Introduction
Retinal pigment epithelial (RPE) cells are a highly
specific cell monolayer localized between the choroid and retina
that transports nutrients from the choroid to photoreceptors,
phagocytizes outer segments of the photoreceptor and maintains
retinal adhesion and intraocular pressure balance through water and
ion transport (1,2). The migration, de-differentiation and
proliferation of RPE cells play important roles in the pathogenesis
of proliferative vitreoretinopathy (PVR) (3–7), a
common cause of intraoperative failure following retinal
reattachment surgery, occurring in 5–10% of patients with
rhegmatogenous retinal detachment (8). Specifically, the diffusion of RPE
cells into the intravitreal cavity induces the formation of a
retraction membrane on the retina, resulting in the vitreous
retraction of fibrous tissues and retinal detachment (8–10).
Currently, the only effective strategy for the treatment of
hyperplastic fibrous tissues is surgical detachment; however,
repeated surgeries may result in PVR (11).
The mechanisms underlying the migration and
proliferation of RPE cells remain poorly understood; however, the
role of growth factors and cytokines (3–7,12)
in addition to factor Xa and thrombin (13) has been reported. The hyperplastic
fibrous tissues of PVR are mainly composed of cells, including RPE
cells, Müller glia, fibroblasts and macrophages, as well as an
extracellular matrix (ECM) composed of collagen I and fibronectin
(14,15). In addition, growth factor
expression, including insulin-like growth factor-binding protein-6
(IGFBP-6) (16) and vascular
endothelial growth factor A (VEGF-A) (17), has been observed in the vitreous
of PVR patients. The inhibition of the migration and proliferation
of these cells may be helpful in preventing the recurrence of
retinal detachment (18,19). Although controversial, some
clinicians have used the intravitreal or systemic injection of
steroids to inhibit cell proliferation with poor efficacy (20,21). In addition, although the
inhibition of RPE cell proliferation in vitro has been
achieved using tranilast (22),
genistein (23), ciprofloxacin
(24), vitamin C (25), vitamin E (26), minoxidil (27), hypericin (28), cis-hydroxyproline (29), retinoic acid (25), cis-vitamin A acid,
aclacinomycin A (30),
daunorubicin (31) and
N,N-dimethyl doxorubicin (32),
many of these drugs have side effects, which significantly limit
their wide application in clinical practice.
In recent years, studies have demonstrated that
curcumin, derived from the rhizome of Curcuma longa, may
inhibit the proliferation of endothelial and epithelial cells;
however, few studies have assessed its effects on RPE cells
(33,34). Therefore, this study aimed to
examine the hypothesis that curcumin inhibits RPE cell
proliferation. In addition, the possible mechanisms underlying the
effects of curcumin were analyzed by determining the expression of
pro-apoptotic factors (e.g., p53 and p21WAF1/CIP1) and
proliferating cell nuclear antigen (PCNA).
Materials and methods
In vitro culture of human RPE (hRPE)
cells
Primary RPE cells were obtained from ScienCell
Research Laboratories (Carlsbad, CA, USA) which characterized them
by immunofluorescence staining of cytokeratin-18, -19 and
fibronectin. Cells infected with HIV-1, HCV, mycoplasma, bacteria,
yeast and fungi were excluded. The cells were maintained in
Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Invitrogen,
Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 100
U/ml penicillin and 100 mg/ml streptomycin (all from Invitrogen) at
37°C in a humidified environment with 5% CO2. The cells
were passaged at a ratio of 1:2 when they reached 80% confluence
after 2–3 days in culture.
Cell proliferation assay
The WST-1 kit (Roche, Indianapolis, IN, USA) was
used to detect RPE cell proliferation and viability following the
manufacturer’s instructions. Briefly, the hRPE cells at passage 5
who had achieved a satisfactory growth were used to prepare a
single-cell suspension. The cells were seeded onto 96-well plates
(5,000 cells/well in 100 μl) and incubated at 37°C with 5%
CO2. The cells were treated with 5, 10, 15, 20 μg/ml
curcumin (dissolved in DMSO; Sigma-Aldrich, St. Louis, MO, USA) or
DMSO. After 24, 48 and 72 h, 10 μl of WST-1 solution were added to
each well, followed by incubation for 1–4 h. The optical density
(OD) was measured at 450 nm to determine the optimal concentration
of the curcumin-mediated inhibition of proliferation.
Detection of apoptosis
The Annexin V-FIT apoptosis detection kit I (BD
Pharmingen, San Jose, CA, USA) was used to detect curcumin-induced
apoptosis. In brief, the hRPE cells were treated with 15 μg/ml
curcumin or DMSO (control) for 48 h, digested with EDTA-free
trypsin (Invitrogen) and then washed twice with phosphate-buffered
saline (PBS). Approximately 1–5×105 cells were collected
and re-suspended in 500 μl of binding buffer, 2 μl of Annexin
V-FITC and 5 μl of propidium iodide (PI), followed by incubation in
the dark at room temperature for 5 min. Flow cytometry (using a
flow cytometer; Beckman Coulter, Brea, CA, USA) was performed at an
excitation of 488 nm and an emission of 530 nm to detect the
apoptotic cells. Cells positive for Annexin V-FITC, but negative
for PI were considered apoptotic; those positive for both Annexin
V-FITC and PI were considered necrotic.
Cell cycle analysis
The hRPE cells were treated with 15 μg/ml curcumin
or DMSO (control) for 48 h, digested with EDTA-free trypsin
(Invitrogen), washed twice with PBS and harvested by
centrifugation. Following fixation in 70% ethanol at 4°C overnight,
the cells were washed with 5 ml of PBS and incubated with 500 μl of
PBS containing 100 μg/ml PI (Sigma-Aldrich), 100 μg/ml RNase A
(Sigma-Aldrich) and 0.2% Triton X-100, followed by incubation at
4°C in the dark for 30 min and flow cytometry.
