Introduction
CD5 is a monomeric 67-kDa type I transmembrane
glycoprotein belonging to the scavenger receptor cysteine-rich
(SRCR) family (1), and is
expressed in a variety of immune cells (2). CD5 is expressed at low levels on
immature CD4-CD8-(double negative, DN) thymocytes and becomes
increasingly expressed on CD4+CD8+ (double
positive, DP) and single positive (SP) CD4+ or
CD8+ thymocytes (3).
In the periphery, T cells express high levels of CD5 (4). Although several potential ligands
for CD5 have been identified (5–7),
the physiologically relevant CD5 interactions with their associated
signaling pathways is not completely understood.
CD5 has been shown to physically and functionally
associate with the antigen receptor on T and B cells (8–10).
However, the physiologically relevant and ultimate function of CD5
on antigen receptor signaling remains elusive. Historically, CD5
has been shown to possess a costimulatory function, as
cross-linking of CD5 with antibodies enhances TCR-mediated
activation, proliferation, increases in intracellular
Ca2+, inositol triphosphate, interleukin-2 (IL-2)
secretion, and IL-2R expression (11,12). Previous studies, particularly
those utilizing the CD5 knockout mice have provided considerable
insight into CD5 function. By contrast the studies suggested a
primarily negative role for CD5 in antigen receptor-mediated
signaling (13), although recent
findings demonstrated that soluble human CD5 expressed in mice
enhances experimentally induced autoimmune and anti-tumoral immune
responses (14). Furthermore, the
ability of CD5 to act as a negative regulator of TCR-mediated
signaling has been shown to have a physiological impact on
thymocyte development in terms of thymic selection (15,16). The ability of CD5 to attenuate
TCR-mediated signals suggests that CD5 is important in T-cell
development and represents a mechanism for fine-tuning thymic
selection (3).
Although CD5 does not appear to have any intrinsic
catalytic activity, the cytoplasmic domain of CD5 contains Y378,
Y429, Y441 and Y463 tyrosine residues and several putative
serine/threonine phosphorylation sites (17). Following TCR stimulation the
cytoplasmic domain of CD5 becomes rapidly phosphorylated (15,18) and this is thought to recruit other
signaling molecules. In particular, tyrosine residues 429 and 441
are embedded in an imperfect immunoreceptor tyrosine-based
activation motif (ITAM)-like sequence (19), while tyrosine 378 is contained
within an immunoreceptor tyrosine-based inhibitory motif
(ITIM)-like sequence (20),
suggesting that these sites can act as docking sites for proteins
with SH2 domains. A study on Jurkat T cells has shown that tyrosine
378 in the ITIM-like sequence of CD5 is required for SHP-1
association and is involved in SHP-1 tyrosine phosphatase activity
in mediating the downregulatory activity of CD5 (21). Furthermore, a correlation was
demonstrated between the phosphorylation state of CD5 and the
phosphatase activity of SHP-1, suggesting that CD5 may represent a
substrate for SHP-1 activity (22). It has been reported that
Lyn-mediated SHP-1 binding to CD5 contributes to resistance to
apoptosis of B-cell chronic lymphocytic leukemia cells (23). However, the role of SHP-1 in
mediating the negative regulatory effects of CD5 remains
controversial. For example, CD5-deficient T-cell hybridomas
transfected with a truncated form of CD5 that retains the ITIM-like
sequence (Y378) were unable to negatively regulate TCR responses
(16).
To clarify the functional and physiological
requirement of SHP-1 in the CD5 signaling pathway, we assessed the
ability of CD5 to downregulate TCR signaling and thymic selection
in the context of SHP-1 deficiency. The results showed that
although SHP-1 associates with CD5, the tyrosine phosphorylation
profile of CD5 following TCR stimulation was not different in
SHP-1-deficient viable motheaten (mev), compared
to wild-type thymocytes. The lack of SHP-1 activity also had no
impact on the levels of CD5 surface expression, CD5-associated PTP
activity, and intracellular calcium mobilization profiles following
TCR/CD5 co-crosslinking. Similarly, an analysis of T-cell thymocyte
populations in mev mice expressing an H-Y
transgene as well as a construct mediating T-cell-restricted CD5
overexpression, revealed that the reduction in positive selection
conferred by CD5 overexpression was unaffected by SHP-1 deficiency.
Cumulatively, these observations indicate that CD5 is not an SHP-1
substrate and suggest SHP-1 is not required for and possibly not
involved in CD5-mediated downregualation of TCR signaling.
Materials and methods
Mice
Mice homozygous for the viable motheaten mutation
(mev) were obtained by mating C57BL/6J
mev/+ breeding pairs derived from breeding stock
maintained at the Samuel Lunenfeld Research Institute, Mount Sinai
Hospital (Toronto, ON, Canada). The study was approved by the local
Ethics Committee of Mount Sinai Hospital. Mice carrying an
H-Y-specific TCR transgene, which recognizes the H-Y male-specific
antigen presented on H-2Db (24), were crossed with
mev/+ hetero zygotes/+ heterozygotes and the H-Y
TCR/mev/+ progeny selected and backcrossed with
mev/+ mice to obtain H-Y
TCR/mev homozygotes. For the derivation of CD5
transgenic mice, a huCD2-CD5 transgene was derived as previously
detailed by substituting the murine CD5 coding sequence for the
TCRζ cDNA sequence in the construct ζ-CT108 (25). Founder lines were identified by
Southern blotting, screened for expression of CD5 by Northern
blotting and flow cytometry analysis and the mice then backcrossed
to C57BL/6J through six generations. The mice were then mated with
H-Y TCR transgenic mice to generate H-Y TCR/CD5 transgenics. To
derive H-Y TCR/CD5/mev mice, the H-Y TCR/CD5
transgenics were mated to mev/+ mice and the F1
H-Y TCR/CD5 transgenic viable motheaten heterozygote progeny then
backcrossed with mev/+ mice. The mice were typed
for expression of the H-Y TCR and CD5 transgenes using PCR
amplification with the primer pairs: 5′-CAGACCCTCCT
TGATCCTGGCCCTCCAGT-3′ (forward) and 5′-CAGTCC
GTGGACCAGCCTGATGCTCATGT-3′ (reverse); 5′-GGA
GCACATCAGAAGGGCTGGCTT-3′ (forward) and 5′-CGG
AGATCCTTGGGCAGAAGACCTG-3′ (reverse), respectively. The PCR
amplification cycle (denaturation for 15 sec at 94°C, annealing for
20 sec at 64°C and elongation for 30 sec at 72°C) was repeated 35
times. H-Y TCR and CD5 transgene expression was also confirmed by
surface staining of peripheral blood lymphocytes (26). The mice were studied at the ages
of 2-3 weeks.
Antibodies and reagents
Antibodies used for these studies included
FITC-conjugated anti-CD8 (cat. no. 553031; 1:1,000) and anti-CD5
(cat. no. 553020; 1:1,000) antibodies, PE-conjugated anti-CD4
antibody (cat. no. 557307; 1:1,000), and biotin-conjugated
monoclonal rat anti-mouse CD5 (clone 53–7.3; cat. no. 553018;
1:1,000), anti-TCR (αβ), and anti-CD4 antibodies all obtained from
Pharmingen (La Jolla, CA, USA). Purified monoclonal rat ant-mouse
CD5 (clone 53–7.3) was generously provided by Dr L.A. Herzenberg
(Stanford University, Stanford, CA, USA) or purchased from
Pharmingen. Rat anti-mouse IgG, goat ant-rat IgG and streptavidin
and avidin were obtained from Jackson ImmunoResearch (West Grove,
PA, USA). Anti-phosphotyrosine monoclonal antibody 4G10, protein A
and sheep anti-mouse antibody conjugated to horseradish peroxidase
were purchased from Upstate Biotechnology, Inc. (Lake Placid, NY,
USA). Rabbit polyclonal anti-SHP-1 antibody recognizing the tandem
SH2 domains of SHP-1 was generated in the laboratory as previously
described (27). Polyclonal
anti-CD5 (R5) rabbit serum to the highly conserved peptide
sequence, TASHVDNEYSQPPR, in the CD5 cytoplasmic domain was
generously provided by Drs Greg Appleyard and Bruce Wilkie
(Department of Pathobiology, University of Guelph, Guelph, ON,
Canada). Chemicals used for immunoblotting/immunoprecipitation were
purchased from Sigma Chemical Corp. (St. Louis, MA, USA).
