Introduction
Acute myocardial infarction (AMI) results in
myocardial necrosis and is caused by acute coronary artery disease
and persistent ischemia and hypoxia. There have been many
documented clinical cases of severe and persistent pain behind the
sternum, which cannot be completely relieved by rest and nitrate
esters. AMI is associated with an increase in serum myocardial
enzyme activity and progressive changes in the electrocardiogram,
and can cause arrhythmia, heart failure or shock and is often fatal
(1). This disease is common in
Europe and America, and appoximately 620,000 Americans suffer from
heart disease in the US each year (2).
During the development of AMI, the inflammatory
reaction plays a key role in all types of damage factors, and it
can be viewed as the intermediary link between the generation and
development of various injury factors and damage to cardiac muscle.
Under normal circumstances, inflammatory cells and vascular
endothelial cells do not attach, or hardly attach; however,
following stimulation with inflammatory factors, adhesion molecules
are rapidly activated and help inflammatory cells to attach and
accumulate in endothelial cells; this is followed by the release of
inflammatory mediators and damage to endothelial cells (3).
Matrix metalloproteinases (MMPs) are a family of
enzymes that contain at least 26 members. Previous research has
indicated that MMPs can adjust and control the synthesis and
degradation of the extracellular matrix (ECM), and they are the
main factor contributing to plaque rupture and can thus participate
in ventricular reconstruction (4). MMP-2 and MMP-9 are the most
important members of MMPs, and both are secreted by vascular smooth
muscle cells. The expression of these two MMPs is significantly
increased in patients with atherosclerotic plaque, which can cause
specific degradation of the ECM and may also cause plaque rupture,
thus leading to the development of AMI (5).
Mitogen-activated protein kinases (MAPKs) are
signaling mediators that connect cell-surface receptors to
intracellular critical regulatory targets. There is emerging
evidence indicating that the MAPK cascades regulate a variety of
cellular activities, such as cell growth, survival and death
(6). The MAPK family includes at
least three distinct subgroups, namely extracellular
signal-regulated kinase (ERK), c-Jun N-terminal kinase
(JNK)/stress-activated protein kinase (SAPK, and p38 in mammalian
cells (7). A previous study
demonstrated the transient activation of ERK in cultured rat
neonatal cardiac myocytes following exposure to hydrogen peroxide;
the inhibition of the activation of ERK exacerbated cardiomyocyte
apoptosis (8). Moreover, it has
been demonstrated that the JNK pathway is specifically activated
following the transfection of cultured neonatal rat cardiac
myocytes with recombinant adenoviral vectors expressing a
constitutively active mutant of MKK7 (or JNKK2), an upstream
activator of JNK, which contributed to the induction of
hypertrophic responses (9). As
regards the role of the p38 signaling pathway in cardiomyocyte
apoptosis, a previous study demonstrated that p38 was activated in
neonatal rat cardiomyocytes due to ischemia and that the inhibition
of p38 protected cardiac myocytes from apoptosis (10).
It has also been previously reported that osthole,
the active constituent of Cnidium monnieri extracts, has
anti-inflammatory and hepatic fat-oxidizing properties (11). Osthole has been shown to
effectively inhibit the activation of the MMP-2 promoter and its
enzyme, which may be one of the mechanisms involed in cellular
migration and invasion (12). The
present study is the first, to the best of our knowledge, which
investigated the hypothesis that osthole decreases the expression
of Toll-like receptor (TLR)2 and TLR4, nucleotide-binding
oligomerization domain-containing protein (NOD)1, NOD2, increases
endothelial nitric oxide synthase (eNOS) and decreases
cyclooxygenase-2 (COX-2) expression in a rat model of AMI. We
wished to examine whether these effects are mediated through the
inhibition of inflammatory reactions, a decrease in MMP-2
expression and the activation of MAPK cascades.
Materials and methods
Drugs, reagents and kits
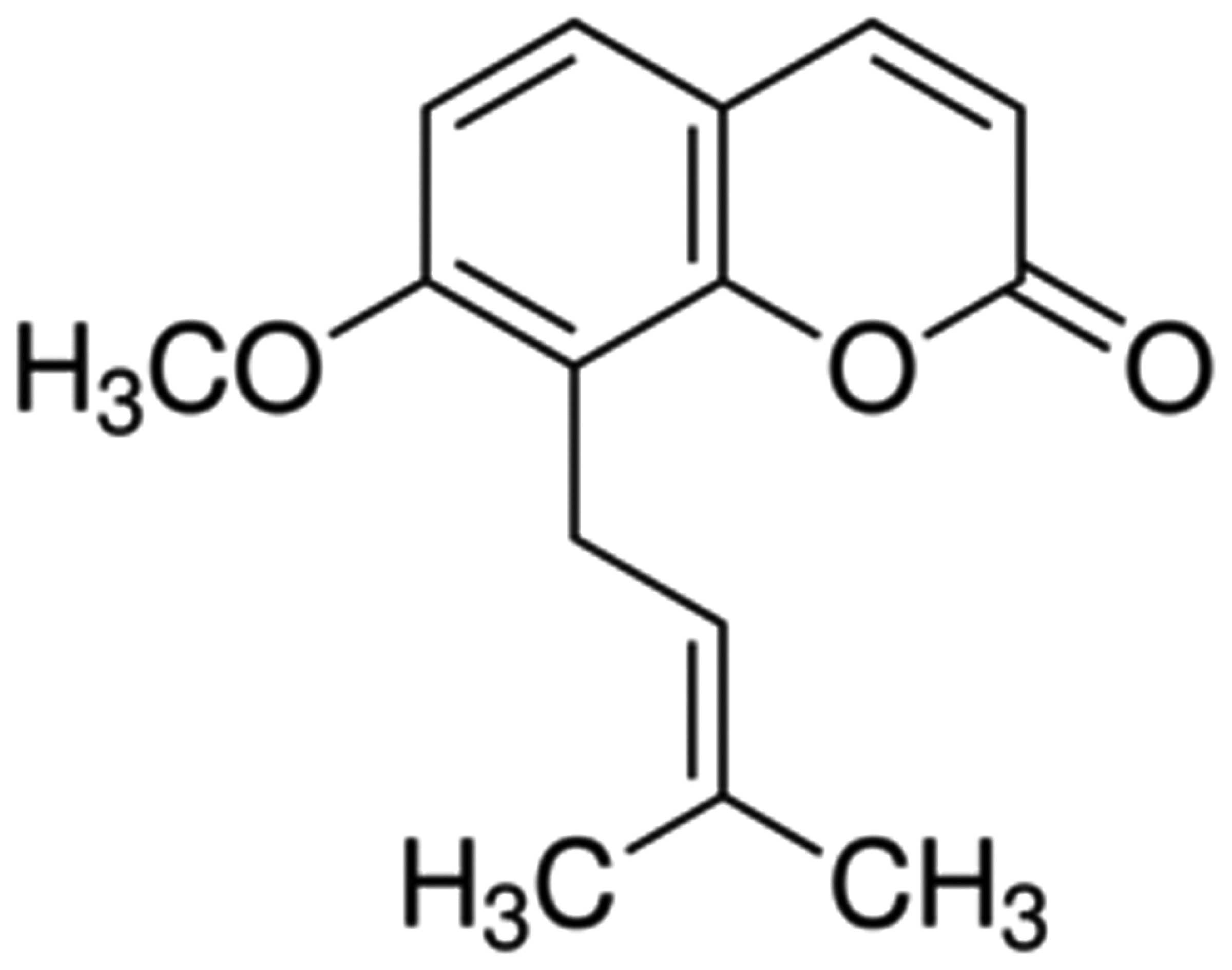
The chemical structure of osthole is depicted in
Fig. 1. Osthole (with a purity of
95%; Sigma-Aldrich Co., St. Louis, MO, USA), was dissolved in
physiological saline. Casein kinase (CK), the MB isoenzyme of
creatine kinase (CK-MB), lactate dehydrogenase (LDH) and cardiac
troponin T (cTnT) commercial kits were purchased from Sangon
Biotech (Shanghai, China). Nuclear factor-κB (NF-κB), tumor
necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 commercial
kits were purchased from Jiancheng Bioengineering (Nanjing, China).
A BCA protein assay kit was purchased from Tiangen (Beijing,
China). Caspase-3 and caspase-9 activity assay kits were purchased
from Sangon Biotech.
Experimental animals
A total of 50 healthy male Wistar rats (weighing
220–250 g) were obtained from the Experimental Animal Center of the
Second Xiangya Hospital of Central South University (Changsha,
China). All experimental procedures were approved by the Second
Xiangya Hospital of Central South University and were performed in
accordance with the ethical standards of our institution. All
experimental rats were allowed to acclimatize in enriched plastic
cages and had free access to food and clean drinking water.