Transmission electron microscopy
Following treatment with 15 μg/ml curcumin for 24,
48 and 72 h, the hRPE cells were harvested and seeded onto
coverslips, which were washed twice with PBS and fixed in 2.5%
glutaraldehyde at room temperature for 1 h and then in 2% osmic
acid. Following washing in 0.1 M PBS followed by distilled water,
the cells were dehydrated in a graded ethanol series. Following
treatment with Poly(diallyl phthalate) (PDAP; Sigma-Aldrich) and
in situ embedding, ultra-thin sections were obtained, which
were stained with 2% uranyl acetate and lead citrate. These
sections were observed under a transmission electron microscope
(JEM-1230; Joel, Tokyo, Japan).
Western blot analysis
Following treatment with 15 μg/ml curcumin for 24,
48 and 72 h, the hRPE cells were harvested and lysed in RIPA lysis
buffer (50 mM Tris, pH 7.4; 150 mM NaCl; 1% Triton X-100; 1% sodium
deoxycholate; 0.1% SDS; sodium orthovanadate; sodium fluoride;
EDTA; leupeptin), and the protein concentration was determined
using the BCA method. Proteins (50 μg) were separated with 12%
SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica,
MA, USA), which were incubated in blocking buffer (TBS, 0.1%
Tween-20, 2% BSA) at room temperature for 1 h. After the membranes
were washed 3 times in TBS (5 min/wash), they were incubated with
the following primary antibodies at room temperature for 1.5 h:
mouse anti-human p21WAF1/CIP1 monoclonal antibody, mouse
anti-human p53 monoclonal antibody, mouse anti-human PCNA
monoclonal antibody (all from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) monoclonal antibody (Cell Signaling Technology, Danvers,
MA, USA). After washing in TBS, the membranes were incubated with
HRP-conjugated secondary antibodies (1:2,000; Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h followed by
chemiluminescence detection with an ECL GST Western Blotting
Detection kit (Santa Cruz Biotechnology, Inc.). Quantity One
software (Bio-Rad Laboratories, Hercules, CA, USA) was used to
determine the OD of the bands, and GAPDH served as an internal
reference.
Statistical analysis
Continuous data are presented as the means ±
standard deviation (SD). Curcumin-induced hRPE inhibition by dose
at a given time or among different time points at a given dose was
compared by one-way analysis of variance (ANOVA) with pair-wise
post-hoc tests using Bonferroni correction. The apoptotic rate,
cell necrotic rate, cell cycle and protein expression between the
control and curcumin-treated groups were compared by an independent
t-test. All data analyses were performed using SPSS statistical
software (version 17.0; SPSS Inc., Chicago, IL, USA). A two-tailed
