Introduction
Pyropia yezoensis (P. yezoensis) is an
important marine algae that is cultivated primarily in China, Japan
and Korea (1). It is exposed to
adverse environmental conditions, such as high light intensity and
oxygen concentration, which lead to the formation of free radicals
and other strong oxidizing agents without causing serious
photodynamic damage (2–4). Consequently, P. yezoensis may
be capable of generating the necessary compounds to protect itself
from external factors, such as pollution, stress and ultraviolet
(UV) radiation.
There are various biologically active phytochemicals
in P. yezoensis, including carotenoids, fatty acids,
polysaccharides, proteins, vitamins, tocopherol and phytocyanins
(5,6), which have shown various medicinal
effects (7,8). In particular, its high protein
content makes P. yezoensis a potential source of bioactive
peptides and an alternative to synthetic drugs, with
antihypertensive (8),
anti-inflammatory (9,10), antioxidant (11–13), anticancer (14) and tissue-healing properties
(15–17).
Bioactive peptides usually contain 3–20 amino acid
residues, and their activities are based on their amino acid
composition and sequence (18).
These short amino acid chains are inactive within the sequence of
the parent protein but can be released during gastrointestinal
digestion, food processing or fermentation. Marine-derived
bioactive peptides have been obtained through enzymatic hydrolysis
and have been shown to drive numerous fundamental metabolic
processes (19–21).
Inflammation is an effective immune response to
foreign stimuli and ultimately results in restoration of normal
function. Macrophages are important mediators of the inflammatory
response (22). Activated
macrophages produce pro-inflammatory enzymes, such as inducible
nitric oxide synthase (iNOS), cyclo-oxygenase-2 (COX-2), and
various pro-inflammatory cytokines, such as tumor necrosis factor-α
(TNF-α) and interleukins (IL-1β and IL-6), which serve as essential
mediators of the inflammatory response (23). However, excessive and uncontrolled
production of these inflammatory mediators and cytokines are
associated with autoimmune disorders, neuropathological diseases,
rheumatoid arthritis, microcirculatory dysfunction and tissue
damage, leading to fatality. Suppression of these inflammatory
mediators may be an effective therapeutic strategy to prevent
diseases caused by inflammatory disorders (24).
Mitogen-activated protein kinases (MAPKs) are a
family of serine/threonine protein kinases that mediate fundamental
biological processes and cellular responses to external stress
signals. Increased activity of MAPK and their involvement in
regulating the synthesis of inflammation mediators at the levels of
transcription and translation makes them potential targets for
anti-inflammatory therapeutics (25). Modulation of the activity of the
protein 38 (p38), extracellular signal-regulated kinase (ERK), and
c-jun NH2-terminal kinase (JNK) signaling cascades on
the MAPK pathways is regarded as an attractive strategy, as they
are capable of reducing the synthesis of pro-inflammatory cytokines
and their signaling.
The increasing incidence of inflammation has led to
the search for proteins and peptides, and their anti-inflammatory
effects have been identified in numerous studies. According to Zhu
et al (26), TC26RFa from
the tree shrew of Tupaia belangeri chinensis may inhibit
inflammatory factor secretion induced by lipopolysaccharides (LPS).
The inhibition of lunasin on pro-inflammatory cytokines via
suppressing the nuclear factor-κB pathway has also been suggested
(22). The egg white peptide was
a promising novel therapeutic approach to treating the inflammatory
bowel disease by reducing the local expression of pro-inflammatory
cytokines (27). However, limited
research on the production of anti-inflammatory peptides from P.
yezoensis has been reported. The present study investigated the
anti-inflammatory properties of PPY1 in LPS-stimulated Raw 264.7
cells as an in vitro model and assessed the
anti-inflammatory mechanism of PPY1 with the potential contribution
to MAPK signaling.