Cell stimulation, Immunoprecipitation and
western blot analysis
Single-cell suspensions of thymocytes
(3×107/condition) obtained from wild-type or
mev mice were resuspended in 150 µl of
phosphate-buffered saline (PBS) and incubated for 30 min at 4°C in
the presence or absence of 2.5 µg biotin-conjugated
anti-mouse TCR antibody. Following several washes to remove any
unbound antibody, the cells were resuspended in 40 µl PBS
and incubated at 37°C for various time points with 50 µg/ml
avidin or 25 µg/ml streptavidin. The cells were then
pelleted by 30-sec centrifugation and lysed in 400 µl of
cold lysis buffer supplemented with protease inhibitors [1% Nonidet
P-40, 50 mM HEPES (pH 7.2), 150 mM NaCl, 50 µM NaF, 50
µM 0-phosphate, 50 µM ZnCl2, 2 mM EDTA, 2
mM Na3VO4, 2 mM PMSF, 10 µg/ml
leupeptin and 10 µg/ml aprotinin] for 30 min on ice. Nuclei
and unlysed cells were removed by centrifugation at 14,000 × g for
10 min at ~4°C and protein concentrations were determined by means
of the bicinchoninic acid (BCA) assay (Pierce Biochemicals,
Rockford, IL, USA). Equal amounts of lysates (300–500 µg)
were incubated for 2 h at 4°C with the appropriate antibody
(anti-CD5, or anti-IgG isotype control) and 30 µl of 50%
protein G Sepharose beads (Pharmacia, Toronto, Canada) was added
and the samples agitated at 4°C for an additional hour.
Immunocomplexes were collected by centrifugation and washed five
times in 1 ml of cold lysis buffer and then boiled for 5 min in
reduced SDS-gel sample buffer. The samples were resolved on 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto nitrocellulose membranes (Bio-Rad
Laboratories, Mississauga, ON, Canada). Blots were blocked for ≥1 h
in TBS-T containing 3% gelatin or 5% non-fat milk, and incubated
for 1 h at room temperature with optimal concentrations of the
primary antibody [anti-CD5 (R5), anti-SHP-1 or anti-phosphotyrosine
4G10]. The blots were then incubated with the appropriate secondary
antibody conjugated to horseradish peroxidase and subjected to
enhanced chemiluminescence (ECL; Amersham Corp., Arlington Heights,
IL, USA). Where indicated, the immunoblots were stripped and
reprobed with anti-CD5 or anti-SHP-1 antibody.
Immunocomplex phosphatase activity
assay
For analysis of CD5-associated phosphatase activity,
anti-CD5 immunoprecipitates were prepared. Briefly, wild-type and
mev-derived thymocytes (1×108),
unstimulated or stimulated with biotinylated antibodies to anti-TCR
and anti-CD4 at 10 µg/ml plus streptavidin at 25
µg/ml for 5 min at 37°C, were lysed into 400 µl of
cold lysis buffer, as described earlier, without sodium
orthovanadate. An equal amount of lysates was then subjected to CD5
immunoprecipitation using anti-CD5 (53–7.3) antibody or rat
anti-mouse IgG isotype control. Immunoprecipitates were incubated
at 37°C for 90 min with 1 mM phosphopeptide RRLIEDAEY-pAARG
(Upstate Biotechnology, Lake Placid, NY, USA) in 10 mM Tris-HCl (pH
7.4) phosphatase buffer. Free phosphate detection was carried out
as specified by the manufacturer. To standardize for the
non-specific phosphatase the activity associated with IgG, relative
phosphatase activity was determined by dividing the measured
absorbance values with those from unstimulated IgG negative
controls.
Calcium measurements
Thymocytes (5×106 cell/ml) were labeled
with Indo-1 (5 µM) and incubated at 37°C in the dark for 30
min. Thymocytes were washed, resuspended in RPMI-1640 containing 2%
fetal bovine serum (FBS) and 10 mM HEPES (pH 7.4), and incubated on
ice with biotinylated anti-TCR alone (2.0 or 0.3 µg), or in
combination with either biotinylated (2.5 µg) or
non-biotinylated anti-CD5 (2.5 µg) for 30 min. After
washing, the cells were resuspended in RPMI-1640 buffer at a
concentration of ×107 cells/ml and stimulated with
streptavidin (5 µg/ml). Goat anti-rat antibody (16
µg) was also used to crosslink samples containing
non-biotinylated anti-CD5 antibody. The cells were analyzed on a
flow cytometer and calcium levels were detected by analysis of the
Indo-1 violet-blue fluorescence ratio.
Flow cytometric analysis
Cells (5×106/sample) were resuspended in
100 µl immunofluorescent staining buffer (PBS containing 1%
BSA and 0.05% sodium azide) and incubated with the appropriate
fluorochrome-conjugated antibodies (FITC-conjugated anti-CD8 or
PE-conjugated anti-CD4) for 30 min at 4°C. For CD5 staining, the
cells were incubated with biotinylated anti-CD5 for 30 min at 4°C,
washed and then incubated with FITC-conjugated streptavidin.
Stained cells were analyzed using a FACScan flow cytometer with
CellQuest software (Becton-Dickinson, San Diego, CA, USA).
Results
SHP-1 interaction with CD5 is increased
following receptor phosphorylation
We previously demonstrated the ability of SHP-1 to
associate with CD5 in activated mouse thymocytes, association that
was somewhat enhanced following CD5 tyrosine phosphorylation,
suggesting that the interaction may be SH2 domain is mediated
(28). Since then there have been
several studies supporting this contention. Bikah et al have
reported observing CD5 association with SHP-1 in resting but not
activated T cells and in BKS-2 B lymphoma cells (29). Sen et al have also reported
that phosphorylated CD5 is associated with SHP-1 in B-1 cells
(30). Recently, Perez-Villar
et al have also shown a constitutive association of CD5 with
SHP-1 in Jurka T cells and PHA-expanded T lymphoblasts, which
increased following TCR stimulation (21). In addition, mapped Tyr-378 in the
ITIM-like sequence of CD5 was found to be essential for SHP-1
binding to CD5 in Jurkat T cells (21). However, the association of SHP-1
with CD5 as well as the SH2 mechanism for mediating SHP-1
interaction has been previously addressed (16,31,32). We therefore examined the
association profile of SHP-1 and CD5 in resting and TCR-stimulated
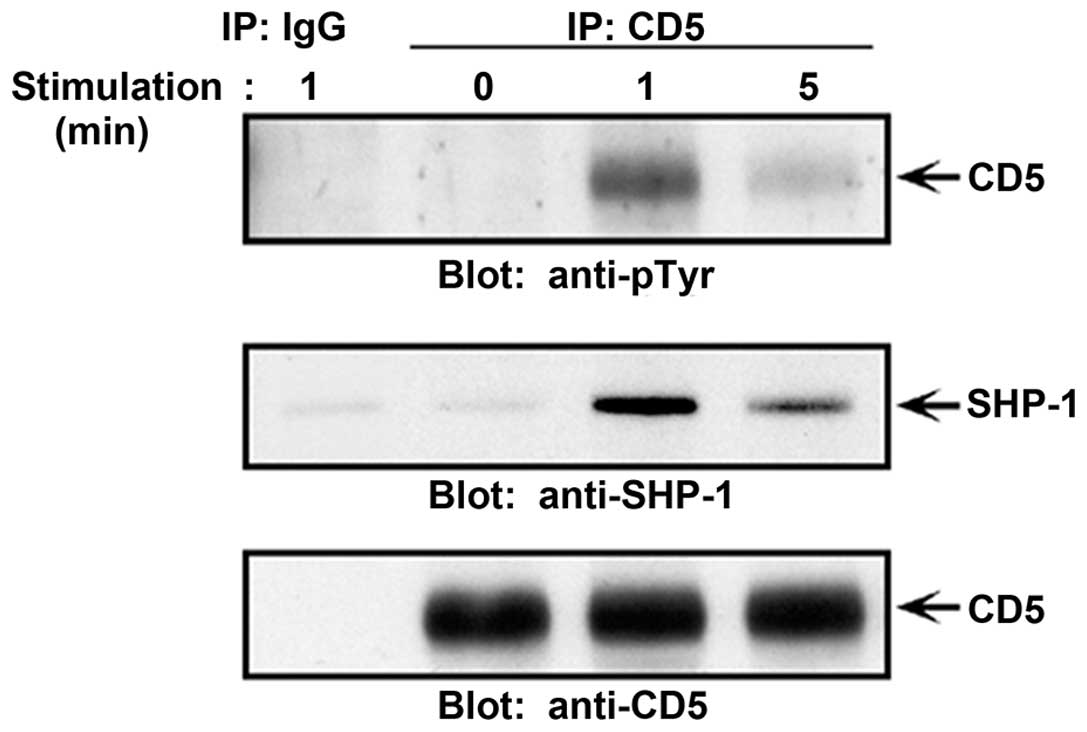
wild-type thymocytes. Immunoprecipitates of CD5 when immunoblotted
with SHP-1 revealed a strong association with SHP-1 following TCR
activation (Fig. 1). The
association of SHP-1 with CD5 occured rapidly following this
stimulation and directly correlated to the level of CD5
phosphorylation (interaction decreased as the level of CD5 was
reduced). Thus, association of SHP-1 with CD5 in thymocytes was not
constitutive but rather dependent on TCR activation and the level
of CD5 tyrosine phosphorylation.