P-value <0.05 indicated a statistically significant
difference.
Results
Inhibitory effects of curcumin on hRPE
cell proliferation in vitro
The curcumin-mediated inhibition of hRPE cell
proliferation was assessed following treatment with different doses
of curcumin after 24, 48 and 72 h. A dose-dependent inhibition in
hRPE cell proliferation was observed at each time point analyzed
(P<0.001) (Table I). Moreover,
the inhibitory effects of curcumin significantly increased with the
increase in the treatment duration at each curcumin concentration
(P<0.001). For both the 48- and 72-h time points, the
proliferation inhibition rate of the cells treated with 15 μg/ml
curcumin was significantly higher than that of the cells treated
with 5 and 10 μg/ml curcumin at the same time points (all
P<0.05). The proliferation inhibition rate of the cells treated
with 20 μg/ml curcumin was significantly higher than that of the
cells treated with 5 and 10 μg/ml curcumin at all 3 time points;
however, it did not differ significantly from that of the cells
treated with 15 μg/ml curcumin (Table
I). Therefore, the dose of 15 μg/ml curcumin was selected for
the subsequent experiments.
 | Table IThe inhibitory effects of curcumin on
the proliferation of cultured human retinal pigment epithelial
(hRPE) cells. |
Table I
The inhibitory effects of curcumin on
the proliferation of cultured human retinal pigment epithelial
(hRPE) cells.
| Curcumin
concentration (μg/ml) | |
|---|
|
| |
|---|
| Time (h) | 5 | 10 | 15 | 20 |
P-valuea |
|---|
| 24 | 5.07±2.66 | 11.53±2.58 |
15.85±2.71c |
21.32±1.67c,d | <0.001 |
| 48 |
11.24±0.44e | 15.44±2.52 |
21.41±2.55c,d |
26.38±1.09c,d,e | <0.001 |
| 72 |
18.85±1.60e,f |
29.69±0.75c,e,f |
39.96±3.88c–f |
40.10±2.11c–f | <0.001 |
|
P-valueb | <0.001 | <0.001 | <0.001 | <0.001 | |
Effects of curcumin on hRPE cell
apoptosis and necrosis
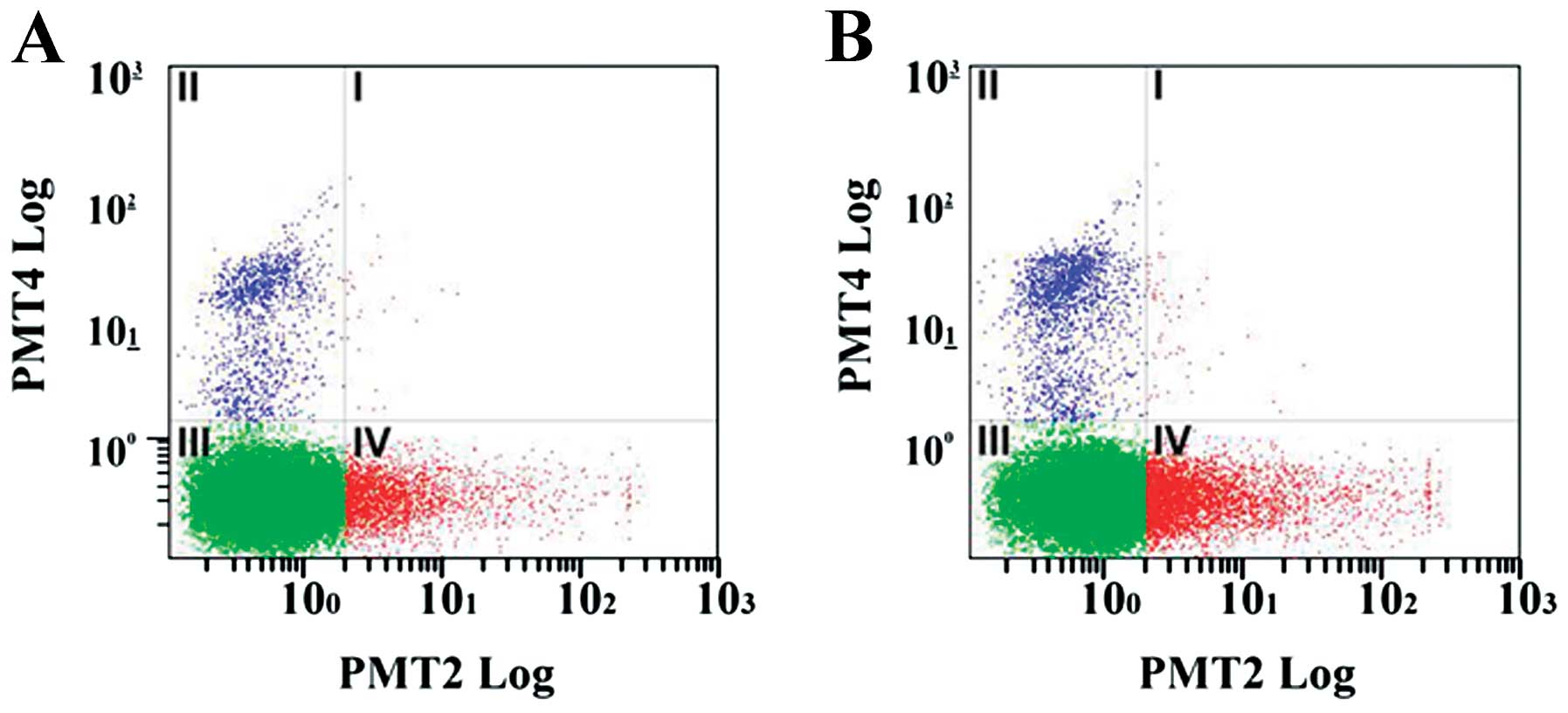
The hRPE cells were treated with 15 μg/ml curcumin
for 48 h after which the proportion of apoptotic and necrotic cells
was determined; representative data are shown in Fig. 1. As shown in Table II, the curcumin-treated group had
a significantly greater proportion of cells in the early apoptotic
phase (13.37 vs. 7.03%; P=0.001), resulting in a significantly
higher overall apoptotic rate (P=0.001) compared to the control
group. However, no significant difference was observed in the
proportion of necrotic cells between the 2 groups (Table II).
 | Table IIThe effects of curcumin on the
apoptosis and necrosis of cultured human retinal pigment epithelial
(hRPE) cells after 48 h. |
Table II
The effects of curcumin on the
apoptosis and necrosis of cultured human retinal pigment epithelial
