Introduction
Perinatal white matter injury (WMI) is the most
common form of brain injury in premature infants, which can lead to
the development of severe long-term neurological sequelae, such as
visual and audio dysfunction, cognitive and behavioral deficits and
even cerebral palsy (CP) (1).
Systemic infection and inflammation during pregnancy principally
cause preterm birth and may play an important role in the
pathogenesis of WMI (2,3). A growing body of clinical and
experimental evidence has indicated that the placental inflammatory
response may lead to the production of excessive inflammatory
mediators, thus leading to blood-brain barrier (BBB) breakdown,
microglial activation, coagulation abnormalities, oligodendrocyte
loss and, eventually, neonatal brain injury (4,5).
However, the precise mechanisms underlying intrauterine
inflammation-induced cerebral WMI remain unclear.
Both inflammatory and coagulation factors are
thought to be the mediators between maternal inflammation and fetal
or neonatal brain injury. Increased levels of pro-inflammatory
cytokines, such as tumor necrosis factor-α (TNF-α), interleukin
(IL)-1β and IL-6, have been observed in neonatal brains with white
matter lesions and in the amniotic fluid of the mothers (6,7).
Several experimental studies have demonstrated that the excessive
production of cerebral pro-inflammatory cytokines may be attributed
to the activation of the mitogen-activated protein kinase (MAPK) or
nuclear factor-κB (NF-κB) signaling pathways in microglia (8,9).
Fibrinogen-like protein 2 (fgl2), a member of the
fibrinogen-related protein superfamily, is a novel prothrombinase
with serine protease activity (10). The membrane-anchored fgl2, mainly
expressed in activated reticuloendothelial cells (i.e., macrophages
and endothelial cells), exerts procoagulant effects by converting
prothrombin into thrombin without activating the classic
coagulation cascade (11). fgl2
is expressed in various types of tissue, including those in the
uterus of pregnant mice (12).
Nevertheless, the placental or cerebral distribution of fgl2 in
rats with or without lipopolysaccharide (LPS) stimulation has not
yet been fully addressed. fgl2 is responsible for fibrin deposition
in a number of inflammatory processes (13–15) and its expression is markedly
upregulated by LPS and certain pro-inflammatory cytokines,
including interferon (IFN)-γ and TNF-α (16). Therefore, it remains to be
elucidated whether fgl2 plays a role in the pathogenesis of
intrauterine inflammation-induced WMI. Furthermore, the correlation
of fgl2 with pro-inflammatory cytokines during this disease process
should be investigated.
Activated protein C (APC) is an endogenous serine
protease with anticoagulant, anti-apoptotic, cytoprotective and
anti-inflammatory activities (17), which can cross an intact BBB to
reach its therapeutic targets in the brain (18). Although the beneficial
pharmacological effects of APC have been demonstrated in a variety
of neurological disorders (19–21), research on the role of APC in
inflammation-induced fetal or neonatal brain injury is rare.
Yesilirmak et al (22)
reported that APC reduced cell death and hypomyelination, and
decreased inflammatory cytokine expression in a rat model of
endotoxin-induced WMI. However, their study focused only on
neonatal rat brains at 7 days of postnatal age, and investigated
neither the fetal brain nor the placental tissue following
intrauterine exposure to inflammatory stimuli (22). Furthermore, the cellular target
and the potential molecular signaling mechanisms of the
cytoprotective effects of APC remain to be determined.
In the present study, we aimed to explore the
potential anti-inflammatory and neuroprotective properties of APC
and its underlying mechanisms of action in a rat model of WMI
induced by maternal exposure to LPS. We determined fgl2 expression,
fibrin deposition and pro-inflammatory cytokine production in the
placenta and the fetal brains following maternal exposure to LPS
with or without APC treatment. We further assessed cerebral
apoptosis, the brain water content, microglial activation,
protease-activated receptor 1 (PAR1) and NF-κB p65 expression in
the fetal brain, and myelination in the neonatal rat brain.
Materials and methods
Ethics statement
The present study was carried out in strict
accordance with the Guide for the Care and Use of Laboratory
Animals established by the US National Institutes of Health. All
experimental procedures were approved by the Center of Experimental
Animals, Tongji Medical College, Huazhong University of Science and
Technology, Wuhan, China (permit number: SYXK 2010-0057). The
animals were anesthetized with pentobarbital. Every effort was made
to minimize the suffering of the animals.
Animals
A total of 60 adult female and 20 male
Sprague-Dawley rats, weighing 230–250 g, were purchased from the
Hunan SJA Laboratory Animal Co., Ltd. (Hunan, China). The animals
were given free access to food and water and were bred at 22°C
under a 12-h light/dark cycle. Females and fertile males were mated
together overnight at a ratio of 3:1, and the full view of sperm in
a vaginal smear the following morning was designated as day 1 of
pregnancy. Pregnant rats were randomly divided into 3 groups
(n=19/group, as 3 female rats were not pregnant) as follows: the
control, LPS and the LPS + APC group. To avoid premature delivery
and to obtain live offspring, an intraperitoneal (i.p.) injection
of 350 μg/kg LPS (Escherichia coli, serotype 055:B5;
Sigma, St. Louis, MO, USA), prepared in saline, was consecutively
administered to the pregnant rats on embryonic day (E)17 and E18
(the LPS group). This dosage schedule was established by improving
the methods of preliminary research (6). The rats in the control group were
injected with an equivalent amount of sterile saline on the same
days. The LPS + APC group was administered an i.p. injection of 0.2
mg/kg APC (Sigma) 30 min after the second LPS injection, as
previously described (22).
Pregnancies were allowed to proceed to term. Following delivery,
the birth weight of the neonatal rats, as well as their body weight
and brain weight on postnatal day (P)1, P3, P7, P10 and P14, were
recorded.
Histopathological and immunohistochemical
analyses
Pregnant rats on E19 and neonatal rats on P14 were
anesthetized by an i.p. injection of pentobarbital (50 mg/kg), and
were transcardially perfused with saline, followed by 4%
paraformaldehyde (PFA) in 0.1 mol/l PBS (pH 7.4). The placentas, as
well as the fetal and neonatal brains were removed and fixed in 4%
PFA for 72 h. After dehydration in ethanol, they were embedded in
paraffin and cut at 4 μm. The placentas were cut at the
maximum cross section and the rat brains were cut through +2.20 to
−3.80 mm from the bregma. The sections were stained with
hematoxylin and eosin (H&E) according to standard protocols or
prepared for immunohistochemistry.
Mouse anti-fgl2 monoclonal antibody (sc-100276;
Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit
anti-fibrinogen/fibrin polyclonal antibody (ab34269; Abcam Inc.,
Cambridge, MA, USA), and mouse anti-myelin basic protein (MBP)
monoclonal antibody (MAB382; Chemicon International, Temecula, CA,
USA) were used as primary antibodies to evaluate fgl2 expression,
fibrin deposition and myelination. The tissue sections were
deparaffinized in xylene, rehydrated through a graded alcohol
series, and then incubated in 3% hydrogen peroxide to inactivate
the endogenous peroxidase. Antigen retrieval was conducted by
heating in 10 mM citrate buffer (pH 6.0) at 100°C in a microwave
oven for 20 min. The slides were incubated with primary antibodies
(dilutions: fgl2, 1:200; fibrinogen/fibrin, 1:200; MBP, 1:100)
overnight at 4°C, subjected to a streptavidin-biotin-horseradish
peroxidase (SABC kit from Boster Biological Technology, Wuhan,
China) according to the manufacturer’s instructions, and then
counterstained with hematoxylin.
The number of fgl2-positive cells in the placental
labyrinth and spongiotrophoblast zone were counted in 4 randomly
selected visual fields of sections from each group (n=4) at x400
magnification by 2 independent blinded observers. The ratio of
fgl2-positive cells against the total number of cells counted was
calculated and averaged.
To evaluate MBP expression, a semi-quantitative
analysis was performed, as previously described (23). The optical density of the
MBP-positive fibers was measured in 4 sections of 4–8 rats per
group at x200 magnification using ImageJ software (http://rsb.info.nih.gov/ij/; NIH, Bethesda, MD,
USA).
Double immunofluorescence staining
Fetal brains on E19 were removed, immediately
immersed in OCT compound (Sakura Finetek USA Inc., Torrance, CA,
USA), snap-frozen in liquid nitrogen and stored at −80°C. Serial
frozen sections of 10 μm thickness were cut coronally
through the cingular cortex (+2.20 to −0.40 mm from the bregma) at
−20°C in a cryostat (Model CM1900; Leica, Wetzlar, Germany),
mounted onto polylysine-coated slides and stored at −80°C until
use.
The brain sections were fixed for 15 min in 4%
phosphate-buffered PFA, washed in PBS and permeabilized with
PBS/0.5% Triton X-100 (Sigma) for 20 min. After blocking in 5% BSA
(Beyotime, Nantong, China) for 2 h at room temperature, the tissue
sections were incubated in polyclonal rabbit anti-Iba-1 antibody (a
specific marker for microglia, 019-19741; dilution 1:250; Wako Pure
Chemical Industries, Osaka, Japan) admixed with monoclonal mouse
anti-fgl2 antibody (sc-100276; dilution 1:200; Santa Cruz
Biotechnology) overnight at 4°C. FITC (111-095-003)- and Cy3
(115-165-003)-conjugated secondary antibodies (dilution 1:1,000;
Jackson ImmunoResearch, West Grove, PA, USA) were then added. The
slides were counterstained with DAPI (10 ng/ml; Molecular Probes;
Invitrogen, Eugene, OR, USA), mounted with fluorescent mounting
medium (Code S3023; DakoCytomation, Inc., Carpinteria, CA, USA),
and examined under a fluorescence microscope (Model BX51, Olympus
Co., Tokyo, Japan).
The number of Iba-1 and fgl2-positive microglia in
the cortex and corpus striatum was counted in 4 non-continuous
sections from 5 independent fetuses per group by 2 observers
blinded to the experimental design. The area counted was 0.6
mm2 (at x400 magnification).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) staining
TUNEL assays were applied to the 10 μm-thick
coronal cryosections obtained from the fetal brains on E19
following the manufacturer’s instructions (Roche, Mannheim,
Germany). TUNEL-positive cells were identified in the
periventricular white matter on both sides of the brain. All nuclei
were counterstained with DAPI. Four images for each brain and
region of interest were acquired, and the TUNEL-positive cells and
all cells in the visual field (0.6 mm2) were counted at
x400 magnification by 2 independent researchers blinded to the
treatment conditions using a fluorescence microscope (Olympus). The
apoptotic index (AI) was determined as follows: AI (%) = (number of
TUNEL-positive cells/total number of the cells counted) x100.
Measurement of brain water content
The brain water content was determined using the
wet/dry weight method on fetal brains on E19. The brain samples
(n=6 per group) were immediately weighed on an electronic
analytical balance (Model PL203; Mettler Toledo, Zurich,
Switzerland) to obtain the wet weight. The brain samples were then
dried in an oven at 100°C for 24 h and reweighed to obtain the dry
weight. The brain water content was calculated according to the
following formula: (wet weight - dry weight)/wet weight x100.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from the whole placental and
fetal brain tissues on E19 using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
After the evaluation of the RNA quantity and quality using a UV
spectrophotometer (Eppendorf, Hamburg, Germany), the RNA was
reverse transcribed into cDNA using a First Strand cDNA Synthesis
kit (Fermentas, Vilnius, Lithuania) on a C1000 Thermal Cycler
(Bio-Rad, Hemel Hempstead, UK) at 42°C for 60 min, followed by
termination at 70°C for 5 min. The resulting cDNA was stored at
−70°C until amplification.
