Introduction
Dojuksan is a traditional herbal prescription
medicine used in Korea and China to treat urinary diseases which
cause symptoms, such as yellowish-red urine, pain in the phallus,
dysuria and stomatitis (1).
Dojuksan is composed of 4 medicinal herbs: Rehmanniae radix,
Akebia caulis, Glycyrrhizae radix and
Phyllostachys folium. Rehmanniae radix
(Scrophulariaceae) is the root of Rehmannia glutinosa
Libosch. To date, there a number of studies have been conducted on
Rehmanniae radix, including its effects on astrocytes
(2), fatigue (3), wound healing (4), anemia (5) and nephropathy (6,7).
The second ingredient, Akebia caulis (Lardizabalaceae), is
the stem of Akebia quinata Decne and has been studied for
its anti-nociceptive and anti-inflammatory effects (8), and for its cytotoxicity (9). The third ingredient,
Glycyrrhizae radix (Leguminosae), is the root of
Glycyrrhiza glabra L. or Glycyrrhiza uralensis Fisch.
and it has been suggested that this reduces stress-induced anxiety
(10), apoptosis (11), hepatitis C virus replication
(12) and inflammation (13). It has also been suggested that it
induces growth-hormone release (14) and acts as an antispasmodic
(15). Finally,
Phyllostachys folium (Gramineae) is the leaf of
Phyllostachys nigra and has been shown to protect retinal
ganglion cells (16), treat
diabetes (17), enhance leukemic
cell differentiation (18) and
inhibit interleukin (IL)-12 (19).
Lipopolysaccharide (LPS) is a well-known and
important pro-inflammatory factor that can cause endotoxemia,
shock, and, eventually, multiple organ dysfunction syndromes
(20). Stimulation with LPS can
induce the expression of pro-inflammatory mediators in macrophages,
such as inducible nitric oxide synthase (iNOS), cyclooxygenase
(COX)-2, and chemokines (21). In
addition, activated macrophages may secrete pro-inflammatory
cytokines, including tumor necrosis factor-α (TNF-α) and IL-1β
(22). Prostaglandin
E2 (PGE2), which is synthesized by the COX
enzyme, is the most abundant prostaglandin in the human body and
plays many biological roles. PGE2 is an important
mediator of inflammatory symptoms, including fever and pain. The
COX enzyme exists in 2 forms: the constitutive COX-1 form and the
inducible cyclooxygenase-2 (COX-2) form (23). It has a variety of effects on
various biological processes, including inflammation, pain,
tumorigenesis, vascular function, neuronal function, female
reproduction, gastric mucosal health and kidney function (24,25). Nitric oxide (NO) is generated by
nitric oxide synthases (NOSs), which catalyze the production of NO
and L-citrulline from L-arginine in the presence of nicotinamide
adenine dinucleotide phosphate (NADPH)-derived electrons and
O2. Compared with neuronal NOS (nNOS) and endothelial
NOS (eNOS), iNOS is expressed in many cell types, including
macrophages, neutrophils, dendritic cells, endothelial cells and
epithelial cells. The NO produced by these reactions is harmful and
plays the role of an effector, for example in macrophages (26,27).
Heme oxygenase-1 (HO-1) is the rate-limiting enzyme
in the catabolism of heme, a process that leads to the formation of
equimolar amounts of the bile pigment biliverdin, free iron and
carbon monoxide (CO) (28,29).
The degradation of heme is considered critical in cellular defense.
It has been suggested that CO contributes significantly to the
anti-inflammatory properties of HO-1 (30). In addition, it is well known that
the nuclear translocation of activated nuclear factor E2-related
factor 2 (Nrf2) is an important event upstream of HO-1 expression.
Nrf2 is required for the expression of some inducible proteins,
such as glutathione S-transferases, quinine reductase and HO-1
(31,32). In a previous study (33), Dojuksan was shown to inhibit
inflammatory mediators in RAW264.7 cells. However, to the best of
our knowledge, no study on the ethanol extracts or beneficial
effects of a fraction from Dojuksan, and its specific
anti-inflammatory mechanisms, has been undertaken thus far. In the
present study, we investigated the effects of an ethanol solvent
extract of Dojuksan and a fraction (by bioassay-guided
fractionation) derived from this extract and investigated the
anti-inflammatory mechanisms associated with Nrf2-dependent HO-1
expression. We used the murine macrophage-like cell line, RAW264.7,
in order to examine the anti-inflammatory effects and mechanisms of
action of Dojuksan extracted using different methods, such as 30%
ethanol extract (DEE) and bioassay-guided fractionation (fraction
DEE-5).
Materials and methods
Preparation of Dojuksan extracts and MeOH
fraction
The herbs that comprise Dojuksan were purchased from
the University Oriental Herbal Drugstore (Iksan, Korea) in August
2011, and a voucher specimen was deposited at the Herbarium of the
College of Pharmacy at Wonkwang University (Iksan, Korea). The
Dojuksan water extract (DWE), and the 30% ethanol extract (DEE)
were both deposited at the Standardized Material Bank for New
Botanical Drugs (Wonkwang University). Dojuksan comprised
Rehmanniae radix (8 g), Akebia caulis (8 g),
Glycyrrhizae radix (8 g) and Phyllostachys folium (8
g). It (32 g) was extracted with either hot water or 30% ethanol (2
liters of each) for 2 h, and the extracts were concentrated in
vacuo to obtain the 30% ethanol extract (NNMBS308). The 30%
ethanol extract was subjected to C18-functionalized
silica gel flash column chromatography and eluted with a stepwise
gradient of 0% (Fr. 1, DEE-1), 20% (Fr. 2, DEE-2), 40% (Fr. 3,
DEE-3), 60% (Fr. 4, DEE-4), 80% (Fr. 5, DEE-5) and 100% (Fr. 6,
DEE-6) (v/v) of MeOH in H2O (4 ml each) (Fig. 4).
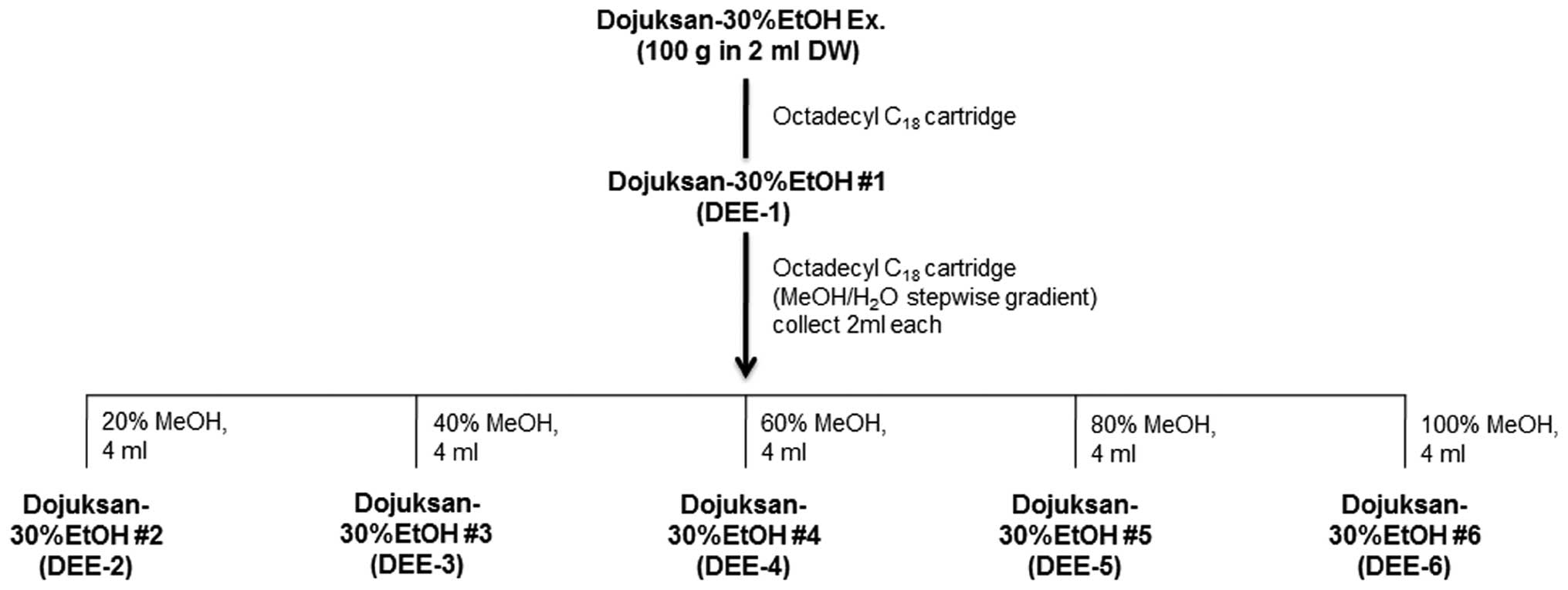 | Figure 4Fractions from 30% ethanol extract.
