Introduction
The control of influenza A virus infection poses a
major social and economical issue. Although neuraminidase
inhibitors have been used as an effective treatment for influenza
infection, the rapid development of drug-resistant variants of the
virus often limits the applicability of these antiviral drugs with
single targets. Thus, there is a constant need for the development
of novel anti-influenza drugs. Considering the potential safety of
natural dietary products, in previous studies, we examined the
antiviral and virucidal activities of various natural products and
their components (1–3, reviewed in ref. 4). We found that caffeic acid inhibits
the multiplication of herpes simplex virus type 1 (HSV-1) in
vitro by interfering mainly with the early stages of HSV-1
multiplication in the infected cells prior to the completion of
viral genome DNA replication, independently of its cytotoxic action
(5).
Caffeic acid (3,4-dihydroxycinnamic acid) is one of
the abundant plant-derived polyphenol compounds with 2 phenolic
hydroxyl groups and is known to have antioxidant activity (6). This compound is the major metabolite
produced by the hydrolyzation of chlorogenic acid, which is
commonly found in a variety of foods, including coffee, fruits,
vegetables and grains. In the present study, we aimed to further
characterize the antiviral activities of caffeic acid against a
variety of viruses with different replication mechanisms. We found
that the reagent effectively inhibited the multiplication of
influenza A virus in MDCK cells, as well as the virus-induced
cytopathic effect (CPE). We present the results of an in
vitro virological characterization of the antiviral activity of
caffeic acid against influenza A virus.
Materials and methods
Cells and viruses
The MDCK cells (obtained from Dr Katsuhisa Nakajima,
Nagoya City University, Nagoya, Japan), HEp-2 cells (obtained from
Dr Takahiro Uchida, Tokushima University, Tokushima, Japan) and
Vero cells (obtained from Dr Kamesaburo Yoshino, University of
Tokyo, Tokyo, Japan) were grown in Eagle’s minimum essential medium
(MEM) (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) containing 5%
fetal bovine serum (FBS) (Euroclone, Pero, Italy). Influenza virus
A/Aichi/68 (H3N2) (obtained from Dr Katsuhisa Nakajima, Nagoya City
University), Sabin strain of poliovirus type 1 (PV-1) (from Dr Akio
Nomoto, University of Tokyo) and HSV-1 strain F (from Dr Bernard
Roizman, Chicago University, Chicago, IL, USA), were used
throughout the experiments. The influenza virus was propagated in
the MDCK cells in MEM supplemented with both 0.1% bovine serum
albumin (BSA) (Wako Pure Chemical Industries, Ltd., Osaka, Japan)
and acetylated trypsin (4 μg/ml) and stored at −80°C until use. The
amounts of the viruses were measured by a plaque assay as
previously described (7–9). Briefly, Vero (for PV-1 and HSV-1) or
MDCK (for influenza virus) cell monolayers were washed once with
Dulbecco’s phosphate-buffered saline without Ca++ and
Mg++ (PBS) and received an aliquot of the virus
suspension in PBS containing 0.5% FBS (for PV-1 and HSV-1) or 0.1%
BSA (for influenza virus), followed by an incubation for 60 min at
room temperature with mechanical rocking on a rocker platform
(Taitec Co., Ltd., Koshigaya, Saitama, Japan). The infected cell
monolayer were incubated at 37°C (for HSV-1 and influenza virus) or
at 35.5 °C (for PV-1) in MEM containing 0.5% FBS and 0.68%
methylcellulose (Nacalai Tesque Inc., Kyoto, Japan) for PV-1 and
HSV-1 or in MEM containing 0.6% Difco agar noble (Becton, Dickinson
and Co., Sparks, MD, USA) and 5.3 μg/ml acetylated trypsin
(Sigma-Aldrich Corp., St. Louis, MO, USA) for influenza virus until
the formation of plaques.
Effect of the reagent on the virus
yields
Caffeic acid, quinic acid and chlorogenic acid were
obtained from Wako Pure Chemical Industiries, Ltd. (Osaka, Japan).
The reagent solution (1.0 or 100 mM) was prepared by dissolving the
reagents in hot water and neutralizing its acidity with 1 N sodium
hydroxide solution, followed by filtration through a Millipore
Dimex membrane (pore size 0.22 μm; Merck Millipore, Billerica, MA,
USA).
Monolayered cells in 35 mm-dishes were infected with
the viruses at an indicated multiplicity of infection (MOI). The
infected cells were further incubated at 37°C (for influenza virus
and HSV-1) or 35.5°C (for PV-1) for the indicated periods of time
in serum-free MEM containing both 0.1% BSA and the indicated
concentrations of the reagent. For the influenza virus, acetylated
trypsin was added to the infected culture and, at the indicated
time point, the culture medium of the infected cells was harvested
and the amount of the progeny virus in the medium was determined by
a plaque assay as previously described (8).
Determination of CPE and apoptotic cell
nuclei
Confluent monolayers of MDCK cells were
mock-infected or infected with the influenza virus and were then
incubated at 37°C for the indicated periods of time in serum-free
MEM containing 0.1% BSA and acetylated trypsin, supplemented with
the indicated concentrations of the reagent. The CPE was determined
by a microscopic observation of the cells; approximate amounts of
rounded cells on cell monolayers were estimated under a
phase-contrast microscope (Eclipse E800 model; Nikon Corp., Tokyo,
Japan).