The relative quantitative (real-time) PCR reaction
was performed on an ABI 7500 real-time PCR system (Applied
Biosystems, Foster City, CA, USA) in a final volume of 25
μl, consisting of 12.5 μl of Maxima SYBR-Green/ROX
qPCR Master Mix (Fermentas), 1.5 μl of forward and reverse
primers, 250 ng of cDNA, and H2O was added to achieve
the final volume. The PCR reaction conditions were 10 min at 95°C,
40 cycles at 95°C for 15 sec, 60°C for 30 sec and 72°C 35 sec. A
stable standard curve was constructed using synthesized
oligonucleotides resembling cDNA fragments in 5-fold decrements as
the template. A melting curve was established to assess the
amplification specificity. β-actin was amplified as an internal
control after analyzing its stability as a housekeeping gene. The
primers used were examined using BLAST from NCBI and are listed in
Table I. All samples were treated
in triplicate. The relative mRNA expression data were analyzed
using the comparative threshold cycle (2−∆∆CT)
method.
 | Table IThe primers used in RT-qPCR. |
Table I
The primers used in RT-qPCR.
| Gene | Primer | Sequence
(5′→3′) | Product length
(bp) |
|---|
| fgl2 | Forward |
TTTACGCCGTGTACGATCAGT | |
| Reverse |
AGGTCGTGGTTGTAATGTCGG | 124 |
| TNF-α | Forward |
GCTACGGGCTTGTCACTCG | |
| Reverse |
GCCACCACGCTCTTCTGTC | 149 |
| IL-1β | Forward |
GTGCTTGGGTCCTCATCCTG | |
| Reverse |
ACTATGGCAACTGTCCCTGAAC | 275 |
| IL-6 | Forward |
CTCTGAATGACTCTGGCTTTG | |
| Reverse |
TTGCCTTCTTGGGACTGATGT | 397 |
| β-actin | Forward |
AAGGAAGGCTGGAAGAGAGC | |
| Reverse |
GGAAATCGTGCGTGACATTA | 180 |
Quantification of cytokine expression by
enzyme-linked immunosorbent assay (ELISA)
Whole placental and fetal brain tissue on E19 was
homogenized in 0.01 mol/l PBS (pH 7.4), containing a protease
inhibitor cocktail (Roche). The homogenates were then centrifuged
at 15,000 x g for 20 min at 4°C. The levels of cytokines in the
supernatant, including those of TNF-α, IL-1β and IL-6, were
quantified using ELISA kits (Dakewei Biotechnology Co., Ltd.,
Shenzhen, China) according to the manufacturer’s instructions. The
protein concentrations of the tissue lysates were measured using
the bicinchoninic acid (BCA) protein assay kit (Beyotime). All
samples were treated in triplicate. The results were expressed as
the concentration of each cytokine per milligram of protein.
Western blot analysis
The proteins from the whole placental and fetal
brain tissue on E19 were extracted in ice-cold
radioimmunoprecipitation assay (RIPA) buffer containing a protease
inhibitor, and the protein concentrations were measured using the
BCA assay kit, according to the manufacturer’s instructions
(Beyotime). Equal amounts of proteins were run on a 10%
SDS-polyacrylamide gel with a 5% stacking gel and electroblotted
onto polyvinylidene difluoride (PVDF) membranes (Millipore,
Billerica, MA, USA). The membranes were blocked in 5% non-fat milk
for 2 h at room temperature and then incubated with primary
antibodies [1:200 dilution for mouse monoclonal anti-PAR1
(sc-13503), anti-NF-κB p65 (sc-8008) and anti-fgl2 (sc-100276;
Santa Cruz Biotechnology); 1:1,000 dilution for mouse monoclonal
anti-β-actin (3700; Cell Signaling Technology, Danvers, MA, USA)]
overnight at 4°C. The membranes were washed 4 times with 0.1%
TBS-Tween-20 solution for 10 min each and then probed with
secondary horseradish peroxidase-conjugated IgG (G-21040; Pierce
Chemical Co., Rockford, IL, USA) at a dilution of 1:200,000 for 2 h
at room temperature. Chemiluminescent detection was performed using
the SuperSignal West Femto Maximum Sensitivity Substrate (Thermo
Fisher Scientific, Rockford, IL, USA). Protein signals were
visualized and densitometrically analyzed using a Kodak 4000MM Pro
imaging system and Molecular Imaging software (Carestream Health,
Toronto, Canada).
Statistical analysis
Values are expressed as the means ± SD. Comparisons
among multiple groups were performed by one-way analysis of
variance (ANOVA) followed by Fisher’s least significant difference
(LSD) or Tamhane’s T2 tests. Statistical analysis was conducted
using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). The
level of statistical significance was defined as P<0.05.
Results
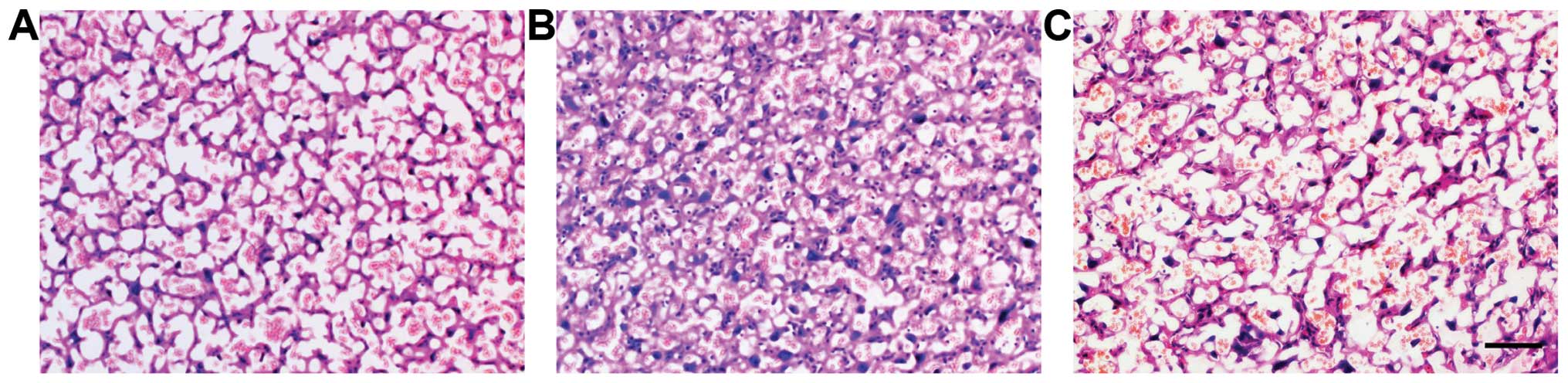
APC ameliorates placental
inflammation
Placental histopathological changes suggestive of
inflammation were detected with H&E staining in all the
experimental groups (Fig. 1). The
control placenta was normal (Fig.
1A). At 24 h after the consecutive application of LPS, the
placental vasculature was significantly engorged with red blood
cells and mesenchymal hyperplasia was evident in the labyrinth of
the placenta, accompanied by the infiltration of large amounts of
neutrophils (Fig. 1B). These
pathological changes receded significantly following treatment with
APC (Fig. 1C).
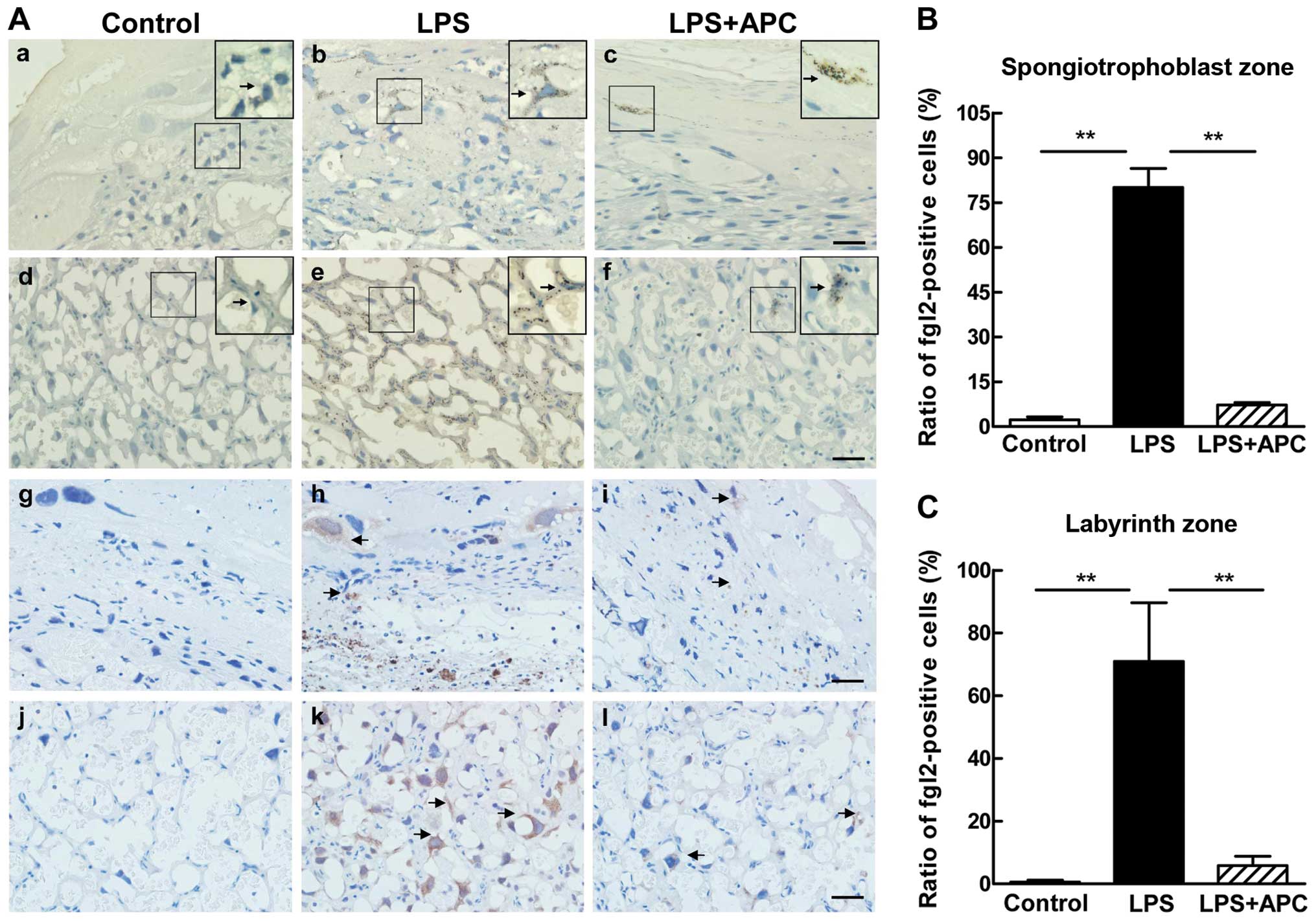
APC downregulates fgl2 and
pro-inflammatory cytokine expression in the placenta
Immunohistochemical staining was used to determine
fgl2 expression and fibrin deposition in the rat placentas on E19
following the different treatments (Fig. 2). Intense staining of fgl2 was
observed in the labyrinth and spongiotrophoblast zone following the
administration of LPS (Fig. 2A
panels b and e), which showed the characteristics of membrane
protein staining. We occasionally observed fgl2-positive cells in
the labyrinth and sporadic staining of the trophoblasts in the
spongiotrophoblast zone in placentas of the LPS + APC-treated rats
(Fig. 2A panels c and f). By
contrast, almost no fgl2 expression was detected in the placentas
of the control group rats (Fig.