(hRPE) cells after 48 h.
| Control group | Curcumin-treated
group | P-valuea |
|---|
| Apoptotic rate (%),
early phase | 7.03±0.37 | 13.37±1.26 | 0.001 |
| Apoptotic rate (%),
mid-late phase | 0.09±0.00 | 0.16±0.09 | 0.307 |
| Apoptotic rate (%),
total | 7.12±0.37 | 13.53±1.18 | 0.001 |
| Cell necrosis rate
(%) | 4.40±0.18 | 5.19±0.75 | 0.154 |
A subsequent analysis of the effects of curcumin on
the hRPE cell ultrastructure after 48 h was undertaken using
transmission electron microscopy (Fig. 2). While the absence of
apoptosis-induced changes in the cellular ultrastructure was noted
in the normal control cells (Fig.
2A), changes associated with the different phases of apoptosis
were observed in the curcumin-treated cells (Fig. 2B–H). Specifically, in early
apoptosis, chromatin margination was detected along with a slight
increase in the number of mitochondria, mitochondrial swelling and
lysosomes (Fig. 2B and C). In
middle-to-late apoptosis, the hRPE cells exhibited nuclear membrane
shrinkage, mitochondrial swelling (Fig. D–F),
as well as a large amount of lysosomes (Fig. 2F). In the late phase of apoptosis,
characteristics similar to those of necrotic cells (i.e., rupture
of the cell membrane, nuclear membrane shrinkage, injured
organelles and cytoplasmic vacuoles) were observed (Fig. 2H).
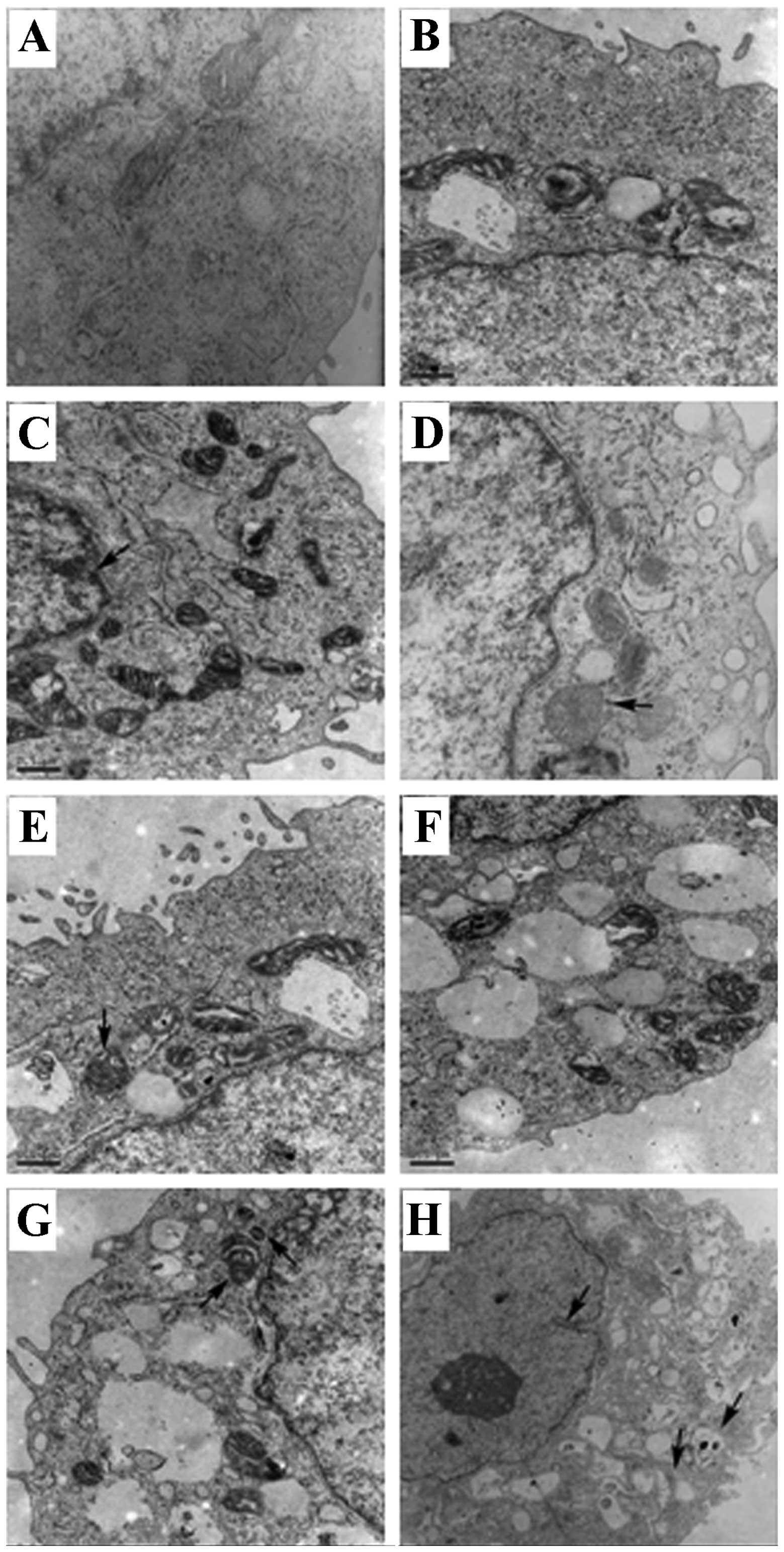 | Figure 2Ultrastructural analysis of human
retinal pigment epithelial (hRPE) cells following treatment with
curcumin. (A) hRPE cells in the normal control group (TEM ×20,000;
scale bar, 0.5 μm). (B and C) hRPE cells in the early phase of
apoptosis (TEM ×20,000; scale bar, 0.5 μm). The arrow in panel C
indicates chromatin margination. (D–G) hRPE cells in the
middle-to-late phase of apoptosis (TEM ×20,000; scale bar, 0.5 μm).
The arrows in panels D and E indicate mitochondrial swelling. The
arrow on the left side of panel G indicates mitochondrial swelling,
and the arrow on the right side of panel G indicates a lysosome.
(H) hRPE cells in the late phase of apoptosis (TEM ×10,000, scale
bar, 1 μm). The arrow on the left side indicates nuclear membrane
shrinkage, and the arrows on the right side indicate damaged,
vesicular organelles. |
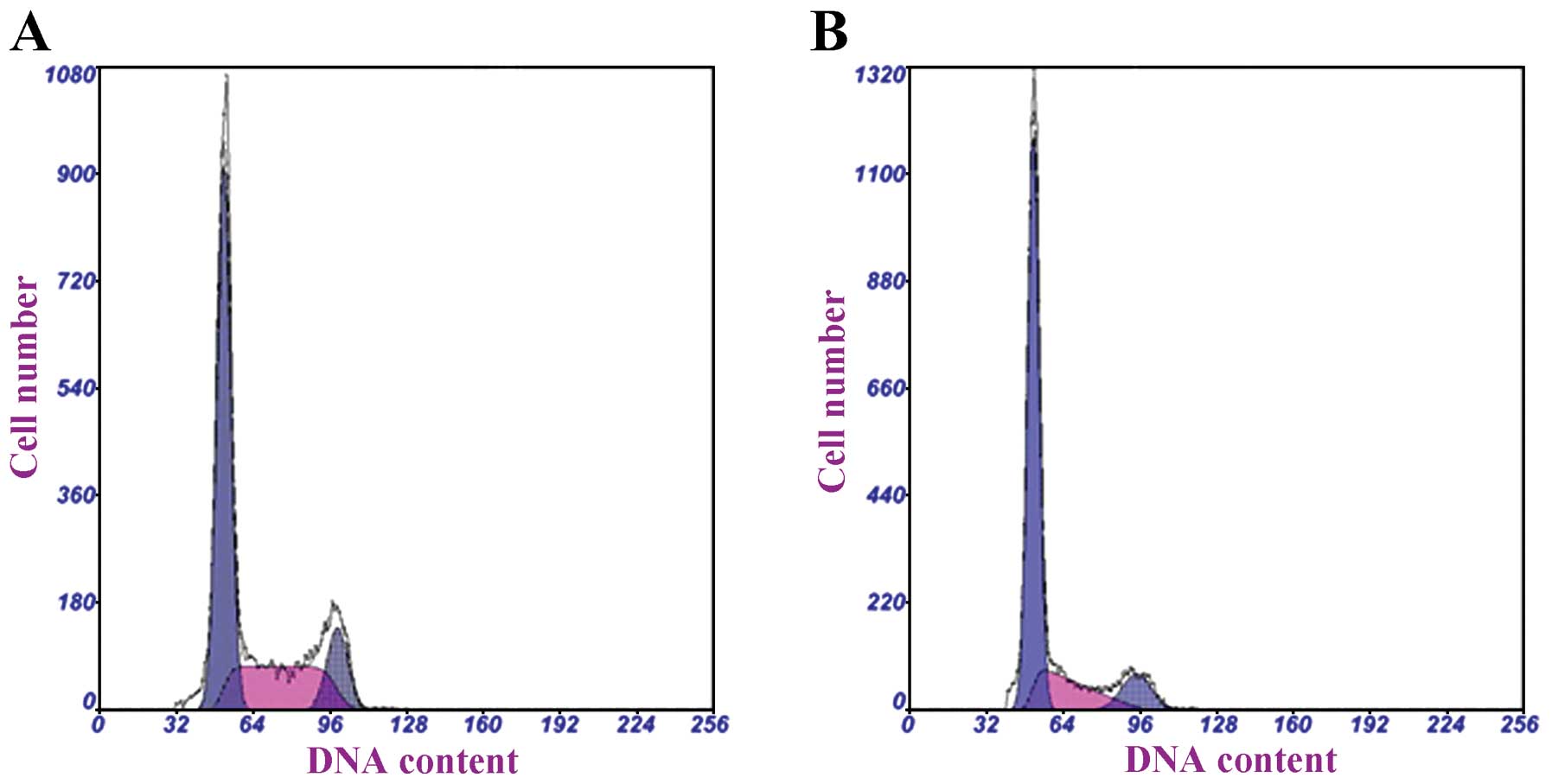
Effects of curcumin on hRPE cell cycle
progression
The cell cycle progression of hRPE cells was then
determined following treatment with 15 μg/ml curcumin for 48 h
(Fig. 3). The proportion of hRPE
cells in the G0/G1 phase was significantly higher in the
curcumin-treated group than in the control group (67.73 vs. 57.17%;
P<0.001) (Table III). A
concomitant decrease in the proportion of curcumin-treated hRPE
cells in the S phase was observed (P=0.010).