Apoptotic cell nuclei were observed after the cells
were fixed with methyl alcohol/acetic acid (3:1) and stained for 10
min with the DNA-binding dye, Hoechst 33258 (0.05 μg/ml), according
to the method previously described in the study by McGarrity
(10).
Results and Discussion
Effects of caffeic acid on the
multiplication of influenza A virus
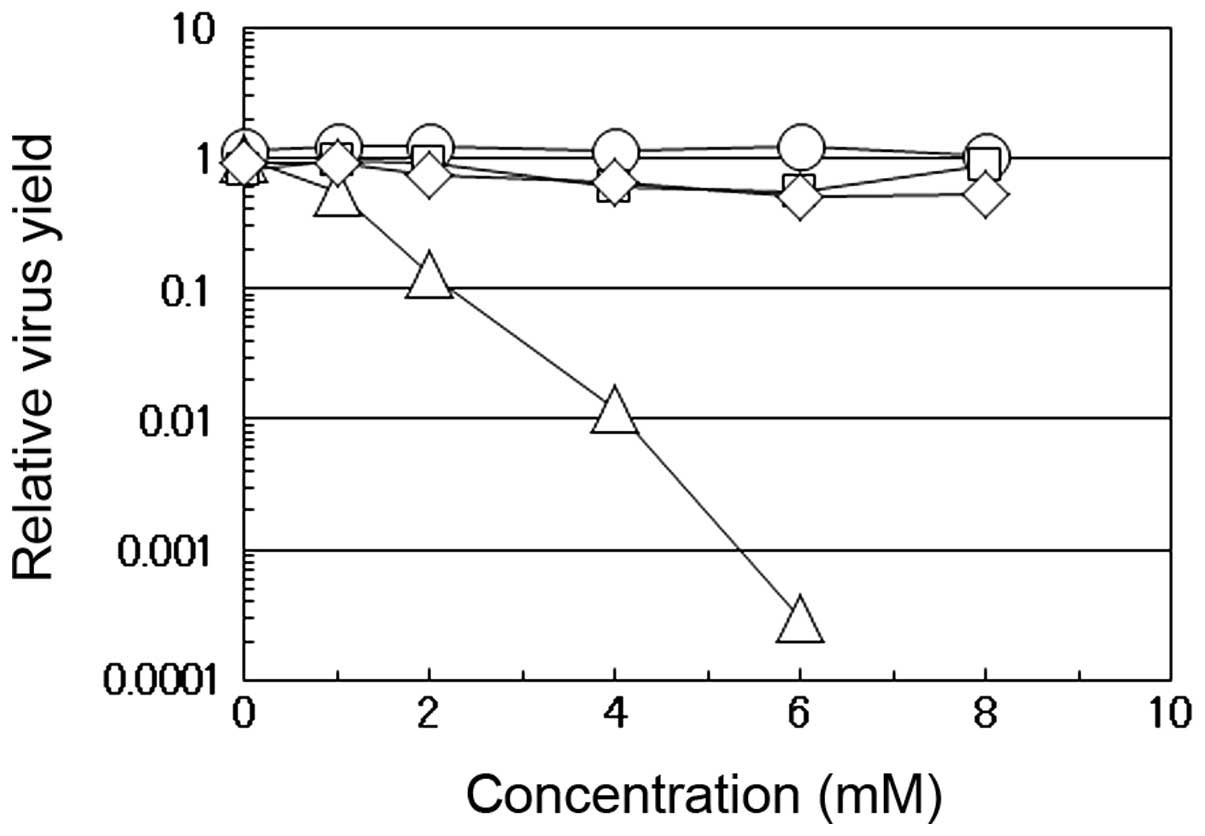
As shown in Fig.
1, we compared the effects of caffeic acid, caffeine, quinic
acid and chlorogenic acid on the multiplication of influenza A
virus in the MDCK cells. Chlorogenic acid is an esterified form of
caffeic acid with quinic acid and is one of the most widely
consumed polyphenols abundant in dietary foods, particularly in
coffee. When the virus was propagated in the presence of the
indicated concentrations of these compounds, the virus yield at 12
h post-infection (p.i.) was compared to the yield in the absence of
the compounds. The yield markedly decreased with the increasing
concentrations of caffeic acid, but not with any of the other 3
compounds. At a dose of 4 mM caffeic acid, the yield of influenza A
virus was approximately 100-fold lower than that in the absence of
the reagent, while, even at higher concentrations (6 or 8 mM),
caffeine, quinic acid or chlorogenic acid had insignificant effects
on the virus yields. Although caffeine did not suppress the
multiplication of influenza A virus even at 8 mM (Fig. 1, cirle), we have previously found
that both caffeic acid and caffeine, but neither chlorogenic acid
nor quinic acid, inhibit the multiplication of HSV-1 in HEp-2 cells
(5), indicating that caffeic acid
suppresses the multiplication of both DNA and RNA viruses, in
contrast to the selective antiviral activity of caffeine which is
effective only against HSV-1 (11).
In addition, in conjunction with the decrease in the
virus yield in the presence of caffeic acid, the virus-induced CPE
of the infected cells was also suppressed; although not completely,
the cell rounding and ballooning, as well as the detachment from
the dish surface in the influenza virus-infected cells in culture
were significantly suppressed even at 12 h p.i. in the presence of
caffeic acid at 4 mM. No such suppression of CPE was observed with
the other 3 compounds (data not shown).