The 30% ethanol extract was subjected to
C18-functionalized silica gel flash column
chromatography and eluted with a stepwise gradient of 0% [Fr. 1,
Dojuksan 30% ethanol extract (DEE)-1], 20% (Fr. 2, DEE-2), 40% (Fr.
3, DEE-3), 60% (Fr. 4, DEE-4), 80% (Fr. 5, DEE-5), and 100% (Fr. 6,
DEE-6) (v/v) of MeOH in H2O (4 ml each). |
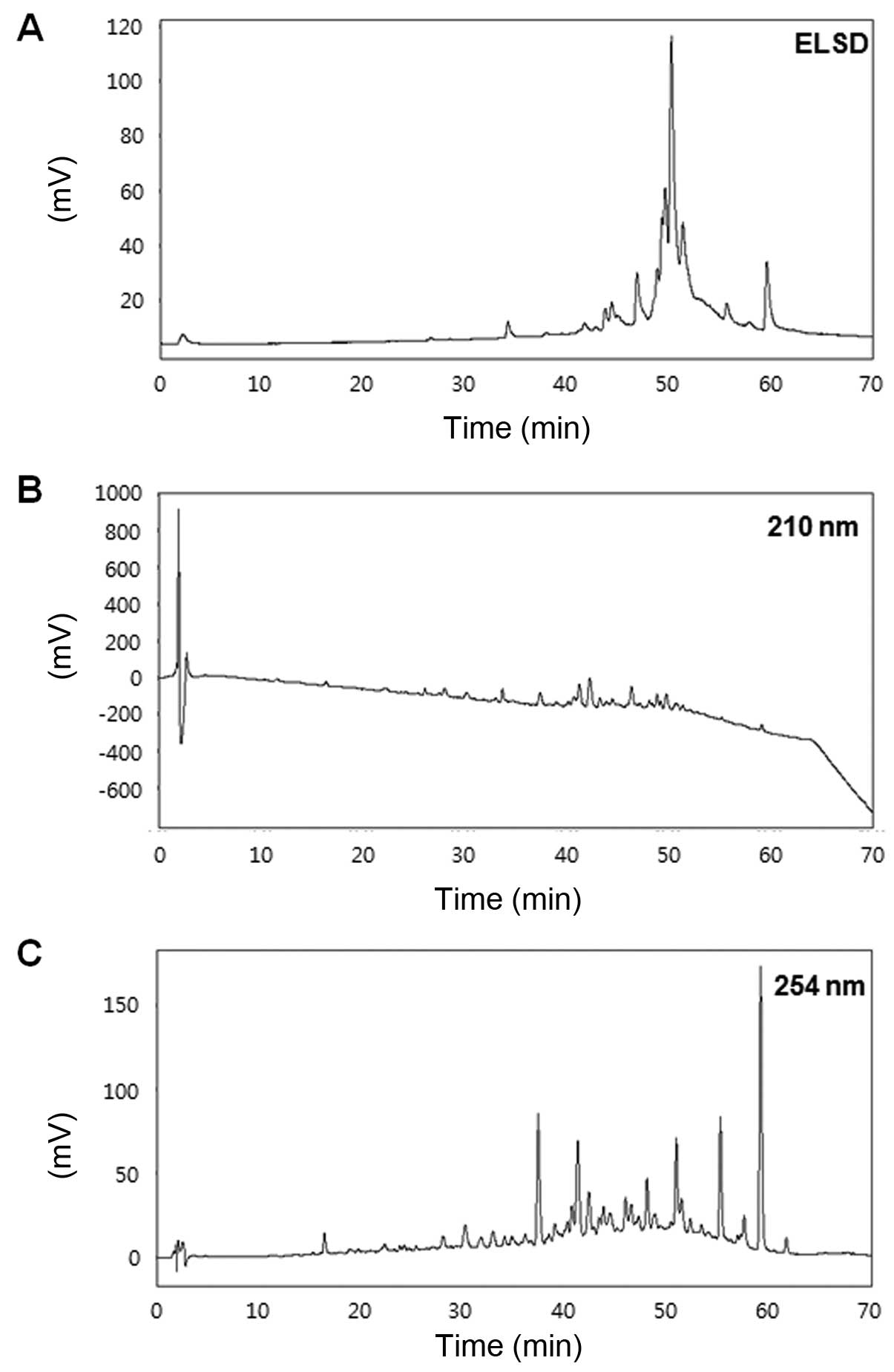
High performance liquid chromatography
(HPLC)
The solvents used for the extraction and flash
column chromatography were reagent grade without further
purification, whereas the solvents used for HPLC were of analytical
grade. Flash column chromatography was performed using YMC
octadecyl-functionalized silica gel (C18). HPLC
(YOUNGLIN-YL9100; Young Lin Instrument Co., Anyang, Korea)
separation was performed using a Shiseido Capcell Pak
C18 column (4.6×250 mm and 5 µm particle size;
Shiseido Co., Ltd., Tokyo, Japan) with a flow rate of 0.7 ml/min,
and an injection volume of 20 µl. The mobile phase was
composed of (A) water and (B) acetonitrile, with an applied
gradient of 5% B increasing to 100% B within 60 min. The column was
cleaned with 100% B for 10 min, and the system was then
equilibrated for 20 min under starting conditions. The detection
wavelengths were adjusted to ELSD, 210 and 254 nm.
Chemicals and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), and all other tissue culture reagents used,
were purchased from Gibco-BRL Co. (Grand Island, NY, USA) unless
otherwise stated. Tin-protoporphyrin IX (SnPP), an inhibitor of HO
activity, was obtained from Porphyrin Products (Logan, UT, USA).
Lipofectamine™ 2000 was purchased from Invitrogen Life Technologies
(Grand Island, NY, USA). Cobalt protophorphyrin IX (CoPP, Cat. no.
C1900) and all other chemicals were obtained from Sigma Chemical
Co. (St. Louis, MO, USA) unless otherwise indicated. RAW264.7 cells
were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA). Small interfering RNA (siRNA) against Nrf2 and
primary antibodies, including those against HO-1 (SC-10789), Nrf2
(SC-722), COX-2 (SC-1745) and iNOS (SC-650), and appropriate
secondary antibodies [anti-goat (SC-2768), anti-mouse (SC-2005) and
anti-rabbit (SC-2004)] for western blot analysis, were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Enzyme-linked immunosorbent assay (ELISA) kits for PGE2,
TNF-α and IL-1β were purchased from R&D Systems (Minneapolis,
MN, USA). TLC silica gel 60 F254 aluminum plates were purchased
from Merck (Darmstadt, Germany).
Cell culture and viability assay
Cell culture and viability were carried out as
previously described (34). The
RAW264.7 cells were maintained at 5×105 cells/ml in DMEM
supplemented with 10% heat-inactivated FBS, penicillin G (100
U/ml), streptomycin (100 mg/ml) and L-glutamine (2 mM), and were
incubated at 37°C in a humidified atmosphere containing 5%
CO2 and 95% air. The effects of various experimental
modulations on cell viability were evaluated by determining
mitochondrial reductase function with an assay based on the
reduction of tetrazolium salt
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
into formazan crystals. The synthesis of formazan is proportional
to the number of functional mitochondria in living cells. For the
determination of cell viability, 50 mg/ml MTT solution were added
to 1 ml cell suspension (1×105 cells/ml in 96-well
plates) for 4 h. The formazan synthesized was dissolved in acidic
2-propanol, and the optical density was measured at 590 nm. The
optical density of the formazan synthesized in the control
(untreated) cells was considered to be 100% cell viability. The
majority of experiments were performed using incubation with LPS
and samples, alone and in combination, unless stated otherwise.
Pretreatment with samples was performed, beginning at 12 h before
the incubation period, and then stimulation with LPS (1
µg/ml) was undertaken.
Determination of nitrite production, and
PGE2, TNF-α and IL-1β assays
The production of nitrite in the conditioned medium
was determined using a method based on the Griess reaction, as
described in a previous study (35). An aliquot (100 µl) of each
supernatant was mixed with the same volume of Griess reagent [0.1%
(w/v) N-(1-naphathyl)-ethylenediamine and 1% (w/v)
sulfanilamide in 5% (v/v) phosphoric acid] for 10 min at room
temperature. The absorbance of the final product was measured
spectrophotometrically at 525 nm using an ELISA plate reader, and
the nitrite concentration in the samples was determined from a
standard curve of sodium nitrite made up in phenol red-free DMEM.
