Introduction
Retinal neovascularization (RNV) is a characteristic
pathological finding of a number of retinal diseases, including
retinopathy of prematurity (ROP), proliferative diabetic
retinopathy (PDR) and retinal vein occlusion (RVO) (1,2).
ROP is a leading cause of vision impairment and blindness in
childhood (3,4). If left untreated, it can lead to
retinal fibrovascularization, and can ultimately cause vitreous
hemorrhage, tractional retinal detachment and vision loss (5). For many years, the treatment of RNV
was limited to laser therapy (6)
and cryotherapy (7) on the
avascular zone, which, while effectively reducing severe vision
loss, also led to serious side-effects (7). Thus, it is important to study RNV
pathogenesis and to develop novel therapies. It is clear from
previous research that some progress has been made in relation to
the pharmacological inhibition of proangiogenic factors (8–11).
However, further investigations are required to provide effective
and non-invasive treatment strategies.
The matricellular protein, CCN family member 1
(CCN1), also known as the cysteine-rich protein 61 (Cyr61), is an
extracellular matrix (ECM)-associated immediate early gene-encoded
protein (12) whose expression is
highly restricted and dynamic at sites of vascularization and
skeletogenesis during development and pathological states (13). CCN1/Cyr61 was the first cloned
member of the CCN family (14–16) and has been reported to mediate
cell adhesion, stimulate chemotaxis, increase growth factor-induced
DNA synthesis, improve cell survival and enhance angiogenesis
(17). CCN1 can also modulate the
activities of several ECM proteins, growth factors and cytokines,
including transforming growth factor-β (TGF-β), tumor necrosis
factor-α (TNF-α), vascular endothelial growth factor (VEGF),
hypoxia-inducible factor-1α (HIF-1α) and bone morphogenetic
proteins (18,19).
The phosphoinositide 3-kinase (PI3K)/AKT signaling
pathway is essential to angiogenesis (20). Recent research has demonstrated
that CCN1/Cyr61 induces the expression of monocyte chemoattractant
protein-1 (MCP-1) through the activation of the focal adhesion
kinase (FAK), PI3K/AKT and nuclear factor (NF)-κB signaling
pathways in chorioretinal vascular endothelial cells (21). In addition, a previous study
indicated that the PI3K/AKT pathway is required for the
hypoxia-induced expression of HIF-1α and VEGF in choroidal
neovascularization (20). It has
also been suggested that targeting the PI3K/AKT pathway may be a
possible treatment strategy for RNV (22).
Therefore, in the present study, we hypothesized
that the CCN1/Cyr61-PI3K/AKT signaling pathway may promote retinal
angiogenesis in ROP. In order to confirm this hypothesis, we
investigated the angiogenic effects of CCN1/Cyr61 in human
umbilical vein endothelial cells (HUVECs). In addition,
CCN1/Cyr61-PI3K/AKT signaling in the retina was evaluated using a
mouse pup model of oxygen-induced retinopathy (OIR).
Materials and methods
siRNA vector construction
siRNA targeting CCN1 (CCN1 siRNA) and scrambled
siRNA were purchased from GenePharma Co., Ltd. (Shanghai, China),
and used according to previously published methods (23,24). The sequences were as follows: CCN1
(Cyr61-homo-553) sense, 5′-GGGAAAGUUUCCAGCCCAACUTT-3′ and
antisense, 5′-AGUUGGGCUGGAAACUUUCCCTT-3′; CCN1 (Cyr61-homo-789)
sense, 5′-GAGGUGGAGUUGACGAGAAACTT-3′ and antisense,
5′-GUUUCUCGUCAACUCCACCUCTT-3′; CCN1 (Cyr61-homo-1072) sense,
5′-GCAAGAAAUGCAGCAAGACCATT-3′ and antisense,
5′-UGGUCUUGCUGCAUUUCUUGCTT-3′; CCN1 (Cyr61-homo-1268) sense,
5′-GAUGAUCCAGUCCUGCAAAUGTT-3′ and antisense,
5′-CAUUUGCAGGACUGGAUCAUCTT-3′; and scrambled siRNA sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. The siRNA were cloned into the
pGPU6/GFP/Neo siRNA expression vector kit (GenePharma, Ltd.) to
create the pGPU6/GFP/Neo-Cyr61 siRNA and the
pGPU6/GFP/Neo-scrambled siRNA plasmids. The plasmids contained the
Bbs1 and BamH1 restriction sites. All plasmids
contained green fluorescent protein (GFP). The Cyr61-homo-1072
construct yielded the optimal results (data not shown), and was
thus used in the following experiments.
Cell culture and exposure to hypoxia
HUVECs were purchased from Cell Systems (Kirkland,
WA, USA) and cultured in Dulbecco's minimum essential medium (DMEM;
HyClone, Logan, UT, USA) with 10% fetal bovine serum (FBS; HyClone,
Thermo Fisher Scientific, Waltham, MA, USA) in a 37°C humidified
atmosphere containing 95% air and 5% CO2. Subconfluent
monolayers of HUVECs from passages 3 to 10 were used in the
following experiments.
The HUVECs were subsequently divided into the
normoxia (20% O2/5% CO2/75% N2)
and hypoxia groups (1% O2/5% CO2/94%
N2). The hypoxia group was further subdivided into the
hypoxia group, the hypoxia-scrambled siRNA group (transiently
transfected with scrambled siRNA) and the hypoxia-CCN1 siRNA group
(transiently transfected with CCN1 siRNA). The plasmids (500
ng/µl) were transiently transfected into the HUVECs using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
The PI3K/AKT inhibitor, LY294002, was used to treat
the cells exposed to hypoxia to determine the effects of inhibiting
this pathway. The cells were cultured under hypoxic conditions in
the presence of LY294002 (Sigma, St. Louis, MI, USA) (40
µmol/l, dissolved in DMSO; the final concentration of DMSO
in the cell culture was 0.1%). The cells were treated with LY294002
for 30 min before being placed in the incubator. An apoptosis
assay, western blot analysis and reverse-transcription quantitative
PCR (RT-qPCR) were performed after 24 h of exposure to hypoxia.
Cell proliferation assay
The cell counting kit-8 (CCK-8) assay (C0038;
Beyotime, Jiangsu, China) was used to evaluate cell proliferation.
The HUVECs were plated in 96-well plates at a density of 2,000
cells/well. Cell proliferation was evaluated each day for 4 days
following transfection. CCK-8 (10 µl) was added to each well
followed by incubation for 2 h at 37°C. After 10 min of vortexing,
the absorbance was measured in a micro-plate reader (Sunrise RC;
Tecan, Mannedorf, Switzerland) at 450 nm.
Apoptosis detection by flow
cytometry
Apoptosis was measured using a fluorescein
isothiocyanate (FITC) Annexin V Apoptosis Detection kit (BD
Biosciences, San Diego, CA, USA) according to the manufacturer's
instructions. The HUVECs were collected, washed in cold
phosphate-buffered saline (PBS), and labeled with 5 µl
Annexin V-FITC and 5 µl propidium iodide (PI), and then
incubated for 15 min at room temperature in the dark. Flow
cytometry was immediately performed and the cells were analyzed
using CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
Annexin V was set as the horizontal axis and PI was set as the
vertical axis. Mechanically damaged cells were located in the upper
left quadrant, late apoptotic or necrotic cells in the upper right
quadrant, dual-negative and normal cells in the lower left
quadrant, and early apoptotic cells in the lower right quadrant of
the flow cytometry dot plot. The apoptotic rate was calculated as
the ratio of early and late apoptotic cells to total cells, as
previously described (25,26).
Immunofluorescence staining
The HUVECs were washed twice with PBS and fixed with
4% paraformaldehyde for 30 min. The cells were then washed with
0.1% Triton X-100 for 10 min and twice with PBS, and were incubated
with 1% bovine serum albumin (BSA) at room temperature for 1 h. The
cells were incubated overnight with the following commercially
available primary antibodies: rabbit anti-Cyr61 polyclonal antibody
(ab24448; Abcam, Cambridge, UK), mouse anti-p-PI3K monoclonal
antibody (sc-12929), or rabbit anti-p-AKT1/2/3 (Ser473) polyclonal
antibody (sc-101629) (both from Santa Cruz Biotechnology Inc.,
Santa Cruz, CA, USA). The cells were washed thrice with PBS. The
primary antibodies were replaced by isotype controls, which were
used as negative controls. The cells were then treated with
fluorescence-conjugated secondary antibody [FITC-conjugated
AffiniPure rabbit anti-goat IgG (H+L; ZF-0314), or
tetramethylrhodamine (TRITC)]-conjugated AffiniPure goat anti-mouse
IgG (H+L; ZF-0313) (Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China) for 1 h and counter-stained with 0.5 µg/ml
4′,6-diamidino-2-phenylindole (DAPI; Beyotime Institute of
Biotechnology, Jiangsu, China) for 5 min. Images were digitally
captured using a confocal laser scanning microscope (FV1000;
Olympus Corp., Tokyo, Japan).
