has been shown
to have an anabolic effect on these cells, may be another factor
(15).
During the following two passages, 4- and 9-fold
higher proliferation rates were observed during the same culture
time in DFEFH medium compared with SKGM-2 medium cultures, and each
passage began with the same number of cells (Fig. 1B). The peak proliferation rate for
myoblasts cultured in SKGM-2 medium was observed at passage 2 after
17 days, while for myoblasts cultured in DFEFH, the peak
proliferation rate was observed at passage 3 after 24 days.
Different proliferation rates induced different (87-fold higher)
cumulative numbers of myoblasts which were obtained from the same
biopsy sample in the DFEFH culture. Furthermore, a doubling time of
21.5 and 30 h for the 2nd and 3rd passages, respectively, for the
cells cultured in DFEFH medium more closely resembled the 18.2 h
observed in vivo following the activation of satellite cells
in a previous study (42) than
the doubling times of 32.3 and 60 h for the 2nd and 3rd passages,
respectively, for cells cultured in SKGM-2 medium (Fig. 1D). The doubling time increased
after the 2nd passage, at day 17, in both culture media, but for
the cells cultured in DFEFH medium, the increase was approximately
50%, whereas for the cells cultured in SKGM-2 medium, the increase
was approximately 100%. This result indicates that the composition
of DFEFH medium provides the stimulation for cell proliferation
throughout the 17 days of culture, resulting in a doubling time
similar to the in vivo proliferation rate of regenerating
muscle cells. To summarize, during the 24 days of culture,
myoblasts cultured in SKGM-2 medium underwent approximately 18
doublings, whereas myoblasts cultured in DFEFH medium underwent
approximately 25 doublings. Additionally, the average purity of
myoblasts cultured in DFEFH medium was greater and more convergent
than that of those cultured in SKGM-2 medium. The high diversity of
CD56 expression in myoblasts cultured in the previous version of
SKGM-2, SKGM, has already been demonstrated (3).
In order to understand how these two culture media
influence the endogenous regulation of myoblast proliferation and
differentiation, we determined the mRNA expression levels of
myostatin during regulation by the myogenic regulatory factors,
Myf5 and myogenin, both in myoblasts cultured in DFEFH medium and
in myoblasts cultured in SKGM-2 medium.
Molecular analysis revealed that the myostatin
expression levels in both populations of cells were 1/4- and 3-fold
lower in the cells cultured in DFEFH compared to those cultured in
SKGM-2 at passages 2 and 3, respectively (Fig. 2A). This difference may be the
result of bFGF, which has been shown to repress the expression of
myostatin at the mRNA level, present in the DFEFH medium but absent
in the commercial SKGM-2 medium (43). This observation provides a more
detailed explanation of the shorter doubling time and the higher
proliferation rate of the cells cultured in DFEFH medium as
myostatin has been shown to block cell cycle progression by
increasing p21 expression, which blocks the release of E2F
(25). A decrease in myostatin
expression levels between passages 2 and 3 was observed in both
media types. However, a greater decrease was observed in myostatin
expression levels in DFEFH medium, indicating that the previously
described negative autoregulation of myostatin expression (44,45) may occur in order to unblock the
differentiation of myoblasts (26,27).
Myf5 expression, confirming the myogenic phenotype
of the cells, was detected at passages 2 and 3 in the cells
cultured in both media types (46,20). As was expected, during the
expansion of the cells, Myf5 expression levels decreased from
passage 2 to 3 in both populations. An approximately 50% higher
expression of Myf5 was observed at passage 2 in the cells cultured
in DFEFH medium compared with those cultured in SKGM-2 medium.
However, the higher expression level of Myf5 in cells that
underwent two more doublings may be the result of a combination of
growth factors that lower myostatin expression in these cells, as
myostatin has been shown to inhibit the mRNA expression of Myf5
(26,27). At passage 3, the expression of
Myf5 was similar in both populations of cells. Although the
difference in myostatin expression was greater at passage 3, the
cells cultured in DFEFH medium underwent four more doublings than
those cells cultured in SKGM-2 medium; thus, fusion potential,
lower myostatin expression levels and lower Myf5 inhibition may
balance an expected decrease in Myf5 expression levels during
subsequent doublings.
Parallel myogenin expression levels were observed in
both populations of cells at the 2nd and 3rd passages, indicating
that the cells differentiated into myoblasts (21,47). However, the myogenin expression
levels in cells cultured in SKGM-2 medium decreased between
passages 2 and 3, indicating that the cells may be entering the
early myocyte stage, whereas the myogenin expression levels in
cells cultured in DFEFH medium increased between passages 2 and 3
and finally decreased at passage 7 (Fig. 2B), indicating that these
conditions may cause the cells to remain at the early
differentiating myoblast stage much longer by decreasing myostatin
expression levels (47).