The levels of PGE2, TNF-α or IL-1β present in each
sample were determined using a commercially available kit from
R&D Systems (Abingdon, UK). The assay was performed according
to the instructions provided by the manufacturer.
Western blot analysis
Western blot analysis was carried out as previously
described (36). Western blot
analysis was performed by lysing the cells in 20 mM Tris-HCl buffer
(pH 7.4) containing a protease inhibitor mixture (0.1 mM PMSF, 5
mg/ml aprotinin, 5 mg/ml pepstatin A and 1 mg/ml chymostatin). The
protein concentration was determined using a Lowry protein assay
kit (P5626; Sigma Chemical Co.). An equal amount of protein for
each sample was resolved by 12 or 7.5% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
electrophoretically transferred onto a Hybond enhanced
chemiluminescence (ECL) nitrocellulose membrane (Bio-Rad, Hercules,
CA, USA). The membrane was blocked with 5%skimmed milk and
sequentially incubated with primary antibody (Santa Cruz
Biotechnology, Inc.) and horseradish peroxidase-conjugated
secondary antibody, followed by ECL detection (Amersham Pharmacia
Biotech, Piscataway, NJ, USA).
Preparation of cytosolic and nuclear
fractions
Preparation of cytosolic and nuclear fractions was
carried out as previously described (37). The cells were collected and washed
with phosphate-buffered saline (PBS) and suspended in 200 µl
lysis buffer [10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM
EGTA, 1 mM DTT, 1 mM PMSF and a protease inhibitor cocktail]. The
cells were allowed to swell on ice for 15 min; subsequently, 12.5
µl 10% NP-40 was added. The tubes were agitated on a vortex
for 10 sec and then centrifuged for 5 min. The resulting
supernatant represented the cytosolic extract. The nuclear pellets
were resuspended in 50 µl ice-cold nuclear extraction buffer
[20 mM HEPES (pH 7.9), 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT,
0.5 mM PMSF and a protease inhibitor cocktail] and incubated on ice
for 1 h with intermittent vortexing. This nuclear extract was
centrifuged for 10 min at 15,000 × g; the resulting supernatant
represented the nuclear fraction.
DNA binding activity of nuclear factor-κB
(NF-κB)
As previously described (38), the DNA-binding activity of NF-κB
in the nuclear extracts was measured using a TransAM kit (Active
Motif, Carlsbad, CA, USA) according to the manufacturer's
instructions. Briefly, 30 µl complete binding buffer (DTT,
herring sperm DNA and binding buffer AM3) was added to each well.
The samples were nuclear extracts of RAW264.7 macrophages that had
been stimulated for 30 min with LPS and specific concentrations of
DEE. Subsequently, the samples were diluted in complete lysis
buffer and added to each well (20 µg nuclear extract diluted
in complete lysis buffer to a final volume of 20 µl). The
plates were incubated for 1 h at room temperature with mild
agitation (100 rpm on a rocking platform). After washing each well
with wash buffer, 100 µl of diluted NF-κB antibody (1:1,000
dilution in 1X antibody-binding buffer) was added to each well, and
the plates were then further incubated, as previously described,
for 1 h. After washing each well with wash buffer, 100 µl
diluted HRP-conjugated antibody (1:1,000 dilution in 1X
antibody-binding buffer) was added to each well followed by
incubation for 1 h, as before. Developing solution (100 µl)
was added to each well and left to react for 5 min, followed by the
addition of a stop solution to each well. Finally, the absorbance
of each sample was read at 450 nm on a spectrophotometer
(microplate reader model 680, serial no. 19590, Bio-Rad) within 5
min of reaction termination.
siRNA transfection of Nrf2
Cells were transiently transfected with Nrf2 siRNA
(Santa Cruz Biotechnology, Inc.) for 6 h using Lipofectamine 2000™
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
instructions, and then incubated in fresh media containing 10% FBS
for 24 h before further manipulation.
Statistical analysis
Data are expressed as the means ± SD of at least 3
independent experiments. To compare three or more groups, one-way
analysis of variance (ANOVA) followed by a Newman-Keuls post hoc
test was used. Statistical analysis was performed using GraphPad
Prism software, version 3.03 (GraphPad Software Inc., San Diego,
CA, USA).
Results
Inhibitory effects of DEE on the
production of pro-inflammatory mediators and cytokines, and the
expression of iNOS and COX-2 in LPS-stimulated RAW264.7 cells
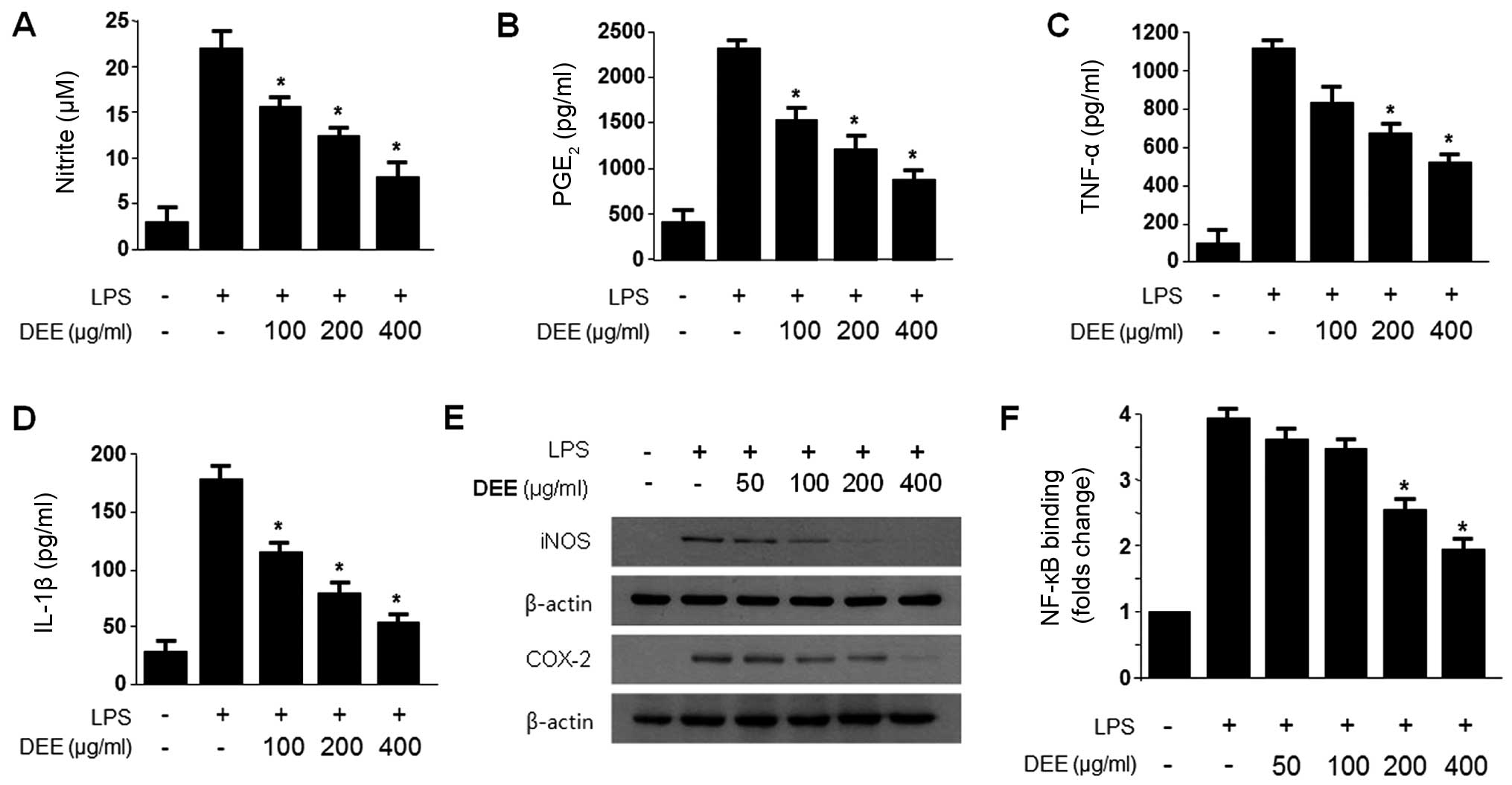
We found that the Dojuksan 30% ethanol extract (DEE)
was significantly more effective in terms of its anti-inflammatory
effects than DWE (data not shown), and that it exerted its
anti-inflammatory effects by inhibiting the expression of the
pro-inflammatory enzymes, iNOS and COX-2, and suppressing the
secretion of the pro-inflammatory cytokines, NO, PGE2,
TNF-α and IL-1β, through the inhibition of NF-κB activation. Since
DEE had more potent anti-inflammatory effects, it was used in our
subsequent experiments. The cytotoxicity of DEE and DEE-5 in the
RAW264.7 cells was determined by MTT assay. We found that cell
viability was not significantly decreased by either DEE or DEE-5 at
concentrations of up to 400 µg/ml (data not shown).
