Introduction
Radiocontrast agents are a type of medical contrast
medium which are used when performing computed-tomography or
angiography to improve visibility and observe vessels and changes
in tissues more clearly. These agents are cleared mainly through
glomerular filtration (1).
However, these agents are associated with adverse effects, mainly
radiocontrast-induced nephropathy (RIN), which is one of the
leading causes of hospital-acquired acute kidney injury (AKI), and
accounts for approximately 10% of all causes (2). Some patients may suffer from stage 3
AKI and may thus require dialysis (3,4).
Once RIN has developed, the mortality rate increases significantly
(4). At least three mechanisms,
renal vasoconstriction, increased oxidative stress and direct renal
tubular toxicity are known to be involved in the pathophysiology of
RIN (5). Clinically, the standard
prophylaxis for RIN is intravenous hydration and/or the oral
administration of N-acetylcysteine (NAC). Intravenous hydration is
mainly used to maintain renal perfusion in order to overcome
radiocontrast-induced renal vasoconstriction. However, hydration is
contraindicated for some cases, such as congestive heart failure
and chronic kidney disease. The antioxidant agent, NAC, acts as
free radical scavenger. However, certain meta-analyses and
randomized trials have demonstrated variable and inconsistent
outcomes (6,7). Therefore, the development of a novel
strategy for the prevention of RIN is required.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a
natural polyphenolic compound found in several plants (e.g., grape
skins) (8). The reno-protective
effects of resveratrol, particularly those regarding diabetic
nephropathy, have been attributed to its antioxidant and
anti-inflammatory effects (9).
Thus, the aim of the present study was to investigate the
protective effects of resveratrol against toxicity induced by the
radiocontrast agent, ioxitalamate, in human renal proximal tubule
epithelial cells in vitro.
Materials and methods
Cell and cell culture
The human renal proximal tubule epithelial cell
line, HK-2, immortalized by transduction with human papilloma virus
16 (HPV-16) E6/E7 genes, was purchased from the Bioresource
Collection and Research Center (BCRC), Hsin-Chu, Taiwan. The HK-2
cells were maintained in keratino-cyte serum-free medium (KSFM)
supplemented with 5 ng/ml recombinant epidermal growth factor and
40 ng/ml bovine pituitary extract (Invitrogen, Carlsbad, CA, USA),
and cultured in 5% CO2 at 37°C in a humidified
incubator, as previously described (10).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The HK-2 cells were seeded in 96-well plates at
1×104 cells/well and used to evaluate cell viability
following treatment with ioxitalamate (Telebrix™; Guerbet, Paris,
France) and/or resveratrol (Tocris Bioscience, Minneapolis, MN,
USA), NAC (Sigma-Aldrich, St. Louis, MO, USA), necrostatin-1
(Nec-1) (BioVision, Inc., Milpitas, CA, USA), sirolimus and
everolimus (both from LC Laboratories, Woburn, MA, USA), and EX-527
and SRT-1720 (both from Selleck Chemicals, Houston, TX, USA). The
HK-2 cells were incubated with complete medium at 37°C overnight
first, then treated with the above-mentioned agent(s) for 48 h.
Subsequently, MTT solution (Sigma-Aldrich) was added to each well
followed by incubation for 4 h. The supernatant was then removed,
and 100 μl of dimethyl sulfoxide (DMSO) (J.T. Baker Chemical
Co., Phillipsburg, NJ, USA) was added to each well. The absorbance
at 565 nm was measured using an enzyme-linked immunosorbent assay
(ELISA) reader and cell viability was calculated according to the
following formula: cell viability = (absorbance of the experimental
group)/(absorbance of reference group) ×100. The reference group
was treated with an equal volume of phosphate-buffered saline (PBS)
as the control. In some experiments (the cytotoxicity of
ioxitalamate plus the autophagy inducers, sirolimus or everolimus,
as well as the cytotoxicity of ioxitalamate plus the SIRT1
inhibitor, EX-527, or the SIRT1 activator, SRT-1720), ioxitalamate
was considered as the control in order to elucidate the mechanisms
involved.
Flow cytometry
Flow cytometry was performed using the Annexin
V-FITC apotosis detection kit (BD Biosciences, San Jose, CA, USA).
Cell death resulting from either apoptosis or necrosis following
treatment with ioxitalamate was analyzed by flow cytometry (BD
Biosciences) with conventional protocols (11). The Cyto-ID® autophagy
detection kit (Enzo Life Sciences, Inc., Farmingdale, NY, USA) was
used for the staining of autophagic vacuoles, as previously
described (12).
DNA damage assay
To evaluate DNA fragmentation in HK-2 cells, the
cell death detection ELISAPlus kit (Roche, Mannheim,
Germany) was used. The HK-2 cells were seeded in 12-well plates at
1×104 cells/well. The supernatant of the culture medium
and cytoplasmic fraction was collected following exposure to
ioxitalamate and/or resveratrol for 48 h. To determine the
occurence of oxidative DNA damage, the OxiSelect™ Oxidative DNA
Damage ELISA kit (Cell Biolabs, Inc., San Diego, CA, USA) was used
for the detection and quantification of 8-hydroxy-2′-deoxyguanosine
(8-OHdG).
Reactive oxygen species (ROS) assay
ROS assay was carried out using the OxiSelect™
Intracellular ROS Assay kit (Cell Biolabs, Inc.). The intensity of
green fluorescence is proportional to the levels of ROS production
in the cytoplasm of HK-2 cells. The cells were photographed using
an Olympus fluorescence microscope (BX43; magnification, ×400) with
a Panasonic DMC-G1 digital camera following exposure to
ioxitalamate and/or resveratrol for 48 h.
Western blot analysis
The HK-2 cells were homogenized in cell lysis buffer
(Cell Signaling Technology, Inc., Danvers, MA, USA), and the
protein concentrations were determined using the Bio-Rad protein
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Conventional procedures were followed, as previously described
(13). Primary antibodies were
purchased from Cell Signaling Technology, Inc. [anti-cleaved
caspase-3 (#9661), anti-survivin (#2808) and anti-LC3B (#2775)],
and GeneTex [Irvine, CA, USA; anti-B-cell lymphoma 2 (Bcl-2;
GTX127958), anti-cellular inhibitor of apoptosis protein (cIAP)1
GTX110087), anti-cIAP2 (GTX113128), anti-B-cell lymphoma-extra
large (Bcl-xL; GTX105661), anti-receptor-interacting protein kinase
3 (RIP3; GTX107574), anti-sirtuin (SIRT)1; GTX61042),
anti-phospho-SIRT1 (GTX61962) and SIRT3 (GTX89507)]. The expression
of α-tubulin (sc-8305) (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) or GAPDH (GTX100118; GeneTex) was used as the internal
standard. To quantify the changes in protein expression, the levels
of intensity were calculated as follows: (immunoreactive intensity
of the target protein)/(immuno-reactive intensity of internal
control) using NIH software (ImageJ v.1.40).
Statistical analysis
Data were analyzed using the Student's t-test,
Mann-Whitney U test, or one-way analysis of variance (ANOVA) based
on individual data, and are presented as the means ± SEM (standard
error of the mean). In all cases, a value of p<0.05 was
considered to indicate a statistically significant difference.