2A panels a and d). Fibrin deposition correlated with the
pattern of fgl2 staining (Fig. 2A
panels g–l). In the LPS-treated rats, fibrin deposits were
prominent in the trophoblasts of the spongiotrophoblast zone and
within the microvascular vessels of the labyrinth zone of the
placentas (Fig. 2A panels h and
k). There was little or no fibrin deposition in the control group
(Fig. 2A panels g and j). The
administration of APC eliminated the deposition of fibrin compared
to the LPS-treated rats (Fig. 2A
panels i and l). The ratio of fgl2-positive cells in the labyrinth
and spongiotrophoblast zone was significantly higher in the LPS
group than in the control or LPS + APC group (P<0.001; Fig. 2B and C).
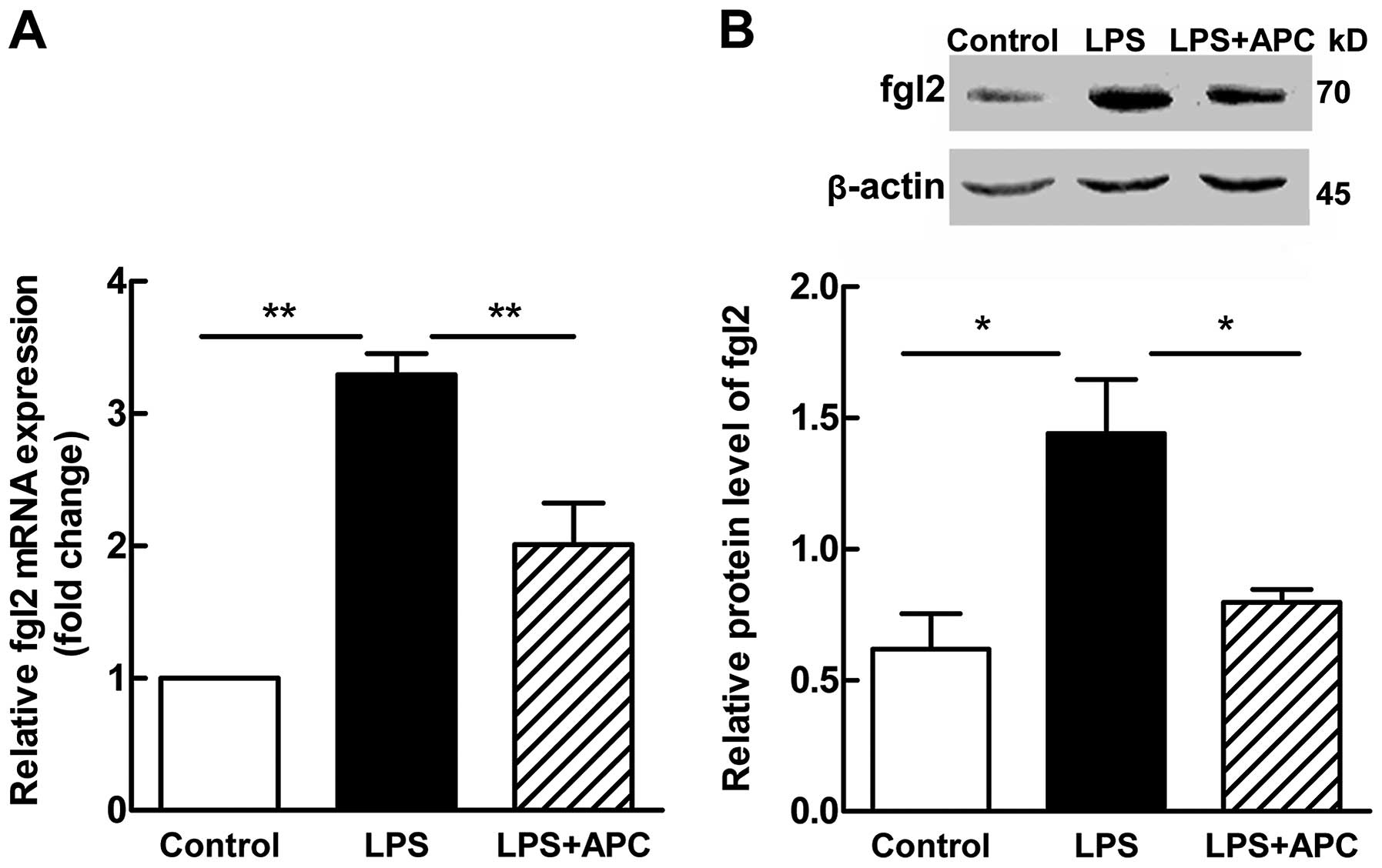
In accordance with the results of
immunohistochemistry, RT-qPCR and western blot analysis revealed a
significant increase in the mRNA (P<0.001; Fig. 3A) and protein (P<0.05; Fig. 3B) expression levels of fgl2 in the
placentas following treatment with LPS. Following treatment with
APC, a significant decrease was observed in the levels of fgl2
(P<0.001 for mRNA expression, Fig.
3A; P<0.05 for protein expression, Fig. 3B). The placental expression of the
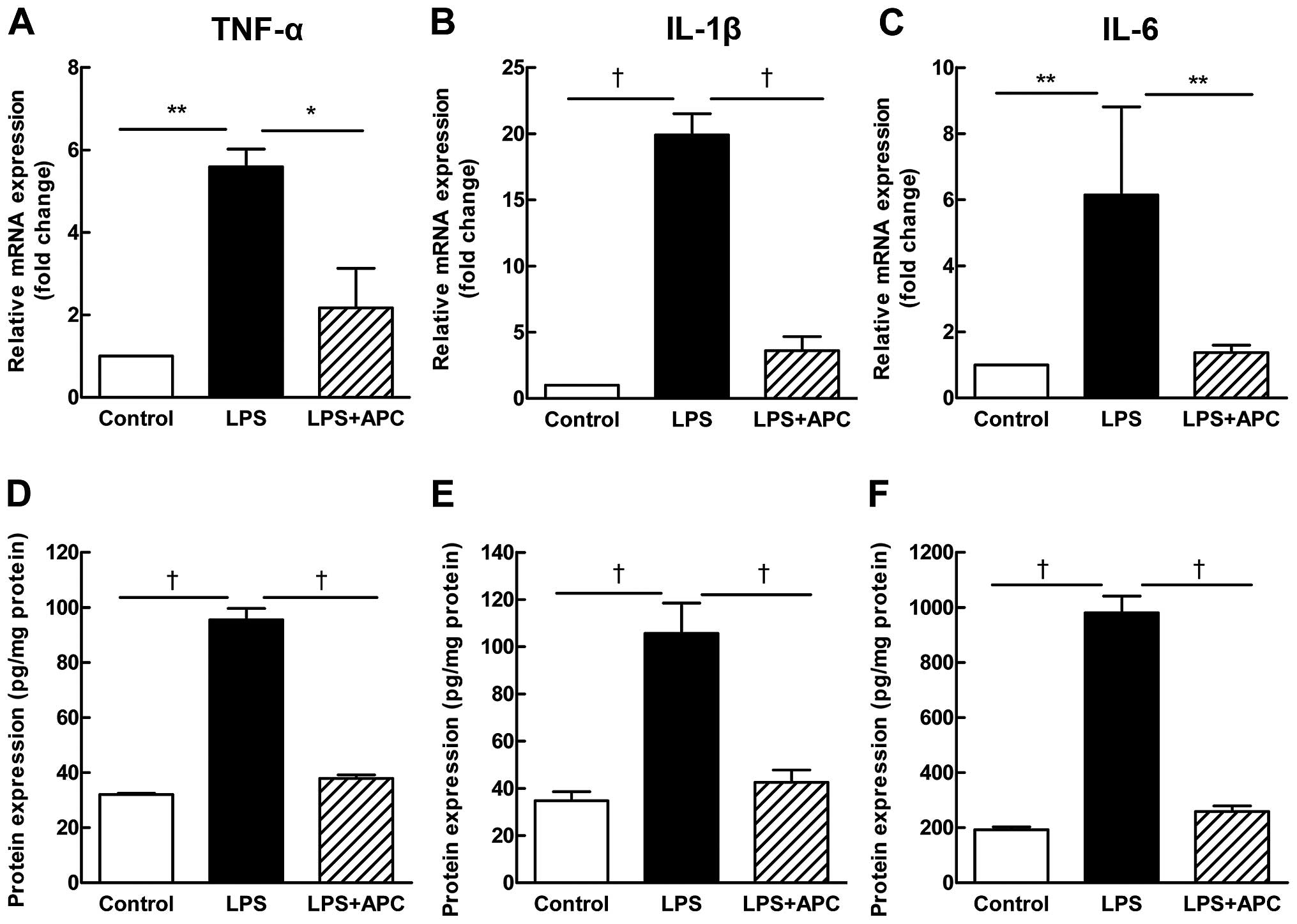
pro-inflammatory cytokines, TNF-α, IL-6 and IL-1β (Fig. 4A–C mRNA expression; and D–F
protein expression), increased significantly after the LPS
injection, and decreased significantly following treatment with
APC.
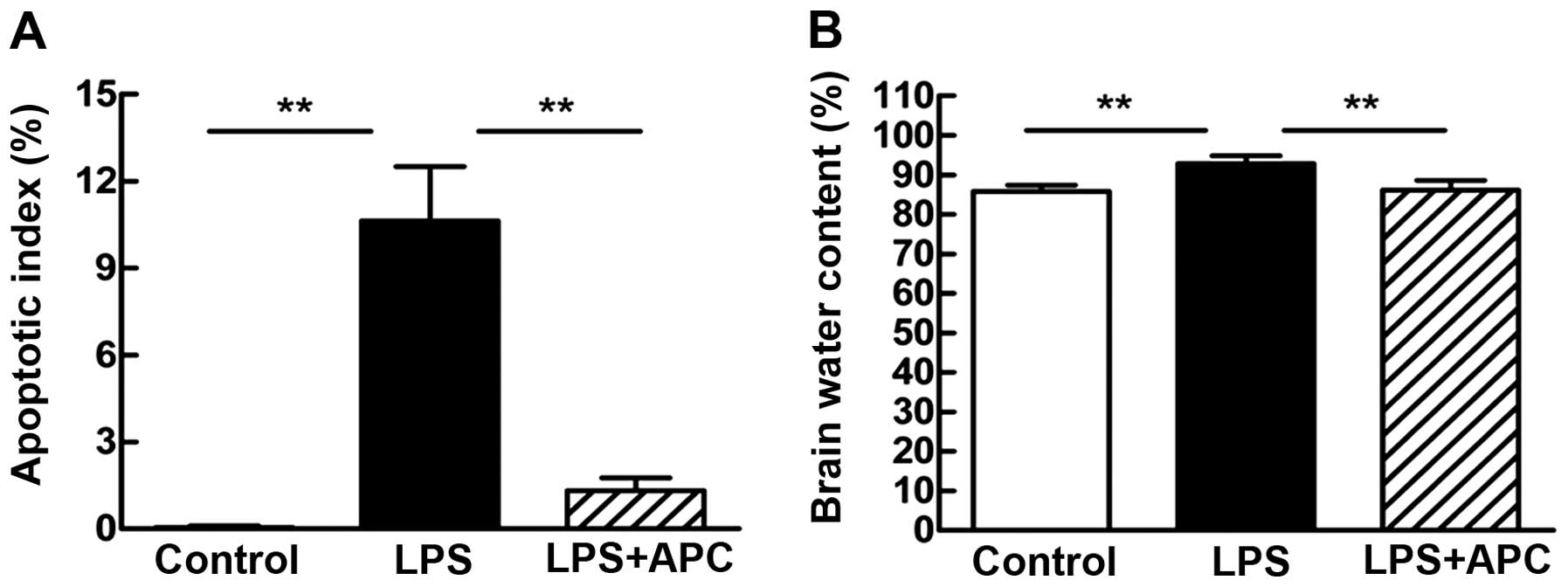
APC attenuates fetal cerebral apoptosis
and brain edema
For the evaluation of fetal brain injury following
maternal exposure to LPS, apoptotic cell death was measured by
TUNEL staining (Fig. 5A). The
apoptotic index (AI) was calculated to quantify the number of
TUNEL-positive cells. The number of TUNEL-positive cells in the
periventricular white matter was significantly increased in the LPS
group (AI = 10.63±1.88%) compared with the control group (AI =
0.05±0.05%; P<0.001, Fig. 5A).
