1. Introduction
Nuclear receptors (NRs) are a superfamily of
transcription factors, which are ligand-dependent and homologous to
steroid hormone receptors. NRs are widely distributed and have
important physiological functions in cell development and
differentiation, circadian rhythm, metabolism and immune
regulation. NRs consist of three components: the steroid hormone
receptors, non-steroid hormone receptors and the orphan nuclear
receptor family. Steroid and non-steroid hormone receptors have
specific ligands, including steroid hormones, thyroid hormones,
retinoic acids and fatty acids. Ligands for orphan NRs have not yet
been determined. Retinoic acid-related orphan receptors (RORs),
also known as nuclear receptor subfamily 1 group F members (NR1F),
are specified by gene sequences, which are homologous to retinoic
acid receptor (RAR) and retinoid X receptor (RXR) which belong to
non-steroid receptors (1,2). RORs include RORα, RORβ and RORγ,
which are also referred to as RORA, RORB and RORC or NR1F1-3,
respectively, and have been cloned from different mammalian
species. The molecular mechanisms and physiological functions of
RORα and RORγ are well established. However, the function of RORβ
needs to be further elucidated. This review focuses on the
structure of RORs and aims to provide an overview of the present
studies on the functions that RORβ has in several biological and
pathological processes, particularly in terms of circadian rhythm
abnormalities and tumorigenesis.
2. Genotyping, molecular structures and
distribution of RORs
RORα contains isoforms RORα 1–4, with only RORα1 and
RORα4 being present in mice. Two isoforms of RORβ are found in mice
and humans (RORβ1 and RORβ2), although studies have reported that
only RORβ1 exists in humans. RORγ also has two isoforms (RORγ1 and
RORγ2). RORγ2, originally found in the immune system, is often
regarded as RORγt and is important in thymocyte development, with
its expression being highly restricted to the thymus. The two
isoforms of RORγ are found in mice and humans (3,4).
RORs share a common modular structure composed of
four functional domains including an amino-terminal A/B domain, a
DNA-binding domain (DBD), a hinge region and a carboxy-terminal
ligand-binding domain (LBD). The A/B domains are highly variable in
sequence between different ROR isoforms (5,6).
The DBD consists of two highly conserved zinc finger motifs
involved in the recognition of ROR response elements (ROREs) that
contain the consensus motif AGGTCA preceded by a 5-bp AT-rich
sequence. The RORs bind to ROREs as a monomer to regulate the
transcription of target genes (7,8).
The hinge region has shorter sequences and is thought to be a
flexible domain that connects the DBD with the LBD. Its main roles
are to maintain the structural stability of RORs and influence
intracellular trafficking and subcellular distribution. The
multifunctional domain LBD, consisting of 12 classic α helices
(H1-12) and two additional α helices (H2′ and H11′), plays multiple
roles in ligand binding, nuclear localization, receptor
dimerization, and serves as an interface for the interaction with
co-activators and co-repressors (9). RORs have two activation domains,
including the ligand-independent activation function-1 domain
(AF-1), which is localized in the N-terminal domain and the
ligand-dependent AF-2, which resides in the C-terminal domain. The
transcriptional activation of AF-1 is commonly very weak, but it
can synergize with AF-2 to produce a more robust upregulation of
gene expression. AF-2, located in H12 and consisting of the PLYKELF
motif, is 100% conserved among RORs and plays a dominant role in
transcriptional regulation. The study on the crystal structure of
RORα suggests that deletion or point mutation in H12, in particular
Y507A, may result in a decrease in transcriptional activity and a
dominant negative RORα (1).
RORs are conservative during evolution. Orthologs of
RORs have been identified in some lower species, such as
Drosophila hormone receptor 3 (DHR3) in Drosophila
melanogaster, caenorhabditis hormone receptor 3 (CHR3) in
Caenorhabditis elegans and manduca hormone receptor 3 (MHR3)
in Manduca sexta (10–12). The DBDs of RORs are highly
conserved and the DBD of RORγ exhibits a 92 and 75% identity with
that of RORβ and RORα, respectively (13). Although the LBD sequence is
moderately conserved and does not have a high degree of homology
(63% for RORα and RORβ, respectively, and 58% for RORα and RORγ,
respectively) (5,14), their secondary structure is very
similar and always contains 12α-helices (H1-H12). H12 is 100%
conserved among RORs and contains the AF2 consensus motif ΦΦXE/DΦΦ
(Φ denotes a hydrophobic amino acid and X denotes any amino acid)
(15), indicating that RORs may
likely have similar molecular functions.
RORα is widely distributed in multiple tissues
including brain, liver, pancreas, kidney, thymus, skeletal muscle,
testis, ovary, lung, skin and fat tissue, and is most highly
expressed in the cerebellum and hypothalamus (16–18). RORγ is mainly expressed in the
thymus, kidney, skeletal muscle, heart, liver, pancreas and testis,
and is particularly highly expressed in immune cells (4,19).