Results
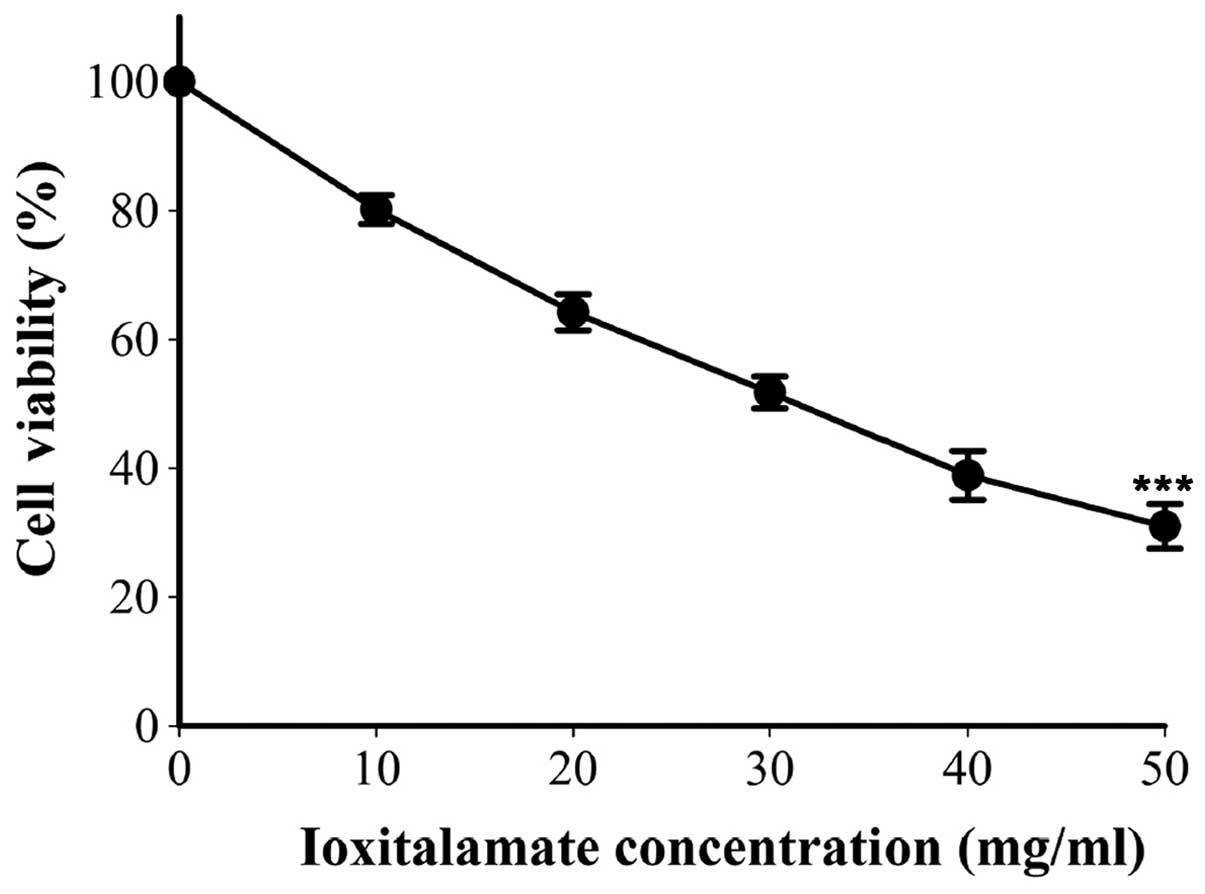
Cytotoxic effects of ioxitalamate on HK-2
cells determined by MTT assay
Cell viability following 48 h of treatment with
ioxi-talamate is shown in Fig. 1.
Cell viability was 80.2±2.2, 64.2±2.8, 51.8±2.5, 38.9±3.8 and
31.0±3.5% following treatment with 10, 20, 30, 40 and 50 mg/dl of
ioxitalamate, respectively (n=8 experiments). Ioxitalamate induced
significant cell cytotoxicity in a concentration-dependent manner
(p<0.001, as shown by one-way ANOVA). Ioxitalamate at 30 mg/ml
was selected for use in our subsequent experiments.
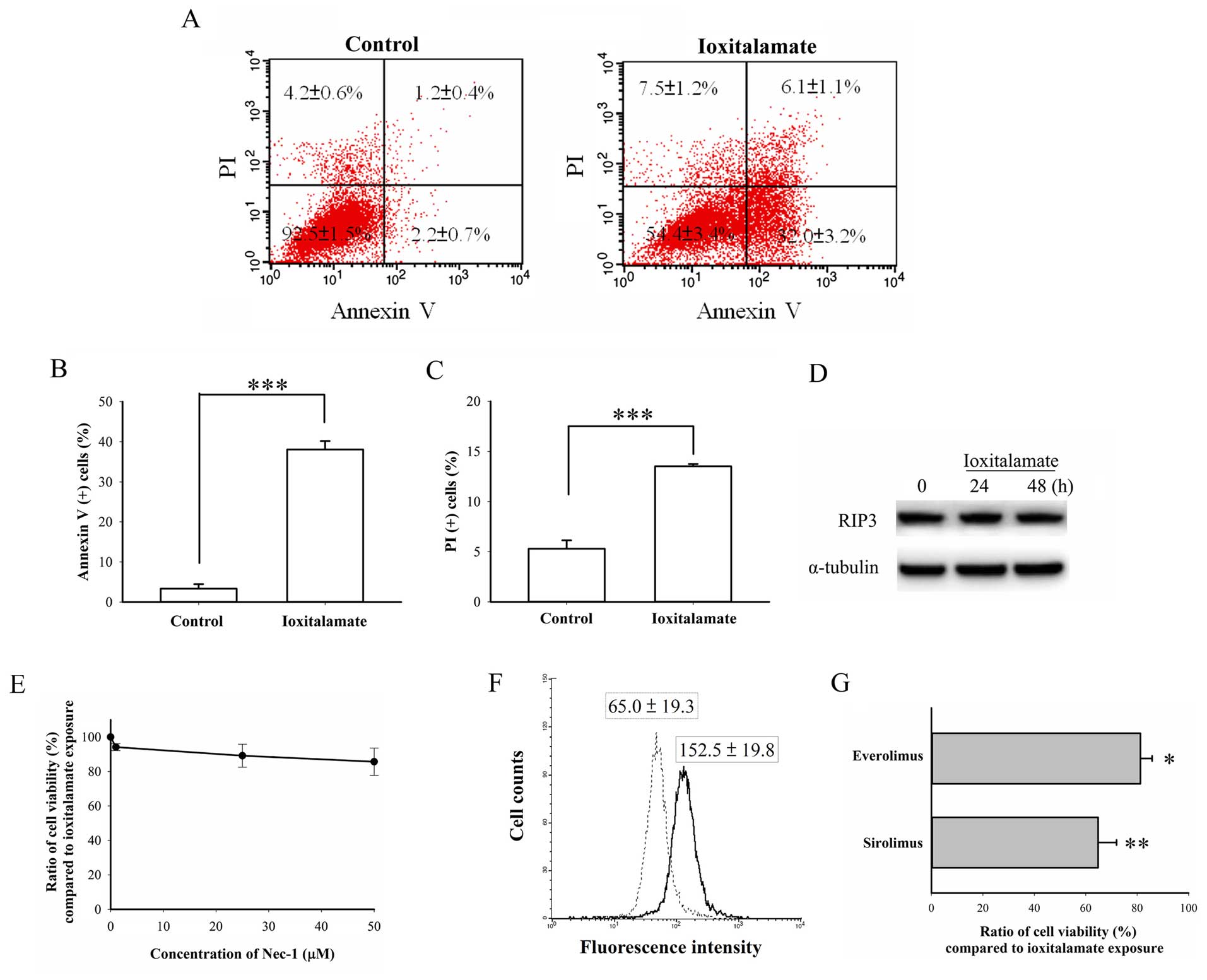
Ioxitalamte induces the apoptosis of HK-2
cells
As shown in Fig.