 | Table IIIThe effects of curcumin on the cell
cycle progression of cultured human retinal pigment epithelial
(hRPE) cells after 48 h. |
Table III
The effects of curcumin on the cell
cycle progression of cultured human retinal pigment epithelial
(hRPE) cells after 48 h.
| Cell cycle
phase | Control group | Curcumin-treated
group | P-valuea |
|---|
| G0/G1 (%) | 57.17±1.17 | 67.73±1.10 | <0.001 |
| S (%) | 32.23±3.47 | 21.47±2.03 | 0.010 |
| G2/M (%) | 10.64±3.41 | 10.83±1.57 | 0.935 |
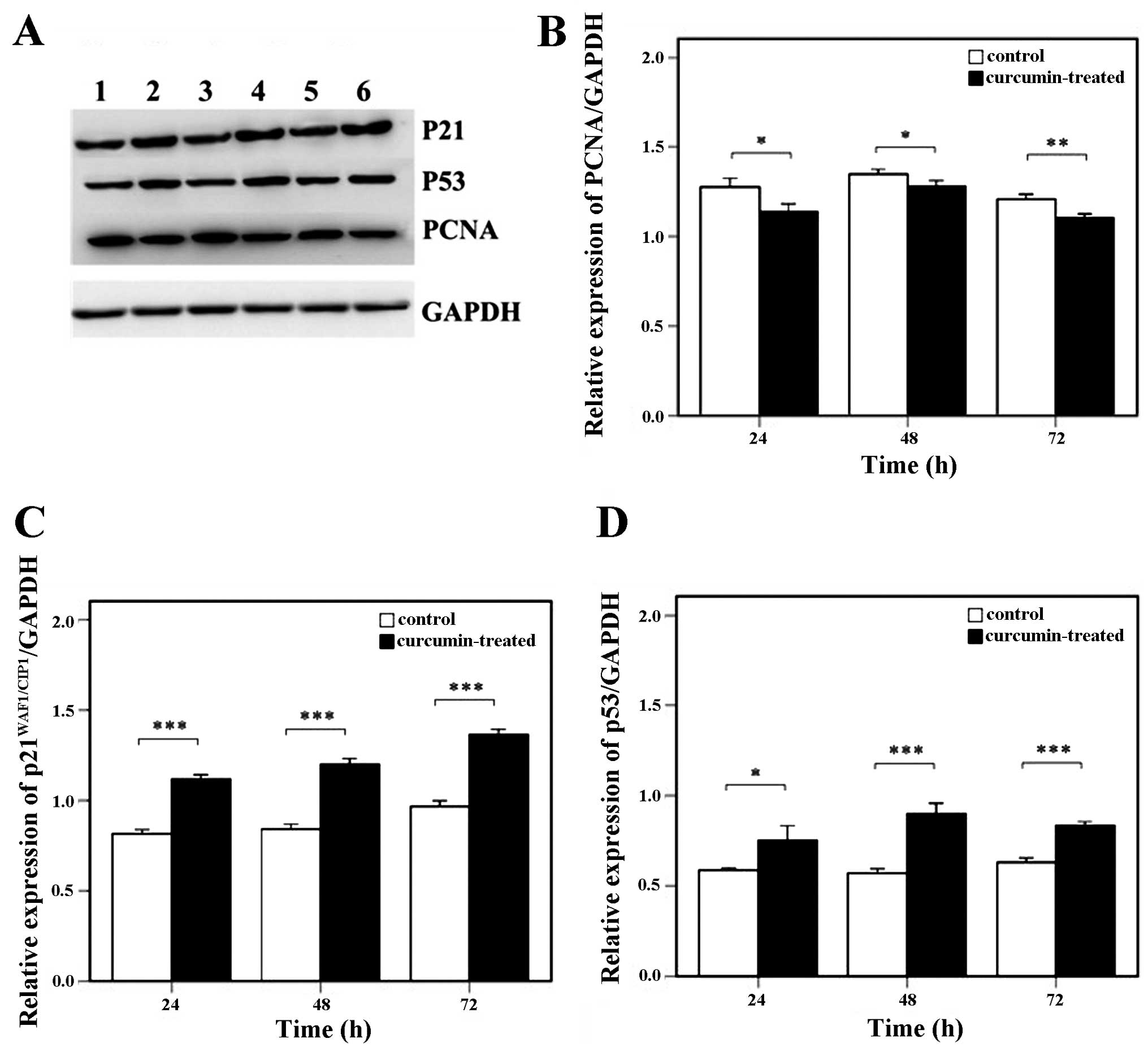
Effects of curcumin on
p21WAF1/CIP1, p53 and PCNA protein expression in hRPE
cells
The hRPE cells were treated with 15 μg/ml curcumin
for 24, 48 and 72 h after which western blot analysis was
undertaken; a representative blot is shown in Fig. 4A. The relative PCNA expression was
significantly decreased in the curcumin-treated group as compared
to the control group at all time points (P<0.05) (Fig. 4B); however, the relative
expression of p21WAF1/CIP1 (Fig. 4C) and p53 (Fig. 4D) was significantly elevated in
response to curcumin treatment (all P<0.05).