Materials and methods
Preparation of peptide
The peptide PPY1, derived from P. yezoensis,
was synthesized by Peptron Inc. (Daejeon, Korea) and the sequence
was K-A-Q-A-D. Purification of PPY1 was performed using the
Shimadzu Prominence HPLC apparatus and controlled using the
software package Class-VP, 6.14 (Shimadzu Corp., Kyoto, Japan). A
C18 column (Shiseido Capcell Pak; Shiseido, Tokyo, Japan) in 0.1%
trifluoroacetic acid (TFA)/water and a gradient of 10–70%
acetonitrile in 0.1% TFA, with a flow rate of 1 nm/min and UV
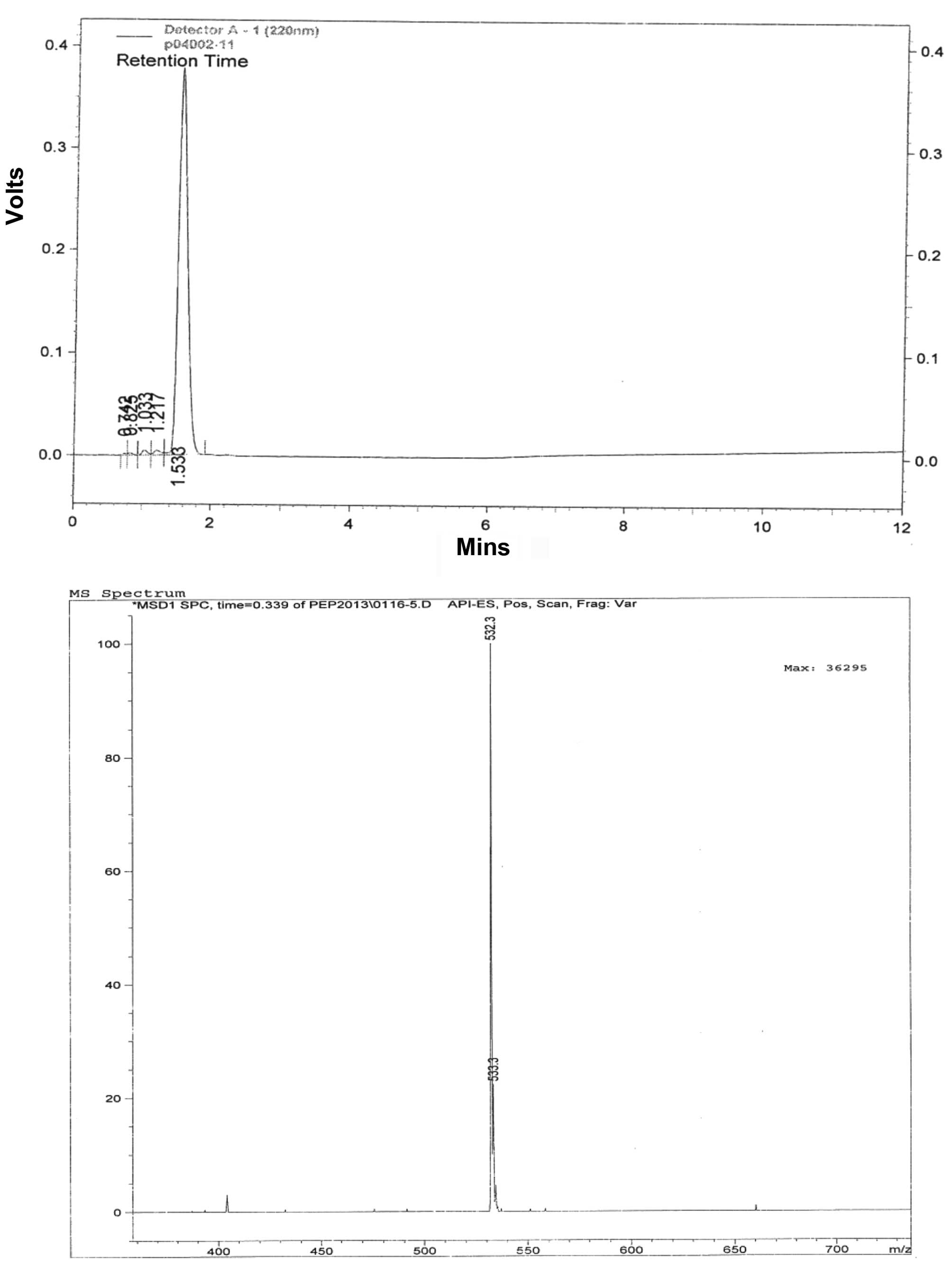
detection at 220 nm, was used. The molecular weight of PPY1 was
determined to be 532 Da (Fig. 1)
using mass spectrometer analysis (HP 1100 series LC/MSD; Agilent
Technologies, Inc., Santa Clara, CA, USA).
Cell culture
The mouse macrophage cell line RAW 264.7 was
obtained from the American Type Culture Collection (Rockville, MD,
USA) and cultured in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum, 50 µg/ml
penicillin, 25 µg/ml amphotericin B, and 50 µg/ml
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2.
Cell proliferation assay
RAW 264.7 cell proliferation was evaluated using the
Cytox™ cell viability assay kit (LPS solution, Daejeon, Korea).
Cells were plated at a density of 1×104 cells/well in
96-well plates overnight and subsequently treated with PPY1 (250,
500 and 1,000 ng/ml) for 24 h. At the end of the treatment period,
10 µl of the cell viability assay solution was added to each
well, and the plate was incubated for 30 min. The absorbance at 450
nm was measured using a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA). The results are expressed as a percentage
of the control.
