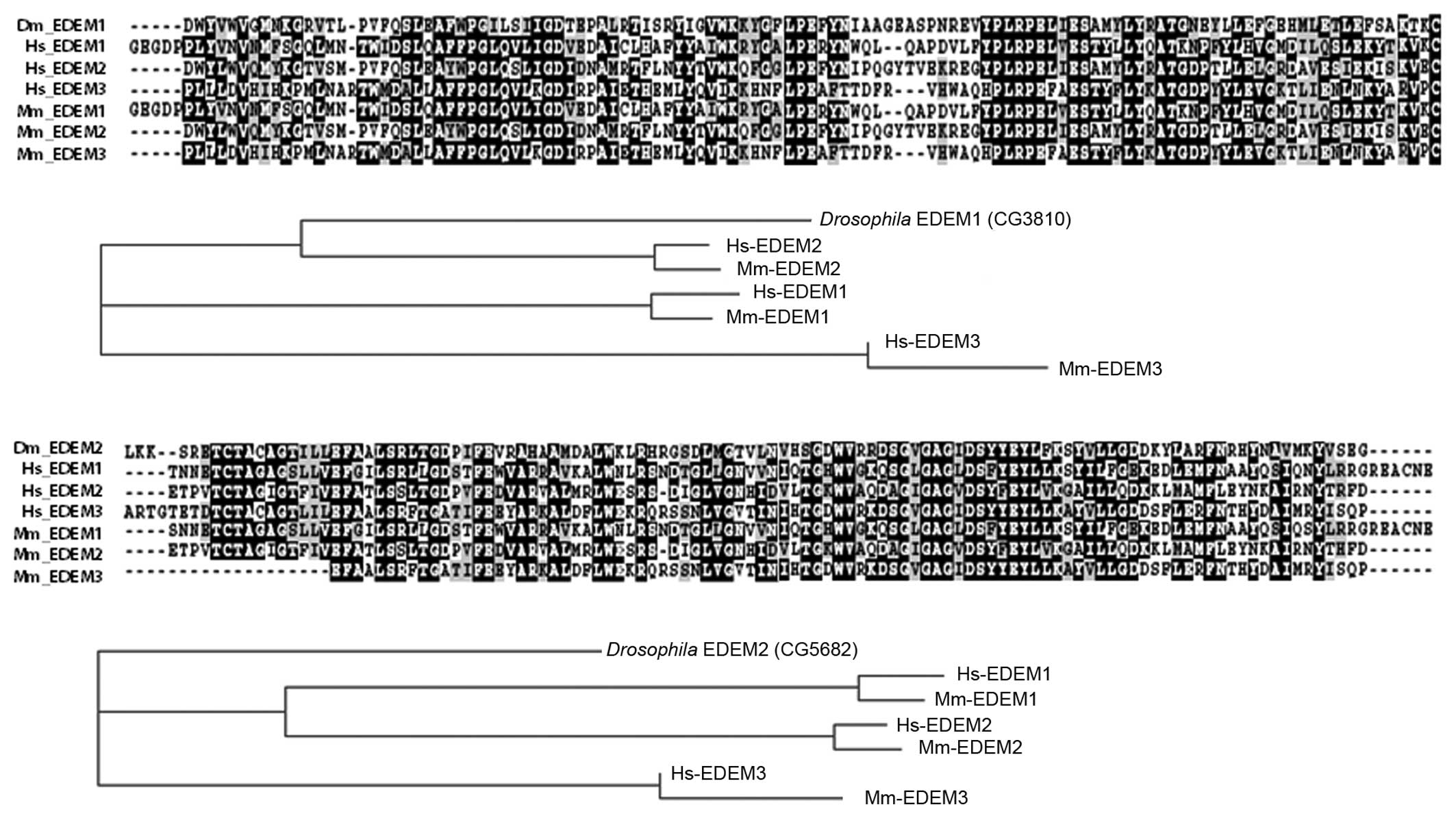
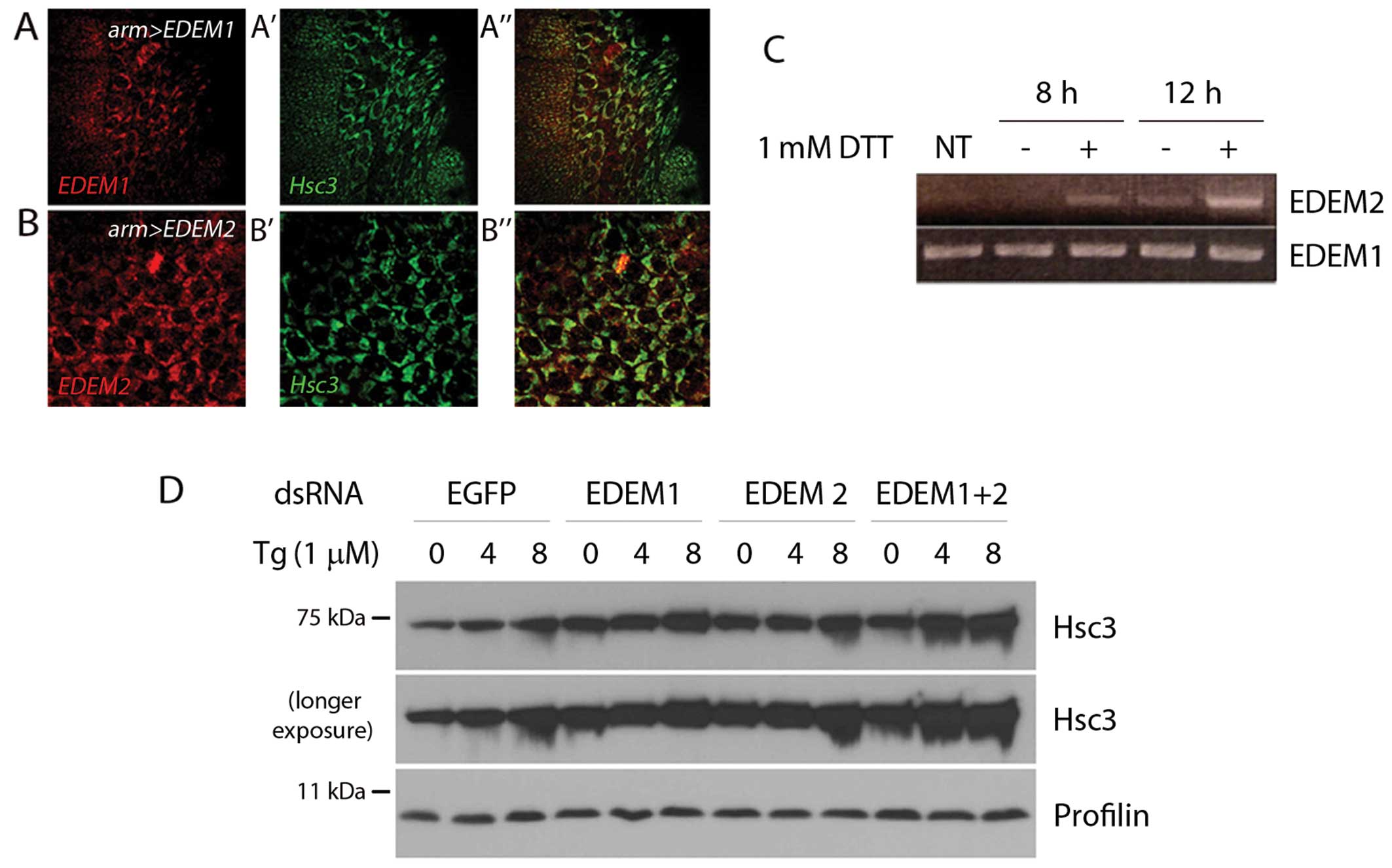
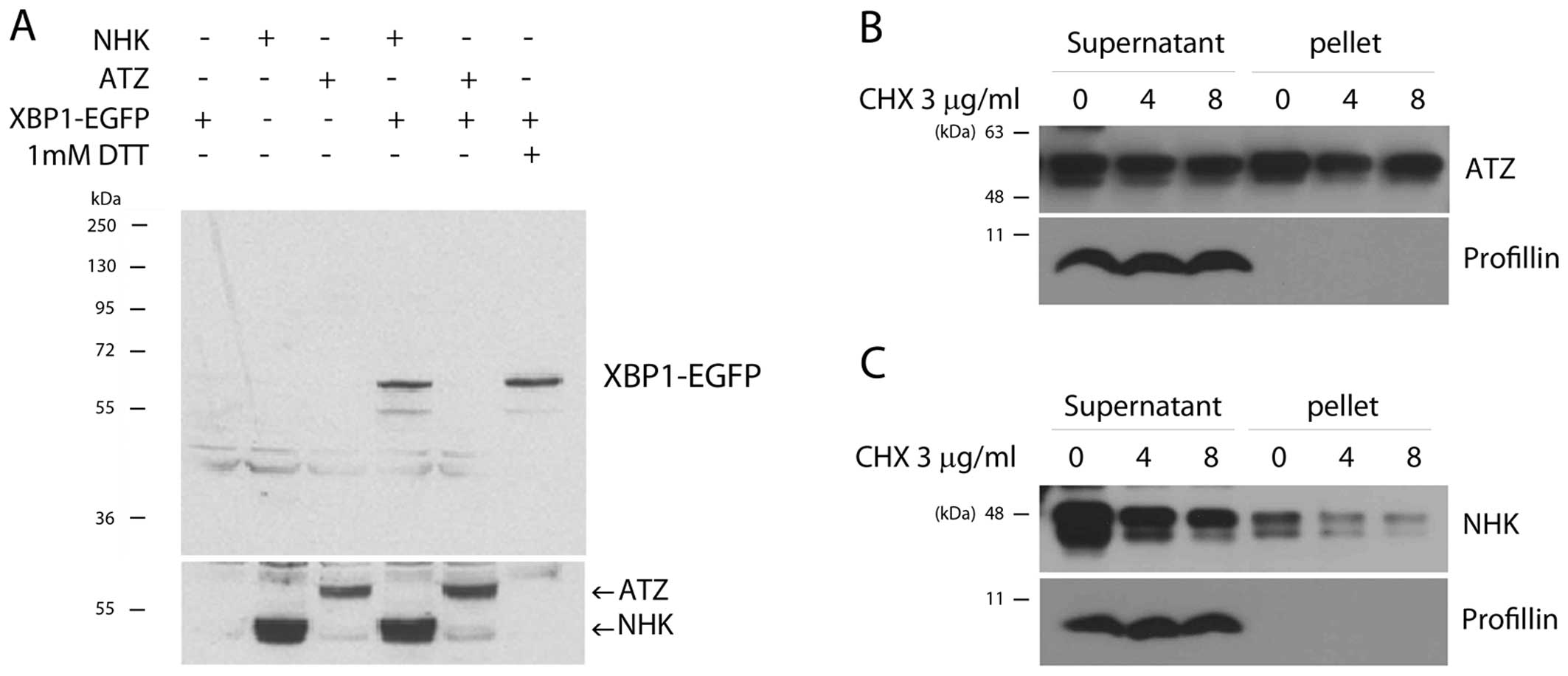
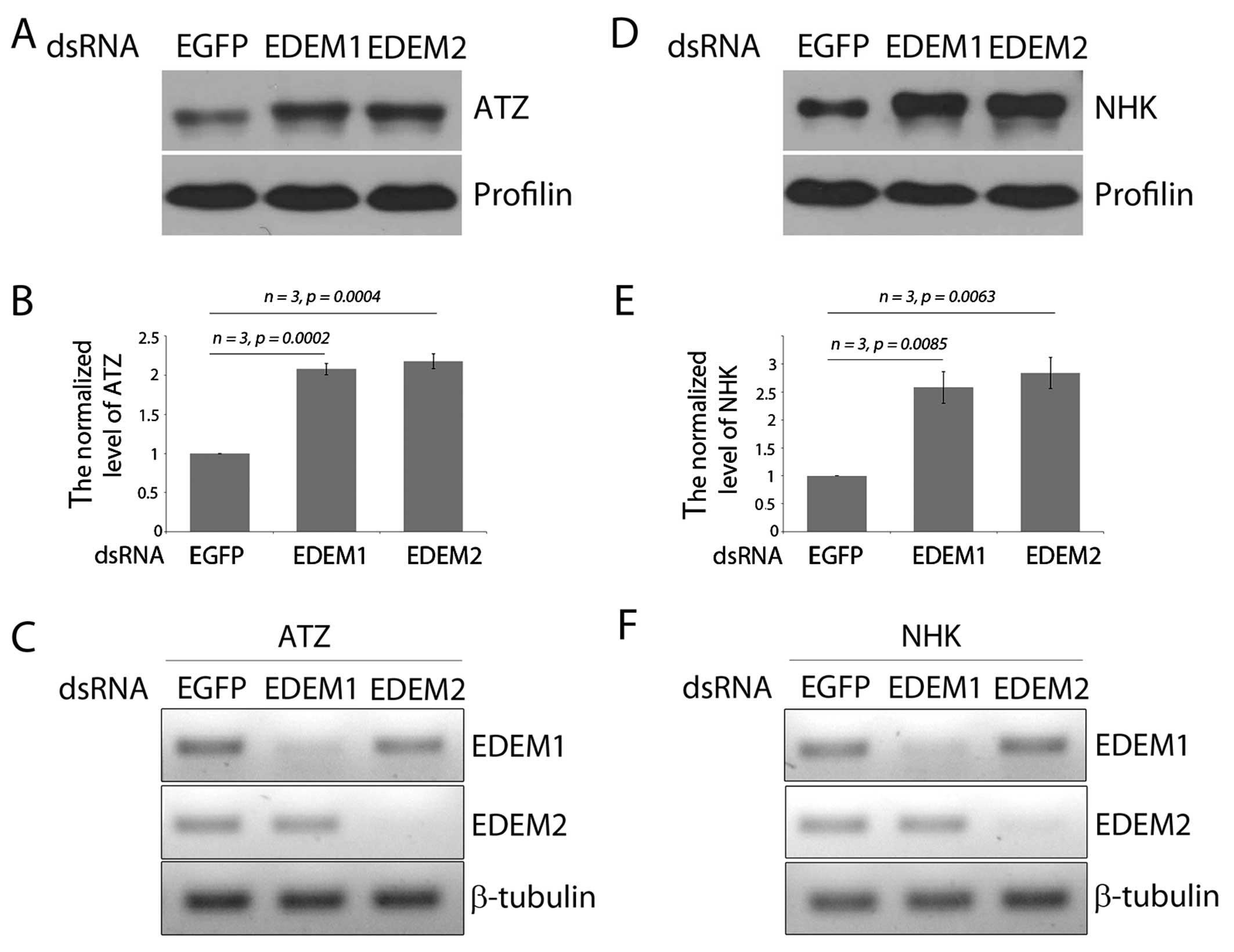
|
1
|
Vembar SS and Brodsky JL: One step at a
time: Endoplasmic reticulum-associated degradation. Nat Rev Mol
Cell Biol. 9:944–957. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molinari M, Calanca V, Galli C, Lucca P
and Paganetti P: Role of EDEM in the release of misfolded
glycoproteins from the calnexin cycle. Science. 299:1397–1400.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oda Y, Hosokawa N, Wada I and Nagata K:
EDEM as an acceptor of terminally misfolded glycoproteins released
from calnexin. Science. 299:1394–1397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clerc S, Hirsch C, Oggier DM, Deprez P,
Jakob C, Sommer T and Aebi M: Htm1 protein generates the N-glycan
signal for glycoprotein degradation in the endoplasmic reticulum. J
Cell Biol. 184:159–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hosokawa N, Tremblay LO, Sleno B, Kamiya
Y, Wada I, Nagata K, Kato K and Herscovics A: EDEM1 accelerates the
trimming of alpha1,2-linked mannose on the C branch of N-glycans.