Animals
Specific pathogen-free healthy C57BL/6J newborn mice
(female or male; n=240; and their mothers) were obtained from the
Animal Laboratory of China Medical University, Shenyang, China and
were housed with their mothers. The room temperature was maintained
at 23±2°C. Light did not exceed 300 lux on a 12 h light-dark
cycle.
All procedures and animal experiments were approved
by the Animal Care and Use Committee of the China Medical
University.
OIR
OIR was induced in the C57BL/6J mice as previously
described in the study by Smith et al (27). Briefly, on postnatal day (P)7, the
pups and their mothers were placed in homemade glass containers
coupled to an RSS-5100 oxygen analyzer (Rex Xinjing Instrument Co.,
Ltd., Shanghai, China). The mice were exposed to hyperoxia (75±2%
O2) for 5 days (P7-P12), and were then re-exposed to
normoxia (room air) for 5 days. The rationale of exposing mice to
hyperoxia and then to normoxia was to emulate a state of relative
hypoxia. Neovascularization occurred when the mice re-exposed to
normoxia and peaked at P17, as previously observed (27). The mice were randomly divided into
4 groups: the normoxia, hyperoxia, hyperoxia-scrambled siRNA and
hyperoxia-CCN1 siRNA groups (n=60/group).
In the normoxia group, the newborn mice were
maintained in room air from P0 to P17. In the hyperoxia group, OIR
was induced by the mice being exposed to hyperoxia (75±2%
O2) for 5 days (P7–P12) and then re-exposed to normoxia
(room air) for 5 days (P12–P17). The same OIR induction protocol
was used in the hyperoxia-scrambled siRNA and hyperoxia-CCN1 siRNA
groups. The mice were administered an intravitreal injection of 1
µl (500 ng/µl) of the scrambled siRNA plasmid or the
CCN1 siRNA plasmid on P11 using a 33-gauge needle attached to a
Hamilton syringe, and were returned to room air on P12, as
previously described (28,29).
The mice in all 4 groups were anesthetized by an intraperitoneal
injection of ketamine hydrochloride (100 mg/kg body weight), and
were then sacrificed by decapitation on P17 in order to collect the
retinas for morphological and pathological examinations, as well as
for mRNA and protein expression analyses. In the present study, the
percentage of GFP-positive cells in the retina was used to assess
the transfection rate 1 day after the intravitreal injection of
siRNA. The transfection rate was approximately 80%. In addition,
after examination it was clear that transfection did not cause
endophthalmitis or retinal detachment (data not shown), suggesting
that the gene transfer was successful and did not affect the
eyes.
Observation of RNV
Retinal vascular patterns were assessed on P17, as
previously described (30). The
eyes were enucleated and fixed with 4% paraformaldehyde for 3 h.
The retinas were then dissected, flat-mounted through 4 incisions
in the center of the disc to divide them into 4 quadrants, as
previously described (31) and
processed for magnesium-activated adenosine diphosphatease (ADPase)
staining. The ADPase-stained retinas were then flat-mounted on
microscope slides with a gelatin-coated cover slip and carefully
examined using an Olympus B201 optical microscope (Olympus Corp.).
For neovascularization grading evaluation under the microscope,
each retina was divided into 12-h clocks in order to assess the
neovascularization clock hour scores, as previously described
(32–34). Three independent reviewers blinded
to grouping assessed the clock hour scores in order to assess the
severity of neovascularization.
Quantification of preretinal
neovascularization
To quantify preretinal neovascularization, the
retinal structures were analyzed on 6-µm hematoxylin and
eosin (H&E)-stained sections, as previously described (35). Briefly, the eyes were enucleated,
fixed and embedded in paraffin. Serial sections (6-µm-thick)
of whole eyes were cut sagittally, through the cornea and parallel
to the optic nerve, and stained with H&E. Vascular cell nuclei,
identified under a light microscope (Olympus B201, Olympus Corp.),
were considered to be associated with new vessels if they were
found on the vitreal side of the internal limiting membrane (ILM),
as previously described (35).
Three independent reviewers blinded to grouping counted the
cells.
Immunohistochemistry
Immunohistochemistry was performed using an SABC
immunohistochemistry kit (Boster Bioengineering Co., Wuhan, China).
Formalin-fixed, paraffin-embedded eye tissue sections
(6-µm-thick) were placed on slides, deparaffinized in xylene
and rehydrated by incubation in graded ethanol baths in PBS.
Endogenous peroxidase was blocked with 3% hydrogen peroxide in
methanol. The sections were then treated with 10% normal goat serum
and incubated overnight with the following commercially available
primary antibodies: rabbit anti-Cyr61 polyclonal antibody (ab24448;
Abcam), mouse anti-p-PI3K monoclonal antibody (sc-12929), or rabbit
anti-p-AKT1/2/3 (Ser473) polyclonal antibody (sc-101629) (both from
Santa Cruz Biotechnology, Inc.) at 4°C. The sections were incubated
with biotinylated horse secondary antibody againts mouse IgG
(ZB-2020; Zhongshan Jinqiao Biotechnology Co., Ltd.) and reacted
with the avidin-biotinylated peroxidase complex. The primary
antibody was replaced with PBS for the negative controls, and
3,3′-diaminobenzidine (DAB) was used as the chromogen. The sections
were counterstained with hematoxylin, dehydrated and mounted.
Images were digitally captured using an Olympus B201 optical
microscope (Olympus).
Western blot analysis
The HUVECS and retinal tissue homogenates were lysed
using 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% Nonidet P-40, 0.5%
sodium deoxycholate and phenylmethylsulfonyl fluoride (PMSF) (all
from Sigma). Cell debris were removed by centrifugation at 12,000
rpm, 4°C, for 20 min. Protein levels were determined by
bicinchoninic acid (BCA) assay (Beyotime Institute of
Biotechnology).
For western blot analysis, protein samples (30
µg) were run on 10% sodium dodecyl sulfate
(SDS)-polyacrylamide gels and transferred onto polyvinylidene
fluoride (PVDF) membranes (Millipore, Billerica, MA, USA).
Non-specific sites on each blot were blocked with 5% BSA diluted in
TBS with 0.05% Tween-20 (TBST) for 2 h. The membranes were
incubated with specific primary antibodies overnight at 4°C [rabbit
anti-Cyr61 polyclonal antibody (ab24448; Abcam), or mouse
anti-p-PI3K monoclonal antibody (sc-12929) or rabbit
anti-p-AKT1/2/3 (Ser473) polyclonal antibody (sc-101629) (Santa
Cruz Biotechnology, Inc.)], followed by incubation with horseradish
peroxidase-conjugated secondary antibody (ZB-5305; Zhongshan
Jinqiao Biotechnology Co., Ltd.) for 60 min. Intensive washing was
performed between incubations. Signals were detected by enhanced
chemiluminescence (Pierce Biotechnology, Rockford, IL, USA).
Protein levels were determined by densitometric scanning of the
protein bands using a GIS-2020 image-processing system (TechNew
Group Co., Ltd., Shanghai, China) and normalized to the intensity
of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) band (using
a rabbit anti-mouse GAPDH polyclonal antibody, bsm-0978M;
Biosynthesis Biotechnology Co., Ltd., Beijing, China).
RT-qPCR
Total RNA was extracted from the HUVECs and retinas
using TRIzol reagent (Invitrogen). The RNA purity was determined
using absorbance at 260 and 280 nm (A260/280). cDNA synthesis was
performed using a reverse transcriptase kit (PrimeScript™ RT
Reagent kit-Perfect Real-Time; Takara Bio, Otsu, Japan), according
to the manufacturer's instructions. The primer sequences used in
the HUVECs and retinas are presented in Table I. Quantitative (real-time) PCR was
performed using the SYBR-Green PCR Master Mix (Premix Ex
Taq™-Perfect Real-Time; Takara Bio) in a total volume of 20
µl on a 7300 Real-Time PCR System (Applied Biosystems,
Foster City, CA, USA). All reactions involved an initial
denaturation at 95°C for 30 sec followed by 50 cycles of 95°C for 5
sec and 60°C for 31 sec. GAPDH was used as the reference gene. The
Ct value was defined as the number of PCR cycles in which the
fluorescence signal exceeded the detection threshold value. First,
the ΔCt value was calculated as follows: Ct gene - Ct GAPDH.