CD5 tyrosine phosphorylation profile is
unchanged in SHP-1-deficient thymocytes
Given that the profile of SHP-1 binding to CD5 was
found to be dependent on the level of CD5 phosphorylation (Fig. 1), together with recent data
demonstrating a correlation between the phosphatase activity of
SHP-1 and the status of CD5 phosphorylation (involving SHP-1 as the
phosphatase responsible for CD5 dephosphorylation) (22), we addressed whether CD5 is a
substrate for SHP-1 activity. Previously it was shown that many
molecules representing direct or indirect potential substrates for
SHP-1 activity, were either constitutively hyper-phosphorylated
and/or exhibited enhanced and prolonged activation-induced tyrosine
phosphorylation profiles in SHP-1 deficient compared to wild-type
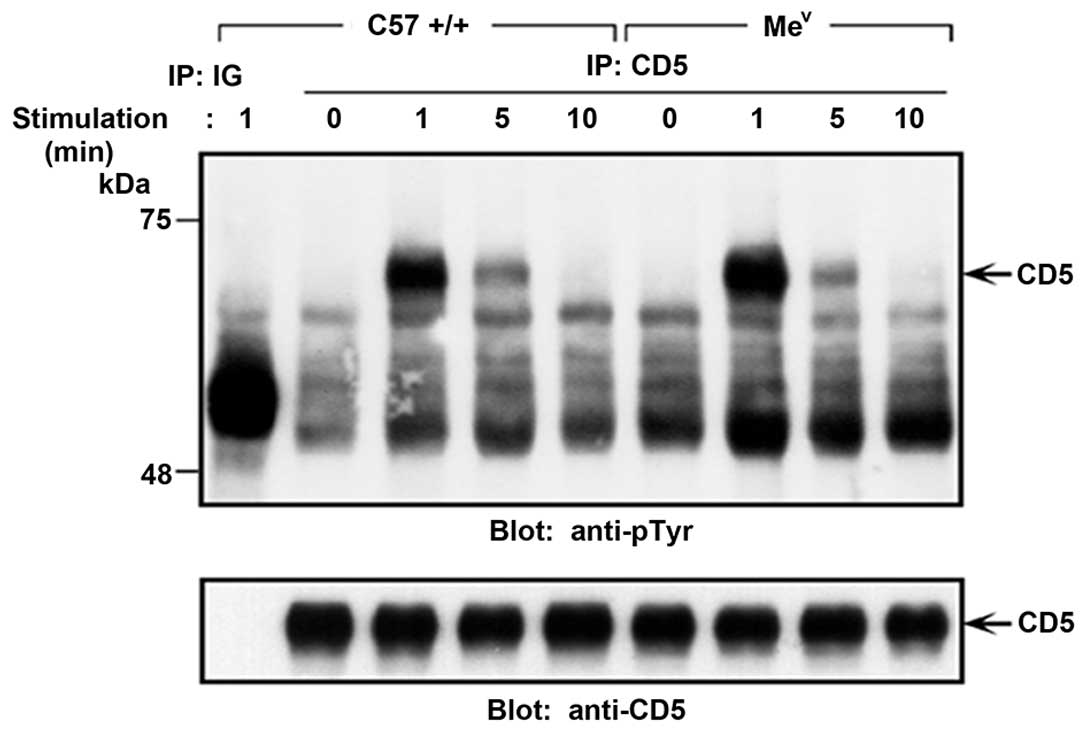
cells (28). Of note, the
tyrosine phosphorylation status of CD5 in the resting and
TCR-stimulated thymocytes from normal compared to SHP-1 deficient
(mev) mice was unchanged (Fig. 2). The lack of constitutive and
hyper-phosphorylated CD5 in the context of SHP-1-deficiency,
together with a normal phosphorylation profile following TCR
stimulation, strongly argues against a role for SHP-1 in the
dephosphorylation of CD5.
