Introduction
Arctium fruit (Arctium lappa L.) has been
used in traditional herbal medicine in East Asia, and is known to
exert various beneficial, such as antioxidant (1), anticancer (2), anti-aging (3), anti-diabetes (4), neuroprotective (5), hepatoprotective (6) and anti-colitis (7) effects. Such effects are associated
with polyphenol compounds, such as arctigenin, arctiin and other
lignan compounds in Arctium lappa fruit (1,2,4,6,8).
However, to the best of our knowledge, the effects of Arctium
lappa fruit or its fermentation product on allergic reactions
have not been reported to date.
The fermentation process for medicinal herbs has
been frequently used for improving the biological effects of the
extracts (9,10). Fermentation using microorganisms,
such as yeast (11), fungi
(12) and bacteria (9,10)
is known to yield metabolites, or to convert glycosylated compounds
into aglycones, one of the active forms.
Anaphylactic shock is known as the type I of
allergic response classes (13),
and is the most important clinically, as anaphylactic shock is
closely associated with the degranulation of mast cells (14). When mast cells are activated by
the antigen-immunoglobulin E (IgE) complex, they are then able to
release a number of allergic inflammatory mediators, such as
histamine, cytokines, prostaglandins and leukotrienes from numerous
granules in a matter of minutes (15). Consequently, tissues or organs
stimulated by allergic inflammatory mediators induce acute
inflammatory responses simultaneously. In signaling cascades
associated with allergic reactions, the FcεRI receptor is known as
the high affinity IgE receptor, and is located on the plasma
membrane of mast cells and basophilic cells (15). Therefore, the activation of the
receptor on mast cells is intimately associated with degranulation
or biosynthesis of the allergic inflammatory mediators in mast
cells.
In present study, we found that Arctium lappa
fruit extract (AFE) exerted mild anti-allergic effects. Based on
this finding, we hypothesized that the fermentation process may
improve the anti-allergic effects of AFE on IgE-activated mast
cells. To investigate anti-allergic effects of fermented AFE
(F-AFE), degranulation was determined by measuring β-hexosaminidase
activity in IgE-activated RBL-2H3 cells. To examine the
anti-allergic and anti-inflammatory effects of F-AFE, the levels of
inflammatory biomarkers, including tumor necrosis factor-α (TNF-α)
and prostaglandin E2 (PGE2) were measured.
Finally, to elucidate the mechanisms and active compounds
responsible for the anti-allergic effects of F-AFE, the FcεRI
signaling cascade was analyzed by immumoblotting analysis, and the
active phytochemical composition of F-AFE was analyzed by
high-performance liquid chromatography (HPLC). Herein, we
demonstrate that F-AFE suppresses IgE-mediated allergic events in
mast cells. Furthermore, our data may aid in the development of a
phytomedicine for allergic diseases.
Materials and methods
Reagents
MEM-α medium, 1X Dulbecco's phosphate-buffered
saline (DPBS), penicillin, streptomycin and fetal bovine serum
(FBS) were purchased from GE Healthcare Life Sciences (HyClone,
Logan, UT, USA). The EZ-Cytox cell viability assay kit was
purchased from DAEIL Lab Service Co. Ltd. (Seoul, Korea). Specific
antibodies against phosphorylated (p-)Lyn (#2731), p-Syk (#2710),
p-phosphoinositide phospholipase C (PLC)γ1/2 (#2821, #3871,
respectively), p-protein kinase C (PKC)δ (#2055), p-extracellular
signal-regulated kinase 1/2 (ERK1/2; #9101), p-c-Jun
N-terminal kinase 1/2 (p-JNK; #9251), p-p38 (#9211), p-Akt
(#9271), p-cytosolic phospholipase A2 (cPLA2;
#2831) and cyclooxygenase (COX)-2 (#4842) were obtained from Cell
Signaling Technology (Beverly, MA, USA). A specific antibody
against p-Fyn (orb128087) was purchased from Biorbyt Ltd.
(Cambridge, UK). A specific antibody against β-actin (sc-47778) was
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The
enzyme immunoassay (EIA) kit for PGE2 was obtained from
Cayman Chemical Co. (Ann Arbor, MI, USA). The enzyme-linked
immunosorbent assay (ELISA) kit for TNF-α was purchased from
e-Bioscience, Inc. (Science Center Drive, San Diego, CA, USA).
4-Nitrophenyl N-acetyl-β-D-glucosaminide (p-NAG),
dinitrophenyl (DNP)-immunoglobulin E (DNP-IgE) and DNP-human serum
albumin (DNP-HSA) were obtained from Sigma-Aldrich (St. Louis, MO,
USA). The standards of arctiin and matairesinol were purchased from
Wuhan ChemFaces Biochemical Co., Ltd. (Wuhan, China). A standard of
arctigenin was obtained from Must Bio-Technology Co., Ltd.
(Chengdu, China). All other chemicals were of analytical grade.
Preparation of extracts of Arctium lappa
fruit or fermented Arctium lappa fruit
AFE or F-AFE were prepared according to a
modification of a process reported previously (16); Arctium lappa fruits were
purchased from the Yeongcheon Oriental Herbal Market (Yeongcheon,
Korea), and were then verified by Dr Ki-Hwan Bae, who holds the
position of Professor Emeritus at the College of Pharmacy, Chungnam
National University (Daejeon, Korea). Arctium lappa fruits
(1 kg) were boiled in distilled water (10 liter) for approximately
3 h at 115°C. The aqueous extract was filtered through a testing
sieve (aperture 500 and 150 μm). AFE was fermented using
Lactobacillus rhamnosus (L. rhamnosus) (KFRI 128,
KCTC 2182) provided from the Korea Food Research Institute.
Briefly, the water extract adjusted to pH 7.0 with 1 N NaOH was
autoclaved for 5 min, inoculated with L. rhamnosus
(1×105–1×107 CFU/ml), and then incubated for
48 h at 37°C for 48 h. F-AFE was filtered through a 60-μm
nylon net filter (Millipore, Billerica, MA, USA), deposited
overnight. The supernatant was lyophilized, and the dried pellet
was then stored at −20°C until use. AFE and F-AFE were dissolved in
10% DMSO solution for use in all the experiments.