Subsequently, the ΔΔCt value was calculated as follows: ΔCt treated
- ΔCt control. Lastly, the 2−ΔΔCt value was calculated
to represent the relative mRNA expression of target genes, as
previously described (36).
 | Table IPrimer sequences for used for
RT-qPCR. |
Table I
Primer sequences for used for
RT-qPCR.
| Gene | Primer sequences
(5′→3′) | Product length
(bp) | Temperature
(°C) |
|---|
| Against HUVECs
GAPDH | F:
GCACCGTCAAGGCTGAGAAC | 138 | 60 |
| R:
TGGTGAAGACGCCAGTGGA | | |
| AKT | F:
TTGCTTTCAGGGCTGCTCA | 230 | 60 |
| R:
TCTTGGTCAGGTGGTGTGATG | | |
| PI3K | F:
CGGTGACTGTGTGGGACTTA | 116 | 60 |
| R:
ACTGATGTAGTGTGTGGCTGT | | |
| Cyr61 | F:
CGAGGTGGAGTTGACGAGAA | 211 | 60 |
| R:
GCACTCAGGGTTGTCATTGGT | | |
| Against mouse
retina GAPDH | F:
CCCATCTATGAGGGTTACGC | 150 | 55 |
| R:
TTTAATGTCACGCACGATTTC | | |
| AKT | F:
AGCAAACAGGCTCACAGGTT | 245 | 55 |
| R:
TAAGTCCTCCCCATGTCCCT | | |
| PI3K | F:
GGCTTGGACCGAATGCT | 143 | 55 |
| R:
TTGTTGAAGGCTGTGGC | | |
| Cyr61 | F:
AGACCCTGTGAATATAACTCCA | 300 | 55 |
| R:
AATTGCGATTAACTCATTGTT T | | |
Statistical analysis
SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA)
was used for statistical analysis. Data are expressed as the means
± standard deviation (SD) of 3 independent experiments. Statistical
significance was evaluated by one-way analysis of variance (ANOVA)
with Fisher's least significant difference (LSD) test for post hoc
analysis. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Silencing of CCN1 by CCN1 siRNA inhibits
HUVEC proliferation and induces HUVEC apoptosis under hypoxic
conditions
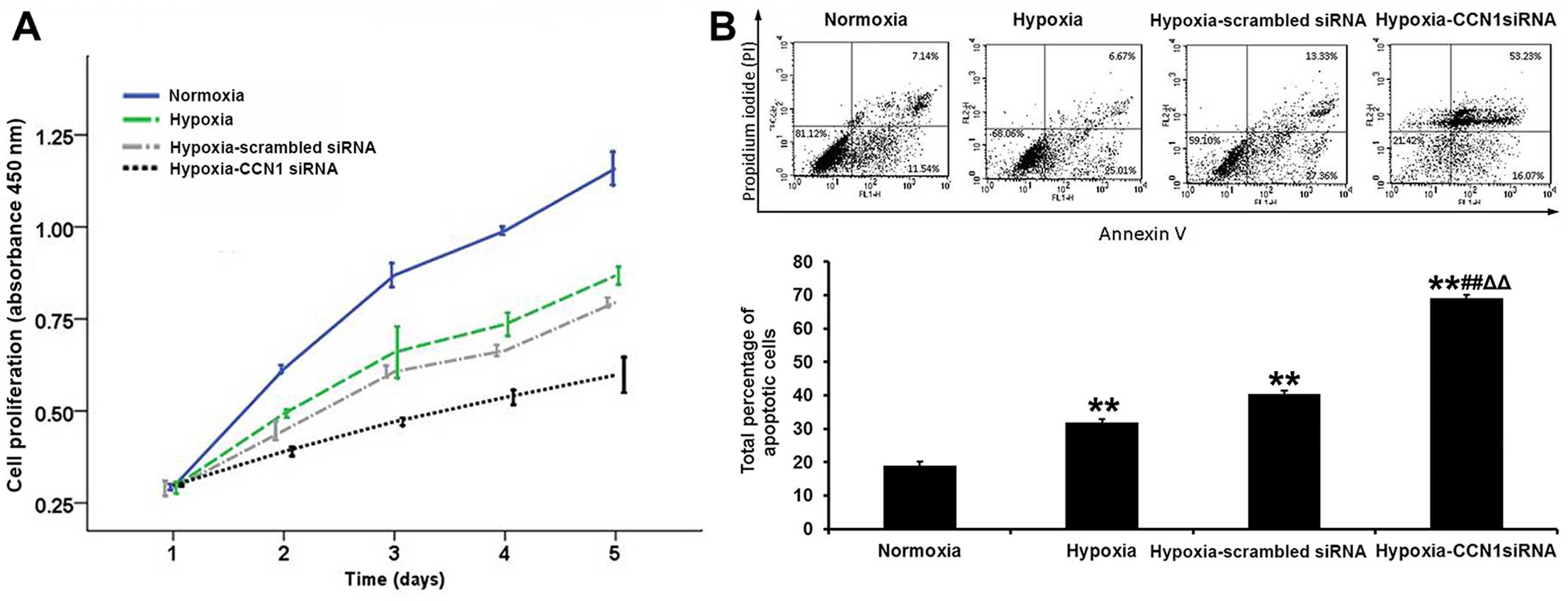
The first step of angiogenesis is endothelial cell
proliferation. The cell growth curves of 24, 48, 72 and 96 h, as
assessed by the CCK-8 method, indicated that the growth rate was
slower in the hypoxia-CCN1 siRNA group than in the hypoxia and
hypoxia-scrambled siRNA groups Fig.
1A). An FITC Annexin V apoptosis detection kit was used to
evaluate the effects of CCN1 siRNA on early and late apoptosis in
the HUVECs. As shown in Fig. 1B,
the early apoptotic rate was decreased, but the late apoptotic rate
was significantly increased in the hypoxia-CCN1 siRNA group
compared with the hypoxia-scrambled siRNA group (total apoptotic
rate, 69.1±1.1 vs. 40.4±1.0%, P<0.01; Fig. 1B). These results indicated that
transfection of the cells with CCN1 siRNA exerted more prominent
anti-proliferative and pro-apoptotic effects on the HUVECs.
Silencing of CCN1 by CCN1 siRNA inhibits
HUVEC proliferation under hypoxic conditions by inhibiting PI3K/AKT
signaling
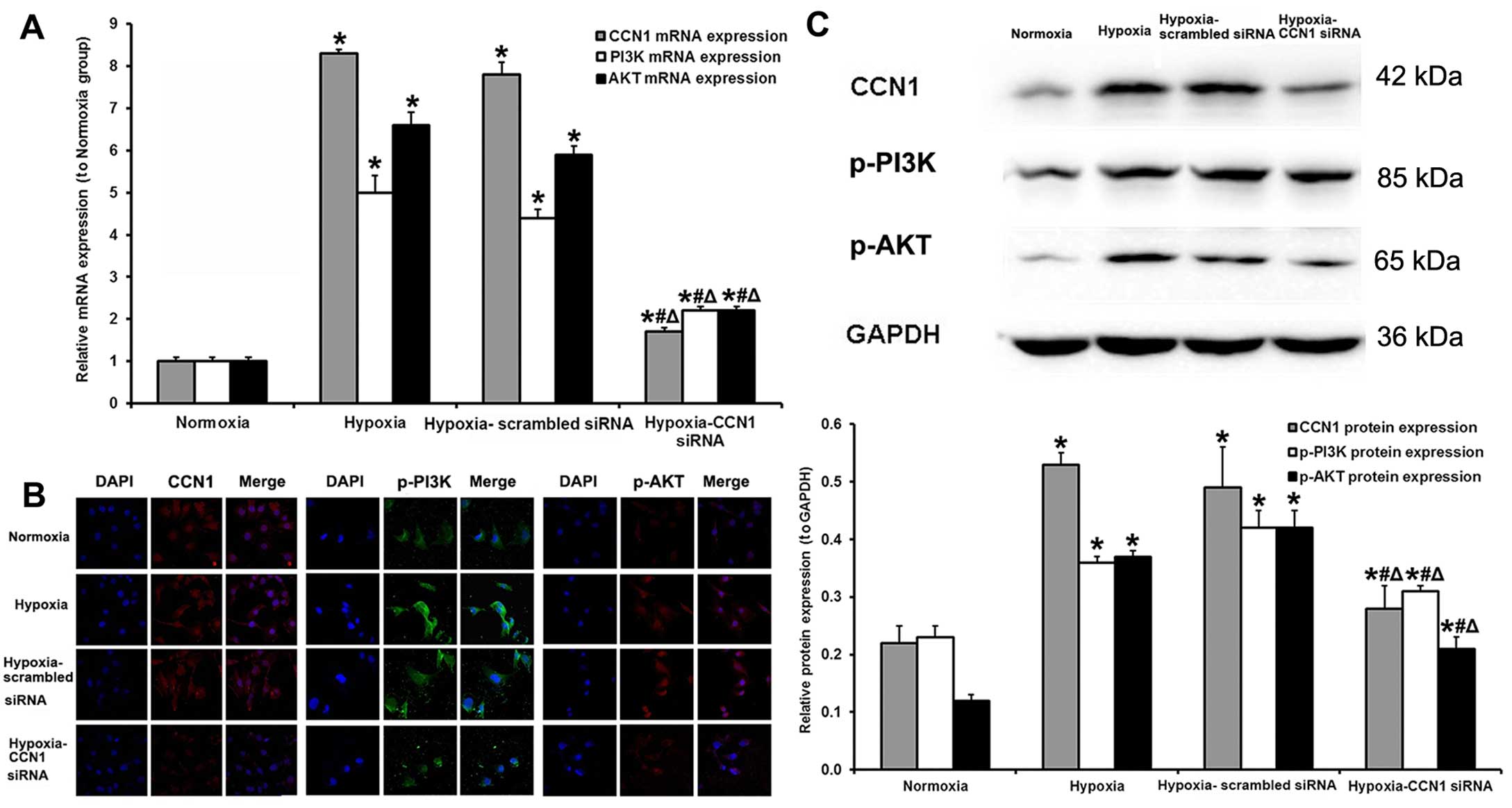
Transfection with CCN1 siRNA decreased the mRNA
expression levels of all 3 factors (CCN1, PI3K and AKT). Compared
with the hypoxia-scrambled siRNA group, the mRNA levels of CCN1,
PI3K and AKT in the hypoxia-CCN1 siRNA group were decreased by
78.2, 50.0 and 62.7%, respectively (Fig. 2A). Immunofluorescence staining
(Fig. 2B) and western blot
analysis (Fig. 2C) revealed that
the protein levels of CCN1, p-PI3K and p-AKT were higher in the
hypoxia (protein, 0.53±0.02, 0.36±0.01 and 0.37±0.01, respectively)
and hypoxia-scrambled siRNA (protein, 0.49±0.07, 0.42±0.03 and
0.42±0.03, respectively) groups compared with the normoxia group
(protein, 0.22±0.03, 0.23±0.02 and 0.12±0.01, respectively; all
P<0.05); however, no significant differences were observed
between the hypoxia and hypoxia-scrambled siRNA groups (all
P>0.05). In addition, the CCN1, p-PI3K and p-AKT protein
expression levels were decreased in the hypoxia-CCN1 siRNA group
compared with the hypoxia and hypoxia-scrambled siRNA groups (all
P<0.05). Compared with the hypoxia-scrambled siRNA group, the
CCN1, p-PI3K and p-AKT protein levels were decreased by 42.9, 26.2
and 50.0%, respectively (all P<0.05; Fig. 2C).