Treatment wit APC significantly reduced the number of
TUNEL-positive cells in the periventricular white matter (AI =
1.33±0.44%; P<0.001, Fig. 5A)
compared with the LPS group.
Brain edema was estimated by measuring the brain
water content of the rats in each treatment group 24 h after the
second dose of LPS (Fig. 5B). The
brain water content of the rats in the LPS group increased
significantly compared to the controls (92.86±2.00 vs. 85.84±1.52%;
P<0.001). Compared with the LPS group, the brain water content
was significantly lower in the LPS + APC group (92.86±2.00 vs.
86.12±2.46%; P<0.001; Fig.
5B).
APC decreases fgl2 and pro-inflammatory
cytokine expression in the fetal brain
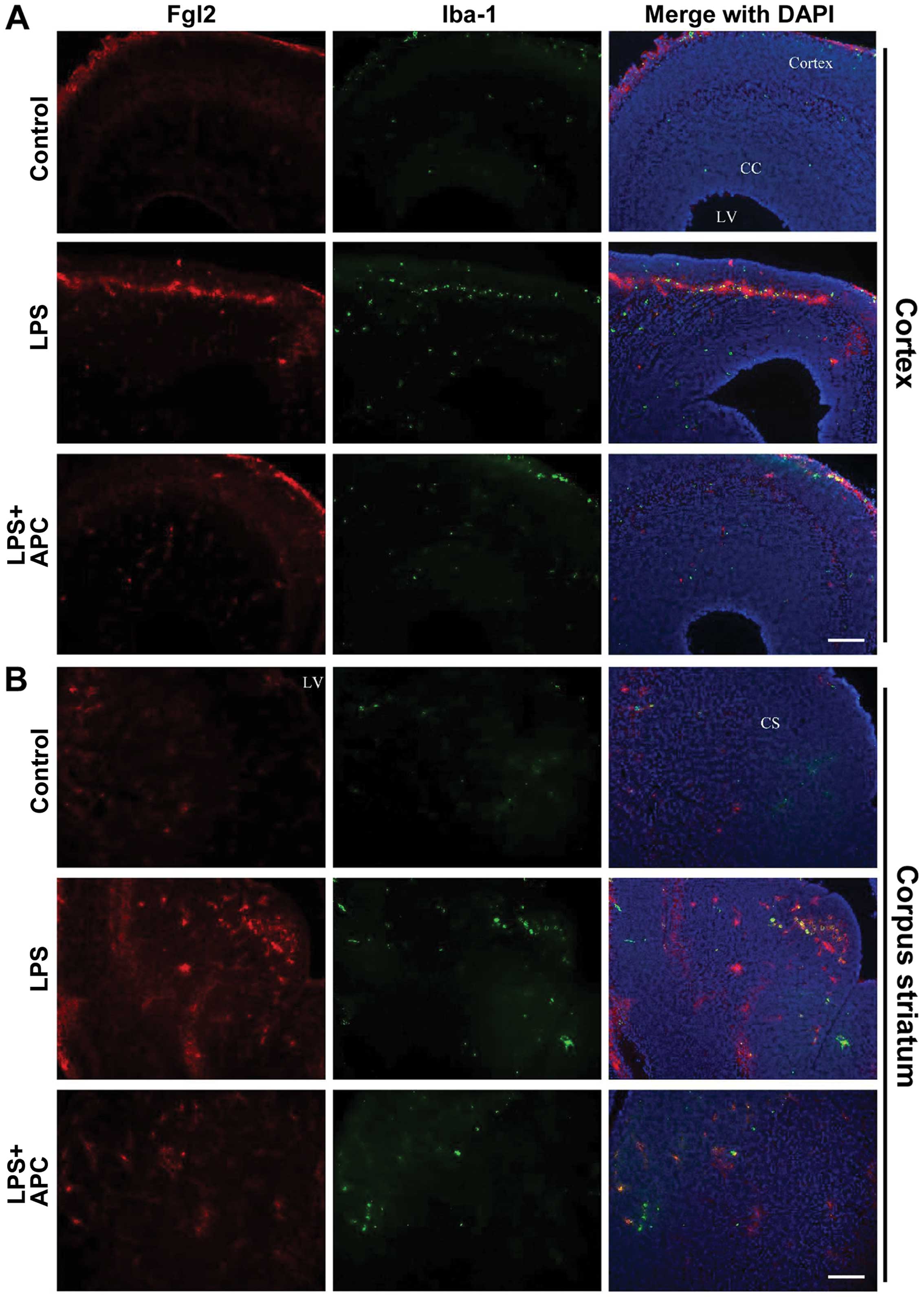
Following maternal exposure to LPS and treatment
with APC, fgl2 expression was detected in the fetal rat brains on
E19 by immunofluorescence staining. As shown in Fig. 6, the fgl2-positive cells were
mostly distributed in the cortex, corpus callosum, striatum and
periventricular region. Double-labeling staining revealed the
co-localization of fgl2 with the microglia marker, Iba-1, in the
cortex (Fig. 6A) and corpus
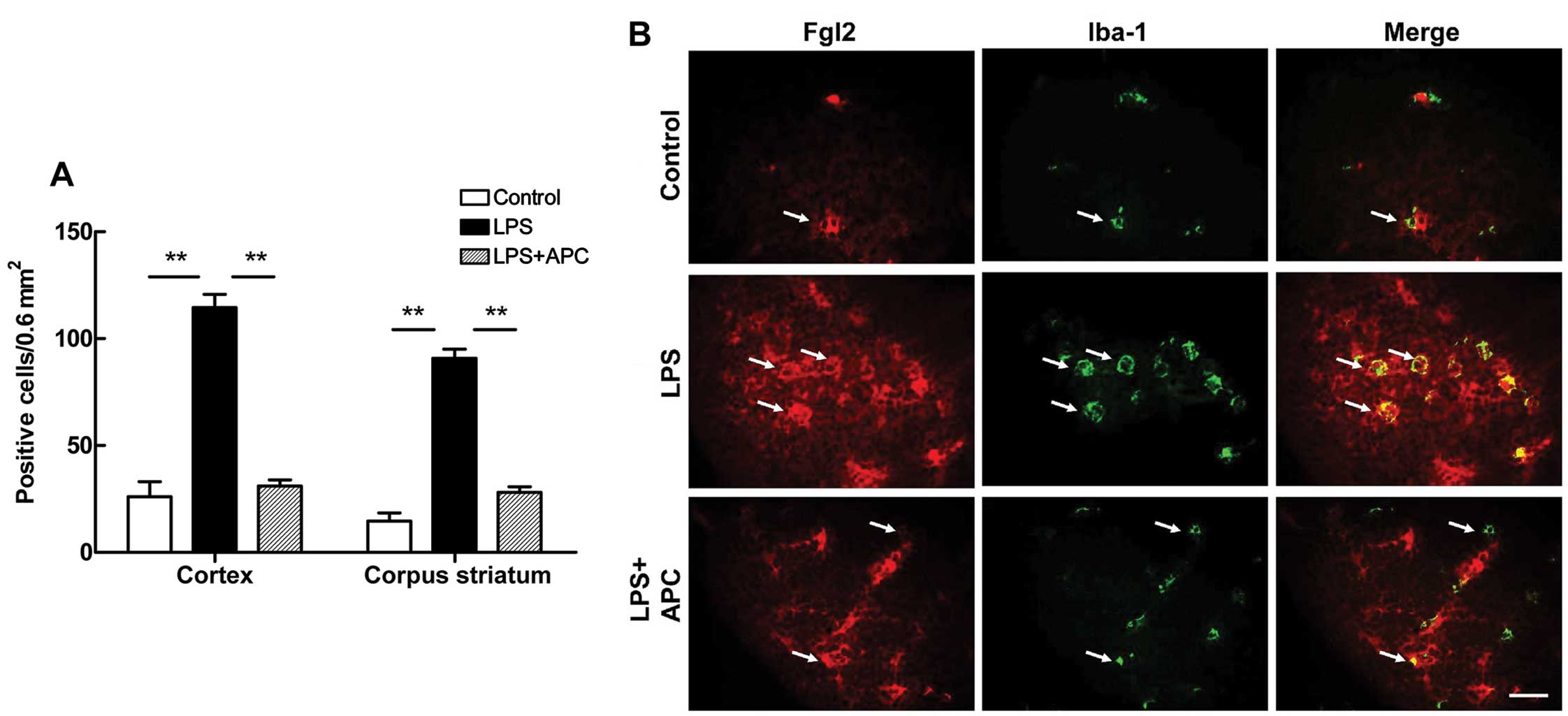
striatum (Fig. 6B). The numerical
densities of double immunopositive cells in the cortex and corpus
striatum were increased significantly following the administration
of LPS (P<0.01, Fig. 7A).
Treatment with APC significantly reversed the increase in
fgl2-positive microglia densities observed in the above regions
(P<0.01, Fig. 7A). In
addition, as shown in Fig. 7B,
resting microglia in a ramified form exhibited a low expression of
fgl2. By contrast, activated microglia became round and enlarged,
with or without short processes following the administration of
LPS, and fgl2 expression was markedly increased, appearing as
strong red fluorescent signals on the green-stained Iba-1-positive
cells (i.e., microglia) (Fig.
7B). Treatment with APC inhibited microglial activation and
fgl2 expression, as indicated by the morphological and numerical
changes.