Subsequently, we pre-treated the RAW264.7 cells with either DEE at
non-cytotoxic concentrations (50–400 µg/ml) for 12 h and
measured the production of NO, PGE2, TNF-α and IL-1β
following stimulation with LPS (1 µg/ml) for 18 h. With the
increasing concentration of DEE, NO production decreased in a
dose-dependent manner until it reached a level close to that of the
control cells (which were not treated with LPS) at 400
µg/ml. We observed a similar effect on the production of
PGE2, TNF-α and IL-1β in the cells treated with DEE
(Fig. 1).
The expression of the pro-inflammatory enzyme, iNOS,
plays a crucial role in immune-activated macrophages through the
production of NO (26,27). In addition, prostaglandins are an
important mediator of the symptoms of inflammation, including fever
and pain. Inducible COX-2 is the major source of prostaglandins
(24,25). Thus, in order to examine the
effects of DEE on the expression of iNOS and COX-2, the RAW264.7
cells were challenged with LPS (1 µg/ml) in combination with
the indicated concentrations of DEE, and the protein expression
levels of iNOS and COX-2 were measured by western blot analysis
(Fig. 1E). DEE decreased both
iNOS and COX-2 protein expression in a concentration-dependent
manner.
NF-κB is a key molecule in an important signaling
pathway involved in inflammatory diseases, and it regulates iNOS
and genes, such as COX-2 (39,40). Thus, to determine whether the
NF-κB pathway is involved in the suppression of inflammatory
responses by DEE, we measured NF-κB binding activity. DEE
significantly decreased NF-κB binding activity in a
concentration-dependent manner (Fig.
1D).
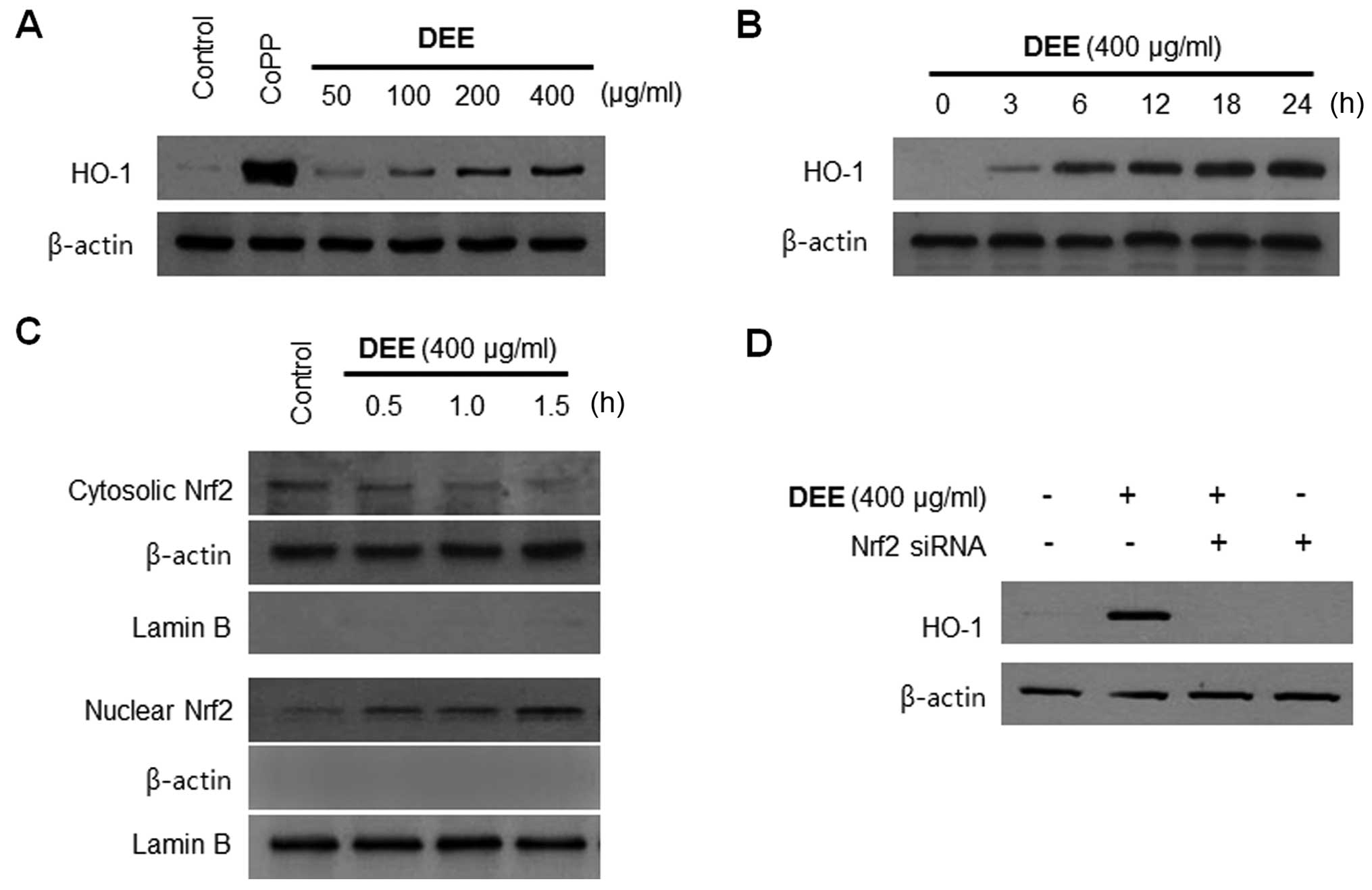
Effects of DEE on HO-1 expression are
mediated by the nuclear translocation of Nrf2 in RAW264.7
cells
We examined whether HO-1 is the key player mediating
the DEE-induced suppression of pro-inflammatory responses. The
RAW264.7 cells were treated with the indicated concentrations of
DEE for 12 h, or with 400 µg/ml of DEE for the indicated
periods of time. We noted that DEE increased HO-1 expression in a
concentration-dependent manner (Fig.
2A), and HO-1 expression began to increase 3 h following
treatment with 400 µg/ml DEE (Fig. 2B). Nrf2 is as an indispensable
regulator of the coordinated induction of phase II enzymes,
including HO-1 (41). Therefore,
we performed western blot analysis to determine whether treatment
with DEE induces the nuclear translocation of Nrf2. When the cells
were incubated with DEE for 0.5, 1.0 or 1.5 h at a concentration of
400 µg/ml, there was a gradual increase in the levels of
nuclear Nrf2, with a concomitant decrease in the cytoplasmic levels
(Fig. 2C). The role of Nrf2 in
DEE-mediated HO-1 expression was confirmed using siRNA against
Nrf2. The RAW264.7 cells were transiently transfected with Nrf2
siRNA, and then treated with 400 µg/ml DEE for 12 h. As
shown in Fig. 2D, transient
transfection with Nrf2 siRNA completely abolished DEE-induced HO-1
expression.