PI3K/AKT inhibition decreases CCN1
expression
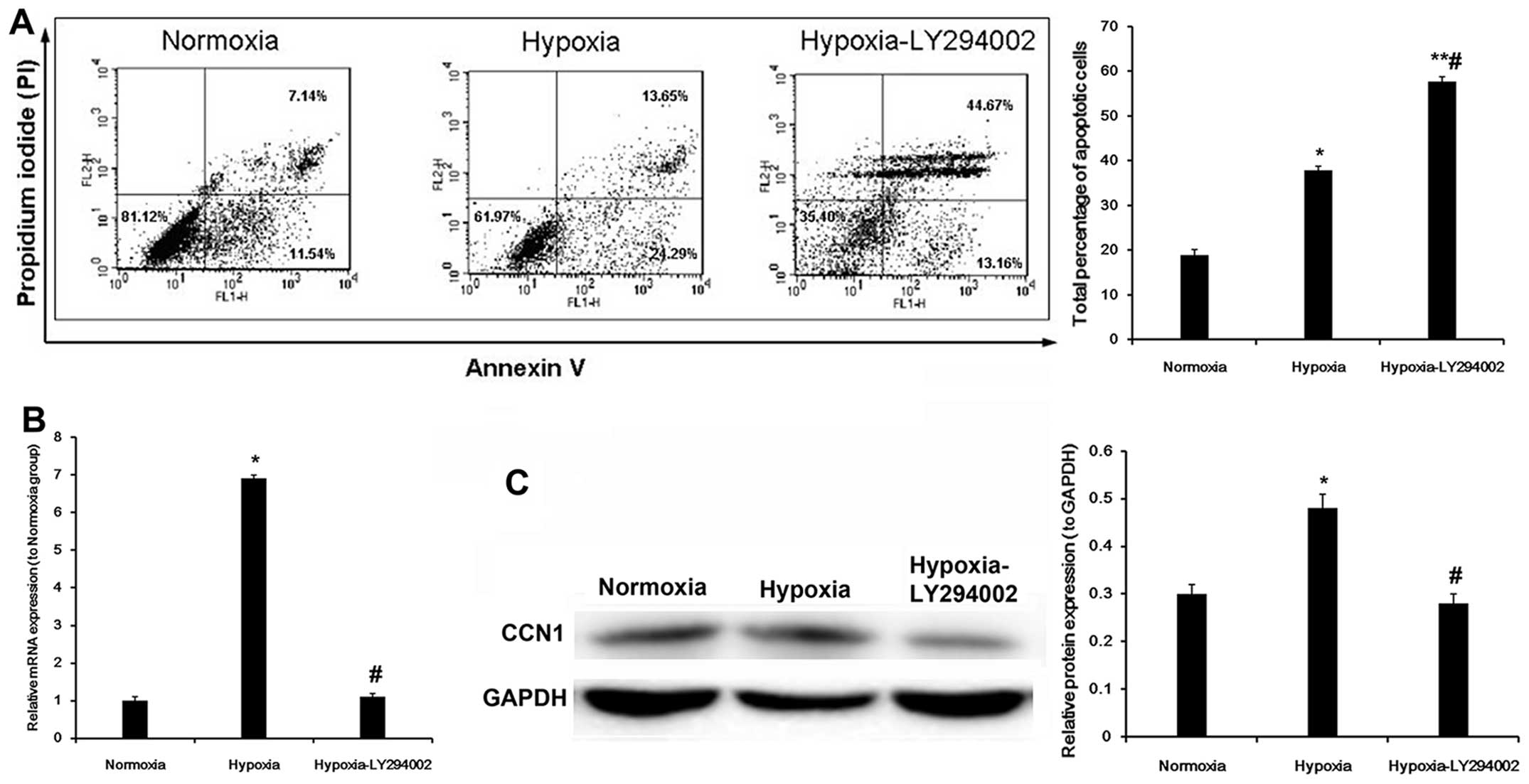
We performed an experiment using LY294002, an
inhibitor of the PI3K/AKT pathway. The results revealed that the
early apoptotic rate was decreased, but that the late apoptotic
rate was significantly increased in the hypoxia-LY294002 group
(total apoptotic rate, 58.1±1.2 vs. 37.9±1.5%, P<0.05) (Fig. 3A). Compared with the hypoxia
group, the mRNA expression of CCN1 in the hypoxia-LY294002 group
was downregulated by 84.1% (P<0.05; Fig. 3B). Compared with the hypoxia
group, treatment with LY294002 decreased CCN1 protein expression in
the cells exposed to hypoxia (P<0.05; Fig. 3C). These results suggest that a
PI3K/AKT inhibitor may be used to decrease CCN1 expression, and
that this process involves an autocrine loop.
Silencing of CCN1 by CCN1 siRNA inhibits
RNV in a mouse pup model of OIR
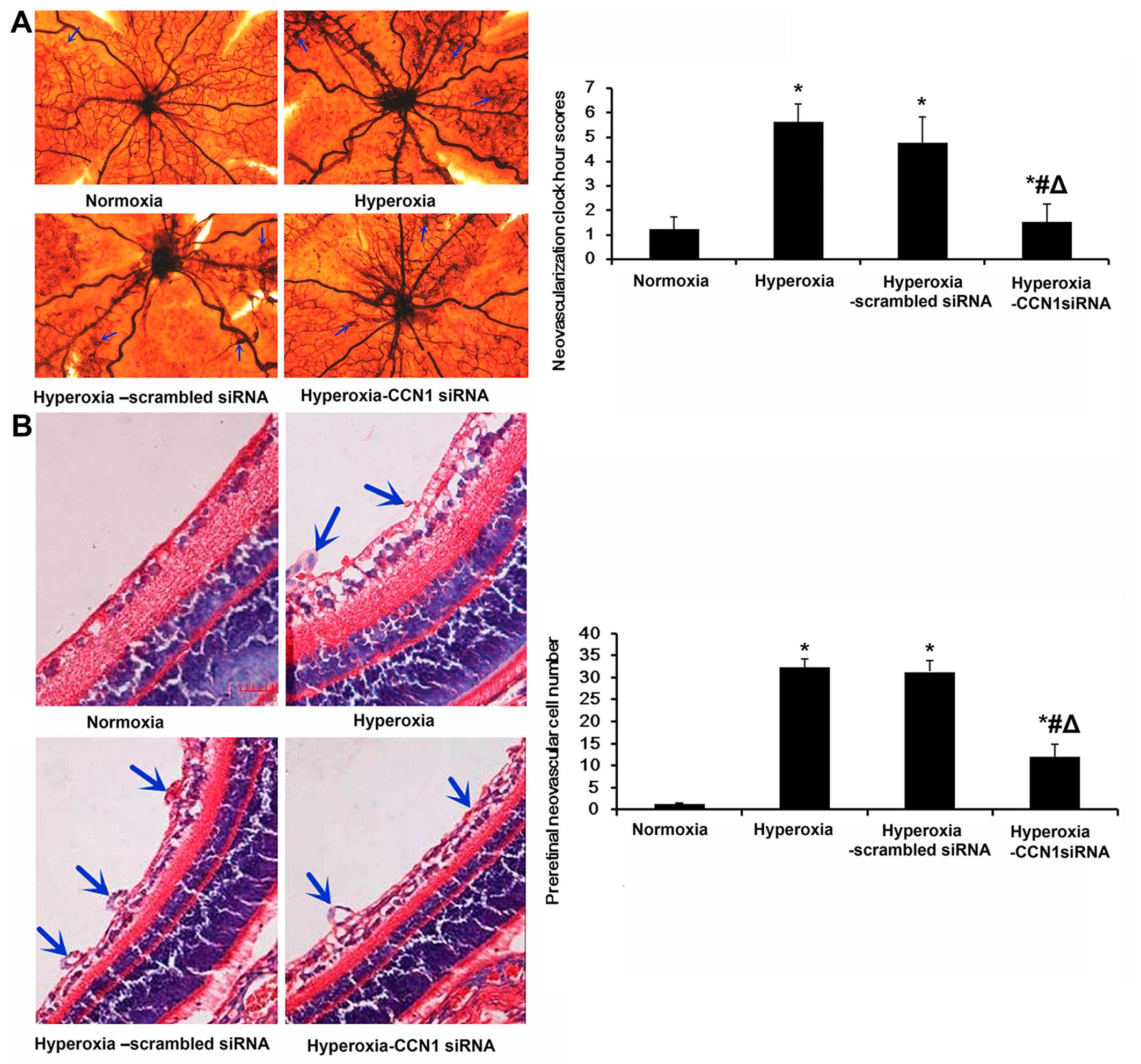
To determine whether the silencing of CCN1 using
CCN1 siRNA suppresses oxygen-induced ischemic RNV, we examined the
retinal vasculature using an ADPase assay in retinal flat-mounts on
P17. In our model of OIR, in the mice treated with CCN1 siRNA,
alterations in vessel morphology and distribution were observed (in
the flat mount image; Fig. 4A).