Moreover, our data demonstrate that the culture
medium composition influences the morphology of the expanded cells
(Fig. 3A). Among the small
spindle-like myoblasts cultured in SKGM-2 medium, single elongated
cells appeared at passage 1, and most of the cells became elongated
by passage 2, while the myoblasts cultured in DFEFH medium were
still small, spindle-like cells at the second passage. This
difference was still apparent at passage 3. However, the myoblasts
cultured in DFEFH medium also became elongated and vacuolated at
further passages, which correlated with a decreased proliferation
rate and an increased doubling time. The differences in morphology
may be explained by the difference in the expression levels of the
muscle contractile protein, MYHC-2A, which may be associated with
phenotypic differentiation (29).
MyHC-2A expression was 3-fold and 40% lower at passage 2 and 3,
respectively, in myoblasts cultured in DFEFH medium than in those
cells cultured in SKGM-2 medium (Fig.
3B). Different morphology could be also partly explained by
different purity of myoblasts cultured in different media.
The differentiation potential of the cells in both
media was compared at passage 3, when a substantial number of
myoblasts could be achieved in both media types, showing similar
percentages of cells undergoing fusion. However, the myotubes
created from myoblasts expanded in DFEFH medium contained
considerably more nuclei after 11 days, which may be explained by
the higher purity of the myoblasts culture. The differentiation
potential of myoblasts expanded in DFEFH medium was also verified
at further passages. A marked decrease in fusion potential was
observed after passage 3, leading to an almost complete lack of
fusion at passage 5 and a considerable decrease of nuclei in
created myotubes. The lack of fusion observed at further passages
confirms the recent finding that, with increasing passages,
myoblasts become elongated cells with vesicles containing
cytoplasm, their differentiation potential decreases and they
acquire a different metabolic phenotype (40).
Our results confirm the earlier observations
(2,3) that a high number of myoblasts can be
obtain in an in vitro setting. However, our results also
indicate a considerably higher, up to 5×109, expansion
efficacy, in a considerably shorter time, i.e., 24 days [in
contrast to 1×109 in 46 days after a 0.6–1.9 g biopsy
(2), 3×108 in 18 days
after a 2 g biopsy (3), or
3×108 in 25 days after a 0.2 g biopsy], which may be
caused by biopsy weight, growth factor composition and seeding
density (39). We also confirm
the findings of earlier reports showing that further expansion
results in obtaining myoblasts that contain vacuoles and are unable
to fuse (40).
The present study also suggests that conditions
enabling the growth of up to 5×109 of myoblasts in 24
days present fusion potential in elderly patients. Additionally, it
shows that the expansion of the cells over 23 generations results
in the rapid decrease of proliferation potential, an increase in
doubling time, the appearance of vacuoles in cells and the loss of
fusion potential.
Setting standard conditions for the medium
composition of myoblast expansion for clinical purposes by
manipulating the intrinsic control of proliferation and
differentiation may be of value. For the clinical transplantation
of functionally competent cells for successful cell therapy and the
unnecessary costs of prolonged cultures, the maximal number of
doublings, rather than passages, that cells can undergo in in
vitro conditions should be set.
Acknowledgements
Part of this study was presented by Jarocha D,
Stangel- Wójcikiewicz K, Basta A and Majka M at the American
Society of Gene and Cell Therapy 15th Annual Meeting in
Pennsylvania (USA) in May 2012 [Mol Ther 20 (Suppl 1): 172; P444];
the study was entitled ‘Efficient myoblast expansion for urinary
incontinence treatment’. The present study was supported by grants
awarded to J.W.K. (MNiSW grant N N407 048538) and was also
partially funded by Jagiellonian University (K/ZDS002269).
Abbreviations:
|
SKGM-2
|
skeletal muscle cell growth
medium-2
|
|
MDCs
|
muscle-derived cells
|
|
HGF
|
hepatocyte growth factor
|
|
bFGF
|
basic fibroblast growth factor
|
|
EGF
|
epidermal growth factor
|
|
PDGF
|
platelet-derived growth factor
|
|
TGFβ
|
tumor growth factor-β
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
PBS
|
phosphate-buffered saline
|
|
FBS
|
fetal bovine serum
|
References
|
1
|
Peters K, Kaufman M, Dmochowski R, et al:
1340 Autologous muscle derived cell therapy for the treatment of
female stress urinary incontinence: A multi-center experience. J
Urol. 185:e535–e536. 2011. View Article : Google Scholar
|
|
2
|
Baj A, Bettaccini AA, Casalone R, et al:
Culture of skeletal myoblasts from human donors aged over 40 years:
dynamics of cell growth and expression of differentiation markers.