2A, the number of early apoptotic cells (Annexin
V-positive/PI-negative) increased from 2.2±0.7% in the controls to
32.0±3.2% (p=0.0008, n=3 experiments) following treatment with
ioxitalamate for 48 h and reached statistical significance. The
number of necrotic cells (Annexin V-negative/PI-positive) also
increased from 4.2±0.6 in the controls to 7.5±1.2% in the
ioxitalamate-treated cells, but this increase was not statistically
significant (p=0.07). The number of Annexin V-positive/PI-positive
cells, indicative of necrotic and late apoptotic cells,
significantly increased from 1.2±0.4 in the controls to 6.1±1.1% in
the ioxitalamate-treated cells (p=0.02). The total number of
Annexin V-positive cells also significantly increased from 3.3±1.1
in the controls to 38.1±2.1% in the ioxitalamate-treated cells
(p=0.0001; Fig. 2B), while the
total number of PI-positive cells increased from 5.3±0.8 in the
controls to 13.5±0.2% in the ioxitalamate-treated cells (p=0.0007;
Fig. 2C). RIP3 is a marker of
necroptosis, a programmed form of necrosis contributing to AKI
(14). In this study, we did not
observe any changes in RIP3 expression, suggesting that necrosis,
not necroptosis, had occurred following treatment with ioxitalamate
(Fig. 2D). In addition, the
necroptosis inhibitor, Nec-1, did not reverse ioxitalamate-induced
cytotoxicity (p=0.312, as shown by one-way ANOVA, n=3 experiments)
(Fig. 2E). Therefore, apoptosis,
and not necrosis played a predominant role in ioxitalamate-induced
cytotoxicity in HK-2 cells.
Autophagy is also involved in renal protection
(15). Thus, in this study, we
also determined the autophagic status following treatment with
ioxitalamate. Surprisingly, flow cytometric analysis revealed a
significantly increased autophagic flux following 48 h of treatment
with ioxitalamate (p=0.03, n=3 experiments; Fig. 2F). To confirm that this phenomenon
was a compensatory result or autophagic death, the cells were
treated with the autophagy inducers, sirolimus and everolimus,
so-called mammalian target of rapamycin (mTOR) inhibitors, in
addition to ioxitalamate for 48 h. Both sirolimus and everolimus
(both at 1 μM) significantly aggravated
ioxitala-mate-induced cytotoxicity (p=0.008 and 0.01, respectively;
n=3 experiments) (Fig. 2G).
Therefore, ioxitalamate also induced autophagic death.
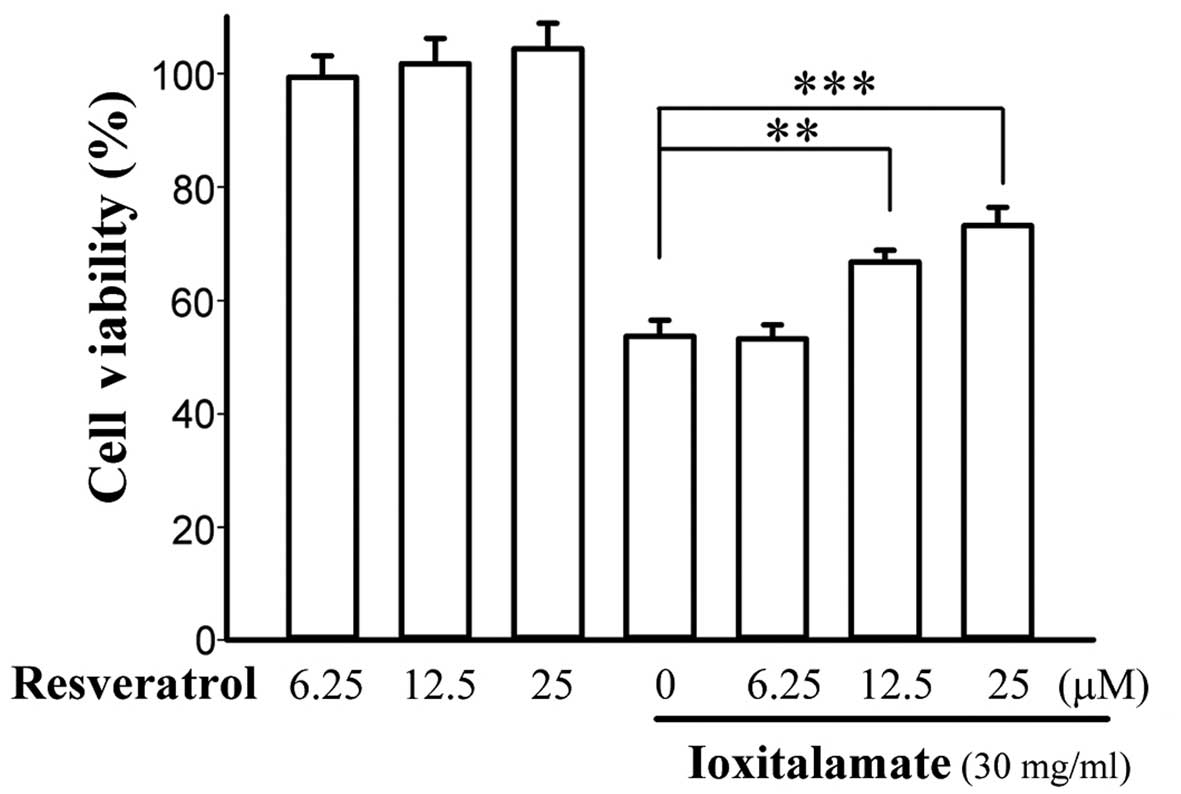
Resveratrol alleviates cytotoxicity
induced by ioxitalamate in HK-2 cells
Cell viability following 48 h of treatment with
ioxitalamate (30 mg/ml) in combination with resveratrol is shown in
Fig. 3. Resveratrol alone did not
influence the viability of the HK-2 cells. Ioxitalamate induced
significant cytotoxicity in a concentration-dependent manner
(p<0.001, as shown by one-way ANOVA, n=5 experiments). However,
treatment with resveratrol at 12.5 and 25 μM increased cell
viability from 53.7±2.8 to 66.8±2.1 and 73.2±3.2%, respectively
(p=0.006 and 0.002, respectively). The difference in cell viability
between treatment with 12.5 and 25 μM of resveratrol did not
reach statistical significance (p=0.14). Therefore, resveratrol at
12.5 μM was selected for use in subsequent experiments.