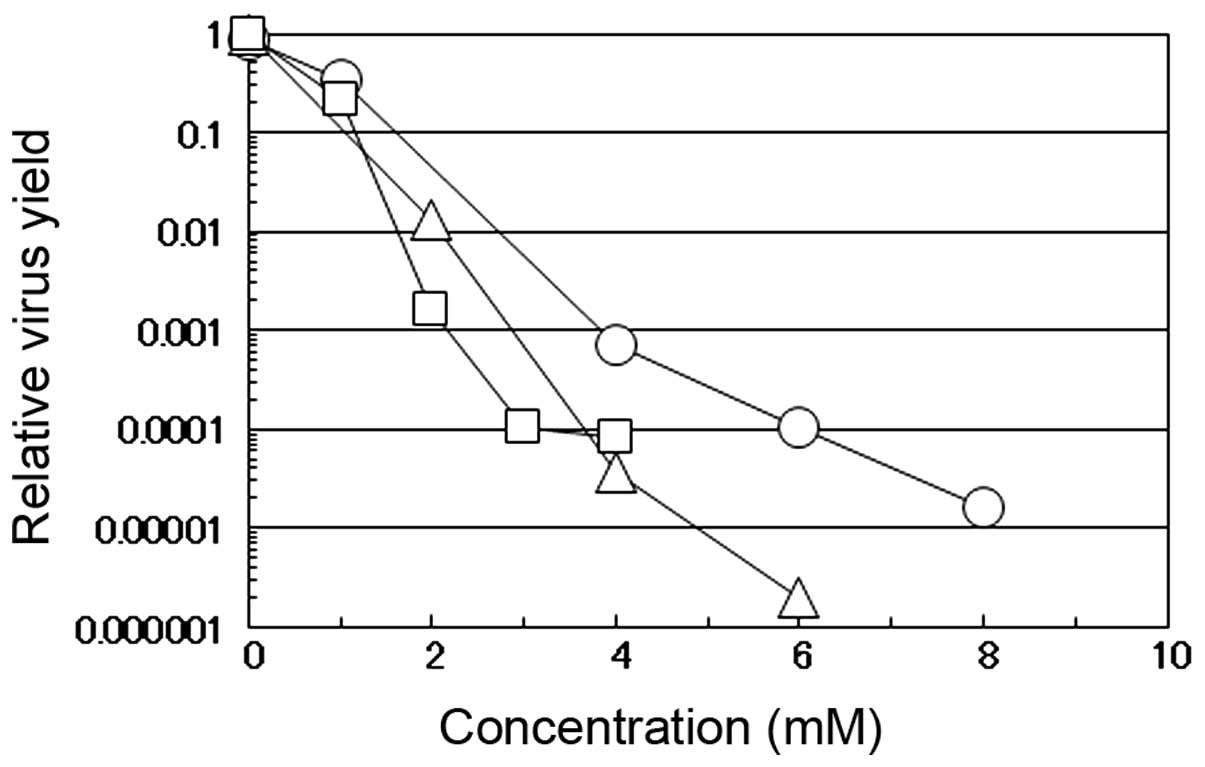
The antiviral effects of caffeic acid against
influenza A virus were compared with those against other DNA and
RNA viruses (i.e., HSV-1 and PV-1, respectively). These 3 viruses
have a different genome structure and replication strategy in the
infected cells; HSV-1 has a double-stranded DNA genome which
replicates in the nucleus (12)
and PV-1 has a positive-stranded RNA genome which replicates in the
cytoplasm (13), while influenza
A virus has a negative-stranded RNA genome which replicates in the
nucleus of the infected cells (14). When the PV-1-infected or
HSV-1-infected HEp-2 cells or influenza virus-infected MDCK cells
were incubated in the medium-containing various concentrations of
caffeic acid, the yield of the respective virus markedly decreased
in a concentration-dependent manner; these effects were more
prominent for PV-1 and influenza A virus than for HSV-1 (Fig. 2). These results confirm that
caffeic acid can inhibit both DNA and RNA viruses regardless of
cell type and suggest that RNA viruses are more sensitive to the
reagent than DNA viruses. The results also indicate that both
enveloped (influenza virus and HSV-1) and non-enveloped (PV-1)
viruses are sensitive to caffeic acid. It may be noteworthy that,
although PV-1 induced massive CPE in the infected HEp-2 cells, CPE
induced by PV-1 infection was also markedly suppressed by the
addition of caffeic acid (data not shown), similar to the effects
observed by infection with the influenza virus.