RORβ was initially identified as a member of the orphan nuclear
receptor family by Carlberg et al (3). It has been mapped to human
chromosome 9q21.13, the RORβ gene, which comprises 10 exons
and spans approximately a region of 188 kb of the genome (Table I). RORβ1 and RORβ2 share the
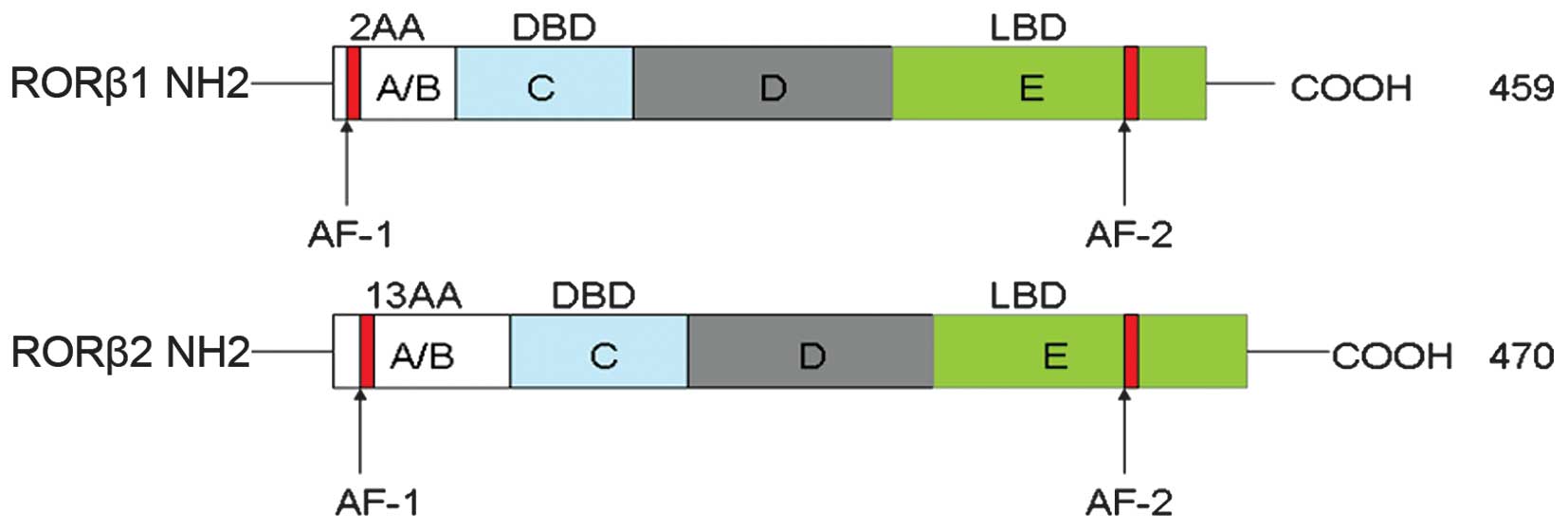
common DBD and LBD, but are characterized by a different A/B
domain, which, respectively, contains 2 and 13 amino acids. The
N-terminal 2–13 amino acids of RORβ1 are replaced by an arginine.
RORβ1 and RORβ2 consist of 459 and 470 amino acids, respectively
(Fig. 1). It is likely that RORβ1
and RORβ2 originate from the same gene by either alternative
splicing or transcription from an alternative promoter. RORβ was
primarily detected by northern blot analysis and its expression was
restricted to the central nervous system, in particular in regions
involved in the modulation of circadian rhythms, such as
suprachiasmatic nucleus (SCN), pineal gland, and retina (13,20–22). Recently, with the increasing
sensitivity of detection, RORβ has been found outside the nervous
system such as normal bone tissue, pancreatic and endometrial
cancer, and uterine leiomyosarcoma (23). The expression of RORβ in normal
intestinal epithelial cells and intestinal tumors has been
identified using the methods of mRNA view and qPCR. In addition,
intestinal tumor tissues show a lower level of RORβ when compared
with paralleled adjacent intestinal tissues. In the pathological
state, the expression profile of RORβ is altered, indicating that
the distribution of RORβ may be more widespread than is currently
known. RORβ1 and RORβ2 differ from each other as regards their
distribution. RORβ1 is highly expressed in cerebral cortex layer
IV, thalamus, and hypothalamus, while comparatively low expressed
in retina and pineal gland. By contrast, RORβ2 is the predominant
isoform in retina and pineal gland, and little is found in the
cerebral areas. When compared with RORβ1, the level of RORβ2 mRNA
oscillates robustly with true circadian rhythmicity and is elevated
to reach its maximal value at night. RORβ1 plays a leading role in
serving sensory input integration, while RORβ2 mainly affects
circadian rhythmicity. The different A/B domain may contribute to
their different functions.
 | Table ICharacteristics of ROR isoforms. |
Table I
Characteristics of ROR isoforms.
| Human chromosomal
localization | Genome region
(kb) | Exons | Expression | Physiological
function |
|---|
| RORα 15q22.2 | 730 | 12 | Brain, liver,
lung
Skin, pancreas, kidney and thymus | Brain/Purkinje cell
development
Bone metabolism
Lymphocyte development
Circadian rhythm |
| RORβ 9q21.3 | 188 | 10 | Brain, retina,
pineal gland, bone, colon, epididymus | Circadian
rhythm
Bone metabolism
Retinal neurogenesis |
| RORγ 1q21.3 | 24 | 11 | Thymus, kidney,
heart, liver, skeletal muscle and pancreas | Lymph-node
organogenesis
Thymopoiesis
Circadian rhythm
Mesenchymal differentiation |
3. Ligands identification and
protein-protein interactions for RORβ
RORβ is a ligand-dependent transcription factor,
although the identification of its functional ligands has not been
determined. Melatonin was initially presumed as a natural ligand of
RORβ due to its rhythmic synthesis and activity. RORβ can regulate
circadian rhythms, and the mRNA level of RORβ coincides with
melatonin production in the retina and pineal gland (22,24). However, subsequent studies have
not confirmed this finding. Masana et al (25) found that the level of pineal
melatonin exhibited a robust and significant diurnal rhythm with
low levels during the day and high levels during the night in
RORβ (C3H)+/+, RORβ (C3H)+/−
and RORβ (C3H) −/− mice, indicating that RORβ
does not affect the production of pineal melatonin and melatonin is
not involved in the role of RORβ-regulating circadian rhythm. In
the study on the X-ray structure of LBD of RORβ, stearic acid was
found to bind to RORβ and act as a filler and stabilizer rather
than a functional ligand. Later studies (1,26)
demonstrated that all-trans-retinoic acid (ATRA) and synthetic
retinoic acid (ALRT) 1550 reversibly combined to LBD of RORβ with a
high affinity, which effectively reduced the transcriptional
activity mediated by GAL4-RORβ in vitro. They are
functionally equivalent to an inverse agonist. However, the effect
of ATRA on RORβ activity is cell-type-dependent. In the neuronal
cell lines HT22 and Neuro2A, ATRA antagonized transactivation by
RORβ, while in cells such as NIH3T3, 293, and P19, ATRA showed no
effect. ATRA and ALRT 1550 are believed to be bona fide ligands for
RORβ, but whether they can regulate the transcriptional activity of
full-length RORβ and its target genes remains to be proven.