Induction of AMI and group design
The rats were operated on after being
intraperitoneally anesthetized using sodium pentobarbitone (40
mg/kg). The model of AMI was created by ligating the left anterior
descending coronary artery, as previously described (13). The successful establishment of the
rat model of AMI was confirmed by the presence of regional cyanosis
on the myocardial surface and represented by the elevation of the
ST-segment. All experimental rats were randomly divided into 5
groups as follows (n=10 in each group): i) the sham-operated group;
ii) the vehicle-treated group; iii) the group treated with 1 mg/kg
osthole; iv) the group treated with 3 mg/kg osthole and v) the
group treated with 10 mg/kg osthole. The rats in the
osthole-treated groups were treated with various doses of osthole
(1, 3 and 10 mg/kg) once a day for 4 weeks. The dosage and dosage
frequency were determined on the basis of the findings of a
previous study (14). Rats in the
sham-operated group were not operated upon. Rats in the
vehicle-treated group underwent the procedure of AMI after being
injected with the same volume of physiological saline. Rats in the
osthole-treated group underwent the procedure of AMI after being
injected with osthole though the tail veil. The rats were treated
with physiological saline or osthole once a day for 4 weeks. The
rats were operated on (occlusion of the coronary artery) 30 min
after the final injection. The coronary artery was occluded with a
1–2 mm of 5-0 silk suture for encircling the left anterior
descending coronary artery below the left atrial appendage.
Measurement of infarct size
The hearts of the experimental rats were immediately
measured through the aorta and washed with physiological saline.
The coronary artery was occluded and after 6 h, the left ventricle
was placed in a freezer at −80°C for 5–10 min. Subsequently, it was
sliced into 2-mm-thick sections in order to measure the infarct
size. The infarct size of the rats was measured using 1%
2,3,5-triphenyltetrazolium chloride (1.5%; Sigma-Aldrich Co.) at
37°C for 30 min in the dark (15,16). The area of the heart without color
was regarded as the ischemic heart muscle, and the area which was
colored brick red was regarded as the normal myocardium. The size
of the infarct area was measured by calculating the volume and
weight as a percentage of the left ventricle.
Measurement of levels of plasma cardiac
marker enzymes
Serum samples of rats were extracted from the vena
cava following the occlusion of the coronary artery for 6 h. Serum
samples were then centrifuged at 1,000 × g for 25 min and were
saved for measurement at −20°C. The CK, CK-MB, LDH and cTnT
activities in the rats were analyzed using a series of commercial
kits (Sangon Biotech) according to the manufacturer's
instructions.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
The heart samples were collected, and total RNA was
extracted and purified using TRIzol reagent following the
manufacturer's instructions (Invitrogen, Carlsbad, CA, USA).
Subsequently, 1 µg of total RNA from the heart samples was
used to perform first-strand cDNA synthesis and this first-strand
cDNA was transcribed into cDNA using random hexamers (Promega,
Beijing, China) according to the manufacturer's instructions. The
StepOnePlus real-time PCR system was used to perform quantitative
(real-time) PCR (qPCR) using power SYBR-Green PCR Master Mix
(Applied Biosystems, Foster, CA, USA). Ten microliters of power
SYBR-Green PCR master mix, 1 µl of cDNA, 2 µl of
primers and 7 µl of PCR-grade water were blended and PCR was
then performed. The reactions were performed at 95°C for 10 min,
followed by 35 cycles of 95°C for 30 sec, 58°C for 45 sec, and 72°C
30 sec, 4°C for saving. The sequences for the gene-specific primers
are listed in Table I.
 | Table ISequences of gene-specific primers
used for RT-qPCR. |
Table I
Sequences of gene-specific primers
used for RT-qPCR.
| Gene | Primer sequence
(5′→3′) | Product size
(bp) |
|---|
| TLR2 |
TCTCCCATTTCCGTCTTTTT | 125 |
|
GGTCTTGGTGTTCATTATCTTC | |
| TLR4 |
GAAGCTGGTGGCTGTGGA | 213 |
|
TGATGTAGAACCCGCAAG | |
| NOD1 |
GTCACTGAGGTCCATCTGAAC | 363 |
|
CATCCACTCCTGGAAGAACCT | |
| NOD2 |
CATGTGCTGCTACGTGTTCTC | 226 |
|
CCTGCCACAATTGAAGAGGTG | |
| COX-2 |
CAAATCCTTGCTGTTCCCACCCAT | 173 |
|
GTGCACTGTGTTTGGAGTGGGTTT | |
| β-actin |
AGCGAGCATCCCCCAAAGTT | 284 |
|
GGGCACGAAGGCTCATCATT | |
Western blot analysis
The heart samples were lysed in ice-cold RIPA buffer
(Beyotine Biotechnology, Natong, China) containing 10 mM Tris (pH
8.0), 150 mM NaCl, 10% glycerol, 1% NP-40, 5 mM EDTA and protease
inhibitor cocktail. An equal amount of protein (60 µg) was
separated by electrophoresis on 8 or 10% sodium dodecyl sulfate
(SDS)-polyacrylamide (SDS-PAGE) gels and transferred onto
nitrocellulose membranes (Millipore, Billerica, MA, USA). After
blocking in 5% fat-free milk, the membranes were incubated with the
following primary antibodies: rabbit anti-eNOS (1:300; sc-49055),
rabbit anti-COX-2 (1:500; sc-7951), mouse anti-phosphorylated
(p-)JNK (Thr183/Tyr185) (1:200; sc-293136), rabbit anti-p-p38 MAPK
(Tyr182) (1:200; sc-101758), mouse anti-p-ERK (Tyr204) (1:200;
sc-377400) (all from Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) and mouse anti-β-actin (1:2,000; KC-5A08; Kangchen,
Beijing, China), overnight at 4°C. The membranes were then treated
with horseradish peroxidase-conjugated goat anti-rabbit antibody
(1:5,000; sc-2004) or goat anti-mouse antibody (1:5,000; sc-2005)
(both from Santa Cruz Biotechnology, Inc.) for 2 h. Immunodetection
was carried out with an enhanced chemiluminescence (ECL) kit
(Pierce, Burlingame, CA, USA).
Determination of plasma nitrite
production
Nitric oxide (NO) production was determined by
measuring the plasma supernatant for nitrite using Griess reagent
(Promega). The absorbance was recorded at a wavelength of 540 nm,
and the nitrite concentration was determined using sodium nitrite
as the standard.
Measurement of NF-κB, tumor necrosis
factor-α (TNF-α), IL-1β and IL-6 levels
Whole blood of rats was collected into a serum
separator tube for 30 min after a 6-h ischemic period. Serum
samples were then centrifuged at 1,000 × g for 25 min and were kept
for the measurement at −20°C. A commercial ELISA kit was used to
measure the serum levels of NF-κB, TNF-α, IL-1β and IL-6 in rats
according to the manufacturer's instructions.
Measurement of MMP-2 levels
Following treatment with osthole, MMP-9 markers were
detected by gelatin zymography assays. A total of 50 µg of
samples were absorbed into a new centrifuge tube, and an equal
volume of SDS sample buffer was then added to the centrifuge tube.
The miscible liquids were subjected to 10% SDS-PAGE electrophoresis
on a gel impregnated with 0.1% gelatin. The gel was washed 3 times
for 20 min at room temperature in 2.5% Triton X-100 to remove the
SDS after electrophoresis. The gel was incubated in reaction buffer
at 37°C for 12 h. Subsequently, the gel was stained with 0.05%
Coomassie brilliant blue R-250.
Caspase-3 and caspase-9 activity
assay
Approximately 50 µg cardiac cytosolic protein
was incubated in solution buffer at 37°C for 30 min. Briefly,
caspase-3 and caspase-9 activity was measured using a caspase-3 and
caspase-9 activity assay kit (Sangon Biotech). A total of 10
µl protein cell lysate per sample was added to 80 µl
reaction buffer with 10 µl substrate Asp-Glu-Val-Asp
(DEVD)-p-nitroaniline (pNA) and incubated at 37°C for 4–6 h.
Caspase-3 activation was measured using a microplate reader
(Bio-Rad, Hercules, CA, USA) at an absorbance of 405 nm. The change
in fluorescence (excitation at 400 nm) was detected at a wavelength
of 405 nm.
Statistical analysis
Data are expressed as the means ± SD and were
analyzed using SPSS 17.0 software. Differences between the
experimental groups were analyzed using the Student's t-test.
Values of p<0.05 were considered to indicate statistically
significant differences.