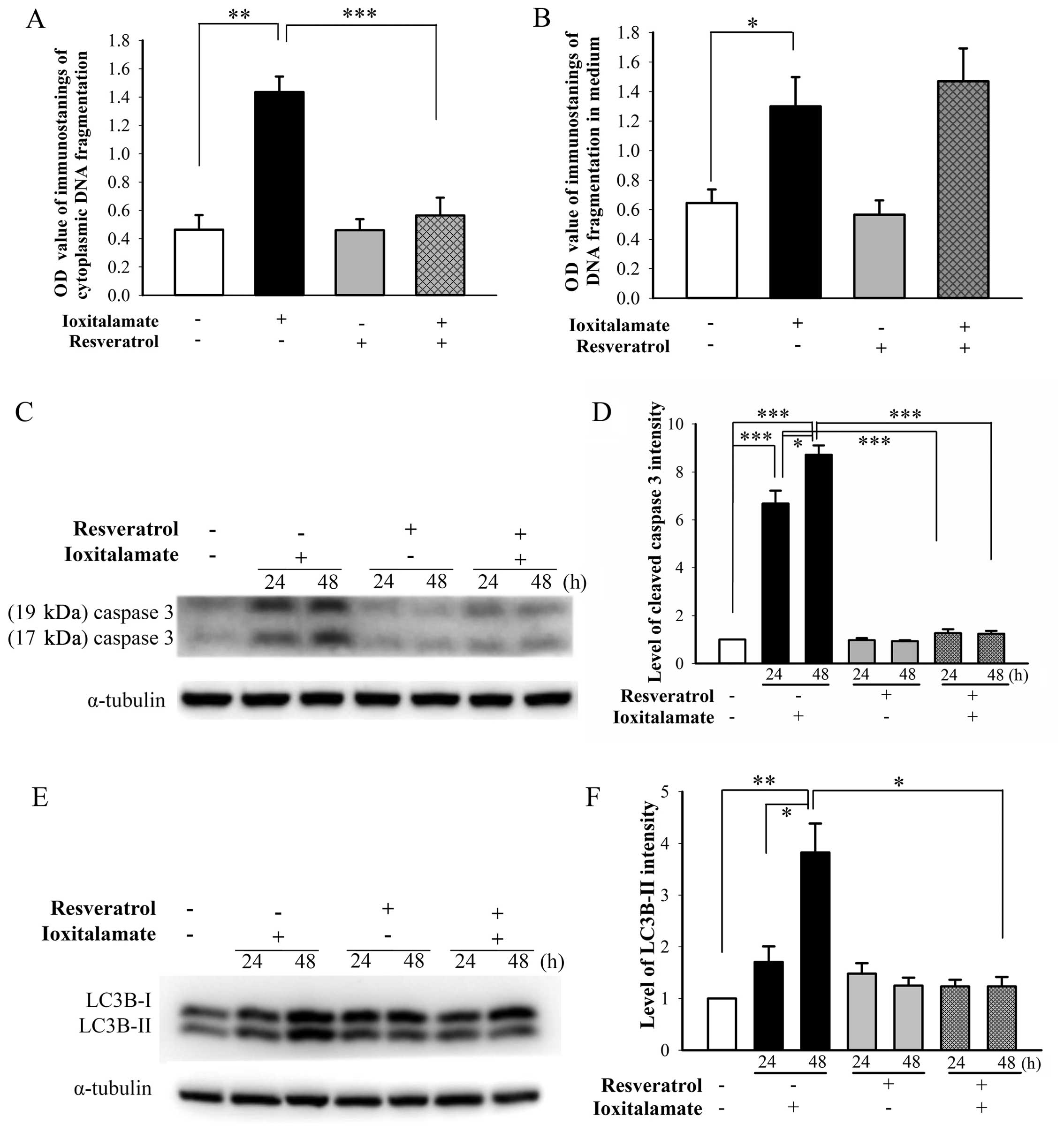
Apoptosis and autophagic death induced by
ioxitalamate are suppressed by resveratrol
A significant increase in cytosolic DNA
fragmentation, indicative of apoptosis, was observed following
treatment with ioxitalamate (30 mg/ml) compared to the controls
(p=0.008, n=5 experiments). Treatment with resveratrol (12.5
μM) significantly suppressed ioxitalamate-induced apoptosis
(p=0.008; Fig. 4A). A significant
increase in DNA fragmentation in the culture medium, indicative of
necrosis, was also observed following treatment with ioxitalamate
compared to the controls (p=0.016; Fig. 4B). However, the difference in DNA
fragmentation in the culture medium of the cells treated with
ioxitalamate plus resveratrol and those treated with ioxitalamate
alone did not reach statistical significance (p=0.584; Fig. 4B).
The activation of caspase-3 plays an important role
in apoptosis (16). The
expression of cleaved caspase-3, the active form of caspase-3 (both
17 and 19 kDa) was markedly upregulated following treatment with
ioxitalamate for 24 h (p=0.01, n=3 experiments) and 48 h (p=0.03).
However, treatment with resveratrol significantly attenuated this
increase (p=0.0002 and 0.0004 at 24 and 48 h, respectively)
(Fig. 4C and D). Resveratrol also
alleviated the ioxitalamate-induced activation of LC3B-II, a marker
of autophagy converted from LC3B-I (p=0.0002 and 0.04 at 24 and 48
h, respectively; n=3 experiments) (Fig. 4E and F).
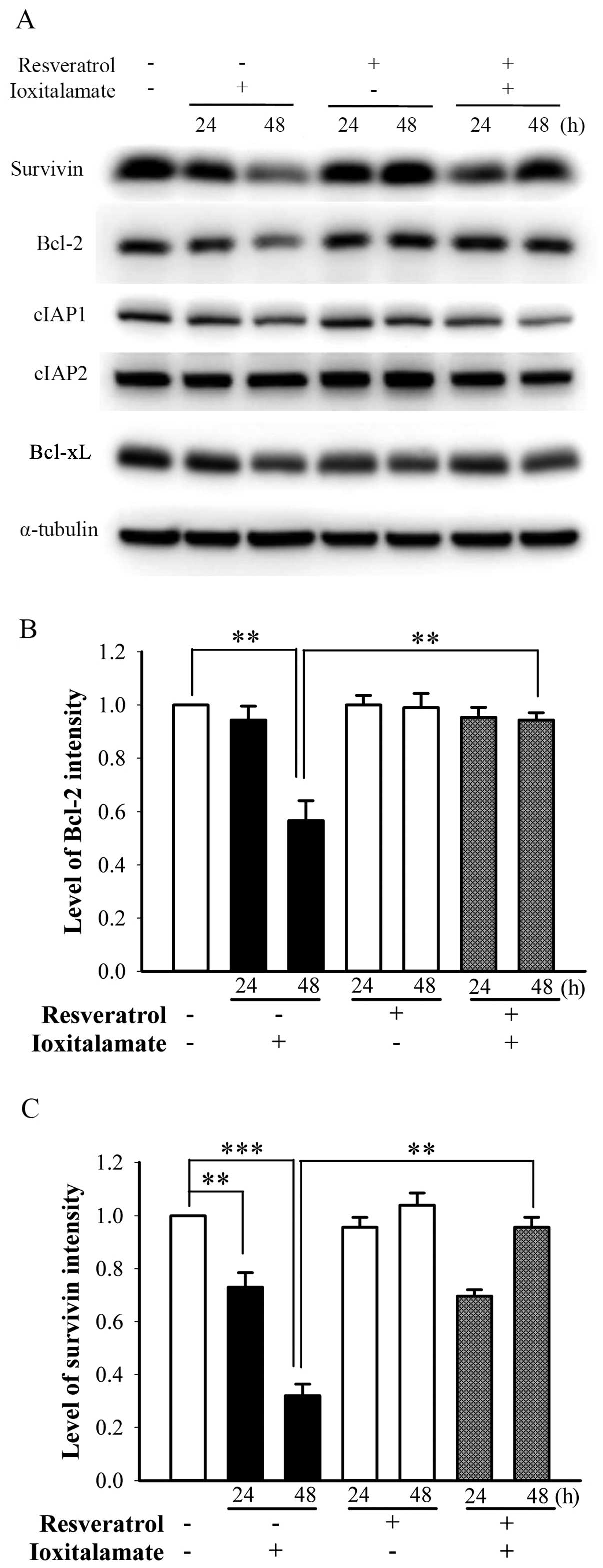
Changes in the expression of
anti-apoptotic proteins following treatment with ioxitalamate
and/or resveratrol
Anti-apoptotic proteins [e.g., survivin (17), Bcl-2 (18), cIAP (11), Bcl-xL (19)] play important roles in the death
of renal tubule epithelial cells. Following treatment with
ioxitalamate for 48 h, the expression of Bcl-2 significantly
decreased (p=0.005, n=3 experiments); however, resveratrol
significantly reversed this decrease in Bcl-2 expression (p=0.01;
Fig. 5A and B). The expression of
survivin was significantly downregulated following treatment with
ioxitalamate for 24 and 48 h (p=0.008 and 0.0001, respectively; n=3
experiments); however, resveratrol reversed this decrease in
survivin expression at 48 h of treatment (p=0.0004) (Fig. 5A and C). The expression levels of
cIAP1, cIAP2 and Bcl-xL were not altered following treatment with
ioxitalamate and/or resveratrol (Fig.