HPLC analysis
HPLC analysis was evaluated following a modification
of a previously described method (17). To analyze the lignan compounds,
HPLC analysis was performed using a Chromeleon 7 system equipped
with a Dionex UltiMate 3000 Pump, an autosampler, a column
compartment and a diode array detector (Thermo Fisher Scientific
Inc., Foster City, CA, USA) and a Phenomenex Luca C18
column (4.6×250 mm, 5 μm; Phenomenex Inc., Torrance, CA,
USA). The lignan chemicals were eluted in a gradient system
composed of solvent A (deionized water) and solvent B
(acetonitrile). The gradient was 20-30-36-45-90-20% of solvent B at
gradient time, tG = 0-20-35-50-53-75 min, column oven
temperature was 30°C and the flow rate was 1.0 ml/min; an injection
volume of 10 μl was applied. The UV/Vis detector was set at
a wavelength range from 190 to 400 nm. The standards of arctiin,
arctigenin or matairesinol and sample solutions (1.0 mg/ml) were
dissolved and diluted in 60% methanol. Calibration curves
constructed using linear least-squares regression were linear over
the concentration range of the standards used (Table I). The relative standard deviation
of the measured concentrations was used to assess the precision. A
comparison of the mean measured concentration versus the
corresponding nominal concentration was used to assess the
accuracy.
 | Table ICalibration curves of three lignan
standards. |
Table I
Calibration curves of three lignan
standards.
| Lignan
compound | Regression
equationa | Correlation
coefficient (r2)b | Linear range
(μg) |
|---|
| Arctiin |
y=0.0916x+0.0737 | 10.000 | 37.5–600.0 |
| Arctigenin |
y=63597x−2277.6 | 10.000 | 12.5–200.0 |
|
Methylarctigenin |
y=119193x−18355 | 10.000 | 5.0–80.0 |
Cell culture
RBL-2H3 cells, originating from rat basophilic
leukemia and known as one of the mast cell lines, were obtained
from the Korean Cell Line Bank (Seoul, Korea), and were cultured in
MEM-α medium containing 10% (v/v) FBS, 100 U/ml penicillin and 100
μg/ml streptomycin at 37°C in a humidified atmosphere of 5%
CO2 as previously described (18). All the experiments included a
control group, which was a vehicle control group (0.1% DMSO).
Cell viability assay
Cell viability was determined by measuring the
mitochondrial-dependent reduction of WST-1 to water-soluble
tetrazolium salt (19). Briefly,
the RBL-2H3 cells were seeded on a 96-well plate (1×104
cells/well) in MEM-α medium with 10% FBS at 37°C overnight. The
cells were washed by 1X DPBS, and then incubated with 50 ng/ml
DNP-IgE for 24 h. The above-mentioned cells, pre-incubated with AFE
(0–2,500 μg/ml) or F-AFE (0–500 μg/ml) for 1 h in
Hank's balanced salt solution (HBSS) containing 0.1% BSA, were
simultaneously mixed with 0.1 μg/ml DNP-HSA and 10 μl
EZ-Cytox reagent, and then incubated for a further 4 h. Cell
viability was determined at 450 nm using a microplate reader
(SpectraMax i3; Molecular Devices, Sunnyvale, CA, USA).
β-hexosaminidase activity assay
β-hexosaminidase activity was evaluated according to
a previously described method (20). The supernatant (25 μl) was
mixed with 50 μl p-NAG (10 mM) in 0.1 M sodium citrate
buffer (pH 4.5) in a 96-well plate, followed by incubation for 1 h
at 37°C. The reaction was terminated by stop buffer (0.1 M sodium
carbonate buffer, pH 10.0). The absorbance was measured at 405 nm
using a microplate reader.
ELISA for determining the levels of
TNF-α
To determine the levels of TNF-α in the culture
media, the IgE-sensitized cells were pre-incubated with F-AFE
(0–500 μg/ml) for 1 h in MEM-α medium with 0.1% FBS for 1 h,
spiked with DNP-HSA, and then incubated for a further 4 h. All
culture media were centrifuged (17,000 × g for 10 min) at 4°C, and
the samples were stored at −80°C until use. TNF-α was detected
using an ELISA kit according to the manufacturer's instructions
(e-Bioscience, Inc.).
EIA for determining the levels of
PGE2
To measure the levels of PGE2 in the
culture media, all culture media were centrifuged (17,000 × g for
10 min) at 4°C, and the samples were stored at −80°C until use.
PGE2 was measured using an EIA kit according to the
manufacturer's instructions (Cayman Chemical Co.).
Immunoblot analysis
Immunoblot analysis was carried out according to a
previously described method (20). PVDF membranes containing blotted
proteins were visualized using a chemiluminescent reaction (ECL
plus kit) and an Imaging system (ChemiDoc Touch Imaging System)
(both from Bio-Rad, Hercules, CA, USA). The levels of target
proteins were compared to those of a loading control (β-actin), and
the results were expressed as a ratio of the density of each
protein identified by a protein standard size marker (BIOFACT Co.,
Ltd., Daejeon, Korea). The density of each band was measured using
ImageJ software (version 1.49v for Windows; NIH, Bethesda, MD,
USA).
Statistical analyses
The experimental results are presented as the means
± SD. One-way analysis of variance (ANOVA) was used for multiple
comparisons (GraphPad Prism version 5.01 for Windows; GraphPad
Software, San Diego, CA, USA). If there was a significant variation
between the treatment groups, the Dunnett's test was applied.
Values of P<0.05 and P<0.01 were considered to indicate
statistically significant differences.
Results
Inhibitory effects of AFE or F-AFE on
IgE-mediated degranulation in RBL-2H3 cells: comparison between AFE
and F-AFE
First, to investigate effects of AFE and F-AFE on
IgE-mediated degranulation in mast cells, the IgE-sensitized
RBL-2H3 cells were incubated with various concentrations of AFE or
F-AFE prior to exposure to the antigen (0.1 μg/ml DNP-HSA).
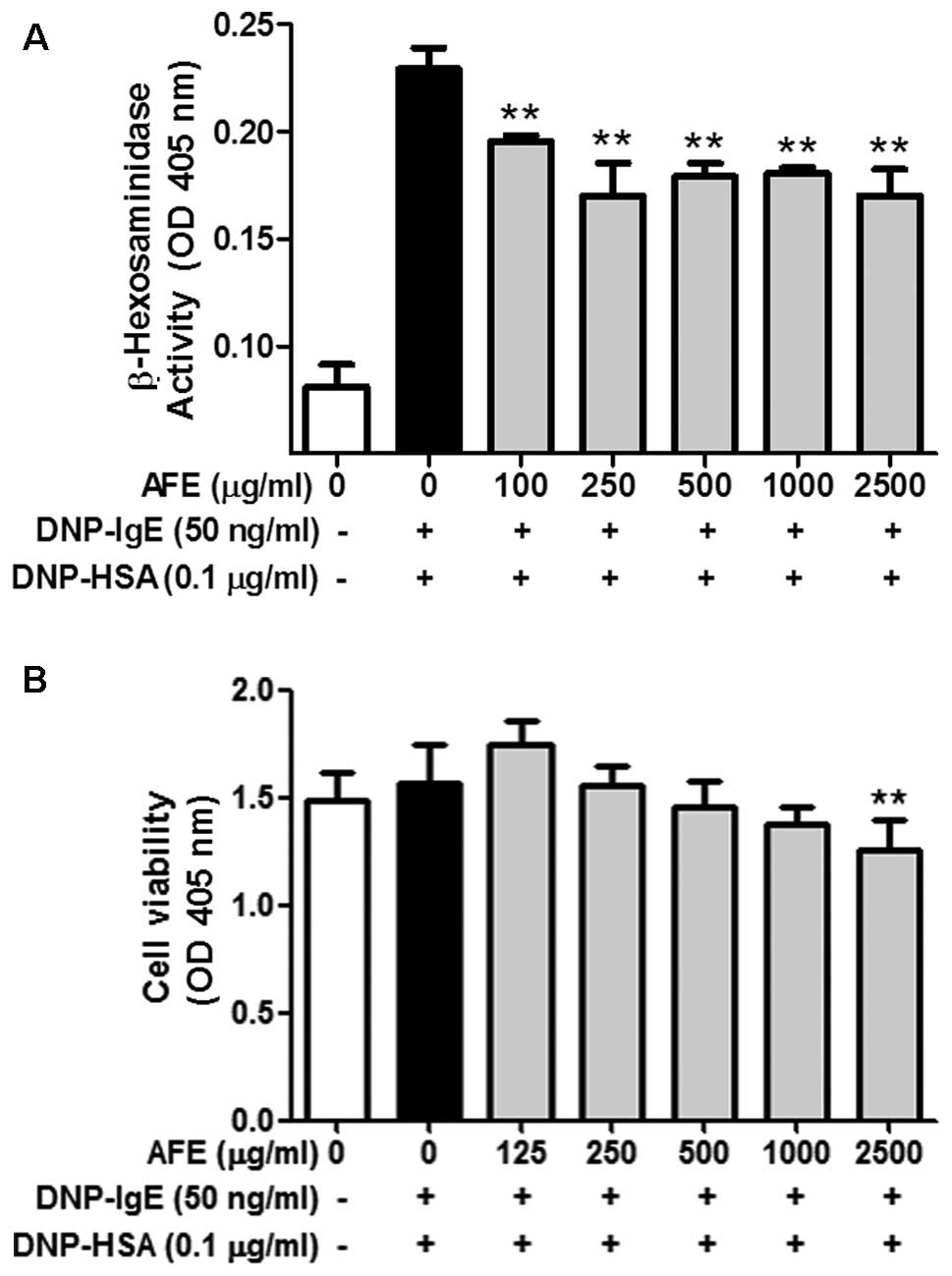
Although AFE weakly inhibited the release of β-hexosaminidase, a
marker of degranulation, from the IgE-activated RBL-2H3 cells until
2,500 μg/ml, the inhibitory effect of AFE was not enhanced
after 250 μg/ml (Fig. 1A).
