In the human gut, a continuous homeostasis is
maintained by the strict regulation of microbial load and the
immune responses against it (1,2).
Under normal physiological conditions, gut microbiota colonize and
contribute to the proper development of the mucosal immune system
(1,2). When the epithelial barrier is
disrupted, they induce an uncontrollable inflammatory condition.
The breakdown of this balance by the dysregulation of immune
responses may increase susceptibility to chronic inflammatory
disorders, such as Crohn’s disease (3,4).
Uncontrolled mucosal inflammatory responses against gut microbiota
due to the disruption of the epithelial barrier hence play
important roles in the pathogenesis of Crohn’s disease (3,4).
Crohn’s disease is a chronic inflammatory disorder associated with
mucosal inflammation of the bowel wall, which is characterized by
repetitive active cycles of the disease state (5). It is histologically characterized by
the massive transmural infiltration of lymphoctes and macrophages
with granulomas (6). Crohn’s
disease can affect the entire gastrointestinal tract although the
most common presentation is the ileum-colon junction.
Pro-inflammatory cytokines, such as tumor necrosis factor α (TNFα),
are pivotal for the development of inflammatory bowel disease
(IBD). Therefore, the downregulation of cytokines and
cytokine-induced inflammatory responses constitute molecular
targets for the development of therapeutic strategies in IBD
(7). Numerous agents are
currently available for the treatment of Crohn’s disease. When
treating patients with Crohn’s disease, therapy is usually aimed at
the effective induction and maintenance of remission, as well as in
reducing therapy-related issues and improving quality of life.
Anti-TNFα therapy is an effective therapy for Crohn’s disease, and
a large proportion of patients show a favorable response to its
therapeutic antibodies (8).
Despite the therapeutic efficacy of anti-TNFα agents, however,
treatment failure is frequently observed. On the other hand, the
macrolide, 7-O-succinyl macrolactin A (SMA), markedly
inhibits the TNFα-induced adhesion of monocytes to epithelial cells
similar to rapamycin, an immunosuppressant macrolide and a
mammalian target of rapamycin (mTOR) inhibitor (9). Importantly, SMA is more effective in
the inhibition of inflammation than 5-aminosalicylic acid, the most
ordinarily prescribed agent for the treatment of IBD (9). SMA also causes the suppression of
TNFα-induced phosphorylation of phosphatidylinositol 3-kinase
(PI3K), AKT and mTOR, similar to the effect of rapamycin (9). Accordingly, managing the
PI3K/AKT/mTOR pathway may be a good therapeutic intervention for
the treatment of Crohn’s disease.
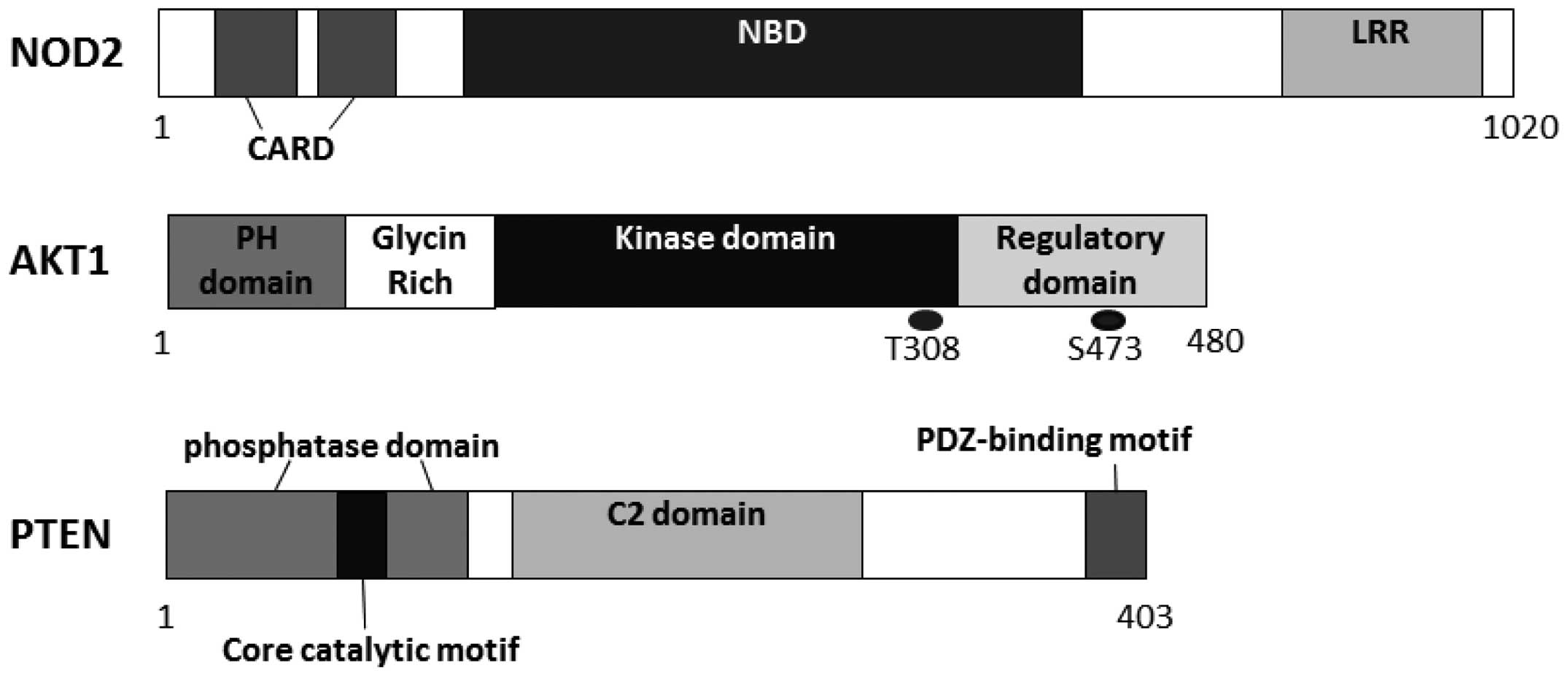
One innovation in Crohn’s disease research is the
identification of nucleotide-binding oligomerization
domain-containing protein 2 (NOD2) as its susceptible gene
(10,11). NOD2 is a member of the