Effects of caffeic acid on virus
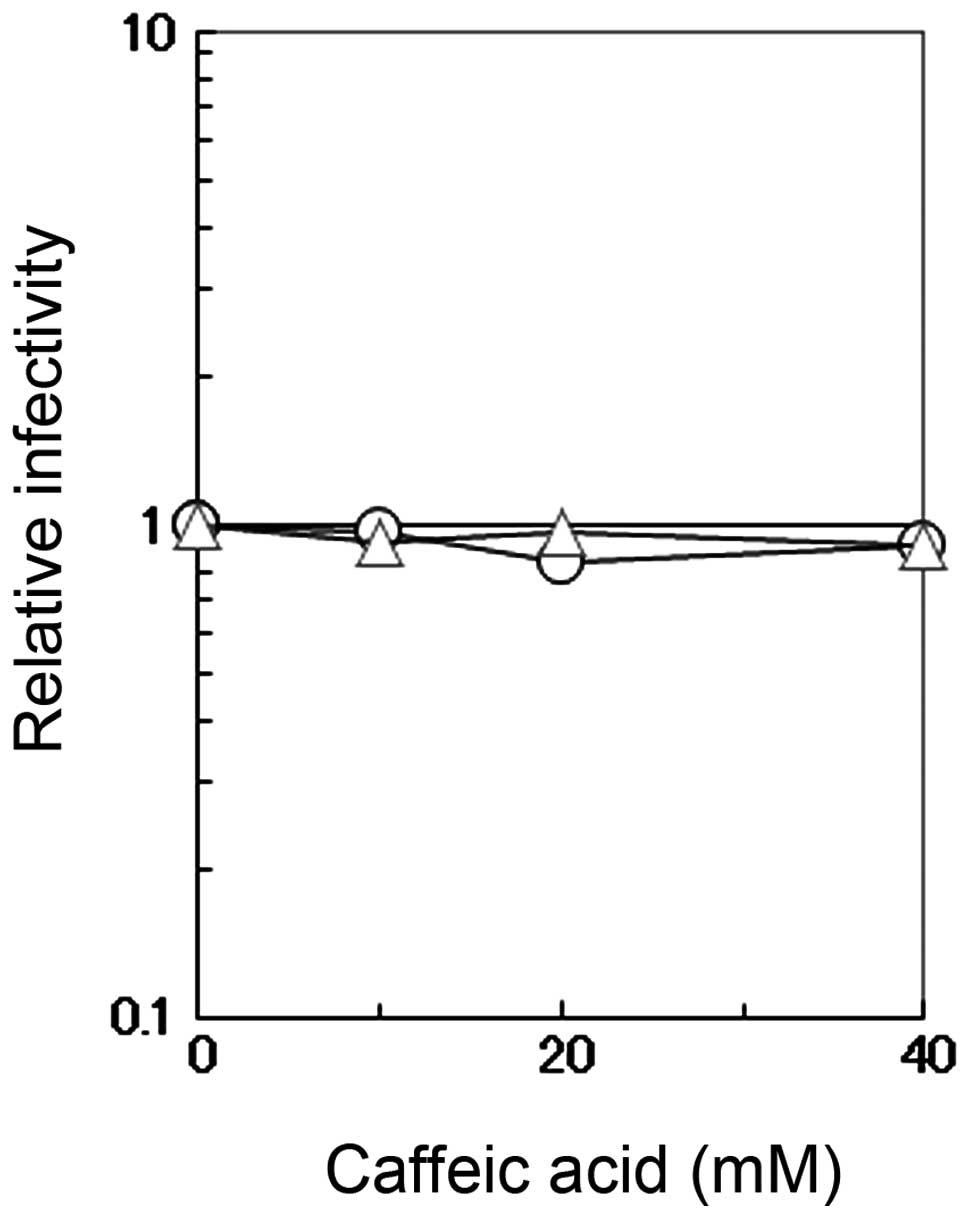
infectivity and cell viability
The direct effects of caffeic acid on the
infectivity of influenza A virus were measured by incubating the
virus in PBS containing 0.1% BSA and various concentrations of
caffeic acid or caffeine. The viral infectivity was not affected by
caffeic acid or caffeine even at the concentration of 40 mM
(Fig. 3). This concentration is
much higher than that used for the antiviral assay described above
(Figs. 1 and 2), indicating that the observed
antiviral activity is not a result of the virucidal effects of
caffeic acid.
In addition, although we have previously found
(5) that caffeic acid has
significant cytotoxic activity during the prolonged incubation of
cells (>24 h), the cytotoxicity was mild and not sufficiently
strong (data not shown) to explain the observed antiviral effect,
particularly by a short incubation period for the antiviral assay
against influenza virus (<12 h). The suppression by caffeic acid
of CPE of influenza virus-infected or PV-1-infected cells also
suggests that the cytotoxic effects of caffeic acid are unlikely to
result in the observed decrease in virus yields by caffeic
acid.