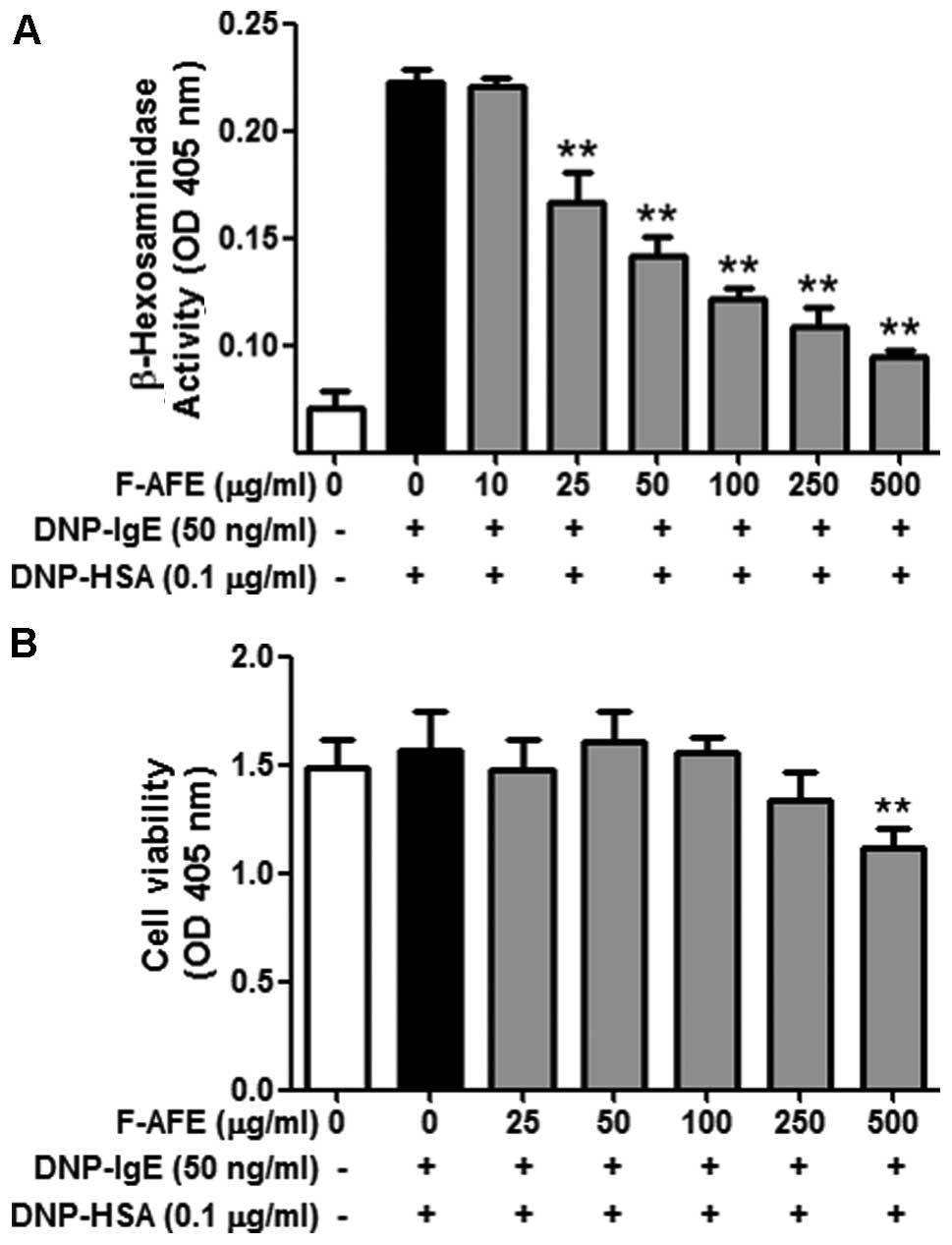
On the other hand, F-AFE markedly inhibited degranulation
(IC50 value, 30.73 μg/ml) in the IgE-activated
RBL-2H3 cells in a dose-dependent manner (Fig. 2A). Notably, neither AFE nor F-AFE
exerted any severe cytotoxic effects at concentrations of up to
1,000 μg/ml and 250 μg/ml, respectively (Figs. 1B and 2B). These results indicate that
fermentation of the Arctium lappa fruit potently enhances
the anti-allergic effects of AFE within non-cytotoxic
concentrations.