nucleotide-binding oligomerization domain and leucine-rich repeat
(LRR)-containing protein (NLR) family of cellular sensors of
pathogens, which is also a member of cytosolic factors related to
the regulator of apoptosis, apoptotic protease activating factor 1
(Apaf-1), a member of a class of disease resistance proteins
(12). Similar to several members
of the NLR family, NOD2 contains a LRR domain on its C-terminal
side and two tandem caspase recruitment domains (CARDs) on its
N-terminal side. The LRR domain has a molecular structure similar
to a domain found in Toll-like receptors (TLRs) (13). NOD2 appears to regulate the host
response to pathogens that may be defective in certain inflammatory
diseases. Genetic variation in NOD2 is associated with
susceptibility to Crohn’s disease (14). NOD2 activates the downstream
signaling pathways, including the nuclear factor-κB (NF-κB)
pathway, which confers responsiveness to lipopolysaccharides and
interacts with a mediator of NF-κB activation (15). NF-κB is found in the cytoplasm in
its inactive form with the inhibitor of NF-κB subunit, which is in
turn regulated by IκB kinase (IKK). The phosphorylation of the
Ser32 and Ser36 residues of IκBa by IKK triggers a signal for the
ubiquitination and degradation of IκBa and then the activation of
NF-κB (16). The activation of
NF-κB by IKK also occurs in association with reactive oxygen
species (ROS) produced by NADPH oxidase in response to the
activation of receptors, such as interleukin (IL)-1 or TNF
(17). Cellular ROS metabolism is
firmly regulated by a variety of proteins involved in the redox
mechanism with PI3K/AKT signaling (18). AKT activates IKK, which in turn
stimulates p38 MAPK in the transduction of signals originating from
IL-1 receptor (19). IKK may be
present in the form of a complex with mTOR. The kinase may also
activate IKK.
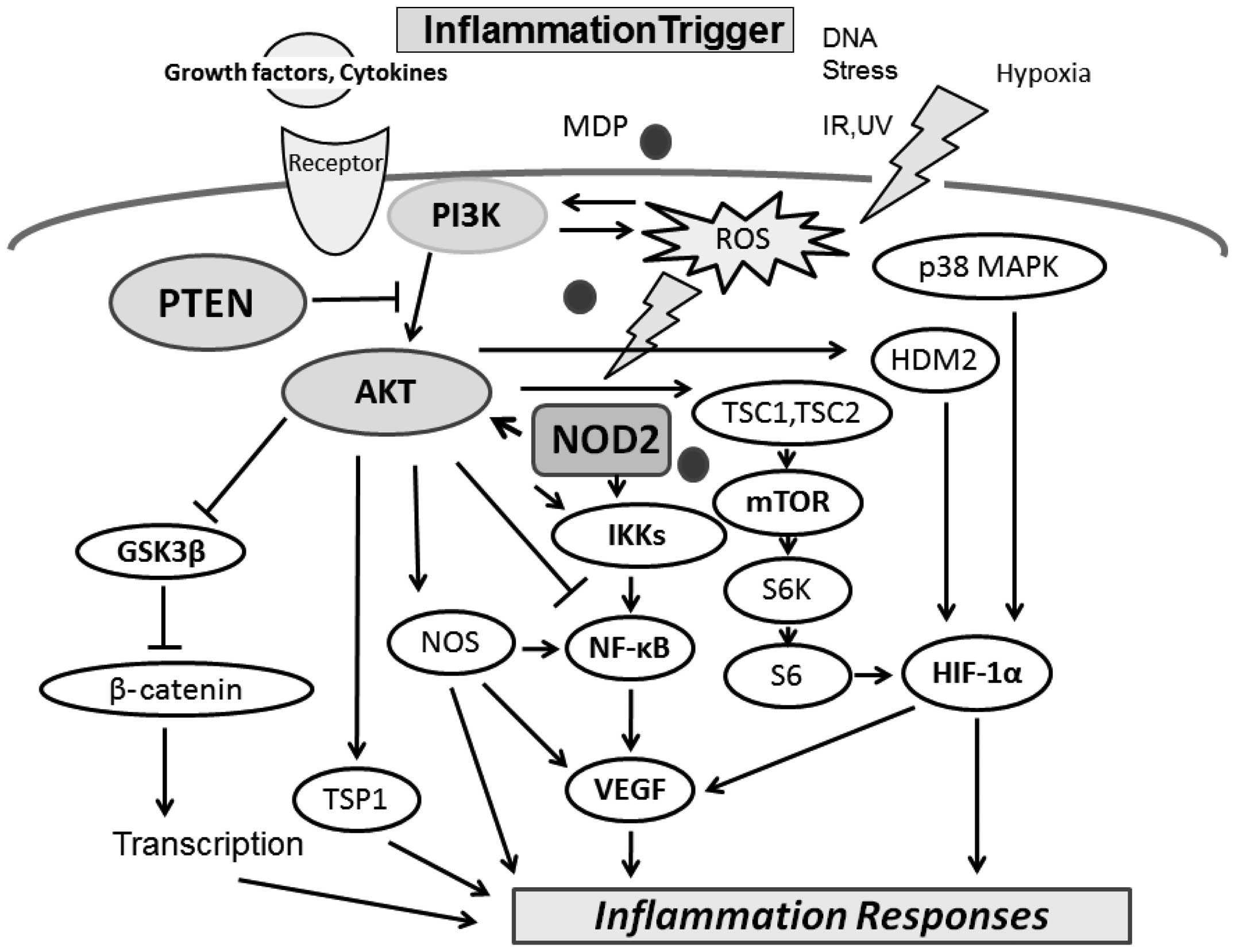
The PI3K/AKT pathway negatively regulates the
NOD2-mediated NF-κB pathway, which may be involved in the
resolution of the inflammatory responses induced by NOD2 activation
(14). Accumulating evidence has
revealed that the PI3K/AKT pathway acts as a pivotal determinant of
cell fate regarding senescence and apoptosis, which is mediated by
intracellular ROS generation (18). Muramyl dipeptide (MDP) is the
minimal bioactive peptidoglycan motif common to all bacteria, which
has been shown to be recognized by NOD2 and induces AKT
phosphorylation (14). NOD2 is
expressed in immune tissue and bacterial peptidoglycan motifs; MDP
activates NOD2, inducing a reduction in AKT Ser473 phosphorylation
(20). ROS also activate PI3K/AKT
and inactivate phosphatase and tensin homolog (PTEN). In addition,
AKT is activated as a result of IL-1β, TNFα or lipopolysaccharide
receptor stimulation. The pharmacological inhibitor of PI3K and
dominant-negative forms of the regulatory subunit of PI3K enhance
NF-κB activation, while constitutive active forms of the catalytic
