Introduction
Dental implants have been widely used to replace
missing teeth. Despite promising results, implant treatment is
associated with a considerable rate of complications, particularly
following the surgical procedures. Nerve injury is one of the most
serious complications of implant surgery (1), which usually involves the sensory
nerves of the mandible, including the lingual, inferior alveolar
and mental nerves. Present treatments for peripheral nerve injury
include pharmacological therapy, physical rehabilitation and
invasive treatments, such as microsurgery; however, these options
require a long-term period for nerve recovery and usually do not
result in satisfactory functional recovery (2). Therefore, there is a great need for
alternative approaches which promote the regeneration of the
damaged neural tissues more effectively and rapidly.
A critical prerequisite for the regeneration of
peripheral nerves following injury is the migration of Schwann
cells (SCs), which are the major glial cell of the peripheral nerve
system (3,4). A number of cytokines, such as nerve
growth factor and erythropoietin, have been reported to promote the
migration of SCs (5,6). However, frequent injections are
required to maintain cytokine levels in order to sustain the
regenerative effects and, therefore, this type of treatment is
inconvenient and costly for the patient (7–9).
To overcome these limitations, new regenerative strategies are
required which are more effective and convenient for the
patient.
Platelet concentrates are increasingly used in
regenerative medicine due to their sustained release of cytokines
from degraded platelets and leukocytes (10–12). These cytokines mainly include
transforming growth factor-β1 (TGF-β1), vascular endothelial growth
factor (VEGF), insulin growth factor (IGF), platelet-derived growth
factor-AB (PDGF-AB), and interleukin-1β (IL-1β) (10–12). Several generations of platelet
concentrates have been developed. Platelet-rich plasma (PRP), the
first generation of platelet concentrates, has been reported to
promote the migration of SCs. However, PRP is prepared by
centrifuging venous blood with the addition of bovine thrombin, an
animal-derived biological product, which may lead to the
transmission of unknown infections (13).
Concentrated growth factor (CGF) belongs to the
latest generation of platelet concentrates (Sacco, 2006;
unpublished data), which is produced by the centrifugation of
venous blood without the addition of any exogenous products and is
therefore free from cross-contamination. Additionally, CGF has a
complex three-dimensional architecture, composed of a platelet,
leukocyte and growth factor-rich fibrin biomaterial (14). It has been principally applied for
the regeneration of alveolar and sinus bone (15). Recently, it has also been found to
increase the proliferation of periodontal ligament cells (16). However, the possible promoting
effect of CGF on the migration of SCs, as well as the associated
molecular mechanisms have not yet been fully investigated. One
suggested mechanism for the promoting effect of CGF on the
migration of SCs is through the activation of integrins.
Integrins, which are cell surface glycoproteins, are
in fact heterodimers of α and β subunits that modulate cell
migration (17). SCs exhibit an
increased expression of integrin β1 following nerve injury
(18). It has been demonstrated
that integrin β1 plays a necessary role in the migration of SCs
promoted by erythropoietin and VEGF (6,19).
However, to the best of our knowledge, there are no studies
available to date on the role of integrin β1 in the CGF-induced
migration of SCs.
Therefore, in this study, we investigated the
following: i) the structure of CGF and its effects on the migration
of SCs and ii) the possible role of integrin β1 in the CGF-induced
migration of SCs. The following hypotheses were examined: i) CGF
possesses properties which allow the sustained release of growth
factors; ii) CGF increases the migration of SCs; iii) integrin β1
and subcellular downstream signaling [namely focal adhesion kinase
(FAK)] are activated following treatment with CGF; and iv) the
short-interfering RNA (siRNA)-mediated downregulation of integrin
β1 decreases the CGF-induced migration of SCs.
Materials and methods
Preparation of CGF extract
In the present study, all the experiments were
performed in accordance with the Guidance Suggestions for the Care
and Use of Laboratory Animals, formulated by the Ministry of
Science and Technology of China. CGF was prepared according to the
following protocol as previously described (14): briefly, 5 ml venous blood was
drawn from the inferior vena cava of Wistar rats (weighing 250 g).
Blood was collected into a sterile tube without any anticoagulants
and immediately centrifuged in a Medifuge centrifuge (Silfradent
S.R.L., Sofia, Italy). Following centrifugation, the blood had
separated into 3 layers due to the different densities of the blood
components. CGF was the middle layer; it was mechanically separated
using scissors and gently compressed into a thin membrane. Each
membrane was soaked in 5 ml fresh Dulbecco's modified Eagle's
medium (DMEM; Gibco, New York, NY, USA) without fetal bovine serum
(FBS) in a 15 ml centrifuge tube (Corning Glassworks, Corning, NY,
USA). The medium, named CGF extract, was collected 7 days later and
centrifuged at 400 × g for 5 min to pellet the platelets and red
blood cells. All the extractions were stored at −80°C for future
use.
Scanning electron microscopy
The CGF membrane was fixed in 2.5% glutaraldehyde
solution and 1% osmium tetroxide (both from Sigma-Aldrich, St.
Louis, MO, USA). It was dehydrated serially in 50, 70, 80, 90 and
100% ethanol. After drying, the sample was coated with gold, and
images were captured under a scanning electron microscope (SEM;
S-3400N; Hitachi High Technologies America, Schaumburg, IL, USA).
Fibrin fiber diameter and percentage porosity were measured on the
SEM images using ImageJ software (version 1.49; Wayne Rasband,
National Institutes of Health, Bethesda, MD, USA). Briefly, the
diameters of 6 randomly selected fibers were measured using the
tool. Percentage porosity was determined by the particle analysis
function in the image analysis software.
Enzyme-linked immunosorbent assay
(ELISA)
We hypothesized that the growth factors, which are
trapped within the fibrin meshes of CGF, are released in a
controlled manner over a long-term period. Thus, we examined the
state of the growth factors released from the CGF extract over
time. Briefly, the CGF membrane was soaked in 5 ml DMEM without FBS
in a 15 ml flacon tube and incubated at 37°C. The medium was
collected on days 1, 3, 5, 7, 9, 11 and 13 and following
collection, 5 ml fresh DMEM was added to each tube. All the
collected extracts were stored at −80°C. TGF-β1 levels were
determined using a commercially available ELISA kit (Elabscience,
Wuhan, China) according to the manufacturer's instructions. All the
assays were performed in triplicate.
Analysis of cell migration using the
scratch wound-healing assay
The RSC96 SCs (obtained from Chinese Academy of
Sciences, Shanghai, China) were seeded into 6-well plates at a
density of 5×105 cells/well. After reaching confluence,
a scratch was made on the cell monolayer using a P10 pipette tip
according to the protocol previously described by Liang et
al (20). Cellular debris was
then washed off using phosphate-buffered saline (PBS). DMEM or CGF
were then added to the culture plates. Images of the cells were
captured using an Olympus DP-50 digital microscope camera (Olympus
Optical Co., Ltd., Tokyo, Japan) immediately after wounding and
again 24 h later. Reference points were made on the outer bottom of
the plates to mark the location of the scratch in order to obtain
the same field. The distance between the edges of the scratch was
measured using cellSens Entry software (Olympus Life Science,
Hamburg, Germany). The migration rate was calculated using the
following equation: migration rate = (D0 −
D24)/D0 ×100, as previously described
(21). D0 is the
distance at the time of making the scratch wound, and
D24 is the corresponding distance 24 h later.