Inhibitory effects of F-AFE on the
release of pro-inflammatory mediators
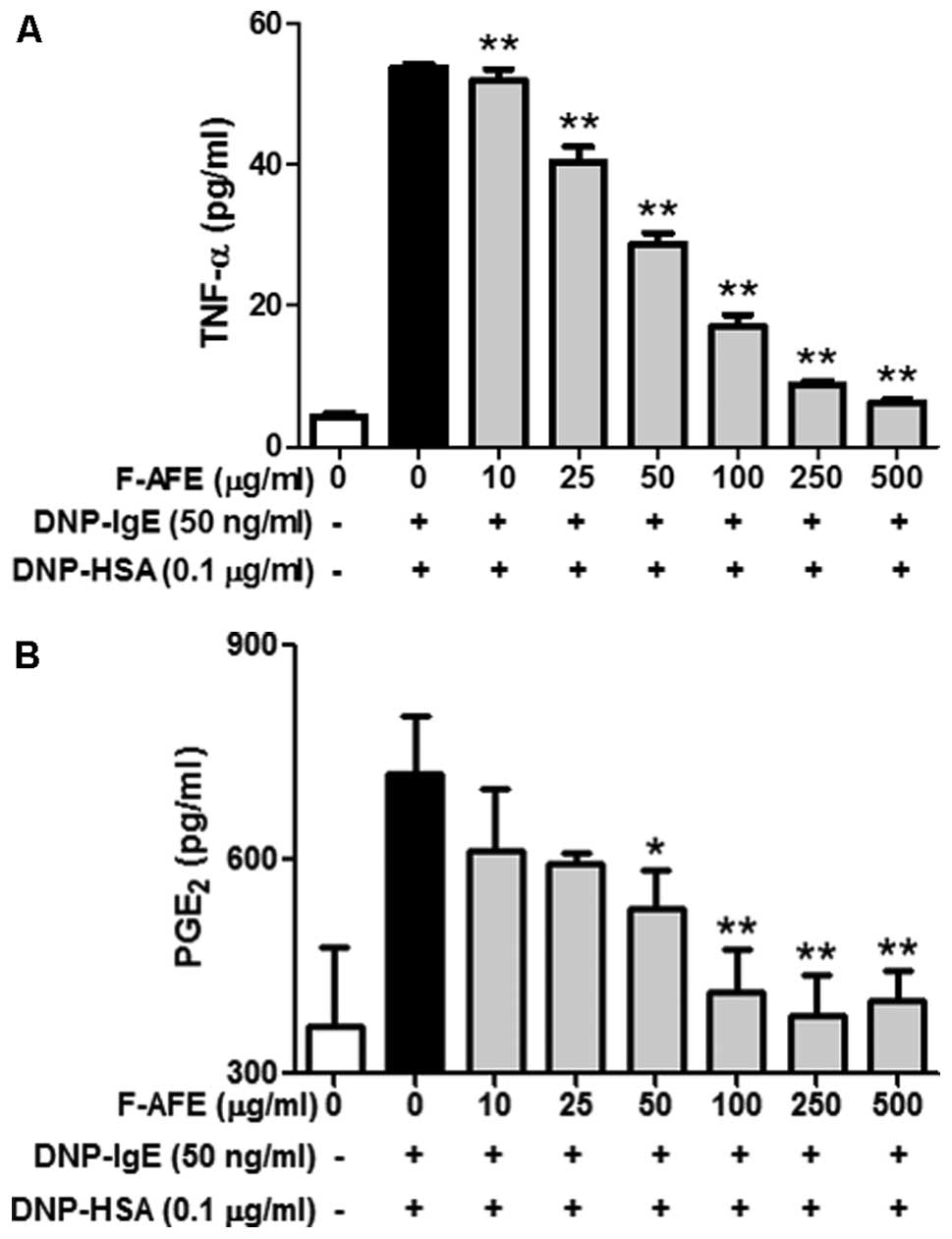
It is known that IgE-activated mast cells are able
to release pro-inflammatory mediators, such as TNF-α, associated
with the initiation of airway inflammation and the generation of
airway hyper-responsiveness in asthma (21), and PGE2, associated
with asthma development and inflammation (22). Therefore, we focused on the
effects of F-AFE on the release of TNF-α and PGE2 from
IgE-activated RBL-2H3 cells. When the IgE-sensitized RBL-2H3 cells
were pre-incubated with F-AFE and stimulated with the antigen,
F-AFE significantly decreased the release of TNF-α with an
IC50 value of 46.96 μg/ml (Fig. 3A) and that of PGE2 with
an IC50 value of 36.27 μg/ml (Fig. 3B). These results suggest that
F-AFE suppresses acute or chronic allergic inflammatory responses
by inhibiting the release of various inflammatory mediators,
including cytokines and lipid mediators from IgE-activated mast
cells.
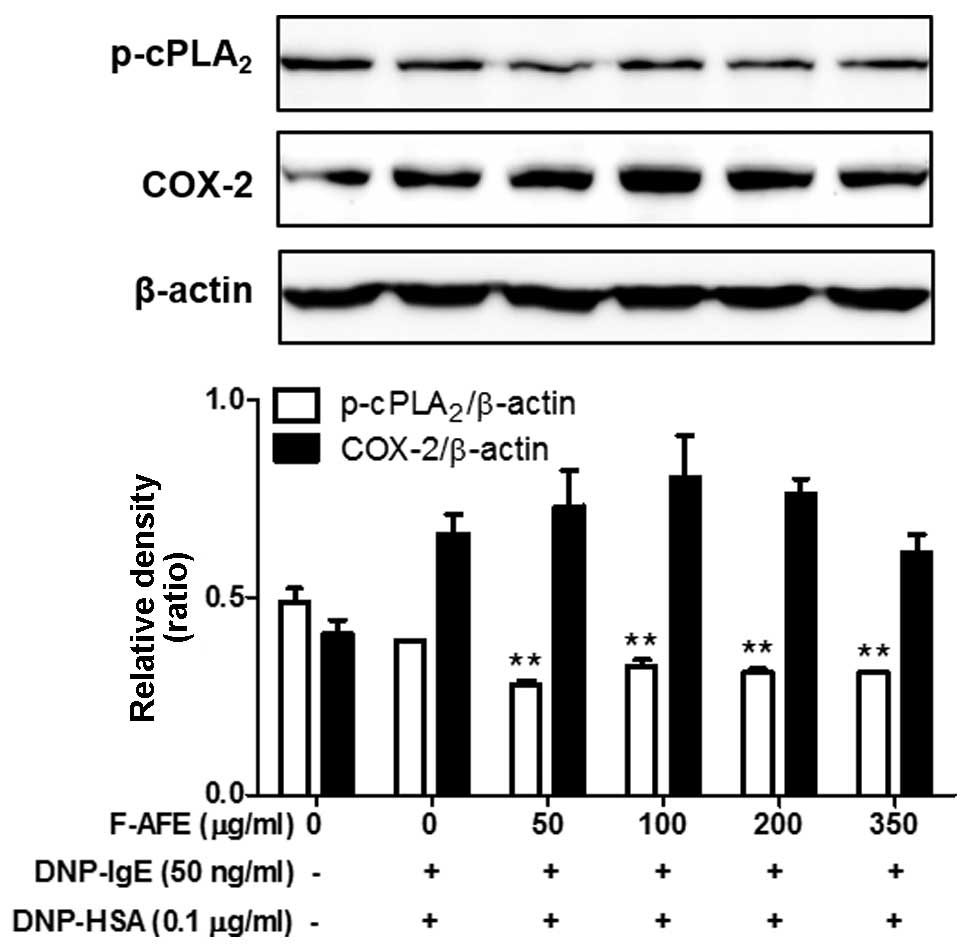
Regulatory effects of F-AFE on the
activation of the arachidonate cascade
We wished to examine the effects of F-AFE on the
formation of pro-inflammatory lipid mediators, as pro-inflammatory
lipid mediators, including PGE2, prostaglandin
D2 (PGD2), leukotriene B4
(LTB4) and leukotriene C4 LTC4)
are known to aggravate allergic diseases (23–26). Thus, we investigated the effects
of F-AFE on the phosphorylation of cPLA2, a
rate-limiting enzyme of eicosanoid synthesis, and the expression of
COX-2, a rate-limiting enzyme of prostaglandins. When the
IgE-sensitized RBL-2H3 cells were incubated with F-AFE prior to
antigen challenge for 4 h, F-AFE partially suppressed the
phosphorylation of cPLA2, but not the expression of
COX-2 (Fig. 4). These findings
indicate that F-AFE regulates the formation of eicosanoids,
including prostaglandins by regulating the activation of
cPLA2 in the arachidonate cascade.