Nitric oxide (NO) measurement
Accumulated nitrite (NO2−) in
the culture media was measured following a modified version of the
assay described by Green et al (28) RAW 264.7 cells were plated at a
density of 1×104 cells/well on a 96-well plate and
incubated overnight. Subsequently, cells were stimulated with LPS
(10 ng/ml) and treated with PPY1 (250, 500 and 1,000 ng/ml). A
volume of 100 µl of the cultured supernatant was plated in a
96-well plate, and 100 µl of Griess reagent was added. The
plate was incubated for 10 min and the absorbance at 540 nm was
measured using a microplate reader.
Determination of intracellular reactive
oxygen species (ROS)
The intracellular ROS were detected using the
ROS-sensitive fluorescent dye, CM-H2DCFDA. Cells were plated at
1×104 cells/well in 96-well plates overnight and were
subsequently treated with PPY1 (250, 500 and 1,000 ng/ml) for 24 h.
Cells were stimulated with LPS (10 ng/ml) during the last 18 h of
treatment with peptides. At the end of the treatment, cells were
washed with cold phosphate-buffered saline (PBS) and incubated with
10 µM CM-H2DCFDA at 37°C for 30 min. Following incubation,
the cells were washed with cold-PBS and the fluorescence
intensities of the stained cells were determined in a FilterMax F5
microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA) using
excitation and emission wavelengths of 485 and 535 nm,
respectively.
Western blot analysis
The whole cell extracts for immunoblotting iNOS,
COX-2, TNF-α, IL-1β and MAPKs were isolated from LPS-stimulated RAW
264.7 cells. A protein sample (35 µg) was loaded in SDS-PAGE
and transferred to a PVDF membrane (Millipore Corp., Billerica, MA,
USA). The membrane was blocked with 1% bovine serum albumin (BSA)
in Tris-buffered saline and Tween 20 (TBS-T) buffer and
subsequently incubated with primary antibodies [iNOS (sc-650),
COX-2 (sc-1745), TNF-α (sc-1350), IL-1β (sc-7884), p-ERK (sc-7383),
ERK (sc-94), p-p38 (sc-7973), p38 (sc-7149), p-JNK (sc-6254), JNK
(sc-7345) GAPDH (sc-25778) (1:1,000 in 1% BSA/TBS-T)] overnight at
4°C. The membranes were then washed twice for 15 min in TBS-T. The
secondary antibody was a horseradish peroxidase (HRP)-conjugated
goat anti-mouse or rabbit antibody [goat anti-mouse IgG-HRP
(sc-2031), goat anti-rat IgG-HRP (sc-2032) (1:10,000 in 1%
BSA/TBS-T)]. Following incubation and repeated washing, the
expression of each protein was visualized using chemiluminescence
substrate (Advansta, Menlo Park, CA, USA) and visualized using the
GeneSys imaging system (SynGene Synoptics, Ltd., London, UK).
Statistical analysis
All the samples were analyzed in triplicate, and the
results are expressed as means ± standard error of the mean. SPSS
(SPCC Inc., Chicago, IL, USA) was used to perform the statistical
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell viability of RAW 264.7 macrophages
cells
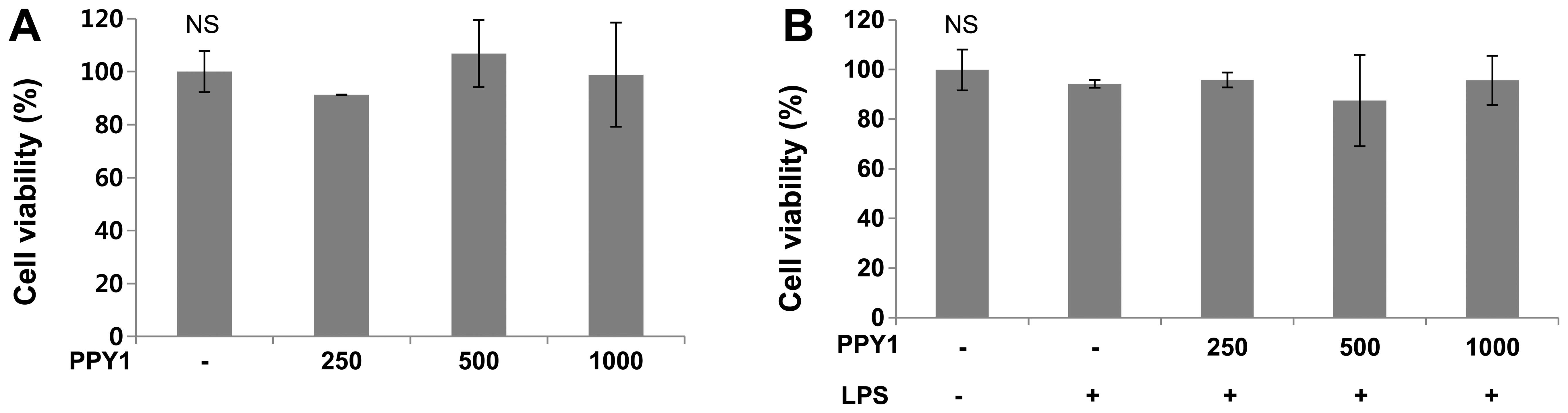
To determine the effect of PPY1 on cell viability,
PPY1 was tested in the Cyto X™ cell viability assay using RAW 264.7
macrophage cells. Proliferation and cytotoxic effects were examined
to establish the appropriate concentration ranges of PPY1 for
analysis in the following experiments (Fig. 2). A range of concentrations
(250–1,000 ng/ml) of PPY1 had no cytotoxic effects on RAW 264.7
cells and could be used in further studies.