J Transl Med. 12:1–10. 2005.PubMed/NCBI
|
|
3
|
Pagani FD, DerSimonian H, Zawadzka A, et
al: Autologous skeletal myoblasts transplanted to ischemia-damaged
myocardium in humans. J Am Coll Cardiol. 41:879–888. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bischoff R: A satellite cell mitogen from
crushed adult muscle. Dev Biol. 115:140–147. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clarke MS, Khakee R and McNeil PL: Loss of
cytoplasmic basic fibroblast growth factor from physiologically
wounded myofibers of normal and dystrophic muscle. J Cell Sci.
106:121–133. 1993.PubMed/NCBI
|
|
6
|
Allen RE, Sheehan SM, Taylor RG, et al:
Hepatocyte growth factor activates quiescent skeletal muscle
satellite cells in vitro. J Cell Physiol. 165:307–312. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tatsumi R, Anderson JE, Nevoret CJ, et al:
HGF/SF is present in normal adult skeletal muscle and is capable of
activating satellite cells. Dev Biol. 194:114–128. 1998. View Article : Google Scholar
|
|
8
|
Johnson SE and Allen RE: Activation of
skeletal muscle satellite cells and the role of fibroblast growth
factor receptors. Exp Cell Res. 219:449–453. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rao SS and Kohtz S: Positive and negative
regulation of d-type cyclin expression in skeletal myoblasts by
basic Fibroblasts growth factor and Transforming growth Factor
beta. J Biol Chem. 270:4093–4100. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheehan SM and Allen RE: Skeletal muscle
satellite cell proliferation in response to members of the
fibroblast growth factor family and hepatocyte growth factor. J
Cell Physiol. 181:499–506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clegg CH, Linkhart TA, Olwin BB, et al:
Growth factor control of skeletal muscle differentiation:
commitment to terminal differentiation occurs in G1 phase and is
repressed by fibroblast growth factor. J Cell Biol. 105:949–956.
1987. View Article : Google Scholar
|
|
12
|
McGeachie JK and Grounds MD: Retarded
myogenic cell replication in regenerating skeletal muscles of old
mice: an autoradiographic study in young and old BALBc and SJL/J
mice. Cell Tissue Res. 280:277–282. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bischoff R: Proliferation of muscle
satellite cells on intact myofibers in culture. Dev Biol.
115:129–139. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allen RE, Dodson MV and Luiten LS:
Regulation of skeletal muscle satellite cell proliferation by
bovine pituitary fibroblast growth factor. Exp Cell Res.
152:154–160. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roe JA, Baba AS, Harper JM, et al: Effects
of growth factors and gut regulatory peptides on nutrient uptake in
ovine muscle cell cultures. Comp Biochem Physiol A Physiol.
110:107–114. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alessandri G, Pagano S, Bez A, et al:
Isolation and culture of human muscle-derived stem cells able to
differentiate into myogenic and neurogenic cell lineages. Lancet.
364:1872–1883. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boudreault P, Tremblay JP, Pépin MF, et
al: Scale-up of a myoblast culture process. J Biotechnol. 91:63–74.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Editor S, Mcinnes RR, La S, et al: The
molecular regulation of myogenesis. Clin Genet. 16:16–25. 2000.
|
|
19
|
Kitzmann M, Carnac G, Vandromme M, et al:
The muscle regulatory factors MyoD and myf-5 undergo distinct cell
cycle-specific expression in muscle cells. J Cell Biol.
142:1447–1459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cooper RN, Tajbakhsh S, Mouly V, et al: In
vivo satellite cell activation via Myf5 and MyoD in regenerating
mouse skeletal muscle. J Cell Sci. 112:2895–2901. 1999.PubMed/NCBI
|
|
21
|
Hasty P, Bradley A, Morris JH, et al:
Muscle deficiency and neonatal death in mice with a targeted
mutation in the myogenin gene. Nature. 364:501–506. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rawls A, Valdez MR, Zhang W, et al:
Overlapping functions of the myogenic bHLH genes MRF4 and MyoD
revealed in double mutant mice. Development. 125:2349–2358.
1998.PubMed/NCBI
|
|
23
|
McPherron AC, Lawler AM and Lee SJ:
Regulation of skeletal muscle mass in mice by a new TGF-beta
superfamily member. Nature. 387:83–90. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCroskery S, Thomas M, Maxwell L, et al:
Myostatin negatively regulates satellite cell activation and
self-renewal. The J Cell Biol. 162:1135–1147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas M, Langley B, Berry C, et al:
Myostatin, a negative regulator of muscle growth, functions by
inhibiting myoblast proliferation. The J Biol Chem.