Glycobiology. 20:567–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hebert DN and Molinari M: Flagging and
docking: Dual roles for N-glycans in protein quality control and
cellular proteostasis. Trends Biochem Sci. 37:404–410. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lomas DA, Evans DL, Finch JT and Carrell
RW: The mechanism of Z alpha 1-antitrypsin accumulation in the
liver. Nature. 357:605–607. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Termine DJ, Moremen KW and Sifers RN: The
mammalian UPR boosts glycoprotein ERAD by suppressing the
proteolytic down-regulation of ER mannosidase I. J Cell Sci.
122:976–984. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Swulius MT, Moremen KW and Sifers
RN: Elucidation of the molecular logic by which misfolded alpha
1-antitrypsin is preferentially selected for degradation. Proc Natl
Acad Sci USA. 100:8229–8234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mast SW, Diekman K, Karaveg K, Davis A,
Sifers RN and Moremen KW: Human EDEM2, a novel homolog of family 47
glycosidases, is involved in ER-associated degradation of
glycoproteins. Glycobiology. 15:421–436. 2005. View Article : Google Scholar
|
|
11
|
Teckman JH and Perlmutter DH: Retention of
mutant alpha(1)-antitrypsin Z in endoplasmic reticulum is
associated with an autophagic response. Am J Physiol Gastrointest
Liver Physiol. 279:G961–G974. 2000.PubMed/NCBI
|
|
12
|
Kamimoto T, Shoji S, Hidvegi T, Mizushima
N, Umebayashi K, Perlmutter DH and Yoshimori T: Intracellular
inclusions containing mutant alpha1-antitrypsin Z are propagated in
the absence of autophagic activity. J Biol Chem. 281:4467–4476.
2006. View Article : Google Scholar
|
|
13
|
Kroeger H, Miranda E, MacLeod I, Pérez J,
Crowther DC, Marciniak SJ and Lomas DA: Endoplasmic
reticulum-associated degradation (ERAD) and autophagy cooperate to
degrade polymerogenic mutant serpins. J Biol Chem. 284:22793–22802.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang MJ and Ryoo HD: Suppression of
retinal degeneration in Drosophila by stimulation of ER-associated
degradation. Proc Natl Acad Sci USA. 106:17043–17048. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hosokawa N, Tremblay LO, You Z, Herscovics
A, Wada I and Nagata K: Enhancement of endoplasmic reticulum (ER)
degradation of misfolded Null Hong Kong alpha1-antitrypsin by human
ER mannosidase I. J Biol Chem. 278:26287–26294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brand AH and Perrimon N: Targeted gene
expression as a means of altering cell fates and generating
dominant phenotypes. Development. 118:401–415. 1993.PubMed/NCBI
|
|
17
|
Ryoo HD, Domingos PM, Kang MJ and Steller
H: Unfolded protein response in a Drosophila model for retinal
degeneration. EMBO J. 26:242–252. 2007. View Article : Google Scholar
|
|
18
|
Wu Y, Whitman I, Molmenti E, Moore K,
Hippenmeyer P and Perlmutter DH: A lag in intracellular degradation
of mutant alpha 1-antitrypsin correlates with the liver disease
phenotype in homozygous PiZZ alpha 1-antitrypsin deficiency. Proc
Natl Acad Sci USA. 91:9014–9018. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Armknecht S, Boutros M, Kiger A, Nybakken
K, Mathey-Prevot B and Perrimon N: High-throughput RNA interference
screens in Drosophila tissue culture cells. Methods Enzymol.
