Introduction
Iron is an essential metal involved in a large array
of biological processes, such as energy metabolism, DNA replication
and other important cellular functions (1–3).
Systemic iron homeostasis is accurately and strictly governed to
ensure a balance between iron absorption, utilization and storage
in mammals (4). Hepcidin is the
central hormone secreted by hepatocytes that plays a fundamental
role in the regulation of iron flow through limiting dietary iron
absorption from the duodenum and reducing iron egress from
macrophages (5). Hepcidin
achieves this effect by binding to its receptor, ferroportin (FPN;
the only known iron exporter thus far), on the plasma membrane of
target cells and inducing its internalization and degradation
(5). The misregulation of
hepcidin gives rise to several types of iron disorders, including
refractory iron deficiency anemia, as well as iron overload-related
diseases, such as hereditary hemochromatosis (HH) and β-thalassemia
(6). HH is characterized by
enhanced iron absorption and, consequently, iron overload in
various organs due to the low hepcidin levels (7). Hepcidin deficiency derives from
genetic mutations in hepcidin itself, or in other genes encoding
its upstream regulators, such as the human hemochromatosis protein
(HFE) and transferrin receptor 2 (TFR2) (6). Insufficient hepcidin levels result
in elevated FPN concentrations and this facilitates dietary iron
absorption and increases iron egress from macrophages (8). As a result, excess iron accumulates
in the liver and other organs, eventually leading to iron overload
and tissue injuries. β-thalassemia is also a type of iron
overload-related disease characterized by low hepcidin levels,
although the mechanisms involved differ somewhat from those leading
to HH (9–11). Therefore, strategies that are able
to increase the circulating hepcidin concentrations may be
promising therapies for iron overload-related diseases, including
HH and β-thalassemia. For this purpose, hepcidin mimics and
agonists are under development (8).
Exhaustive and ongoing efforts are being made in
search of natural botanical compounds for use in the treatment of a
wide spectrum of disorders, including various types of cancer and
hematological diseases. Sometimes drugs derived from natural
compounds often exhibit greater efficacy than drugs manufactured
synthetically. To date, to the best of our knowledge, no extract or
natural botanical compound has been found that can upregulate
hepcidin expression. To this end, in the present study, we assessed
12 pure natural compounds with the potential to modulate iron
metabolism. All these compounds are extracts from traditional
Chinese herbal medicinal plants, and many of them have been
reported to be effective therapeutically for multiple disorders,
including inflammation, oxidative injuries and cardiovascular
diseases (12–20). According to the Chinese
Pharmacopoeia (21), all of these
compounds are capable of improving microcirculation and have the
potential to modulate blood cell formation. Thus, we deliberately
selected these compounds in the present study. Of these compounds,
icariin was found to markedly stimulate hepcidin expression in
vitro and in vivo. Mechanistic experiments indicated
that icariin increased hepcidin transcription through a combination
of the activation of the signal transducer and activator of
transcription 3 (Stat3) and Smad1/5/8 signaling pathways. Our
combined data therefore suggest that icariin represents a promising
therapeutic strategy for restricting iron absorption and iron
egress from macrophages, and hence for ameliorating iron
overload-related diseases by regulating hepcidin-FPN signaling.
Materials and methods
Chemicals and reagents
The following pure natural compounds extracted from
Chinese medicinal plants were purchased from the National
Institutes for Food and Drug Control of China (Beijing, China) with
>99.5% purity: propyl gallate,
C10H12O5; resveratrol,
C14H12O3; astragaloside,
C41H68O14; curcumin,
C21H20O6; paeoniflorin,
C23H28O11; ligustrazine,
C8H12N2·HCl·2H2O;
ferulic acid, C10H10O4;
ginsenoside Rb1, C54H92O23;
wogonin, C16H12O5; liquiritin,
C21H22O9; berberine,
C20H18NO4; icariin,
C33H40O15; epimedin A,
C39H50O19; epimedin B,
C38H48O19; and epimedin C,
C39H50O19. Dimethyl sulfoxide
(DMSO; Solarbio, Beijing, China) was used to dissolve these
compounds, and the final concentration of DMSO in the culture
medium was <0.5%.
Cell culture and treatments
The human hepatocellular carcinoma cell line, HepG2,
and the mouse hepatocellular carcinoma cell line, Hepa 1-6,
obtained from the Institute of Biochemistry and Cell Biology,
Shanghai Institute for Biological Sciences, Chinese Academy of
Sciences were cultured in RPMI-1640 and Dulbecco's modified Eagle's
medium (DMEM), respectively, supplemented with 10% heat-inactivated
fetal bovine serum (FBS) (all from Gibco, Grand Island, NY, USA) at
37°C under a humidified atmosphere with 5% CO2, as
previously described (22,23).
The cells were collected for RNA or protein extraction 24 h after
being subjected to the various treatments (the cells were treated
with the various compounds at concentrations of 5 µM for 24
h). Untreated (control) cells were cultured with the corresponding
DMSO-containing medium.
Cytotoxicity assay
The cytotoxicity of the natural compounds was
evaluated using an MTT assay kit according to the manufacturer's
instructions (Roche Life Science, Basel, Switzerland). Briefly, the
HepG2 and the Hepa 1-6 cells were first serum-starved overnight,
then inoculated into 96-well plates with 5,000 cells/well. The
cells were then subjected to the different treatments. The cells
were cultured for an additional 24 h, and subsequently, 20
µl MTT solution (5 mg/ml) was added to each well, followed
by incubation for a further 4 h. At the end of the incubation
period, 200 µl DMSO were added to each well, and the
absorbance at 490 nm was measured using a microplate reader (Thermo
Fisher Scientific, Waltham, MA, USA).
Luciferase assay
The HepG2 and the Hep 1-6 cells were seeded at
2×105 cells/well in a 24-well plate 12 h prior to
transfection. The cells were then co-transfected with 0.8 µg
of a hepcidin-promoter-luciferase plasmid and 80 ng of
Renilla luciferase plasmid with Lipofectamine 2000 in
accordance with the manufacturer's instructions (Invitrogen,
Carlsbad, CA, USA). The medium was replaced with RPMI-1640 or DMEM
containing 10% FBS 6 h later, and the cells were then treated with
each compound (at 5 µM) for 24 h. Afterwards, the cells were
collected and washed with PBS, and finally lysed in lysis buffer
(Promega, Madison, WI, USA). The cell lysates were then assessed
for luciferase activity using the Dual-Luciferase Reporter assay
system (Promega). The relative firefly luciferase activity for each
sample was normalized to that of Renilla luciferase.
RNA extraction and RT-qPCR
Total RNA was purified from the cells using TRIzol
reagent according to the manufacturer's instructions (Invitrogen
Grand, NY, Island, USA). Total RNA from liver specimens (obtained
from mice, as described below) was also extracted using TRIzol
reagent according to the manufacturer's instructions after the
specimens were pulverized in liquid nitrogen. The mRNA expression
levels were assessed by performing qPCR using SYBR-Green qPCR
Master Mix (Qiagen, Valencia, CA, USA). Glyceraldehyde 3- phosphate
dehydro genase (GAPDH) was used as a housekeeping gene for the
normalization of relative gene expression. The primer sequences
used were as follows: human hepcidin forward,
5′-CCTGACCAGTGGCTCTGTTT-3′ and reverse, 5′-CACATCCCACACTTTGATCG-3′;
human GAPDH forward, 5′-GAAGGTGAAGGTCGGAGT-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′; mouse hepcidin forward,
5′-CTGAGCAGCACCACCTATCTC-3′ and reverse,
5′-TGGCTCTAGGCTATGTTTTGC-3′; and mouse GAPDH forward,
5′-AAGGTCATCCCAGAGCTG′ and reverse, 5′-GCCATGAGGTCCACCACCCT-3′.
Western blot analysis
Following treatment with the compounds, HepG2 cells
were harvested in cold PBS, and total proteins were then extracted
using RIPA lysis buffer (Solarbio) supplemented with protease
inhibitor cocktail (Roche Life Science). Interleukin (IL)-6 was
used as positive control for p-Stat3 detection, which was purchased
from Sigma Aldrich. Proteins were extracted following 24 h of
administration at 100 ng/ml. Equal amounts of protein lysates for
each sample were subjected to SDS-PAGE and western blot analysis
following a standard procedure, as previously described (24,25). The primary antibodies used were as
follows: anti-p-Smad1/5/8 (1:1,000; sc-12353) and anti-Smad1
(1:1,000; sc-7965) antibodies (both from Santa Cruz Biotechnology,
Santa Cruz, CA, USA); anti-p-Stat3 (1:1,000; 9145) and anti-Stat3
(1:1,000; 9139) antibodies (both from Cell Signaling Technology,
Danvers, MA, USA); and anti-GAPDH (1:2,000; G8795; Sigma-Aldrich,
St. Louis, MO, USA). Protein quantification was performed using
ImageJ software (NIH, http://rsb.info.nih.gov/ij/).
Animal experiments
All animal care and experimental procedures were
approved by the Animal Ethics Committee at Xiyuan Hospital, China
Academy of Chinese Medical Sciences (Beijing, China).