275:40235–40243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Langley B, Thomas M, Bishop A, et al:
Myostatin inhibits myoblast differentiation by down-regulating MyoD
expression. The J Biol Chem. 277:49831–49840. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rios R, Carneiro I, Arce CM, et al:
Myostatin is an inhibitor of myogenic differentiation. Am J Physiol
Cell Physiol. 282:993–999. 2002. View Article : Google Scholar
|
|
28
|
Pette D and Staron RS: Myosin isoforms,
muscle fiber types, and transitions. Microsc Res Tech. 50:500–509.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chowdhury SR, Muneyuki Y, Takezawa Y, et
al: Growth and differentiation potentials in confluent state of
culture of human skeletal muscle myoblasts. J Biosci Bioeng.
109:310–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ham RG, St Clair JA, Webster C, et al:
Improved media for normal human muscle satellite cells: serum-free
clonal growth and enhanced growth with low serum. In Vitro Cell Dev
Biol. 24:833–844. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stern-Straeter J, Bran G, Riedel F, et al:
Characterization of human myoblast cultures for tissue engineering.
Int J Mol Med. 21:49–56. 2008.
|
|
32
|
Sheehan SM, Tatsumi R, Temm-Grove CJ, et
al: HGF is an autocrine growth factor for skeletal muscle satellite
cells in vitro. Muscle Nerve. 23:239–245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yanagiuchi A, Miyake H, Nomi M, et al:
Modulation of the microenvironment by growth factors regulates the
in vivo growth of skeletal myoblasts. BJU Int. 103:1569–1573. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kühl U, Ocalan M, Timpl R, et al: Role of
laminin and fibronectin in selecting myogenic versus fibrogenic
cells from skeletal muscle cells in vitro. Dev Biol. 117:628–635.
1986.PubMed/NCBI
|
|
35
|
Ocalan M, Goodman SL, Kühl U, et al:
Laminin alters cell shape and stimulates motility and proliferation
of murine skeletal myoblasts. Dev Biol. 125:158–167. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eberli D, Soker S, Atala A, et al:
Optimization of human skeletal muscle precursor cell culture and
myofiber formation in vitro. Methods. 47:98–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martin SD, Collier FM, Kirkland MA, et al:
Enhanced proliferation of human skeletal muscle precursor cells
derived from elderly donors cultured in estimated physiological
(5%) oxygen. Cytotechnology. 61:93–107. 2009.PubMed/NCBI
|
|
38
|
Blau HM, Chiu C-P and Webster C:
Cytoplasmic activation of human nuclear genes in stable
heterocaryons. Cell. 32:1171–1180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kino-Oka M, Chowdhury SR, Muneyuki Y, et
al: Automating the expansion process of human skeletal muscle
myoblasts with suppression of myotube formation. Tissue Eng Part C
Methods. 15:717–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nehlin JO, Just M, Rustan AC, et al: Human
myotubes from myoblast cultures undergoing senescence exhibit
defects in glucose and lipid metabolism. Biogerontology.
12:349–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Herschorn S, Carr L, Birch C, et al:
Autologous muscle-derived cells as therapy for stress urinary
incotinence: a randomized, blinded trial. Neurourol Urodyn.
29:3072010.
|
|
42
|
Zammit P: Kinetics of myoblast
proliferation show that resident satellite cells are competent to
fully regenerate skeletal muscle fibers. Exp Cell Res 2002.
281:39–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu H-Z, Li Q, Yang X-Y, et al: Expression
of basic fibroblast growth factor results in the decrease of
myostatin mRNA in murine C2C12 myoblasts. Acta Biochim Biophys Sin
(Shanghai). 38:697–703. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu X, Topouzis S, Liang L-F, et al:
Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by
the inhibitory Smad7 by a negative feedback mechanism. Cytokine.
26:262–272. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Forbes D, Jackman M, Bishop A, et al:
Myostatin auto-regulates its expression by feedback loop through
Smad7 dependent mechanism. J Cell Physiol. 206:264–272. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cornelison DD and Wold BJ: Single-cell
analysis of regulatory gene expression in quiescent and activated
mouse skeletal muscle satellite cells. Dev Biol. 191:270–283. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bentzinger CF, Wang YX and Rudnicki M:
Building muscle: molecular regulation of myogenesis. Cold Spring
Harb Perspect Biol. 4:1–16. 2012. View Article : Google Scholar : PubMed/NCBI
|