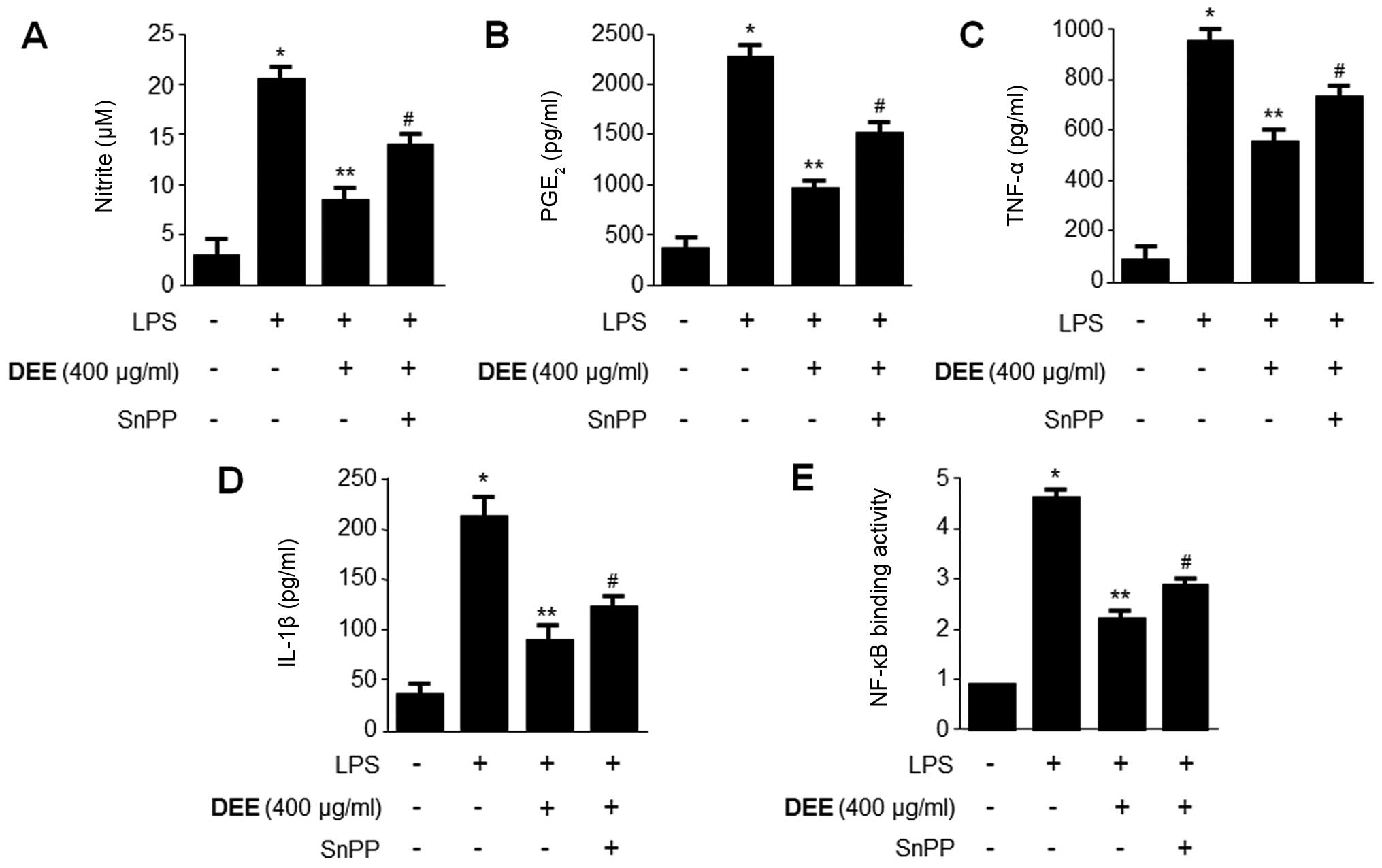
Effects of HO-1 expression on the
inhibition of pro-inflammatory mediators, cytokines and NF-κB
activity by DEE in LPS-stimulated RAW264.7 cells
To confirm the suppressive effects of HO-1 on
pro-inflammatory mediators, cytokines and the NF-κB pathway, we
used SnPP, which is a competitive inhibitor of HO activity.
Previously, imidazole-dioxolane compounds have been shown to act as
inhibitors of HO activity (42,43), as well as specific HO isoenzymes
(44). In the present study,
RAW264.7 cells were pre-treated with DEE (400 µg/ml) for 12
h in the absence or presence of SnPP (20 µM). The inhibitory
effects of DEE on the LPS-induced production of NO,
PGE2, TNF-α and IL-1β, as well as NF-κB binding
activity, were partially reversed by SnPP (Fig. 3).
Comparison of Dojuksan fractions by
HPLC
To further explore the differences in the
anti-inflammatory effects of DEE, we generated 6 different
fractions of DEE using a C18 cartridge with a stepwise
elution of MeOH in H2O (Fig. 4), as the HPLC chromatograms of DEE
showed unclear patterns (data not shown). We then performed a
comparative analysis of the HPLC chromatograms of each of the 6
fractions of DEE (Fig. 5). Data
from the HPLC analysis of DEE-5 was obtained in the form of
chromatograms by monitoring detector responses at ELSD, 210 and 254
nm (Fig. 5). Of the 6 fractions,
DEE-5 induced the greatest decrease in NO production in the
LPS-stimulated RAW264.7 cells (data not shown). Therefore, we
further examined the anti-inflammatory effects of the Dojuksan
ethanol extract using DEE-5.
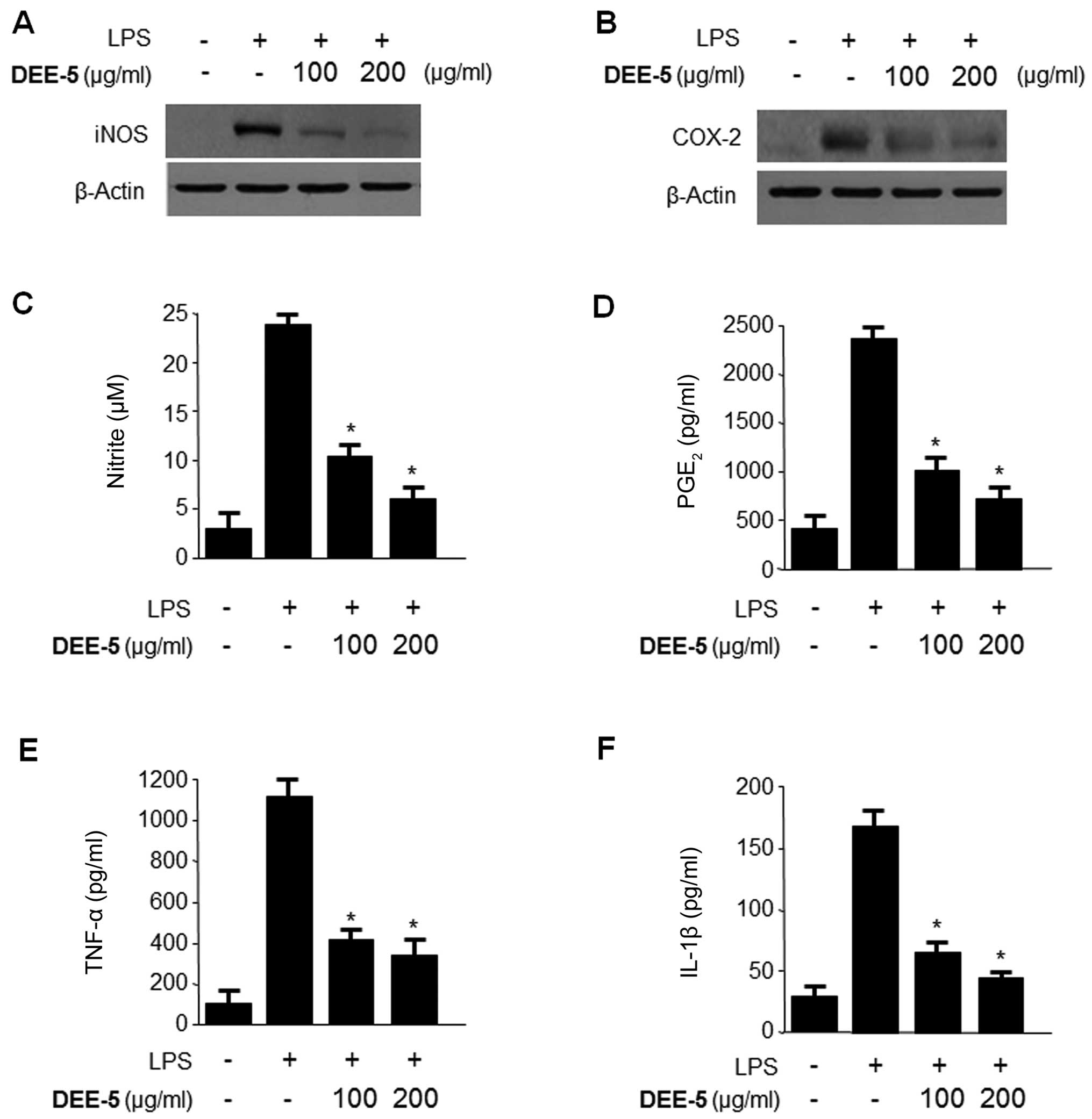
Effects of DEE-5 on the inhibition of
pro-inflammatory mediators and cytokines in LPS-stimulated RAW264.7
cells
To confirm the inhibitory effects of DEE-5 on
pro-inflammatory mediators and cytokines, we pre-treated the
RAW264.7 cells with DEE-5 (100 or 200 µg/ml) for 12 h and
then measured the protein expression of iNOS and COX-2, as well as
the production of NO, PGE2, TNF-α and IL-1β following
stimulation with LPS (1 µg/ml) for 18 h. DEE-5 significantly
decreased the protein expression of iNOS and COX-2, and the
production of NO, PGE2, TNF-α and IL-1β (Fig. 6).