subunit of PI3K inhibit the NF-κB activation and their target genes
(14). AKT is inhibited by
protein phosphatase-2A (PP2A), which in turn may be inactivated by
ROS (19,21). On the other hand, ROS induction is
accompanied by the activation of PI3K. AKT substrates include IKKα,
NOS, tuberous sclerosis complex (TSC)1 and 2, caspase-9, mouse
double minute 2 homolog (MDM2) and glycogen synthase kinase (GSK)
3β (21,22). Accordingly, the pathway has
central signaling elements in a diverse array of cellular
functions, including proliferation, migration and inflammation
responses. It is therefore reasonable that the dysregulation of the
PI3K/AKT pathway has been implicated in the induction and/or
progression of a variety of disease states.
Functional PI3K heterodimers consist of a regulatory
subunit, such as p85 and a catalytic subunit, such as p110. Each
PI3K forms a family which can be divided into three classes based
on its structure, distribution and mechanism of activation
(23). Class I PI3Ks are further
divided into class IA and IB based on their different adaptors,
which are activated by receptor tyrosine kinases (RTKs) and by the
G-protein-coupled receptors, respectively (24). One of the substrates for class I
PI3Ks is phosphatidylinositol 4,5-bisphosphate (PIP2), resulting in
the production of phosphatidylinositol 3,4,5-trisphosphate (PIP3).
AKT is a downstream target of PI3Ks, which belongs to the AGC
family of protein kinases (25).
Human AKT has three homologous members known as AKT1, AKT2 and AKT3
(26), which contain three
functionally different sites, i.e., a pleckstrin homology (PH)
domain, a central catalytic domain and a C-terminal hydrophobic
motif (27). The binding of PI3K
products to the PH domain results in AKT translocation to the
plasma membrane where it is activated via phosphorylation by
certain upstream protein kinases, such as the
phosphoinositide-dependent kinase 1 (PDK1). PIP3 binds to PDK1 via
the PH domains. PDK1 then phosphorylates at Thr308 of AKT1 in its
kinase domain. For the full activation of AKT, further
phosphorylation of Ser473 at AKT1 by PDK2 is required (28). AKT then moves to the cytoplasm and
nucleus, where it phosphorylates several downstream targets to
regulate several cellular functions. For example, AKT inhibits
GTPase-activating protein (GAP) activity by phosphorylating TSC2,
which leads to activation of the mTOR complex (29). mTOR mediates the phosphorylation
of the ribosomal protein S6 kinase, leading to the release of the
translation initiation factor, eukaryotic translation initiation
factor 4E (eIF4E) (30). GSK3β is
also a downstream target of AKT and is a serine/threonine kinase
itself. GSK3β was originally identified to play a key role in the
regulation of glycogen synthesis in response to insulin receptor
stimulation (31), which has been
shown to be involved in cellular proliferation, apoptosis and
circadian entrainment, in addition to the regulation of
glycogenesis (32).