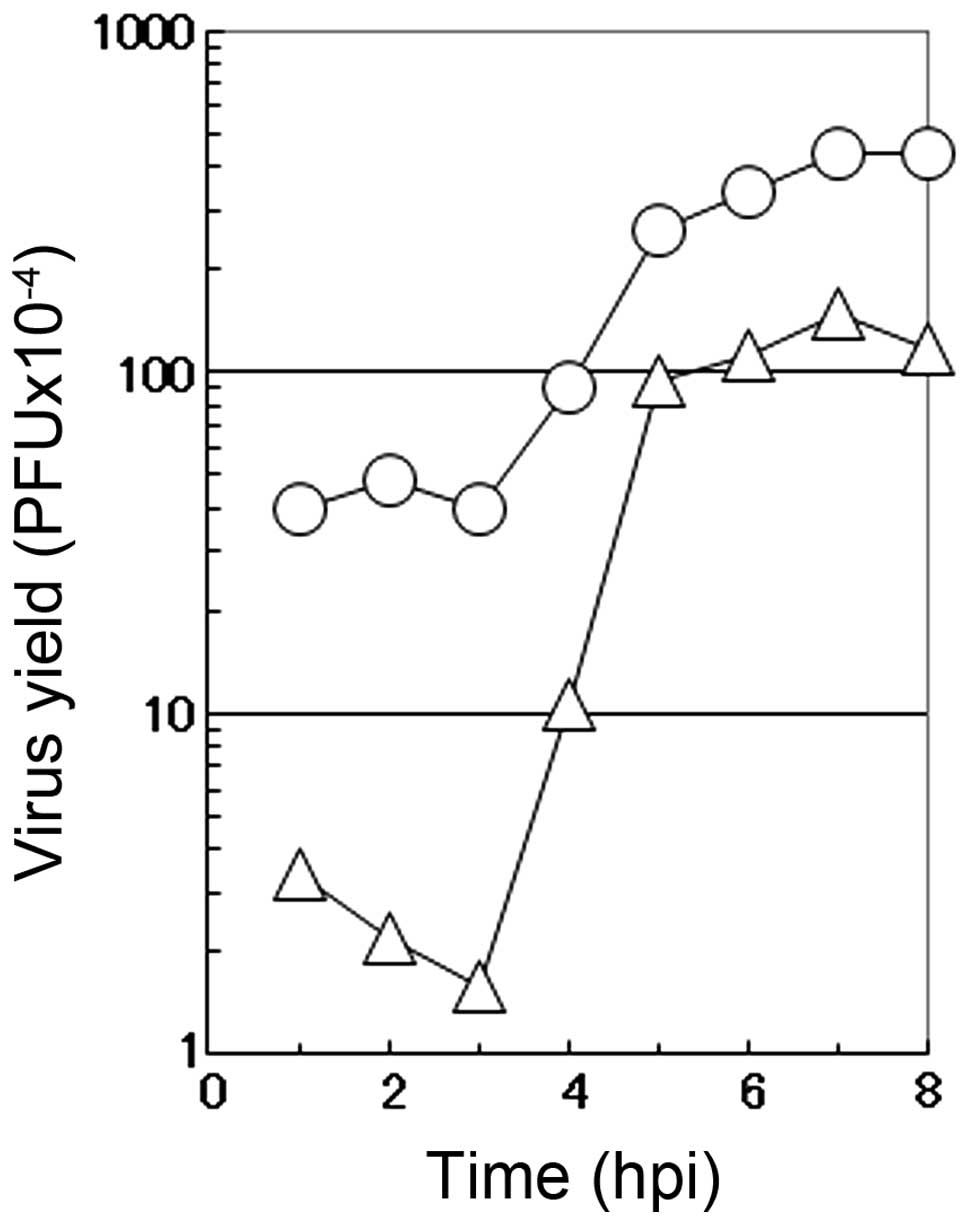
One-step growth curve in the presence of
caffeic acid
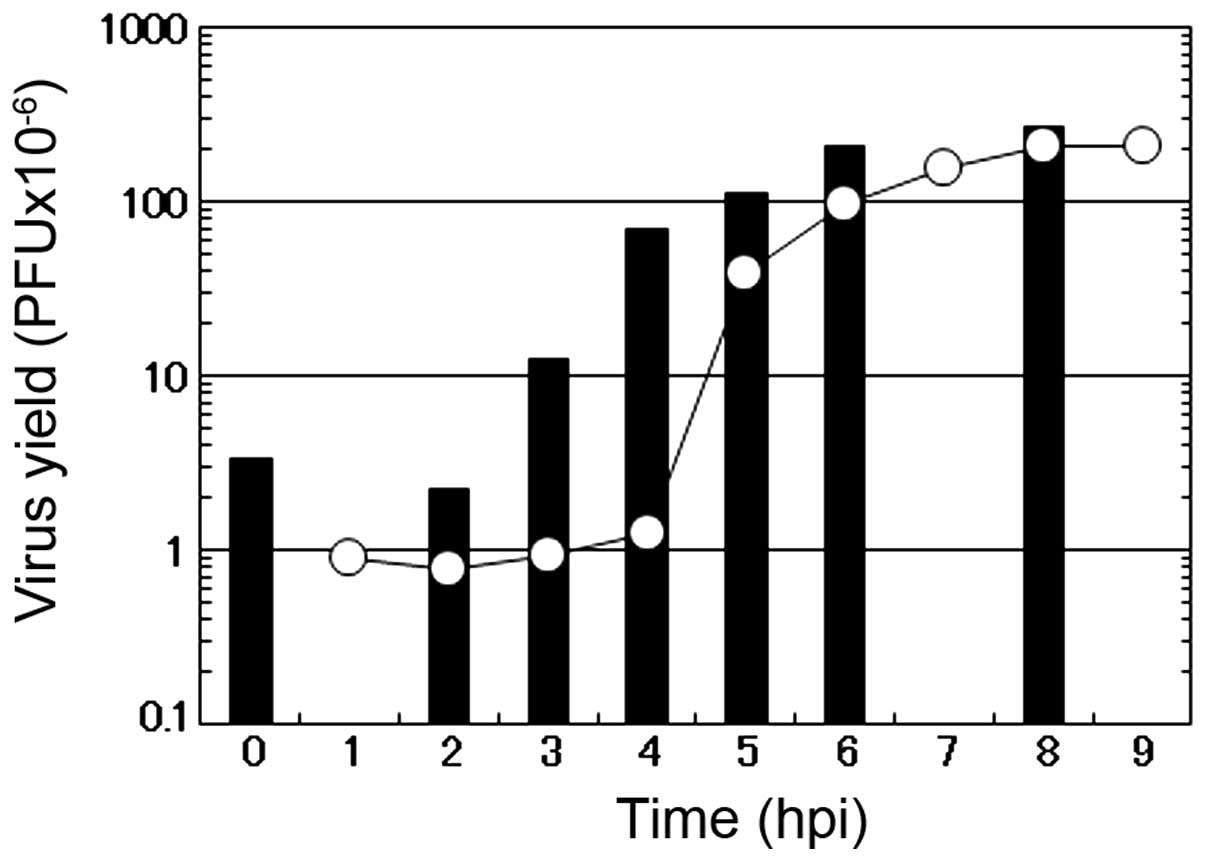
To characterize the mode of the antiviral action of
caffeic acid on the multiplication of influenza A virus, a one-step
growth curve was examined in the presence of 2 mM caffeic acid. In
the absence of the reagent (circle), the number of infectious
progeny viruses began to increase at 3 h p.i. and reached the
plateau at 7 to 8 h p.i. in the culture medium of the infected MDCK
cells (Fig. 4). Under these
conditions (in the absence of the reagent), CPE by the virus
infection was hardly observed before 6 h p.i., when a small number
of round cells appeared in the infected cultures. At 6 h p.i., the
number of rounded cells increased with time, followed by the
gradual detachment of the cells from the dish surface (data not
shown). At 10 h p.i., the majority of the infected cells became
rounded and only a limited fraction of the infected cells remains
attached to the dish.