Furthermore, ATRA and ALRT 1550 can also bind to RORγ and inhibit
its transcriptional activity, but not to RORα. Cholesterol,
identified as a putative RORα ligand, neither binds to nor
modulates RORβ activity (26).
Based on the finding of the crystal structure study that the AF-2
side of ligand-binding pocket (LBP) is strictly hydrophobic, it was
predicted that hydrophobic molecules with a carboxylic head are
more likely to be ligand candidates for RORβ (9). On the issue of synthetic ligands,
significant progress has been made as regards RORα and RORγ, while
little is known with regard to RORβ. Studies on its specific
ligands may provide insight into our understanding of RORβ
(15).
Nuclear receptor transcriptional activation domain
AF-1 and AF-2 are important in protein-protein interactions
(27). Co-activators such as the
RNA co-activator SRA and the RNA-binding DEAD-box protein (p72/p68)
interact with AF-1 (28). The
co-activators acting on AF-2 including the BRG1 complex, the p160
steroid receptor co-activator (SRC) family, CBP/p300, and the
TRAP/DRIP/ARC complex enhance the receptor's transcriptional
activity through histone acetylation or methylation. By contrast,
co-repressors such as the nuclear receptor co-repressor (NCoR) and
its closely associated protein, the silencing mediator for retinoid
and thyroid hormone receptor (SMRT) repress gene transcription
through deacetylation by histone deacetylases (HDACs) (29). Transcriptional regulation by RORs
is mediated via an interaction with co-repressors such as NCoR or
co-activator complexes such as SRC-1, indicating that RORs can act
as repressors and activators of transcription (30). RORβ, which contains AF-1 and AF-2,
also has those molecular functions. It was also found that RORβ
proteins in humans and mouse have acetylation sites K27 and K98,
respectively. Thus, the regulation of target genes by RORβ is
likely to be more complex.
To determine the target genes regulated by RORβ,
Greiner et al (31) cloned
a 27-kDa protein consisting of 229 amino acid residues from a cDNA
library derived from mouse brain by using the method of yeast
two-hybrid screen and designated it as neuronal interacting factor
X1 (NIX1). NIX1 was originally identified as a neuronal-specific
cofactor and is exclusively expressed in the brain including in
neurons of the dentate gyrus, amygdala, thalamus, hypothalamus and
several brainstem nuclei. A subgroup of NRs such as RAR and TR but
not RXR or steroid hormone receptors can interact with NIX1.
Furthermore, RAR and TR only interact with NIX1 in the presence of
their cognate ligands, whereas NIX1 does not bind to RXR in the
absence or presence of the ligand. There is no similarity of
protein domains of NIX1 with other nuclear receptor cofactors
except for two nuclear receptor-binding LXXLL motifs, which are
also identified as nuclear location signals. One is located within
the C terminus (amino acids 192–196) and another is in an opposite
orientation within the central part of the protein (amino acids
87–91) (31). Previous findings
have demonstrated that LXXLL is required for the ligand-dependent
binding of transcriptional co-activators to NRs (32,33). Greiner et al (31) found that NIX1 directly combined
RORβ in vitro and in vivo, and specifically inhibited
its activity in a dose-dependent manner. They also identified a
minimal protein fragment spanning amino acids 61–99, which is both
necessary and sufficient for the interaction between NIX1 and RORβ.
Additionally, the fragment contains a nuclear receptor-binding
motif in an inverted orientation (LLQAL, amino acids 87–91), which
is required for the binding of NRs by NIX1. The AF-2 core motif of
RORβ located at amino acid residues 445–451 is necessary for the
interaction with NIX1. This finding is consistent with studies
showing that elimination or deletion of AF-2 may cause the molecule
to behave as a constitutive repressor or inactivate the protein
(27,31,34).
NRIP2 is a protein derived from humans and it is
homologous to NIX1. Compared with NIX1, NRIP2 has an extra 50 amino
acids on the N terminal and is mainly distributed in the cytoplasm,
which is different from NIX1. It has been found that NRIP2 and NIX1
belong to the aspartyl protease family. They are aspartyl
endopeptidases and homologous to DNA damage-inducible protein
(Ddi). Ddi1-related aspartyl proteases are believed to contain
human homologues such as Ddi1 and Ddi2 and neuron-specific NRs
NIX1, NRIP2 and NRIP3. However, the difference is that Ddi1
possesses three domains: a retroviral aspartyl-protease domain
(RVP), an NH2-terminal ubiquitin-like domain (UBL), and a
COOH-terminal ubiquitin-associated domain (UBA) and it is a
ubiquitin receptor (35). NRIP2
lacks the three domains and contains only one conservative D-S/T-G
fingerprint. The abovementioned findings suggest that NRIP2
functionally may be irrelevent to its ubiquitinated target
proteins. RORβ is likely to be one of the major substrates for
aspartyl endopeptidases NRIP2.
It is predicted that there are numerous interacting
proteins for RORβ via UniProtKB, MINT, STRING and I2D, such as
Nm23-H1(NME1), Nm23-H2(NME2), NCOA1 and MAP6. Nm23 and MAP6 are
associated with cytoskeletal movement, suggesting that RORβ may be
involved in the regulation of cytoskeletal movement.
4. Physiological functions of RORs
Although RORs have similar structures comprising
four homologous functional domains, there are obvious differences
in physiological functions, as well as in the expression between
RORs. RORα, RORβ and RORγ regulate circadian rhythms and RORα plays
the central role (36). RORα has
a key role in the development of the cerebellum, particularly in
the regulation of the maturation and survival of Purkinje cells and
the formation of bone. In RORα−/− mice, Purkinje and
granule cells are decreased and this results in cerebellar atrophy
(17). RORγ is required for
lymph-node organogenesis and the formation of multiple lymphoid
tissues such as Peyer's patches, crypto patches, and isolated
lymphoid follicles in the intestine. RORγ−/− mice are
deficient in lymph nodes. Moreover, RORγ promotes the
differentiation of T cells and the development of lymphoid
tissue-inducer (LTi) cells (37).