PPY1 inhibits NO and ROS production in
LPS-stimulated RAW 264.7 macrophage cells
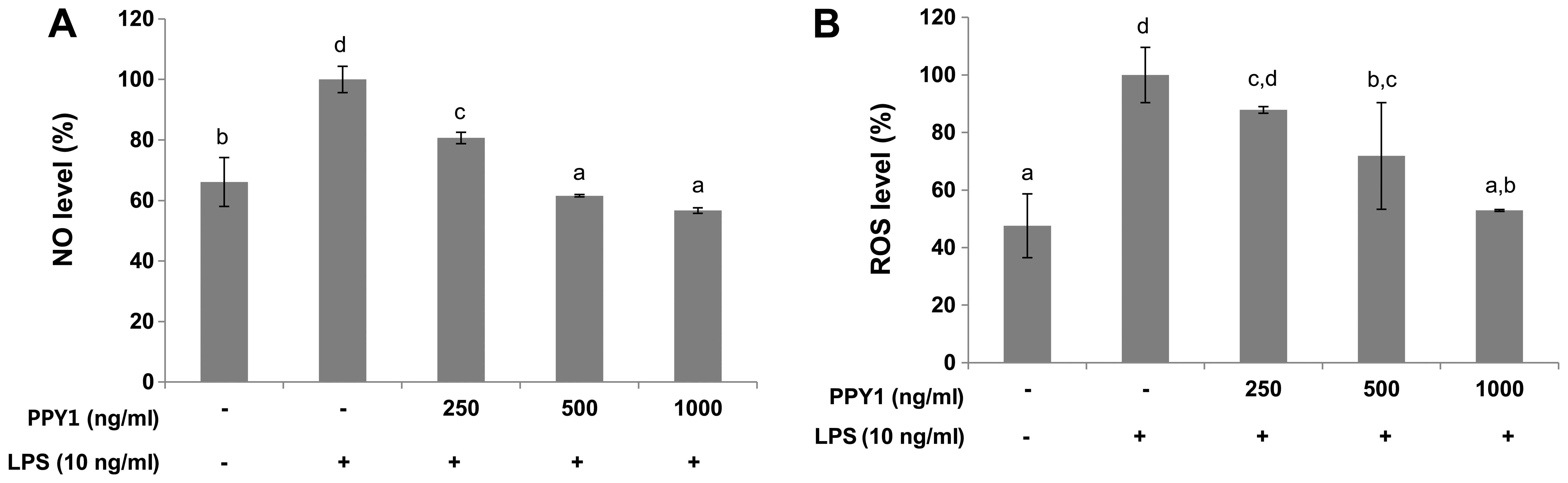
NO has a central role in inflammation, and its level
can be quantified by a simple procedure. RAW 264.7 cells were
treated with LPS (10 ng/ml) for 24 h to examine the inhibitory
effect of PPY1 on LPS-stimulated NO production. The LPS treatment
significantly (P<0.05) increased NO production compared with the
control group, whereas PPY1 co-treatment significantly (P<0.05)
suppressed NO production in a dose-dependent manner (Fig. 3A). Treatment of cells with 1,000
ng/ml of PPY1 inhibited NO release by 66.67%. NO induction can be
directly correlated with iNOS expression and other pro-inflammatory
cytokines. The latter can be considered a molecular target for
therapeutic compounds with anti-inflammatory action.
Whether PPY1 inhibited ROS generation in
LPS-stimulated RAW 264.7 cells using CM-H2DCFDA was also examined,
which has been widely used to study oxidative stress in macrophages
(29). The fluorescence intensity
corresponding to ROS produced by 10 ng/ml LPS-stimulated cells
significantly shifted compared with that of non-stimulated cells.