Pharmacologic modulators directed against components
of intracellular signaling pathways have been developed to improve
therapeutic performance. As the regulation of the components in the
PI3K/AKT/PTEN pathway is thought to correlate with disease
prognosis and drug resistance, it is considered to be a promising
target for therapy. A number of pharmacological inhibitors of this
pathway have already been developed to improve therapy (40). Usually, the PI3K/AKT/GSK3β
activation is maintained by extracellular signals. A metabolite of
guanosine released from activated T lymphocytes and macrophages is
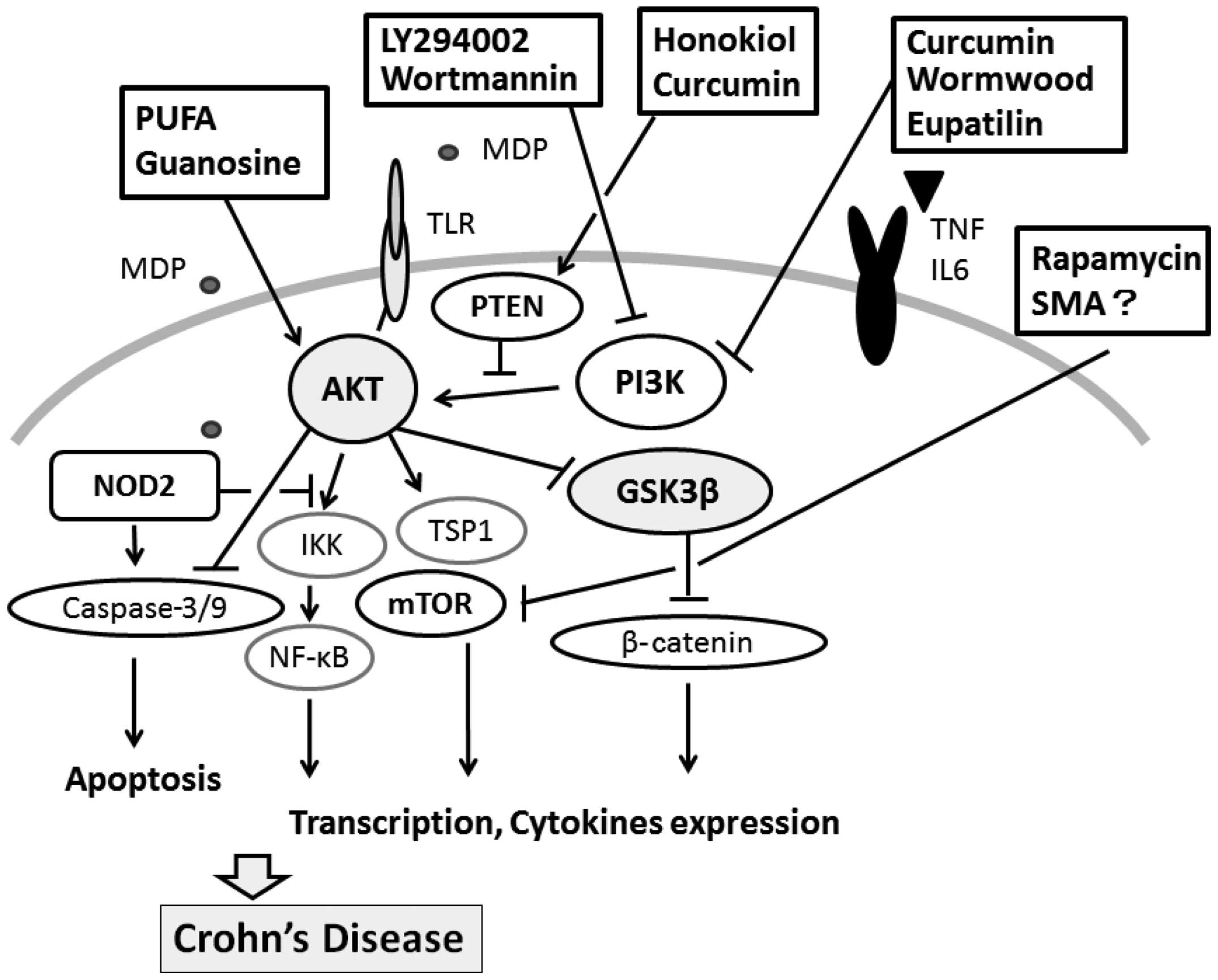
increased in patients with Crohn’s disease (41). It has been shown that guanosine
increases AKT and GSK3β phosphorylation (42); this suggests that it plays an
important role in Crohn’s disease. The cell-protective effect of
guanosine is abolished by blocking the AKT pathway with LY294002
(43). Both LY294002 and
wortmannin are the best characterized PI3K inhibitors which prevent
ATP from binding to the active portion (44). They are low molecular weight
compounds and are also cell-permeable. In addition, they enhance
the phosphorylation of NF-κB p65 on Ser529 and Ser536 residues;
this results in enhanced p65 transactivational activity (14). Furthermore, the inhibition of PI3K
by these pharmacological inhibitors prevents the inactivation of
GSK3β (45), suggesting that the
negative regulation of PI3K/AKT on NF-κB activation is mediated
through the inactivation of GSK3β. LY294002 blocks not only PI3K
activity, but also mTOR to the same extent as PI3K. Some mTOR
inhibitors suppress hypoxia-inducible factor 1α (HIF-1α) and
vascular endothelial growth factor (VEGF), which initiate an
inhibitory effect on the progression of inflammation (46). PI3K inhibition with LY294002 is
reversible, while wortmannin irreversibly inhibits PI3K (44,47). N-cadherin overexpression in bowel
stricture formation in Crohn’s disease may be silenced by LY294002
(48). In addition, LY294002 has
been shown to reduce the production of chemokine-induced ROS in
phagocytes (49). It has been
reported that serum withdrawal kills human U937 monocyte cells by
elevating cellular ROS levels, which occurs through PI3K activation
(50). mTOR inhibitors are the
most developed class of compounds including rapamycin and its
derivatives, which bind to FK506-binding protein 12 (FKBP12)
(51,52). Subsequently, the rapamycin/FKBP12
complex binds mTORC1 and prevents downstream signaling (53). ATP-competitive mTOR inhibitors
suppress the activity of both mTORC1 and mTORC2 (54). Autophagy, which is a cellular
process implicated in the clearance of intracellular bacteria, has
been highlighted as a key feature in the pathogenesis of Crohn’s
disease. Rapamycin is a drug used to upregulate autophagy (55). The use of rapamycin in the
treatment refractory Crohn’s disease has been reported (55), offering a promising new
therapeutic strategy for the treatment of IBD (56). The suppression of PTEN may
increase PIP3, thus leading to the activation of PI3K/AKT
signaling. However, in general, the administration of PI3K/AKT/mTOR
inhibitors can give rise to potentially life-threatening adverse
effects, such as pneumonitis (57).
A variety of signals including growth factors and
nutrients leads to PI3K/AKT pathway activation and inhibition
(Fig. 3). In addition, several
gene transcriptions of the components in the pathway are regulated
by dietary polyunsaturated fatty acids (PUFAs) (58). Potential therapeutic strategies
exploit the observation that defects in critical processes required
for maintaining cellular homeostasis produce a metabolic situation
characterized by Crohn’s disease. It would be of significance to
define appropriate strategies to achieve benefits from dietary
supplements to control the activities of PI3K/AKT pathway
molecules, including the expression of pro-inflammatory cytokines.