RORβ is necessary for the proliferation and differentiation of
retinal cells in addition to the maintenance of normal circadian
rhythms. At birth, the retina of RORβ−/− mice appears
very similar to that of wild-type mice with regard to morphology,
but in adulthood it is disorganized and lacks the normal layer
structure. The degeneration of retina occurs during the first weeks
after birth and eventually results in blindness (24). RORβ−/− mice also
exhibit behavioral changes such as reduced anxious behaviors,
increased exploratory activities, changes in motor function
occurring such as ‘duck gait’ decline in male reproductive
capacity, and olfactory dysfunction (25). Recent studies have found that RORβ
plays a role outside the neural system (38–41).
RORβ and retinal neurogenesis
There are two main continuous processes involved in
retinal neurogenesis. One is the proliferation of retinal
progenitors, which promote the growth of retina, and the other is
the differentiation of the various neuronal and glial cell types
that constitute the histology of the mature retina. RORβ has been
found to be expressed in retinal progenitor cells but not in
ganglion cells during embryonic development and it is highly
expressed in the retina during embryonic and postnatal development,
indicating that RORβ plays an important role in the maintenance of
retinal progenitor phenotype (21) Overexpression of RORβ in retinal
progenitors by biolistic transfection causes an increase in the
number of large cell clones. The transcription factor Chx10, which
is believed to influence retinal progenitor proliferation, is the
upstream molecule of RORβ and can upregulate the transcriptional
activity of RORβ. RORβ expression was markedly decreased when the
genetic defect of Chx10 was present. Therefore, the role of RORβ in
regulating retinal progenitor proliferation may be dependent on
Chx10 (21).
RORβ is a critical transcription factor regulating
rod differentiation. Rods and cones are two different types of
photoreceptor cells. Rods mediate dim light vision while cones
mediate daylight and color vision. The leucine zipper protein Nrl,
which is restricted to be expressed in rod precursors, blocks cone
differentiation when ectopically expressed in cones (42). Jia et al (43) reported that decreased rods and
overproduced cones were observed in RORβ−/− mice, which
were deficient in outer segments and expression of Nrl, and
re-expression of Nrl in RORβ−/− mice converted cones to
rod-like cells. As the upstream regulator of Nrl in the rod
transcriptional pathway, RORβ is a key transcription factor for rod
differentiation. Amacrine and horizontal cells are critical for
integrating visual information. Ptf1a and Foxn4 are two
early-acting factors that are essential for the generation of
amacrine and horizontal cells. RORβ1 has been shown to promote the
differentiation of amacrine and horizontal cells and
synergistically induced expression of Ptf1a with Foxn4. Ectopic
expression of RORβ1 in neonatal retina promoted amacrine cell
differentiation (38).
RORβ and osteogenesis
Osteoblastic bone formation essentially involves
several highly complex processes including osteoblastic
differentiation, maturation and mineralization. Numerous
transcription factors are involved in these processes. RORβ was
identified to act as an osteogenic repressor in regulating bone
formation. It is overexpressed in primary mouse and human bone
tissue, especially in undifferentiated osteoblastic cultures but
downregulated during osteoblastic differentiation. Roforth et
al (44) showed that RORβ was
significantly upregulated (>50-fold) in osteoblastic precursor
cells isolated from the bone marrow of aged osteoporotic mice
(18–22 months old), but markedly downregulated during osteoblastic
differentiation of MC3T3-E1 osteoblasts. The following mechanisms
are considered to be involved when RORβ suppresses osteogenesis
(39,44). i) RORβ inhibits Runx2 activity.
Runx2 is the key transcription factor driving expression of the
osteoblastic phenotype and its deletion in mice results in a
complete deficiency of an ossified skeleton (45). The Runx2 target genes osteocalcin
and osterix are reduced in mouse osteoblastic MC3T3-E1 cells,
however, the exact mechanism regarding how RORβ mediates Runx2
inhibition remains to be determined. It is most likely that the
protein interaction between RORβ and Runx2 inhibits normal
functions of Runx2. ii) Target genes of RORβ disrupt osteoblastic
extracellular matrix (ECM) production. ECM is required for the
deposition of bone mineral by providing a supporting structure and
its production is modulated by the activities of several growth
factors and cytokines, such as transforming growth factor-β (TGF-β)
and bone morphogenetic proteins (BMPs) (46). TGF-β inhibitor decorin (DCN) as
well as the matrix gla protein (MGP), an inhibitor of bone
formation by sequestrating BMP2, are upregulated in RORβ-expressing
cells. These results indicate that RORβ possibly disrupt ECM
production by upregulating the ECM inhibitors. iii) RORβ promotes
cell proliferation by activating the mitogen-activated protein
kinase (MAPK) signaling pathway.
RORβ and tumorigenesis
Relatively less evidence has been accumulated
concerning the association between RORβ and tumors. In a study on
79 patients with endometrial cancer and 12 patients with stage I
serous endometrial cancer, Risinger et al (47) analyzed the transcriptional
expression profile of oligonucleotide microarray from laser
microdissection on epithelial gland cells, and reported that in
endometrial cancer and serous endometrial cancer, RORβ, which
showed a high-level expression in the endometrium in healthy pre-
or post-menopausal women, was significantly downregulated when
compared with the 12 samples of healthy postmenopausal women.