SHP-1 deficiency does not alter thymic
CD5 surface expression
CD5 expression is closely regulated throughout
T-cell development. CD5 is expressed at low levels on immature
CD4−CD8− (DN) thymocytes and becomes
increasingly expressed on CD4+CD8+ (DP) and
single-positive (SP) CD4+ or CD8+ thymocytes
(3), with the mature peripheral T
cells expressing high levels (33). Recently, Azzam et al showed
that CD5 expression was regulated by the strength and avidity of
TCR signals (3). The lack of any
observable differences in the thymic CD5 tyrosine phosphorylation
profiles in mev mice led us to examine thymic CD5
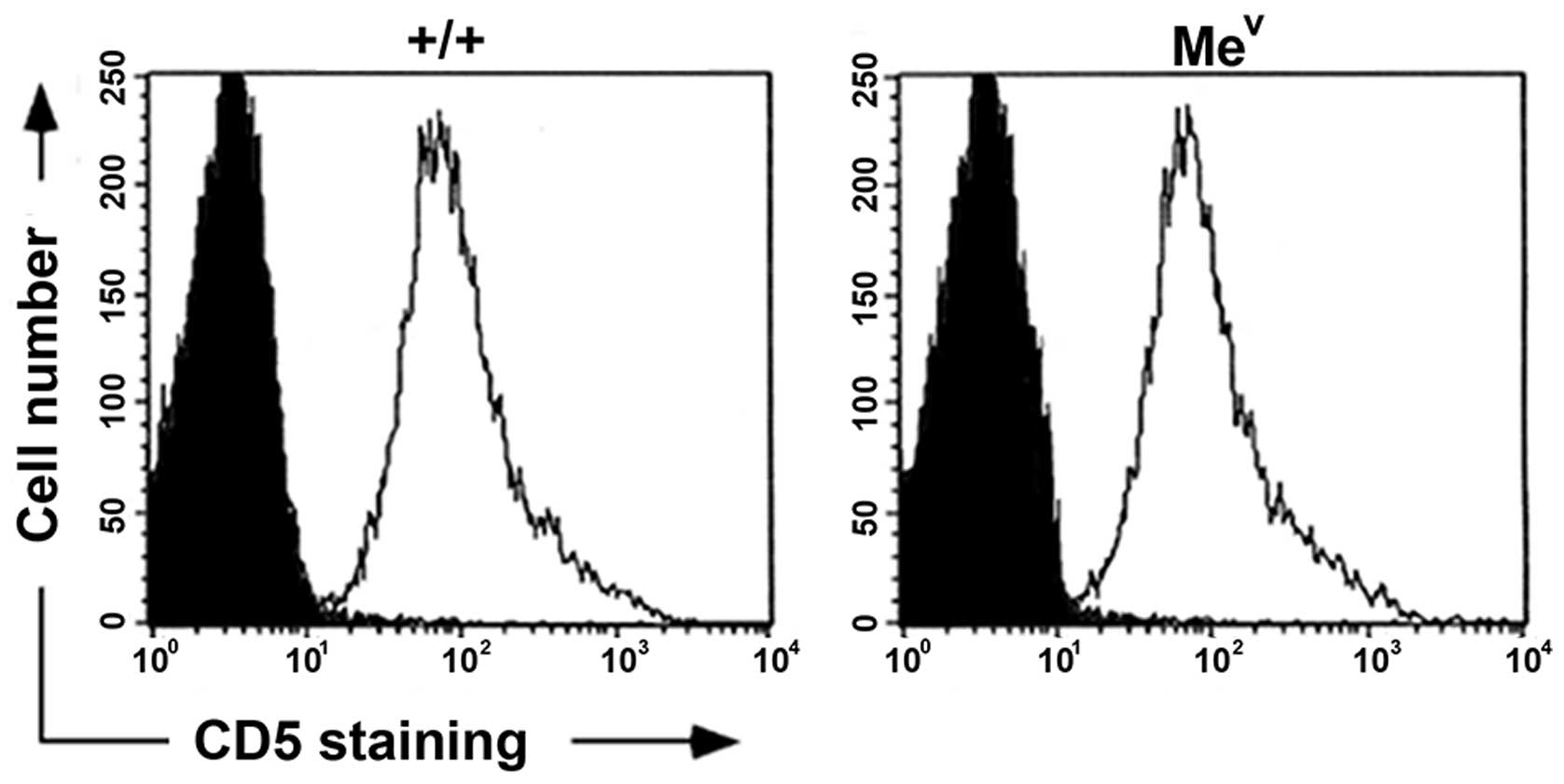
surface expression levels. In contrast to the findings in
peripheral T cells isolated from SHP-1-deficient mice, which
express elevated basal levels of CD5 (34), we detected equivalent CD5 surface
levels in mev compared to wild-type thymocytes
(Fig. 3). Therefore, the lack of
SHP-1 did not affect levels of CD5 surface expression during early
T-cell development.
SHP-1 deficiency does not significantly
alter CD5-associated PTP activity
Previous studies in the human T-cell Jurkat lymphoma
cell line demonstrated a moderate tyrosine phosphatase activity
associated with CD5 immunoprecipitates (21). This CD5-associated PTP activity
was shown to substantially increase following TCR-stimulation
(21). Perez-Villar et al
elucidated the molecular basis for this CD5-associated phosphatase
activity and attributed this function to the interaction of CD5
with SHP-1 but not SHP-2 (21).
Supporting this view are recent data by Sen et al, examining
CD5-associated PTP activity in wild-type B-1 cells (30). Sen et al also found CD5 to
be associated with PTP activity, which could be completely
eliminated by the prior immunodepletion of SHP-1 but not SHP-2,
supporting the hypothesis that the phosphatase activity associated
with CD5 was derived mainly from SHP-1 (30). Given that CD5 is not a direct
substrate for SHP-1 action, the functional association of SHP-1
with CD5 supports a model whereby the negative regulatory function
of CD5 is mediated by the recruitment of SHP-1 into the
antigen-receptor complex (21,28–30). By contrast, Gary-Gouy et al
suggest that the effect of CD5, at least, on BCR signaling was
independent of SHP-1. In that study, the authors did not show any
physical interaction with SHP-1, SHP-2 or SHIP concluding that
other inhibitory phosphatases may exist that carry out the negative
function of CD5 (31). Given
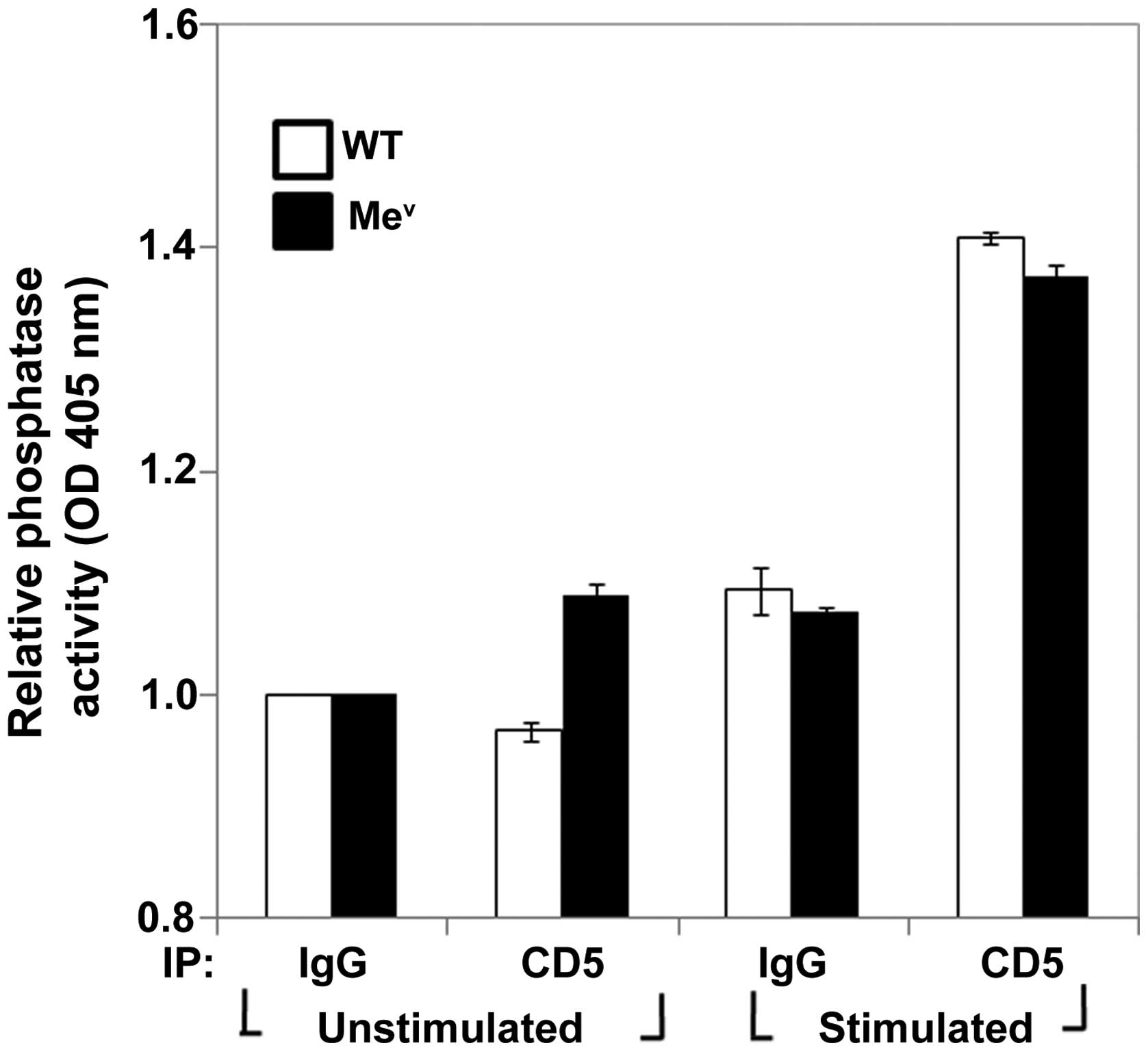
those findings, we examined and compared CD5-associated PTP
activity from wild-type and SHP-1-deficient (mev)
thymocytes. The results showed that SHP-1-deficient thymocytes also
retained wild-type compared to CD5-associated PTP activity
(Fig. 4). This PTP activity (as
in the case of wild-type) increased following TCR stimulation.