Dietary supplementation of fish oil attenuates
lipopolysaccharide-induced bowel inflammation (59). Fish oil increases AKT1 mRNA
expressiin and decreases Forkhead Box O (FOXO)1 and FOXO4 mRNA
expression (60). Fish oil also
increases the phosphorylation of AKT and FOXO1. In addition,
n-3-PUFAs in fish oil exert an inhibitory effect on
pro-inflammatory cytokines thus affecting many inflammatory
diseases (61,62). In fact, linoleic acid has
demonstrated efficacy as an immune modulator and anti-inflammatory
compound that moderates Crohn’s disease (63). Fish oil reduces the plasma levels
of TNFα and prostaglandin E2 concentrations (64). Moreover, fish oil downregulates
the mRNA expression of TLR4 and its downstream signaling molecule,
myeloid differentiation factor 88 (MyD88), TNFα receptor-associated
factor 6 (TRAF6), NF-κB p65 and NOD2 (65,66). By suppressing pro-inflammatory
cytokine production via the regulation of NOD2 signaling, fish oil
may therefore improve the symptoms of Crohn’s disease, possibly
through the maintenance of PI3K/AKT signaling.
Several herbs may also be promising. Curcumin is an
active ingredient derived from the root of the Curcuma longa
plant, which has been used as a traditional Chinese herb for the
treatment of various inflammatory diseases (67). Treatment with curcumin has been
shown to significantly attenuate myocarditis and improve heart
histopathology (68). Of note,
curcumin administration reduces the expression of pro-inflammatory
cytokines, such as TNFα, IL-1β and IL6. Curcumin treatment also
inhibits the activation of NF-κB in a PI3K/AKT pathway-dependent
manner, indicating that curcumin exerts a protective effect against
inflammatory response by inhibiting the PI3K/AKT/NF-κB pathway
(68). Hence, curcumin may have a
therapeutic use in the prevention and treatment of Crohn’s disease
(69). Wormwood (Artemisia
absinthium) also accelerates healing in patients with Crohn’s
disease (70) and has a positive
effect on their mood and quality of life (71). Eupatilin, a flavonoid from
wormwood, inhibits PI3K activity, causing a direct effect on the
phosphorylation of downstream AKT and p70S6K (72). Licorice is a common Chinese
medicinal herb with anti-tumor activity, which induces autophagy
through the inhibition of the PI3K/AKT/mTOR pathway (73). Honokiol is has been demonstrated
to attenuate PI3K/AKT/mTOR signaling through the upregulation of
PTEN expression (74,75). Curcumin also restores PTEN
expression (76). By contrast, a
component of the herb rosemary inhibits the expression of PTEN in
the K562 myeloid cell line (77).
An important mediator implicated in the regulation
of several diseases is PI3K/AKT/PTEN signaling. Efforts to exploit
pharmacological modulators of the cascade that show efficacy and
safety are in progress. It is unlikely that the regulation of a
single signaling pathway will be a cure for Crohn’s disease.
However, the combination of regulators and conventional
chemotherapeutic drugs may prove to be an effective therapeutic
option for patients with this disease. Disorders are characterized
by multiple signaling abnormalities and deregulated pathways may be
redundant. It is difficult to find the correct combinations of
accurate targets. The precise involvement of
PI3K/AKT/GSK3β/mTOR/PTEN in disease signaling has not yet been
fully elucidated. PTEN appears to act as a regulator of basal PIP3
levels that maintains the levels below a threshold for signaling
activation, suggesting that the levels of PIP3 may have an
appropriate level zone. That is the reason why both activators and
inhibitors of the PI3K/AKT pathway may contribute to the treatment
of Crohn’s disease. Although further research is required to
examine the safety and efficacy of regulators, the indicated
compounds appear to possess promising therapeutic activities. An
understanding of the intracellular mechanisms may provide
innovative insight into the development of therapeutic approaches.
To further optimize therapeutic regimens, research should also
focus on the combination of regulators of PI3K/AKT signaling and
regulators directed against other signal transduction molecules.
Further studies are warranted to assess the safety and efficacy of
these regulators using large-scale cohorts of patients with
IBD.
This study was supported by Grants-in-Aid from the
Ministry of Education, Culture, Sports, Science and Technology in
Japan.
|
1
|
Cénit MC, Matzaraki V, Tigchelaar EF and
Zhernakova A: Rapidly expanding knowledge on the role of the gut
microbiome in health and disease. Biochim Biophys Acta. pii:
S0925-4439(14)00151-00153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taschuk R and Griebel PJ: Commensal
microbiome effects on mucosal immune system development in the
ruminant gastrointestinal tract. Anim Health Res Rev. 13:129–141.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nepal S, Navaneethan U, Bennett AE and
Shen B: De novo inflammatory bowel disease and its mimics after
organ transplantation. Inflamm Bowel Dis. 19:1518–1527. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cromer WE, Mathis JM, Granger DN,
Chaitanya GV and Alexander JS: Role of the endothelium in
inflammatory bowel diseases. World J Gastroenterol. 17:578–593.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Lent AU and D’Haens GR: Management of
postoperative recurrence of Crohn’s disease. Dig Dis. 31:222–228.
2013. View Article : Google Scholar
|
|
6
|
Irié T, Maeda Y, Aida T, Sumitani K,
Nagumo M and Tachikawa T: Multiple granulomatous inflammation in
the minor salivary glands: a proposed new entity, allergic
granulomatous sialadenitis. Pathol Int. 54:850–853. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blandizzi C, Gionchetti P, Armuzzi A,
Caporali R, Chimenti S, Cimaz R, Cimino L, Lapadula G, Lionetti P,
Marchesoni A, Marcellusi A, Mennini FS, Salvarani C and Girolomoni
G: The role of tumour necrosis factor in the pathogenesis of
immune-mediated diseases. Int J Immunopathol Pharmacol. 27(Supple
1): S1–S10. 2014.
|
|
8
|
de Boer NKh, Löwenberg M and Hoentjen F:
Management of Crohn’s disease in poor responders to adalimumab.