Western blot analysis
The protein expression of integrin β1 and
phosphorylated (p-)FAK was examined by western blot analysis. The
RSC96 SCs were treated with DMEM or CGF extract supplemented with
10% FBS (Gibco, Melbourne, Australia). At 1, 2 and 3 days, the
cells were washed twice using PBS, and then lysed in ice-cold lysis
buffer [radioimmunoprecipitation assay buffer with 1%
phenylmethylsulfonyl fluoride (Biyuntian, Beijing, China)] on ice
for 1 h. The cell lysates were collected and centrifuged at
approximately 400 × g. The protein concentrations were determined
using the bicinchoninic acid (BCA) assay. Equal amounts of protein
(40 µg) were loaded onto a 10% SDS-polyacrymide gel and
electrophoretically transferred onto polyvinylidene fluoride
membranes (Millipore, Bedford, MA, USA). After blocking in
Tris-buffered saline with 0.05% Tween-20 (TBST) containing 5%
skimmed powdered milk for 90 min, the membranes were washed thrice
with TBST and then incubated with primary antibodies against either
integrin β1 (polyclonal antibody, rabbit anti-rat, 1:500, Cat. no.
bs-0486R; Bioss, Wuhan, China) or p-FAK (polyclonal antibody,
rabbit anti-rat, 1:1,000, cat no. AP0437; ABclonal, Cambridge, MA,
USA) overnight at 4°C. The samples were eluted 4 times with TBST
and then incubated with horseradish peroxidase (HRP)-conjugated
secondary antibodies (goat anti-rabbit, 1:1,000, cat. no. AS014;
ABclonal). An ECL chemiluminescence assay was used for the
chemiluminescence-based immunodetection of HRP. The intensities of
the bands (representative of protein levels) were determined using
ImageJ software (version 1.49; Wayne Rasband, National Institutes
of Health). β-actin was used to normalize target proteins.
Construction of plasmid vector expressing
integrin β1 siRNA
During RNA intereference (RNAi), siRNAs may serve as
guides for the enzymatic cleavage of complementary RNAs, thereby
inhibiting sequence-specific gene expression. RNAi is an effective
tool for the analysis of gene function (22). In the this study, a plasmid vector
synthesizing siRNA was constructed in order to suppress the gene
expression of integrin β1 in the SCs. Briefly, siRNA were
synthesized by Sangon Biotech (Shanghai, China). The siRNAs with
the targeting sequences GAAGGGTTGCCAACCAAGT, GACATGGATGCTTACTGCA
and GGTGGCTTTGATGCAATCA were labeled as siRNA-1, siRNA-2 and
siRNA-3, respectively. All the siRNAs were annealed and ligated
into the pRNA-H1.1 plasmid between the HindIII and the
BamHI sites. The normal plasmid without siRNA was used as a
control, labeled as siRNA-N. A volume of 20 µl of the
recombinant plasmids was transformed into 100 µl E.
coli strain JM109, and the cells were then plated onto
Luria-Bertani (LB) plates containing 10 g of tryptone, 5 g of yeast
extract, 5 g of NaCl, 100 mg of ampicillin, and 15 g of agar per
liter and incubated at 37°C. The colony was picked up and the
plasmids were extracted. All the plasmids were verified by DNA
sequence analysis.
Integrin β1 gene silencing
The RSC96 cells were cultured in dishes to obtain
70–90% confluence without antibiotics. The cells were then
transfected with siRNA-1, siRNA-2, siRNA-3 or siRNA-N, using
transfection reagent (Lipofectamine 2000; Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's instructions. The medium was
replaced with DMEM supplemented with 10% FBS 4 h after
transfection. The protein expression of integrin β1 after gene
silencing was determined using western blot analysis as described
above.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression of integrin β1 following the
application of siRNA was analyzed by RT-qPCR. Total RNA was
extracted using TRIzol reagent (Invitrogen) and was then reverse
transcribed using the RevertAid kit (Takara Bio, Otsu, Japan)
according to the manufacturer's instructions. SYBR-Green qPCR Super
Mixture (Takara Bio) was used to assess gene expression on an
Exicycler 96 Real-time PCR system (Bioneer, Daejeon, Korea). The
samples were normalized to β-actin. The following primer sequences
were used: rat integrin β1 forward, 5′-TTGCCAACCAAGTGACATA-3′ and
reverse, 3′-GGTAGTCTTCAGCCCTCTT-5′; and rat β-actin forward,
5′-GGAGATTACTGCCCTGGCTCCTAGC-3′ and reverse,
3′-GGCCGGACTCATCGTACTCCTGCTT-5′. The following amplification
conditions were used: 95°C for 10 min followed by 40 cycles of 95°C
for 10 sec, 60°C for 20 sec and 72°C for 30 sec. All the samples
were run in triplicate in each experiment. Changes in gene
expression were calculated using the 2−ΔΔCt method.
Transwell migration assay
The migration of the RSC96 SCs following integrin β1
gene silencing was examined using 6.5 mm Transwell chambers with 8
µm pores (Corning Costar, Corning, NY, USA). CGF or DMEM
supplemented with 20% FBS was added to the lower wells to act as a
chemoattractant. The cells were trypsinized, washed in PBS and then
added to the upper Transwell chambers (1×104 cells/well)
and allowed to migrate for 24 h. The non-migrated cells were
removed from the upper chambers using a cotton swab. The migrated
cells were fixed with paraformaldehyde, stained with 0.5% crystal
violet (Amresco, Solon, OH, USA), and then counted in 5 fields/well
under a microscope (×200 magnification).