Eight-week-old mice were used in this study. Wild-type (Wt) ICR
mice were purchased from the Laboratory Animal Technology Co., Ltd.
(Beijing, China), and housed in a central specific pathogen-free
(SPF) facility at the Beijing Xiyuan Hospital. Hepcidin-deficient
[Hamp1−/− or Hamp1-knockout (KO)] mice were originally
provided by Dr Sophie Vaulont [Institut National de la Santé et de
la Recherche Médicale (INSERM)] (26) and have been back-crossed into the
C57BL/6 background (27). To
deplete iron, the Hamp1−/− mice were placed on a
low-iron diet (4 ppm iron) 1 week prior to the administration of
the compounds. This method was developed to reduce the serum iron
content in the Hamp1−/− mice in order to more clearly
demonstrate changes in serum iron levels following the
administration of the compounds. The mice were randomly grouped
with 8 mice in each group (n=8). The compounds were administered
intraperitoneally to the mice at a dose of 100 mg/kg body weight.
The control mice received PBS containing DMSO at the relevant
concentration. For the short-term treatment experiments, the mice
were sacrificed 6 or 48 h after the injection of the compounds.
With respect to the long-term treatments, the compounds were
administered to the mice every 2 days, and the mice were sacrificed
on days 4, 8 or 16. Lipopolysaccharide (LPS) was used as a positive
control and was purchased from Sigma-Aldrich. Mice were
administrated with LPS at 100 µg/mouse for 6 h. Blood was
collected, and liver and spleen specimens from each mouse were
isolated and individually separated. A small section of each liver
specimen was quickly frozen in liquid nitrogen and then stored at
−80°C for future RNA extraction.
Iron parameters
The serum iron levels were determined using a serum
iron detection kit according to the manufacturer's instructions
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Hepatic and spleen iron contents were determined following the
established protocols in our laboratory, as previously described
(25). Briefly, liver and spleen
specimens were first digested with an acid solution at 65°C for 72
h. Thereafter, chromagen working solution was added to each
supernatant. Finally, the absorption at 535 nm was measured using a
microplate reader (Thermo Fisher Scientific).
Histological examination
The liver specimens were fixed in 10% buffered
formaldehyde, and embedded in paraffin. The deparaffinized sections
were stained with hematoxylin and eosin (H&E), following a
standard protocol, as previously described (25,28,29).
Statistical analysis
The SPSS 17.0 statistics package was used to analyze
the experimental data. One-way analysis of variance (ANOVA) was
used to differentiate the mean differences among groups compared to
the control group. The differences between 2 groups were determined
using an independent Student's t-test. All experimental data are
presented as the means ± SD. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Screening of natural compounds for
modulating hepcidin expression
The liver (where hepatocytes secrete hepcidin) is
the predominant organ for the secretion of hepcidin into the
circulation in order to regulate iron homeostasis (30). Thus, in this study, we used
liver-derived cell lines. HepG2 and Hepa 1-6 are established
hepatocyte cell lines representative of human cells and mouse
cells, respectively. In an effort to investigate the potential role
of natural compounds in the modulation of hepcidin expression, we
screened 12 pure natural products using the dual hepcidin
luciferase assay. The reporter construct was developed by our
research group with the human hepcidin promoter fragment (1.6 kb)
upstream of the firefly luciferase gene, as previousy described
(31). The screening assay was
performed in both the HepG2 and Hepa 1-6 cells. Prior to screening,
cytotoxicity was evaluated by MTT assay. All these compounds
exhibited no significant toxicity to both the HepG2 and Hepa 1-6
cells, with only a mild reduction in cell viability observed at
higher concentrations of berberine, curcumin, icariin and wogonin
(data not shown). Thus, we used a concentration of 5 µM in
the initial screening assay, and cell viability was not impaired by
any of the compounds (data not shown). Following treatment with the
compounds for 24 h, hepcidin-luciferase activity was measured. As
shown in Table I, propyl gallate,
resveratrol, berberine, icariin and wogonin were observed to
enhance hepcidin-luciferase activity by >2-fold in the HepG2
cells, compared to the untreated controls (P<0.05).
Astragaloside, ligustrazine, ginsenoside Rb1 and liquiritin were
found to enhance hepcidin-luciferase activity by approximately 50%
in the HepG2 cells compared with the untreated controls
(P<0.05). However, curcumin, paeoniflorin and ferulic acid
failed to significantly alter hepcidin-luciferase activity in the
HepG2 cells (P>0.05). Analogously, in the Hepa 1-6 cells,
berberine, icariin and wogonin promoted hepcidin-luciferase
activity by >2-fold compared with the untreated controls
(P<0.05), which was consistent with the results observed in the
HepG2 cells. Ferulic acid, ginsenoside Rb1 and liquiritin were able
to stimulate hepcidin-luciferase activity by approximately 50% in
the Hepa 1-6 cells in comparison with the untreated cells
(P<0.05). The remaining compounds (propyl gallate, resveratrol,
astragaloside, curcumin, paeoniflorin and ligustrazine) failed to
significantly alter the hepcidin-luciferase activity in the Hepa
1-6 cells (P>0.05). Taken together, these findings indicated
that berberine, icariin and wogonin exerted a robust stimulatory
effect on hepcidin transcription in both the HepG2 and Hepa 1-6
cells. Thus, these 3 compounds were selected for use in our
subsequent experiments.
 | Table IChanges in relative luciferase
activity in cells treated with the various compounds compared to
the untreated controls |
Table I
Changes in relative luciferase
activity in cells treated with the various compounds compared to
the untreated controls
| Compounds | HepG2 | Hepa 1-6 |
|---|
| Propyl gallate | ↑↑↑↑a | – |
| Resveratrol | ↑↑↑↑a | – |
| Astragaloside | ↑a | – |
| Curcumin | – | – |
| Paeoniflorin | – | – |
| Ligustrazine | ↑a | – |
| Ferulic acid | – | ↑a |
| Ginsenoside
Rb1 | ↑a | ↑a |
| Liquiritin | ↑a | ↑a |
| Berberine | ↑↑↑↑a | ↑↑↑↑a |
| Icariin | ↑↑↑↑a | ↑↑↑↑a |
| Wogonin | ↑↑↑↑a | ↑↑↑↑a |
Validation of screening data using
RT-qPCR
To confirm the screening results described above,
RT-qPCR was employed to determine the hepcidin mRNA levels
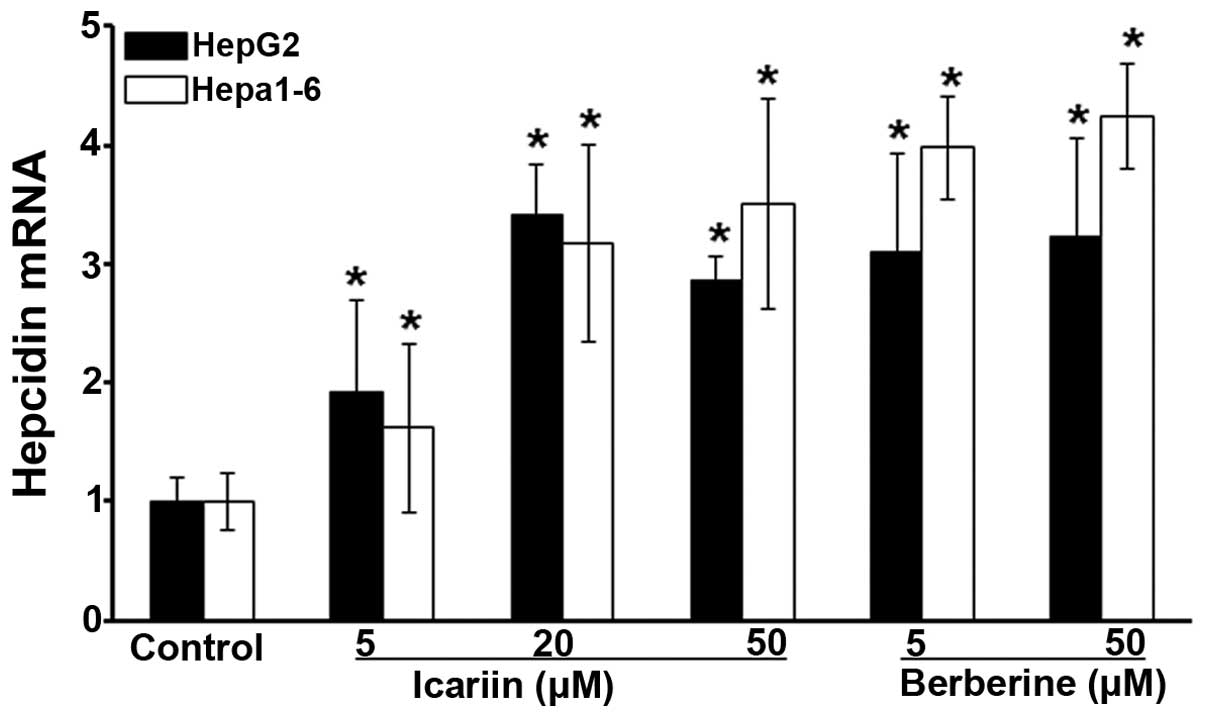
following treatment with the selected compounds. As shown in
Fig. 1, hepcidin expression in
the HepG2 cells was increased by approximately 2-fold by icariin at
a concentration of 5 µM, and was further increased at
concentrations of 20 and 50 µM by approximately 3-fold,
compared with the untreated cells (P<0.05). Similarly, hepcidin
expression in the Hepa 1-6 cells was induced by icariin (at
concentrations of 5, 20 and 50 µM) in a dose-dependent
manner, with a maximum increase observed at the concentration of 50
µM of approximately 3-fold compared to the controls
(Fig. 1; P<0.05). Analogously,
berberine increased hepcidin expression in the HepG2 cells by
>3-fold at both 5 and 50 µM compared with the untreated
cells (Fig. 1; P<0.05). In
parallel to the HepG2 cells, hepcidin expression was elevated by
approximately 4-fold in the Hepa 1-6 cells by berberine at both 5
and 50 µM (Fig. 1;
P<0.05). By contrast, wogonin failed to alter hepcidin
expression, and this was revealed by RT-qPCR (data not shown).