Regulatory effects of F-AFE on the
activation of the FcεRI cascade
Since F-AFE reduced the rate-limiting enzymes of the
arachidonate cascade in the late phase (4 h), we further
investigated the rate-limiting proteins and intermediate proteins
in the FcεRI cascade in the early phase (10 min). The activation of
the arachidonate cascade is involved in the IgE-mediated activation
of the FcεRI cascade in mast cells (20,27). Thus, we wished to determine
whether F-AFE regulates the activation of rate-limiting proteins
and/or intermediate proteins in the IgE-mediated FcεRI cascade.
When the IgE-sensitized RBL-2H3 cells were stimulated with the
antigen (DNP-HSA) for 10 min after the cells were incubated with
F-AFE for 1 h, F-AFE inhibited the phosphorylation of Lyn and Fyn,
as well as that of Syk (Fig. 5A).
In addition, F-AFE significantly reduced the phosphorylation of
intermediate proteins, the downstream targets of Syk, such as ERK,
JNK, p38 and Akt (Fig. 5C).
Additionally, F-AFE significantly suppressed the phosphorylation of
PLCγ1/2 and PKCδ, which are involved in the degranulation process
of mast cells (Fig. 5B). These
findings indicate that the anti-allergic effects of F-AFE are
associated with the regulation of the activation of the FcεRI
cascade through the inhibition of the activation of Lyn, Fyn and
Syk in mast cells.
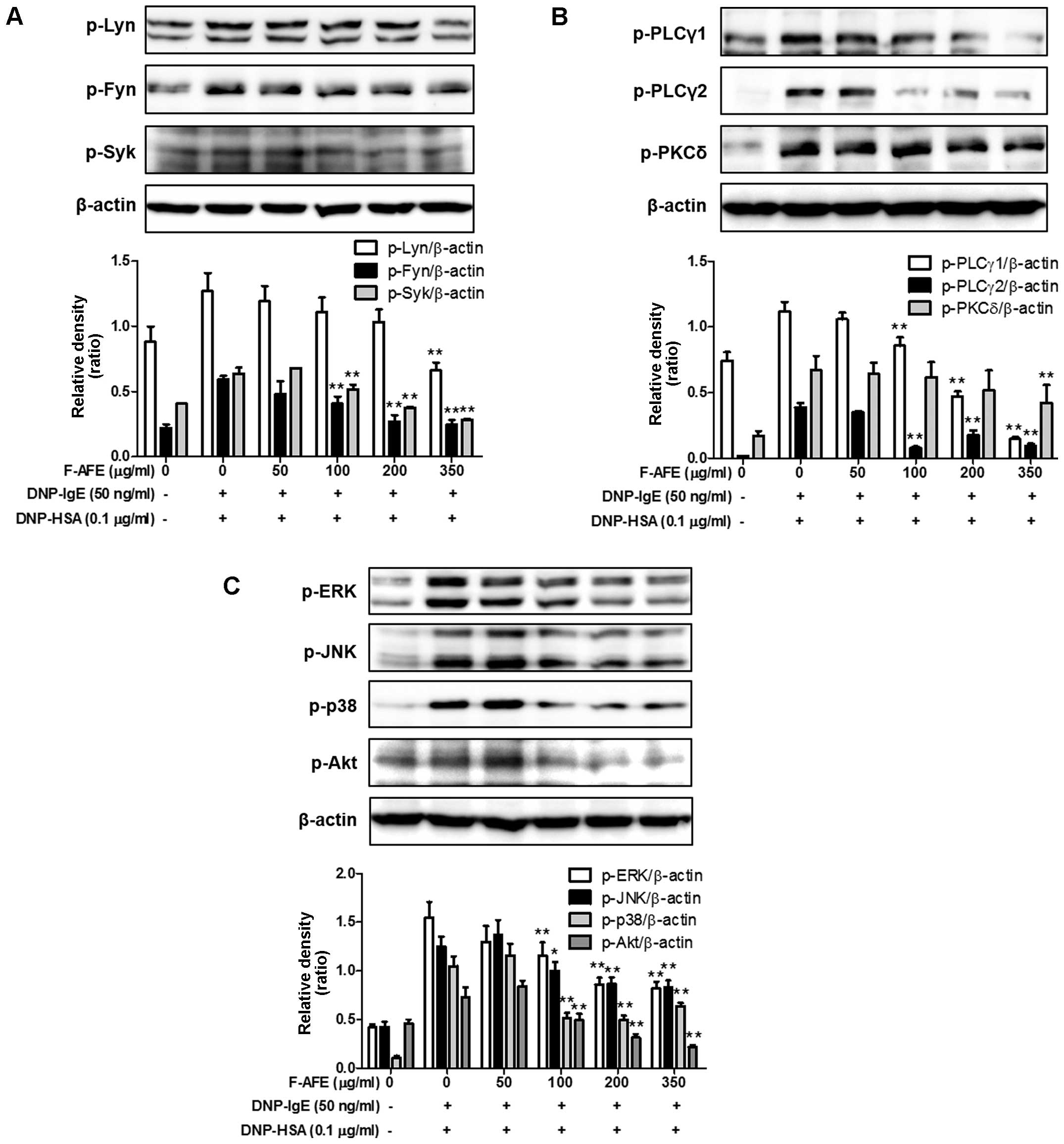 | Figure 5Effects of fermented Arctium
lappa fruit extract (F-AFE) on the phosphorylation of
intermediate proteins in the FcεRI cascade. immunoglobulin E
(IgE)-sensitized RBL-2H3 cells were exposed to F-AFE (0–350
μg/ml) for 1 h, and then stimulated with dinitrophenyl-human
serum albumin (DNP-HSA) (0.1 μg/ml) for 10 min. The
above-mentioned cells were rinsed with 1X DPBS, and lysed with cell
lysis buffer. The expression of p-Lyn, p-Fyn, p-Syk, p-PLCγ1,
p-PLCγ2, p-PKCδ, p-ERK, p-JNK, p-p38, p-Akt, or β-actin was
determined as described in the Materials and methods. Similar
results were obtained from 3 independent experiments.
*P<0.05 and **P<0.01 vs.