Therefore, our findings suggested that there is a redundancy with
respect to SHP-1 function, or more likely, that there are other
phosphatases besides SHP-1 that are responsible for mediating the
negative modulatory effects of CD5 in thymocytes.
TCR/CD5-mediated calcium response is
dependent on the method of CD5 stimulation
Biochemical observations thus far in SHP-1-deficient
thymocytes suggest that CD5 does not require SHP-1 activity, as
there were no differences in the CD5 phosphorylation status, CD5
surface expression or CD5-associated PTP activity. However, these
findings still do not exclude a possible requirement of SHP-1 in
CD5 signaling and function. One of the earliest biochemical events
to occur following TCR stimulation is the enhanced mobilization of
calcium (35–38). Studies examining CD5 function have
identified an important role for CD5 signaling in the calcium
pathway (11,15). Peripheral T-cell co-stimulation
through anti-CD5 antibodies has been shown to increase
TCR/CD3-induced intracellular Ca2+ concentration
(39), and this increase is
entirely due to an influx of extracellular calcium (40-42). By contrast, CD5 acts as a negative
regulator of antigen receptor-mediated calcium mobilization in
thymocytes and B-1 cells (15,29) since thymocytes and B-1 cells from
CD5-deficient mice exhibit a moderate increase in Ca2+
mobilization following antigen receptor activation (15,29). The differences suggest that CD5
possesses a dual function, providing either positive or negative
modulatory signals depending on the cell type and maturational
stage (43). However, the calcium
mobilization profiles associated with CD5 and antigen receptor
stimulation depend on the method of antibody crosslinking. In
particular, co-crosslinking the antigen-receptor with CD5 compared
to a separate crosslinking of the two receptors generates a
qualitatively different calcium mobilization profile that can be
explained in a manner consistent with a negative regulatory
function for CD5 (21,30,31).
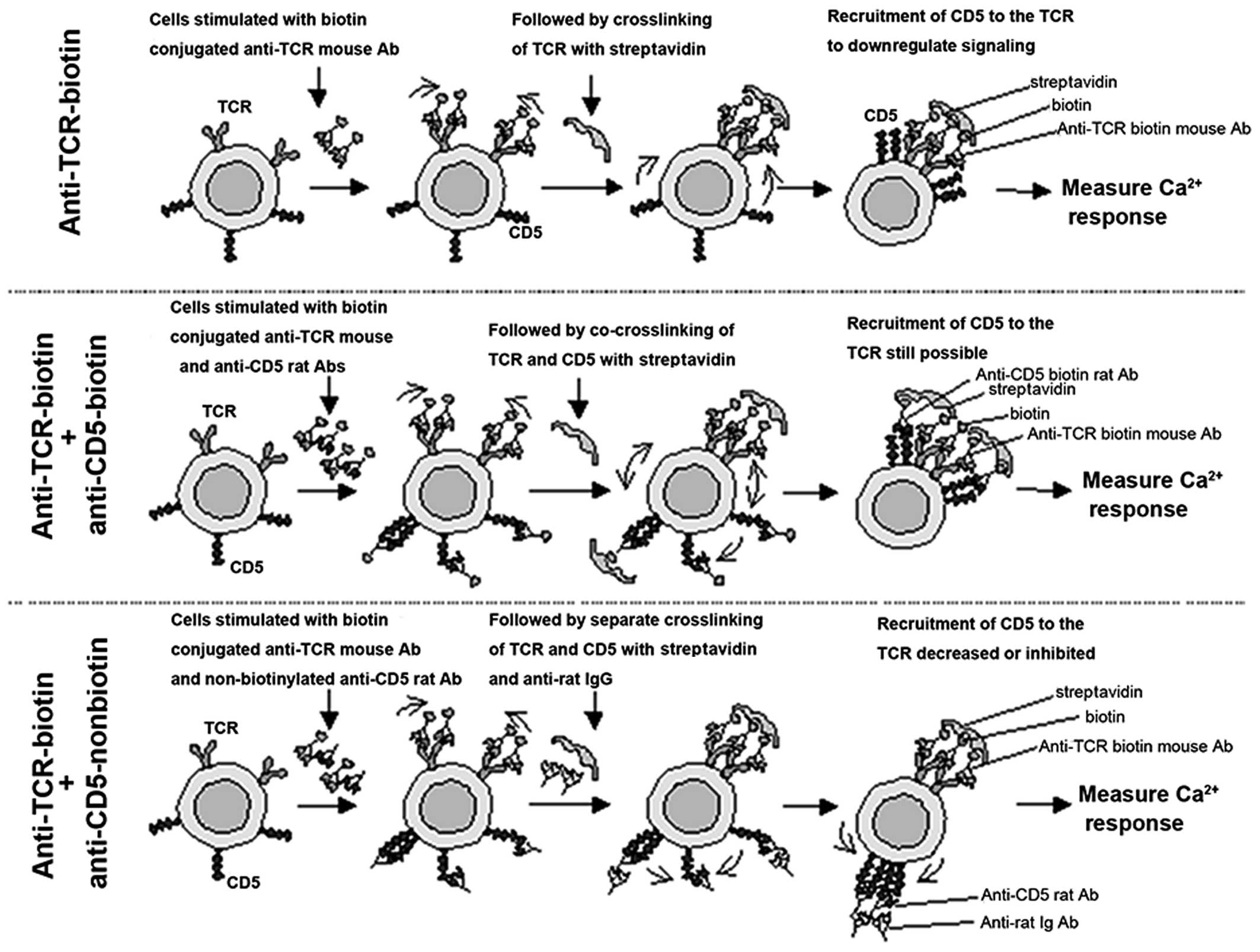
Therefore, to examine the functional contribution of
SHP-1 in CD5/TCR-mediated Ca2+ mobilization, we produced
a stimulation protocol based on those studies (Fig. 5). As shown in the top panel of
Fig. 5, TCR crosslinking induces
a rapid recruitment of CD5 to the TCR/CD3 complex, as has already
been demonstrated by co-capping studies (44). Similarly, the coligation of TCR
and CD5 resulted in a similar or increased recruitment of CD5 to
the TCR complex (Fig. 5, middle
panel), as this treatment has been reported to reduce the
Ca2+ influx when compared to the ligation of TCR/CD3
alone (21). By contrast, when
the TCR and CD5 were separately cross linked (Fig. 5, bottom panel) the recruitment of
CD5 to the TCR complex was inhibited or significantly decreased
(44). In keeping with the
negative role of CD5, separate crosslinking of the antigen receptor
and CD5 has been demonstrated to increase the Ca2+
mobilization and proliferative response in B-1 cells, and this has