Effects of DEE-5 on HO-1 expression and
the inhibition of pro-inflammatory mediators and cytokines in
RAW264.7 cells
We also examined whether DEE-5 is able to induce the
expression of HO-1. The RAW264.7 cells were treated with the
indicated concentrations of DEE-5 for 12 h, and we observed a
significant increase in HO-1 expression at a concentration of
100–200 µM (Fig. 7A). The
role of Nrf2 in DEE-5-mediated HO-1 expression was confirmed using
siRNA against Nrf2. The RAW264.7 cells were transiently transfected
with Nrf2 siRNA, and then treated with 200 µg/ml DEE-5 for
12 h. As shown in Fig. 7B,
transient transfection with Nrf2 siRNA completely abolished
DEE-5-induced HO-1 expression. To confirm the suppressive effects
of HO-1 on pro-inflammatory mediators and cytokines, we used SnPP,
which is a competitive inhibitor of HO activity. The RAW264.7 cells
were pre-treated with DEE-5 (200 µg/ml) for 12 h in the
absence or presence of SnPP. The inhibitory effects of DEE-5 on
LPS-stimulated NO, PGE2, TNF-α, and IL-1β production
were partially reversed by SnPP (Fig.
7C–F).
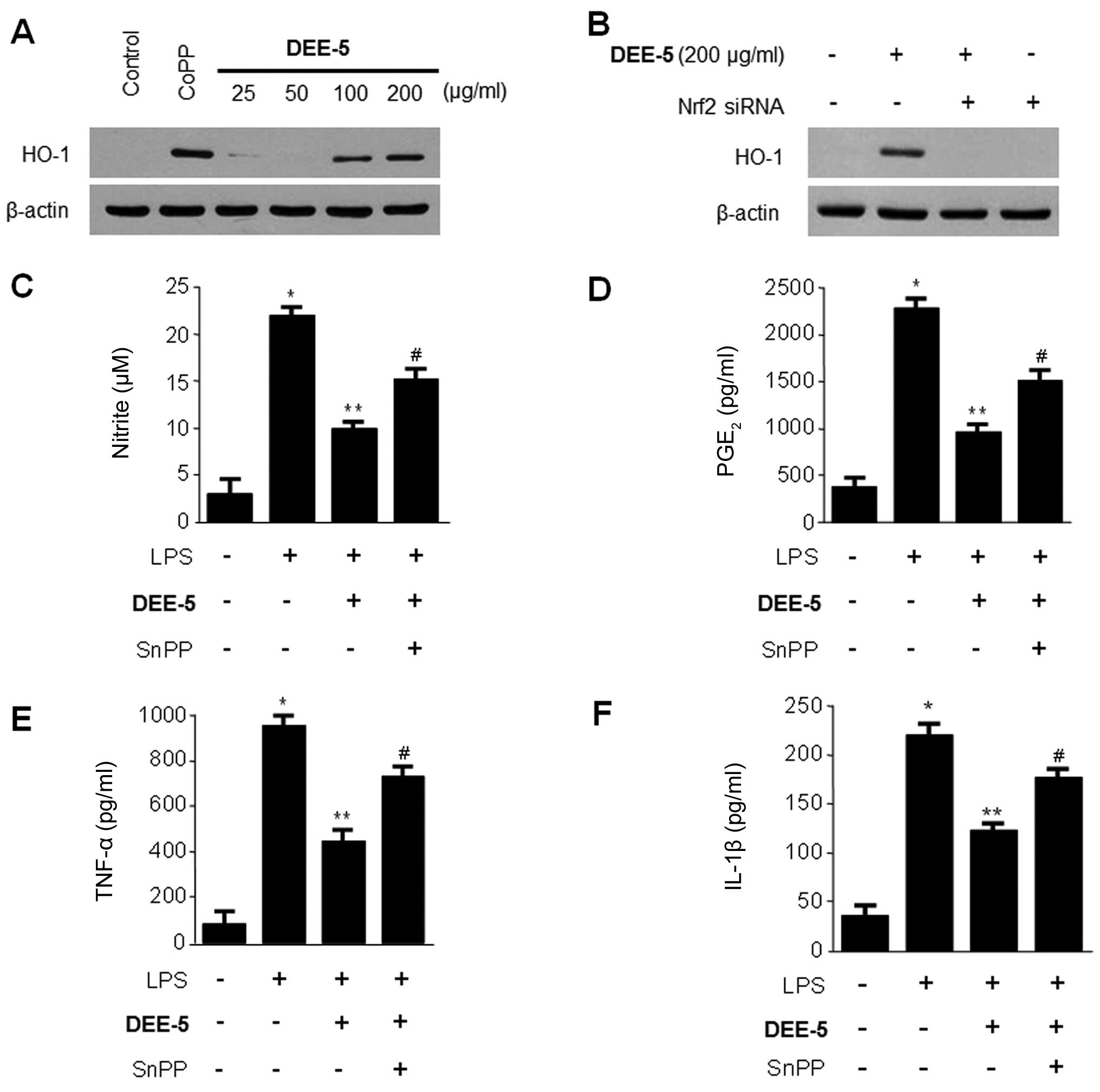 | Figure 7Effects of Dojuksan 30% ethanol
extract (DEE)-5 on (A and B) heme oxygenase-1 (HO-1) expression and
(C) effects of Tin-protoporphyrin IX (SnPP; HO-1 inhibitor) on the
DEE-5-mediated inhibition of nitrite, (D) prostaglandin
E2 (PGE2), (E) tumor necrosis factor-α
(TNF-α), and (F) interleukin-1β (IL-1β) production in RAW264.7
cells. (A) The cells were incubated for 12 h with the indicated
concentrations of DEE-5. The positive control, the HO-1 inducer
cobalt protoporphyrin IX (CoPP), increased the expression of HO-1
at 20 µM. (B) RAW264.7 cells were transiently transfected
with nuclear factor E2-related factor 2 (Nrf2) siRNA, and then
treated with 200 µg/ml DEE-5 for 12 h. Western blot analysis
of HO-1 expression was performed as described in the Materials and
methods, and representative blots of three independent experiments
are shown. Cells were pretreated with DEE-5 (200 µg/ml) for
12 h in the presence or absence of SnPP (50 µM), and then
stimulated with lipopolysaccharide (LPS) (1 µg/ml) for 18 h.
The concentrations of (C) nitrite, (D) PGE2, (E) TNF-α,
(F) IL-1β were determined as described in Materials and methods.
Data shown represent the mean values of 3 experiments ± SD.
*p<0.05 compared to the control group; **p<0.05
compared to the group treated with LPS alone; #p<0.05
compared to the group treated with DEE-5 and LPS. |
Discussion
Dojuksan is a traditional herbal prescription
medicine used in Korea and China to treat urinary diseases which
cause symptoms, such as yellowish-red urine, pain in the phallus,
dysuria and stomatitis (1). In a
previous study (33), water
extracts of Dojuksan inhibited inflammatory mediators in RAW264.7
cells. To date, and to the best of our knowledge, however, there
have been few studies on Dojuksan, and no comparisons of the
biological efficacy of Dojuksan extracts or its anti-inflammatory
mechanisms have been made. In addition, our data suggested that DEE
exerted a significantly more prominent anti-inflammatory effect
than DWE (data not shown). Based on these findings, in the present
study, we suggest a novel approach to understanding and improving
the therapeutic effects of this traditional herbal formula.
Inflammation is a part of the complex biological
response of the body to harmful stimuli, such as pathogens or
irritants that have the capacity to cause cell damage (45). Inflammation is a protective
attempt to remove injurious stimuli and to initiate the healing
process by the organism. Various symptoms are associated with
inflammation, including flares, fever, swelling, itching and
functional disorders caused by the induction of various
pro-inflammatory mediators (46).
It is well known that NO exerts broad physiological and
pathological effects on a number of tissues, including immune
cells. There are three well-known isoforms of NOS: nNOS, eNOS and
iNOS (47). In lymphoid tissues,
iNOS is the principal isoform of NOS. Macrophages appear to be a
major source of iNOS (26,47,48).
iNOS differs from other NOS isoforms, since it is not
constitutively present, but is instead induced by cytokines, such
as interferon-γ (IFN-γ) and TNF-α, or other immunological stimuli,
including LPS. Prostaglandin endoperoxide H synthase and COX
convert arachidonic acid (AA) to prostaglandin endoperoxide H2.
Synthesized PGH2 is converted to prostaglandins
(PGD2, PGE2 and PGF2α),
prostacyclin (PGI2), or thromboxane A2 by
tissue-specific isomerases (49).
In the present study, we noted that DEE decreased iNOS and COX-2
protein expression and the production of pro-inflammatory
cytokines, namely NO, PGE2 TNF-α and IL-1β, in a
concentration-dependent manner. Moreover, to examine whether NF-κB
DNA-binding activity, a key signaling pathway leading to
pro-inflammatory gene expression, is an upstream target for the
inhibitory effects of DEE, we also examined the appearance of NF-κB
DNA-binding activity. We noted that DEE inhibited the increase in
NF-κB DNA-binding activity in RAW264.7 cells stimulated with LPS in
a dose-dependent manner. In addition, as mentioned above, we found
that DEE exerted a significantly more potent anti-inflammatory
effect than DWE, and exerted its anti-inflammatory effects by
inhibiting the expression of the pro-inflammatory enzymes, iNOS and
COX-2, and suppressing the secretion of the pro-inflammatory
cytokines, NO, PGE2, TNF-α and IL-1β, via the inhibition
of NF-κB activation (data not shown). Therefore, we only used DEE
in all our subsequent experiments.