The ROS level was increased in LPS-stimulated cells (Fig. 3B). However, this increase was
significantly reduced by 52.9% when the cells were treated with
PPY1. These results suggested that PPY1 is effective in suppressing
the production of ROS in macrophages.
Anti-inflammatory effects of PPY1
The expression of inflammatory enzymes iNOS and
COX-2 was strongly induced in LPS-stimulated Raw 264.7 cells. The
effects of PPY1 on iNOS and COX-2 expression were examined by
western blot analysis. The overexpression in LPS-stimulated RAW
264.7 cells was suppressed by PPY1 in a dose-dependent manner at
both levels. As the quantities of iNOS protein correlated with NO
accumulation, these results suggest that PPY1 inhibited NO
production by reducing iNOS protein expression. Cytokines are also
produced during inflammation, and these are indicative of
inflammatory progression. In response to LPS, TNF-α and IL-1β were
significantly upregulated. Treatment with PPY1 considerably
inhibited the LPS induction of TNF-α and IL-1β in a dose-dependent
way (Fig. 4).
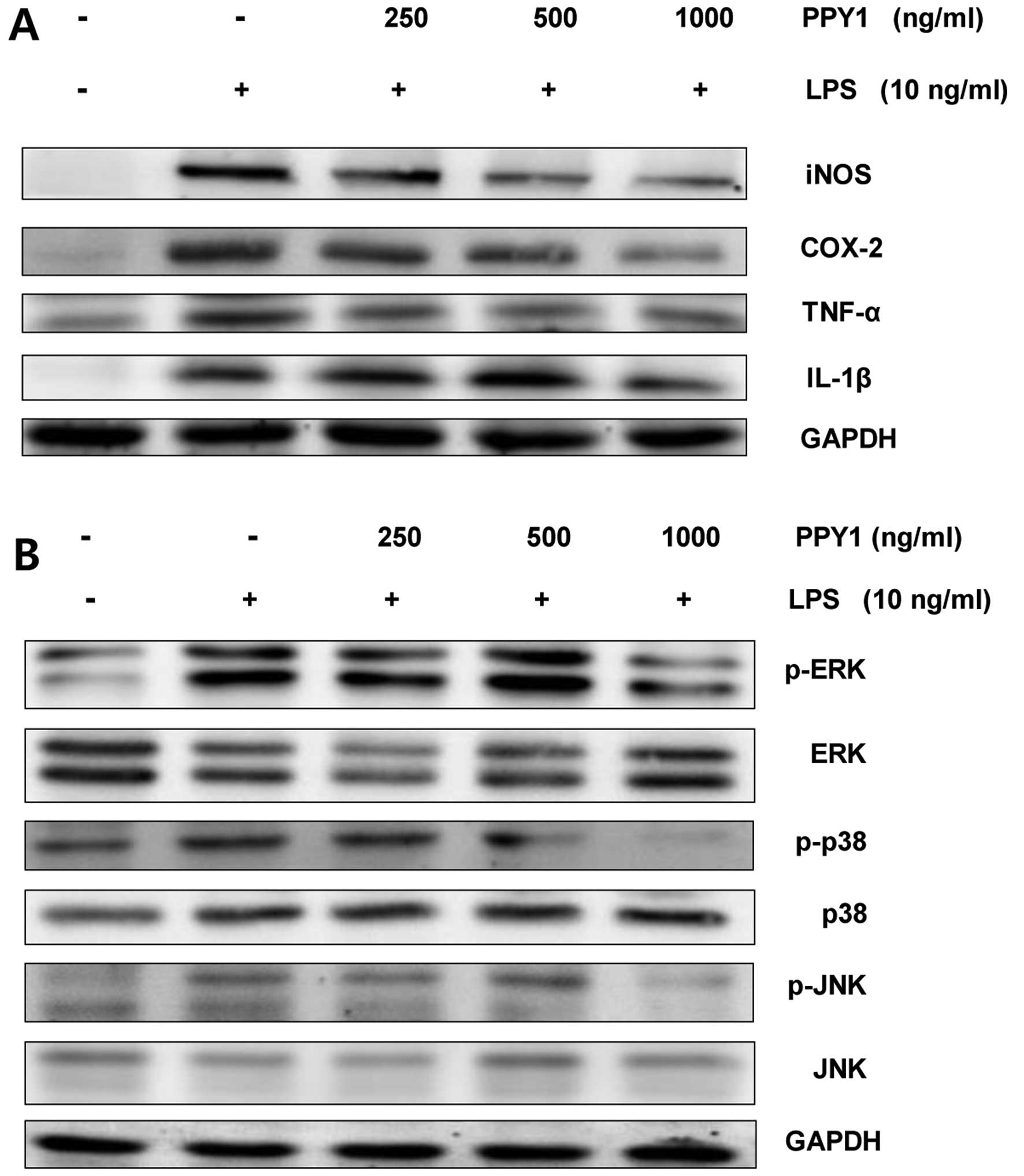 | Figure 4Effect of the bioactive peptide from
P. yezoensis (PPY1) on the level of (A) pro-inflammatory
mediators and cytokines, and (B) MAPK signaling in LPS-stimulated
RAW 264.7 macrophages. RAW 264.7 cells were stimulated with LPS (10
ng/ml) and treated with PPY1 (250, 500 and 1,000 ng/ml). The whole
cell sample (35 µg) was loaded in SDS-PAGE and transferred
to a PVDF membrane. The membrane was prepared and analyzed by
immunoblotting using pro-inflammatory mediators (anti-iNOS,
anti-COX-2, anti-TNF-α and anti-IL-1β) and MAPKs (anti-phospho ERK,
anti-ERK, anti-phospho p38, anti-p38, anti-phospho JNK and
anti-JNK). GAPDH was the loading control for immunoblotting. MAPK,
mitogen-activated protein kinase; LPS, lipopolysaccharide; iNOS,
inducible nitric oxide synthase; COX-2, cyclooxygenase-2; TNF-α,
tumor necrosis factor-α; IL, interleukin; ERK, extracellular
signal-regulated kinase; JNK, c-jun NH2-terminal
kinase. |
Effects of PPY1 on MAPK signaling
As MAPK signaling has a critical role in the
response of cells to various cytokines and stresses, the effects of
PPY1 were investigated on the MAPK pathways. The phosphorylation of
ERK, p38 and JNK was induced by LPS stimulation. Co-treatment of
PPY1 significantly reduced the level of p-ERK1/2, p-JNK, and p-p38
levels in LPS-stimulated RAW 264.7 cells (Fig. 4). The results show that inhibition
of pro-inflammatory mediator expression by PPY1 in LPS-stimulated
RAW 264.7 cells was associated with downregulation of ERK, p38, and
JNK phosphorylation.
Discussion
There is increasing interest in the natural products
obtained from marine resources, which are regarded as the last