been explained to occur as a result of CD5 sequestration from the
BCR (29,30). We hypothesized that if SHP-1 is
required in the CD5-mediated calcium mobilization pathway, then the
lack of SHP-1 activity may significantly affect the calcium influx
profiles in thymocytes from SHP-1-deficient mev
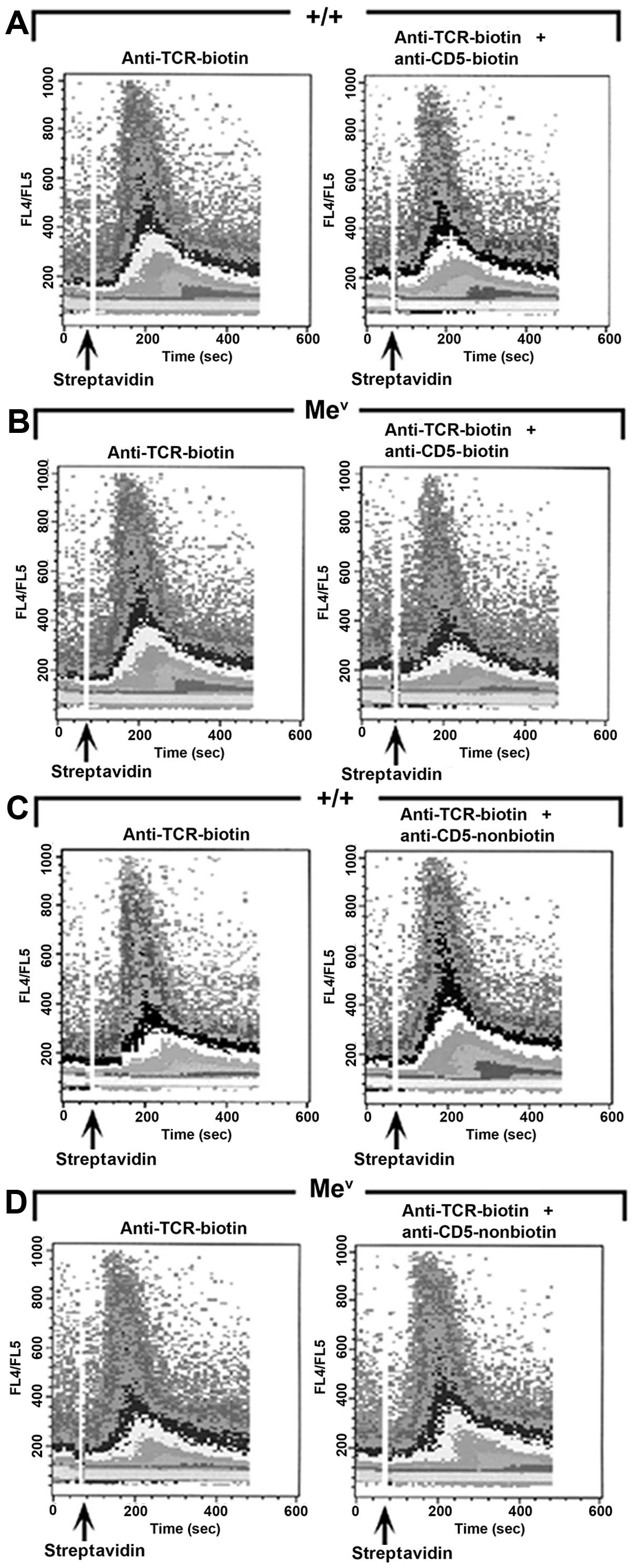
compared to wild-type mice. As expected, the co-ligation of TCR and
CD5 resulted in a slightly decreased or unchanged calcium influx in
wild-type thymocytes when compared to the ligation of TCR alone
(Figs. 6A and 5). Similarly, the inhibitory effects of
CD5 were intact and readily observable in the mev
mice (Fig. 6B). Although, the
initial upswing in calcium influx occurred at relatively the same
time after the addition of the crosslinking agent (~40 sec), there
was a definite decrease in the slope and amount of Ca2+
influx in thymocytes from SHP-1-deficient mice following CD5 and
TCR coligation versus TCR ligation alone (Fig. 6B), suggesting that the CD5
receptor retains functionality even in the absence of SHP-1
activity. We also examined, whether the calcium mobilization in
thymocytes could be enhanced by sequestering or inhibiting the
recruitment of CD5 to the antigen receptor complex (Fig. 5), as previously demonstrated by
Sen et al in B-1 cells (30). The separate crosslinking of the
TCR and CD5 resulted in a moderate increase in calcium
mobilization, as witnessed by a definite change in the slope and
amount of Ca2+ influx, in the wild-type and
mev thymocytes when compared to the ligation of
TCR alone (Fig. 6C and D). Our
results support a negative regulatory role for CD5 in thymocytes,
which is consistent with the observations made in CD5-deficient
thymocytes (15), and addresses
the involvement of SHP-1 in CD5 signaling.
CD5 levels influence positive and
negative selection in the thymus
A selection in thymocytes from CD5-deficient,
α/β-TCR transgenic mice has been shown to be altered in a manner
consistent with enhanced TCR signaling (15). As another tool for exploring the
functional and physiological role of CD5 in thymocyte development,
Azzam et al (3) generated
transgenic mice in which there is a T cell-specific, CD2
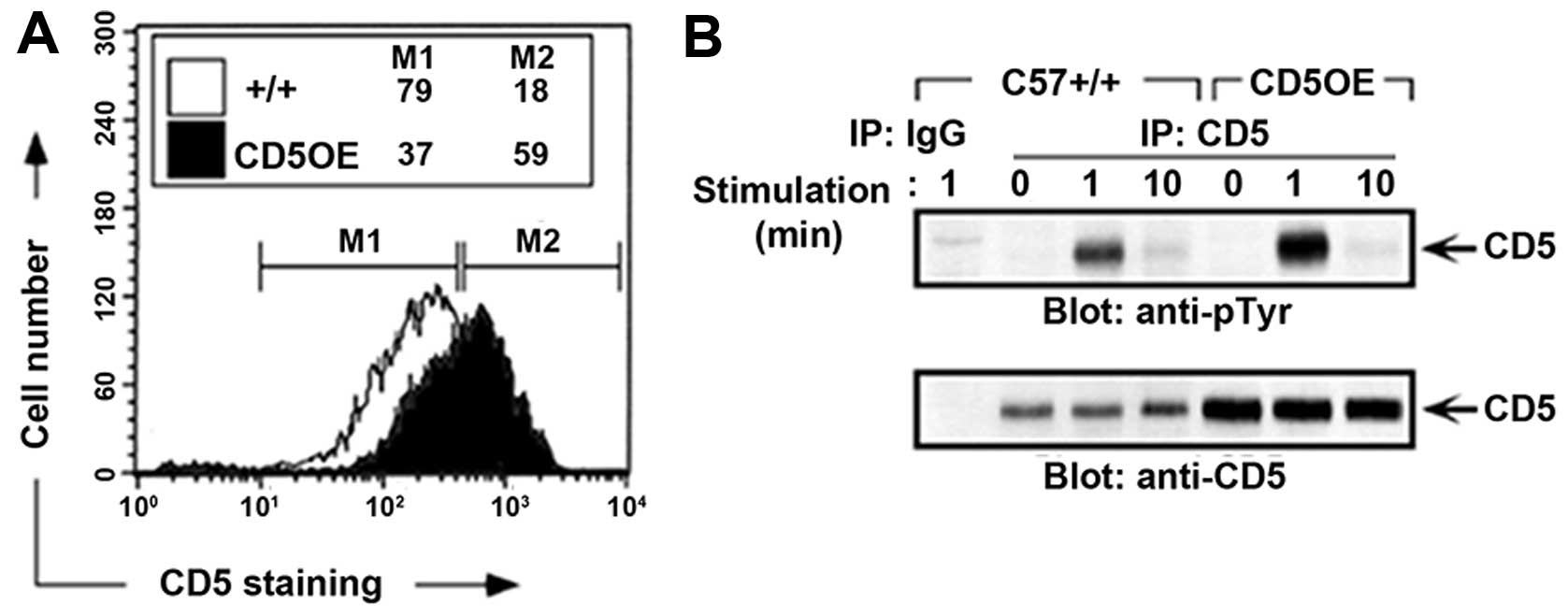
promoter/enhancer driven CD5 overexpression (CD5OE). CD5 surface
expression in thymocytes from CD5OE mice have an ~2.5-fold increase
in CD5 expression as compared to wild-type mice (Fig. 7). This increase in CD5 surface
expression does not alter the kinetics of TCR-mediated CD5
phosphorylation, except for the observed increase in intensity of
CD5 phosphorylation which is explained by the increase in CD5
protein levels (Fig. 7B). This
observation suggests that the increased CD5 in the CD5OE mice is
fully functional and participates in the CD5 signaling pathway. The
CD5OE mice were previously bred onto the H-Y TCR transgenic
background and shown to manifest a decrease in the positive and
negative selection, thus corroborating the inhibitory role for CD5
in modulating signaling thresholds in T-cell selection (26).