However, Davidson et al (40) found that RORβ was upregulated in
the primary leiomyosarcoma of uterus. Matijevic and Pavelic
(48) demonstrated that Toll-like
receptor 3 (TLR3) suppressed the expression of RORβ in the
metastatic pharyngeal cancer cell line Detroit 562. RORβ was
upregulated by chloroquine inhibiting TLR3 expression and
downregulated by siRNA silencing TLR3, suggesting that the
upregulation of RORβ was TLR3-dependent. In a recent study, we
observed that RORβ was decreased in colorectal cancer when compared
with paralleled para-cancerous tissues, but until recently the
detailed expression levels of RORβ in other tumors are still
largely unelucidated.
The molecular mechanisms of how RORβ affects tumor
formation and progression remain unclear. RORβ has similar
functionality with RORα due to the high homology between ROR
molecules. It has been identified that RORα plays a role in the
regulation of the Wnt pathway; thus, RORβ may be involved in this
process. The Wnt signaling pathway is closely associated with tumor
growth and development, which has been evidenced in multiple
tumors, such as colon, liver, gastric, lung, ovarian, and
endometrial cancer. The Wnt signaling pathway includes canonical
and non-canonical pathways. The canonical Wnt pathway is also known
as the Wnt/β-catenin pathway. Secreted Wnt molecules such as Wnt1,
Wnt3a and Wnt8 bind to the Frizzled (Fzd) and low-density
receptor-related protein 5/6 (LRP5/6) co-receptor, regulate
downstream TCF/LEF family gene transcription, and affect cell
behavior. Under normal circumstances, most of the β-catenin
participating in the cytoskeleton regulation is sequestrated in the
cytoplasm by E-cadherin located on the cell membrane, and the
remaining small component of cytoplasmic β-catenin binds to APC,
GSK3β and Axin. In the absence of canonical Wnt signal, β-catenin
forms degradable complexes with the three molecules, which
activates the phosphorylation of β-catenin and leads to its
degradation by Trcp ubiquitination; thus, β-catenin is maintained
at a low level in the cytoplasm. In the presence of Wnt signal, Wnt
molecules bind to the transmembrane receptor Fzd, which induces its
combination with the intracellular protein Dsv, resulting in the
inactivation of Dsv-GSK3β-APC complexes, preventing β-catenin
ubiqui tination and blocking its degradation. Subsequently, free
β-catenin is accumulated and translocated into the nucleus and
binds with the DNA-binding protein transcription factors to
activate the target gene transcription. The non-canonical Wnt
pathway includes Wnt/Ca2+ and JNK-mediated planar cell
polarity pathway, which is mainly involved in cytoskeleton
rearrangement and cell polarity. Wnt5a and Wnt11 can activate the
Wnt/Ca2+ signaling pathway (49). The non-canonical Wnt pathway
commonly negatively regulates the activity of the canonical Wnt
pathway (50). Lee et al
(51) found that in colorectal
cancer, phosphorylated RORαS35 by PKCα showed an enhanced
combination with β-catenin and was able to bind to the promoter
region of β-catenin, preventing its transcription and reducing the
activity of the Wnt/β-catenin pathway. Wnt5a can also increase the
phosphorylation of RORα and reduce the Wnt3a-induced expression of
cyclin D1 and c-Myc. RORβ and RORα contain four domains, and this
homology suggests that RORβ may share similar tumor-suppressive
mechanisms with RORα by inhibiting the Wnt pathway to affect
tumorigenesis.
RORβ-induced circadian rhythm
abnormalities and tumorigenesis
Circadian rhythms are the daily cycles of
biochemistry, behavioral and physiological changes regulated by the
endogenous circadian clock, which plays an important role in the
physiological function and behavior of the body (52–54). A series of physiological processes
including sleep, body temperature, energy metabolism, cell cycle
and hormone secretion are controlled by circadian rhythms. The
association between circadian rhythm abnormalities and tumori
genesis has drawn increasing attention. Circadian rhythms of
mammals are mainly controlled by hypothalamic SCN and are
independent of the light-sensitive system. Destruction of SCN can
cause rhythm abnormalities in experimental animals and sleep
disorders in patients. Circadian behaviors can be restored in
SCN-ablated rodents following re-implantation of the perinatal SCN
into the brain. Core clock components generally refer to the genes
that are essential for the generation and regulation of circadian
rhythms in individual cells and organisms, which primarily include
the period and cryptochrome families. There are three subtypes of
mammalian period including Per1, Per2 and Per3 and two subtypes of
cryptochrome including Cry1 and Cry2. Transcription factors CLOCK
and BMAL1 form a heterodimer and bind to the E-box region of the
promoter of Per and Cry to promote their transcription and
expression. When Per and Cry reach a certain level, their
transcription mediated by BMAL1-CLOCK complexes is in turn
suppressed by direct interaction. This leads to decreased levels of
Per and Cry and to a new loop of activation and suppression
(55,56). NPAS2, the homolog of CLOCK, can
also form a heterodimer with BMAL1 and activate the transcription
of circadian genes by binding to the E-box sequences (57). Previous findings suggest that
histone deacetylase SIRT1 is involved in the regulation of
circadian rhythms by regulating the activity of the histone
acetyltransferase of CLOCK. SIRT1 is essential for circadian
transcription of several core clock genes, such as Per2,
Cry1, Bmal1 and Rorγ. SIRT1 binds CLOCK-BMAL1
in a circadian manner and promotes the deacetylation and
degradation of Per2 (58,59).
Circadian rhythm abnormalities are associated with
tumorigenesis and tumor development. The IARC suggested that
abnormal circadian rhythm caused by shift work was one of the major
carcinogenic factors leading to human cancers. The effects of
abnormal circadian rhythms on tumorigenesis have been evaluated in
pilots, flight attendants, shift workers and animal experiments.
Epidemiological studies have shown increased incidences of prostate
cancer and acute myeloid leukemia in pilots (60,61). The incidence of endometrial cancer
is much higher in women who work in shifts day and night for >20
years as compared to those who work on normal schedules (62). Similarly, night working women are
also prone to breast cancer (63). It is reported that in colorectal
cancer, the incidence is significantly increased in women working
at night >3 days a month for ≥15 years, and the survival time of
patients with regular rhythms is 5-fold higher than patients with
circadian rhythm disorders (64).