remaining reservoir for materials to meet future therapeutic
requirements (30). In the
present study, the bioactive peptide (PPY1) from the marine algae,
P. yezoensis, was investigated for the presence of a potent
anti-inflammatory agent. P. yezoensis is the diverse group
of photosynthetic marine organisms that has adapted to surviving in
highly complex and competitive environments, including severe
salinity levels, temperature variations, low light intensities, and
low and high tides (31).
Therefore, it is assumed that specific peptides from P.
yezoensis can serve in prominent biological activities,
particularly those involving anti-inflammatory effects. Our
previous studies demonstrated the effects of a peptide from P.
yezoensis on cell proliferation and the associated signaling
pathway in IEC-6 and MCF-7 cells (32–35). Our study was inconsistent with the
general finding that short peptides with 2-−10 amino acids exert
more bioactive properties than their parent native proteins or
large polypeptides. The present study developed PPY1 as a bioactive
peptide. PPY1 was composed of five amino acids (K-A-Q-A-D) by
enzymatic hydrolysis from P. yezoensisa. Anti-inflammatory
activity, by suppression of inflammatory cytokines, was observed.
The result was similar to the study reporting that the function of
any peptide is mostly dependent on its specific amino acid
composition (36), and these
amino acids containing His, Ala, and Lys have been shown to have
strong activity against intracellular NO and ROS production
(37). The present results also
suggest that PPY1 may contribute to preventing inflammation through
inhibition of the expression of the inflammatory mediators, iNOS,
COX-2, in LPS-stimulated RAW 264.7 macrophages without having any
effect on cell viability. TNF-α and IL-1β have been described as
important inflammatory cytokines, with upregulation linked to the
pathogenesis of numerous infectious and inflammatory diseases,
including cancer (38). MAPKs
regulate various inflammatory and immune responses, including
LPS-induced expression of COX-2 and iNOS in macrophages. The
inhibition of MAPK family members, ERK, p38 and JNK, blocks the
production of pro-inflammatory cytokines, which resulted from the
suppression of the phosphorylation of these MAPK members.
Therefore, the results of the present study have
promising implications for biomedical research and warrant further
in vivo studies. Future study should verify the role of
these bioactive peptides in the prevention and treatment of other
inflammatory-related disorders, such as degenerative diseases and
aging.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
2012R1A6A1028677).