Results
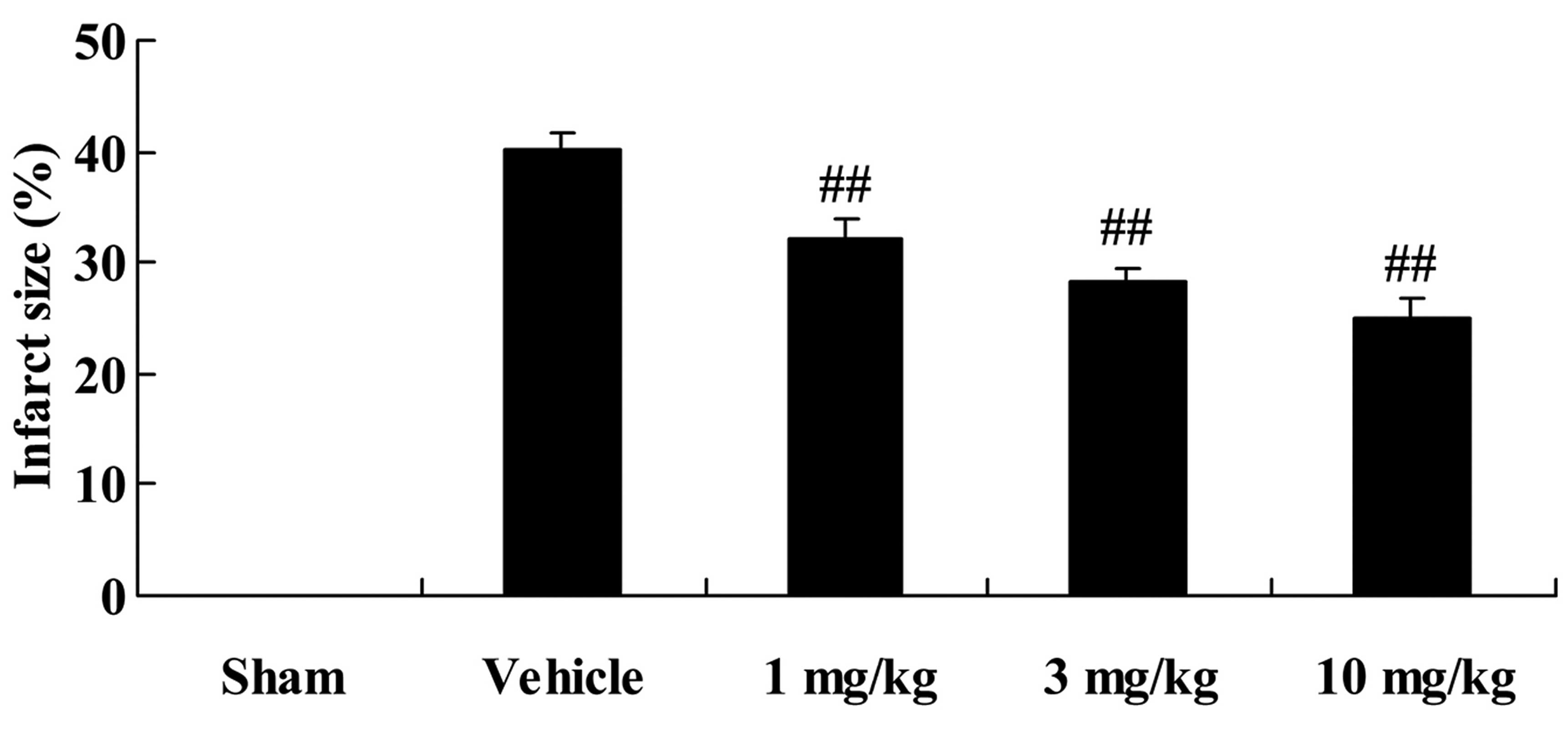
Effects of osthole on myocardial infarct
size
The chemical structure of osthole is illustrated in
Fig. 1. Firstly, we examined the
effects of osthole on the myocardial infarct size in the rats with
AMI. After the operation, the rats with AMI were treated with
various doses (1, 3 and 10 mg/kg) of osthole for 4 weeks. As shown
in Fig. 2, treatment with 1, 3
and 10 mg/kg of osthole significantly inhibited the myocardial
infarct size of the rats with AMI (p<0.01), compared with the
vehicle-treated group (Fig.
2).
Effects of osthole on the levels of
plasma cardiac marker enzymes
We examined the effects of osthole on the levels of
plasma cardiac marker enzymes. CK, CK-MB, LDH and cTnT activities
were measured to analyze its curative effects on AMI. CK, CK-MB,
LDH and cTnT activities were higher in the vehicle-treated group
compared to the sham-operated group (p<0.01; Table II). However, treatment with
osthole significantly inhibited the CK, CK-MB, LDH and cTnT
activities in the rats with AMI in a dose-dependent manner
(p<0.05 and p<0.01) compared to the vehicle-treated group
(Table II).
 | Table IIEffects of osthole on plasma cardiac
marker enzymes. |
Table II
Effects of osthole on plasma cardiac
marker enzymes.
| Groups | CK (U/ml) | CK-MB (IU/l) | LDH (U/l) | cTnT (U/ml) |
|---|
| Sham | 0.24±0.03 | 79.27±6.72 | 1836.63±292.12 | 0.07±0.04 |
| Vehicle | 0.58±0.05a | 189.00±9.31a |
3713.25±367.83a | 0.32±0.06a |
| 1 mg/kg | 0.41±0.04b | 111.75±7.45c |
3055.25±330.39b | 0.15±0.04c |
| 3 mg/kg | 0.31±0.04c | 94.47±10.99c |
2541.25±365.82c | 0.13±0.07c |
| 10 mg/kg | 0.24±0.03c | 83.60±8.32c |
2256.13±301.77c | 0.12±0.03c |
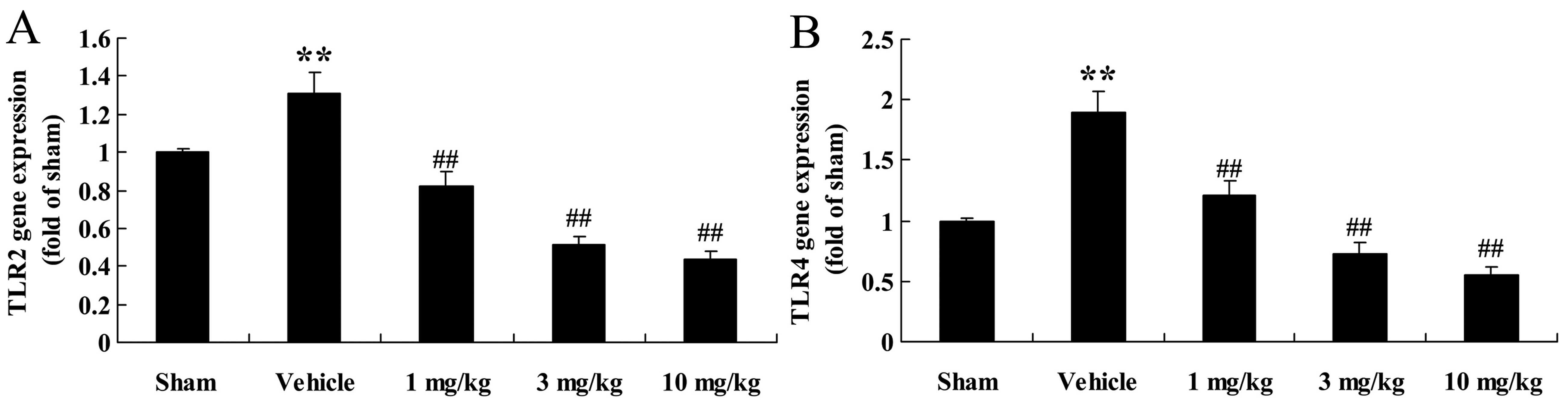
Effects of osthole on TLR2 and TLR4 gene
expression
Our results revealed that treatment with osthole
markedly decreased the mRNA expression levels of TLR2 and TLR4 in
the rats with AMI compared to the vehicle-treated group (Fig. 3).
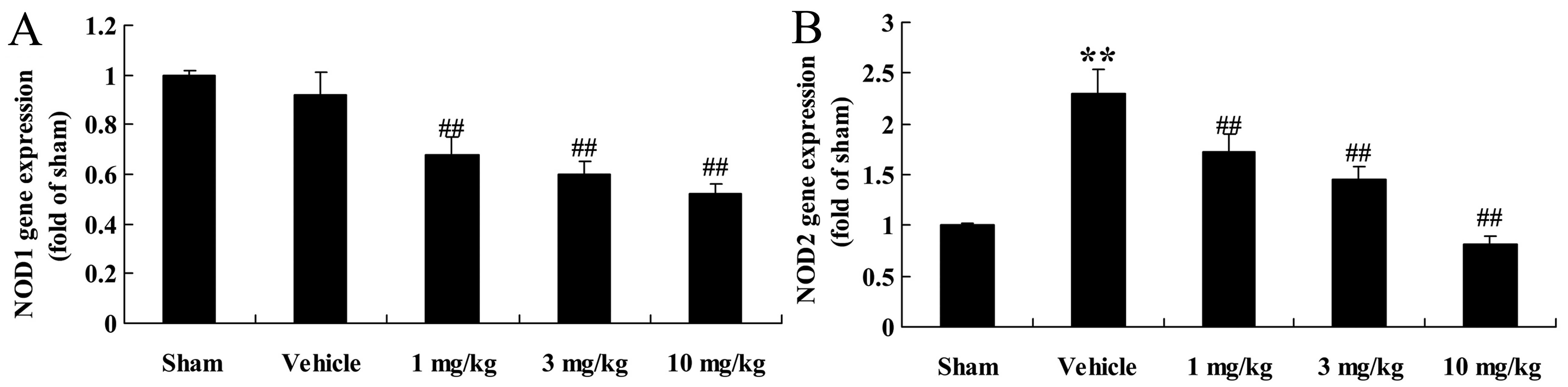
Effects of osthole on NOD1 and NOD2 gene
expression
After 4 weeks of osthole treatment, RT-qPCR was
performed to measure the mRNA expression levels of NOD1 and NOD2 in
the rats with AMI. It should be noted that there was no significant
difference in the mRNA expression of NOD1 between the sham-operated
group and the vehicle-treated group (Fig. 4A). Following treatment of the rats
with AMI with osthole at the doses of 1, 3 and 10 mg/kg, NOD1 mRNA
expression was significantly decreased in a dose-dependent manner
(p<0.01), compared to the vehicle-treated group (Fig. 4A). As regards NOD2 mRNA
expression, a signficant increase was observed in the
vehicle-treated group (p<0.01), in comparison with the
sham-operated group, although NOD2 expression was significantly
(p<0.01) downregulated in a dose-dependant manner following
treatment with osthole (Fig. 4B).