Keith et al (65) have
suggested that circadian rhythm was a more important carcinogenic
factor than the family history of breast cancer.
Evidence has shown that key genes that regulate
circadian rhythms are aberrantly expressed in tumor tissues.
Abnormal expression of the clock gene has been found in tumor
cells, for instance, in breast, ovarian, endometrial and prostate
cancer, while for chronic myelogenous leukemia the expression of
Per/Cry gene is downregulated by the promoter methylation
(60,66,67). In a recent study, it was shown
that circadian genes can function as tumor-suppressor genes
(68). Tumor cell proliferation
was suppressed following the overexpression of Per1 or Per2 and
promoted after these genes were silenced in cultured breast and
prostate cancer cell lines (69,70), although the molecular mechanisms
remain unclear. Genes that regulate circadian regulation are
important in the regulation of cell cycle and apoptosis. Aberrant
gene expression leads to gene instability, accelerates tumor cell
proliferation, and thereby increases the incidence of cancer. It is
believed that cancer is a circadian rhythm disorder-related
disease. Filipski and Levi (71)
found that modulation of the circadian rhythm played a role in the
regulation of liver tumor develop ment. SCN ablation or
experimental chronic jetlag (CJL) promoted tumor growth and CJL
inhibited or altered the expression of cell cycle genes and the
rhythm of the clock gene in rat liver. The incidence of
diethylnitrosamine-induced liver cancer was increased in jet-lagged
mice and meal timing eliminated abnormal rhythms caused by CJL and
retarded tumor growth.
Circadian rhythms are regulated by RORs. Clock genes
including Cry1, BMAL1, CLOCK and NPAS2
are identified to contain ROREs; for example, the promoter region
of BMAL1 contains two ROREs (41,72). RORα and RORγ promote the
transcription of BMAL1 by binding with ROREs, of which RORα4
shows the greatest transcriptional activation effect on BMAL1.
Although there is little evidence supporting the regulatory effects
of RORβ on clock genes, RORs possess structural homology and when
compared with RORα and RORγ, a high expression of RORβ is
intensively confined to the SCN, pineal gland, and retina, which
are the major elements responsible for the regulation of circadian
rhythms. The nighttime peak level of mRNA of RORβ2 has been
detected in the pineal gland and retina whose expression shows a
significant circadian rhythm. Moreover, RORβ−/− mice are
endowed with circadian rhythm abnormalities (24,25). The mechanisms of how RORβ-induced
circadian rhythm abnormalities promote tumorigenesis and tumor
development may become a new direction for future investigations on
tumor etiology.
5. Conclusion
RORβ, as an important member of orphan nuclear
receptor family, plays important regulatory roles in the
maintenance of a variety of physiological processes and
physiological rhythms. Circadian rhythm abnormalities are
increasingly being considered as a novel incentive for
tumorigenesis. Therefore, to elucidate the molecular mechanisms of
RORβ, regulating tumor-associated circadian rhythm abnormalities
may yield important clinical benefits on effective intervention and
blocking of abnormal circadian rhythm-induced tumorigenesis.
Acknowledgments
This study was supported by the grant from the
Science and Technology Agency of Zhejiang province
(2013C33129).
References
|
1
|
Solt LA, Griffin PR and Burris TP: Ligand
regulation of retinoic acid receptor-related orphan receptors:
implications for development of novel therapeutics. Curr Opin
Lipidol. 21:204–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Becker-André M, André E and DeLamarter JF:
Identification of nuclear receptor mRNAs by RT-PCR amplification of
conserved zinc-finger motif sequences. Biochem Biophys Res Commun.
194:1371–1379. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carlberg C, Hooft van Huijsduijnen R,
Staple JK, DeLamarter JF and Becker-André M: RZRs, a new family of
retinoid-related orphan receptors that function as both monomers
and homodimers. Mol Endocrinol. 8:757–770. 1994.PubMed/NCBI
|
|
4
|
Jetten AM and Ueda E: Retinoid-related
orphan receptors (RORs): roles in cell survival, differentiation
and disease. Cell Death Differ. 9:1167–1171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jetten AM, Kurebayashi S and Ueda E: The
ROR nuclear orphan receptor subfamily: critical regulators of
multiple biological processes. Prog Nucleic Acid Res Mol Biol.
69:205–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giguère V: Orphan nuclear receptors: from
gene to function. Endocr Rev. 20:689–725. 1999.PubMed/NCBI
|
|
7
|
Gawlas K and Stunnenberg HG: Differential
transcription of the orphan receptor RORbeta in nuclear extracts
derived from Neuro2A and HeLa cells. Nucleic Acids Res.
29:3424–3432. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gawlas K and Stunnenberg HG: Differential
binding and transcriptional behaviour of two highly related orphan
receptors, ROR alpha(4) and ROR beta(1). Biochim Biophys Acta.
1494:236–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stehlin C, Wurtz JM, Steinmetz A, et al:
X-ray structure of the orphan nuclear receptor RORbeta
ligand-binding domain in the active conformation. EMBO J.
20:5822–5831. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sullivan AA and Thummel CS: Temporal
profiles of nuclear receptor gene expression reveal coordinate
transcriptional responses during Drosophila development. Mol
Endocrinol. 17:2125–2137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palli SR, Ladd TR and Retnakaran A:
Cloning and characterization of a new isoform of Choristoneura
hormone receptor 3 from the spruce budworm. Arch Insect Biochem
Physiol. 35:33–44. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiruma K and Riddiford LM: Differential
control of MHR3 promoter activity by isoforms of the ecdysone
receptor and inhibitory effects of E75A and MHR3. Dev Biol.