Discussion
Given the anti-proliferative effects of curcumin for
epithelial and endothelial cells, its effects on hRPE cell
proliferation were determined in the present study. Similarly,
curcumin inhibited hRPE cell proliferation in a dose-dependent
manner. The anti-proliferative response by curcumin was mediated in
part by increased apoptosis, as well as G0/G1 cell cycle arrest. No
induction of necrosis was observed. Furthermore, curcumin increased
p21 and p53 protein expression, while decreasing PCNA protein
levels.
Curcumin is a diarylheptanoid extracted from the
rhizome of Curcuma longa. In vitro studies have
demonstrated the antioxidant (35), anti-inflammatory (36), anti-angiogenic (37) and anti-proliferative (38) effects of curcumin in a variety of
cells. In the present study, curcumin significantly inhibited the
proliferation of hRPE cells in vitro in a dose- and
time-dependent manner. This is consistent with the cytotoxic
effects of curcumin in hRPE cells reported by Hollborn et al
(33).
As the WST-1 assay only indirectly reflects cell
viability without directly assessing cell proliferation or cell
death, we also assessed the effects of curcumin on apoptosis and
necrosis. Although no significant induction of necrosis was
observed, treatment with 15 μg/ml curcumin for 48 h significantly
increased the apoptotic rate, which was confirmed by transmission
electron microscopy that detected ultrastructural changes
consistent with the early and middle-to-late phases of apoptosis.
Hollborn et al (33)
reported increased hRPE cell necrosis in response to >10 μM
curcumin. Differences in the concentration of curcumin used (>10
μM vs. 15 μg/ml), as well as the duration of culture (6 and 24 vs.
48 h) may account for the discrepant results with respect to
necrosis.
In the present study, curcumin increased the
expression of p53, which plays a role in the mitochondrial
apoptotic pathway, as well as the expression of its downstream
factor, p21WAF1/CIP1. This result is consistent with
that of a previous study that reported the curcumin-induced
apoptosis of retinal vascular endothelial cells (39). In hRPE cells and ARPE19 cells,
curcumin has been shown to induce apoptosis through the
mitochondrial apoptotic pathway (40). These results suggest that curcumin
increases mitochondrial outer membrane permeability (MOMP), leading
to the release of p53, which in turn elevates MOMP and thereby
initiates apoptosis. Given the importance of the p53 pathway and
its negative regulator, murine double minute 2 (MDM2), in PVR
pathogenesis (41), further
studies are required to assess this possibility in detail.
Furthermore, curcumin-induced p53 and p21WAF1/CIP1
expression was detected at the same time point, suggesting that p53
initiates the expression of p21WAF1/CIP1. In a previous
study, in hRPE cells treated with H2O2 for 5
min, an increased p21WAF1/CIP1 expression ensued that of
p53 (42). Therefore, we
hypothesized that treatment with curcumin may induce the release of
p53 from the mitochondria at extremely early time points; however,
prolonged curcumin treatment may induce the synthesis of p53 and
p21WAF1/CIP1. As p53 suppresses VEGF, a growth factor
relevant to PVR pathogenesis (17) and negatively regulated by curcumin
(33), further studies are
required to assess the expression of additional p53 downstream
factors in response to curcumin, apart from p53.
In addition to inducing apoptosis, both p53 and
p21WAF1/CIP1 mediate cell cycle arrest. p53 arrests
cells in the G1 phase to impair DNA repair or induce apoptosis, and
p21WAF1/CIP1 interacts with cyclin-dependent kinase
(CDK)2 to arrest cells in the G1 phase, inhibiting DNA replication
and mitosis (43–45). Adenovirus-mediated
p21WAF1/CIP1 overexpression in retinal vascular
endothelial cells inhibits their proliferation and tube formation
(46). In the present study,
curcumin induced G0/G1 phase arrest in the hRPE cells, which is
consistent with that observed in human umbilical vein endothelial
cells (HUVECs) (47). Moreover,
the proportion of hRPE cells in the S phase was reduced with
curcumin treatment, while the number of cells in the G2/M phase
remained unaltered. These results were confirmed by evaluating the
expression of PCNA, an important marker of cell proliferation
involved in DNA synthesis, repair, regulation of the cell cycle,
chromosomal rearrangement and DNA methylation. As a co-factor of
DNA polymerase δ, PCNA participates in DNA synthesis and
replication and regulates entry into the S phase; its expression is
increased in the G1 phase, peaking in the S phase (48,49). In the present study, PCNA levels
decreased in the hRPE cells treated with curcumin. This
downregulation may be attributed to its increased interaction with
p21WAF1/CIP1, which inhibits CDK activity, blocks
retinoblastoma protein (Rb) phosphorylation and arrests cells
before the S phase (50). Further
studies are required to assess the mechanisms and factors through
which curcumin inhibits cell cycle progression in greater detail,
including assessing Krüppel-like factor 5 (KLF5), an inhibitor of
aberrant cell cycle progression in epithelial cells which functions
in part through the regulation of p21WAF1/CIP1 (51).
In addition to hRPE cell proliferation, hyperplastic
fibrous tissues of PVR contain ECM components, including collagen I
and sometimes collagen II (14).
Further studies are required to determine the effects of curcumin
on ECM components in PVR.
In conclusion, taken together, our data demonstrate
that curcumin effectively inhibits the proliferation of primary
hRPE cells in a time- and dose-dependent manner. The cytotoxic
effects of curcumin are attributed to reduced cell cycle
progression and the induction of apoptosis, which may be related to
the p53 and p21WAF1/CIP1 upregulation and the decrease
in PCNA expression. We hypothesize that the p53 signaling pathway
is involved in the anti-proliferative effects of curcumin on hRPE
cells. Further in vivo studies are required to fully explore
the therapeutic potential of curcumin for PVR.
Abbreviations:
|
ANOVA
|
analysis of variance
|
|
ECM
|
extracellular matrix
|
|
FBS
|
fetal bovine serum
|
|
OD
|
optical density
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
PVR
|
proliferative vitreoretinopathy
|
|
RPE cells
|
retinal pigment epithelial cells
|
|
SD
|
standard deviation
|
References
|
1
|
Rizzolo LJ, Peng S, Luo Y and Xiao W:
Integration of tight junctions and claudins with the barrier
functions of the retinal pigment epithelium. Prog Retin Eye Res.