Statistical analysis
Data are expressed as the means ± standard deviation
and were analyzed using one-way analysis of variance (ANOVA) with
the Tukey HSD comparison test or using the independent-samples
t-test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Structural characterization of CGF
The biological properties of CGF were evaluated as a
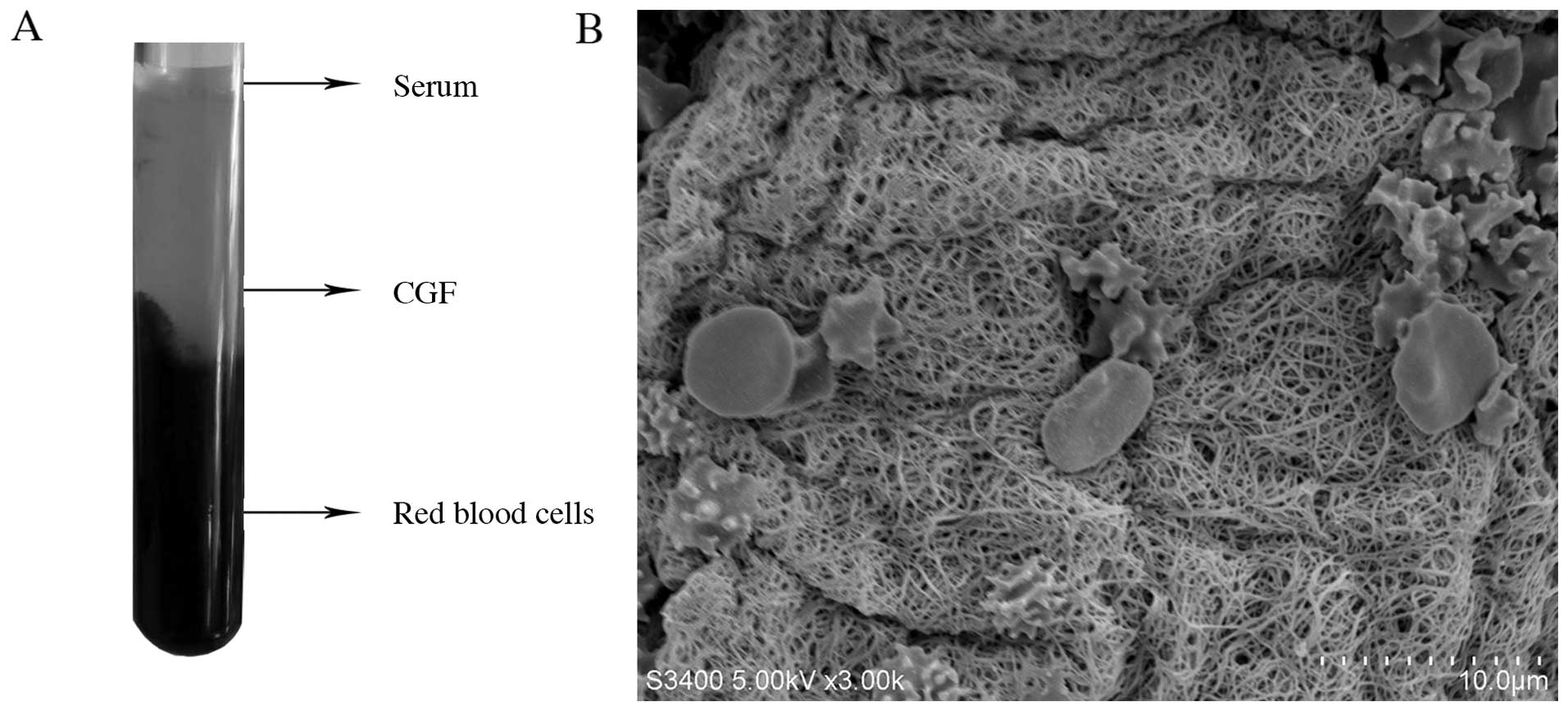
first step. CGF was generated by centrifuging whole blood. There
were 3 layers following centrifugation (Fig. 1A): the upper layer is a clear
fluid which is the blood serum; the middle layer is CGF which is
composed of a large and dense polymerized fibrin block with
aggregated platelets and growth factors; the bottom layer is
composed of red blood cell debris. CGF was mechanically separated
using scissors and then compressed into a thin membrane. Surface
parameters such as topography and hardness markedly affect cell
behavior and the release of growth factors (10,12,23). SEM analysis (Fig. 1B) revealed that CGF had a
fiber-like appearance. The diameter of the fibers was 0.36±0.14
µm and the percentage porosity was 40.44±2.97%. The fibrin
fiber network in CGF was sparse, with many platelet aggregates or
red blood cells trapped in the spaces between the fibrin
fibers.
Release of growth factors
TGF-β1 is one of the growth factors secreted by
platelets (24) and has
neuroprotective effects (25,26). Thus, the secretion of TGF-β1 was
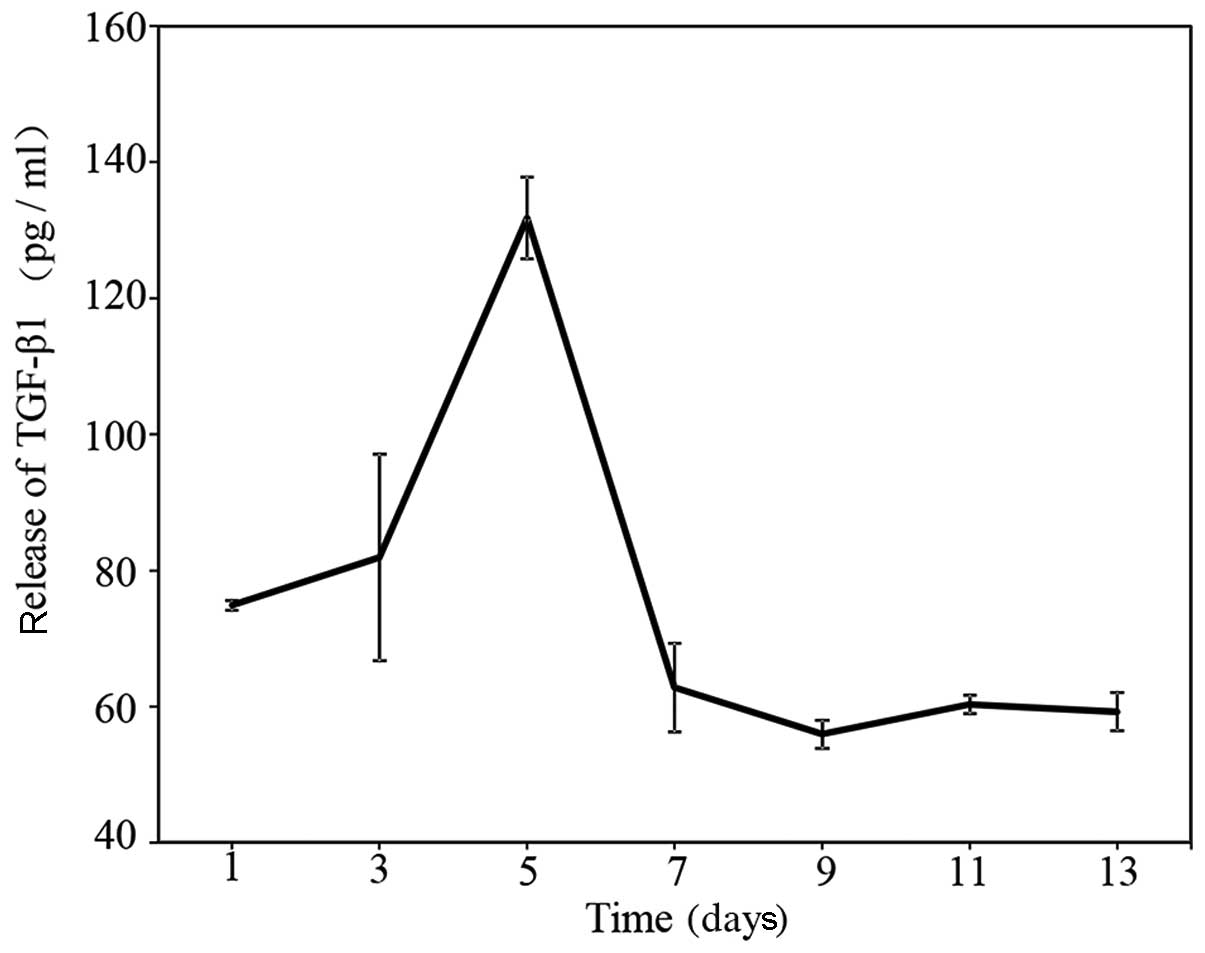
measured by ELISA in the present study. In a sustained-release
test, it was found that CGF slowly released TGF-β1 for up to 13
days (Fig. 2). The level of
TGF-β1 increased from 75 pg/ml on day 1 to 130 pg/ml on day 7.