Compared with the hyperoxia group (5.60±0.73), the retinas from the
hyperoxia-CCN1 siRNA group had less severe neovascular tufts and
regions of non-perfusion, vascular tortuosity and less irregular
expansion (1.53±0.72, P<0.05); these values were still slightly
higher than in the normoxia group (1.23±0.49, P<0.05), but much
lower than in the hyperoxia-scrambled siRNA group (4.76±1.04,
P<0.05) (Fig. 4A).
To further confirm the effects of CCN1 siRNA on RNV,
we quantified the number of preretinal neovascular cells, a
characteristic of OIR (37).
Preretinal neovascular cells growing in the vitreous humor were
counted on 10 non-continuous cross-sections from each eye,
according to a previously established method (35). As shown in Fig. 4B, the numbers of preretinal
neovascular cells in the retinas from the hyperoxia group
(32.5±1.8) and the hyperoxia-scrambled siRNA group (31.4±2.6) were
significantly higher than those in the retinas from the normoxia
group (1.3±0.2) (both P<0.05; Fig.
4B). Moreover, the numbers of preretinal neovascular cells in
the hyperoxia-CCN1 siRNA group (12.0±2.8) were significantly lower
than those in the retinas from the hyperoxia and
hyperoxia-scrambled siRNA groups (both P<0.05), confirming the
anti-neovascularization effects of the silencing of CCN1 (by CCN1
siRNA) on the retina.
Silencing of CCN1 by CCN1 siRNA inhibits
RNV by inhibiting PI3K/AKT signaling in a mouse pup model of
OIR
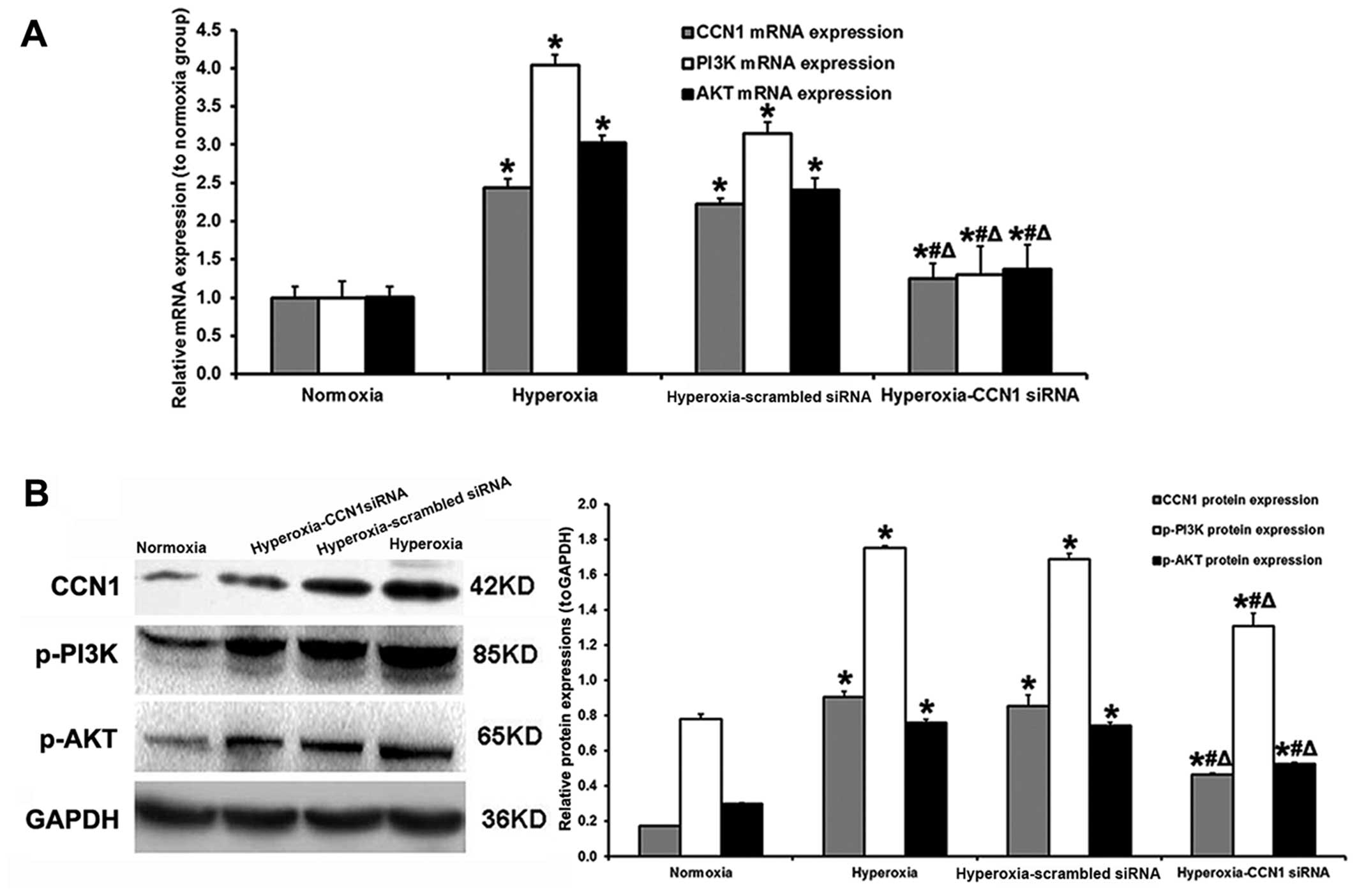
RT-qPCR was used to measure the CCN1, PI3K and AKT
mRNA expression levels in the retinal samples. In the hyperoxia and
hyperoxia-scrambled siRNA groups, the CCN1 (+244 and +122%,
respectively), PI3K (+404 and +215%, respectively) and AKT (+202
and +140%, respectively) expression levels were increased compared
with the normoxia group (all P<0.05; Fig. 5A). Compared with the
hyperoxia-scrambled siRNA group, the administration of CCN1 siRNA
decreased the CCN1, PI3K and AKT mRNA expression levels (−43.7,
−58.7 and −42.9%, respectively, all P<0.05; Fig. 5A).
Western blot analysis revealed similar results in
the retinal samples. In the hyperoxia and hyperoxia-scrambled siRNA
groups, the CCN1 (+429 and +406%, respectively), p-PI3K (+124 and
+115%, respectively) and p-AKT (+153 and +147%, respectively)
protein expression levels were increased compared with those in the
normoxia group (all P<0.05; Fig.
5B). Compared with the hyperoxia-scrambled siRNA group, the
silencing of CCN1 by CCN1 siRNA decreased the CCN1, PI3K and AKT
protein expression levels (−45.3, −22.5 and −28.4%, respectively,
all P<0.05; Fig. 5B).