The addition of 2 mM caffeic acid at the beginning
of virus multiplication induced a marked decrease in the number of
infectious viruses in the early stage of the virus multiplication,
followed by an accelerated increase in the number of the progeny
viruses (Fig. 4). These results
showed that the length of the eclipse period of the formation of
the progeny virus in the influenza virus-infected cells was not
affected by caffeic acid, although the number of infectious viruses
produced in this period was greatly reduced, followed by a recovery
of the formation of infectious viruses at the middle stage of the
virus multiplication (3 to 5 h p.i.). These results suggest that
caffeic acid mostly inhibits the multiplication of influenza virus
in the early stages of viral replication in the infected MDCK
cells.
In addition, the one-step growth experiment revealed
that caffeic acid suppressed not only virus multiplication, but
also CPE induced by the infection with influenza virus. The onset
of CPE by viral infection was delayed in accordance with the
suppression of progeny virus production; a small number of the
rounded cells appeared at 8 h p.i. and, at 10 h p.i., a significant
fraction (approximately 20%) of the infected cells became rounded
and the number of the detached cells was clearly lower than that in
the absence of caffeic acid (data not shown).
Effect of caffeic acid on the induction
of apoptosis
As described above, the presence of caffeic acid in
the culture medium effectively suppressed the virus-induced
cytotoxic effects in the influenza A virus-infected culture.
Previously, we found that the infection with influenza virus
induces apoptosis in the infected cells and compared the kinetics
of the induction of apoptosis in the influenza virus-infected cells
with that of the formation of progeny viruses (8). When the virus-infected cells in the
absence of the reagent were fixed and stained with the DNA-binding
dye, Hoechst 33258, apoptotic cell nuclei with characteristic
morphology (i.e., condensation and fragmentation of cellular
chromatin) appeared at 6 h p.i. in some (1–2%) of the infected
cells and the number of cells with these apoptotic nuclei increased
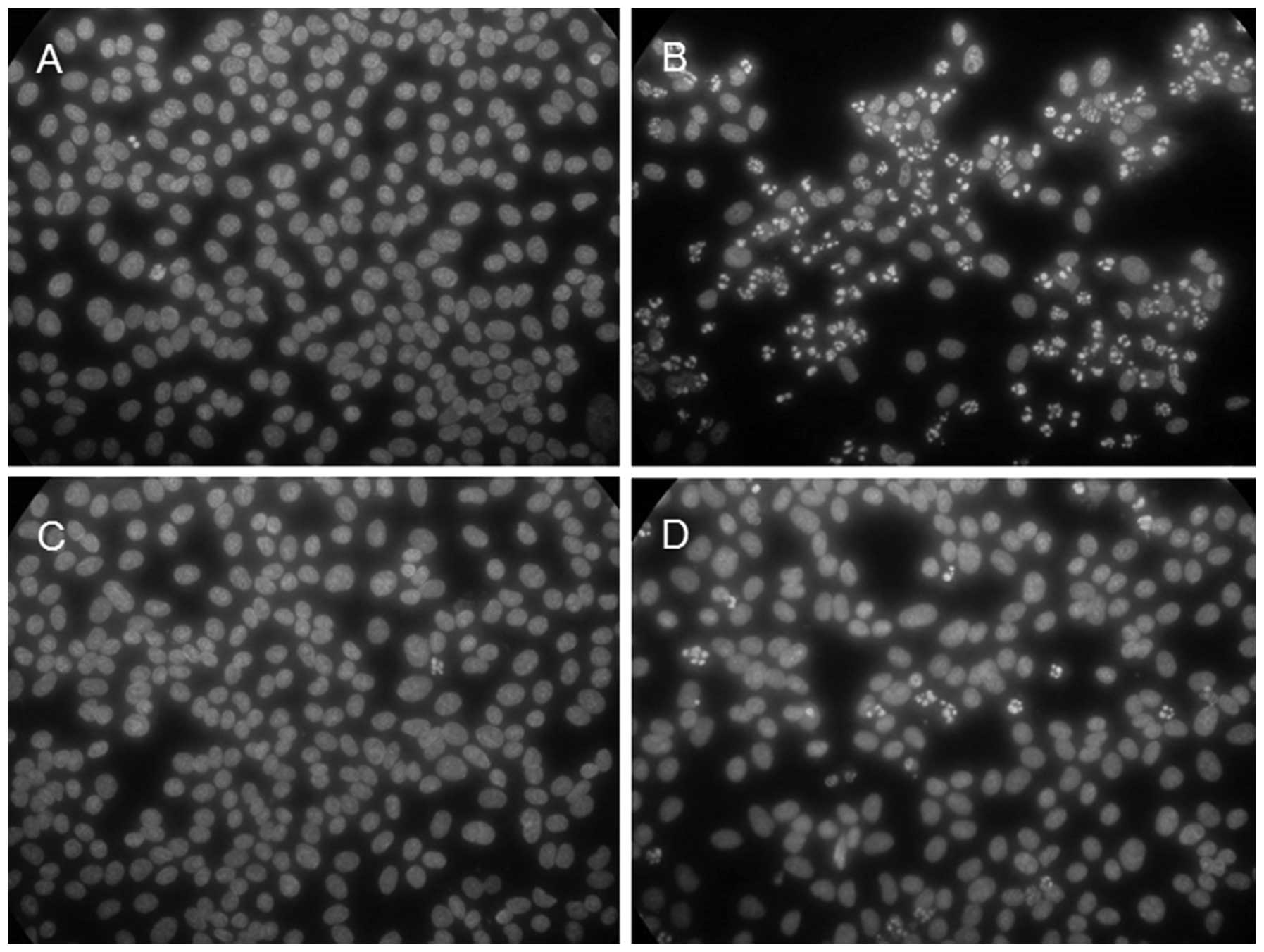
with time. As shown in Fig. 5,
the majority (approximately 70% or more) of the cells showed
apoptotic nuclei at 8 h p.i. (Fig.
5B) in contrast to the mock-infected cells (Fig. 5A). In agreement with the observed
suppression of the CPE by caffeic acid, the virus-induced apoptosis
was greatly suppressed in the presence of the reagent; only a few
(1–5%) of the cells showed apoptotic nuclei at 8 h p.i. (Fig. 5D). Fig. 5 also shows that incubation with
caffeic acid alone did not induce apoptosis (Fig. 5C), in agreement with the lack of
notable cytotoxicity of the reagent.