30:296–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sparrow JR, Hicks D and Hamel CP: The
retinal pigment epithelium in health and disease. Curr Mol Med.
10:802–823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He S, Chen Y, Khankan R, et al: Connective
tissue growth factor as a mediator of intraocular fibrosis. Invest
Ophthalmol Vis Sci. 49:4078–4088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaczmarek R and Misiuk-Hojlo M:
Patomechanisms in proliferative vitreoretinopathy. Klin Oczna.
113:64–67. 2011.PubMed/NCBI
|
|
5
|
Lei H, Rhéaume MA and Kazlauskas A: Recent
developments in our understanding of how platelet-derived growth
factor (PDGF) and its receptors contribute to proliferative
vitreoretinopathy. Exp Eye Res. 90:376–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lei H, Rhéaume MA, Velez G, Mukai S and
Kazlauskas A: Expression of PDGFRalpha is a determinant of the PVR
potential of ARPE19 cells. Invest Ophthalmol Vis Sci. 52:5016–5021.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Umazume K, Barak Y, McDonald K, Liu L,
Kaplan HJ and Tamiya S: Proliferative vitreoretinopathy in the
Swine-a new model. Invest Ophthalmol Vis Sci. 53:4910–4916. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asaria RH and Charteris DG: Proliferative
vitreoretinopathy: developments in pathogenesis and treatment.
Compr Ophthalmol Update. 7:179–185. 2006.PubMed/NCBI
|
|
9
|
Leiderman YI and Miller JW: Proliferative
vitreoretinopathy: pathobiology and therapeutic targets. Semin
Ophthalmol. 24:62–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sebag J: Shaken not stirred.
Ophthalmology. 108:1177–1178. 2001. View Article : Google Scholar
|
|
11
|
Kim IK and Arroyo JG: Mechanisms in
proliferative vitreoretinopathy. Ophthalmol Clin North Am.
15:81–86. 2002. View Article : Google Scholar
|
|
12
|
Wickham L and Charteris DG: Glial cell
changes of the human retina in proliferative vitreoretinopathy. Dev
Ophthalmol. 44:37–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bastiaans J, van Meurs JC, van
Holten-Neelen C, et al: Factor Xa and thrombin stimulate
proinflammatory and profibrotic mediator production by retinal
pigment epithelial cells: a role in vitreoretinal disorders?
Graefes Arch Clin Exp Ophthalmol. 251:1723–1733. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feist RM Jr, King JL, Morris R,
Witherspoon CD and Guidry C: Myofibroblast and extracellular matrix
origins in proliferative vitreoretinopathy. Graefes Arch Clin Exp
Ophthalmol. 252:347–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Halper J and Kjaer M: Basic components of
connective tissues and extracellular matrix: elastin, fibrillin,
fibulins, fibrinogen, fibronectin, laminin, tenascins and
thrombospondins. Adv Exp Med Biol. 802:31–47. 2014. View Article : Google Scholar
|
|
16
|
Zhu W, Wu Y, Cui C, Zhao HM, Ba J, Chen H
and Yu J: Expression of IGFBP6 in proliferative vitreoretinopathy
rat models and its effects on retinal pigment epithelial-J cells.
Mol Med Rep. 9:33–38. 2014.
|
|
17
|
Azzolini C, Pagani IS, Pirrone C, et al:
Expression of VEGF-A, Otx homeobox and p53 family genes in
proliferative vitreoretinopathy. Mediators Inflamm.
2013:8573802013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou Q, Tang J, Wang Z, et al: Inhibitory
effect of microRNA-34a on retinal pigment epithelial cell
proliferation and migration. Invest Ophthalmol Vis Sci.
54:6481–6488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu S, Jiang Z, Huang Z, Chen X, Qian X,
Gao Q and Zheng H: Migration of retinal pigment epithelium cells is
regulated by protein kinase Calpha in vitro. Invest Ophthalmol Vis
Sci. 54:7082–7090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahmadieh H, Feghhi M, Tabatabaei H,
Shoeibi N, Ramezani A and Mohebbi MR: Triamcinolone acetonide in
silicone-filled eyes as adjunctive treatment for proliferative
vitreoretinopathy: a randomized clinical trial. Ophthalmology.
115:1938–1943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheema RA, Peyman GA, Fang T, Jones A,
Lukaris AD and Lim K: Triamcinolone acetonide as an adjuvant in the
surgical treatment of retinal detachment with proliferative
vitreoretinopathy. Ophthalmic Surg Lasers Imaging. 38:365–370.
2007.PubMed/NCBI
|
|
22
|
Yasukawa T, Kimura H, Dong J, Tabata Y,
Miyamoto H, Honda Y and Ogura Y: Effect of tranilast on
proliferation, collagen gel contraction, and transforming growth
factor beta secretion of retinal pigment epithelial cells and
fibroblasts. Ophthalmic Res. 34:206–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krott R, Lebek J, Grisanti S, Esser P and
Heimann K: Antiproliferative Wirkung von Genistein auf kultivierte
retinale Pigmentepithelzellen vom Schwein. Ophthalmologica.
214:296–300. 2000.(In German).
|
|
24
|
Koutsandrea CN, Miceli MV, Peyman GA,
Farahat HG and Niesman MR: Ciprofloxacin and dexamethasone inhibit
the proliferation of human retinal pigment epithelial cells in
culture. Curr Eye Res. 10:249–258. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu WC, Hu DN, Mehta S and Chang YC:
Effects of retinoic acid on retinal pigment epithelium from excised
membranes from proliferative vitreoretinopathy. J Ocul Pharmacol
Ther. 21:44–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakamoto T, Hinton DR, Kimura H, Spee C,
Gopalakrishna R and Ryan SJ: Vitamin E succinate inhibits
proliferation and migration of retinal pigment epithelial cells in
vitro: therapeutic implication for proliferative vitreoretinopathy.
Graefes Arch Clin Exp Ophthalmol. 234:186–192. 1996. View Article : Google Scholar
|
|
27
|
Handa JT, Murad S and Jaffe GJ: Inhibition
of cultured human RPE cell proliferation and lysyl hydroxylase
activity by hydroxy derivatives of minoxidil. Invest Ophthalmol Vis
Sci. 35:463–469. 1994.PubMed/NCBI
|
|
28
|
Gao Q and Ge J: The inhibition of
Ca2+influx induced by hypericin in cultured human
retinal pigment epithelial cells analyzed by confocal imaging.