5A).
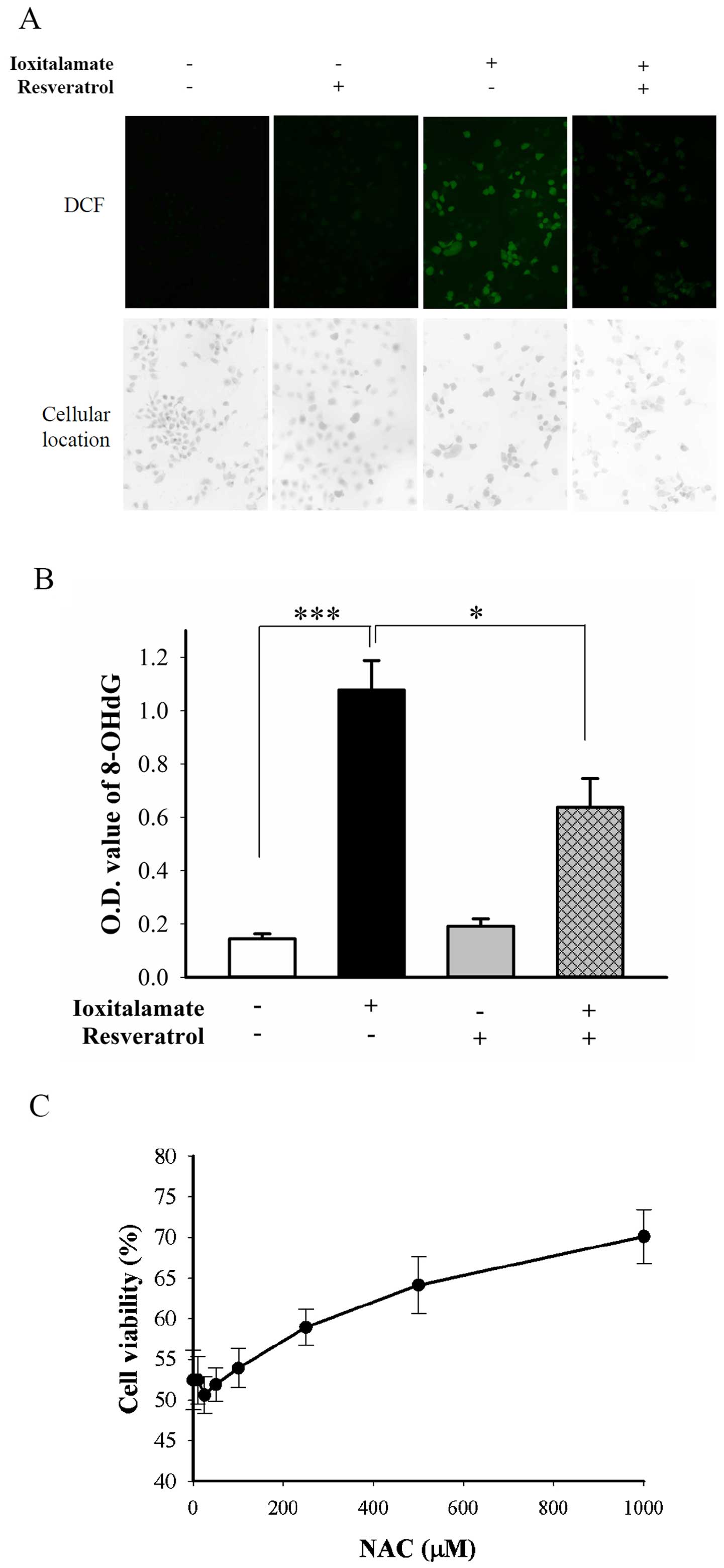
Resveratrol alleviates
ioxitalamate-induced ROS generation without SIRT1 and SIRT3
activation
The protective effects of resveratrol are mediated
through at least two mechanisms, direct antioxidant activity and
SIRT1 activation (8). ROS,
indicated by green fluorescence from
2′,7′-dichlorodihydro-fluorescein (DCF), were abundantly generated
following treatment with ioxitalamate for 48 h. Resveratrol
suppressed the production of ROS induced by ioxitalamate (Fig. 6A). DNA fragmentation may result
from oxidative stress (20).
Ioxitalamate significantly induced oxidative DNA damage, as shown
by the detection of the formation of 8-OHdG (p=0.0002, n=4
experiments; Fig. 6B). However,
resveratrol significantly suppressed the ioxitalamate-induced
formation of 8-OHdG (p=0.01; Fig.
6B). We also determined the therapeutic effects of the ROS
scavenger, NAC. NAC also significantly reduced ioxitalamate-induced
cytotoxicity in the HK-2 cells (p=0.001, as shown by one-way ANOVA,
n=3 experiments) (Fig. 6C).
However, a high concentration of NAC (1 mM) was required to
increase cell viability form 52.4±3.7 to 70.2±3.3%.
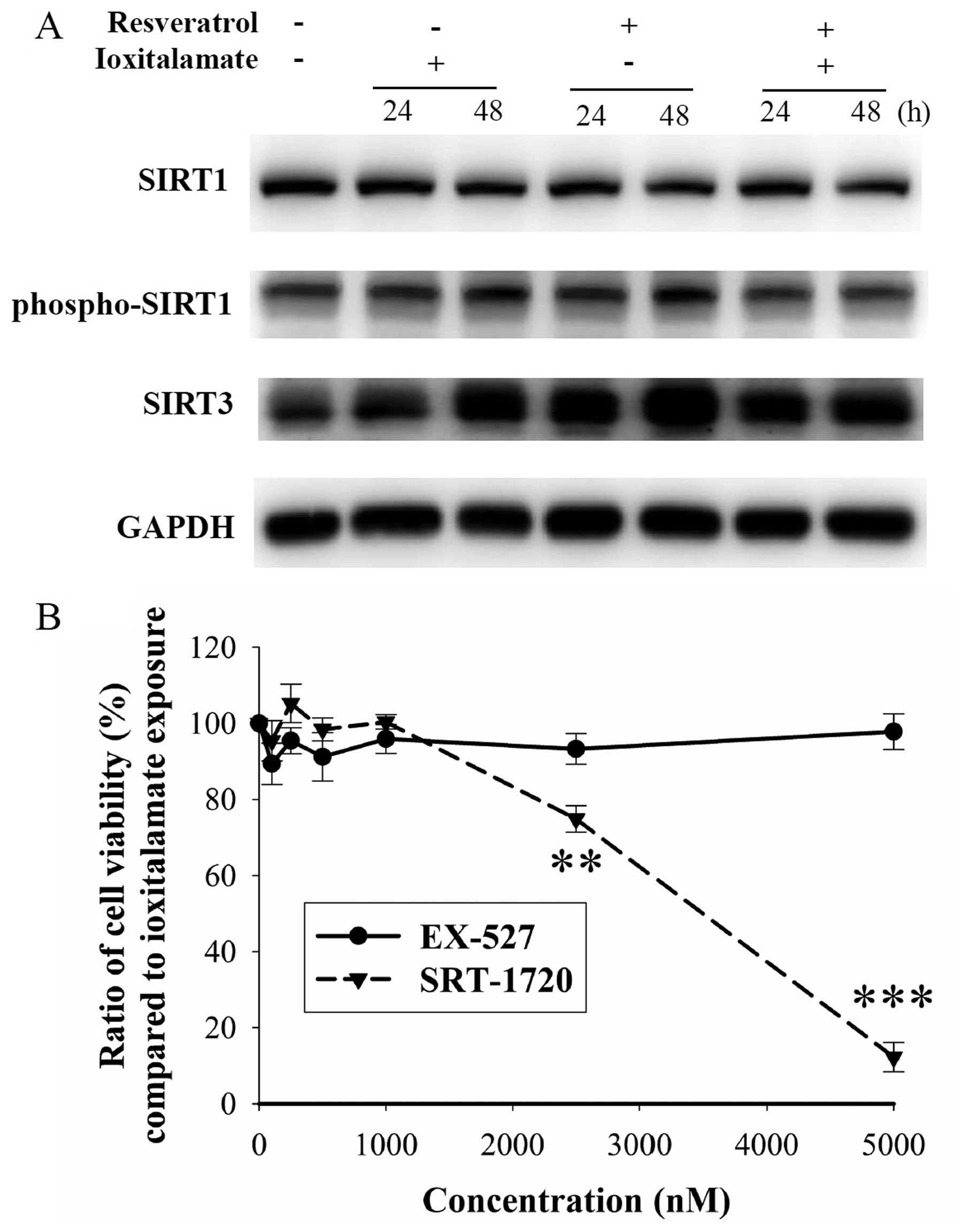
Furthermore, we examined the expression of SIRT1,
which is associated with reno-protection in AKI (21). The expression of SIRT1 and
phospho-SIRT1 was not altered following treatment with ioxitalamate
and/or resveratrol (Fig. 7A).