Target of caffeic acid in the influenza
virus multiplication
Hatada et al (15) reported that viral RNA replication
occurs between 3 and 6 h p.i. in the influenza virus-infected MDCK
cells and, then, the formation of progeny viruses takes place in
conjunction with the envelopment of RNA nucleocapsids at the plasma
membrane, followed by the release of the virus to the culture
medium (14). In our study, the
formation of progeny viruses began at approximately 4 h p.i. and
was completed at 7 to 8 h p.i. under our experimental conditions
(Fig. 4).
To further examine the target of the antiviral
action of caffeic acid on the multiplication process of influenza
virus, the ‘time of addition experiment’ was carried out. As shown
in Fig. 6, 4 mM caffeic acid was
added to the infected culture at various time points after
infection and the virus yield at the end of virus multiplication
(black bar) was compared to the virus yield at the time of the
addition of the reagent (open circle). The amounts of progeny virus
(black bar) were markedly suppressed when the infected cells
received the reagent in the early stage of the infection, such as
at 0, 2 or 3 h p.i. When the cells received caffeic acid at 3 h
p.i., a notable degree of the suppression of the progeny virus
formation was observed; however, the degree of the suppression
became less and less prominent with the delay of the addition of
the reagent to the infected cell culture. For example, the amount
of the infectious viruses at 3 and 4 h p.i. was 0.95 and 1.26,
respectively (open circles at 3 and 4 h p.i.). However, when
caffeic acid was added at these time points and cell culture was
continued, the final virus yields at 9 h p.i. were 12.4 and 70.8,
respectively, meaning that, by the addition of the reagent at 4 h
p.i., but not at 3 h p.i., progeny virus formation continued
significantly after the addition of the reagent. Although 70.8 was
clearly less than the virus yield without the addition of caffeic
acid (i.e., 207), it was far greater than the amount of the
infectious virus formed at 4 h p.i. (i.e., 1.26). Thus, it is
evident that, although the addition of caffeic acid greatly affects
the progeny virus formation, it does not notably suppress the
formation by the addition at 4 h p.i. or at a later stage.
Taking the results from the study by Hatada et
al (15) into consideration,
it is suggested that i) caffeic acid strongly interferes with the
virus multiplication when added before the onset of RNA replication
(i.e., 3 h p.i.); ii) it affects the formation of progeny virus to
a limited extent when added after the onset of RNA replication (4 h
p.i. or later). These results are in agreement with the results
shown in Fig. 4 and confirm that
the main target of caffeic acid is during the early stage of virus
multiplication.