Thus, the administration of osthole at the doses of 1, 3 and 10
mg/kg reversed the increase in NOD2 mRNA expression in the rats
with AMI (Fig. 4B).
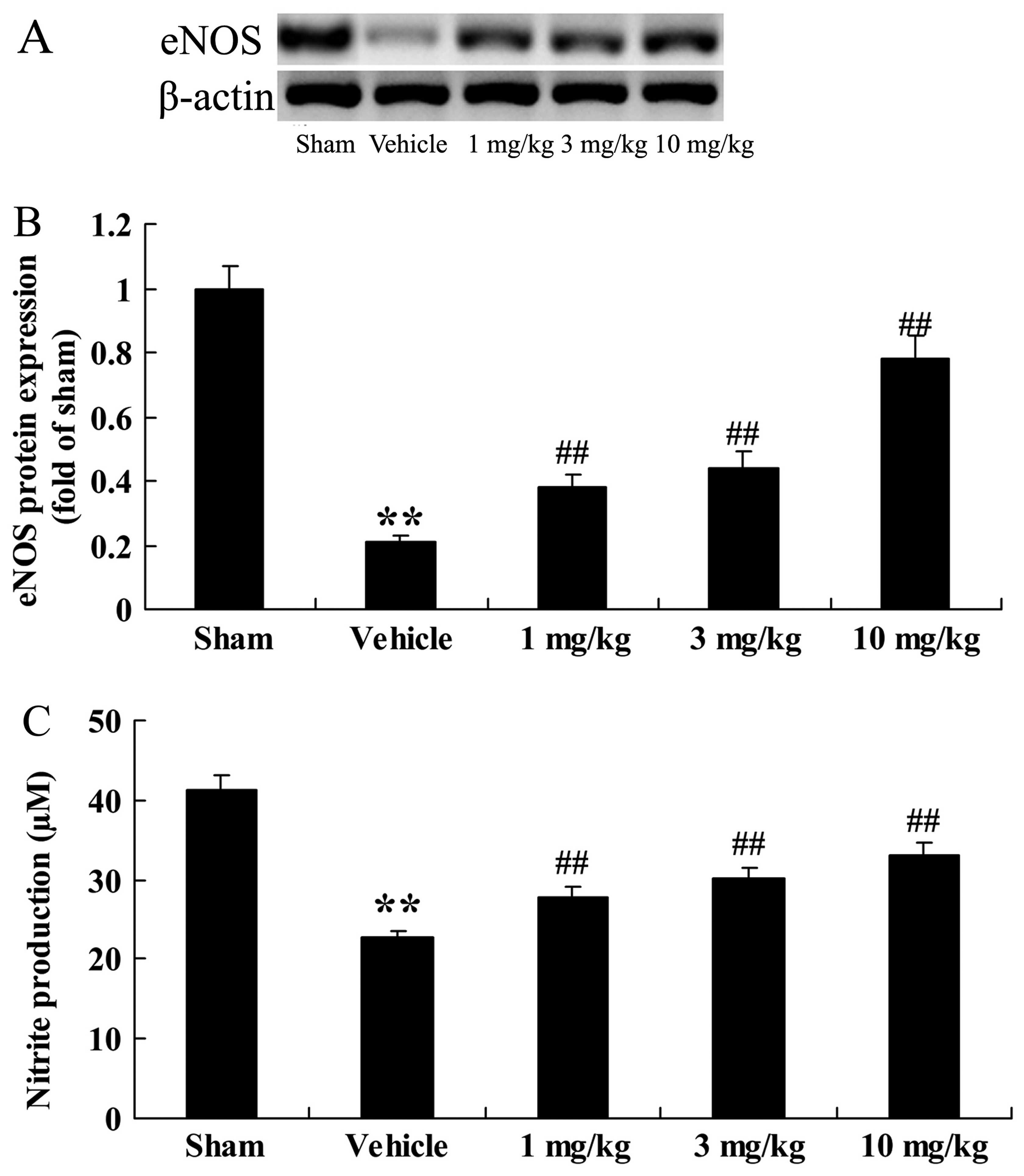
Effects of osthole on eNOS
expression
We examined the effects of osthole on eNOS
expression in the rats with AMI; eNOS protein expression and
production were examined in the rats in the different groups. As
depicted in Fig. 5A and B,
following the induction of AMI, eNOS protein expression markedly
decreased in the vehicle-treated group (p<0.01) in comparison
with the sham-operated group. Notably, compared to the
vehicle-treated group, treatment with osthole at 1, 3 and 10 mg/kg
significantly increased eNOS protein expression in the rats with
AMI (p<0.01; Fig. 5A and B).
We also noted that nitrite production was higher in the
sham-operated group (Fig. 5C).
Moreover, following treatment with osthole (1, 3 and 10 mg/kg), a
marked increase in nitrite production was noted (p<0.01)
compared to the vehicle-treated group.
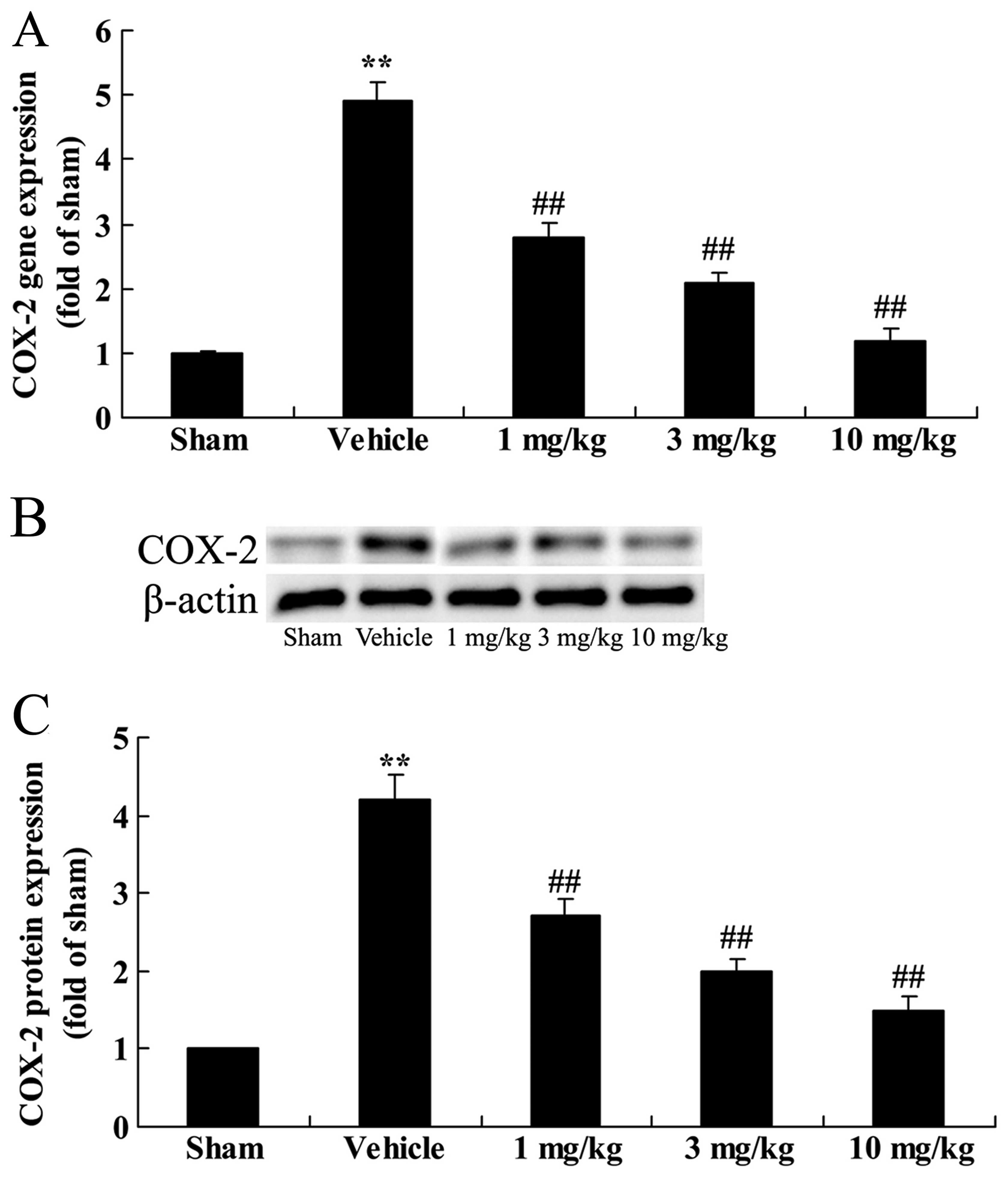
Effects of osthole on COX-2
expression
To examine the effects of osthole on COX-2
expression in the rats with AMI, we measured the expression levels
of COX-2 at the mRNA and protein level in the different groups. The
results revealed that the rats with AMI exhibited an increase in
COX-2 expression at both the mRNA (gene) and protein level in
comparison to the sham-operated group (Fig. 6). However, treatment with osthole
(1, 3 and 10 mg/kg) significantly inhibited the upregulation of
COX-2 expression in the rats with AMI (p<0.01), compared to the
vehicle-treated group.