DNP-HSA-treated group. (A) p-Lyn, p-Fyn and p-Syk; (B) p-PLCγ1,
p-PLCγ2 and p-PKCδ; (C) p-ERK, p-JNK, p-p38 and p-Akt. |
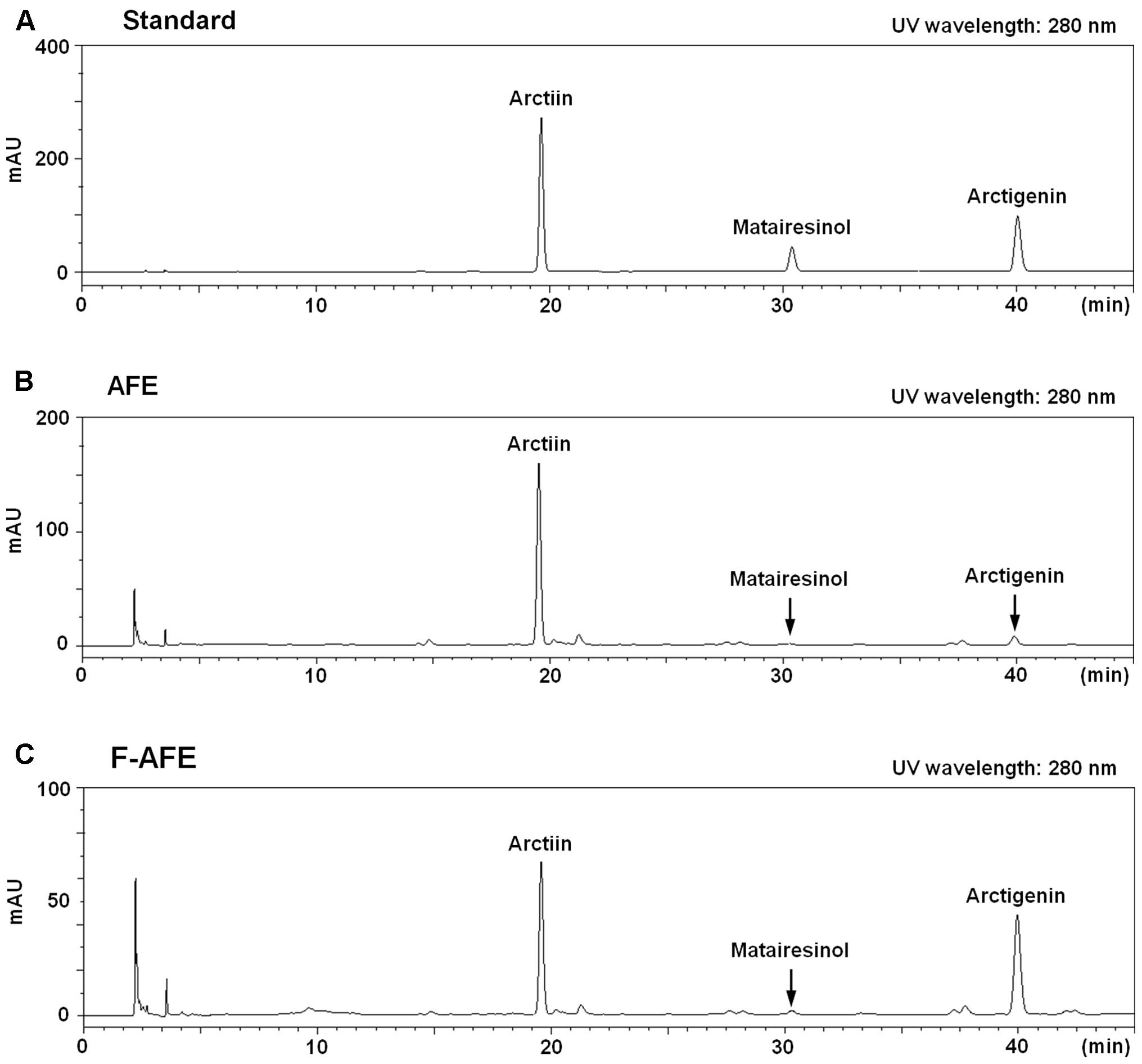
Identification of lignan compounds, one
of the major components, in F-AFE: comparison between AFE and
F-AFE
Finally, to substantiate what is behind the
anti-allergic effects of F-AFE, we analyzed and compared the
chemical composition of F-AFE and AFE using an HPLC system. It is
known as that Arctium lappa fruit contains a number of
lignan compounds, such as arctiin, arctigenin and other lignan
compounds (1,2,4,6,8).
Based on the findings of these previous studies, we analyzed the
amounts of the 3 major compounds: arctiin, matairesinol and
arctigenin. The retention times for the peaks of the arctiin,
matairesinol or arctigenin standard were 19.64, 30.38 or 40.06 min,
respectively, as shown on an HPLC chromatogram (Fig. 6A). The peaks of matairesinol and
arctigenin in F-AFE were increased, and the peak of arctiin in
F-AFE was decreased on the HPLC chromatogram (Fig. 6B and C). Table II summarizes the amounts of
arctiin, matairesinol and arctigenin in AFE and F-AFE. These
results suggest that fermentation using L. rhamnosus
elevates the levels of aglycones, such as arctigenin in AFE through
conversion from lignan glycosides, such as arctiin. Taken together,
our findings indicate that F-AFE has the potential for use as an
anti-allergic remedy, and that aglycones including arctigenin in
AFE are responsible for the anti-allergic effects.
 | Table IIComposition of AFE or F-AFE: lignan
compounds. |
Table II
Composition of AFE or F-AFE: lignan
compounds.
| Lignan compounds
|
|---|
| Arctiin | Arctigenin |
Methylarctigenin |
|---|
| AFE | 338.79±0.01 | 14.69±0.01 | 1.80±0.37 |
| F-AFE | 144.31±0.05 | 88.35±0.07 | 2.99±0.22 |
Discussion
Arctium lappa fruit has long been used in
traditional herbal medicine in East Asia, and has been shown to
exert various beneficial effects, such as antioxidant (1), anticancer (2), anti-aging (3), anti-diabetic (4), neuroprotective (5), hepatoprotective (6) and anti-colitis (7) effects. Such effects of the
Arctium lappa fruit are caused by lignan compounds (1–7).
However, to the best of our knowledge, the effects of the
Arctium lappa fruit on allergic reaction have not been
reported to date.
Various fermented foods have been consumed for
centuries worldwide. In addition, several fermented foods have also
been used in traditional medicine. Currently, the application of
the fermentation process using microorganisms, such as fungi, yeast
and bacteria has been often used to improve the beneficial effects
of medicinal herbs (9–12). Fermentation is known for yielding
metabolites, or converting glycosylated compounds to aglycones, one
of the active forms. The fermentation process is known to yield
metabolites of microorganisms, or convert glycosides to aglycones
(11,12,28). Nevertheless, the application of
the fermentation process for the Arctium lappa fruit has
been not reported to date, to the best of our knowledge.
Generally, Arctium lappa fruit is known to
contain various lignan compounds, such as arctiin, arctigenin and
methylarctigenin (1,2,4,6,8,29).