In this study, we demonstrate that caffeic acid
effectively inhibits the multiplication of influenza A virus. This
antiviral effect of caffeic acid is unlikely to be the secondary
results of cytotoxicity of the reagent or the direct inactivation
of the virus (virucidal effect), but is more likely due to the
specific interaction of caffeic acid to cellular and/or viral
proteins involved in viral genome replication processes. Both the
results in Figs. 4 and 6 reveal that caffeic acid mainly
interferes with the multiplication of influenza A virus in the
early stage of viral multiplication, possibly at the step(s) for a
preparation of the replication of viral genome RNA in the infected
cells. Caffeic acid has been known to directly interact with
proteins. For example, Kang et al (16) found a specific binding of caffeic
acid to Fyn kinase, one of the members of the non-receptor protein
kinase family, resulting an inhibition of the enzyme activity and
Trnkova et al (17) also
examined a binding constant of caffeic acid to bovine serum
albumin. These direct interactions between caffeic acid and
proteins suggest that caffeic acid inhibits virus multiplication by
directly binding and interacting with certain enzyme(s) necessary
for preparing viral RNA replication in influenza virus-infected
cells.
In addition, the results of our study on the
morphological alteration of the infected cells and cell nuclei
(Fig. 5) revealed that, in
addition to the inhibition of virus multiplication, caffeic acid
suppressed the induction of cytopathogenic changes of the infected
cells, such as CPE and apoptosis, confirming the weak cytotoxicity
of the reagent. The observed antiviral activity without notable
cytotoxicity supports a potential application of caffeic acid or
its derivatives as an anti-influenza viral drug.
Acknowledgements
The authors thank Dr Tsutomu Arakawa for his
stimulative discussions and assistance with the editing of the
manuscript. This study was supported in part by research grants
from All Japan Coffee Association.
References
|
1
|
Uozaki M, Yamasaki H, Katsuyama Y, Higuchi
M, Higuchi T and Koyama AH: Antiviral effect of octyl gallate
against DNA and RNA viruses. Antiviral Res. 73:85–91. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Utsunomiya H, Ichinose M, Uozaki M,
Tsujimoto K, Yamasaki H and Koyama AH: Antiviral activities of
coffee extracts in vitro. Food Chem Toxicol. 46:1919–1924. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uozaki M, Ikeda K, Tsujimoto K, Nishide M,
Yamasaki H, Khamsri B and Koyama AH: Antiviral effects of
dehydroascorbic acid. Exp Ther Med. 1:983–986. 2010.PubMed/NCBI
|
|
4
|
Arakawa T, Yamasaki H, Ikeda K, Ejima D,
Naito T and Koyama AH: Antiviral and virucidal activities of
natural products. Curr Med Chem. 16:2485–2497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ikeda K, Tsujimoto K, Uozaki M, Nishide M,
Suzuki Y, Koyama AH and Yamasaki H: Inhibition of multiplication of
herpes simplex virus by caffeic acid. Int J Mol Med. 28:595–598.
2011.PubMed/NCBI
|
|
6
|
Rice-Evans CA, Miller NJ and Paganga G:
Structure-antioxidant activity relationships of flavonoids and
phenolic acids. Free Radic Biol Med. 20:933–956. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koyama AH and Uchida T: The effect of
ammonium chloride on the multiplication of herpes simplex virus
type 1 in Vero cells. Virus Res. 13:271–282. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurokawa M, Koyama AH, Yasuoka S and
Adachi A: Influenza virus overcomes apoptosis by rapid
multiplication. Int J Mol Med. 3:527–530. 1999.PubMed/NCBI
|
|
9
|
Koyama AH, Irie H, Ueno F, Ogawa M, Nomoto
A and Adachi A: Suppression of apoptotic and necrotic cell death by
poliovirus. J Gen Virol. 82:2965–2972. 2001.PubMed/NCBI
|
|
10
|
McGarrity GJ: Detection of contamination.
Methods Enzymol. 58:18–29. 1979. View Article : Google Scholar
|
|
11
|
Murayama M, Tsujimoto K, Uozaki M,
Katsuyama Y, Yamasaki H, Utsunomiya H and Koyama AH: Effect of
caffeine on the multiplication of DNA and RNA viruses. Mol Med Rep.
1:251–255. 2008.PubMed/NCBI
|
|
12
|
Roizman B and Knipe DM: Herpes simplex
virus and their replication. Fields Virology. Fields BN, Knipe DM
and Howley PM: 4th edition. Lippincott-Raven; New York: pp.
2399–2460. 2001
|
|
13
|
Racaniello VR: Picornaviridae: The viruses
and their replication. Fields Virology. Fields BN, Knipe DM and
Howley PM: 4th edition. Lippincott-Raven; Philadelphia: pp.
685–722. 2001
|
|
14
|
Lamb RA and Kruchikokug RM:
Orthomyxoviridae: The viruses and their replication. Fields
Virology. Fields BN, Knipe DM and Howley PM: 4th edition.
Lippincott-Raven; Philadelphia: pp. 1487–1530. 2001
|
|
15
|
Hatada E, Hasegawa M, Mukaigawa J, Shimizu
K and Fukuda R: Control of influenza virus gene expression:
quanatitative analysis of each viral RNA species in infected cells.
J Biochem. 105:537–546. 1989.PubMed/NCBI
|
|
16
|
Kang NJ, Lee KW, Shin BJ, Jung SK, Hwang
MK, Bode AM, Heo YS, Lee HJ and Dong Z: Caffeic acid, a phenolic
phytochemical in coffee, directly inhibits Fyn kinase activity and
UVB-induced COX-2 expression. Carcinogenesis. 30:321–330. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trnkova L, Bousova I, Kubicek V and Drsata
J: Binding of naturally occurring hydroxycinnamic acids to bovine
serum albumin. Nat Sci. 2:563–570. 2010.
|