One of the physiologically relevant functions of CD5
is to modulate TCR-mediated signals involved in T-cell development
(3). Thus, we examined the
ability of CD5 to modulate the selection process in the absence of
SHP-1 activity to establish the physiological relevance of SHP-1 in
CD5 signaling. In addition to other authors, we previously
identified a role for SHP-1 in raising the signaling threshold
required for both positive and negative selection (26,34). Since CD5 and SHP-1 downregulate
signals involved in T-cell selection, we hypothesized that CD5
overexpression would not be able to downregulate selection in the
absence of SHP-1 activity, if SHP-1 is essential for CD5
function.
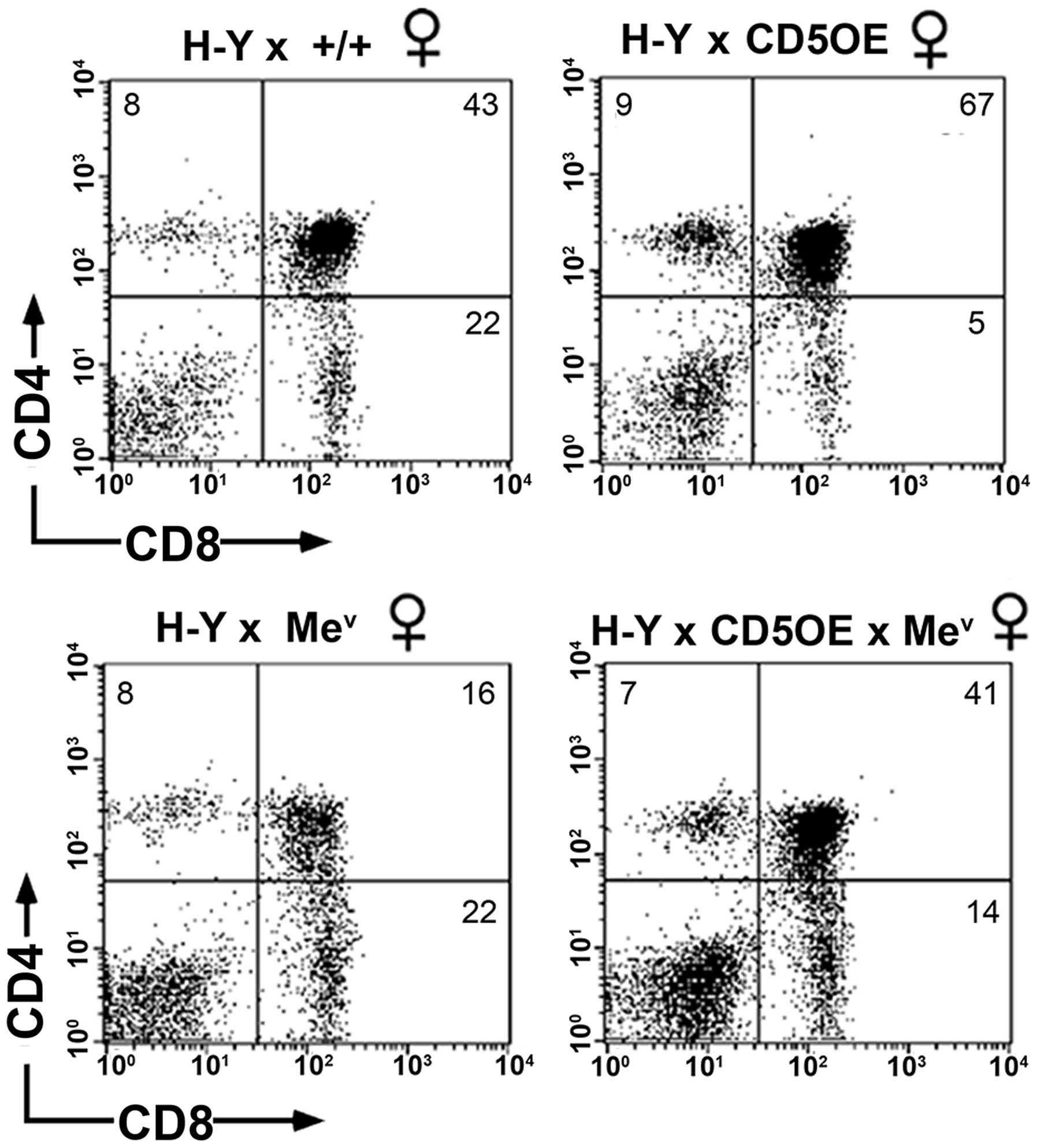
Positive selection is decreased in
wild-type and SHP-1 deficient CD5OE H-Y transgenic females
As shown earlier [Zhang et al (26), unpublished data], the inhibitory
effect of CD5 overexpression (CD5OE) on positive selection was
readily detected in female thymocytes from H-Y TCR/CD5OE compared
to HY-TCR transgenic mice (Fig.
8, upper panel). CD5 overexpression results in an increase in
the representation of DP cells as well as a decrease in the number
of SP CD8+ cells. The overexpression of CD5 in
SHP-1-deficient mev H-Y TCR transgenic mice also
increased the size of the DP population and decreased the number of
SP CD8+ cells compared to mev H-Y TCR
transgenic mice (Fig. 8, lower
panel). Therefore, the lack of SHP-1 activity does not impact on
the ability of CD5 to increase the threshold for TCR signaling.
This finding provides evidence in favor of an SHP-1-independent CD5
receptor capable of downregulating positive selection.