Along with examining its antioxidant effects, recent
studies have also demonstrated the anti-inflammatory effects of
HO-1 reaction in a number of inflammatory models (37,50). The anti-inflammatory effects of
HO-1 are mediated by inhibiting the production of pro-inflammatory
cytokines and chemokines, such as TNF-α, IL-1β and IL-6, in
activated macrophages (51). HO-1
and its product, carbon monoxide, can also suppress the expression
of pro-inflammatory COX-2 and iNOS, thereby reducing COX-2-drived
PGE2 and iNOS-derived NO production (52). Therefore, we suggest that
regulating the degree of generation of oxidative stress and
macrophage activation via the upregulation of HO-1 is an important
aspect to be considered when formulating a strategy for treating
inflammatory diseases. To determine whether HO-1, which is involved
in both antioxidant and anti-inflammatory activities, is the key
player in the DEE-mediated suppression of pro-inflammatory
responses, we treated RAW264.7 cells with DEE and examined both tje
concentration- and time-dependent effects. The expression of HO-1
increased as the concentration of DEE increased, and began to
increase 3 h following treatment with 400 µg/ml DEE. To
confirm the suppressive effects of HO-1 on pro-inflammatory
mediators, we examined the effects of SnPP, which is a competitive
inhibitor of HO-1 activity. The suppressive effects of DEE on
LPS-induced NO, PGE2, TNF-α and IL-1β production were
partially reversed by SnPP. These results indicate that the
inhibition of the pro-inflammatory mediators resulted, at least
partially, from a stimulatory effect of DEE on HO-1. In addition,
it has previously been noted that Nrf2 is an indispensable
regulator of the coordinated induction of phase II enzyme genes,
including HO-1 (41). Under
normal homeostatic conditions, Nrf2 is retained in the cytoplasm
via its interaction with Keap1 and is degraded by the proteasome.
Under oxidative conditions, or as a result of specific stimuli,
Nrf2 is released from Keap1, translocates to the nucleus, forms a
heterodimer with small Maf proteins, and recognizes and binds to a
cis-acting antioxidant response element (ARE), where it eventually
recruits the transcriptional machinery, including RNA polymerase
II, necessary for the transcription of its target genes (31,32,52). In the present study, there was a
gradual increase in Nrf2 levels in the nuclear fractions of the
DEE-treated RAW264.7 cells, whereas the cytoplasmic levels were
concomitantly decreased. This supports the hypothesis that
Nrf2-mediated HO-1 expression contributes to the inhibitory effects
of DEE on the production of pro-inflammatory mediators via the
NF-κB pathway.
To further clarify the effects of Dojuksan, we also
examined potential differences in the effects of DWE and DEE and
examined whether the anti-inflammatory efficacy of the subfractions
differed. We therefore generated 6 different fractions from DEE,
and chromatographic analysis led to additional experiments using
the 80% MeOH fractions of DEE-5. Of the fractions, DEE-5 was
responsible for the most significantly reduced NO production in the
LPS-stimulated RAW264.7 cells (data not shown). Therefore, we
further examined the anti-inflammatory effects of Dojuksan extracts
using DEE-5. DEE-5 markedly suppressed the protein expression of
iNOS and COX-2, and the production of NO, PGE2, TNF-α
and IL-1β. Furthermore, DEE-5 induced HO-1 protein expression, and
the suppressive effects of DEE-5 on LPS-induced NO,
PGE2, TNF-α and IL-1β production were partially reversed
by SnPP. These findings support the hypothesis that the induction
of HO-1 contributes to the inhibitory effects of DEE-5 on the
production of pro-inflammatory mediators.
In this study, we determined that the choice of
extraction solvent affects the biological activity of Dojuksan, a
traditional herbal formula. In conclusion, our results indicated
that the Dojuksan 30% ethanol extract, and its fraction, exerted
its anti-inflammatory effects through Nrf2-mediated HO-1
expression, and that DEE likely has greater potential therapeutic
applications than the water extract. We also suggest that the use
of different extraction solvents or bioassay-guided fractionation
is an option for improving the therapeutic efficacy of this
traditional herbal formula.
Acknowledgments
This study was supported by Wonkwang University in
2013.
References
|
1
|
Sung SH, Lee SY, Kang OH, Kwon DY, Chong
MS and Lee KN: Anti-allergic activity of Dojuk-San ethanol extract.
Korean J Orient Physiol Pathol. 25:438–444. 2011.
|
|
2
|
Kim HM, An CS, Jung KY, Choo YK, Park JK
and Nam SY: Rehmannia glutinosa inhibits tumour necrosis
factor-alpha and interleukin-1 secretion from mouse astrocytes.
Pharmacol Res. 40:171–176. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan W, Yu KQ, Liu YY, Ouyang MZ, Yan MH,
Luo R and Zhao XS: Anti-fatigue activity of polysaccharides extract
from Radix Rehmanniae Preparata. Int J Biol Macromol. 50:59–62.
2012. View Article : Google Scholar
|
|
4
|
Lau TW, Lam FF, Lau KM, Chan YW, Lee KM,
Sahota DS, Ho YY, Fung KP, Leung PC and Lau CB: Pharmacological
investigation on the wound healing effects of Radix Rehmanniae in
an animal model of diabetic foot ulcer. J Ethnopharmacol.
123:155–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin AS, Qian K, Usami Y, Lin L, Itokawa H,
Hsu C, Morris-Natschke SL and Lee KH: 5-Hydroxymethyl-2-furfural, a
clinical trials agent for sickle cell anemia, and its
mono/di-glucosides from classically processed steamed Rehmanniae
Radix. J Nat Med. 62:164–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee HS, Kim ST and Cho DK: Effects of
Rehmanniae radix water extract on renal function and renin
secretion rate in unanesthetized rabbits. Am J Chin Med.
21:179–186. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yokozawa T, Kim HY and Yamabe N:
Amelioration of diabetic nephropathy by dried Rehmanniae Radix (Di
Huang) extract. Am J Chin Med. 32:829–839. 2004. View Article : Google Scholar
|
|
8
|
Choi J, Jung HJ, Lee KT and Park HJ:
Antinociceptive and anti-inflammatory effects of the saponin and
sapogenins obtained from the stem of Akebia quinata. J Med Food.
8:78–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung HJ, Lee CO, Lee KT, Choi J and Park
HJ: Structure-activity relationship of oleanane disaccharides
isolated from Akebia quinata versus cytotoxicity against cancer
cells and NO inhibition. Biol Pharm Bull. 27:744–747. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park HJ, Shim HS, Kim H, Kim KS, Lee H,
Hahm DH and Shim I: Effects of Glycyrrhizae radix on repeated
restraint stress-induced neurochemical and behavioral responses.
Korean J Physiol Pharmacol. 14:371–376. 2010. View Article : Google Scholar
|
|
11
|
Zhang SP, Zhou YJ, Liu Y and Cai YQ:
Effect of liquiritigenin, a flavanone existed from Radix
glycyrrhizae on pro-apoptotic in SMMC-7721 cells. Food Chem
Toxicol. 47:693–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sekine-Osajima Y, Sakamoto N, Nakagawa M,
Itsui Y, Tasaka M, Nishimura-Sakurai Y, Chen CH, Suda G, Mishima K,
Onuki Y, et al: Two flavonoids extracts from Glycyrrhizae radix
inhibit in vitro hepatitis C virus replication. Hepatol Res.
39:60–69. 2009. View Article : Google Scholar
|
|
13
|
Shin EM, Zhou HY, Guo LY, Kim JA, Lee SH,
Merfort I, Kang SS, Kim HS, Kim S and Kim YS: Anti-inflammatory
effects of glycyrol isolated from Glycyrrhiza uralensis in
LPS-stimulated RAW264.7 macrophages. Int Immunopharmacol.