Clin Exp Gastroenterol. 7:83–92. 2014.
|
|
9
|
Park S, Regmi SC, Park SY, Lee EK, Chang
JH, Ku SK, Kim DH and Kim JA: Protective effect of 7-O-succinyl
macrolactin A against intestinal inflammation is mediated through
PI3-kinase/Akt/mTOR and NF-κB signaling pathways. Eur J Pharmacol.
735:184–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hugot JP, Chamaillard M, Zouali H, Lesage
S, Cézard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M,
Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P,
Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M and Thomas G:
Association of NOD2 leucine-rich repeat variants with
susceptibility to Crohn’s disease. Nature. 411:599–603. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogura Y, Bonen DK, Inohara N, Nicolae DL,
Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH,
Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G
and Cho JH: A frameshift mutation in NOD2 associated with
susceptibility to Crohn’s disease. Nature. 411:603–606. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eckmann L and Karin M: NOD2 and Crohn’s
disease: loss or gain of function? Immunity. 22:661–667. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsai WH, Huang DY, Yu YH, Chen CY and Lin
WW: Dual roles of NOD2 in TLR4-mediated signal transduction and
-induced inflammatory gene expression in macrophages. Cell
Microbiol. 13:717–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao L, Lee JY and Hwang DH: The
phosphatidylinositol 3-kinase/Akt pathway negatively regulates
Nod2-mediated NF-kappaB pathway. Biochem Pharmacol. 75:1515–1525.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hasegawa M, Fujimoto Y, Lucas PC, Nakano
H, Fukase K, Núñez G and Inohara N: A critical role of RICK/RIP2
polyubiquitination in Nod-induced NF-kappaB activation. EMBO J.
27:373–383. 2008. View Article : Google Scholar
|
|
16
|
Nomura F, Kawai T, Nakanishi K and Akira
S: NF-kappaB activation through IKK-i-dependent I-TRAF/TANK
phosphorylation. Genes Cells. 5:191–202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Terra X, Palozza P, Fernandez-Larrea J,
Ardevol A, Blade C, Pujadas G, Salvado J, Arola L and Blay MT:
Procyanidin dimer B1 and trimer C1 impair inflammatory response
signalling in human monocytes. Free Radic Res. 45:611–619. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakanishi A, Wada Y, Kitagishi Y and
Matsuda S: Link between PI3K/AKT/PTEN pathway and NOX protein in
diseases. Aging Dis. 5:203–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Korbecki J, Baranowska-Bosiacka I,
Gutowska I and Chlubek D: The effect of reactive oxygen species on
the synthesis of prostanoids from arachidonic acid. J Physiol
Pharmacol. 64:409–421. 2013.PubMed/NCBI
|
|
20
|
Tamrakar AK, Schertzer JD, Chiu TT, Foley
KP, Bilan PJ, Philpott DJ and Klip A: NOD2 activation induces
muscle cell-autonomous innate immune responses and insulin
resistance. Endocrinology. 151:5624–5637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hales EC, Taub JW and Matherly LH: New
insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling
axis: targeted therapy of γ-secretase inhibitor resistant T-cell
acute lymphoblastic leukemia. Cell Signal. 26:149–161. 2014.
View Article : Google Scholar
|
|
22
|
Johnson SE, Shah N, Bajer AA and LeBien
TW: IL-7 activates the phosphatidylinositol 3-kinase/AKT pathway in
normal human thymocytes but not normal human B cell precursors. J
Immunol. 180:8109–8117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okumura N, Yoshida H, Kitagishi Y,
Murakami M, Nishimura Y and Matsuda S: PI3K/AKT/PTEN signaling as a
molecular target in leukemia angiogenesis. Adv Hematol.
2012:8430852012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Theodoropoulou M and Stalla GK:
Somatostatin receptors: from signaling to clinical practice. Front
Neuroendocrinol. 34:228–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao XH, Buggey J and Kimmel AR:
Chemotactic activation of Dictyostelium AGC-family kinases AKT and
PKBR1 requires separate but coordinated functions of PDK1 and
TORC2. J Cell Sci. 123:983–992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kirkegaard T, Witton CJ, Edwards J, et al:
Molecular alterations in AKT1, AKT2 and AKT3 detected in breast and
prostatic cancer by FISH. Histopathology. 56:203–211. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robertson GP: Functional and therapeutic
significance of Akt deregulation in malignant melanoma. Cancer
Metastasis Rev. 24:273–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hodgkinson CP, Sale EM and Sale GJ:
Characterization of PDK2 activity against protein kinase B gamma.
Biochemistry. 41:10351–10359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bartolomé A, Guillén C and Benito M: Role
of the TSC1-TSC2 complex in the integration of insulin and glucose
signaling involved in pancreatic beta-cell proliferation.
Endocrinology. 151:3084–3094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jastrzebski K, Hannan KM, Tchoubrieva EB,
Hannan RD and Pearson RB: Coordinate regulation of ribosome
biogenesis and function by the ribosomal protein S6 kinase, a key
mediator of mTOR function. Growth Factors. 25:209–226. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brand C, Cipok M, Attali V, Bak A and
Sampson SR: Protein kinase Cdelta participates in insulin-induced
activation of PKB via PDK1. Biochem Biophys Res Commun.
349:954–962. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim YM, Seo YH, Park CB, Yoon SH and Yoon
G: Roles of GSK3 in metabolic shift toward abnormal anabolism in
cell senescence. Ann N Y Acad Sci. 1201:65–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Downes CP, Perera N, Ross S and Leslie NR:
Substrate specificity and acute regulation of the tumour suppressor
phosphatase, PTEN. Biochem Soc Symp. 69–80. 2007.PubMed/NCBI
|
|
34
|
Kong D and Yamori T: Advances in
development of phosphatidylinositol 3-kinase inhibitors. Curr Med
Chem. 16:2839–2854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Y, Wang SM, Wu JC and Huang SH:
Effects of PPARgamma agonists on cell survival and focal adhesions
in a Chinese thyroid carcinoma cell line. J Cell Biochem.