Subsequently, the level slowly decreased to 60 pg/ml on day 13.
CGF promotes cell migration
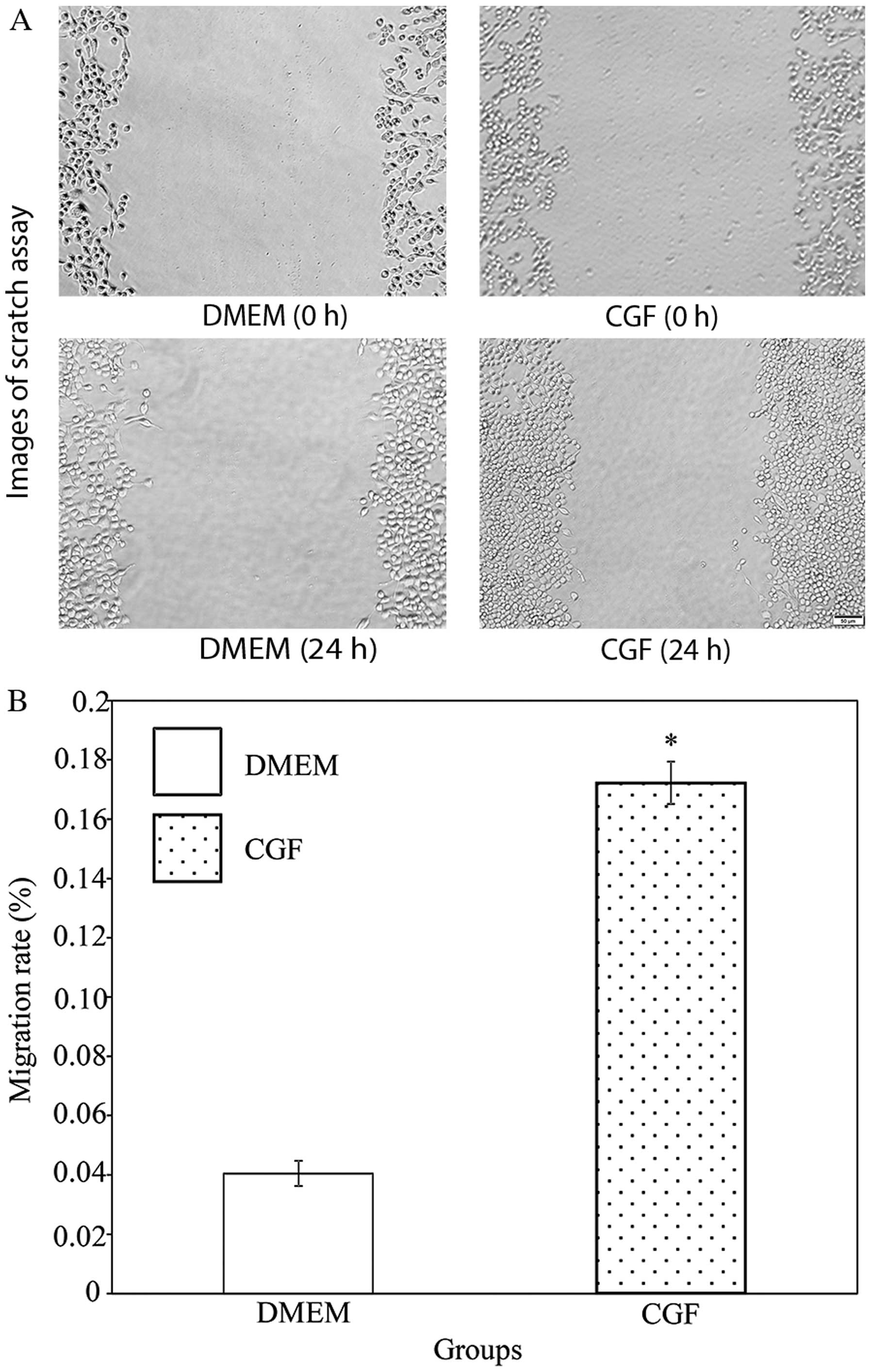
To examine the effects of CGF on the migration of
RSC96 SCs, a scratch wound-healing assay was performed (Fig. 3). The scratch assay is a
straightforward and economical method used to observe cell
migration in vitro (20).
It imitates, to a certain extent, the migration of cells in
vivo. CGF significantly increased the migration rate of the
RSC96 SCs in comparison with DMEM. The migration rate of the cells
in the CGF group was 4.25-fold greater than that of the cells in
the DMEM group (P<0.05).