8:1524–1532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HY, Jung DY, Ha H, Kang SS, Kim JS and
Kim C: Induction of growth hormone release by Glycyrrhizae radix on
rat. J Biochem Mol Biol. 40:979–985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato Y, Akao T, He JX, Nojima H, Kuraishi
Y, Morota T, Asano T and Tani T: Glycycoumarin from Glycyrrhizae
radix acts as a potent antispasmodic through inhibition of
phosphodiesterase 3. J Ethnopharmacol. 105:409–414. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee HJ, Kim KA, Kang KD, Lee EH, Kim CY,
Um BH and Jung SH: The compound isolated from the leaves of
Phyllostachys nigra protects oxidative stress-induced retinal
ganglion cells death. Food Chem Toxicol. 48:1721–1727. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung SH, Lee JM, Lee HJ, Kim CY, Lee EH
and Um BH: Aldose reductase and advanced glycation endproducts
inhibitory effect of Phyllostachys nigra. Biol Pharm Bull.
30:1569–1572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SH, Kim TS, Lee HJ and Yoo JC:
Enhancement of 1,25-dihydroxyvitamin D3- and all-trans retinoic
acid-induced differentiation of human leukemia HL-60 cells by
Phyllostachys nigra var. henonis Immunopharmacol Immunotoxicol.
29:119–129. 2007. View Article : Google Scholar
|
|
19
|
Kim SH, Kim TS, Kim SJ, Seong CN, Lee OH,
Lee HJ and Yoo JC: Inhibition of interleukin-12 production in mouse
macrophages via suppression of nuclear factor-kappaB binding
activity by Phyllostachys nigra var. henonis Immunopharmacol
Immunotoxicol. 29:131–139. 2007. View Article : Google Scholar
|
|
20
|
Sachithanandan N, Graham KL, Galic S,
Honeyman JE, Fynch SL, Hewitt KA, Steinberg GR and Kay TW:
Macrophage deletion of SOCS1 increases sensitivity to LPS and
palmitic acid and results in systemic inflammation and hepatic
insulin resistance. Diabetes. 60:2023–2031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee H, Bae S, Choi BW and Yoon Y:
WNT/β-catenin pathway is modulated in asthma patients and
LPS-stimulated RAW264.7 macrophage cell line. Immunopharmacol
Immunotoxicol. 34:56–65. 2012. View Article : Google Scholar
|
|
22
|
Karpurapu M, Wang X, Deng J, Park H, Xiao
L, Sadikot RT, Frey RS, Maus UA, Park GY, Scott EW and Christman
JW: Functional PU.1 in macrophages has a pivotal role in NF-κB
activation and neutrophilic lung inflammation during endotoxemia.
Blood. 118:5255–5266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suh GY, Jin Y, Yi AK, Wang XM and Choi AM:
CCAAT/enhancer-binding protein mediates carbon monoxide-induced
suppression of cyclooxygenase-2. Am J Respir Cell Mol Biol.
35:220–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Samuelsson B, Morgenstern R and Jakobsson
PJ: Membrane prostaglandin E synthase-1: a novel therapeutic
target. Pharmacol Rev. 59:207–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simmons DL, Botting RM and Hla T:
Cyclooxygenase isozymes: the biology of prostaglandin synthesis and
inhibition. Pharmacol Rev. 56:387–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bogdan C: Nitric oxide and the immune
response. Nat Immunol. 2:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobayashi Y: The regulatory role of nitric
oxide in proinflammatory cytokine expression during the induction
and resolution of inflammation. J Leukoc Biol. 88:1157–1162. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tenhunen R, Marver HS and Schmid R: The
enzymatic conversion of heme to bilirubin by microsomal heme
oxygenase. Proc Natl Acad Sci USA. 61:748–755. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tenhunen R, Marver HS and Schmid R: The
enzymatic catabolism of hemoglobin: stimulation of microsomal heme
oxygenase by hemin. J Lab Clin Med. 75:410–421. 1970.PubMed/NCBI
|
|
30
|
Motterlini R, Haas B and Foresti R:
Emerging concepts on the anti-inflammatory actions of carbon
monoxide-releasing molecules (CO-RMs). Med Gas Res. 2:282012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alam J, Stewart D, Touchard C, Boinapally
S, Choi AM and Cook JL: Nrf2, a Cap'n'Collar transcription factor,
regulates induction of the heme oxygenase-1 gene. J Biol Chem.
274:26071–26078. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jaiswal AK: Regulation of genes encoding
NAD(P)H:quinone oxidoreductases. Free Radic Biol Med. 29:254–262.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JE, Kim SB, Kang OH, Shin IS, Kang SH,
Lee SH and Kwon DY: Inhibitory effect of water extract from
Dojuksan on LPS-induced proinflammatory cytokines production in
RAW264.7 cells. Kor J Herbology. 28:53–60. 2013. View Article : Google Scholar
|
|
34
|
Choi HG, Lee DS, Li B, Choi YH, Lee SH and
Kim YC: Santamarin, a sesquiterpene lactone isolated from Saussurea
lappa, represses LPS-induced inflammatory responses via expression
of heme oxygenase-1 in murine macrophage cells. Int
Immunopharmacol. 13:271–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Titheradge MA: The enzymatic measurement
of nitrate and nitrite. Methods Mol Biol. 100:83–91. 1998.
|
|
36
|
Jeong GS, Lee DS and Kim YC:
Cudratricusxanthone A from Cudrania tricuspidata suppresses
pro-inflammatory mediators through expression of anti-inflammatory
heme oxygenase-1 in RAW264.7 macrophages. Int Immunopharmacol.
9:241–246. 2009. View Article : Google Scholar
|
|
37
|
Lee DS, Kim KS, Ko W, Li B, Keo S, Jeong
GS, Oh H and Kim YC: The neoflavonoid latifolin isolated from MeOH
extract of Dalbergia odorifera attenuates inflammatory responses by
inhibiting NF-κB activation via Nrf2-mediated heme oxygenase-1
expression. Phytother Res. 28:1216–1223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim KS, Cui X, Lee DS, Sohn JH, Yim JH,
Kim YC and Oh H: Anti-inflammatory effect of neoechinulin a from
the marine fungus Eurotium sp SF-5989 through the suppression of
NF-κB and p38 MAPK pathways in lipopolysaccharide-stimulated
RAW2647 macrophages. Molecules. 18:13245–13259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bonizzi G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim HG, Shrestha B, Lim SY, Yoon DH, Chang
WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, et al: Cordycepin
inhibits lipopolysaccharide-induced inflammation by the suppression
of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage
cells. Eur J Pharmacol. 545:192–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar
|
|
42
|
Sugishima M, Higashimoto Y, Oishi T,
Takahashi H, Sakamoto H, Noguchi M and Fukuyama K: X-ray
crystallographic and biochemical characterization of the inhibitory
action of an imidazole-dioxolane compound on heme oxygenase.
Biochemistry. 46:1860–1867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vlahakis JZ, Kinobe RT, Bowers RJ, Brien
JF, Nakatsu K and Szarek WA: Imidazole-dioxolane compounds as
isozyme-selective heme oxygenase inhibitors. J Med Chem.
49:4437–4441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kinobe RT, Ji Y, Vlahakis JZ, Motterlini
R, Brien JF, Szarek WA and Nakatsu K: Effectiveness of novel
imidazole-dioxolane heme oxygenase inhibitors in renal proximal
tubule epithelial cells. J Pharmacol Exp Ther. 323:763–770. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ferrero-Miliani L, Nielsen OH, Andersen PS
and Girardin SE: Chronic inflammation: importance of NOD2 and NALP3
in interleukin-1beta generation. Clin Exp Immunol. 147:227–235.
2007.PubMed/NCBI
|
|
46
|
Mariathasan S and Monack DM: Inflammasome
adaptors and sensors: intracellular regulators of infection and
inflammation. Nat Rev Immunol. 7:31–40. 2007. View Article : Google Scholar
|
|
47
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liew FY and Cox FE: Nonspecific defence
mechanism: the role of nitric oxide. Immunol Today. 12:A17–A21.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
O'Banion MK: Cyclooxygenase-2: Molecular
biology, pharmacology, and neurobiology. Crit Rev Neurobiol.
13:45–82. 1999.PubMed/NCBI
|
|
50
|
Lee DS, Ko W, Quang TH, Kim KS, Sohn JH,
Jang JH, Ahn JS, Kim YC and Oh H: Penicillinolide A: a new
anti-inflammatory metabolite from the marine fungus Penicillium sp
SF-5292. Mar Drugs. 11:4510–4526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee DS, Li B, Im NK, Kim YC and Jeong GS:
4,2′,5′-Trihydroxy-4′-methoxychalcone from Dalbergia odorifera
exhibits anti-inflammatory properties by inducing heme oxygenase-1
in murine macrophages. Int Immunopharmacol. 16:114–121. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Otterbein LE, Bach FH, Alam J, Soares M,
Tao Lu H, Wysk M, Davis RJ, Flavell RA and Choi AM: Carbon monoxide
has anti-inflammatory effects involving the mitogen-activated
protein kinase pathway. Nat Med. 6:422–428. 2000. View Article : Google Scholar : PubMed/NCBI
|