Resveratrol is also known as a SIRT3 activator (22). Cisplatin-induced tubular damage
may result from the downregulation of SIRT3 (23). In our study, SIRT3 was upregulated
following treatment with expression was increased even further.
However, there was no difference observed in the expression of
SIRT3 between the cells treated with ioxitalamate alone and those
treated with ioxitalamate plus resveratrol (Fig. 7A). No changes in cell viability
were observed following treatment with the SIRT1 inhibitor, EX-527
(p=0.123 in one-way ANOVA, n=3 experiments). By contrast, the SIRT1
activator, SRT-1720, reduced cell viability compared to
ioxitalamate treatment (p<0.001, as shown by one-way ANOVA, n=3
experiments), particularly at a high concentration (p=0.002 and
0.00002 at 2,500 and 5,000 nM, respectively) (Fig. 7B). There are no available specific
SIRT3 activators and inhibitors. These results indicated that
activation of SIRT1 and SIRT3 played no roles in these effects.
Discussion
The present study demonstrated that the
radiocontrast agent, ioxitalamate, exerted cytotoxic effects on
HK-2 human renal proximal tubule epithelial cells in vitro.
Resveratrol alleviated ioxitalamate-induced oxidative stress and
apoptosis by reversing the downregulation in Bcl-2 and survivin
expression. The proximal tubule is particularly vulnerable to
extensive damage along the segments by a number of toxins,
including radiocontrast agents (24). Ioxitalamate has been reported to
exert more potent cytotoxic effects than other radiocontrast agents
in an in vitro study using the porcine proximal tubular
epithelial cell line, LLC-PK1 (25). Ioxitalamate is a high-osmolar
ionic monomer radiocontrast agent. In a previous study, iodine
alone and hyperosmolar mannitol solution did not elicit significant
cytotoxicity on human embryonic kidney (HEK 293) cells, porcine
proximal renal tubular (LLC-PK1) and Madin-Darby canine kidney
(MDCK) distal tubular renal cells in vitro (26). However, the HK-2 human renal
proximal tubule epithelial cell line is a more suitable model than
LLC-PK1 for in vitro toxicity studies to determine
drug-induced nephrotoxicity in humans (27). To the best of our knowledge, the
investigation of RIN (not ioxitalamate) using HK-2 cells has only
been reported in one study to date (28). Therefore, the use of ioxitalamate
to examine the therapeutic effects of resveratrol on HK-2 cells is
novel.
Not only apoptosis, but also necrosis is induced as
a result of cellular damage induced by radiocontrast agents
(29). However, no necrosis was
noted in the LLC-PK1 in vitro model of RIN (30). Again, the HK-2 in vitro
model may be more compatible with the in vivo condition of
RIN. In a previous study, necroptosis occurred in a murine
ischemia/reperfusion injury-based approach of iomeprol-induced
nephropathy, and Nec-1 prevented RIN (31). Liang et al reported that
necroptosis contributed to damage to HK-2 cells in an ATP-depleted
model (32). However, necroptosis
was not observed in this study, and the necroptosis inhibitor,
Nec-1, did not reduce ioxitalamate-induced cytotoxicity. If
possible, contrast agent-induced necroptosis should be confirmed in
human specimens. Besides, perhaps different stimuli cause different
patterns of death, even in the same cells. Perhaps the preventive
effects of reveratrol against ioxitalamate-induced damage are not
sufficient as resveratrol did not prevent the ioxitalamate-induced
necrosis. Currently, the most toxic radiocontrast agent,
ioxitalamate, is not often used for intravenous infusion. The
protective effects of resveratrol, particularly in combination with
standard hydration therapy, against the nephrotoxicity induced by
other radiocontrast agents should be investigated in the
future.
The inhibitor of apoptosis protein, survivin, plays
an important role in the regulation of cellular division and
survival, particularly in embryonic and cancer cells. Lechler et
al examined the expression of survivin in human proximal
tubular cells, particularly at the apical membrane (33). In a previous study, the
upregulation of survivin expression in renal proximal tubule
epithelial cells resulted in the functional and structural recovery
of the kidneys from AKI (17). In
AKI, the expression of Bcl-2 in proximal renal tubular cells is
also decreased, and any management which aims to increase Bcl-2
expression reduces the apoptosis of renal proximal tubule
epithelial cells and improves renal function (34). The changes in the immunoreactivity
of survivin were more prominent than those of Bcl-2 in our study.
This suggests that survivin plays a more important role in the
ioxitalamate-induced apoptosis of HK-2 cells. To the best of our
knowledge, to date, no agent has been reported to increase both
Bcl-2 and survivin expression in AKI. The reverse effects on both
Bcl-2 and survivin expression may explain the prominent
anti-apoptotic effects of resveratrol in our study. In cancer
cells, resveratrol has been shown to induce apoptosis through the
downregulation of Bcl-2 (35) and
survivin (36). It is possible
that resveratrol exerts antioxidant effects in normal cells and
pro-oxidant effects in cancer cells. One explanation is the
'Warburg effect' (37). The
glycolytic pathway is not the primary resource of energy generation
and the accumulation of lactate develops in normal cells, promoting
physiological pH, to maintain DNA integrity with copper bound to
DNA bases hidden in a tight conformation. Resveratrol cannot easily
access the protected copper ions and therefore cannot chelate
and/or reduce the metal ion in the normal DNA configuration. In
cancer cells, the primary energy generating mechanism is the
glycolytic pathway that leads to the accumulation of lactate due to
the hypoxic environment. Such a low pH may induce base rotation in
DNA, particularly copper bound N7 guanine. Resveratrol then attacks
exposed copper, becomes oxidized itself through a copper peroxide
mechanism to induce DNA strand breakage in cancer cells (38).