CGF increases integrin β1 protein
expression
Integrin β1 is essential for the migration of SCs
(6,19). FAK is a cytoplasmic tyrosine
kinase that plays critical roles in integrin-mediated signal
transductions, and it is phosphorylated following activation
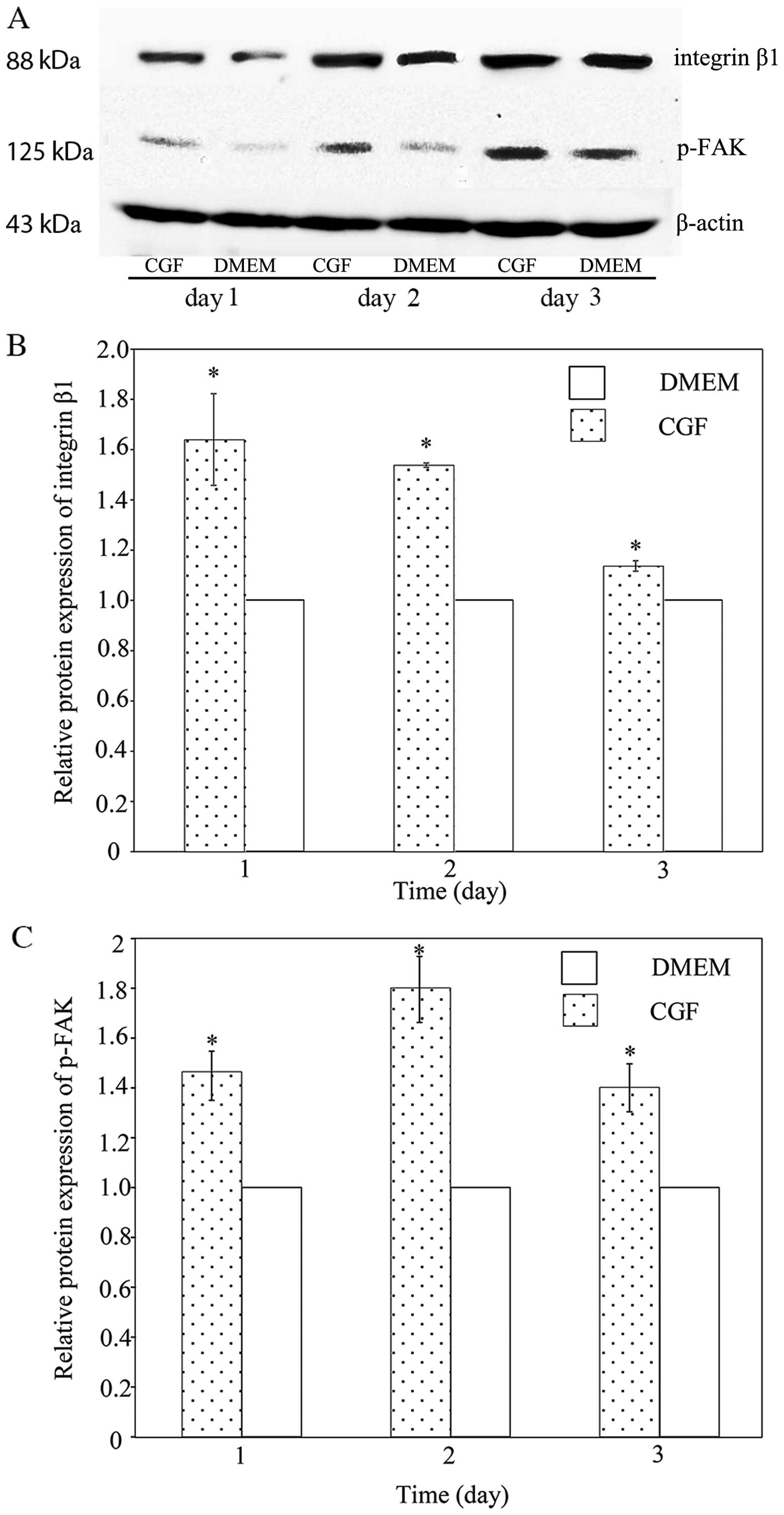
(27). Fig. 4 shows the protein expression of
integrin β1 and p-FAK. There was a statistically significant
(P<0.05) increase in the integrin β1 and p-FAK levels following
treatment with CGF compared with DMEM. The integrin β1 levels
increased 1.62-fold on day 1, 1.54-fold on day 2, and 1.14-fold on
day 3. The p-FAK levels were increased 1.46-fold on day 1,
1.80-fold on day 2 and 1.40-fold on day 3. These results suggest
that integrin β1 is involved in the CGF-induced migration of SCs,
and that the FAK signaling pathway is activated.
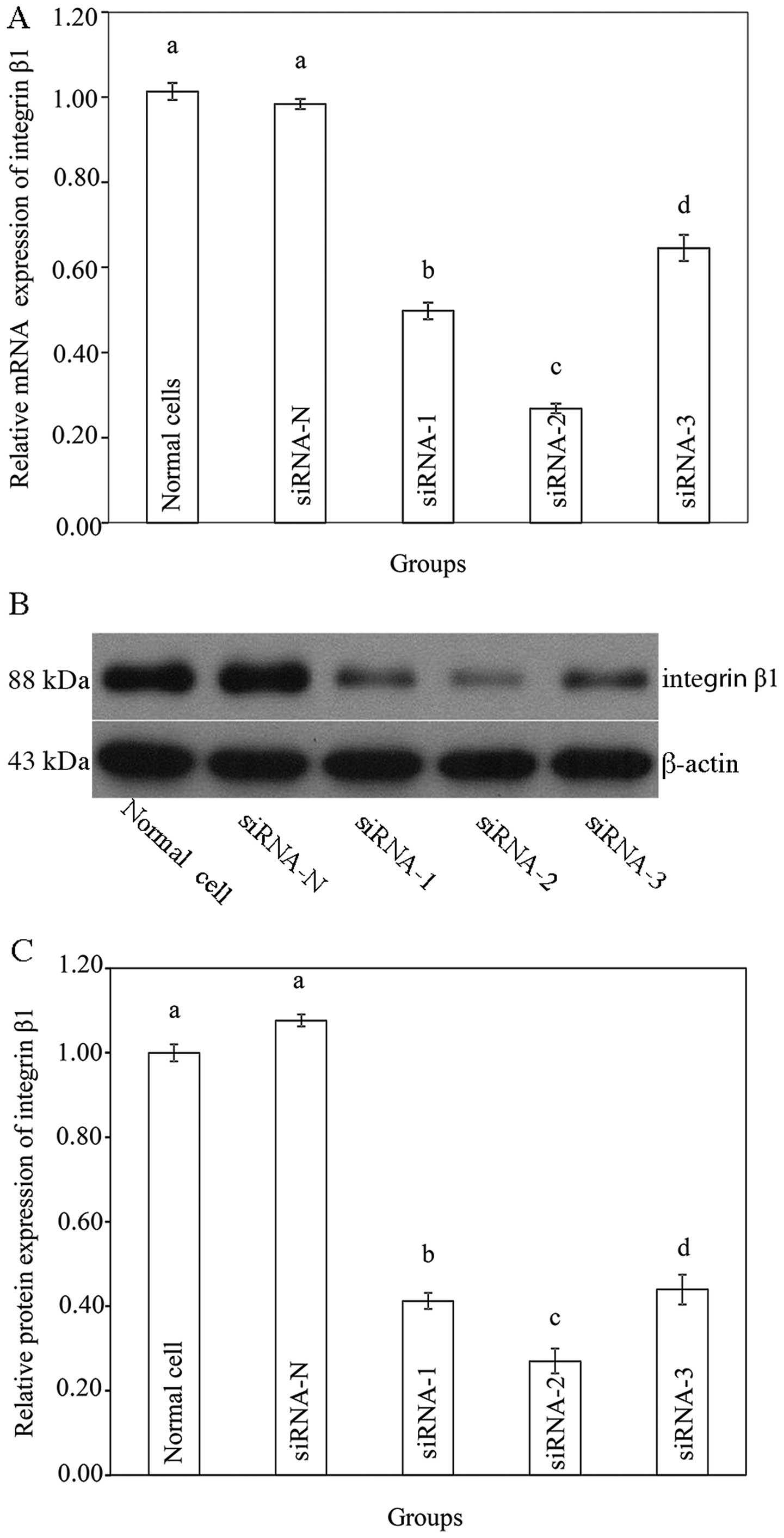
Silencing of integrin β1 using siRNA
In order to reveal whether integrin β1 plays an
essential role in the CGF-induced migration of SCs, integrin β1 was
silenced by different siRNAs. As shown in Figure 5A, no significant change in the
integrin β1 mRNA level was observed in the siRNA-N group compared
with that the normal (untransfected) cells. Transfection with
siRNA-1 decreased the integrin β1 mRNA level by approximately 50%,
transfection with siRNA-2 decreased the integrin β1 mRNA level by
70–80% and transfection with siRNA-3 reduced the integrin β1 mRNA
level by 30–40%. Fig. 5B and C
show the protein expression of integrin β1 following transfection.