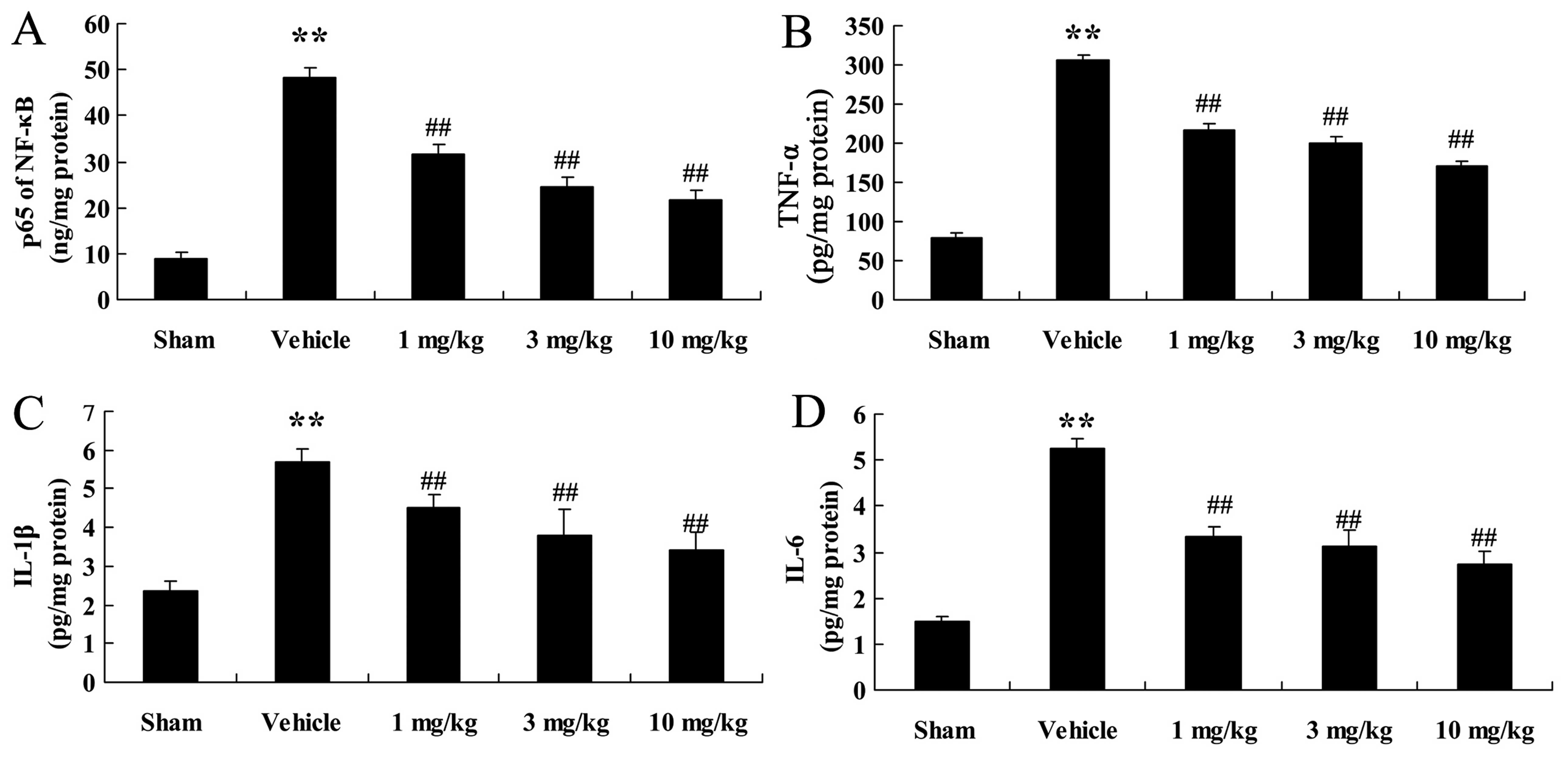
Effects of osthole on the activities of
NF-κB, TNF-α, IL-1β and IL-6
We quantified the activities of NF-κB, TNF-α, IL-1β
and IL-6 in the different groups of rats. As illustrated in
Fig. 7, NF-κB, TNF-α, IL-1β and
IL-6 activities in the rats with AMI were significantly increased
(p<0.01), in comparison wtih the sham-operated rats. However,
the levels of these inflammatory factors were significantly reduced
following treatment with osthole (1, 3 and 10 mg/kg; p<0.01;
Fig. 7).
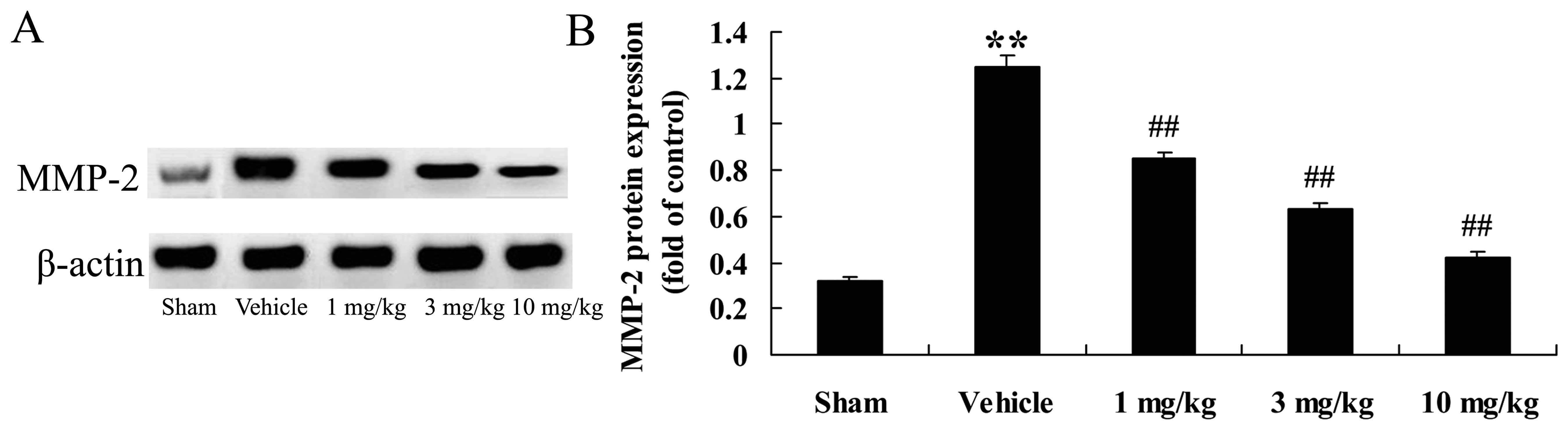
Effects of osthole on MMP-2 protein
expression
In the present study, we examined the effects of
osthole oin the inflammatory reaction. We measured MMP-2 protein
expression in the different groups of rats. Compared to the
sham-operated group, the rats with AMI (vehicle-treated group)
exhibited a significant increase in MMP-2 protein expression
(p<0.01; Fig. 8). This
AMI-induced increase in MMP-2 protein expression was reversed by
treatment with osthole (p<0.01; Fig. 8).