Autophagy, so-called type II cell death, was
triggered by RIN in our study. The cell death-promoting role of
autophagy has also been shown in renal tubular cells (39). However, autophagy plays a
protective role in cisplatin-induced renal tubular damage (40). Autophagy is a double-edged sword
(41). The discrepancy among
these studies is generally believed to be due to the different
experimental conditions used. Autophagy can be either protective or
detrimental in renal tubule cells, depending on the different types
of injury (15). In this study,
ioxitalamate-induced autophagy played a pro-apoptotic role, as the
autopahy inducers, sirolimus and everolimus, potentiated
ioxitalamate-induced cytotoxicity. By contrast, sirolimus has been
shown to alleviate cisplatin induced nephropathy (42). Sirolimus and everolimus bind to
FKBP12 and inhibit mTOR, a key regulator of cell proliferation,
growth, apoptosis and survival in response to growth factors and
cytokines (43). For example,
sirolimus has been shown to inhibit the growth factor-induced
proliferation of renal proximal tubule cells and promote apoptosis
by blocking the survival effects of the same growth factors
mediated by the inhibition of p70S6k (44). Clinically, the deterioration of
renal function has been observed in some patients using mTOR
inhibitors (45). To determine
the definite role of autohagy in ioxitalamate-induced cytotoxicity,
specific genetic approaches, such as the knockdown or transfection
of related targets should be used.
Ioxitalamate induces renal tubule cell apoptosis via
ROS production (46). The
reno-protective effects of resveratrol through antioxidant activity
have been also confirmed (8).
Thus, it is not surprising that resveratrol alleviated
ioxitalamate-induced ROS production in HK-2 cells. To date, the
administration of NAC is still the standard treatment used to
prevent RIN. Liu et al evaluated 9 randomized trials which
suggested that NAC helps to prevent the decline in renal function
induced by RIN (47). However,
not all of the trials were consistent with the protective effects
of NAC. The meta-analysis by Nallamothou et al included 20
trials with 2,195 patients who met the inclusion criteria, but the
results were not significant as regards the benefits in patients
treated with NAC (48). Perhaps
the dosage of NAC used is not sufficient to promote the antioxidant
effects in the renal proximal tubule in these clinical studies.
Zhang et al demonstrated the cytoprotective effects of NAC
at 1 mM against ROS-induced cytotoxicity in HK-2 cells (49), and we found that NAC alleviated
ioxitalamate-induced cytotoxicity at the same concentration.
Compared to NAC, resveratrol was a robust scavenger of ROS in our
study.
Resveratrol is a SIRT activator. However, the
expression of SIRT1 was not altered following treatment with 12.5
μM resve-ratrol in the present study. It is reasonable that
resveratrol only activates SIRT1 effectively at a concentration
>200 μM (50). In
addition, no changes in cell viability were observed following
treatment with the SIRT1 inhibitor, EX-527, suggesting that SIRT1
does not play a role in ioxitalamate-induced cyto-toxicity in HK-2
cells. Beyond the anti-apoptotic effects of SIRT1 via the loss of
Ac K382 mark on p53, SIRT1 may also diminish NF-κB transcription
and a decrease in pro-survival gene product in some condition
(51). This can explain the fact
that the SIRT1 activator, SRT-1720, potentiated
ioxitalamate-induced cytotoxicity. Resveratrol indeed activated
SIRT3 in the present study, and the concentration was lower than
that used in another in vitro study (52). The reason that ioxitalamate
upregulated SIRT3 expression in our study remains unknown. The
determination of the role of SIRT3 in RIN should be conducted
through specific genetic approaches of knockdown or transfection in
the future.
In conclusion, the reno-protective effects of
resveratrol against ioxitalamate-induced nephropathy was
demonstrated by a decrease in ROS production and the suppression of
apoptosis in HK-2 human renal proximal tubule epithelial cells.
Further in vivo investigations of the therapeutic effects of
resveratrol against RIN are warranted in the future.
Acknowledgments
This study was supported by a Grant-in-Aid from Tzu
Chi General Hospital, Hualien, Taiwan, R.O.C.
References
|
1
|
Deray G: Dialysis and iodinated contrast
media. Kidney Int Suppl. 100:S25–S29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tepel M, Aspelin P and Lameire N:
Contrast-induced nephropathy: a clinical and evidence-based
approach. Circulation. 113:1799–1806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai HM, Aronow WS, Chugh SS, Pudasaini B,
Goel A and Garrick R: Risk factors for hemodialysis and mortality
in patients with contrast-induced nephropathy. Am J Ther.
20:607–612. 2013. View Article : Google Scholar
|
|
4
|
Neyra JA, Shah S, Mooney R, Jacobsen G,
Yee J and Novak JE: Contrast-induced acute kidney injury following
coronary angiography: a cohort study of hospitalized patients with
or without chronic kidney disease. Nephrol Dial Transplant.
28:1463–1471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong PC, Li Z, Guo J and Zhang A:
Pathophysiology of contrast-induced nephropathy. Int J Cardiol.
158:186–192. 2012. View Article : Google Scholar
|
|
6
|
Pattharanitima P and Tasanarong A:
Pharmacological strategies to prevent contrast-induced acute kidney
injury. BioMed Res Int. 2014:2369302014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quintavalle C, Donnarumma E, Fiore D,
Briguori C and Condorelli G: Therapeutic strategies to prevent
contrast-induced acute kidney injury. Curr Opin Cardiol.
28:676–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kitada M and Koya D: Renal protective
effects of resveratrol. Oxid Med Cell Longev. 2013:5680932013.
View Article : Google Scholar :
|
|
9
|
Saldanha JF, Leal VO, Stenvinkel P,
Carraro-Eduardo JC and Mafra D: Resveratrol: why is it a promising
therapy for chronic kidney disease patients? Oxid Med Cell Longev.
2013:9632172013. View Article : Google Scholar
|
|
10
|
Ryan MJ, Johnson G, Kirk J, Fuerstenberg
SM, Zager RA and Torok-Storb B: HK-2: an immortalized proximal
tubule epithelial cell line from normal adult human kidney. Kidney
Int. 45:48–57. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren X, Han HJ, Lee YJ, Lee SH, Ng HY, Chae
KJ and Kim IS: Proapoptotic effect of a micropollutant
(tris-(2-chloroethyl)-phosphate) at environmental level in primary
cultured renal proximal tubule cells. J Water Health. 10:522–530.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang YT, Cheng CC, Lin TC, Chiu TH and
Lai PC: Therapeutic potential of sepantronium bromide YM155 in
gemcitabine-resistant human urothelial carcinoma cells. Oncol Rep.
31:771–780. 2014.
|
|
13
|
Huang YT, Lai PC, Wu CC, Hsu SH, Cheng CC,
Lan YF and Chiu TH: BDNF mediated TrkB activation is a survival
signal for transitional cell carcinoma cells. Int J Oncol.
36:1469–1476. 2010.PubMed/NCBI
|
|
14
|
Linkermann A, De Zen F, Weinberg J,
Kunzendorf U and Krautwald S: Programmed necrosis in acute kidney
injury. Nephrol Dial Transplant. 27:3412–3419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livingston MJ and Dong Z: Autophagy in
acute kidney injury. Semin Nephrol. 34:17–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Chen JK, Conway EM and Harris RC:
Survivin mediates renal proximal tubule recovery from AKI. J Am Soc
Nephrol. 24:2023–2033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki C, Isaka Y, Shimizu S, Tsujimoto Y,
Takabatake Y, Ito T, Takahara S and Imai E: Bcl-2 protects tubular
epithelial cells from ischemia reperfusion injury by inhibiting
apoptosis. Cell Transplant. 17:223–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lorz C, Justo P, Sanz AB, Egido J and
Ortíz A: Role of Bcl-xL in paracetamol-induced tubular epithelial
cell death. Kidney Int. 67:592–601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ott U, Aschoff A, Fünfstück R, Jirikowski
G and Wolf G: DNA fragmentation in acute and chronic rejection
after renal transplantation. Transplant Proc. 39:73–77. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hasegawa K, Wakino S, Yoshioka K,
Tatematsu S, Hara Y, Minakuchi H, Sueyasu K, Washida N, Tokuyama H,
Tzukerman M, et al: Kidney-specific overexpression of Sirt1
protects against acute kidney injury by retaining peroxisome
function. J Biol Chem. 285:13045–13056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Fu LL, Wen X, Wang XY, Liu J,
Cheng Y and Huang J: Sirtuin-3 (SIRT3), a therapeutic target with
oncogenic and tumor-suppressive function in cancer. Cell Death Dis.