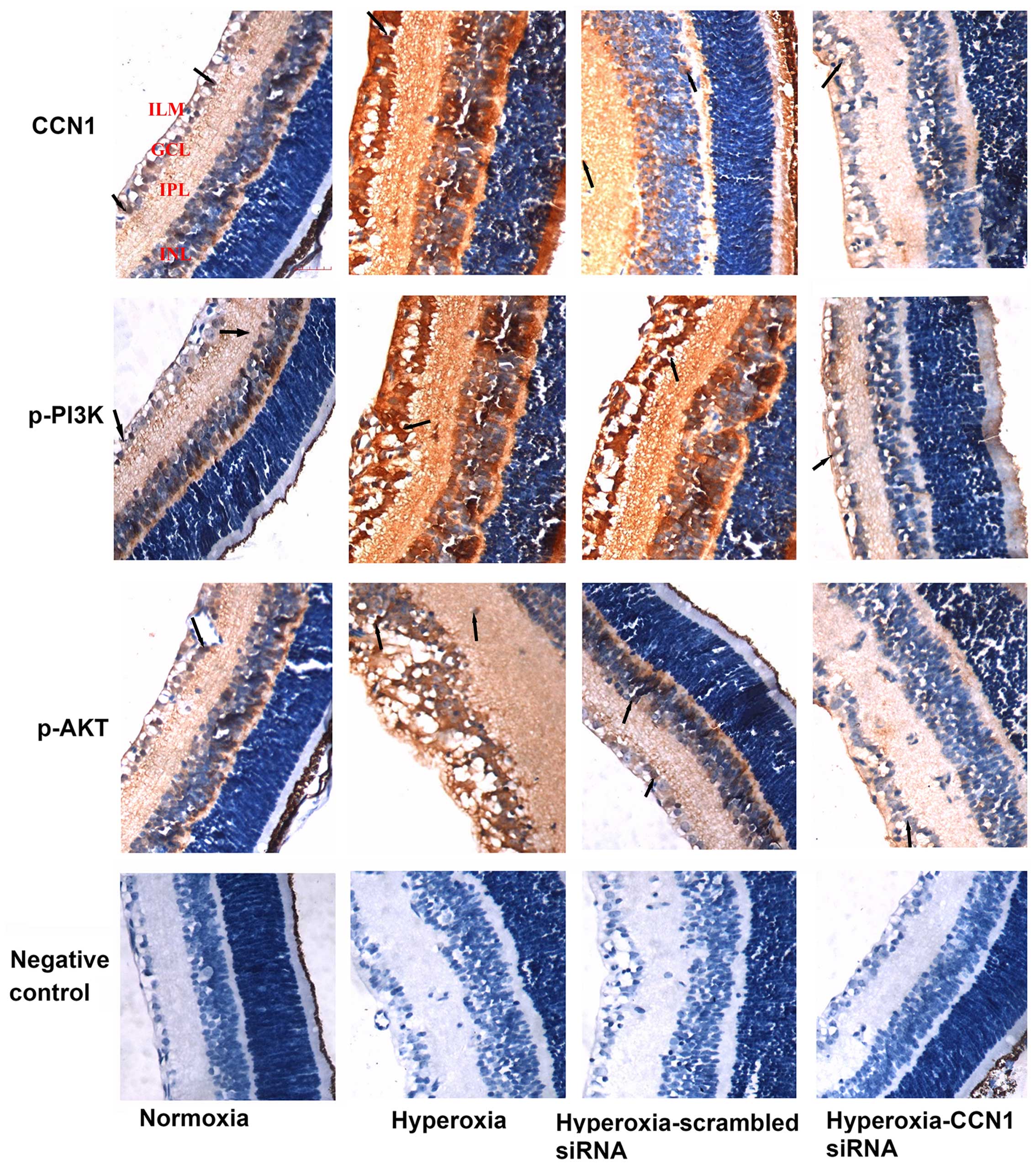
Immunohistochemistry was also performed to
investigate the localization and expression levels of CCN1, p-PI3K
and p-AKT (Fig. 6).
Immunohistochemistry of the retinal sections revealed that the
CCN1, p-PI3K and p-AKT expression levels were weakly detected only
in the ganglion cell layer (GCL) and inner plexiform layer (IPL) of
the normoxia group, whereas in the hyperoxia and
hyperoxia-scrambled siRNA groups, they were strongly detected in
the GCL, IPL, inner nuclear layer (INL) and outer plexiform layer
(OPL), with neovascularization breaking through the ILM. However,
the expression of CCN1, p-PI3K and p-AKT in the hyperoxia-CCN1
siRNA group was low in the GCL, IPL with less neovascularization
breaking through the ILM compared with the hyperoxia and
hyperoxia-scrambled siRNA groups.
Discussion
The aim of the present study was to assess the
angiogenic effects of CCN1/Cyr61 in HUVECs, as well as the effects
of CCN1/Cyr61-PI3K/AKT signaling in the retinas of mouse pups with
OIR. CCN1 knockdown decreased CCN1, PI3K and AKT mRNA and protein
expression, inhibited HUVEC proliferation and induced HUVEC
apoptosis under hypoxic conditions. CCN1 knockdown resulted in less
severe neovascularization in the eyes of the mouse pups with OIR.
Exposure to hypoxia increased the number of preretinal neovascular
cells, as well as CCN1, PI3K and AKT protein and mRNA expression.
CCN1 silencing also decreased the number of hypoxic preretinal
neovascular cells, as well as CCN1, PI3K and AKT mRNA and protein
expression.
Previous studies have directly (38–41), as well as indirectly (29,42) demonstrated that CCN1/CYR61
promotes chorioretinal angiogenesis in vitro via endothelial
cell proliferation, migration and the formation of tubular
structures, and that CYR61 plays a role in the formation of new
blood vessels in the retina. All these processes begin with
endothelial cell proliferation. The potent pro-angiogenic
properties of CCN1 have previously been demonstrated in rat models
of ischemic retinopathy (29,31) and in relation to different tumor
cell types (37,43,44). As hyperoxia and subsequent
angiogenesis play important roles in tumor development, a high CCN1
expression is associated with more aggressive tumor invasion. In
experiments using HUVECs, CCN1 has been shown to induce endothelial
cell proliferation (14–16,45). Accordingly, the present study
demonstrated that the silencing of CCN1 using CCN1 siRNA
significantly inhibited endothelial cell proliferation and promoted
endothelial cell apoptosis, thus interfering with angiogenesis, as
observed in the retinas of the mouse pups with OIR. However, these
experiments were not designed to determine whether apoptosis
prevented angiogenesis, or whether apoptosis was induced as
angiogenesis was inhibited. These results suggest that the
CCN1/Cyr61 levels play a role in cell proliferation and apoptosis.
This hypothesis is supported by the findings of previous studies
which showed that endothelial cell proliferation is the first step
in angiogenesis and must occur before cells can migrate and begin
to form tubes (18,42). However, a recent study suggested
that CCN1 itself may be pro-apoptotic (46). This discrepancy may be due to a
number of factors, including the animal model, cell lines, studied
tissues or the methods used to determine apoptosis. Further studies
are thus warranted in order to investigate these issues.
PI3K/AKT activation is both necessary and sufficient
in itself to promote angiogenesis (47,48). The inhibition of the PI3K/AKT
pathway usually results in successful anti-angiogenic and
anti-tumor effects (49). In
addition, it has been previously reported that CCN1 induces
PI3K/AKT expression in different types of cells, such as breast
cancer cells, gastric cancer cells, renal cell carcinoma and glioma
cells (37,50–52). In the present study, we examined
angiogenesis-related signaling pathways to further elucidate the
underlying mechanisms of the anti-angiogenic properties of
silencing CCN1. We demonstrated that the silencing of CCN1 by CCN1
siRNA inhibited the activation of the PI3K/AKT pathway, which is
consistent with the decreased angiogenesis observed in the mouse
pups administered CCN1 siRNA. In addition, our results demonstrated
that cells exposed to hypoxia and treated with LY294002, a PI3K
inhibitor, had a lower early apoptotic rate but a higher late
apoptotic rate compared with the cells exposed to hypoxia not
treated with the inhibitor. In addition, the CCN1 mRNA and protein
expression levels were markedly decreased following the silencing
of CCN1. This suggested that angiogenesis was prevented by
endothelial cell apoptosis and that PI3K is involved in the
process.
We observed that exposure to hypoxia increased the
mRNA and protein levels of CCN1 in vitro and in vivo
via the phosphorylation of PI3K/AKT. In our mouse pup model of OIR,
immunohistochemical analysis indicated that the expression levels
of CCN1, p-PI3K and p-AKT in the neovascular tufts of the retinas
and retinal angiogenesis were prevented by an intravitreal
injection of CCN1 siRNA. The silencing of CCN1 by CCN1 siRNA
effectively and specifically downregulated CCN1 expression in the
retinas and HUVECs. CCN1 siRNA induced a significant inhibition of
PI3K/AKT and also induced apoptosis. This study thus provides
evidence that an increased CCN1 expression contributes to increased
levels of PI3K/AKT and angiogenesis in retinas with OIR and HUVECs,
and that CCN1 may be a target for the treatment of angiogenic
retinopathy. Our findings are consistent with those of previously
published studies, indicating that Cyr61 enhances gastric cancer
invasion via PI3K/AKT (37,51,52). The results of the present study
support the hypothesis that the CCN1/Cyr61-PI3K/AKT signaling
pathway plays an important role in the development of ROP.
However, we observed that endothelial cell
proliferation, retinal angiogenesis and the expression levels of
CCN1, PI3K and AKT were not completely inhibited by CCN1 siRNA.