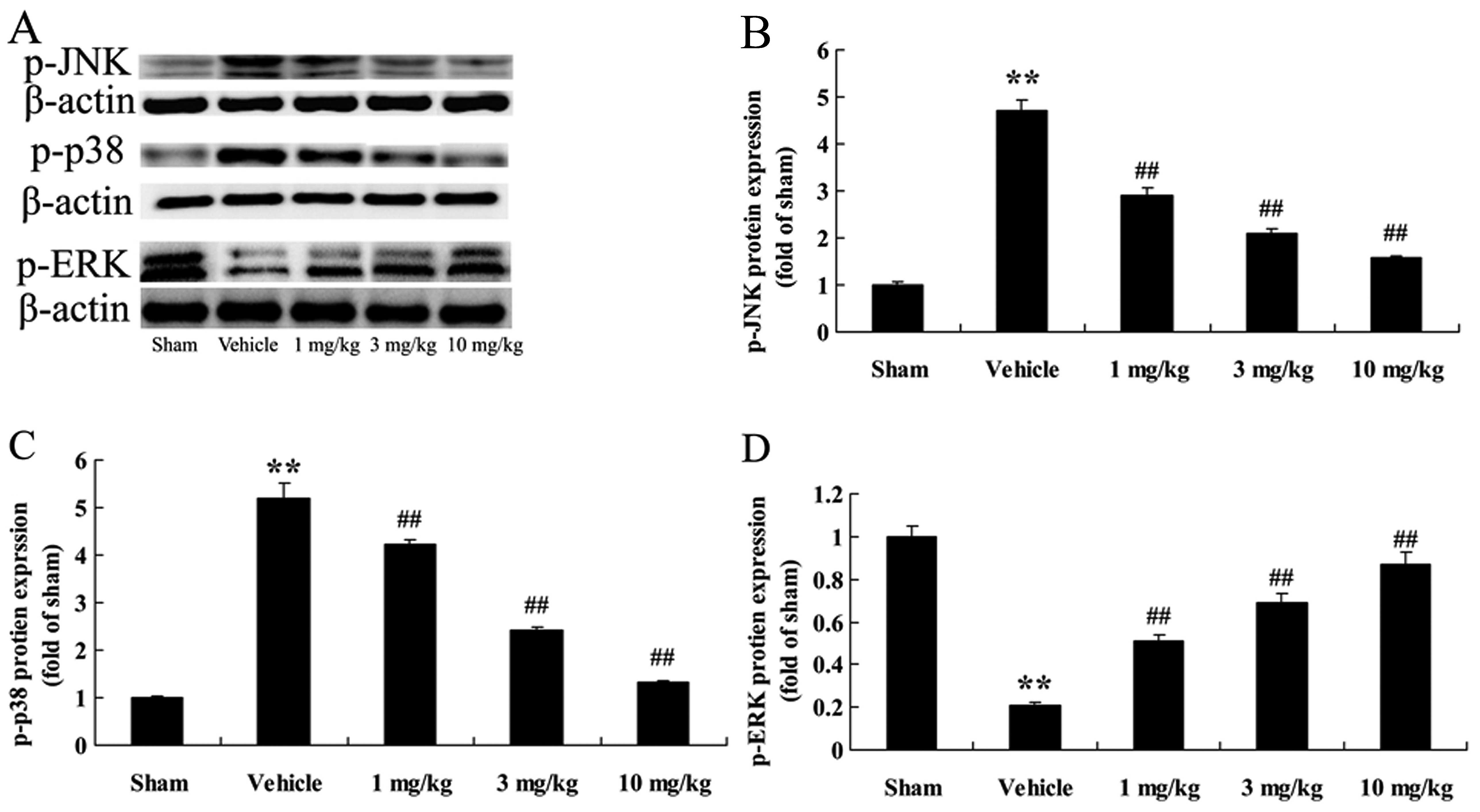
Effects of osthole on the phosphorylation
of MAPK (JNK, p38 MAPK and ERK1/2) proteins
In order to further explore the cardioprotective
mechanisms of action of osthole, the phosphorylation levels of JNK,
p38 MAPK and ERK1/2 (p-JNK, p-p38 and p-ERK1/2) were examined by
western blot analysis. We found that compared with the
sham-operated group, the rats with AMI (vehicle-treated group)
exhibited an increase in p-JNK and p-p38 protein expression
(p<0.01), in comparison with the sham-operated group (Fig. 9A–C); however, treatment with
osthole reduced p-JNK and p-p38 expression in a dose-dependant
manner. We noted that p-ERK1/2 protein expression in the
vehicle-treated group was effectively suppressed, compared to the
sham-operated group (Fig. 9A and
D). However, treatment with osthole (1, 3 and 10 mg/kg)
significanlty increased p-ERK1/2 epxression (p<0.01) compared to
the vehicle-treated group. As expected, pre-treatment with osthole
reversed the effects of AMI on MAPK signaling pathways in the rats
with AMI rats.
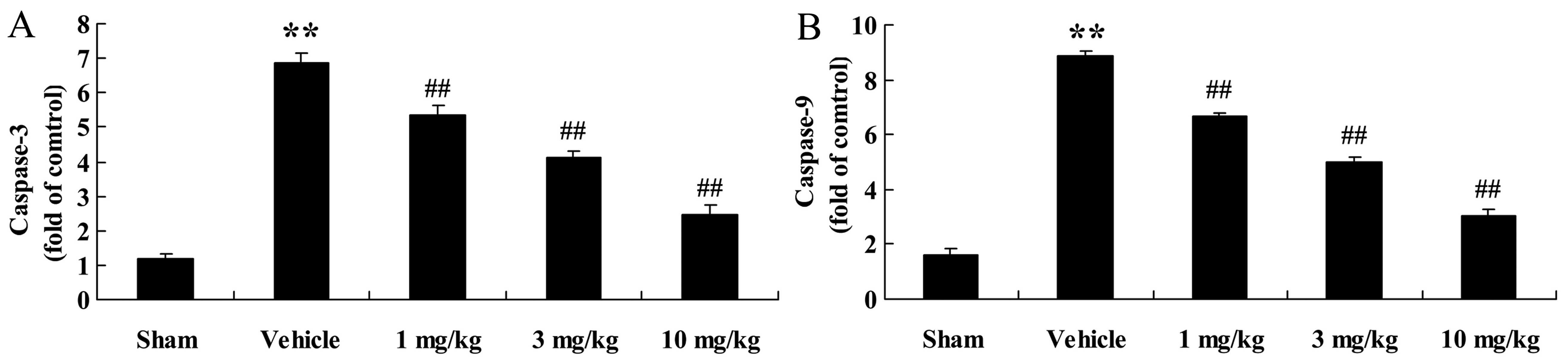
Effects of osthole on caspase-3 and
caspase-9 activities
The apoptotic pathway was also examined in the
present study. We observed a significant increase in caspase-3 and
caspase-9 activities in the rats with AMI (p<0.01) compared to
the sham-operated group (Fig.
10). However, these effects were significantly reversed by
treatment with osthole.
Discussion
In patients with AMI, the reduction of myocardial
cell viability and ventricular remodeling are the main factors
responsible for heart failure and even death in the long-term
(17). With the widespread
application of emergency thrombolysis, emergency interventional
therapy and coronary bypass surgery, the mortality rate of patients
suffering from AMI has been greatly reduced and the number of
survivors has increased significantly (18). In this study, we found that
treatment with osthole markedly reduced the levels of CK, CK-MB,
LDH and cTnT in the serum of rats with AMI. In particular, Wei
et al reported that osthole was capable of reducing
hyperalgesia due to lumbar disc herniation (19). In the present study, the infarct
size of rats with AMI was significantly reduced following treatment
with osthole; however, the exact mechanisms involved require
further investigation. This reduction in the infarct size, however,
indicates that osthole exerts protective effects on heart tissue
and may protect against AMI (as shown in our rat model).
TLRs are an integral component of the inflammation
process. TLR2 and TLR4 are the most common and most well
characterized as regards inflammatory responses in AMI. They are
expressed predominantly in monocytes/macrophages and neutrophils.
TLR2 is involved in the recognition of inflammatory reactions in
AMI and plays an important role in the immune system. The
peptidoglycan subunits are recognized by the NOD family proteins,
in particular by NOD1 and NOD 2. NOD1 and NOD2 are involved
primarily in mediating antibacterial and anti-inflammatory
defenses. They are intracellular proteins involved in innate
immunity and are associated with chronic inflammatory diseases in
humans. In the present study, we demonstrated that there was a
marked increase in TLR2, TLR4 and NOD2 gene expression in the rats
with AMI compared to the sham-operated rats, and this effect was
suppressed in a dose-dependent manner by treatment with osthole,
implying that the cardioprotective effects of osthole are achieved
through mediating TLR2/4 and NOD1/2 expression.
Previous research has demonstrated that NO exerts a
cardioprotective effect against AMI. The effects of NO on
myocardial infarction are associated with both its pro-and
anti-apoptotic properties depending on the source of NO (20). NO is generated from the
guanidinium group of L-arginine in an NADPH-dependent reaction
catalyzed by NO synthase (NOS). At present, there are three known
isoforms of NOS, including neuronal NOS (nNOS or NOS1), inducible
NOS (iNOS or NOS2) and endothelial cell NOS (eNOS or NOS3). Of
these isoforms, eNOS is not only expressed in the endocardium and
endothelium of the coronary vasculature, but is also found in
cardiac myocytes and in specialized cardiac conduction systems,
such as sinoatrial and atrioventricular nodal tissues (21). It is widely accepted that NO
produced from eNOS in the heart plays a critical role in coronary
vasodilation and the inhibition of mitochondrial O2
consumption. A previous study indicated that myocardial injury was
exacerbated in eNOS−/− mice following ischemic insults,
suggesting a protective effect of eNOS-derived NO (22). In the present study, we found that
osthole significantly and dose-dependently promoted the activation
of eNOS and decreased COX-2 expression in rats with AMI. A previous
study also demonstrated that osthole relaxed the pulmonary arteries
via the Akt-eNOS-NO signaling pathway in rats (23). Yang et al also reported
that osthole attenuated focal segmental glomerulosclerosis by
inhibiting NF-κB-mediated COX-2 expression (24).