No significant change was observed in the siRNA-N group. Among the
targeted siRNA groups, transfection with siRNA-2 significantly
reduced the protein expression of integrin β1 by almost 70%. Thus,
siRNA-2 was selected for use in the subsequent experiments.
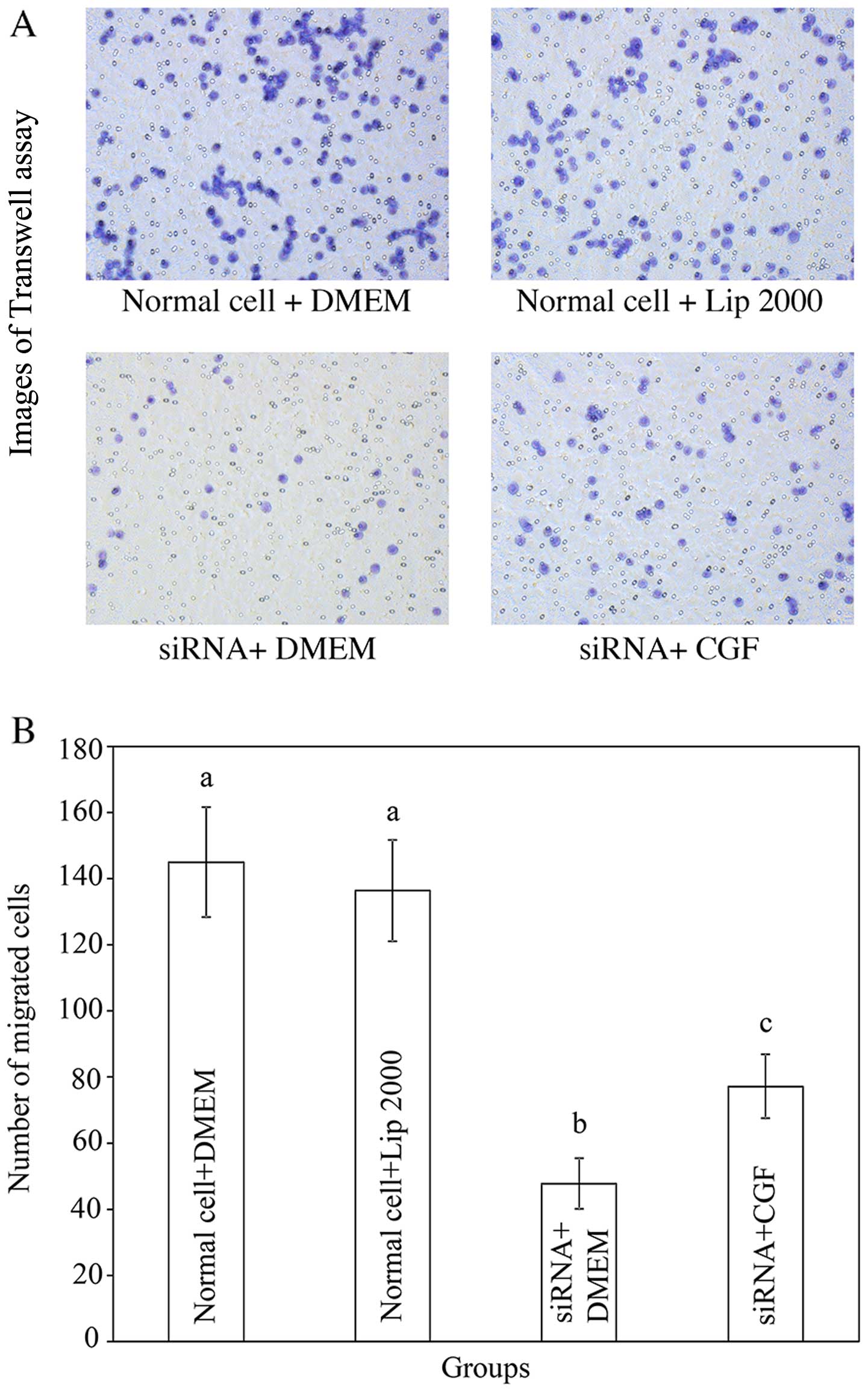
Transwell migration assay
Transwell migration assays were performed to detect
changes in cell migration after integrin β1 was downregulated by
siRNA-2. Fig. 6 shows that
Lipofectamine 2000, which was used as a transfection reagent, did
not affect the migration of the SCs. Following the application of
siRNA-2, the cell migration rates were decreased significantly by
67% in the DMEM-treated group, and by 50% in the CGF-treated group.
Moreover, the migration rate of the cells in the CGF treatment
group was significantly higher than that of the cells in the DMEM
treatment group. This demonstrates that CGF promotes the migration
of SCs even after integrin β1 silencing, indicating that integrin
β1 plays only a partial role in the CGF-induced migration of SCs,
and that other pathways are also involved in this process.
Discussion
Dental implants are used to reconstruct occlusion.
Dental implant surgery itself, however, may damage the nerves. One
of the major challenges in nerve regeneration studies is to
identify agents capable of promoting the migration of SCs (28–30). CGF is a promising candidate which
has shown several advantages. It is prepared from autologous venous
blood without the addition of anticoagulant; thus, the clinical use
of CGF is safe and does not elicit immune-rejection or an
inflammatory response (15).
Moreover, the preparation of CGF is rapid, simple, convenient and
economical. CGF contains a number of growth factors, including
TGF-β1, VEGF, IGF, PDGF-AB and IL-1β which all affect the migration
of diverse cell types (31–34). To the best of our knowledge, the
present study represents the first study on the structural
characterization of CGF, the effect of CGF on the migration of SCs
and the role of integrin β1 and FAK in the CGF-induced migration of
SCs.
The structure of the fibrin mesh is known to affect
the release of growth factors from platelet concentrates (10,12); thus, the surface morphology of CGF
was initially examined under a SEM in this study. The fibrin matrix
is composed of three-dimensional polymer networks with interwoven
fibers. This type of structure protects against the degradation of
the platelets and controls the release of growth factors trapped
within (10,12). This is supported by our results,
which showed that the release of TGF-β1 lasted for at least 13
days. Honda et al reported similar results; CGF slowly
released cytokines for 9-13 days (24). However, the peak concentration of
TGF-β1 in the study by Honda et al was much higher than that
in our study. This may be due to the following reasons: i) the
difference in blood volume used in the experiments. Honda et
al used 10 ml blood to prepare CGF, twice the amount that we
used, and CGF was soaked in 1 ml of PBS, just one fifth of what we
used; ii) the level of growth factors differs between species. Das
et al reported that the TGF-β1 level in rat serum was
15.8±3.32 pg/ml (35), whereas
Kropf et al reported higher levels of TGF-β1 in human serum
ranging from 24.2 to 257 ng/ml (36).
The adequate migration of SCs is crucial for the
regenerative process following peripheral nerve injury. RSC96 is a
spontaneously transformed rat SC line derived from the long-term
culture of primary rat SCs. Due to difficulties in the isolation
and culture of primary SCs, the RSC96 cell line has been employed
successfully in previous studies (28,30). In the present study, the RSC96
cell line was cultured and treated with CGF. CGF extract was used
as conditioned medium in our experiments.