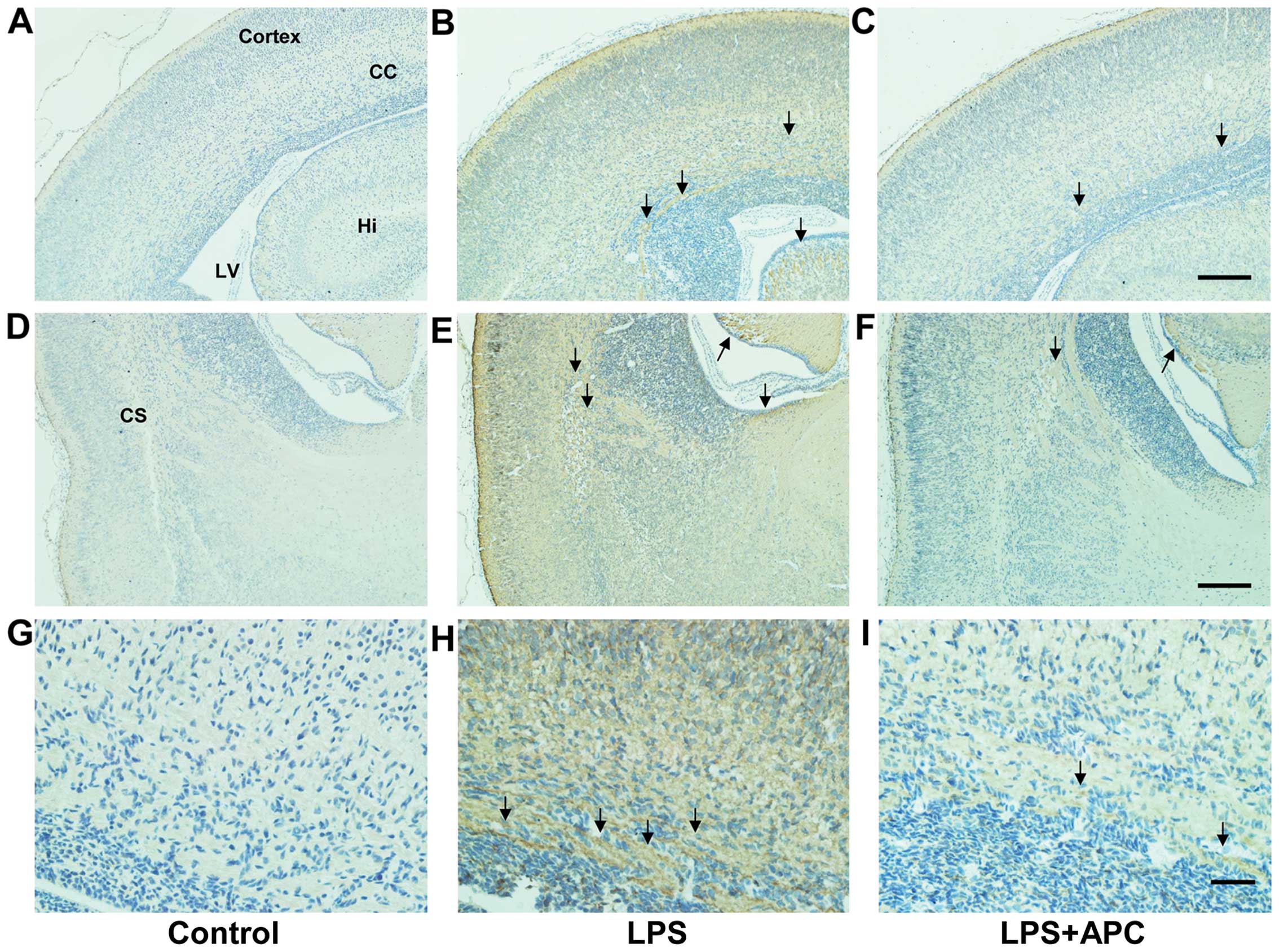
Immunohistochemistry revealed marked fibrin
deposition in the cortex, corpus callosum, hippocampus, striatum
and periventricular region in the brains of the LPS-treated rats
compared to those of the control rats, which lacked fibrin
deposition. However, fibrin deposition was significantly decreased
in those areas following treatment with APC (Fig. 8).
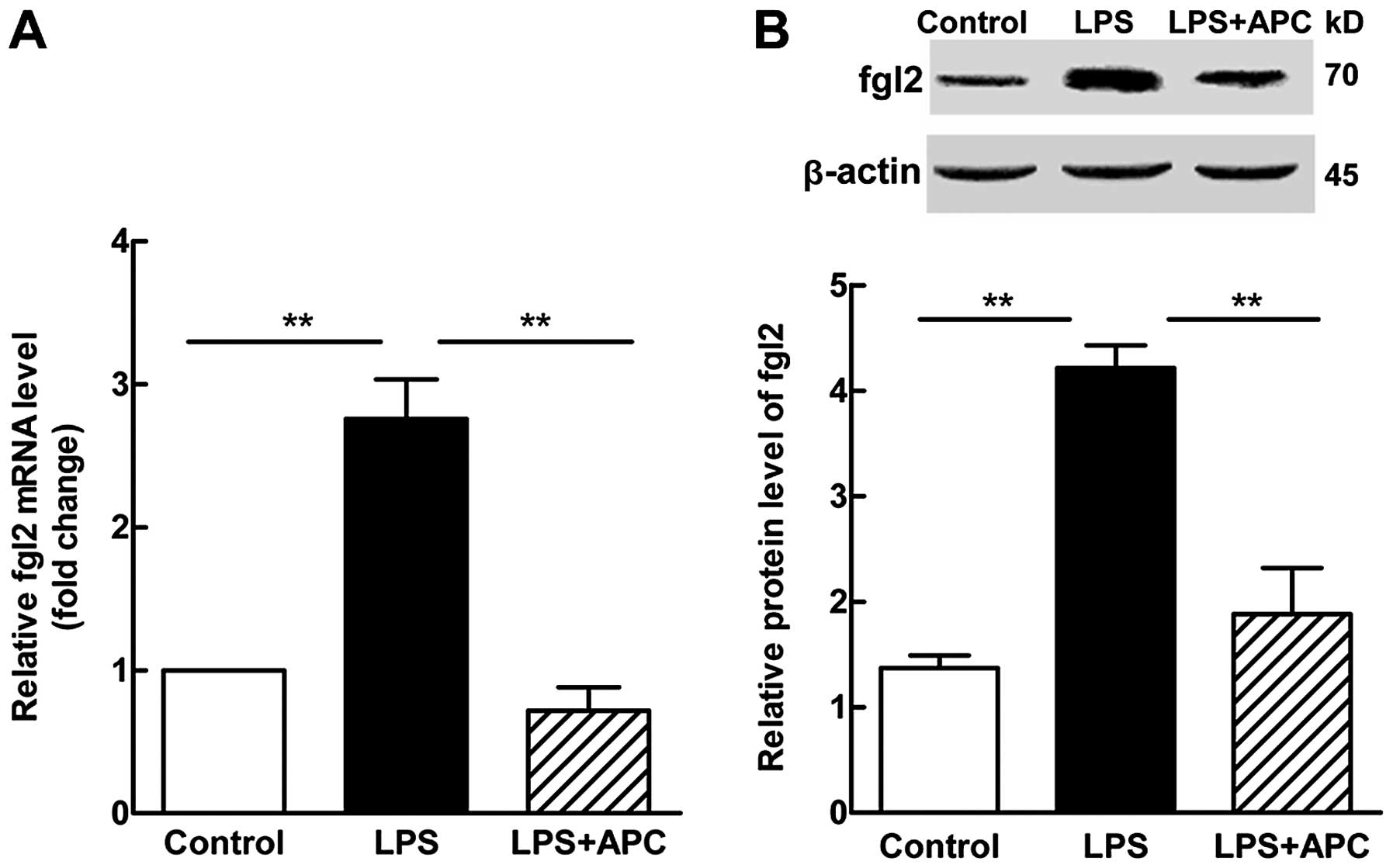
Both the mRNA and protein expression levels of fgl2
increased by >2-fold (P<0.001, Fig. 9) in the fetal brains following the
administration of LPS. Treatment with APC resulted in a lower fgl2
mRNA expression (P<0.001 vs. LPS; Fig. 9A) and this translated to a
significant decrease in the fgl2 protein level (P<0.001 vs. LPS;
Fig. 9B).
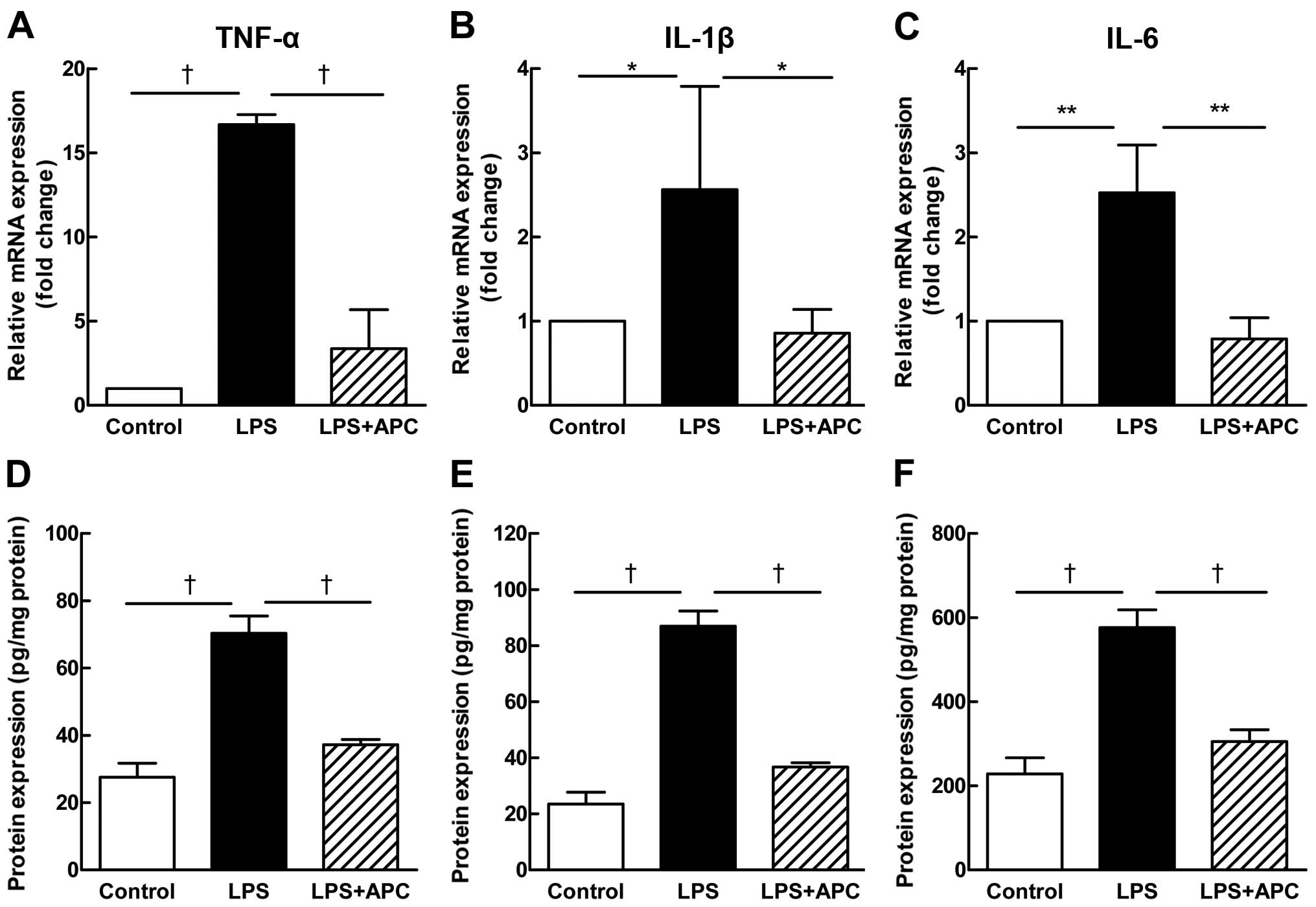
The administration of LPS significantly upregulated
the mRNA levels of the pro-inflammatory cytokines, TNF-α, IL-6 and
IL-1β, in the fetal rat brains, while treatment with APC reduced
these levels significantly (Fig.