In particular, arctigenin is known as one of the major lignan
compounds (29), and is a major
effective component (5,7,30)
in the Arctium lappa fruit. These data demonstrate that AFE
possesses weak anti-allergic properties. By contrast, as shown in
this study, F-AFE, fermented using L. rhamnosus, exerted
potent anti-allergic effects, as shown by cultured cell-based
assays. F-AFE also contains elevated levels of aglycones, such as
arctigenin, as shown by HPLC analysis. Therefore, F-AFE possesses
an advantage in comparison with AFE, as F-AFE is much more potent
than AFE in suppressing the biomarkers for degranulation
(β-hexosaminidase) and inflammation (TNF-α and PGE2) at
several dozen microgram per milliliter levels. Actually, F-AFE
contains 6-fold higher levels of arctigenin than AFE, whereas the
acrtiin level was decreased in F-AFE by approximately 57.40%
compared to AFE. This indicates that the anti-allergic effects of
F-AFE may be caused by the elevated levels of arctigenin. Although
F-AFE at 500 μg/ml significantly had a low cytotoxicity,
F-AFE at the same concentration suppressed the release of
β-hexosaminidase, TNF-α and PGE2. These results suggest
that F-AFE at only 500 μg/ml may inhibit the growth of mast
cells or induces the apoptosis of mast cells. To conclude, F-AFE
(at 500 μg/ml) may not only directly inhibit the allergic
response in IgE-activated mast cells, but may also induce a
decrease in the number of mast cells. Such effects of F-AFE may
contribute to the enhancement of its anti-allergic effects.
As regards the intracellular mechanisms responsible
for the anti-allergic effects of F-AFE, one possible mechanism is
associated with a direct suppression of a signaling cascade of
FcεRI receptor, a high affinity IgE receptor (31). The degranulation in IgE-activated
mast cells correlates with the activation of the FcεRI receptor,
located on the extracellular plasma membrane of mast cells or
basophils (31). When
IgE-activated mast cells liberates granules to the extracellular
space, a number of pro-inflammatory mediators, such as TNF-α,
histamine, prostaglandins and leukotrienes are released from the
cells (15), and these mediators
then induce acute and chronic inflammation. Therefore, the key
function of an anti-allergic action is to regulate the activation
of the FcεRI cascade. In this regard, Fyn, Lyn and Syk are known as
rate-limiting factors of the FcεRI signaling cascade (32,33). Their activation induces the
elevation of intracellular Ca2+ levels and the
activation of the mitogen-activated protein kinase family, such as
ERK, JNK and p38 (32).
Therefore, Fyn, Lyn and Syk are the key signaling proteins of FcεRI
cascade in the early phase. In the present study, the inhibitory
effects of F-AFE on the phosphorylation of Lyn, Fyn or Syk may
support the notion that one of the anti-allergic targets for F-AFE
may be Lyn, Fyn or Syk. In support of this hypothesis, in our
study, F-AFE significantly inhibited the phosphorylation of ERK,
JNK, p38 and Akt. Additionally, F-AFE also reduced the
phosphorylation of PLCγ1/2 and PKCδ in a concentration-dependent
manner. It is known that the degranulation of IgE-activated mast
cells is involved in the activation of the Syk/PLCγ/PKCδ pathway in
the FcεRI cascade (20).
Separately, as previously demonsrated, the
production of eicosanoids, including prostaglandins and
leukotrienes in IgE-activated mast cells is intimately related with
the activation of the FcεRI cascade (20,22). Eicosanoids, such as
PGE2, PGD2, LTB4 and
LTC4 are able to induce or aggravate acute and chronic
inflammation in allergic diseases, such as asthma and allergic
rhinitis (34). Therefore, the
formation of eicosanoids in IgE-activated mast cells is another
target of the anti-allergic actions of F-AFE. In this study, F-AFE
not only reduced the levels of PGE2, which is involved
in asthma development and inflammation related with interleukin
(IL)-4 and IL-5 (23), but also
suppressed the phosphorylation of cPLA2 in IgE-activated
RBL-2H3 cells. This suggests that F-AFE inhibits the biosynthesis
of prostaglandins through the inhibition of cPLA2, a
rate-limiting enzyme of the arachidonate cascade. Besides, the
inhibitory effects of F-AFE on the activation of cPLA2
may support the reduction of leukotriene biosynthesis in
IgE-activated mast cells. Such effects of F-AFE may contribute to
the improvement of its anti-allergic effects on allergic
diseases.
In conclusion, this study demonstrates that AFE or
F-AFE exert anti-allergic effects, and that the fermentation
process enhances the anti-allergic effects of AFE by converting
arctiin, a lignan glycoside, to arctigenin, an aglycone. These
findings reveal a novel feature of F-AFE in allergic reactions. The
mechanisms responsible for its anti-allergic effects may involve
various targets, such as Lyn, Fyn, Syk and cPLA2, as
well as pro-inflammatory mediators. Such effects may be due to
several compounds in the Arctium lappa fruit, and may aid in
the development of a functional food or a preventive agent for
allergic diseases.
Acknowledgments
This study was supported by Grant K15280 from the
Korea Institute of Oriental Medicine, Ministry of Education,
Science and Technology (MEST), Republic of Korea.
References
|
1
|
Liu J, Cai YZ, Wong RN, Lee CK, Tang SC,
Sze SC, Tong Y and Zhang Y: Comparative analysis of caffeoylquinic
acids and lignans in roots and seeds among various burdock (Arctium
lappa) genotypes with high antioxidant activity. J Agric Food Chem.
60:4067–4075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsumoto T, Hosono-Nishiyama K and Yamada
H: Antiproliferative and apoptotic effects of butyrolactone lignans
from Arctium lappa on leukemic cells. Planta Med. 72:276–278. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Knott A, Reuschlein K, Mielke H, Wensorra
U, Mummert C, Koop U, Kausch M, Kolbe L, Peters N, Stäb F, et al:
Natural Arctium lappa fruit extract improves the clinical signs of
aging skin. J Cosmet Dermatol. 7:281–289. 2008. View Article : Google Scholar
|
|
4
|
Xu Z, Wang X, Zhou M, Ma L, Deng Y, Zhang
H, Zhao A, Zhang Y and Jia W: The antidiabetic activity of total
lignan from Fructus Arctii against alloxan-induced diabetes in mice
and rats. Phytother Res. 22:97–101. 2008. View Article : Google Scholar
|
|
5
|
Lee IA, Joh EH and Kim DH: Arctigenin
isolated from the seeds of Arctium lappa ameliorates memory
deficits in mice. Planta Med. 77:1525–1527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang YN, Huang XY, Feng ZM, Jiang JS and
Zhang PC: Hepatoprotective activity of twelve novel 7′-hydroxy
lignan glucosides from Arctii Fructus. J Agric Food Chem.