272:510–521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flores MV, Hall C, Jury A, Crosier K and
Crosier P: The zebrafish retinoid-related orphan receptor (ror)
gene family. Gene Expr Patterns. 7:535–543. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jetten AM: Recent advances in the
mechanisms of action and physiological functions of the
retinoid-related orphan receptors (RORs). Curr Drug Targets Inflamm
Allergy. 3:395–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Solt LA, Kojetin DJ and Burris TP: The
REV-ERBs and RORs: molecular links between circadian rhythms and
lipid homeostasis. Future Med Chem. 3:623–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tosini G, Davidson AJ, Fukuhara C,
Kasamatsu M and Castanon-Cervantes O: Localization of a circadian
clock in mammalian photoreceptors. FASEB J. 21:3866–3871. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vogel MW, Sinclair M, Qiu D and Fan H:
Purkinje cell fate in staggerer mutants: agenesis versus cell
death. J Neurobiol. 42:323–337. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ino H: Immunohistochemical
characterization of the orphan nuclear receptor ROR alpha in the
mouse nervous system. J Histochem Cytochem. 52:311–323. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang HS, Angers M, Beak JY, et al: Gene
expression profiling reveals a regulatory role for ROR alpha and
ROR gamma in phase I and phase II metabolism. Physiol Genomics.
31:281–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
André E, Gawlas K and Becker-André M: A
novel isoform of the orphan nuclear receptor RORbeta is
specifically expressed in pineal gland and retina. Gene.
216:277–283. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chow L, Levine EM and Reh TA: The nuclear
receptor transcription factor, retinoid-related orphan receptor
beta, regulates retinal progenitor proliferation. Mech Dev.
77:149–164. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baler R, Coon S and Klein DC: Orphan
nuclear receptor RZRbeta: cyclic AMP regulates expression in the
pineal gland. Biochem Biophys Res Commun. 220:975–978. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mühlbauer E, Bazwinsky-Wutschke I, Wolgast
S, Labucay K and Peschke E: Differential and day-time dependent
expression of nuclear receptors RORalpha, RORbeta, RORgamma and
RXRalpha in the rodent pancreas and islet. Mol Cell Endocrinol.
365:129–138. 2013. View Article : Google Scholar
|
|
24
|
André E, Conquet F, Steinmayr M, Stratton
SC, Porciatti V and Becker-André M: Disruption of retinoid-related
orphan receptor beta changes circadian behavior, causes retinal
degeneration and leads to vacillans phenotype in mice. EMBO J.
17:3867–3877. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Masana MI, Sumaya IC, Becker-André M and
Dubocovich ML: Behavioral characterization and modulation of
circadian rhythms by light and melatonin in C3H/HeN mice homozygous
for the RORbeta knockout. Am J Physiol Regul Integr Comp Physiol.
292:R2357–R2367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stehlin-Gaon C, Willmann D, Zeyer D, et
al: All-trans retinoic acid is a ligand for the orphan nuclear
receptor ROR beta. Nat Struct Biol. 10:820–825. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wärnmark A, Treuter E, Wright AP and
Gustafsson JA: Activation functions 1 and 2 of nuclear receptors:
molecular strategies for transcriptional activation. Mol
Endocrinol. 17:1901–1909. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watanabe M, Yanagisawa J, Kitagawa H, et
al: A subfamily of RNA-binding DEAD-box proteins acts as an
estrogen receptor alpha coactivator through the N-terminal
activation domain (AF-1) with an RNA coactivator, SRA. EMBO J.
20:1341–1352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishihara E, O’Malley BW and Xu J: Nuclear
receptor coregulators are new players in nervous system development
and function. Mol Neurobiol. 30:307–325. 2004. View Article : Google Scholar
|
|
30
|
Kurebayashi S, Nakajima T, Kim SC, et al:
Selective LXXLL peptides antagonize transcriptional activation by
the retinoid-related orphan receptor RORgamma. Biochem Biophys Res
Commun. 315:919–927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Greiner EF, Kirfel J, Greschik H, et al:
Differential ligand-dependent protein-protein interactions between
nuclear receptors and a neuronal-specific cofactor. Proc Natl Acad
Sci USA. 97:7160–7165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heery DM, Hoare S, Hussain S, Parker MG
and Sheppard H: Core LXXLL motif sequences in CREB-binding protein,
SRC1, and RIP140 define affinity and selectivity for steroid and
retinoid receptors. J Biol Chem. 276:6695–6702. 2001. View Article : Google Scholar
|
|
33
|
Torchia J, Rose DW, Inostroza J, et al:
The transcriptional co-activator p/CIP binds CBP and mediates
nuclear-receptor function. Nature. 387:677–684. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Glass CK, Rose DW and Rosenfeld MG:
Nuclear receptor coactivators. Curr Opin Cell Biol. 9:222–232.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gabriely G, Kama R, Gelin-Licht R and
Gerst JE: Different domains of the UBL-UBA ubiquitin receptor,
Ddi1/Vsm1, are involved in its multiple cellular roles. Mol Biol
Cell. 19:3625–3637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akashi M and Takumi T: The orphan nuclear
receptor RORalpha regulates circadian transcription of the
mammalian core-clock Bmal1. Nat Struct Mol Biol. 12:441–448. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eberl G, Marmon S, Sunshine MJ, Rennert
PD, Choi Y and Littman DR: An essential function for the nuclear
receptor RORgamma(t) in the generation of fetal lymphoid tissue
inducer cells. Nat Immunol. 5:64–73. 2004. View Article : Google Scholar
|
|
38
|
Liu H, Kim SY, Fu Y, et al: An isoform of
retinoid-related orphan receptor beta directs differentiation of
retinal amacrine and horizontal interneurons. Nat Commun.
4:18132013. View Article : Google Scholar
|
|
39
|
Roforth MM, Khosla S and Monroe DG:
Identification of Rorβ targets in cultured osteoblasts and in human
bone. Biochem Biophys Res Commun. 440:768–773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Davidson B, Abeler VM, Forsund M, et al:
Gene expression signatures of primary and metastatic uterine
leiomyosarcoma. Hum Pathol. 45:691–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jetten AM: Retinoid-related orphan
receptors (RORs): critical roles in development, immunity,
circadian rhythm, and cellular metabolism. Nucl Recept Signal.