Another noteworthy finding of this study was the
significant effect of CGF on the increased migration of SCs, which
has not been reported previously, to the best of our knowledge.
This CGF-induced increase in the migration of SCs may possibly be
mediated by the growth factors released by CGF, as those growth
factors identified in CGF independently promote the migration of a
wide variety of cell types. For example, previous research has
found that TGF facilitates the migration of cancer cells (31), VEGF stimulates the migration of
endothelial cells (32) and PDGF
enhances the migration of retinal pigment epithelial cells
(33). IGF-I has also been shown
to stimulate the directional migration of numerous peripheral cells
(34). Apart from these growth
factors, Li et al suggested that chemokines released by
leukocytes trapped in CGF, as well as the soluble fibrin components
may also play roles in cell migration as chemoattractant (37).
In order to reveal the underlying mechanism
responsible for the effects of CGF, an experiment to determine
integrin β1 expression levels was conducted as it plays a role in
the migration of SCs (6,19). In this study, the expression of
integrin β1 and its downstream molecule FAK were increased in the
SCs following CGF treatment, indicating that the CGF-induced cell
migration was associated with the integrin β1 pathway. The possible
mechanisms involved may be the cross-talk between growth factor
receptor and integrin. There are several types of growth factor
receptors on SCs, including the IGF receptor (38), the PDGF receptor (38) and the fetal liver kinase-1 (flk-1)
receptor (39). It has been
reported that there is cross-activation between growth factor
receptor and integrin (40);
therefore, the activation of growth factor receptor may induce
integrin activation. Thus, the growth factors in CGF may bind to
the corresponding growth factor receptors and then activate
integrin β1 in order to modulate the migration of SCs.
In order to investigate whether integrin β1 plays an
essential role in the CGF-induced migration of SCs, integrin β1 was
silenced by siRNA, which interferes in the expression of specific
RNA. Following integrin β1 gene silencing, the migratory ability of
the SCs in the CGF-treated group was still significantly higher
than that of the SCs in the DMEM-treated group. These results
suggested that integrin β1 did not play an essential role in the
CGF-induced migration of SCs. There may be some other integrins
involved in this process, since there are several types of integrin
expressed on SCs, such as integrin α5 (41), integrin α6, and integrin α7
(42). Similar results obtained
by other researchers, have demonstrated that the integrin α5
antibody did not affect the growth factor-induced migration of
smooth muscle cells (43).
The capacity of the sustained release of growth
factors, the promotion of SC migration, autologous nature, easy
preparation and safety, without the risk associated with allogeneic
products renders CGF a promising biomaterial for its clinical
application for nerve regeneration. In order to elucidate the exact
mechanisms involved in the promoting effects of CGF on the cell
migration process, the assessment of the effects of other integrins
on the CGF-induced migration of SCs must be performed in future
studies. In addition, it is important to investigate the in
vivo effects of CGF on nerve regeneration. It is also necessary
to perform further neural electrophysiological studies using animal
models prior to examining the clinical application of CGF in
peripheral nerve regeneration following injury.
Acknowledgments
Financial support from the Jilin Province Science
Foundation (grant no. 2014Z044), the Funding for Top Talents among
PhD students in Jilin University, the National Natural Science
Foundation of China (Youth Scholar Program, grant no. 81400487),
the Jilin Province Science Foundation for Youths (grant no.
20150520043JH), and the Excellent Youth Scholars of Bethune Medical
Department in Jilin University (grant no. 2013208064), is
gratefully acknowledged.
References
|
1
|
Juodzbalys G, Wang HL, Sabalys G,
Sidlauskas A and Galindo-Moreno P: Inferior alveolar nerve injury
associated with implant surgery. Clin Oral Implants Res.
24:183–190. 2013. View Article : Google Scholar
|
|
2
|
Kim YT, Pang KM, Jung HJ, Kim SM, Kim MJ
and Lee JH: Clinical outcome of conservative treatment of injured
inferior alveolar nerve during dental implant placement. J Korean
Assoc Oral Maxillofac Surg. 39:127–133. 2013. View Article : Google Scholar
|
|
3
|
Gaudet AD, Popovich PG and Ramer MS:
Wallerian degeneration: gaining perspective on inflammatory events
after peripheral nerve injury. J Neuroinflammation. 8:1102011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torigoe K, Tanaka HF, Takahashi A, Awaya A
and Hashimoto K: Basic behavior of migratory Schwann cells in
peripheral nerve regeneration. Exp Neurol. 137:301–308. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anton ES, Weskamp G, Reichardt LF and
Matthew WD: Nerve growth factor and its low-affinity receptor
promote Schwann cell migration. Proc Natl Acad Sci USA.
91:2795–2799. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inoue G, Gaultier A, Li X, Mantuano E,
Richardson G, Takahashi K and Campana WM: Erythropoietin promotes
Schwann cell migration and assembly of the provisional
extracellular matrix by recruiting beta1 integrin to the cell
surface. Glia. 58:399–409. 2010.
|
|
7
|
Perrin LA, June JE, Rosebury W, Robertson
A, Kovesdi I, Bruder JT, Kessler PD, Keiser JA and Gordon D:
Increased revascularization efficacy after administration of an
adenovirus encoding VEGF(121). Gene Ther. 11:512–521. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whalen GF, Shing Y and Folkman J: The fate
of intravenously administered bFGF and the effect of heparin.
Growth Factors. 1:157–164. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edelman ER, Mathiowitz E, Langer R and
Klagsbrun M: Controlled and modulated release of basic fibroblast
growth factor. Biomaterials. 12:619–626. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schär MO, Diaz-Romero J, Kohl S, Zumstein
MA and Nesic D: Platelet-rich concentrates differentially release
growth factors and induce cell migration in vitro. Clin Orthop
Relat Res. 473:1635–1643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dohan Ehrenfest DM, de Peppo GM, Doglioli
P and Sammartino G: Slow release of growth factors and
thrombo-spondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold
standard to achieve for all surgical platelet concentrates
technologies. Growth Factors. 27:63–69. 2009. View Article : Google Scholar
|
|
12
|
Dohan Ehrenfest DM, Bielecki T, Jimbo R,
Barbé G, Del Corso M, Inchingolo F and Sammartino G: Do the fibrin
architecture and leukocyte content influence the growth factor
release of platelet concentrates? An evidence-based answer
comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte-
and platelet-rich fibrin (L-PRF). Curr Pharm Biotechnol.
13:1145–1152. 2012. View Article : Google Scholar
|
|
13
|
Kawase T: Platelet-rich plasma and its
derivatives as promising bioactive materials for regenerative
medicine: basic principles and concepts underlying recent advances.