10A–C). The protein concentrations of the cytokines were lower
in the fetal rat brains (Fig.
10D–F) than in the placentas (Fig. 4D–F), yet demonstrated the same
changing trend as in the placenta.
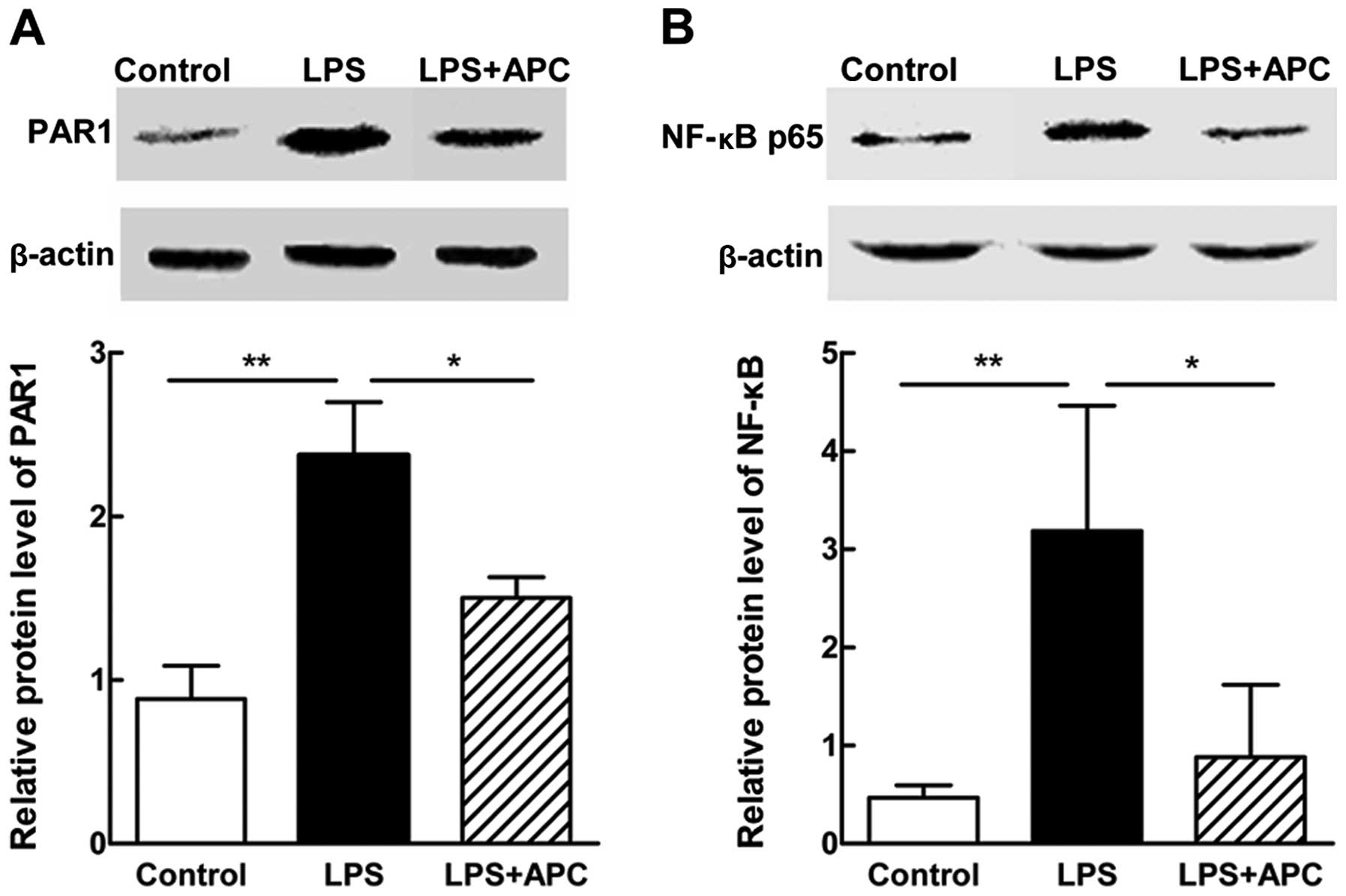
APC inhibits PAR1 and NF-κB p65
expression in the fetal brain
To explore the possible critical mediators or
signaling cascades involved in this process, we measured the
protein expression levels of PAR1 and NF-κB p65 in the fetal rat
brains on E19 by western blot analysis. As shown in Fig. 11, a significant elevation in the
protein expression levels of PAR1 and NF-κB p65 was noted after the
LPS injection, and was downregulated following treatment with
APC.
APC improves fetal growth restriction and
neonatal brain weight loss
To determine whether a mild LPS challenge can
restrict fetal growth or affect neonatal brain development, we
evaluated the birth weight, as well as the brain and body weight of
the rats on P1, P3, P7, P10 and P14. There was a significant
decrease in the birth weight of the rats in the LPS group compared
with the control group (P<0.001, Fig. 12A). The birth weight of the pups
in the LPS + APC group was significantly higher than that of the
pups in the LPS group (P<0.001, Fig. 12A). The brain weight of the pups
from either the control or the LPS + APC group were significantly
higher and kept increasing above those of the pups from the LPS
group on P1 to P14 (Fig. 12B).
Accordingly, the brain/body weight ratios of the pups in the LPS
group were significantly lower than those of the pups in the
control or LPS + APC group (Fig.
12C).
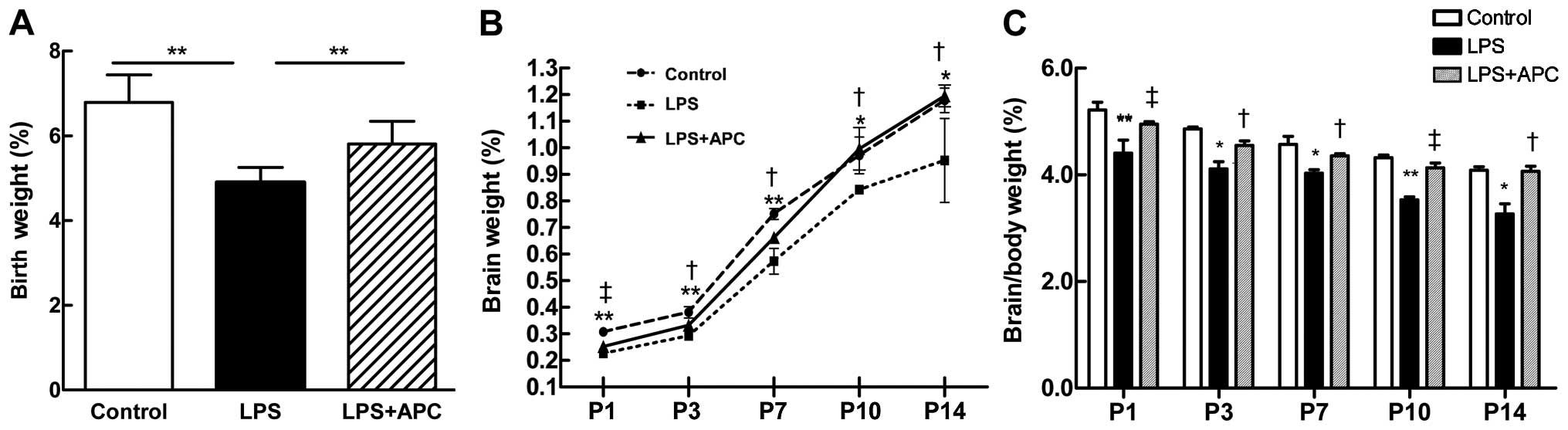 | Figure 12Activated protein C (APC) ameliorates
fetal growth restriction and neonatal brain weight loss following
maternal exposure to lipopolysaccharide (LPS). (A) Birth weight.
Data are presented as the means ± SD (n=46–59 per group);
*P<0.01 vs. LPS group. (B) Neonatal brain weight on
postnatal day (P)1, P3, P7, P10 and P14. The round, tetragonum and
triangle symbols refer to the control, LPS and LPS + APC group,
respectively. (C) Ratios of brain/body weight on P1, P3, P7, P10
and P14. The white, black, and gray bars refer to the control, LPS
and LPS + APC group, respectively,. *P<0.05,
**P<0.01 control vs. LPS group;
†P<0.05, ‡P<0.01 APC vs. LPS group.
Data are presented as the means ± SD (n=5). |
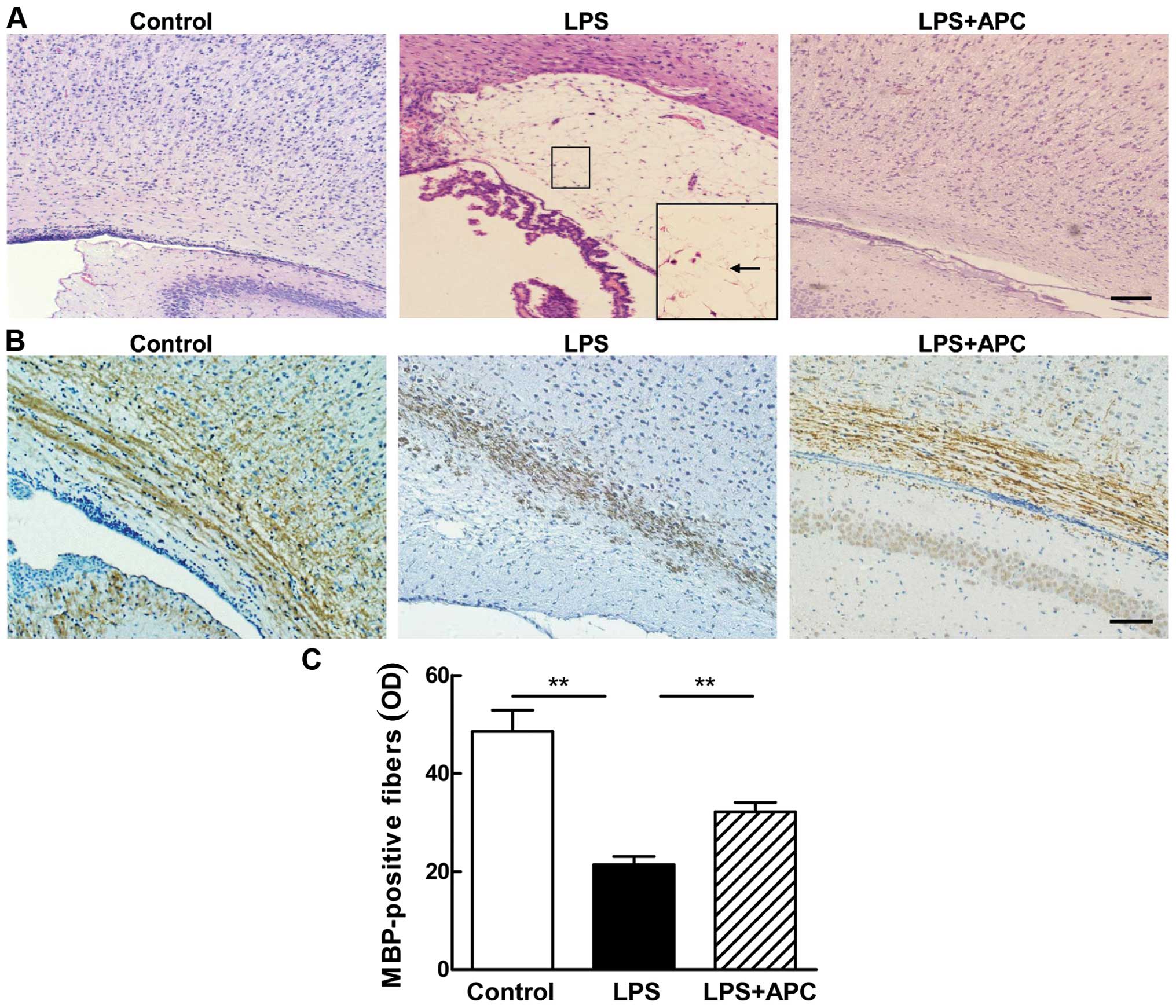
APC reduces hypomyelination and WMI in
the neonatal rat brain
H&E staining (Fig. 13A) revealed structural
disturbances in the periventricular area in the surviving neonatal
rat brains following the LPS injection, including cell loss, tissue
edema and even white matter cyst formation [periventricular
leukomalacia (PVL)]. These disorders were not observed in the
control or LPS + APC group (Fig.