This phenomenon is likely related to a number of factors, including
different plasmid vectors, transfection efficiencies (53,54) and several other growth factors
such as VEGF, basic fibroblast growth factor (bFGF), interleukin-8
(IL-8), c-Jun and HIF-1α (55–59). Further studies are thus required
to define the precise association of these growth factors with
CCN1, and their involvement in retinopathies.
CCN1 expression is regulated by mitogenic factors
such as VEGF, fibroblast growth factor (FGF) and platelet-derived
growth factor (PDGF) (60). CCN1
is downregulated during tissue involution, in avascular tissues and
in conditions associated with vascular obliteration, underlining
the role of this protein in the vasculature (31). Forkhead box O3A (FOXO3a) inhibits
smooth muscle cell proliferation via CCN1 inhibition (61). CCN1 activates the Ras pathway
through the MAPK and AKT signaling pathways and enhances the
expression of regulatory proteins that promote cell cycle
progression (62,63). However, there is a possibility of
an autocrine loop since VEGF is partly under CCN1 regulation
(64). HIF plays a crucial role
in the cell response to hypoxia. In tumor cells, VEGF is
transactivated by HIF-1α and plays a role in tumor progression and
invasion (65). In gastric
cancer, CCN1 promotes tumor progression via the HIF-1α-dependent
upregulation of plasminogen activator inhibitor-1 (PAI-1) (52). In the present study, VEGF and HIF
were not examined. Nevertheless, our results demonstrated that the
silencing of CCN1 decreased cell proliferation, and that similar
effects were achieved using a PI3K/AKT inhibitor, suggesting that
AKT is involved. However, many aspects of CCN1 signaling remain
unknown and thus further studies are warranted (66).
In conclusion, our results demonstrated that CCN1
plays an important role in HUVECs under hypoxic conditions and
retinal apoptosis/angiogenesis in ROP via PI3K/AKT signaling. Thus,
we suggest that CCN1 is a potential target for the prevention and
treatment of ROP. This study also provides strong support for the
view that siRNA therapy may play an important role in future
therapeutic strategies.
Acknowledgments
We would like to thank Dr Jijing Pang, from the
Department of Ophthalmology, University of Florida, for kindly
providing the intravitreal injection needle. This study was
supported by the National Natural Science Foundation of China (no.
81371045 to X.C.) and the Liaoning Province Technology Foundation
of China (no. 2010225034 to X.C.).
References
|
1
|
Li Z, He T, Du K, Xing YQ, Yan Y, Chen Z,
Zhang H and Shen Y: Overexpression of 15-lipoxygenase-1 in
oxygen-induced ischemic retinopathy inhibits retinal
neovascularization via downregulation of vascular endothelial
growth factor-A expression. Mol Vis. 18:2847–2859. 2012.PubMed/NCBI
|
|
2
|
Nowak-Sliwinska P, Storto M, Cataudella T,
Ballini JP, Gatz R, Giorgio M, van den Bergh H, Plyte S and
Wagnières G: Angiogenesis inhibition by the maleimide-based small
molecule GNX-686. Microvasc Res. 83:105–110. 2012. View Article : Google Scholar
|
|
3
|
Gergely K and Gerinec A: Retinopathy of
prematurity - epidemics, incidence, prevalence, blindness. Bratisl
Lek Listy. 111:514–517. 2010.
|
|
4
|
Wang F, Bai Y, Yu W, Han N, Huang L, Zhao
M, Zhou A, Zhao M and Li X: Anti-angiogenic effect of KH902 on
retinal neovascularization. Graefes Arch Clin Exp Ophthalmol.
251:2131–2139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartnett ME and Penn JS: Mechanisms and
management of retinopathy of prematurity. N Engl J Med.
367:2515–2526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phelps DL: Retinopathy of prematurity.
Pediatr Rev. 16:50–56. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
No authors listed. Cryotherapy for
Retinopathy of Prematurity Cooperative Group: Multicenter trial of
cryotherapy for retinopathy of prematurity. 3 1/2-year outcome -
structure and function. Arch Ophthalmol. 111:339–344. 1993.
View Article : Google Scholar
|
|
8
|
Xia XB, Xiong SQ, Song WT, Luo J, Wang YK
and Zhou RR: Inhibition of retinal neovascularization by siRNA
targeting VEGF(165). Mol Vis. 14:1965–1973. 2008.PubMed/NCBI
|
|
9
|
Yan Y, He T, Shen Y, Chen X, Diao B, Li Z,
Liu Q and Xing YQ: Adenoviral 15-lipoxygenase-1 gene transfer
inhibits hypoxia-induced proliferation of retinal microvascular
endothelial cells in vitro. Int J Ophthalmol. 5:562–569.
2012.PubMed/NCBI
|
|
10
|
Mintz-Hittner HA: Treatment of retinopathy
of prematurity with vascular endothelial growth factor inhibitors.
Early Hum Dev. 88:937–941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martínez-Castellanos MA, Schwartz S,
Hernández-Rojas ML, Kon-Jara VA, García-Aguirre G, Guerrero-Naranjo
JL, Chan RV and Quiroz-Mercado H: Long-term effect of
antiangiogenic therapy for retinopathy of prematurity up to 5 years
of follow-up. Retina. 33:329–338. 2013. View Article : Google Scholar
|
|
12
|
Hanna M, Liu H, Amir J, Sun Y, Morris SW,
Siddiqui MA, Lau LF and Chaqour B: Mechanical regulation of the
proangiogenic factor CCN1/CYR61 gene requires the combined
activities of MRTF-A and CREB-binding protein histone
acetyltransferase. J Biol Chem. 284:23125–23136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jun JI and Lau LF: Taking aim at the
extracellular matrix: CCN proteins as emerging therapeutic targets.
Nat Rev Drug Discov. 10:945–963. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brigstock DR: The connective tissue growth
factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family.
Endocr Rev. 20:189–206. 1999.PubMed/NCBI
|
|
15
|
Brigstock DR: The CCN family: a new
stimulus package. J Endocrinol. 178:169–175. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brigstock DR: Regulation of angiogenesis
and endothelial cell function by connective tissue growth factor
(CTGF) and cysteine-rich 61 (CYR61). Angiogenesis. 5:153–165. 2002.
View Article : Google Scholar
|
|
17
|
Yan L and Chaqour B: Cysteine-rich protein
61 (CCN1) and connective tissue growth factor (CCN2) at the
crosshairs of ocular neovascular and fibrovascular disease therapy.
J Cell Commun Signal. 7:253–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi J, Lin A, Shrier E, Lau LF, Grant MB
and Chaqour B: Degradome products of the matricellular protein CCN1
as modulators of pathological angiogenesis in the retina. J Biol
Chem. 288:23075–23089. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CC and Lau LF: Functions and
mechanisms of action of CCN matricellular proteins. Int J Biochem
Cell Biol. 41:771–783. 2009. View Article : Google Scholar :
|
|
20
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1alpha and VEGF in
laser-induced rat choroidal neovascularization. Invest Ophthalmol
Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar
|
|
21
|
You JJ, Yang CH, Yang CM and Chen MS:
Cyr61 induces the expression of monocyte chemoattractant protein-1
via the integrin ανβ3, FAK, PI3K/Akt, and NF-κB pathways in retinal
vascular endothelial cells. Cell Signal. 26:133–140. 2014.
View Article : Google Scholar
|
|
22
|
Sasore T, Reynolds AL and Kennedy BN:
Targeting the PI3K/Akt/mTOR pathway in ocular neovascularization.
Adv Exp Med Biol. 801:805–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo H, Lv Y, Tian T, Hu TH, Wang WJ, Sui
X, Jiang L, Ruan ZP and Nan KJ: Downregulation of p57 accelerates
the growth and invasion of hepatocellular carcinoma.
Carcinogenesis. 32:1897–1904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han Z, Yang Q, Liu B, Wu J, Li Y, Yang C
and Jiang Y: MicroRNA-622 functions as a tumor suppressor by
targeting K-Ras and enhancing the anticarcinogenic effect of
resveratrol. Carcinogenesis. 33:131–139. 2012. View Article : Google Scholar
|
|
25
|
Aubry JP, Blaecke A, Lecoanet-Henchoz S,
Jeannin P, Herbault N, Caron G, Moine V and Bonnefoy JY: Annexin V
used for measuring apoptosis in the early events of cellular
cytotoxicity. Cytometry. 37:197–204. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith LE, Wesolowski E, McLellan A, Kostyk
SK, D'Amato R, Sullivan R and D'Amore PA: Oxygen-induced
retinopathy in the mouse. Invest Ophthalmol Vis Sci. 35:101–111.
1994.PubMed/NCBI
|
|
28
|
Masuda I, Matsuo T, Yasuda T and Matsuo N:
Gene transfer with liposomes to the intraocular tissues by
different routes of administration. Invest Ophthalmol Vis Sci.