98:1021–1035. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leslie NR, Batty IH, Maccario H, Davidson
L and Downes CP: Understanding PTEN regulation: PIP2, polarity and
protein stability. Oncogene. 27:5464–5476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sheppard K, Kinross KM, Solomon B, Pearson
RB and Phillips WA: Targeting PI3 kinase/AKT/mTOR signaling in
cancer. Crit Rev Oncog. 17:69–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi BH, Kim CG, Lim Y, Shin SY and Lee
YH: Curcumin down-regulates the multidrug-resistance mdr1b gene by
inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett.
259:111–118. 2008. View Article : Google Scholar
|
|
39
|
Li L, Wei XH, Pan YP, Li HC, Yang H, He
QH, Pang Y, Shan Y, Xiong FX, Shao GZ and Zhou RL: LAPTM4B: a novel
cancer-associated gene motivates multidrug resistance through
efflux and activating PI3K/AKT signaling. Oncogene. 29:5785–5795.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu XF, Xu R, Ouyang ZJ, Qian C, Shen Y, Wu
XD, Gu YH, Xu Q and Sun Y: Beauvericin ameliorates experimental
colitis by inhibiting activated T cells via downregulation of the
PI3K/Akt signaling pathway. PLoS One. 8:e830132013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Husain N, Tokoro K, Popov JM, Naides SJ,
Kwasny MJ and Buchman AL: Neopterin concentration as an index of
disease activity in Crohn’s disease and ulcerative colitis. J Clin
Gastroenterol. 47:246–251. 2013. View Article : Google Scholar
|
|
42
|
Das A, Xi L and Kukreja RC: Protein kinase
G-dependent cardioprotective mechanism of phosphodiesterase-5
inhibition involves phosphorylation of ERK and GSK3beta. J Biol
Chem. 283:29572–29585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Molz S, Dal-Cim T, Budni J,
Martín-de-Saavedra MD, Egea J, Romero A, del Barrio L, Rodrigues
AL, López MG and Tasca CI: Neuroprotective effect of guanosine
against glutamate-induced cell death in rat hippocampal slices is
mediated by the phosphatidylinositol-3 kinase/Akt/glycogen synthase
kinase 3β pathway activation and inducible nitric oxide synthase
inhibition. J Neurosci Res. 89:1400–1408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Imai Y, Yamagishi H, Ono Y and Ueda Y:
Versatile inhibitory effects of the flavonoid-derived PI3K/Akt
inhibitor, LY294002, on ATP-binding cassette transporters that
characterize stem cells. Clin Transl Med. 1:242012. View Article : Google Scholar
|
|
45
|
Chanoit G, Lee S, Xi J, Zhu M, McIntosh
RA, Mueller RA, Norfleet EA and Xu Z: Exogenous zinc protects
cardiac cells from reperfusion injury by targeting mitochondrial
permeability transition pore through inactivation of glycogen
synthase kinase-3beta. Am J Physiol Heart Circ Physiol.
295:H1227–H1233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jacot JL and Sherris D: Potential
therapeutic roles for inhibition of the PI3K/AKT/mTOR pathway in
the pathophysiology of diabetic retinopathy. J Ophthalmol.
2011:5898132011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gross ER, Peart JN, Hsu AK, Auchampach JA
and Gross GJ: Extending the cardioprotective window using a novel
delta-opioid agonist fentanyl isothiocyanate via the PI3-kinase
pathway. Am J Physiol Heart Circ Physiol. 288:H2744–H2749. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Burke JP, Cunningham MF, Sweeney C,
Docherty NG and O’Connell PR: N-cadherin is overexpressed in
Crohn’s stricture fibroblasts and promotes intestinal fibroblast
migration. Inflamm Bowel Dis. 17:1665–1673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kuehn HS, Swindle EJ, Kim MS, Beaven MA,
Metcalfe DD and Gilfillan AM: The phosphoinositide
3-kinase-dependent activation of Btk is required for optimal
eicosanoid production and generation of reactive oxygen species in
antigen-stimulated mast cells. J Immunol. 181:7706–7712. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee SB, Cho ES, Yang HS, Kim H and Um HD:
Serum withdrawal kills U937 cells by inducing a positive mutual
interaction between reactive oxygen species and phosphoinositide
3-kinase. Cell Signal. 17:197–204. 2005. View Article : Google Scholar
|
|
51
|
Huang S: A new clue to explain resistance
to mTOR inhibitors. Cell Cycle. 11:8442012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Alvarado Y, Mita MM, Vemulapalli S,
Mahalingam D and Mita AC: Clinical activity of mammalian target of
rapamycin inhibitors in solid tumors. Target Oncol. 6:69–94. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dibble CC and Manning BD: Signal
integration by mTORC1 coordinates nutrient input with biosynthetic
output. Nat Cell Biol. 15:555–564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guo Y and Kwiatkowski DJ: Equivalent
benefit of rapamycin and a potent mTOR ATP-competitive inhibitor,
MLN0128 (INK128), in a mouse model of tuberous sclerosis. Mol
Cancer Res. 11:467–473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Massey DC, Bredin F and Parkes M: Use of
sirolimus (rapamycin) to treat refractory Crohn’s disease. Gut.