TNF-α, IL-1β and IL-6 are closely related to disease
severity and the prognosis of patients (25). In a previous study, it has been
verified that, in atherosclerosis, TNF-α, IL-1, IL-8 and other
inflammatory factors are activated through NF-κB. It was verified
that activated NF-κB exists in smooth muscle cells, macrophages and
endothelial cells in atherosclerosis (26). Therefore, it is clear that
activated NF-κB participates in the process of ventricular
remodeling. In the present study, we demonstrated that osthole
reduced the activities of NF-κB, TNF-α, IL-1β and IL-6 in AMI rats.
A previous study found that osthole ameliorated renal
ischemia-reperfusion injury by restraining the inflammatory
response (27). In another
previous study, osthole was also reported to weaken the
inflammatory reaction following permanent middle cerebral artery
occlusion in rats (28).
MMPs are secreted by myocardial cells, vascular
endothelial cells, smooth muscle cells, foam cells and other cells,
including the protease family that depends on Ca2+ and
Zn2+. It has been demonstrated that MMPs are proteases
which adjust and control the ECM, and they can promote the
degradation of the ECM, accelerate the development of atheromatous
plaque and lead to the formation of unstable plaque (29,30). Prior research has indicated that
MMP-2 reflects the status of atherosclerotic plaque, which plays an
important role in a series of processes of occurrences and the
development of AMI (31). In the
present study, we demonstrated that treatment with osthole
decreased the expression of MMP-2. Similarly, osthole was
previously found to reduce the levels of MMP-2 and MMP-9 in the
A549 human lung cancer cells (32). Moreover, Yang et al
reported that osthole effectively inhibited the MMP-2 promoter in
breast cancer cells (33).
Emerging evidence implies that MAPK signaling
cascades are involved in cardiac myocyte apoptosis. The MAPK family
contains three major subgroups, namely ERK, JNK and p38 MAPK. These
are activated in response to myocardial infarction. It is generally
believed that the inhibition of the ERK pathway enhances
ischemia/reoxygen ation-induced apoptosis in cultured cardiac
myocytes and exacerbates myocardial injury in isolated rat hearts
following ischemia-reperfusion (7). Liu et al (34) also reported that the
phosphorylation of ERK was decreased in a rat model of AMI, and
that treatment with baicalin potently suppressed this reduction.
The present study demonstrated a marked decrease in p-ERK levels in
rats with AMI, which is consistent with the results of previous
studies (34) and, more
importantly, there was an evident increase in the phosphorylated
levels of ERK following treatment with osthole. This implies that
the activation of the ERK cascade should be regarded as a critical
signaling pathway for the cardoioprotective effect of osthole in
rats with AMI. By contrast, it has been demonstrated that the
activation of JNK and p38 MAPK exerts deleterious effects on
post-ischemic myocardial apoptosis and cardiac function recovery
(35). It was previously found
that the inhibition of JNK1 markedly suppressed
reoxygenation-induced apoptosis in rat cardiac myocytes (36). However, the activation of JNK by
transfection with MKK7 in cardiomyocytes, an upstream activator of
JNK, has been shown to induce myocardial hypertrophy, but not
apoptosis (9). In terms of p38
MAPK, the inhibition of the cardiac p38 MAPK pathway by SB203580, a
specific inhibitor, was shown to delay ischemic cell death in the
pig myocardium (37). In line
with these findings, our data demonstrated a marked elevation in
p-JNK (the activated form of JNK) and p-p38 (the activated form of
p38) in rats with AMI. Furthermore, treatment with osthole
significantly diminished the expression of p-JNK and p-p38 in the
rats with AMI. Taken together, the activation of ERK and the
suppression of JNK, as well as p38 signaling pathways are conceived
of as one of the most important cardioprotective mechanisms of
osthole against myocardial infarction-induced cardiomyocyte
apoptosis in rats.
Myocardial infarction often occurs when the
myocardial blood supply is interrupted suddenly or persistently,
and this contributes to marked cardiomyocyte apoptosis. Caspases
are evolutionarily conserved cysteinyl proteases which play a
pivotal role in the initiation and execution of cellular apoptosis
(38); caspase-3 serves as an
executioner molecule and is the most abundant caspase under normal
and pathological conditions. A previous study illustrated that
caspase-3 activated caspase-activated DNase, and this eventually
led to DNA fragmentation and cell loss (39). In addition, a marked elevation in
caspase-3 has been observed in Wistar rats with
isoproterenol-induced AMI (40).
The results from our current study revealed that the levels of
caspase-3 in the infarcted rat hearts were altered and that
treatment with osthole downregulated caspase-3 in the rats with
AMI. It is plausible that the beneficial effects of osthole are
associated with its regulation of caspase-3, finally exerting
anti-apoptotic effects on infarcted rat hearts. Of note, treatment
with osthole reduced caspase-3 and caspase-9 activities, which
implies that osthole protects myocardial cells and decreases
cellular apoptosis. In a previous study, it was demonstrated that
osthole significantly reduced the activity of caspase-3 in PC12
cells (41). Ding et al
emphasized that osthole inhibited the proliferation of human
osteosarcoma cells through the expression of Bcl-2, Bax and
caspase-3 (42). Zheng et
al also illustrated that osthole ameliorated renal
ischemia-reperfusion injury in rats by decreasing caspase-3
activity (43).
In conclusion, this study demonstrated the
cardioprotective effects of osthole, and that its cardioprotective
effects may be associated with the inhibition of inflammatory
reactons, the decrease in MMP-2 expression and the activation of
p-ERK. These findings suggest that osthole has the potential to be
used as a drug for the treatment of AMI.
References
|
1
|
Bradshaw PJ, Trafalski S, Hung J, Briffa
TG and Einarsdóttir K: Outcomes after first percutaneous coronary
intervention for acute myocardial infarction according to patient
funding source. BMC Health Serv Res. 14:4052014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al:
Heart disease and stroke statistics - 2014 update: a report from
the American Heart Association. Circulation. 129:e28–e292. 2014.
View Article : Google Scholar
|
|
3
|
Wollenweber T, Roentgen P, Schäfer A,
Schatka I, Zwadlo C, Brunkhorst T, Berding G, Bauersachs J and
Bengel FM: Characterizing the inflammatory tissue response to acute
myocardial infarction by clinical multimodality noninvasive
imaging. Circ Cardiovasc Imaging. 7:811–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Könnecke H and Bechmann I: The role of
microglia and matrix metalloproteinases involvement in
neuroinflammation and gliomas. Clin Dev Immunol. 2013:9141042013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Squire IB, Evans J, Ng LL, Loftus IM and
Thompson MM: Plasma MMP-9 and MMP-2 following acute myocardial
infarction in man: correlation with echocardiographic and
neurohumoral parameters of left ventricular dysfunction. J Card
Fail. 10:328–333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schaeffer HJ and Weber MJ:
Mitogen-activated protein kinases: specific messages from
ubiquitous messengers. Mol Cell Biol. 19:2435–2444. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee
JC, Feuerstein GZ, Thomas H, Maleeff B and Ohlstein EH: Inhibition
of extracellular signal-regulated kinase enhances
Ischemia/Reoxygenation-induced apoptosis in cultured cardiac
myocytes and exaggerates reperfusion injury in isolated perfused
heart. Circ Res. 86:692–699. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aikawa R, Komuro I, Yamazaki T, Zou Y,
Kudoh S, Tanaka M, Shiojima I, Hiroi Y and Yazaki Y: Oxidative
stress activates extracellular signal-regulated kinases through Src
and Ras in cultured cardiac myocytes of neonatal rats. J Clin
Invest. 100:1813–1821. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Su B, Sah VP, Brown JH, Han J and
Chien KR: Cardiac hypertrophy induced by mitogen-activated protein
kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase
in ventricular muscle cells. J Biol Chem. 273:5423–5426. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mackay K and Mochly-Rosen D: An inhibitor
of p38 mitogen-activated protein kinase protects neonatal cardiac
myocytes from ischemia. J Biol Chem. 274:6272–6279. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nam HH, Jun DW, Jeon HJ, Lee JS, Saeed WK
and Kim EK: Osthol attenuates hepatic steatosis via decreased
triglyceride synthesis not by insulin resistance. World J
Gastroenterol. 20:11753–11761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen R, Xue J and Xie M: Osthole regulates
TGF-β1 and MMP-2/9 expressions via activation of PPARα/γ in
cultured mouse cardiac fibroblasts stimulated with angiotensin II.