62:9095–9102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu X, Yang Y, Dou Y, Ye J, Bian D, Wei Z,
Tong B, Kong L, Xia Y and Dai Y: Arctigenin but not arctiin acts as
the major effective constituent of Arctium lappa L. fruit for
attenuating colonic inflammatory response induced by dextran
sulfate sodium in mice. Int Immunopharmacol. 23:505–515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boldizsár I, Füzfai Z, Tóth F, Sedlák E,
Borsodi L and Molnár-Perl I: Mass fragmentation study of the
trimethylsilyl derivatives of arctiin, matairesinoside, arctigenin,
phylligenin, matairesinol, pinoresinol and methylarctigenin: Their
gas and liquid chromatographic analysis in plant extracts. J
Chromatogr A. 1217:1674–1682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh YC, Cho WK, Oh JH, Im GY, Jeong YH,
Yang MC and Ma JY: Fermentation by Lactobacillus enhances
anti-inflammatory effect of Oyaksungisan on LPS-stimulated RAW
264.7 mouse macrophage cells. BMC Complement Altern Med. 12:172012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim A, Im M, Hwang YH, Yang HJ and Ma JY:
Jaeumganghwa-tang induces apoptosis via the mitochondrial pathway
and Lactobacillus fermentation enhances its anticancer activity in
HT1080 human fibrosarcoma cells. PLoS One. 10:e01278982015.
View Article : Google Scholar
|
|
11
|
Kim JH, Park TS, Yang SH, Suh JW and Shim
SM: Microbial bioconversion and processing methods enhance the
phenolic acid and flavonoids and the radical scavenging capacity of
Smilax china L. leaf. J Sci Food Agric. Mar 5–2015.Epub ahead of
print. View Article : Google Scholar
|
|
12
|
Sheih IC, Fang TJ, Wu TK, Chang CH and
Chen RY: Purification and properties of a novel phenolic
antioxidant from Radix astragali fermented by Aspergillus oryzae
M29. J Agric Food Chem. 59:6520–6525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uzzaman A and Cho SH: Chapter 28:
Classification of hypersensitivity reactions. Allergy Asthma Proc.
33(Suppl 1): S96–S99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Theoharides TC and Kalogeromitros D: The
critical role of mast cells in allergy and inflammation. Ann NY
Acad Sci. 1088:78–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gilfillan AM and Tkaczyk C: Integrated
signalling pathways for mast-cell activation. Nat Rev Immunol.
6:218–230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chung TH, Kang TJ, Cho WK, Im GY, Lee GS,
Yang MC, Cho CW and Ma JY: Effectiveness of the novel herbal
medicine, KIOM-MA, and its bioconversion product, KIOM-MA128, on
the treatment of atopic dermatitis. Evid Based Complement Alternat
Med. 2012:7629182012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Pan Q, Han XY, Wang J, Tan RQ, He
F, Dou DQ and Kang TG: Simultaneous determination of arctiin and
its metabolites in rat urine and feces by HPLC. Fitoterapia.
86:6–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morita Y and Siraganian RP: Inhibition of
IgE-mediated histamine release from rat basophilic leukemia cells
and rat mast cells by inhibitors of transmethylation. J Immunol.
127:1339–1344. 1981.PubMed/NCBI
|
|
19
|
Ishiyama M, Tominaga H, Shiga M, Sasamoto
K, Ohkura Y and Ueno K: A combined assay of cell viability and in
vitro cytotoxicity with a highly water-soluble tetrazolium salt,
neutral red and crystal violet. Biol Pharm Bull. 19:1518–1520.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoo JM, Kim NY, Seo JM, Kim SJ, Lee SY,
Kim SK, Kim HD, Lee SW and Kim MR: Inhibitory effects of mulberry
fruit extract in combination with naringinase on the allergic
response in IgE-activated RBL-2H3 cells. Int J Mol Med. 33:469–477.
2014.
|
|
21
|
Russo C and Polosa R: TNF-alpha as a
promising therapeutic target in chronic asthma: A lesson from
rheumatoid arthritis. Clin Sci (Lond). 109:135–142. 2005.
View Article : Google Scholar
|
|
22
|
Yoo JM, Sok DE and Kim MR: Anti-allergic
action of aged black garlic extract in RBL-2H3 cells and passive
cutaneous anaphylaxis reaction in mice. J Med Food. 17:92–102.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van der Pouw Kraan TC, Boeije LC, Smeenk
RJ, Wijdenes J and Aarden LA: Prostaglandin-E2 is a
potent inhibitor of human interleukin 12 production. J Exp Med.
181:775–779. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ford-Hutchinson AW, Bray MA, Doig MV,
Shipley ME and Smith MJ: Leukotriene B, a potent chemokinetic and
aggregating substance released from polymorphonuclear leukocytes.
Nature. 286:264–265. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arima M and Fukuda T: Prostaglandin
D2 and T(H)2 inflammation in the pathogenesis of
bronchial asthma. Korean J Intern Med. 26:8–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nettis E, D'Erasmo M, Di Leo E, Calogiuri
G, Montinaro V, Ferrannini A and Vacca A: The employment of
leukotriene antagonists in cutaneous diseases belonging to
allergological field. Mediators Inflamm. 2010:6281712010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawakami Y, Kitaura J, Satterthwaite AB,
Kato RM, Asai K, Hartman SE, Maeda-Yamamoto M, Lowell CA, Rawlings
DJ, Witte ON, et al: Redundant and opposing functions of two
tyrosine kinases, Btk and Lyn, in mast cell activation. J Immunol.
165:1210–1219. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi SY, Hwang JH, Park SY, Jin YJ, Ko HC,
Moon SW and Kim SJ: Fermented guava leaf extract inhibits
LPS-induced COX-2 and iNOS expression in mouse macrophage cells by
inhibition of transcription factor NF-kappaB. Phytother Res.
22:1030–1034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu H, Zhang Y, Sun Y, Wang X, Zhai Y, Sun
Y, Sun S, Yu A, Zhang H and Wang Y: Determination of the major
constituents in fruit of Arctium lappa L. by matrix solid-phase
dispersion extraction coupled with HPLC separation and fluorescence
detection. J Chromatogr B Analyt Technol Biomed Life Sci.
878:2707–2711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao F, Wang L and Liu K: In vitro
anti-inflammatory effects of arctigenin, a lignan from Arctium
lappa L., through inhibition on iNOS pathway. J Ethnopharmacol.
122:457–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Theoharides TC, Alysandratos KD, Angelidou
A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng
Z, Miniati A, et al: Mast cells and inflammation. Biochim Biophys
Acta. 1822:21–33. 2012. View Article : Google Scholar :
|
|
32
|
Roth K, Chen WM and Lin TJ: Positive and
negative regulatory mechanisms in high-affinity IgE
receptor-mediated mast cell activation. Arch Immunol Ther Exp
(Warsz). 56:385–399. 2008. View Article : Google Scholar
|
|
33
|
Gomez G, Gonzalez-Espinosa C, Odom S, Baez
G, Cid ME, Ryan JJ and Rivera J: Impaired FcepsilonRI-dependent
gene expression and defective eicosanoid and cytokine production as
a consequence of Fyn deficiency in mast cells. J Immunol.
175:7602–7610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fanning LB and Boyce JA: Lipid mediators
and allergic diseases. Ann Allergy Asthma Immunol. 111:155–162.
2013. View Article : Google Scholar : PubMed/NCBI
|