References
|
1
|
Niwa K: Genetic analysis of artificial
green and red mutants of Porphyra yezoensis Ueda (Bangiales,
Rhodophyta). Aquaculture. 308:6–12. 2012. View Article : Google Scholar
|
|
2
|
Wang Y, Cai C, Li B, Liu C and He P:
Photodynamic effect of two kinds of phycobiliproteins on human
liver cancer cell line SMMC-7721 in vitro. Sheng Wu Gong Cheng Xue
Bao. 25:1417–1423. 2009.In Chinese. PubMed/NCBI
|
|
3
|
Kim S, You DH, Han T and Choi EM:
Modulation of viability and apoptosis of UVB-exposed human
keratinocyte HaCaT cells by aqueous methanol extract of laver
(Porphyra yezoensis). J Photochem Photobiol B. 141:301–307. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ryu J, Park SJ, Kim IH, Choi YH and Nam
TJ: Protective effect of porphyra-334 on UVA-induced photoaging in
human skin fibroblasts. Int J Mol Med. 34:796–803. 2014.PubMed/NCBI
|
|
5
|
Cian RE, Caballero MS, Sabbag N, González
RJ and Drago SR: Bio-accessibility of bioactive compounds (ACE
inhibitors and antioxidants) from extruded maize products added
with a red seaweed Porphyra columbina. LWT-Food Sci Technol.
55:51–58. 2014. View Article : Google Scholar
|
|
6
|
Nakano T, Watanabe M, Sato M, Sato M and
Takeuchi M: Characterization of catalase from the seaweed Porphyra
yezoensis. Plant Sci. 104:127–133. 1995. View Article : Google Scholar
|
|
7
|
Cian RE, Martínez-Augustin O and Drago SR:
Bioactive properties of peptides obtained by enzymatic hydrolysis
from protein by products of Porphyra columbina. Food Res Int.
49:364–372. 2012. View Article : Google Scholar
|
|
8
|
Qu W, Ma H, Pan Z, Luo L, Wang Z and He R:
Preparation and antihypertensive activity of peptides from Porphyra
yezoensis. Food Chem. 123:14–20. 2010. View Article : Google Scholar
|
|
9
|
Jiang Z, Hama Y, Yamaguchi K and Oda T:
Inhibitory effect of sulphated polysaccharide porphyran on nitric
oxide production in lipopolysaccharide-stimulated RAW264.7
macrophages. J Biochem. 151:65–74. 2012. View Article : Google Scholar
|
|
10
|
Shin ES, Hwang HJ, Kim IH and Nam TJ: A
glycoprotein from Porphyra yezoensis produces anti-inflammatory
effects in liposaccharide-stimulated macrophages via the TLR4
signaling pathway. Int J Mol Med. 28:809–815. 2011.PubMed/NCBI
|
|
11
|
Toyosaki T and Iwabuchi M: New antioxidant
protein in seaweed (Porphyra yezoensis Ueda). Int J Food Sci Nutr.
60(Suppl 2): 46–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi JW, Kim YM, Park SJ, Kim IH and Nam
TJ: Protective effect of Porphyra yezoensis glycoprotein on
D-galactosamine induced cytotoxicity in Hepa 1c1c7 cells. Mol Med
Rep. 11:3914–3919. 2015.PubMed/NCBI
|
|
13
|
Isaka S, Cho K, Nakazono S, Abu R, Ueno M,
Kim D and Oda T: Antioxidant and anti-inflammatory activities of
porphyran isolated from discolored nori (Porphyra yezoensis). Int J
Biol Macromol. 74:68–75. 2015. View Article : Google Scholar
|
|
14
|
Eitsuka T, Nakagawa K, Igarashi M and
Miyazawa T: Telomerase inhibition by sulfoquinovosyldiacylglycerol
from edible purple laver (Porphyra yezoensis). Cancer Lett.
212:15–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Saga N and Mikami K: Effects of cell
wall synthesis on cell polarity in the red alga Porphyra yezoensis.
Plant Signal Behav. 3:1126–1128. 2008. View Article : Google Scholar
|
|
16
|
Hwang HJ, Kwon MJ, Kim IH and Nam TJ:
Chemoprotective effects of a protein from the red algae Porphyra
yezoensis on acetaminophen-induced liver injury in rats. Phytother
Res. 22:1149–1153. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo TT, Xu HL, Zhang LX, Zhang JP, Guo YF,
Gu JW and He PM: In vivo protective effect of Porphyra yezoensis
polysaccharide against carbon tetrachloride induced hepatotoxicity
in mice. Regul Toxicol Pharmacol. 49:101–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vo TS, Ryu B and Kim SK: Purification of
novel anti-inflammatory peptides from enzymatic hydrolysate of the
edible microalgal Spirulina maxima. J Funct Foods. 5:1336–1346.