J Pharm Pharm Sci. 16:732–741. 2013.
|
|
13
|
Yu HP, Liu FC, Tsai YF and Hwang TL:
Osthole attenuates hepatic injury in a rodent model of
trauma-hemorrhage. PLoS One. 8:e659162013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XY, Dong WP, Bi SH, Pan ZG, Yu H,
Wang XW, Ma T, Wang J and Zhang WD: Protective effects of osthole
against myocardial ischemia/reperfusion injury in rats. Int J Mol
Med. 32:365–372. 2013.PubMed/NCBI
|
|
15
|
Hoda MN, Li W, Ahmad A, Ogbi S, Zemskova
MA, Johnson MH, Ergul A, Hill WD, Hess DC and Sazonova IY:
Sex-independent neuroprotection with minocycline after experimental
thromboembolic stroke. Exp Transl Stroke Med. 3:162011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoda MN, Siddiqui S, Herberg S,
Periyasamy-Thandavan S, Bhatia K, Hafez SS, Johnson MH, Hill WD,
Ergul A, Fagan SC, et al: Remote ischemic perconditioning is
effective alone and in combination with intravenous tissue-type
plasminogen activator in murine model of embolic stroke. Stroke.
43:2794–2799. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kilickesmez KO, Bingöl G, Bulut L, Sinan
UY, Abaci O, Ersanli M and Gurmen T: Relationship between serum
endothelin-1 level and spontaneous reperfusion in patients with
acute myocardial infarction. Coron Artery Dis. 26:37–41. 2015.
View Article : Google Scholar
|
|
18
|
Dziewierz A, Rakowski T and Dudek D:
Abciximab in the management of acute myocardial infarction with
ST-segment elevation: evidence-based treatment, current clinical
use, and future perspectives. Ther Clin Risk Manag. 10:567–576.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei M, Mo SL, Nabar NR, Chen Y, Zhang JJ,
He QL, Zou XN, Liu XG, Sun LB and Zhou SF: Modification of rat
model of sciatica induced by lumber disc herniation and the
anti-inflammatory effect of osthole given by epidural
catheterization. Pharmacology. 90:251–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang G, Fang Z, Liu Y, Zhang H, Shi X, Ji
Q, Lin Q and Lin R: Protective effects of Chinese traditional
medicine buyang huanwu decoction on myocardial injury. Evid Based
Complement Alternat Med. 2011:9303242011. View Article : Google Scholar :
|
|
21
|
Balligand JL and Cannon PJ: Nitric oxide
synthases and cardiac muscle. Autocrine and paracrine influences.
Arterioscler Thromb Vasc Biol. 17:1846–1858. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sumeray MS, Rees DD and Yellon DM: Infarct
size and nitric oxide synthase in murine myocardium. J Mol Cell
Cardiol. 32:35–42. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao L, Lu P, Li Y, Yang L, Feng H, Huang
Y, Zhang D, Chen J and Zhu D: Osthole relaxes pulmonary arteries
through endothelial phosphatidylinositol 3-kinase/Akt-eNOS-NO
signaling pathway in rats. Eur J Pharmacol. 699:23–32. 2013.
View Article : Google Scholar
|
|
24
|
Yang SM, Chan YL, Hua KF, Chang JM, Chen
HL, Tsai YJ, Hsu YJ, Chao LK, Feng-Ling Y, Tsai YL, et al: Osthole
improves an accelerated focal segmental glomerulosclerosis model in
the early stage by activating the Nrf2 antioxidant pathway and
subsequently inhibiting NF-κB-mediated COX-2 expression and
apoptosis. Free Radic Biol Med. 73:260–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stein A, Mohr F, Laux M, Thieme S, Lorenz
B, Cetindis M, Hackl J, Groha P, Demetz G, Schulz S, et al:
Erythropoietin-induced progenitor cell mobilisation in patients
with acute ST-segment-elevation myocardial infarction and
restenosis. Thromb Haemost. 107:769–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Turner NA, Das A, Warburton P, O'Regan DJ,
Ball SG and Porter KE: Interleukin-1alpha stimulates
proinflammatory cytokine expression in human cardiac
myofibroblasts. Am J Physiol Heart Circ Physiol. 297:H1117–H1127.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng Y, Lu M, Ma L, Zhang S, Qiu M and Ma
X: Osthole ameliorates renal ischemia-reperfusion injury by
inhibiting inflammatory response. Urol Int. 91:350–356. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chao X, Zhou J, Chen T, Liu W, Dong W, Qu
Y, Jiang X, Ji X, Zhen H and Fei Z: Neuroprotective effect of
osthole against acute ischemic stroke on middle cerebral ischemia
occlusion in rats. Brain Res. 1363:206–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang KF, Huang PH, Chiang CH, Hsu CY, Leu
HB, Chen JW and Lin SJ: Usefulness of plasma matrix
metalloproteinase-9 level in predicting future coronary
revascularization in patients after acute myocardial infarction.
Coron Artery Dis. 24:23–28. 2013. View Article : Google Scholar
|
|
30
|
Periasamy S, Mo FE, Chen SY, Chang CC and
Liu MY: Sesamol attenuates isoproterenol-induced acute myocardial
infarction via inhibition of matrix metalloproteinase-2 and -9
expression in rats. Cell Physiol Biochem. 27:273–280. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai F, Xiao GL and Pan J: Correlation
between serum MMP-2 and MMP-9 in patients with acute myocardial
infarction before and after PCI. Xi Bao Yu Fen Zi Mian Yi Xue Za
Zhi. 25:716–718. 2009.In Chinese. PubMed/NCBI
|
|
32
|
Xu XM, Zhang Y, Qu D, Feng XW, Chen Y and
Zhao L: Osthole suppresses migration and invasion of A549 human
lung cancer cells through inhibition of matrix metalloproteinase-2
and matrix metallopeptidase-9 in vitro. Mol Med Rep. 6:1018–1022.
2012.PubMed/NCBI
|
|
33
|
Yang D, Gu T, Wang T, Tang Q and Ma C:
Effects of osthole on migration and invasion in breast cancer
cells. Biosci Biotechnol Biochem. 74:1430–1434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu X, Gu J, Fan Y, Shi H and Jiang M:
Baicalin attenuates acute myocardial infarction of rats via
mediating the mitogen-activated protein kinase pathway. Biol Pharm
Bull. 36:988–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shimada K, Nakamura M, Ishida E, Kishi M
and Konishi N: Roles of p38- and c-jun NH2-terminal kinase-mediated
pathways in 2-methoxyestradiol-induced p53 induction and apoptosis.
Carcinogenesis. 24:1067–1075. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hreniuk D, Garay M, Gaarde W, Monia BP,
McKay RA and Cioffi CL: Inhibition of c-Jun N-terminal kinase 1,
but not c-Jun N-terminal kinase 2, suppresses apoptosis induced by
ischemia/reoxygenation in rat cardiac myocytes. Mol Pharmacol.
59:867–874. 2001.PubMed/NCBI
|
|
37
|
Barancik M, Htun P, Strohm C, Kilian S and
Schaper W: Inhibition of the cardiac p38-MAPK pathway by SB203580
delays ischemic cell death. J Cardiovasc Pharmacol. 35:474–483.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang W, Xu J and Li L, Wang P, Ji X, Ai H,
Zhang L and Li L: Neuroprotective effect of morroniside on focal
cerebral ischemia in rats. Brain Res Bull. 83:196–201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanaka M, Mokhtari GK, Terry RD, Balsam
LB, Lee KH, Kofidis T, Tsao PS and Robbins RC: Overexpression of
human copper/zinc superoxide dismutase (SOD1) suppresses
ischemia-reperfusion injury and subsequent development of graft
coronary artery disease in murine cardiac grafts. Circulation.
110(Suppl 1): II200–II206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo J, Li HZ, Wang LC, Zhang WH, Li GW,
Xing WJ, Wang R and Xu CQ: Increased expression of calcium-sensing
receptors in atherosclerosis confers hypersensitivity to acute
myocardial infarction in rats. Mol Cell Biochem. 366:345–354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shokoohinia Y, Hosseinzadeh L, Moieni-Arya
M, Mostafaie A and Mohammadi-Motlagh HR: Osthole attenuates
doxorubicin-induced apoptosis in PC12 cells through inhibition of
mitochondrial dysfunction and ROS production. BioMed Res Int.
2014:1568482014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ding Y, Lu X, Hu X, Ma J and Ding H:
Osthole inhibits proliferation and induces apoptosis in human
osteosarcoma cells. Int J Clin Pharmacol Ther. 52:112–117. 2014.
View Article : Google Scholar
|
|
43
|
Zheng Y, Lu M, Ma L, Zhang S, Qiu M and
Wang Y: Osthole ameliorates renal ischemia-reperfusion injury in
rats. J Surg Res. 183:347–354. 2013. View Article : Google Scholar : PubMed/NCBI
|