Odontology. 103:126–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodella LF, Favero G, Boninsegna R,
Buffoli B, Labanca M, Scarì G, Sacco L, Batani T and Rezzani R:
Growth factors, CD34 positive cells, and fibrin network analysis in
concentrated growth factors fraction. Microsc Res Tech. 74:772–777.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sohn DS, Heo JU, Kwak DH, Kim DE, Kim JM,
Moon JW, Lee JH and Park IS: Bone regeneration in the maxillary
sinus using an autologous fibrin-rich block with concentrated
growth factors alone. Implant Dent. 20:389–395. 2011.PubMed/NCBI
|
|
16
|
Yu B and Wang Z: Effect of concentrated
growth factors on beagle periodontal ligament stem cells in vitro.
Mol Med Rep. 9:235–242. 2014.
|
|
17
|
Hynes RO: Integrins: versatility,
modulation, and signaling in cell adhesion. Cell. 69:11–25. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taskinen HS, Heino J and Röyttä M: The
dynamics of beta 1 integrin expression during peripheral nerve
regeneration. Acta Neuropathol. 89:144–151. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Byzova TV, Goldman CK, Pampori N, Thomas
KA, Bett A, Shattil SJ and Plow EF: A mechanism for modulation of
cellular responses to VEGF: activation of the integrins. Mol Cell.
6:851–860. 2000.PubMed/NCBI
|
|
20
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: a convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Felice F, Zambito Y, Belardinelli E,
Fabiano A, Santoni T and Di Stefano R: Effect of different chitosan
derivatives on in vitro scratch wound assay: a comparative study.
Int J Biol Macromol. 76:236–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu JY, DeRuiter SL and Turner DL: RNA
interference by expression of short-interfering RNAs and hairpin
RNAs in mammalian cells. Proc Natl Acad Sci USA. 99:6047–6052.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Reed DA, Min L, Gopinathan G, Li S,
Dangaria SJ, Li L, Geng Y, Galang MT, Gajendrareddy P, et al:
Lyophilized platelet-rich fibrin (PRF) promotes craniofacial bone
regeneration through Runx2. Int J Mol Sci. 15:8509–8525. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Honda H, Tamai N, Naka N, Yoshikawa H and
Myoui A: Bone tissue engineering with bone marrow-derived stromal
cells integrated with concentrated growth factor in Rattus
norvegicus calvaria defect model. J Artif Organs. 16:305–315. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li M, Zhang P, Li H, Zhu Y, Cui S and Yao
D: TGF-β1 is critical for Wallerian degeneration after rat sciatic
nerve injury. Neuroscience. 284:759–767. 2015. View Article : Google Scholar
|
|
26
|
Luo H, Zhang Y, Zhang Z and Jin Y: The
protection of MSCs from apoptosis in nerve regeneration by TGFβ1
through reducing inflammation and promoting VEGF-dependent
angiogenesis. Biomaterials. 33:4277–4287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao X and Guan JL: Focal adhesion kinase
and its signaling pathways in cell migration and angiogenesis. Adv
Drug Deliv Rev. 63:610–615. 2011. View Article : Google Scholar :
|
|
28
|
Chang HM, Shyu MK, Tseng GF, Liu CH, Chang
HS, Lan CT, Hsu WM and Liao WC: Neuregulin facilitates nerve
regeneration by speeding Schwann cell migration via
ErbB2/3-dependent FAK pathway. PLoS One. 8:e534442013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perlin JR, Lush ME, Stephens WZ,
Piotrowski T and Talbot WS: Neuronal Neuregulin 1 type III directs
Schwann cell migration. Development. 138:4639–4648. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang YM, Shih YT, Chen YS, Liu CL, Fang
WK, Tsai CH, Kuo WW, Lai TY and Huang CY: Schwann cell migration
induced by earthworm extract via activation of PAs and MMP2/9
mediated through ERK1/2 and p38. Evid Based Complement Alternat
Med. 2011:3954582011. View Article : Google Scholar :
|
|
31
|
Takai E, Tsukimoto M, Harada H, Sawada K,
Moriyama Y and Kojima S: Autocrine regulation of TGF-β1-induced
cell migration by exocytosis of ATP and activation of P2 receptors
in human lung cancer cells. J Cell Sci. 125:5051–5060. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morales-Ruiz M, Fulton D, Sowa G, Languino
LR, Fujio Y, Walsh K and Sessa WC: Vascular endothelial growth
factor-stimulated actin reorganization and migration of endothelial
cells is regulated via the serine/threonine kinase Akt. Circ Res.
86:892–896. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chan CM, Chang HH, Wang VC, Huang CL and
Hung CF: Inhibitory effects of resveratrol on PDGF-BB-induced
retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and
MAPK pathways. PLoS One. 8:e568192013. View Article : Google Scholar
|
|
34
|
Maucksch C, McGregor AL, Yang M, Gordon
RJ, Yang M and Connor B: IGF-I redirects doublecortin-positive cell
migration in the normal adult rat brain. Neuroscience. 241:106–115.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Das SK, Dhanya L, Varadhan S, Mukherjee S
and Vasudevan DM: Effects of chronic ethanol consumption in blood:
a time dependent study on rat. Indian J Clin Biochem. 24:301–306.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kropf J, Schurek JO, Wollner A and
Gressner AM: Immunological measurement of transforming growth
factor-beta 1 (TGF-beta1) in blood; assay development and
comparison. Clin Chem. 43:1965–1974. 1997.PubMed/NCBI
|
|
37
|
Li Q, Pan S, Dangaria SJ, Gopinathan G,
Kolokythas A, Chu S, Geng Y, Zhou Y and Luan X: Platelet-rich
fibrin promotes periodontal regeneration and enhances alveolar bone
augmentation. Biomed Res Int. 2013:6380432013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ammoun S, Schmid MC, Ristic N, Zhou L,
Hilton D, Ercolano E, Carroll C and Hanemann CO: The role of
insulin-like growth factors signaling in merlin-deficient human
schwannomas. Glia. 60:1721–1733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sondell M, Lundborg G and Kanje M:
Vascular endothelial growth factor has neurotrophic activity and
stimulates axonal outgrowth, enhancing cell survival and Schwann
cell proliferation in the peripheral nervous system. J Neurosci.
19:5731–5740. 1999.PubMed/NCBI
|
|
40
|
Mahabeleshwar GH, Feng W, Reddy K, Plow EF
and Byzova TV: Mechanisms of integrin-vascular endothelial growth
factor receptor cross-activation in angiogenesis. Circ Res.
101:570–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wakatsuki S, Araki T and Sehara-Fujisawa
A: Neuregulin-1/glial growth factor stimulates Schwann cell
migration by inducing α5 β1 integrin-ErbB2-focal adhesion kinase
complex formation. Genes Cells. 19:66–77. 2014. View Article : Google Scholar
|
|
42
|
Pellegatta M, De Arcangelis A, D'Urso A,
Nodari A, Zambroni D, Ghidinelli M, Matafora V, Williamson C,
Georges-Labouesse E, Kreidberg J, et al: α6β1 and α7β1 integrins
are required in Schwann cells to sort axons. J Neurosci.
33:17995–18007. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Itoh H, Nelson PR, Mureebe L, Horowitz A
and Kent KC: The role of integrins in saphenous vein vascular
smooth muscle cell migration. J Vasc Surg. 25:1061–1069. 1997.
View Article : Google Scholar : PubMed/NCBI
|