Based on these results, the stimulatory effects of icariin and
berberine on hepcidin expression were further demonstrated by the
results of RT-qPCR, and we therefore selected these two compounds
for further detailed evaluation.
Icariin and berberine regulate hepcidin
expression through Stat3 and Smad1/5/8 signaling
To elucidate the molecular mechanisms responsible
for the promotion of hepcidin expression by icariin and berberine,
we explored the signaling pathways involved in the regulation of
hepcidin expression. Although the regulation of hepcidin expression
is rather complex, two signaling pathways are known to
predominantly control hepcidin expression under normal and
pathological conditions: the Stat3 pathway and the Smad1/5/8
pathway (6,8). Upon activation of one or both of
these pathways, hepcidin expression is expected to rise, which
inhibits dietary iron absorption, as well as iron egress from
macrophages (6,8). Thus, in this study, we examined the
activation of the Stat3 pathway and the Smad1/5/8 pathway in HepG2
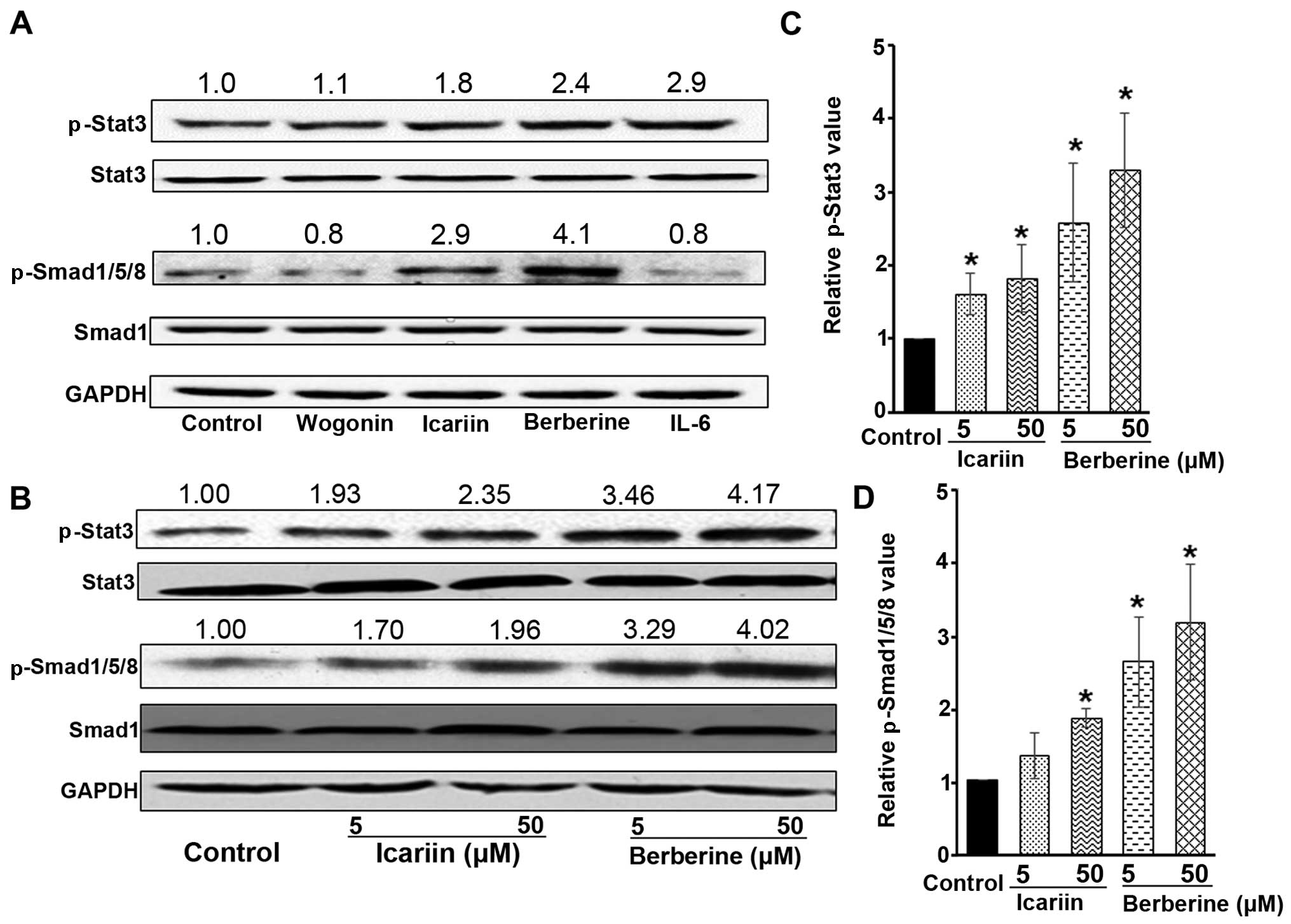
cells following treatment with icariin or berberine. As shown in
Fig. 2A, both icariin and
berberine were found to markedly increase the p-Stat3 and
p-Smad1/5/8 levels compared to the untreated control cells. Since
IL-6 has been shown to increase hepcidin expression by activating
the Stat3 signaling pathway (32,33), it was used in this study as a
positive control to increase the p-Stat3 level (Fig. 2A). In addition, a compound (namely
wogonin) that did not alter hepcidin expression was used as a
negative control (Fig. 2A).
Furthermore, the dose-dependent effects of icariin
and berberine on Stat3 and Smad1/5/8 were examined. As illustrated
in Fig. 2B, icariin was observed
to increase the level of p-Stat3, particularly at 50 µM.
Icariin was also found to increase the level of p-Smad1/5/8,
relative to the untreated cells, particularly at 50 µM
(Fig. 2B). The quantitative
analysis of Stat3 phosphorylation and Smad1/5/8 phosphorylation is
shown in Fig. 2C and D,
respectively. These observations suggest that icariin upregulates
hepcidin expression through both the Stat3 and Smad1/5/8 pathways.
Moreover, icariin at a higher concentration (at 50 µM)
exhibited a greater ability to activate the Stat3 and Smad1/5/8
pathways than at the lower concentration (at 5 µM), in
parallel to the greater induction of hepcidin expression observed
with 50 µM icariin (Fig.
1). Similar to icariin, berberine was also demonstrated to
activate both the Stat3 and Smad1/5/8 pathways, as indicated by a
significant increase in the levels of p-Stat3 and p-Smad1/5/8
(Fig. 2A–D). It should be noted
that neither icariin nor berberine altered the basal levels of
total Stat3 and Smad1, as shown by the results of western blot
analysis (Fig. 2A and B). Taken
together, these results demonstrated that the promotion of hepcidin
expression by icariin and berberine was due to the activation of
both the Stat3 and Smad1/5/8 pathways; namely, these two signaling
pathways jointly promoted hepcidin expression in hepatocytes in
response to treatment with icariin and berberine. It should also be
noted that it is not possible to exclude the involvement of other
pathways which may also have been affected by icariin and
berberine.