13A).
The LPS-induced impairment of myelination in the
developing rat brain was demonstrated by a reduction in the number
of MBP-stained auxiliary fibers with fewer processes and shortened
fragmentation in the periventricular white matter (Fig. 13B). The density of MBP-positive
fibers in the periventricular white matter of the pups in the LPS
group was significantly lower than that of the pups in the control
group (P<0.001, Fig. 13C).
APC attenuated hypomyelination, as evidenced by robust MBP staining
(Fig. 13B) and marked MBP
density (Fig. 13C) in the brains
of rat in the LPS + APC group.
Discussion
Despite the fact that considerable progress has been
made in the research of the pathophysiology of intrauterine
inflammation-induced WMI, facultative therapeutic options are
limited. In the present study, we investigated the protective
effects of APC and the underlying molecular mechanisms in perinatal
brain injury in rats induced by maternal exposure to LPS. To the
best of our knowledge, the present study was the first to evaluate
the placental and cerebral expression of fgl2 in rats in
vivo. We observed a coordinated downregulation in fgl2
expression, fibrin deposition and in pro-inflammatory cytokine
(TNF-α, IL-6 and IL-1β) production in both the placenta and fetal
brain following treatment with APC. APC also ameliorated cerebral
edema and cell death in the developing fetuses, as well as
hypomyelination in the neonatal rat pups. The neuroprotective
effects of APC may involve the inhibition of the PAR1 and NF-κB
pathway. However, further studies are required to confirm this.
Multiple models of intrauterine inflammation have
been established to mimic the white matter injuries in the
developing brain. The systemic use of LPS, a cell wall component of
Gram-negative bacteria, creates an inflammatory state in the
maternal tissue and, eventually, in the fetal tissue without a
conspicuous infection. Herein, we employed a rat model of WMI by
maternal exposure to LPS during the late gestational period. We
observed high rates of apoptosis and severe brain edema in the
LPS-treated fetal rat brains. The majority of these fetuses
suffered miscarriage or death before birth, and a few surviving
ones exhibited impaired development and neurogenesis in regard to
their low birth weight and failure to gain brain weight. Prenatal
maternal APC treatment reversed these changes in the present study.
These results suggest that APC attenuates cerebral apoptosis and
sustains the integrity of BBB in inflammation-induced brain injury.
However, the mechanisms involved remain unclear and require further
investigation.
Pro-inflammatory cytokines are important mediators
of inflammatory injury. In accordance with previously published
data (24), our model showed that
maternal exposure to LPS evoked a vigorous pro-inflammatory
cytokine response in both the placenta and the fetal brain. The
levels of TNF-α, IL-6 and IL-1β increased more markedly in the
placenta than in the brain. This finding may be attributed to the
attenuated cytokine production in the premature fetus or the
partial passage of placental cytokines through the placental-fetal
barrier (25). Moreover, the
inflammatory response may activate certain components of the
coagulation cascade. fgl2 is a novel prothrombinase with double
procoagulant and pro-inflammatory properties (13). Th1 cytokine-driven macrophage
activation upregulates the expression of the membrane-bound fgl2,
leading to thrombin-triggered inflammation (15). Jia et al (26) demonstrated that TNF-α promoted
fgl2 expression by activating the NF-κB and p38 MAPK pathways, and
Clark et al (27)
implicated that an increased fgl2 expression induced
cytokine-enhanced abortions through fibrin deposition. Thus, it can
be hypothesized that pro-inflammatory cytokines lead to fgl2
upregulation, thrombin generation and fibrin deposition. In the
present study, we observed a synchronous elevation in fgl2
epxression in the rat placenta and fetal brain accompanied by an
increase in the expression of pro-inflammatory cytokines following
LPS injection, and an upregulation in fg12 expression that was
associated with increased fibrin staining. These observations
suggest a crosslink between inflammation and coagulation. Elevated
levels of pro- and anti-coagulant factors, as well as
pro-inflammatory cytokines and chemokines, have been previously
observed in term newborns who later developed CP (28). In the present study, the prenatal
administration of APC significantly reduced the LPS-induced
increase in fgl2 expression and fibrin deposition, as well as the
increase in the expression of the pro-inflammatory cytokines,
TNF-α, IL-6 and IL-1β, in the placenta and fetal brain, suggesting
the favorable anti-coagulant and anti-inflammatory effects of APC.
Rezaie (29) demonstrated that
APC regulated anti-coagulant and anti-inflammatory signaling
pathways by recognizing its different substrates and cofactors.
Injuries of the oligodendrocyte lineage are crucial
to the pathogenesis of WMI in the developmental context. The
oligodendrocyte precursor cells go through a range of
differentiation stages to become mature oligodendrocytes, which are
distinguished from oligodendrocyte progenitor cells (OPCs) and
immature oligodendrocytes by the selective expression of MBP.
Premyelinating oligodendrocytes are highly vulnerable to
excitotoxic, oxidative and inflammatory insults, and may fail to
evolve into mature myelin-producing cells, resulting in
hypomyelination (2). In the
present study, we observed weak MBP staining and even localized
multiple cystic lesions with the loss of cellular elements in the
periventricular white matter of rat brains on P14 following the LPS
injection. Programmed cell death was thought to be part of
myelination disturbances. The loss of mature oligodendrocytes
caused by apoptosis in the region of white matter has been
documented in fetal rats with maternal inflammation exposure
(30). However, it has been
suggested that, instead of the induction of apoptosis, the delayed
development and differentiation arrest of OPCs are mandatory for
hypomyelination (31). The
impaired development and maturation of oligodendrocytes have been
found in an oligodendrocyte-microglia co-culture system upon LPS
stimulation (32). APC has been
reported to exert anti-apoptotic effects on neural cells (17). Consistent with the previous
results of Yesilirmak et al (22), we found that treatment with APC
reduced cell death and the loss of MBP-expressing fibers in the
periventricular region, thus, ameliorating white matter lesions.
However, further research needs to be conducted to confirm whether
APC protects inflammation-exposed newborn brains from
hypomyelination and WMI by inhibiting oligodendrocyte apoptosis or
by maintaining the microglia-related oligodendrocyte
maturation.
Microglia are the resident immune cells in the
brain, which share the origin of macrophages and serve as scavenger
cells in the central nervous system (CNS). They appear at a
somewhat early stage and ‘settle down’ in the CNS mainly during the
second or third trimester of fetal development (33). In the present study, we found
traces of microglia as early as the 19th day of gestation.
Microglia are morphologically and functionally dynamic cells that,
once activated, can change from a highly ramified to an unramified
or amoeboid form, travel to sites of injury, trigger
neuroinflammation and evoke damage to cerebral white matter
(1). Our results corroborate
previously published findings (34), as increased densities of
unramified or intermediate microglia were observed in areas of
white matter suffering from inflammatory injury. fgl2 has been
shown to be expressed on activated macrophages (11), and we identified its
co-localization with the specific microglia marker, Iba-1. A range
of receptors are expressed on microglia, including PAR1, which is
implicitly involved in APC signaling. PAR1 activation contributes
to microglial proliferation and activation by increasing microglial
CD40 expression and CD40 ligand-induced TNF-α secretion (35). NF-κB transduces one of the
downstream signaling pathways of PAR1 in microglia and may
contribute to the overexpression of inflammatory mediators in
cerebral WMI (36). In the
present study, APC significantly inhibited the protein levels of
PAR-1 and NF-κB p65, the release of pro-inflammatory cytokines and
the microglial expression of fgl2. Thus, it can be hypothesized
that fgl2 participates in the LPS-triggered microglial activation
by interacting with the PAR1 and NF-κB signaling pathways, and APC
may reduce fgl2 expression through the inhibition of inflammatory
mediator production and the blocking of NF-κB signaling (17). While the whole-tissue expression
of the p65 subunit may not thoroughly represent the contribution of
NF-κB in the present study, the detection of the nuclear
translocation of p65 or p55 is necessary to evaluate NF-κB
activation. Moreover, in the future, we aim to perform further
studies using fgl2 knockout mice in order to verify and expand the
biological function and mechanism of action of fgl2 in
neuroinflammation.
In recent years, a growing number of research
studies has demonstrated the beneficial effects of APC on various
organs (20,37,38). Research on the placental transfer
of APC in mice (39) has
suggested the potential of the antenatal maternal use of APC to
protect the fetal brain. As reported by Yesilirmak et al
(22), prenatal maternal APC
treatment indeed reduced the endotoxin-induced WMI in the
developing rat brain. The extent to which APC crosses the placental
barrier and actually acts on the fetus remains controversial.
However, considering the same changing trend in fgl2 and
pro-inflammatory cytokine expression in the fetal brain as in the
placenta, it can be hypothesized that APC exerts its
neuroprotective effects by inhibiting the LPS-induced placental
inflammatory responses. In addition, the key membrane signaling
receptor modulating the pleiotropic biological activities of APC in
the CNS is PAR1, which can be cleaved and activated by APC and
triggers numerous intracellular signaling cascades (40). APC signaling is inhibited by PAR1
cleavage-blocking antibodies, whereas non-specific PAR1
desensitization, but not PAR1 antibodies, prevents direct PAR1
agonist signaling (41). The
genetic inactivation of PAR1 using Par1−/− mice
abrogates the ability of APC to suppress IL-6 production by
LPS-activated macrophages (42).
The major complication of APC therapy is severe bleeding due to its
anticoagulant activity. Accordingly, newly developed APC analogs
with reduced anticoagulant activity, but intact cell-signaling
activities may help to diminish the risk of bleeding and preserve
its cytoprotective effects and pharmacologic benefits (43), which may provide a translational
potential for the treatment of WMI.