5:e10472014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morigi M, Perico L, Rota C, Longaretti L,
Conti S, Rottoli D, Novelli R, Remuzzi G and Benigni A: Sirtuin
3-dependent mitochondrial dynamic improvements protect against
acute kidney injury. J Clin Invest. 125:715–726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heyman SN, Brezis M, Epstein FH, Spokes K,
Silva P and Rosen S: Early renal medullary hypoxic injury from
radio-contrast and indomethacin. Kidney Int. 40:632–642. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heinrich MC, Kuhlmann MK, Grgic A,
Heckmann M, Kramann B and Uder M: Cytotoxic effects of ionic
high-osmolar, nonionic monomeric, and nonionic iso-osmolar dimeric
iodinated contrast media on renal tubular cells in vitro.
Radiology. 235:843–849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romano G, Briguori C, Quintavalle C, Zanca
C, Rivera NV, Colombo A and Condorelli G: Contrast agents and renal
cell apoptosis. Eur Heart J. 29:2569–2576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gunness P, Aleksa K, Kosuge K, Ito S and
Koren G: Comparison of the novel HK-2 human renal proximal tubular
cell line with the standard LLC-PK1 cell line in studying
drug-induced nephrotoxicity. Can J Physiol Pharmacol. 88:448–455.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zager RA, Johnson AC and Hanson SY:
Radiographic contrast media-induced tubular injury: evaluation of
oxidant stress and plasma membrane integrity. Kidney Int.
64:128–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goldenberg I and Matetzky S: Nephropathy
induced by contrast media: pathogenesis, risk factors and
preventive strategies. CMAJ. 172:1461–1471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heinrich MC, Scheer M, Heckmann M, Kuefner
MA and Uder M: Iodixanol induces apoptotic and antiproliferative
effects but no necrotic cell death in renal proximal tubular cells
in vitro. Rofo. 181:349–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Linkermann A, Heller JO, Prókai A,
Weinberg JM, De Zen F, Himmerkus N, Szabó AJ, Bräsen JH, Kunzendorf
U and Krautwald S: The RIP1-kinase inhibitor necrostatin-1 prevents
osmotic nephrosis and contrast-induced AKI in mice. J Am Soc
Nephrol. 24:1545–1557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang X, Chen Y, Zhang L, Jiang F, Wang W,
Ye Z, Liu S, Yu C and Shi W: Necroptosis, a novel form of
caspase-independent cell death, contributes to renal epithelial
cell damage in an ATP-depleted renal ischemia model. Mol Med Rep.
10:719–724. 2014.PubMed/NCBI
|
|
33
|
Lechler P, Wu X, Bernhardt W, Campean V,
Gastiger S, Hackenbeck T, Klanke B, Weidemann A, Warnecke C, Amann
K, et al: The tumor gene survivin is highly expressed in adult
renal tubular cells: implications for a pathophysiological role in
the kidney. Am J Pathol. 171:1483–1498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Havasi A and Borkan SC: Apoptosis and
acute kidney injury. Kidney Int. 80:29–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pozo-Guisado E, Merino JM, Mulero-Navarro
S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A and
Fernandez-Salguero PM: Resveratrol-induced apoptosis in MCF-7 human
breast cancer cells involves a caspase-independent mechanism with
downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 115:74–84.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu ML and Zhang SJ: Effects of
resveratrol on the protein expression of survivin and cell
apoptosis in human gastric cancer cells. J BUON. 19:713–717.
2014.PubMed/NCBI
|
|
37
|
Muqbil I, Beck FW, Bao B, Sarkar FH,
Mohammad RM, Hadi SM and Azmi AS: Old wine in a new bottle: the
Warburg effect and anticancer mechanisms of resveratrol. Curr Pharm
Des. 18:1645–1654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng LF, Wei QY, Cai YJ, Fang JG, Zhou B,
Yang L and Liu ZL: DNA damage induced by resveratrol and its
synthetic analogues in the presence of Cu (II) ions: mechanism and
structure-activity relationship. Free Radic Biol Med. 41:1807–1816.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao X, Liu G, Shen H, Gao B, Li X, Fu J,
Zhou J and Ji Q: Liraglutide inhibits autophagy and apoptosis
induced by high glucose through GLP-1R in renal tubular epithelial
cells. Int J Mol Med. 35:684–692. 2015.PubMed/NCBI
|
|
40
|
Periyasamy-Thandavan S, Jiang M, Wei Q,
Smith R, Yin XM and Dong Z: Autophagy is cytoprotective during
cisplatin injury of renal proximal tubular cells. Kidney Int.
74:631–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kreuzaler P and Watson CJ: Killing a
cancer: what are the alternatives? Nat Rev Cancer. 12:411–424.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang M, Wei Q, Dong G, Komatsu M, Su Y
and Dong Z: Autophagy in proximal tubules protects against acute
kidney injury. Kidney Int. 82:1271–1283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schmelzle T and Hall MN: TOR, a central
controller of cell growth. Cell. 103:253–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lieberthal W, Fuhro R, Andry CC, Rennke H,
Abernathy VE, Koh JS, Valeri R and Levine JS: Rapamycin impairs
recovery from acute renal failure: role of cell-cycle arrest and
apoptosis of tubular cells. Am J Physiol Renal Physiol.
281:F693–F706. 2001.PubMed/NCBI
|
|
45
|
Marti HP and Frey FJ: Nephrotoxicity of
rapamycin: an emerging problem in clinical medicine. Nephrol Dial
Transplant. 20:13–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hsu SP, Tsai TJ and Chien CT: Ioxitalamate
induces renal tubular apoptosis via activation of renal efferent
nerve-mediated adrenergic signaling, renin activity, and reactive
oxygen species production in rats. Toxicol Sci. 114:149–158. 2010.
View Article : Google Scholar
|
|
47
|
Liu R, Nair D, Ix J, Moore DH and Bent S:
N-acetylcysteine for the prevention of contrast-induced
nephropathy. A systematic review and meta-analysis. J Gen Intern
Med. 20:193–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nallamothu BK, Shojania KG, Saint S, Hofer
TP, Humes HD, Moscucci M and Bates ER: Is acetylcysteine effective
in preventing contrast-related nephropathy? A meta-analysis. Am J
Med. 117:938–947. 2004. View Article : Google Scholar
|
|
49
|
Zhang F, Lau SS and Monks TJ: The
cytoprotective effect of N-acetyl-L-cysteine against ROS-induced
cytotoxicity is independent of its ability to enhance glutathione
synthesis. Toxicol Sci. 120:87–97. 2011. View Article : Google Scholar :
|
|
50
|
Borra MT, Smith BC and Denu JM: Mechanism
of human SIRT1 activation by resveratrol. J Biol Chem.
280:17187–17195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yeung F, Hoberg JE, Ramsey CS, Keller MD,
Jones DR, Frye RA and Mayo MW: Modulation of NF-kappaB-dependent
transcription and cell survival by the SIRT1 deacetylase. EMBO J.
23:2369–2380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen T, Li J, Liu J, Li N, Wang S, Liu H,
Zeng M1, Zhang Y and Bu P: Activation of SIRT3 by resveratrol
ameliorates cardiac fibrosis and improves cardiac function via the
TGF-beta/Smad3 pathwa. Am J Physiol Heart Circ Physiol.
308:H424–H434. 2015. View Article : Google Scholar
|