Icariin regulates hepatic hepcidin
expression in mice
To determine whether the in vitro effects of
icariin and berberine can be reproduced in vivo, we
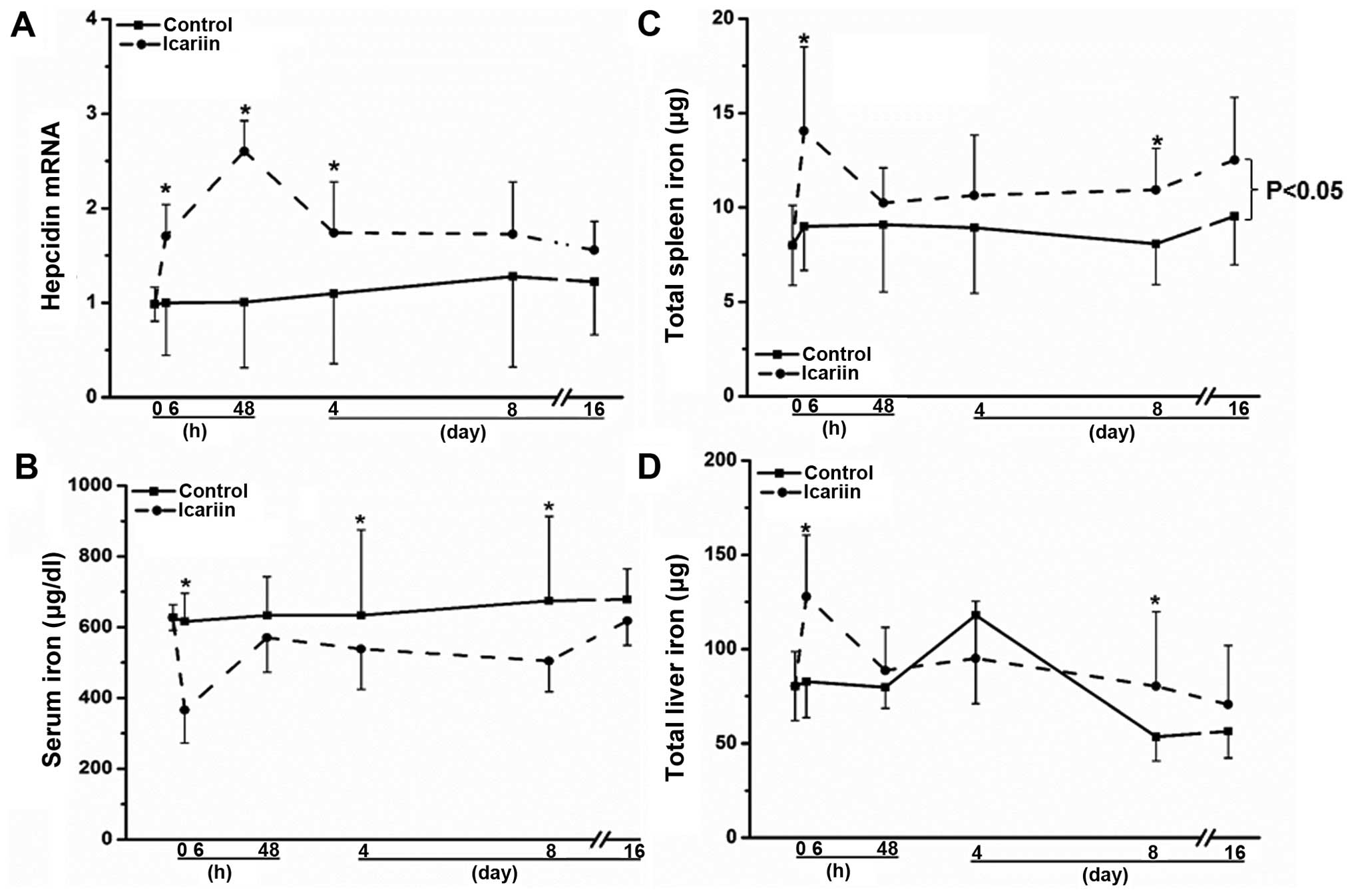
administered both compounds to mice. As shown in Fig. 3A, consistent with the in
vitro results, icariin enhanced hepatic hepcidin mRNA
expression by approximately 50% (P<0.05) 6 h following the
administration of icariin, and a maximum induction (>2.5-fold,
P<0.05) was observed on day 2 post-icariin administration,
compared with the untreated mice (Fig. 3A). The stimulatory effects on
hepatic hepcidin expression declined at later time points (Fig. 3A). As a result of alterations in
hepatic hepcidin expression, serum iron levels were correspondingly
altered, as shown in Fig. 3B. The
serum iron concentration was markedly reduced at 6 h by
approximately 40% in the mice treated with icariin, compared with
the untreated mice (Fig. 3B;
P<0.05). The serum iron concentration was consecutively lower
following treatment with icariin compared to the controls on days
2, 4 and 8 (P<0.05), reaching similar levels to those of the
untreated mice on day 16 (Fig.
3B). Subject to the changes in hepatic hepcidin levels, the
spleen iron concentrations were also altered accordingly. As shown
in Fig. 3C, the total spleen iron
levels were continuously higher following treatment with icariin
than those of the untreated mice from 6 h to the end of the
administration on day 16 (P<0.05). By contrast, the changes in
total hepatic iron levels were less significantly altered upon
icariin administration than the spleen iron levels, although they
were also higher compared to the controls (Fig. 3D; P>0.05); these results are in
agreement with those of previous studies showing the constant
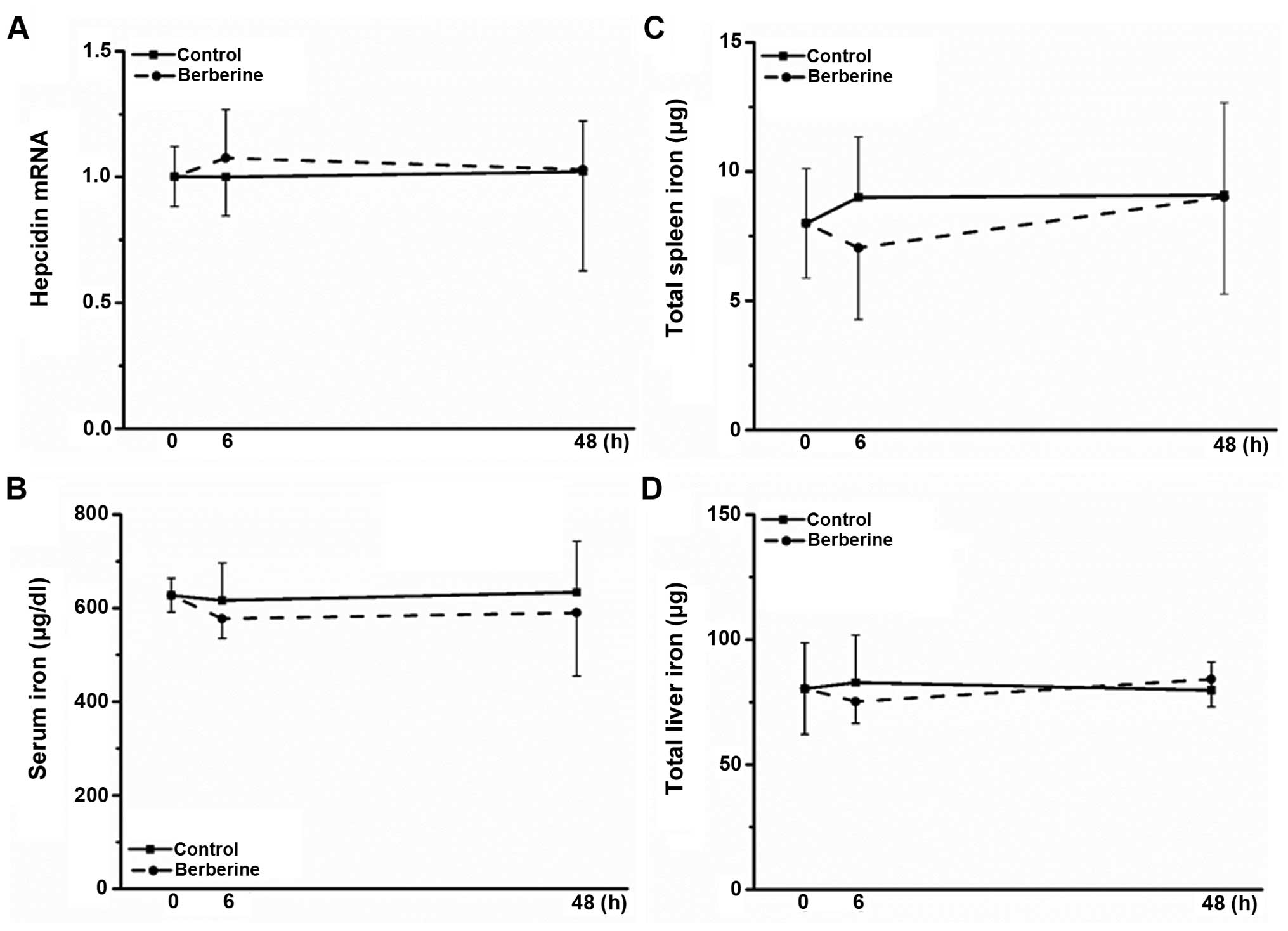
hepatic iron content in response to external stimuli (28,29). Notably, berberine did not promote
hepcidin expression in mice (Fig.
4A), and no significant alterations in serum, liver and spleen
iron levels were observed (Fig.
4B–D); the in vivo results obtained for hepcidin
expression were inconsistent with the in vitro results
described above.
To further investigate our in vivo findings,
ligustrazine (which showed a limited ability to alter hepcidin
expression in vitro, as shown in Table I) was administered to the mice as
a control. Ligustrazine did not alter the hepcidin expression
levels or the body iron status (data not shown). Furthermore, LPS
was used as a positive control to induce hepcidin expression in the
mice, as previously described (29). It has been previously established
that LPS increases hepcidin expression through an inflammatory
mechanism (34–36). Exposure to LPS significantly
increased hepatic hepcidin expression and there were corresponding
alterations in body iron status (data not shown). These data
further supported our findings regarding the icariin-mediated
regulation of hepcidin expression.