In conclusion, the present study demonstrates the
neuroprotective effects of APC against inflammation-induced white
matter lesions, thus suggesting its potential for use as a
therapeutic strategy for intrauterine inflammation-induced neonatal
brain injury. APC not only attenuates fetal neuroinflammation and
the associated secondary WMI in the developing brain, but also
reduces the fgl2 expression, fibrin deposition and pro-inflammatory
cytokine production in the placenta. Although further studies are
required to elucidate the underlying mechanisms, our results
provide new insight into the association between the placental
inflammatory response and neuroinflammation in an adverse
intrauterine environment of inflammatory exposure.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81471519 and
81401277), the Program for Changjiang Scholars and Innovative
Research Team in University (PCSIRT1131), the National Key Science
Projects Program of the Ministry of Science and Technology of China
(no. 2012BAI09B04), the Natural Science Foundation of Hubei
Province of China (no. 2012FFB02524) and the Fundamental Research
Funds for the Central Universities (HUST: 01-18-540-106).
Abbreviations:
|
APC
|
activated protein C
|
|
LPS
|
lipopolysaccharide
|
|
WMI
|
white matter injury
|
|
PVL
|
periventricular leukomalacia
|
|
fgl2
|
fibrinogen-like protein 2
prothrombinase
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-1β
|
interleukin-1β
|
|
IL-6
|
interleukin-6
|
|
PAR1
|
protease-activated receptor 1
|
|
NF-κB
|
nuclear factor κB
|
|
CNS
|
central nervous system
|
|
MBP
|
myelin basic protein
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling
|
|
H&E
|
hematoxylin and eosin
|
References
|
1
|
Deng W: Neurobiology of injury to the
developing brain. Nat Rev Neurol. 6:328–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Volpe JJ: Brain injury in premature
infants: a complex amalgam of destructive and developmental
disturbances. Lancet Neurol. 8:110–124. 2009. View Article : Google Scholar :
|
|
3
|
Favrais G, van de Looij Y, Fleiss B, et
al: Systemic inflammation disrupts the developmental program of
white matter. Ann Neurol. 70:550–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuypers E, Ophelders D, Jellema RK,
Kunzmann S, Gavilanes AW and Kramer BW: White matter injury
following fetal inflammatory response syndrome induced by
chorioamnionitis and fetal sepsis: lessons from experimental ovine
models. Early Hum Dev. 88:931–936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leclerc J, Pu Q, Corseaux D, Haddad E,
Decoene C, Bordet R, Six I, Jude B and Vallet B: A single endotoxin
injection in the rabbit causes prolonged blood vessel dysfunction
and a procoagulant state. Crit Care Med. 28:3672–3678. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai Z, Pan ZL, Pang Y, Evans OB and Rhodes
PG: Cytokine induction in fetal rat brains and brain injury in
neonatal rats after maternal lipopolysaccharide administration.
Pediatr Res. 47:64–72. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoon BH, Jun JK, Romero R, Park KH, Gomez
R, Choi JH and Kim IO: Amniotic fluid inflammatory cytokines
(interleukin-6, interleukin-1(beta), and tumor necrosis
factor-(alpha)), neonatal brain white matter lesions, and cerebral
palsy. Am J Obstet Gynecol. 177:19–26. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee J, Chan SL and Mattson MP: Adverse
effect of a presenilin-1 mutation in microglia results in enhanced
nitric oxide and inflammatory cytokine responses to immune
challenge in the brain. Neuromolecular Med. 2:29–45. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao Q, Li P, Lu J, Dheen ST, Kaur C and
Ling EA: Nuclear factor NF-κB/p65 responds to changes in the Notch
signaling pathway in murine BV-2 cells and in amoeboid microglia in
postnatal rats treated with the γ-secretase complex blocker DAPT. J
Neurosci Res. 88:2701–2714. 2010.PubMed/NCBI
|
|
10
|
Levy GA, Liu M, Ding J, Yuwaraj S,
Leibowitz J, Marsden PA, Ning Q, Kovalinka A and Phillips MJ:
Molecular and functional ana1ysis of the human prothrombinase gene
(hfgl2) and its role in viral hepatitis. Am J Pathol.
156:1217–1225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan CW, Chan MW, Liu M, Fung L, Cole EH,
Leibowitz JL, Marsden PA, Clark DA and Levy GA: Kinetic analysis of
a unique direct prothrombinase, fgl2, and identification of a
serine residue critical for the prothrombinase activity. J Immunol.
168:5170–5177. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Phillippe M, Diamond AK, Sweet LM,
Oppenheimer KH and Bradley DF: Expression of coagulation-related
protein genes during LPS-induced preterm delivery in the pregnant
mouse. Reprod Sci. 18:1071–1079. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Melnyk MC, Shalev I, Zhang J, et al: The
prothrombinase activity of FGL2 contributes to the pathogenesis of
experimental arthritis. Scand J Rheumatol. 40:269–278. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ning Q, Sun Y, Han M, et al: Role of
fibrinogen-like protein 2 prothrombinase/fibroleukin in
experimental and human allograft rejection. J Immunol.
174:7403–7411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu G, Sun Y, Foerster K, Manuel J, Molina
H, Levy GA, Gorczynski RM and Clark DA: LPS-induced murine
abortions require C5 but not C3, and are prevented by upregulating
expression of the CD200 tolerance signaling molecule. Am J Reprod
Immunol. 60:135–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu M, Mendicino M, Ning Q, et al:
Cytokine-induced hepatic apoptosis is dependent on
FGL2/fibroleukin: the role of Sp1/Sp3 and STAT1/PU.1 composite cis
elements. J Immunol. 176:7028–7038. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zlokovic BV and Griffin JH: Cytoprotective
protein C pathways and implications for stroke and neurological
disorders. Trends Neurosci. 34:198–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deane R, LaRue B, Sagare AP, Castellino
FJ, Zhong Z and Zlokovic BV: Endothelial protein C
receptor-assisted transport of activated protein C across the mouse
blood-brain barrier. J Cereb Blood Flow Metab. 29:25–33. 2009.
View Article : Google Scholar
|
|
19
|
Li B, Yu D and Xu Z: Activated protein C
inhibits amyloid β production via promoting expression of ADAM-10.
Brain Res. 1545:35–44. 2014. View Article : Google Scholar
|
|
20
|
Wang Y, Zhao Z, Chow N, Rajput PS, Griffin
JH, Lyden PD and Zlokovic BV: Activated protein C analog protects
from ischemic stroke and extends the therapeutic window of
tissue-type plasminogen activator in aged female mice and
hypertensive rats. Stroke. 44:3529–3536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yesilirmak DC, Kumral A, Tugyan K, Cilaker
S, Baskin H, Yilmaz O, Duman N and Ozkan H: Effects of activated
protein C on neonatal hypoxic ischemic brain injury. Brain Res.
1210:56–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yesilirmak DC, Kumral A, Baskin H, et al:
Activated protein C reduces endotoxin-induced white matter injury
in the developing rat brain. Brain Res. 1164:14–23. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olivier P, Fontaine RH, Loron G, et al:
Melatonin promotes oligodendroglial maturation of injured white
matter in neonatal rats. PLoS One. 4:e71282009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bell MJ, Hallenbeck JM and Gallo V:
Determining the fetal inflammatory response in an experimental
model of intrauterine inflammation in rats. Pediatr Res.
56:541–546. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zaretsky MV, Alexander JM, Byrd W and
Bawdon RE: Transfer of inflammatory cytokines across the placenta.
Obstet Gynecol. 103:546–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia P, Wang J, Wang L, et al: TNF-α
upregulates fgl2 expression in rat myocardial ischemia/reperfusion
injury. Microcirculation. 20:524–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clark DA, Ding JW, Yu G, Levy GA and
Gorczynski RM: Fgl2 prothrombinase expression in mouse trophoblast
and decidua triggers abortion but may be countered by OX-2. Mol Hum
Reprod. 7:185–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leviton A and Dammann O: Coagulation,
inflammation, and the risk of neonatal white matter damage. Pediatr
Res. 55:541–545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rezaie AR: Regulation of the protein C
anticoagulant and anti-inflammatory pathways. Curr Med Chem.
17:2059–2069. 2010. View Article : Google Scholar
|
|
30
|
Bell MJ and Hallenbeck JM: Effects of
intrauterine inflammation on developing rat brain. J Neurosci Res.
70:570–579. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
French HM, Reid M, Mamontov P, Simmons RA
and Grinspan JB: Oxidative stress disrupts oligodendrocyte
maturation. J Neurosci Res. 87:3076–3087. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brehmer F, Bendix I, Prager S, et al:
Interaction of inflammation and hyperoxia in a rat model of
neonatal white matter damage. PLoS One. 7:e490232012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karperien A, Ahammer H and Jelinek HF:
Quantitating the subtleties of microglial morphology with fractal
analysis. Front Cell Neurosci. 7:32013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Verney C, Pogledic I, Biran V,
Adle-Biassette H, Fallet-Bianco C and Gressens P: Microglial
reaction in axonal crossroads is a hallmark of noncystic
periventricular white matter injury in very preterm infants. J
Neuropathol Exp Neurol. 71:251–264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suo Z, Wu M, Ameenuddin S, Anderson HE and
Zoloty JE: Participation of protease-activated receptor-1 in
thrombin-induced microglial activation. J Neurochem. 80:655–666.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan TM, Sun Y, Zhan CY and Yu HM:
Intrauterine infection/inflammation and perinatal brain damage:
role of glial cells and Toll-like receptor signaling. J
Neuroimmunol. 229:16–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gabre J, Chabasse C, Cao C, Mukhopadhyay
S, Siefert S, Bi Y, Netzel-Arnett S, Sarkar R and Zhang L:
Activated protein C accelerates venous thrombus resolution through
heme oxygenase-1 induction. J Thromb Haemost. 12:93–102. 2014.
View Article : Google Scholar :
|
|
38
|
Puig F, Fuster G, Adda M, Blanch L and
Farre R: Barrier-protective effects of activated protein C in human
alveolar epithelial cells. PLoS One. 8:e569652013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishii S, Mochizuki T, Nagao T, Kudo S,
Fujita A, Taniguchi K, Kondo S and Kiyoki M: Pharmacokinetics of
human activated protein C. 2nd communication: tissue distribution
study of a lyophilized purified human activated protein C after
single or repeated intravenous administration in male mice and
placental transfer and milk passage study after intravenous
administration in pregnant and lactating mice.
Arzneimittelforschung. 45:644–656. 1995.PubMed/NCBI
|
|
40
|
Crawley JT and Efthymiou M: Cytoprotective
effect of activated protein C: specificity of PAR-1 signaling. J
Thromb Haemost. 6:951–953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Riewald M, Petrovan RJ, Donner A, Mueller
BM and Ruf W: Activation of endothelial cell protease activated
receptor 1 by the protein C pathway. Science. 296:1880–1882. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao C, Gao Y, Li Y, Antalis TM, Castellino
FJ and Zhang L: The efficacy of activated protein C in murine
endotoxemia is dependent on integrin CD11b. J Clin Invest.
120:1971–1980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mosnier LO, Yang XV and Griffin JH:
Activated protein C mutant with minimal anticoagulant activity,
normal cytoprotective activity, and preservation of thrombin
activatable fibrinolysis inhibitordependent cytoprotective
functions. J Biol Chem. 282:33022–33033. 2007. View Article : Google Scholar : PubMed/NCBI
|