7:e0032009.PubMed/NCBI
|
|
42
|
Oh EC, Khan N, Novelli E, Khanna H,
Strettoi E and Swaroop A: Transformation of cone precursors to
functional rod photoreceptors by bZIP transcription factor NRL.
Proc Natl Acad Sci USA. 104:1679–1684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jia L, Oh EC, Ng L, et al:
Retinoid-related orphan nuclear receptor RORbeta is an early-acting
factor in rod photoreceptor development. Proc Natl Acad Sci USA.
106:17534–17539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roforth MM, Liu G, Khosla S and Monroe DG:
Examination of nuclear receptor expression in osteoblasts reveals
Rorbeta as an important regulator of osteogenesis. J Bone Miner
Res. 27:891–901. 2012. View Article : Google Scholar
|
|
45
|
Komori T, Yagi H, Nomura S, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Munger JS, Harpel JG, Gleizes PE, Mazzieri
R, Nunes I and Rifkin DB: Latent transforming growth factor-beta:
structural features and mechanisms of activation. Kidney Int.
51:1376–1382. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Risinger JI, Allard J, Chandran U, et al:
Gene expression analysis of early stage endometrial cancers reveals
unique transcripts associated with grade and histology but not
depth of invasion. Front Oncol. 3:1392013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Matijevic T and Pavelic J: The dual role
of TLR3 in metastatic cell line. Clin Exp Metastasis. 28:701–712.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kimmel AR: An orphan nuclear receptor
finds a home. Mol Cell. 37:155–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
McDonald SL and Silver A: The opposing
roles of Wnt-5a in cancer. Br J Cancer. 101:209–214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee JM, Kim IS, Kim H, et al: RORalpha
attenuates Wnt/beta-catenin signaling by PKCalpha-dependent
phosphorylation in colon cancer. Mol Cell. 37:183–195. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gery S and Koeffler HP: The role of
circadian regulation in cancer. Cold Spring Harb Symp Quant Biol.
72:459–464. 2007. View Article : Google Scholar
|
|
53
|
Kettner NM, Katchy CA and Fu L: Circadian
gene variants in cancer. Ann Med. 46:208–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fu L and Kettner NM: The circadian clock
in cancer development and therapy. Prog Mol Biol Transl Sci.
119:221–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ueda HR, Hayashi S, Chen W, et al:
System-level identification of transcriptional circuits underlying
mammalian circadian clocks. Nat Genet. 37:187–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shearman LP, Sriram S, Weaver DR, et al:
Interacting molecular loops in the mammalian circadian clock.
Science. 288:1013–1019. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Reick M, Garcia JA, Dudley C and McKnight
SL: NPAS2: an analog of clock operative in the mammalian forebrain.
Science. 293:506–509. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nakahata Y, Kaluzova M, Grimaldi B, et al:
The NAD+-dependent deacetylase SIRT1 modulates
CLOCK-mediated chromatin remodeling and circadian control. Cell.
134:329–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Asher G, Gatfield D, Stratmann M, et al:
SIRT1 regulates circadian clock gene expression through PER2
deacetylation. Cell. 134:317–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rana S and Mahmood S: Circadian rhythm and
its role in malignancy. J Circadian Rhythms. 8:32010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pukkala E, Aspholm R, Auvinen A, et al:
Cancer incidence among 10,211 airline pilots: a Nordic study. Aviat
Space Environ Med. 74:699–706. 2003.PubMed/NCBI
|
|
62
|
Viswanathan AN, Hankinson SE and
Schernhammer ES: Night shift work and the risk of endometrial
cancer. Cancer Res. 67:10618–10622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Koppes LL, Geuskens GA, Pronk A, Vermeulen
RC and de Vroome EM: Night work and breast cancer risk in a general
population prospective cohort study in The Netherlands. Eur J
Epidemiol. 29:577–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Schernhammer ES, Laden F, Speizer FE, et
al: Night-shift work and risk of colorectal cancer in the nurses’
health study. J Natl Cancer Inst. 95:825–828. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Keith LG, Oleszczuk JJ and Laguens M:
Circadian rhythm chaos: a new breast cancer marker. Int J Fertil
Womens Med. 46:238–247. 2001.PubMed/NCBI
|
|
66
|
Zhu Y, Stevens RG, Hoffman AE, et al:
Epigenetic impact of long-term shiftwork: pilot evidence from
circadian genes and whole-genome methylation analysis. Chronobiol
Int. 28:852–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shih MC, Yeh KT, Tang KP, Chen JC and
Chang JG: Promoter methylation in circadian genes of endometrial
cancers detected by methylation-specific PCR. Mol Carcinog.
45:732–740. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hwang-Verslues WW, Chang PH, Jeng YM, et
al: Loss of core-pressor PER2 under hypoxia up-regulates
OCT1-mediated EMT gene expression and enhances tumor malignancy.
Proc Natl Acad Sci USA. 110:12331–12336. 2013. View Article : Google Scholar
|
|
69
|
Yang X, Wood PA, Oh EY, Du-Quiton J,
Ansell CM and Hrushesky WJ: Down regulation of circadian clock gene
Period 2 accelerates breast cancer growth by altering its daily
growth rhythm. Breast Cancer Res Treat. 117:423–431. 2009.
View Article : Google Scholar
|
|
70
|
Gery S, Virk RK, Chumakov K, Yu A and
Koeffler HP: The clock gene Per2 links the circadian system to the
estrogen receptor. Oncogene. 26:7916–7920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Filipski E and Levi F: Circadian
disruption in experimental cancer processes. Integr Cancer Ther.
8:298–302. 2009. View Article : Google Scholar
|
|
72
|
Kumaki Y, Ukai-Tadenuma M, Uno KD, et al:
Analysis and synthesis of high-amplitude Cis-elements in the
mammalian circadian clock. Proc Natl Acad Sci USA. 105:14946–14951.
2008. View Article : Google Scholar : PubMed/NCBI
|