392:55–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen Y, Ballar P and Fang S: Ubiquitin
ligase gp78 increases solubility and facilitates degradation of the
Z variant of alpha-1-anti-trypsin. Biochem Biophys Res Commun.
349:1285–1293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olivari S, Galli C, Alanen H, Ruddock L
and Molinari M: A novel stress-induced EDEM variant regulating
endoplasmic reticulum-associated glycoprotein degradation. J Biol
Chem. 280:2424–2428. 2005. View Article : Google Scholar
|
|
22
|
Hosokawa N, Wada I, Hasegawa K, Yorihuzi
T, Tremblay LO, Herscovics A and Nagata K: A novel ER
alpha-mannosidase-like protein accelerates ER-associated
degradation. EMBO Rep. 2:415–422. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coelho DS, Cairrão F, Zeng X, Pires E,
Coelho AV, Ron D, Ryoo HD and Domingos PM: Xbp1-independent Ire1
signaling is required for photoreceptor differentiation and
rhabdomere morphogenesis in Drosophila. Cell Rep. 5:791–801. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hidvegi T, Schmidt BZ, Hale P and
Perlmutter DH: Accumulation of mutant alpha1-antitrypsin Z in the
endoplasmic reticulum activates caspases-4 and -12, NFkappaB, and
BAP31 but not the unfolded protein response. J Biol Chem.
280:39002–39015. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lawless MW, Greene CM, Mulgrew A, Taggart
CC, O’Neill SJ and McElvaney NG: Activation of endoplasmic
reticulum-specific stress responses associated with the
conformational disease Z alpha 1-antitrypsin deficiency. J Immunol.
172:5722–5726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van ’t Wout EF, Dickens JA, van Schadewijk
A, et al: Increased ERK signalling promotes inflammatory signalling
in primary airway epithelial cells expressing Z α1-antitrypsin. Hum
Mol Genet. 23:929–941. 2014. View Article : Google Scholar
|
|
27
|
Hirsch C, Blom D and Ploegh HL: A role for
N-glycanase in the cytosolic turnover of glycoproteins. EMBO J.
22:1036–1046. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Choudhury P, Cabral CM and Sifers
RN: Oligosaccharide modification in the early secretory pathway
directs the selection of a misfolded glycoprotein for degradation
by the proteasome. J Biol Chem. 274:5861–5867. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teckman JH, Gilmore R and Perlmutter DH:
Role of ubiquitin in proteasomal degradation of mutant
alpha(1)-antitrypsin Z in the endoplasmic reticulum. Am J Physiol
Gastrointest Liver Physiol. 278:G39–G48. 2000.PubMed/NCBI
|
|
30
|
Perlmutter DH: Liver injury in
alpha1-antitrypsin deficiency: An aggregated protein induces
mitochondrial injury. J Clin Invest. 110:1579–1583. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin L, Schmidt B, Teckman J and Perlmutter
DH: A naturally occurring nonpolymerogenic mutant of alpha
1-antitrypsin characterized by prolonged retention in the
endoplasmic reticulum. J Biol Chem. 276:33893–33898. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parfrey H, Mahadeva R and Lomas DA:
Alpha(1)-antitrypsin deficiency, liver disease and emphysema. Int J
Biochem Cell Biol. 35:1009–1014. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gelbart WM and Emmert DB: FlyBase high
throughput expression pattern data. http://flybase.org/reports/FBrf0221009.htmlurisimpleflybase.org/reports/FBrf0221009.html.
2013
|
|
34
|
Sone M, Zeng X, Larese J and Ryoo HD: A
modified UPR stress sensing system reveals a novel tissue
distribution of IRE1/XBP1 activity during normal Drosophila
development. Cell Stress Chaperones. 18:307–319. 2013. View Article : Google Scholar :
|