2013. View Article : Google Scholar
|
|
19
|
Mohamed S, Hashim SN and Rahman HA:
Seaweeds: A sustainable functional food for complementary and
alternative therapy. Trends Food Sci Technol. 23:83–96. 2012.
View Article : Google Scholar
|
|
20
|
Kim SK and Wijesekara I: Development and
biological activities of marine-derived bioactive peptides. J Funct
Foods. 2:1–9. 2010. View Article : Google Scholar
|
|
21
|
Millán-Linares MC, Bermúdez B, Yust MM,
Millan F and Pedroche J: Anti-inflammatory activity of lupine
(Lupinus angustifolius L.) protein hydrolysates in THP-1-derived
macrophages. J Funct Foods. 8:224–233. 2014. View Article : Google Scholar
|
|
22
|
Hernández-Ledesma B, Hsieh CC and de Lumen
BO: Antioxidant and anti-inflammatory properties of cancer
preventive peptide lunasin in RAW 264.7 macrophages. Biochem
Biophys Res Commun. 390:803–808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liew FY: The role of innate cytokines in
inflammatory response. Immunol Lett. 85:131–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baeuerle PA and Baltimore D: NF-κ B: Ten
years after. Cell. 87:13–20. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy - from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Duan Z, Mo G, Shen C, Lv L, Chen W
and Lai R: A novel 26RFa peptide containing both analgesic and
anti-inflammatory functions from Chinese tree shrew. Biochimie.
102:112–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee M, Kovacs-Nolan J, Archbold T, Fan MZ,
Juneja LR, Okubo T and Mine Y: Therapeutic potential of hen egg
white peptides for the treatment of intestinal inflammation. J
Funct Foods. 1:161–169. 2009. View Article : Google Scholar
|
|
28
|
Green LC, Wagner DA, Glogowski J, Skipper
PL, Wishnok JS and Tannenbaum SR: Analysis of nitrate, nitrite, and
[15N]nitrate in biological fluids. Anal Biochem.
126:131–138. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu MZ, Lee WS, Han JM, Oh HW, Park DS,
Tian GR, Jeong TS and Park HY: Antioxidant and anti-inflammatory
activities of N-acetyldopamine dimers from Periostracum Cicadae.
Bioorg Med Chem. 14:7826–7834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samarakoon K and Jeon YJ:
Bio-functionalities of proteins derived from marine algae. Food Res
Int. 48:948–960. 2012. View Article : Google Scholar
|
|
31
|
Plaza M, Cifuentes A and Ibáñez E: In the
search of new functional food ingredients from algae. Trends Food
Sci Technol. 19:31–39. 2008. View Article : Google Scholar
|
|
32
|
Lee MK, Kim IH, Choi YH and Nam TJ: A
peptide from Porphyra yezoensis stimulates the proliferation of
IEC-6 cells by activating the insulin-like growth factor I receptor
signaling pathway. Int J Mol Med. 35:533–538. 2015.
|
|
33
|
Lee MK, Kim IH, Choi YH, Choi JW, Kim YM
and Nam TJ: The proliferative effects of Pyropia yezoensis peptide
on IEC-6 cells are mediated through the epidermal growth factor
receptor signaling pathway. Int J Mol Med. 35:909–914.
2015.PubMed/NCBI
|
|
34
|
Park SJ, Ryu J, Kim IH, Choi YH and Nam
TJ: Induction of apoptosis by a peptide from Porphyra yezoensis:
Regulation of the insulin-like growth factor I receptor signaling
pathway in MCF-7 cells. Int J Oncol. 45:1011–1016. 2014.PubMed/NCBI
|
|
35
|
Park SJ, Ryu J, Kim IH, Choi YH and Nam
TJ: Activation of the mTOR signaling pathway in breast cancer MCF 7
cells by a peptide derived from Porphyra yezoensis. Oncol Rep.
33:19–24. 2015.
|
|
36
|
Wang B, Li L, Chi CF, Ma JH, Luo HY and Xu
YF: Purification and characterisation of a novel antioxidant
peptide derived from blue mussel (Mytilus edulis) protein
hydrolysate. Food Chem. 138:1713–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He R, Girgih AT, Malomo SA, Ju X and Aluko
RE: Antioxidant activities of enzymatic rapeseed protein
hydrolysates and the membrane ultrafiltration fractions. J Funct
Foods. 5:219–227. 2013. View Article : Google Scholar
|
|
38
|
Xie C, Kang J, Li Z, Schauss AG, Badger
TM, Nagarajan S, Wu T and Wu X: The açaí flavonoid velutin is a
potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and
IL-6 production through inhibiting NF-κB activation and MAPK
pathway. J Nutr Biochem. 23:1184–1191. 2012. View Article : Google Scholar
|