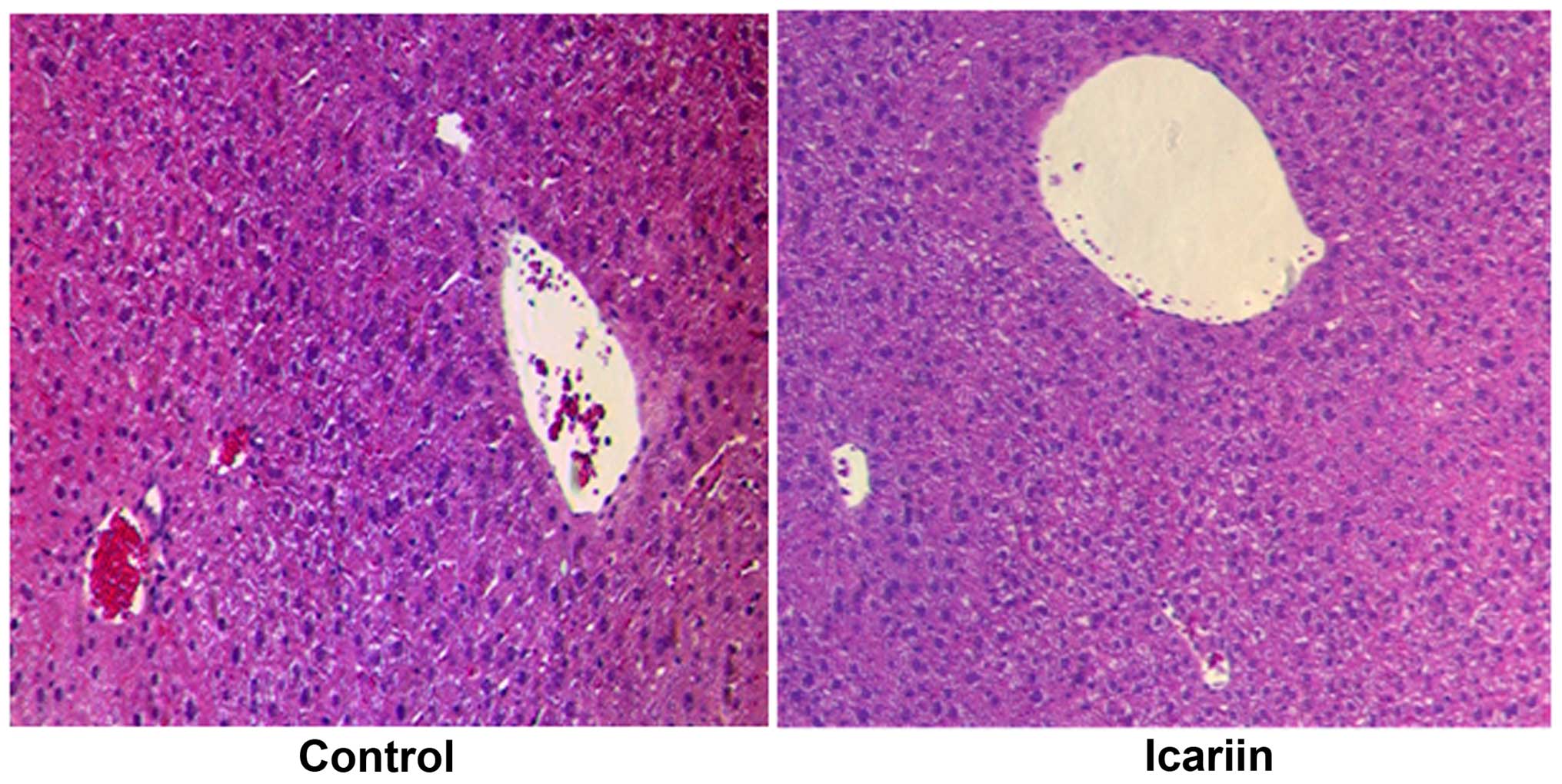
No significant hepatotoxicity was observed in the
liver specimens from the mice administered icariin. As shown in
Fig. 5, no noticeable alterations
(no disordered hepatic cords or enlarged central veins) were
observed in the liver specimens obtained from the mice treated with
icariin for 48 h. This piece of evidence based on histological
examination indicated the safety of icariin for in vivo
administration.
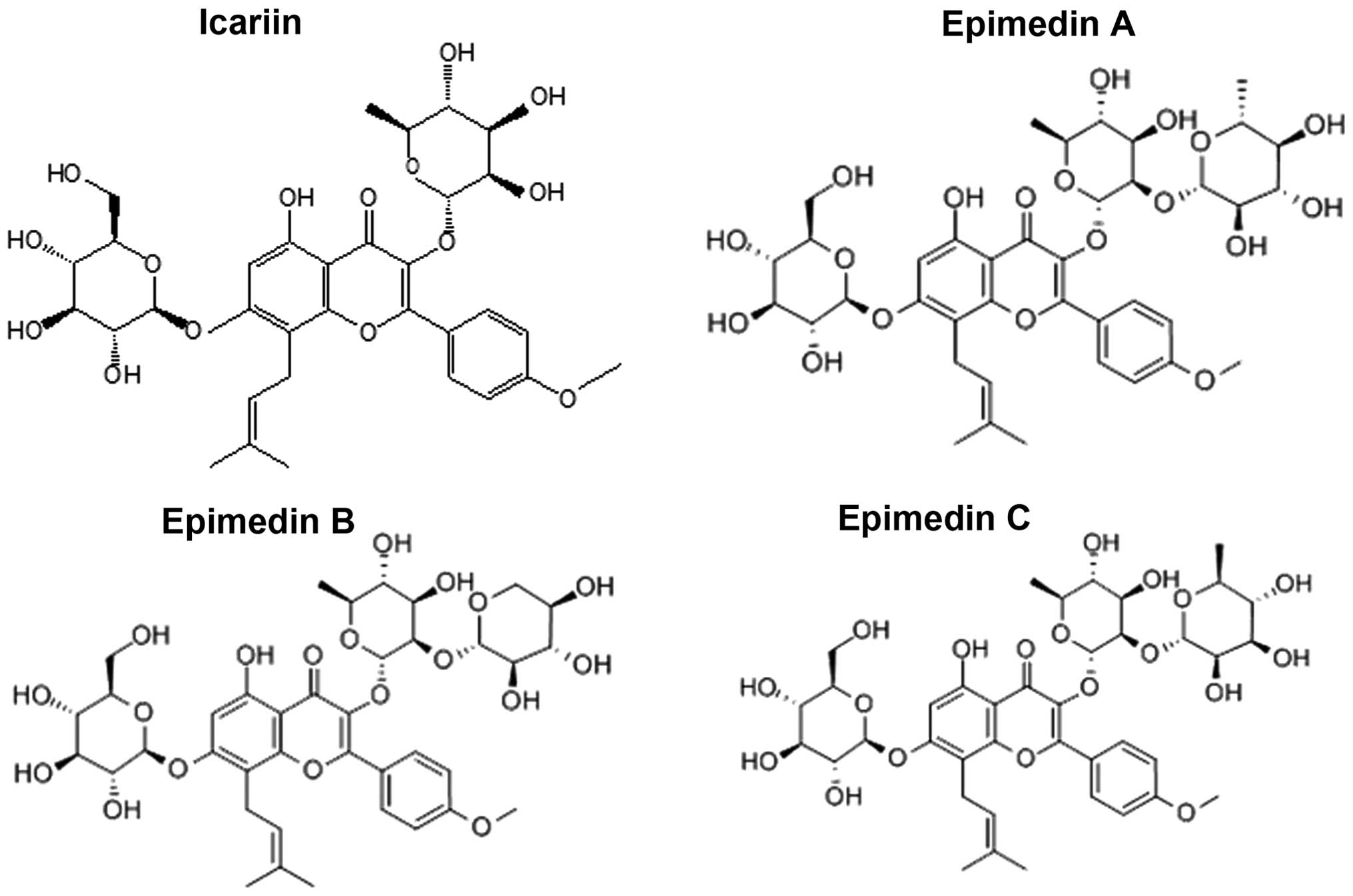
To further examine the regulatory effects of icariin
on hepcidin expression, 3 analogues of icariin, epimedin A, B and C
(which harbor structural similarities to icariin, as depicted in
Fig. 6) were also administered to
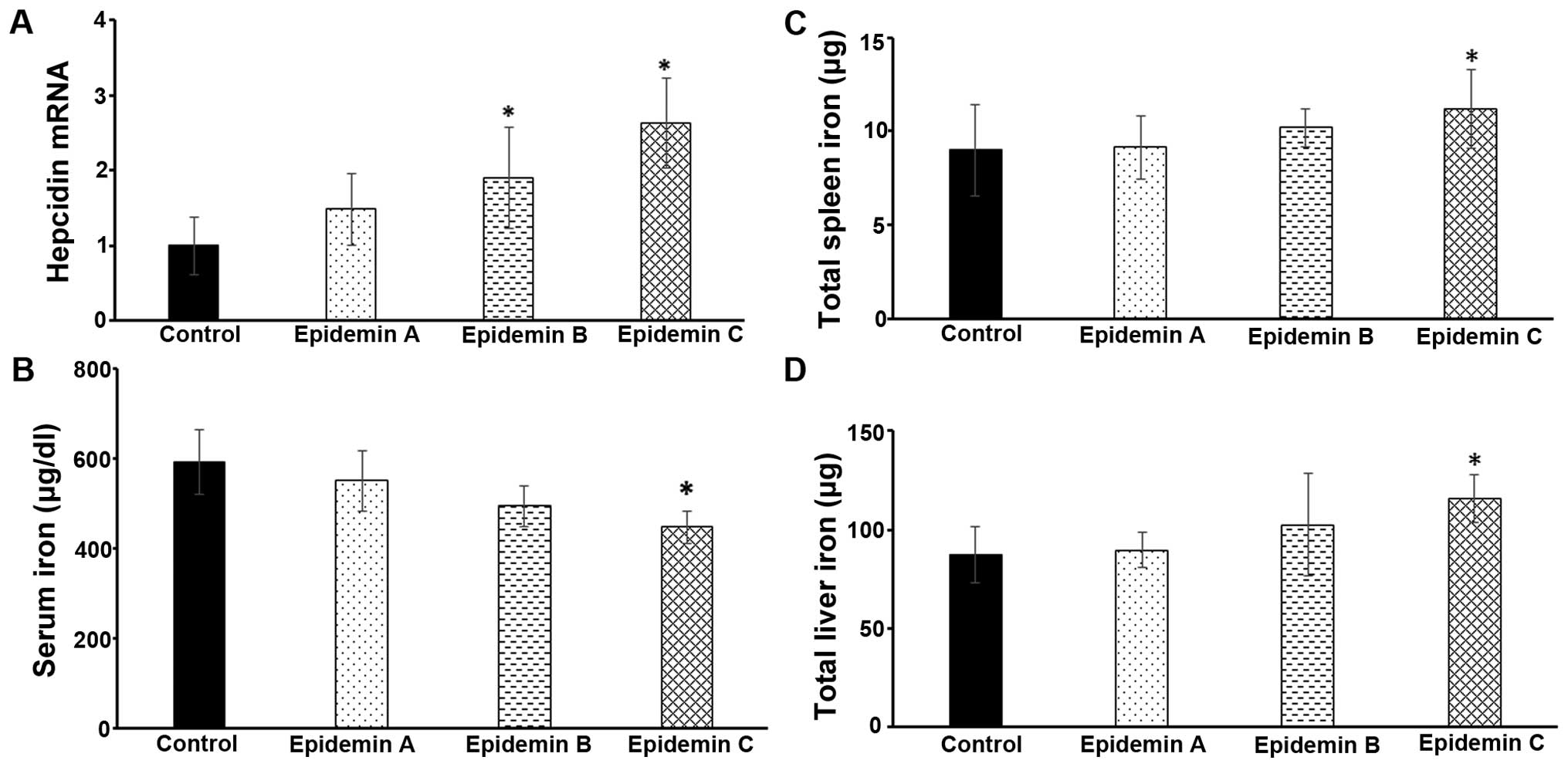
the mice. As shown in Fig. 7A,
epimedin A, B and C all significantly increased hepatic hepcidin
expression, particularly epimedin B and C (P<0.05). As a result,
the serum, spleen and hepatic iron levels were altered accordingly,
with more potent effects being observed following the
administration of epimedin C (Fig.
7B–D). Collectively, these results suggested that icariin and
its analogues exhibited a robust ability to modulate hepatic
hepcidin concentrations in mice, which were associated with
corresponding alterations in systemic iron levels.
Icariin fails to alter body iron content
in Hamp1−/− mice