57:1294–1296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yin H, Li X, Zhang B, Liu T, Yuan B, Ni Q,
Hu S and Gu H: Sirolimus ameliorates inflammatory responses by
switching the regulatory T/T helper type 17 profile in murine
colitis. Immunology. 139:494–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Albiges L, Chamming’s F, Duclos B, Stern
M, Motzer RJ, Ravaud A and Camus P: Incidence and management of
mTOR inhibitor-associated pneumonitis in patients with metastatic
renal cell carcinoma. Ann Oncol. 23:1943–1953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Waters SM, Coyne GS, Kenny DA and Morris
DG: Effect of dietary n-3 polyunsaturated fatty acids on
transcription factor regulation in the bovine endometrium. Mol Biol
Rep. 41:2745–2755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ghosh S, DeCoffe D, Brown K, Rajendiran E,
Estaki M, Dai C, Yip A and Gibson DL: Fish oil attenuates omega-6
polyunsaturated fatty acid-induced dysbiosis and infectious colitis
but impairs LPS dephosphorylation activity causing sepsis. PLoS
One. 8:e554682013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tyagi A, Kumar U, Reddy S, Santosh VS,
Mohammed SB, Ehtesham NZ and Ibrahim A: Attenuation of colonic
inflammation by partial replacement of dietary linoleic acid with
α-linolenic acid in a rat model of inflammatory bowel disease. Br J
Nutr. 108:1612–1622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang Z, Zhang C, Wang H, Zhao J, Liu L,
Lee J, He Y and Zheng Q: n-3 polyunsaturated fatty acids prevents
atrial fibrillation by inhibiting inflammation in a canine sterile
pericarditis model. Int J Cardiol. 153:14–20. 2011. View Article : Google Scholar
|
|
62
|
Nauroth JM, Liu YC, Van Elswyk M, Bell R,
Hall EB, Chung G and Arterburn LM: Docosahexaenoic acid (DHA) and
docosapentaenoic acid (DPAn-6) algal oils reduce inflammatory
mediators in human peripheral mononuclear cells in vitro and paw
edema in vivo. Lipids. 45:375–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bassaganya-Riera J, Hontecillas R, Horne
WT, Sandridge M, Herfarth HH, Bloomfeld R and Isaacs KL: Conjugated
linoleic acid modulates immune responses in patients with mild to
moderately active Crohn’s disease. Clin Nutr. 31:721–727. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gravaghi C, La Perle KM, Ogrodwski P, Kang
JX, Quimby F, Lipkin M and Lamprecht SA: Cox-2 expression, PGE(2)
and cytokines production are inhibited by endogenously synthesized
n-3 PUFAs in inflamed colon of fat-1 mice. J Nutr Biochem.
22:360–365. 2011. View Article : Google Scholar
|
|
65
|
Liu HQ, Qiu Y, Mu Y, Zhang XJ, Liu L, Hou
XH, Zhang L, Xu XN, Ji AL, Cao R, Yang RH and Wang F: A high ratio
of dietary n-3/n-6 polyunsaturated fatty acids improves
obesity-linked inflammation and insulin resistance through
suppressing activation of TLR4 in SD rats. Nutr Res. 33:849–858.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao
L, Sizemore N and Hwang DH: Reciprocal modulation of Toll-like
receptor-4 signaling pathways involving MyD88 and
phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated
fatty acids. J Biol Chem. 278:37041–37051. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Aggarwal BB, Gupta SC and Sung B:
Curcumin: an orally bioavailable blocker of TNF and other
pro-inflammatory biomarkers. Br J Pharmacol. 169:1672–1692. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Song Y, Ge W, Cai H and Zhang H: Curcumin
protects mice from coxsackievirus B3-induced myocarditis by
inhibiting the phosphatidylinositol 3 kinase/Akt/nuclear factor-κB
pathway. J Cardiovasc Pharmacol Ther. 18:560–569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fontani F, Marcucci T, Picariello L,
Tonelli F, Vincenzini MT and Iantomasi T: Redox regulation of
MMP-3/TIMP-1 ratio in intestinal myofibroblasts: effect of
N-acetylcysteine and curcumin. Exp Cell Res. 323:77–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Krebs S, Omer TN and Omer B: Wormwood
(Artemisia absinthium) suppresses tumour necrosis factor alpha and
accelerates healing in patients with Crohn’s disease-a controlled
clinical trial. Phytomedicine. 17:305–309. 2010. View Article : Google Scholar
|
|
71
|
Omer B, Krebs S, Omer H and Noor TO:
Steroid-sparing effect of wormwood (Artemisia absinthium) in
Crohn’s disease: a double-blind placebo-controlled study.
Phytomedicine. 14:87–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Son JE, Lee E, Seo SG, Lee J, Kim JE, Kim
J, Lee KW and Lee HJ: Eupatilin, a major flavonoid of Artemisia,
attenuates aortic smooth muscle cell proliferation and migration by
inhibiting PI3K, MKK3/6, and MKK4 activities. Planta Med.
79:1009–1016. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yo YT, Shieh GS, Hsu KF, Wu CL and Shiau
AL: Licorice and licochalcone-A induce autophagy in LNCaP prostate
cancer cells by suppression of Bcl-2 expression and the mTOR
pathway. J Agric Food Chem. 57:8266–8273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yang JY, Della-Fera MA, Rayalam S and
Baile CA: Enhanced effects of xanthohumol plus honokiol on
apoptosis in 3T3-L1 adipocytes. Obesity (Silver Spring).
16:1232–1238. 2008. View Article : Google Scholar
|
|
75
|
Liu H, Zang C, Emde A, et al: Anti-tumor
effect of honokiol alone and in combination with other anti-cancer
agents in breast cancer. Eur J Pharmacol. 591:43–51. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Roy S, Yu Y, Padhye SB, Sarkar FH and
Majumdar AP: Difluorinated-curcumin (CDF) restores PTEN expression
in colon cancer cells by down-regulating miR-21. PLoS One.
8:e685432013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yoshida H, Okumura N, Kitagishi Y,
Nishimura Y and Matsuda S: Ethanol extract of rosemary repressed
PTEN expression in K562 culture cells. Int J Appl Biol Pharm
Technol. 2:316–322. 2011.
|