Ophthalmic Res. 37:128–135. 2005.
|
|
29
|
Yoo JS, Sakamoto T, Spee C, et al:
cis-Hydroxyproline inhibits proliferation, collagen synthesis,
attachment, and migration of cultured bovine retinal pigment
epithelial cells. Invest Ophthalmol Vis Sci. 38:520–528. 1997.
|
|
30
|
Schmidt JF and Loeffler KU: Toxicity and
antiproliferative effect of aclacinomycin A on RPE cells in vitro.
Curr Eye Res. 15:1112–1116. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang YS, Hui YN and Wiedemann P: Role of
apoptosis in the cytotoxic effect mediated by daunorubicin in
cultured human retinal pigment epithelial cells. J Ocul Pharmacol
Ther. 18:377–387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Steinhorst UH, Chen EP, Machemer R and
Hatchell DL: N,N-dimethyladriamycin for treatment of experimental
proliferative vitreoretinopathy: efficacy and toxicity on the
rabbit retina. Exp Eye Res. 56:489–495. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hollborn M, Chen R, Wiedemann P,
Reichenbach A, Bringmann A and Kohen L: Cytotoxic effects of
curcumin in human retinal pigment epithelial cells. PLoS One.
8:e596032013. View Article : Google Scholar
|
|
34
|
Woo JM, Shin DY, Lee SJ, et al: Curcumin
protects retinal pigment epithelial cells against oxidative stress
via induction of heme oxygenase-1 expression and reduction of
reactive oxygen. Mol Vis. 18:901–908. 2012.PubMed/NCBI
|
|
35
|
Rong S, Zhao Y, Bao W, et al: Curcumin
prevents chronic alcohol-induced liver disease involving decreasing
ROS generation and enhancing antioxidative capacity. Phytomedicine.
19:545–550. 2012. View Article : Google Scholar
|
|
36
|
Katsori AM, Chatzopoulou M, Dimas K,
Kontogiorgis C, Patsilinakos A, Trangas T and Hadjipavlou-Litina D:
Curcumin analogues as possible anti-proliferative and
anti-inflammatory agents. Eur J Med Chem. 46:2722–2735. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mohan R, Sivak J, Ashton P, et al:
Curcuminoids inhibit the angiogenic response stimulated by
fibroblast growth factor-2, including expression of matrix
metalloproteinase gelatinase B. J Biol Chem. 275:10405–10412. 2000.
View Article : Google Scholar
|
|
38
|
Abusnina A, Keravis T, Yougbare I, Bronner
C and Lugnier C: Anti-proliferative effect of curcumin on melanoma
cells is mediated by PDE1A inhibition that regulates the epigenetic
integrator UHRF1. Mol Nutr Food Res. 55:1677–1689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Premanand C, Rema M, Sameer MZ, Sujatha M
and Balasubramanyam M: Effect of curcumin on proliferation of human
retinal endothelial cells under in vitro conditions. Invest
Ophthalmol Vis Sci. 47:2179–2184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alex AF, Spitznas M, Tittel AP, Kurts C
and Eter N: Inhibitory effect of epigallocatechin gallate (EGCG),
resveratrol, and curcumin on proliferation of human retinal pigment
epithelial cells in vitro. Curr Eye Res. 35:1021–1033. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pastor-Idoate S, Rodríguez-Hernandez I,
Rojas J, et al: The T309G MDM2 gene polymorphism is a novel risk
factor for proliferative vitreoretinopathy. PLoS One. 8:e822832013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jin GF, Hurst JS and Godley BF: Hydrogen
peroxide stimulates apoptosis in cultured human retinal pigment
epithelial cells. Curr Eye Res. 22:165–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mahyar-Roemer M and Roemer K: p21
Waf1/Cip1 can protect human colon carcinoma cells against
p53-dependent and p53-independent apoptosis induced by natural
chemopreventive and therapeutic agents. Oncogene. 20:3387–3398.
2001. View Article : Google Scholar
|
|
44
|
Tian H, Wittmack EK and Jorgensen TJ:
p21WAF1/CIP1antisense therapy radiosensitizes human
colon cancer by converting growth arrest to apoptosis. Cancer Res.
60:679–684. 2000.
|
|
45
|
Xu GW, Nutt CL, Zlatescu MC, Keeney M,
Chin-Yee I and Cairncross JG: Inactivation of p53 sensitizes U87MG
glioma cells to 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res.
61:4155–4159. 2001.PubMed/NCBI
|
|
46
|
Han J, Yuan Z and Yan H: Inhibitory effect
of adenoviral vector-mediated delivery of p21WAF1/CIP1on
retinal vascular endothelial cell proliferation and tube formation
in cultured Rhesus monkey cells (RF/6A). Curr Eye Res. 38:670–673.
2013.PubMed/NCBI
|
|
47
|
Wei SC, Lin YS, Tsao PN, Wu-Tsai JJ, Wu CH
and Wong JM: Comparison of the anti-proliferation and
apoptosis-induction activities of sulindac, celecoxib, curcumin,
and nifedipine in mismatch repair-deficient cell lines. J Formos
Med Assoc. 103:599–606. 2004.PubMed/NCBI
|
|
48
|
Kirchmaier AL: Ub-family modifications at
the replication fork: Regulating PCNA-interacting components. FEBS
Lett. 585:2920–2928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Strzalka W and Ziemienowicz A:
Proliferating cell nuclear antigen (PCNA): a key factor in DNA
replication and cell cycle regulation. Ann Bot. 107:1127–1140.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cazzalini O, Perucca P, Riva F, et al:
p21CDKN1A does not interfere with loading of PCNA at DNA
replication sites, but inhibits subsequent binding of DNA
polymerase delta at the G1/S phase transition. Cell Cycle.
2:596–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang Y, Tarapore RS, Jarmel MH, Tetreault
MP and Katz JP: p53 mutation alters the effect of the esophageal
tumor suppressor KLF5 on keratinocyte proliferation. Cell Cycle.
11:4033–4039. 2012. View Article : Google Scholar : PubMed/NCBI
|