To confirm the above-mentioned finding that icariin
regulates iron homeostasis by altering hepatic hepcidin expression,
we used Hamp1−/− mice, as previously described (26). Hamp1−/− mice do not
produce functional hepcidin, and these mice thus develop severe
iron overload in the serum and organs after weaning. To more
clearly demonstrate changes in serum iron levels in the animals
that were already iron-loaded, we depleted body iron by placing
these mice on a low-iron diet. In other words, this regime would in
fact increase the sensitivity of these Hamp1−/−mice to
the administration of the compounds. Following iron depletion, we
observed a significant reduction in the serum iron levels in the
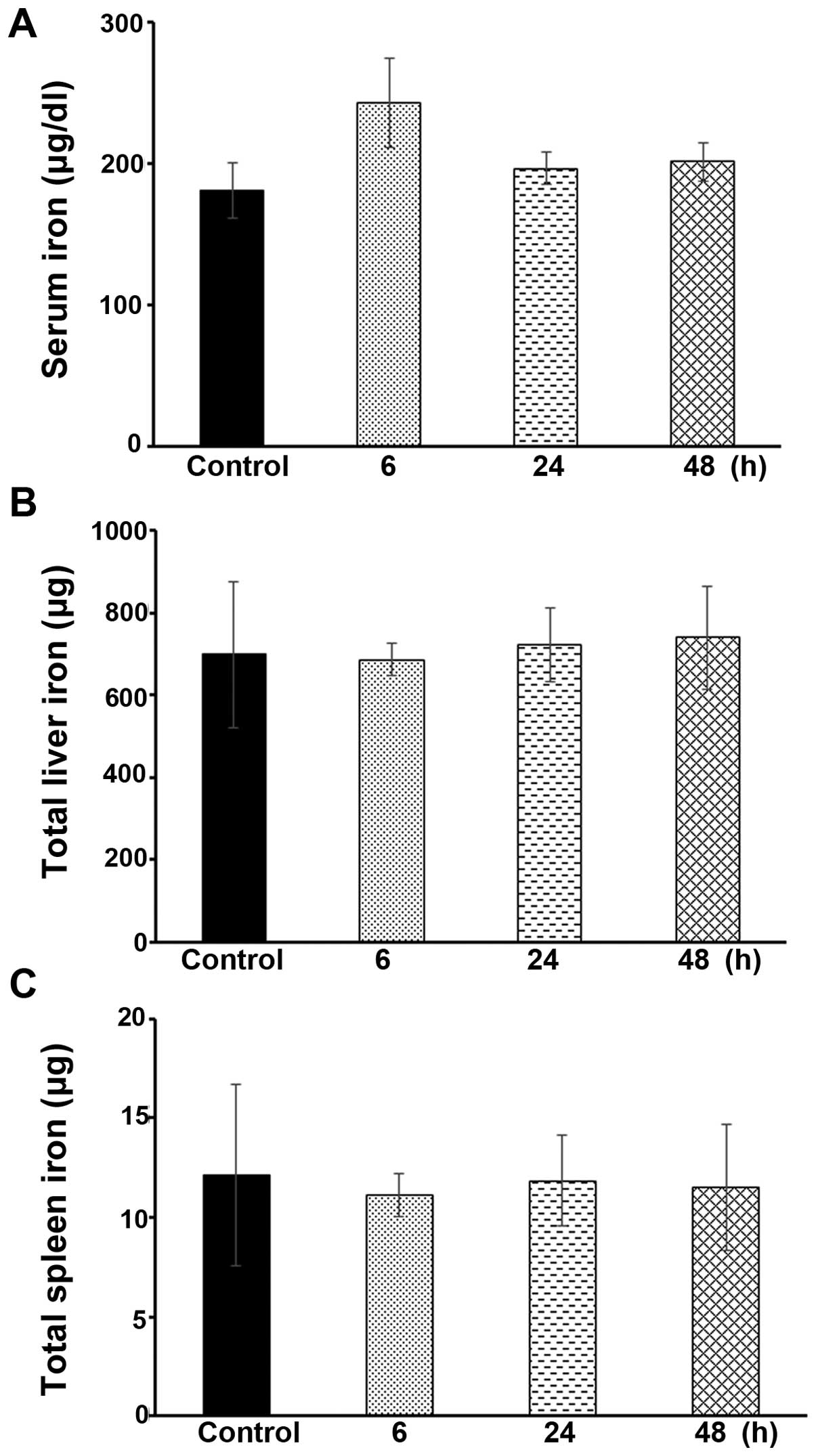
Hamp1−/− mice (data not shown). As shown in Fig. 8A, the serum iron levels were not
significantly altered in the Hamp1−/− mice following the
administration of icariin at the same dose as that administered to
the Wt ICR mice, as described above, i.e., at 100 mg/kg body
weight, for 6, 24 or 48 h (P>0.05). Moreover, there were no
significant changes observed in the total hepatic or spleen iron
levels in the icariin-treated Hamp1−/− mice, compared
with the untreated control mice (Fig.
8B and C). These data further demonstrate that icariin
predominantly targets hepatic hepcidin expression in order to
modulate iron homeostasis.