37:1914–1920. 1996.PubMed/NCBI
|
|
29
|
You JJ, Yang CH, Chen MS and Yang CM:
Cysteine-rich 61, a member of the CCN family, as a factor involved
in the pathogenesis of proliferative diabetic retinopathy. Invest
Ophthalmol Vis Sci. 50:3447–3455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Q, Zhang J, Guan Y, Zhang S, Zhu C,
Xu GT and Wang L: Suppression of retinal neovascularization by the
iNOS inhibitor aminoguanidine in mice of oxygen-induced
retinopathy. Graefes Arch Clin Exp Ophthalmol. 247:919–927. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hasan A, Pokeza N, Shaw L, Lee HS, Lazzaro
D, Chintala H, Rosenbaum D, Grant MB and Chaqour B: The
matricellular protein cysteine-rich protein 61 (CCN1/Cyr61)
enhances physiological adaptation of retinal vessels and reduces
pathological neovascularization associated with ischemic
retinopathy. J Biol Chem. 286:9542–9554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lambert V, Lecomte J, Hansen S, Blacher S,
Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart
JM, et al: Laser-induced choroidal neovascularization model to
study age-related macular degeneration in mice. Nat Protoc.
8:2197–2211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ecoiffier T, Yuen D and Chen L:
Differential distribution of blood and lymphatic vessels in the
murine cornea. Invest Ophthalmol Vis Sci. 51:2436–2440. 2010.
View Article : Google Scholar :
|
|
34
|
Barnett JM, McCollum GW, Fowler JA, Duan
JJ, Kay JD, Liu RQ, Bingaman DP and Penn JS: Pharmacologic and
genetic manipulation of MMP-2 and -9 affects retinal
neovascularization in rodent models of OIR. Invest Ophthalmol Vis
Sci. 48:907–915. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park K, Chen Y, Hu Y, Mayo AS, Kompella
UB, Longeras R and Ma JX: Nanoparticle-mediated expression of an
angiogenic inhibitor ameliorates ischemia-induced retinal
neovascularization and diabetes-induced retinal vascular leakage.
Diabetes. 58:1902–1913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Smith LE: Pathogenesis of retinopathy of
prematurity. Acta Paediatr Suppl. 91:26–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grote K, Salguero G, Ballmaier M, Dangers
M, Drexler H and Schieffer B: The angiogenic factor CCN1 promotes
adhesion and migration of circulating CD34+ progenitor cells:
potential role in angiogenesis and endothelial regeneration. Blood.
110:877–885. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leu SJ, Lam SC and Lau LF: Pro-angiogenic
activities of CYR61 (CCN1) mediated through integrins alphavbeta3
and alpha6beta1 in human umbilical vein endothelial cells. J Biol
Chem. 277:46248–46255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kireeva ML, Latinkić BV, Kolesnikova TV,
Chen CC, Yang GP, Abler AS and Lau LF: Cyr61 and Fisp12 are both
ECM-associated signaling molecules: Activities, metabolism, and
localization during development. Exp Cell Res. 233:63–77. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kireeva ML, Mo FE, Yang GP and Lau LF:
Cyr61, a product of a growth factor-inducible immediate-early gene,
promotes cell proliferation, migration, and adhesion. Mol Cell
Biol. 16:1326–1334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang X, Yu W and Dong F: Cysteine-rich 61
(CYR61) is up-regulated in proliferative diabetic retinopathy.
Graefes Arch Clin Exp Ophthalmol. 250:661–668. 2012. View Article : Google Scholar
|
|
43
|
Meyuhas R, Pikarsky E, Tavor E, Klar A,
Abramovitch R, Hochman J, Lago TG and Honigman A: A Key role for
cyclic AMP-responsive element binding protein in hypoxia-mediated
activation of the angiogenesis factor CCN1 (CYR61) in Tumor cells.
Mol Cancer Res. 6:1397–1409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leask A: CCN1: A novel target for
pancreatic cancer. J Cell Commun Signal. 5:123–124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Perbal B: CCN proteins: multifunctional
signalling regulators. Lancet. 363:62–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Su BC and Mo FE: CCN1 enables Fas
ligand-induced apoptosis in cardiomyoblast H9c2 cells by disrupting
caspase inhibitor XIAP. Cell Signal. 26:1326–1334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gerszten RE, Friedrich EB, Matsui T, Hung
RR, Li L, Force T and Rosenzweig A: Role of phosphoinositide
3-kinase in monocyte recruitment under flow conditions. J Biol
Chem. 276:26846–26851. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zheng ZZ and Liu ZX: Activation of the
phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates
CD151-induced endothelial cell proliferation and cell migration.
Int J Biochem Cell Biol. 39:340–348. 2007. View Article : Google Scholar
|
|
49
|
Quan Y, Wang N, Chen Q, Xu J, Cheng W, Di
M, Xia W and Gao WQ: SIRT3 inhibits prostate cancer by
destabilizing oncoprotein c-MYC through regulation of the
PI3K/Aktpathway. Oncotarget. 6:26494–26507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Long QZ, Zhou M, Liu XG, Du YF, Fan JH, Li
X and He DL: Interaction of CCN1 with αvβ3 integrin induces
P-glycoprotein and confers vinblastine resistance in renal cell
carcinoma cells. Anticancer Drugs. 24:810–817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin BR, Chang CC, Chen LR, Wu MH, Wang MY,
Kuo IH, Chu CY, Chang KJ, Lee PH, Chen WJ, et al: Cysteine-rich 61
(CCN1) enhances chemotactic migration, transendothelial cell
migration, and intravasation by concomitantly up-regulating
chemokine receptor 1 and 2. Mol Cancer Res. 5:1111–1123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin MT, Kuo IH, Chang CC, Chu CY, Chen HY,
Lin BR, Sureshbabu M, Shih HJ and Kuo ML: Involvement of
hypoxia-inducing factor-1alpha-dependent plasminogen activator
inhibitor-1 up-regulation in Cyr61/CCN1-induced gastric cancer cell
invasion. J Biol Chem. 283:15807–15815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hanrahan F, Humphries P and Campbell M:
RNAi-mediated barrier modulation: synergies of the brain and eye.
Ther Deliv. 1:587–594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Roy S, Nasser S, Yee M, Graves DT and Roy
S: A long-term siRNA strategy regulates fibronectin overexpression
and improves vascular lesions in retinas of diabetic rats. Mol Vis.
17:3166–3174. 2011.PubMed/NCBI
|
|
55
|
Kuwabara K, Ogawa S, Matsumoto M, Koga S,
Clauss M, Pinsky DJ, Lyn P, Leavy J, Witte L and Joseph-Silverstein
J: Hypoxia-mediated induction of acidic/basic fibroblast growth
factor and platelet-derived growth factor in mononuclear phagocytes
stimulates growth of hypoxic endothelial cells. Proc Natl Acad Sci
USA. 92:4606–4610. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kunz M, Hartmann A, Flory E, Toksoy A,
Koczan D, Thiesen HJ, Mukaida N, Neumann M, Rapp UR, Bröcker EB and
Gillitzer R: Anoxia-induced up-regulation of interleukin-8 in human
malignant melanoma. A potential mechanism for high tumor
aggressiveness. Am J Pathol. 155:753–763. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yamashita K, Discher DJ, Hu J, Bishopric
NH and Webster KA: Molecular regulation of the endothelin-1 gene by
hypoxia. Contributions of hypoxia-inducible factor-1, activator
protein-1, GATA-2, AND p300/CBP. J Biol Chem. 276:12645–12653.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Woo KJ, Lee TJ, Park JW and Kwon TK:
Desferrioxamine, an iron chelator, enhances HIF-1alpha accumulation
via cyclooxy-genase-2 signaling pathway. Biochem Biophys Res
Commun. 343:8–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
You JJ, Yang CM, Chen MS and Yang CH:
Regulation of Cyr61/CCN1 expression by hypoxia through cooperation
of c-Jun/AP-1 and HIF-1α in retinal vascular endothelial cells. Exp
Eye Res. 91:825–836. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chaqour B and Goppelt-Struebe M:
Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins.
FEBS J. 273:3639–3649. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee HY, Chung JW, Youn SW, Kim JY, Park
KW, Koo BK, Oh BH, Park YB, Chaqour B, Walsh K and Kim HS: Forkhead
transcription factor FOXO3a is a negative regulator of angiogenic
immediate early gene CYR61, leading to inhibition of vascular
smooth muscle cell proliferation and neointimal hyperplasia. Circ
Res. 100:372–380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen CC, Chen N and Lau LF: The angiogenic
factors Cyr61 and connective tissue growth factor induce adhesive
signaling in primary human skin fibroblasts. J Biol Chem.
276:10443–10452. 2001. View Article : Google Scholar
|
|
63
|
Murphy LO, MacKeigan JP and Blenis J: A
network of immediate early gene products propagates subtle
differences in mitogen-activated protein kinase signal amplitude
and duration. Mol Cell Biol. 24:144–153. 2004. View Article : Google Scholar :
|
|
64
|
Mo FE, Muntean AG, Chen CC, Stolz DB,
Watkins SC and Lau LF: CYR61 (CCN1) is essential for placental
development and vascular integrity. Mol Cell Biol. 22:8709–8720.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
66
|
Chaqour B: Molecular control of vascular
development by the matricellular proteins CCN1 (Cyr61) and CCN2
(CTGF). Trends Dev Biol. 7:59–72. 2013.
|