Discussion
The primary therapies for iron overload-related
diseases are bloodletting (phlebotomy) and iron chelation. However,
these therapies have significant limitations. For instance,
repeated phlebotomy may lead to anemia (37) and long-term administration of iron
chelators may also cause infections, gastrointestinal disorders and
skin damage (38). Therefore,
increasing hepcidin expression is a potential alternative strategy
to relieve iron overload in HH and β-thalassemia, and also to
diminish the severity of β-thalassemia by reducing ineffective
erythropoiesis. Hepcidin analogue peptides (minihepcidins) have
been investigated for their ability to relieve iron overload in
hepcidin-KO mice, although they are not orally available (27). It has been demonstrated that
genistein, a small molecule, increases hepcidin expression through
Stat3-dependent and Smad4-dependent pathways in HepG2 cells
(39). A recent chemical screen
also identified 16 small molecules that induce hepcidin expression
in human HepG2 cells, although animal studies were not performed
(40). In comparison with
proteins and synthesized chemicals, natural compounds from
medicinal plants have novel features, including replete supply,
high stability and low toxicity (41,42). A previous study suggested that
some medicinal plant extracts may repress hepcidin expression
(43). Among the 16 medicinal
plant extracts tested in that study (43), Caulis Spatholobi (CS; also known
as Jixueteng, the stem of Spatholobus suberectus Dann)
exhibited the most potent inhibitory effect on hepcidin expression.
However, to the best of our knowledge, no natural compounds or
extracts from traditional Chinese medicinal plants have been
demonstrated to increase hepcidin levels thus far. In the present
study, we screened 12 pure natural compounds extracted from
traditional Chinese medicinal plants, namely, propyl gallate,
resveratrol, astragaloside, curcumin, paeoniflorin, ligustrazine,
ferulic acid, ginsenoside Rb1, wogonin, liquiritin, berberine and
icariin to examine their effects on hepcidin expression. According
to the Chinese Pharmacopoeia (21), these compounds may have the
ability to improve blood supply, implying that they may potentially
enhance red blood cell formation and alter iron metabolism. In this
study, we set up a pipeline to identify compounds that have the
potential to modulate hepcidin expression. Firstly, we used a
luciferase reporter system to screen these compounds in two cell
lines. The results indicated that only wogonin, icariin and
berberine promoted hepcidin expression by >2-fold in both cell
lines. Thereafter, wogonin, icariin and berberine were subjected to
RT-qPCR analysis. The results revealed that only icariin and
berberine, but not wogonin (data not shown), significantly
increased hepcidin expression in both cell lines. In subsequent
animal experiments, only icariin, but not berberine, increased
hepatic hepcidin expression. The icariin analogues epimedin A, B
and C also increased hepcidin expression. Epimedin A has the
hydroxyl in the rhamnose C-2 position substituted by glucose,
whereas in epimedin B and C, xylose and rhamnose, respectively, are
substituted at this position. Our combined data suggested that the
parental flavone framework likely dictates the structure-activity
relationship (SAR) and that the glycosylation derivatives may
affect the SAR and consequently affect the way in which the
compound regulates hepcidin expression.
We further investigated the molecular mechanisms
responsible for the stimulatory effects of icariin on hepcidin
expression. The Stat3 and Smad1/5/8 signaling pathways are two
important pathways which regulate hepatic hepcidin expression under
physiological and pathological conditions (4,44).
We found that both the Stat3 and Smad1/5/8 pathways were activated
by icariin. Furthermore, in support of the data obtained from the
animal experiments, the iron levels in the Hamp1−/− mice
did not respond to icariin, suggesting that icariin affects iron
levels through a hepcidin-dependent mechanism. Additionally,
icariin at the dose used did not cause noticeable toxicity to the
liver. Icariin is a flavonol glycoside purified from plants of the
genus Epimedium (45), and
previous studies have documented that it has multiple functions
which include roles in reducing oxidative stress, limiting
apoptosis, protecting the cardiovascular system and promoting
angiogenesis (46–49). The present study is the first, to
the very best of our knowledge, to reveal that icariin regulates
iron metabolism by modulating hepcidin expression.
In conclusion, this study demonstrated that icariin
and icariin-like natural compounds purified from traditional
Chinese medicinal plants enhanced hepatic hepcidin transcription.
At the molecular level, icariin was demonstrated to simultaneously
activate Stat3 signaling and Smad1/5/8 signaling, increasing
hepcidin expression. Experiments using mice confirmed the
stimulatory effect of icariin on hepatic hepcidin. In addition,
according to previous studies on natural Chinese medicinal herbs
(50–52), the compounds similar to icariin
would be rapidly eliminated with a short half-life if administered
orally to animals. By contrast, these compounds revealed great
bioavailability with a long half-life when administered
intraperitoneally (53).
Therefore, these results demonstrate that natural compounds (such
as icariin) may be developed into therapies (e.g., injection) which
ameliorate iron overload by restricting dietary iron absorption and
iron egress from macrophages in iron overload-related diseases with
hepcidin deficit. This pilot study represents a promising strategy
for the treatment of iron overload-related diseases (such as HH and
β-thalassemia) through the administration of natural compounds,
particularly extracts from traditional Chinese medicinal
plants.
Abbreviations:
|
HH
|
hereditary hemochromatosis
|
|
Hamp1−/−
|
hepcidin-deficient
|
|
FPN
|
ferroportin
|
|
HFE
|
human hemochromatosis protein
|
|
TFR2
|
transferrin receptor 2
|
|
DMSO
|
dimethyl sulfoxide
|
|
FBS
|
fetal bovine serum
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
Wt
|
wild-type
|
|
LPS
|
lipopolysaccharide
|
|
H&E
|
hematoxylin and eosin
|
Acknowledgments
The present study was supported by a grant under the
National '973' Program (grant no. 2014CB932000), the Strategic
Priority Research Program of the Chinese Academy of Sciences (grant
no. XDB14000000) and grants from the National Natural Science
Foundation of China (grant nos. 21425731, 21377159, 21321004,
21207152 and 21407169). We thank Dr Sophie Vaulont and Dr Tomas
Ganz for providing the Hamp−/− mice. We would like to
thank Dr Jun Liu for making suggestions on icariin-like compounds.
We would also like to thank all the laboratory members for their
great assistance with the experiments and reagents.
References
|
1
|
Hentze MW, Muckenthaler MU and Andrews NC:
Balancing acts: molecular control of mammalian iron metabolism.
Cell. 117:285–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Y and Brosh RM Jr: DNA helicase and
helicase-nuclease enzymes with a conserved iron-sulfur cluster.
Nucleic Acids Res. 40:4247–4260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brzóska K, Meczyńska S and Kruszewski M:
Iron-sulfur cluster proteins: electron transfer and beyond. Acta
Biochim Pol. 53:685–691. 2006.PubMed/NCBI
|
|
4
|
Evstatiev R and Gasche C: Iron sensing and
signalling. Gut. 61:933–952. 2012. View Article : Google Scholar
|
|
5
|
Ganz T and Nemeth E: Hepcidin and
disorders of iron metabolism. Annu Rev Med. 62:347–360. 2011.
View Article : Google Scholar
|
|
6
|
Ganz T and Nemeth E: Hepcidin and iron
homeostasis. Biochim Biophys Acta. 1823:1434–1443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Camaschella C: Understanding iron
homeostasis through genetic analysis of hemochromatosis and related
disorders. Blood. 106:3710–3717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ganz T: Systemic iron homeostasis. Physiol
Rev. 93:1721–1741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanno T, Bhanu NV, Oneal PA, Goh SH,
Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, et al:
High levels of GDF15 in thalassemia suppress expression of the iron
regulatory protein hepcidin. Nat Med. 13:1096–1101. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanno T, Porayette P, Sripichai O, Noh SJ,
Byrnes C, Bhupatiraju A, Lee YT, Goodnough JB, Harandi O, Ganz T,
et al: Identification of TWSG1 as a second novel erythroid
regulator of hepcidin expression in murine and human cells. Blood.
114:181–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kautz L, Jung G, Valore EV, Rivella S,
Nemeth E and Ganz T: Identification of erythroferrone as an
erythroid regulator of iron metabolism. Nat Genet. 46:678–684.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar A, Ekavali, Chopra K, Mukherjee M,
Pottabathini R and Dhull DK: Current knowledge and pharmacological
profile of berberine: an update. Eur J Pharmacol. 761:288–297.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghosh S, Banerjee S and Sil PC: The
beneficial role of curcumin on inflammation, diabetes and
neurodegenerative disease: a recent update. Food Chem Toxicol.
83:111–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mancuso C and Santangelo R: Ferulic acid:
pharmacological and toxicological aspects. Food Chem Toxicol.
65:185–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of astragaloside IV: a literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang YR and Chen KJ: Pharmacological
roles of ligustrazine in cardio-/cerebrovascular systems and its
progress in researches of clinical application. Zhongguo Zhong Xi
Yi Jie He Za Zhi. 33:707–711. 2013.In Chinese. PubMed/NCBI
|
|
17
|
Chen Y, Huang JH, Ning Y and Shen ZY:
Icariin and its pharmaceutical efficacy: research progress of
molecular mechanism. Zhong Xi Yi Jie He Xue Bao. 9:1179–1184.
2011.In Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun LR, Cao X, Hou FQ, Zhu XH and Gao TM:
Progressive studies of paeoniflorin. Zhongguo Zhong Yao Za Zhi.
33:2028–2032. 2008.In Chinese.
|
|
19
|
Jia JM, Wang ZQ, Wu LJ and Wu YL: Advance
of pharmacological study on ginsenoside Rb1. Zhongguo Zhong Yao Za
Zhi. 33:1371–1377. 2008.In Chinese. PubMed/NCBI
|
|
20
|
Tai MC, Tsang SY, Chang LY and Xue H:
Therapeutic potential of wogonin: a naturally occurring flavonoid.
CNS Drug Rev. 11:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China China. Medical
Science; Press, Beijing: 2010
|
|
22
|
Zhang W, Yan ZF, Gao JH, Sun L, Huang XY,
Liu Z, Yu SY, Cao CJ, Zuo LJ, Chen ZJ, et al: Role and mechanism of
microglial activation in iron-induced selective and progressive
dopaminergic neurodegeneration. Mol Neurobiol. 49:1153–1165. 2014.
View Article : Google Scholar
|
|
23
|
Liu W, Zhang S, Wang L, Qu C, Zhang C,
Hong L, Yuan L, Huang Z, Wang Z, Liu S and Jiang G: CdSe quantum
dot (QD)-induced morphological and functional impairments to liver
in mice. PLoS One. 6:e244062011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Wang Z, Xu M, Wang X, Liu R, Liu
Q, Zhang Z, Xia T, Zhao J, Jiang G, et al: Nanosilver incurs an
adaptive shunt of energy metabolism mode to glycolysis in tumor and
nontumor cells. ACS Nano. 8:5813–5825. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Chen Y, Guo W, Yuan L, Zhang D,
Xu Y, Nemeth E, Ganz T and Liu S: Disordered hepcidin-ferroportin
signaling promotes breast cancer growth. Cell Signal. 26:2539–2550.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lesbordes-Brion JC, Viatte L, Bennoun M,
Lou DQ, Ramey G, Houbron C, Hamard G, Kahn A and Vaulont S:
Targeted disruption of the hepcidin 1 gene results in severe
hemochromatosis. Blood. 108:1402–1405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramos E, Ruchala P, Goodnough JB, Kautz L,
Preza GC, Nemeth E and Ganz T: Minihepcidins prevent iron overload
in a hepcidin-deficient mouse model of severe hemochromatosis.
Blood. 120:3829–3836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Suragani RN, Han A, Zhao W, Andrews
NC and Chen JJ: Deficiency of heme-regulated eIF2alpha kinase
decreases hepcidin expression and splenic iron in
HFE−/− mice. Haematologica.
93:753–756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu S, Suragani RN, Wang F, Han A, Zhao W,
Andrews NC and Chen JJ: The function of heme-regulated eIF2alpha
kinase in murine iron homeostasis and macrophage maturation. J Clin
Invest. 117:3296–3305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park CH, Valore EV, Waring AJ and Ganz T:
Hepcidin, a urinary antimicrobial peptide synthesized in the liver.
J Biol Chem. 276:7806–7810. 2001. View Article : Google Scholar
|
|
31
|
Hou Y, Zhang S, Wang L, Li J, Qu G, He J,
Rong H, Ji H and Liu S: Estrogen regulates iron homeostasis through
governing hepatic hepcidin expression via an estrogen response
element. Gene. 511:398–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nemeth E, Rivera S, Gabayan V, Keller C,
Taudorf S, Pedersen BK and Ganz T: IL-6 mediates hypoferremia of
inflammation by inducing the synthesis of the iron regulatory
hormone hepcidin. J Clin Invest. 113:1271–1276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wrighting DM and Andrews NC: Interleukin-6
induces hepcidin expression through STAT3. Blood. 108:3204–3209.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pigeon C, Ilyin G, Courselaud B, Leroyer
P, Turlin B, Brissot P and Loréal O: A new mouse liver-specific
gene, encoding a protein homologous to human antimicrobial peptide
hepcidin, is overexpressed during iron overload. J Biol Chem.
276:7811–7819. 2001. View Article : Google Scholar
|
|
35
|
Poli M, Asperti M, Naggi A, Campostrini N,
Girelli D, Corbella M, Benzi M, Besson-Fournier C, Coppin H,
Maccarinelli F, et al: Glycol-split nonanticoagulant heparins are
inhibitors of hepcidin expression in vitro and in vivo. Blood.
123:1564–1573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee P, Peng H, Gelbart T and Beutler E:
The IL-6- and lipopolysaccharide-induced transcription of hepcidin
in HFE-, transferrin receptor 2-, and beta
2-microglobulin-deficient hepatocytes. Proc Natl Acad Sci USA.
101:9263–9265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adams PC and Barton JC: How I treat
hemochromatosis. Blood. 116:317–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pennell DJ, Porter JB, Cappellini MD,
El-Beshlawy A, Chan LL, Aydinok Y, Elalfy MS, Sutcharitchan P, Li
CK, Ibrahim H, et al: Efficacy of deferasirox in reducing and
preventing cardiac iron overload in beta-thalassemia. Blood.
115:2364–2371. 2010. View Article : Google Scholar
|
|
39
|
Zhen AW, Nguyen NH, Gibert Y, Motola S,
Buckett P, Wessling-Resnick M, Fraenkel E and Fraenkel PG: The
small molecule, genistein, increases hepcidin expression in human
hepatocytes. Hepatology. 58:1315–1325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gaun V, Patchen B, Volovetz J, Zhen AW,
Andreev A, Pollastri MP and Fraenkel PG: A chemical screen
identifies small molecules that regulate hepcidin expression. Blood
Cells Mol Dis. 53:231–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kennedy DO and Wightman EL: Herbal
extracts and phytochemicals: plant secondary metabolites and the
enhancement of human brain function. Adv Nutr. 2:32–50. 2011.
View Article : Google Scholar :
|
|
42
|
Ziegler G, Ploch M, Miettinen-Baumann A
and Collet W: Efficacy and tolerability of valerian extract LI 156
compared with oxazepam in the treatment of non-organic insomnia - a
randomized, double-blind, comparative clinical study. Eur J Med
Res. 7:480–486. 2002.
|
|
43
|
Guan Y, An P, Zhang Z, Zhang F, Yu Y, Wu
Q, Shi Y, Guo X, Tao Y and Wang F: Screening identifies the Chinese
medicinal plant Caulis Spatholobi as an effective HAMP expression
inhibitor. J Nutr. 143:1061–1066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bartnikas TB and Fleming MD: A tincture of
hepcidin cures all: the potential for hepcidin therapeutics. J Clin
Invest. 120:4187–4190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma H, He X, Yang Y, Li M, Hao D and Jia Z:
The genus Epimedium: an ethnopharmacological and phytochemical
review. J Ethnopharmacol. 134:519–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu CQ, Liu BJ, Wu JF, Xu YC, Duan XH, Cao
YX and Dong JC: Icariin attenuates LPS-induced acute inflammatory
responses: involvement of PI3K/Akt and NF-kappaB signaling pathway.
Eur J Pharmacol. 642:146–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li WW, Gao XM, Wang XM, Guo H and Zhang
BL: Icariin inhibits hydrogen peroxide-induced toxicity through
inhibition of phosphorylation of JNK/p38 MAPK and p53 activity.
Mutat Res. 708:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song YH, Cai H, Gu N, Qian CF, Cao SP and
Zhao ZM: Icariin attenuates cardiac remodelling through
down-regulating myocardial apoptosis and matrix metalloproteinase
activity in rats with congestive heart failure. J Pharm Pharmacol.
63:541–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chung BH, Kim JD, Kim CK, Kim JH, Won MH,
Lee HS, Dong MS, Ha KS, Kwon YG and Kim YM: Icariin stimulates
angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent
signal pathways in human endothelial cells. Biochem Biophys Res
Commun. 376:404–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen Y, Wang J, Jia X, Tan X and Hu M:
Role of intestinal hydrolase in the absorption of prenylated
flavonoids present in Yinyanghuo. Molecules. 16:1336–1348. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Y, Zhao YH, Jia XB and Hu M:
Intestinal absorption mechanisms of prenylated flavonoids present
in the heat-processed Epimedium koreanum Nakai (Yin Yanghuo). Pharm
Res. 25:2190–2199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu M, Liu H, Lu X, Li C, Xiong Z and Li
F: Simultaneous determination of icariin, icariside II and osthole
in rat plasma after oral administration of the extract of Gushudan
(a Chinese compound formulation) by LC-MS/MS. J Chromatogr B Analyt
Technol Biomed Life Sci. 860:113–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu W, Sui ZG and Zhong W: Pharmacokinetics
of icariin and its two metabolites in rats. The Third China
Pharmacist Assembly. 1–5. 2011.
|