Negative selection is decreased in
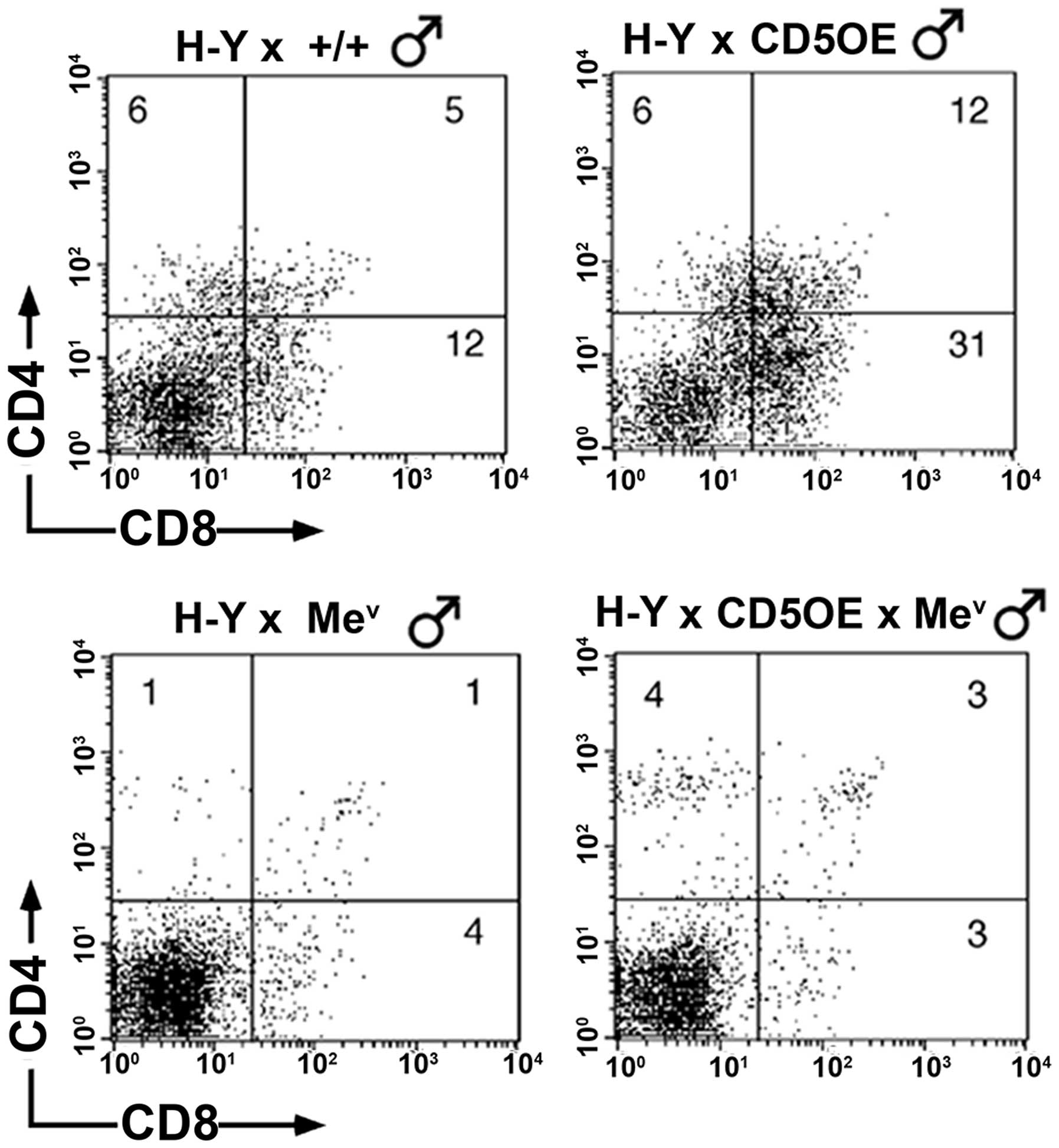
wild-type and SHP-1 deficient CD5OE H-Y transgenic males
As previously shown (26), the inhibitory effect of CD5
overexpression (CD5OE) on negative selection was also readily
detected in the current study, as revealed by the increased
representation of DP and SP CD8+ cells in the thymuses
of H-Y TCR/CD5 compared to H-Y TCR mice (Fig. 9, upper panel). Nevertheless, we
previously showed that the introduction of the
mev mutation nullifies the effects of CD5
overexpression, suggesting that SHP-1 deficiency is able to
counteract the inhibitory effects of CD5 overexpression on negative
selection (26), and raising the
possibility that SHP-1 activity is required for CD5 to realize its
inhibitory effects on negative selection. Careful re-examination of
these data in light of the findings presented above for positive
selection, suggests that the lack of any obvious and marked
decrease in negative selection conferred by CD5 overexpression is
due to the inability of CD5 to compensate for the increased
signaling (and negative selection) inherent in SHP-1 deficiency,
rather than due to a lack of CD5 function. Supporting this
hypothesis is the finding that negative selection in the thymi of
mev H-Y TCR/CD5OE transgenic mice reveal a
slight, albeit reproducible increase in the DP and SP
CD4+hi populations when compared with
mev H-Y TCR mice (Fig. 9 lower panel). The rescue of SP
CD4+ cells observed in the mev H-Y TCR
mice is noteworthy, particularly in light of the study by Page,
which found that CD5 was able to block MHC class II-dependent
negative selection of CD4+ cells rather than
CD8+ cells. The findings suggest that the effects of CD5
may be less profound on MHC class I-dependent negative selection
(45). It is therefore possible
that the enhanced TCR signaling imbued by SHP-1 deficiency
(26), results in the initial
rescue of a small population of CD4+ cells (even in the
absence of MHC class II antigen presentation) manifesting the
appropriate signals necessary for positive selection. Subsequently,
a majority of these cells undergo negative selection for the same
reasons as outlined above and it is only with CD5 overexpression
that this CD4+ population is rescued in the
mev H-Y TCR male mice (Fig. 9). One explanation for this result
is that when the analysis is restricted to a clonotypic TCR, a
partial reduction in signaling ability readily decreases the
signals from the positive selection interactions below the required
threshold. By contrast, when the negative selection stimulus is
strong enough, a partial reduction in signaling ability may not be
sufficient to interfere with this process. Thus, CD5 effects on
positive and negative T-cell selection are realized independently
of SHP-1.
Discussion
The role of CD5 signaling and function remains
elusive, although considerable evidence favors a negative
regulatory role for CD5 in thymocyte and B-1 cell signaling
(13). Previous results have
identified and implicated several cell effectors in the CD5
signaling pathway. For instance, in B cells CD5 constitutively
induces multiple signaling pathways such as extracellular
signal-regulated kinases (ERK1/2), phosphatidylinositol 3-kinase
(PI3K)/mammalian target of rapamycin (mTOR) and calcineurin-NFAT
signaling pathways (46).
However, the physiological and functional relevance of these
pathways to CD5 function are not completely understood.
The majority of studies examining the role of SHP-1
in CD5 signaling have been carried in vitro. To the best of
our knowledge, this is the first study to examine the relevance of
SHP-1 in CD5 signaling, by utilizing primary thymocytes from
SHP-1-deficient mev mice. Furthermore, given that
CD5 and SHP-1 negatively regulate thymocyte development (26,47), we have utilized an H-Y
TCR/CD5-overexpressing mouse model to elucidate the physiological
and functional importance of SHP-1 for mediating CD5-induced
downregulation of both positive and negative selection.
Given that the association of SHP-1 is increased
following CD5 phosphorylation, suggests an SH2-mediated binding
mechanism. The cytoplasmic domain of CD5 contains an ITAM-like
sequence, as well as sequences similar to motifs proposed as SHP-1
binding sites (47). By contrast,
studies by Gary-Gouy et al (31) and Peña-Rossi et al
(16) assessed the importance of
SHP-1 association for CD5 function. When Tyr-378 within the
ITIM-like sequence is omitted from the CD5 chimera, the negative
regulatory functions of CD5 were unaffected despite any observable
association with SHP-1. Similarly, CD5-deficient T-cell hybridomas
transfected with a truncated form of CD5 that retained the
ITIM-like sequence (Y378) were unable to negatively regulate TCR
responses or associate with SHP-1 (16). The findings suggest that the
ITIM-like sequence is not required for CD5 function as well as
questions the direct requirement of SHP-1 in the CD5 pathway. Based
on these findings, the interaction of SHP-1 with CD5 may be
indirect through other proteins that are recruited to
phosphorylated CD5. For example p56 Lck and the p85 regulatory
subunit of PI3K have been shown to associate with phosphorylated
CD5 following TCR activation as well as SHP-1 (48–50).
The reported interaction of SHP-1 with CD5 has led
to the speculation that CD5 is a substrate for CD5 activity. In
particular, studies examining CD2 and CD3 signaling in Jurkat T
cells found that the activity of SHP-1 increased following CD2
stimulation and decreased following TCR/CD3 activation (22). Furthermore, this change in SHP-1
activity correlated with the CD5 phosphorylation status, suggesting
that SHP-1 may specifically dephosphorylate CD5 (22). However, we did not detect any
significant changes in the CD5 phosphorylation profile of
SHP-1-deficient mice suggesting that SHP-1 is not the sole or major
phosphatase responsible for CD5 dephosphorylation.
Gary-Gouy et al in B lymphoma cell lines
suggested that the effect of CD5 at least on BCR signaling, is
independent of SHP-1 (31). In
order to determine whether SHP-1 is the major phosphatase
associated with CD5 in thymocytes, we immunoprecipitated CD5 from
resting and TCR-stimulated thymocytes from both normal and SHP-1
deficient (mev) mice. Our results suggest that
the in vitro phosphatase activity associated with CD5 is
unaffected by SHP-1 deficiency. In agreement with data from
Gary-Gouy et al (31) and
Peña-Rossi et al (16),
our findings, in thymocytes, also shed light on the importance of
SHP-1 activity for CD5 function. The presence of CD5-associated PTP
activity in mev suggests that there is a
redundancy with respect to SHP-1 function, or more likely, there
are other phosphatases capable of mediating the negative modulatory
effects of CD5.
To establish the functional importance of SHP-1 to
CD5-mediated negative signaling we examined the ability of CD5 to
modulate TCR-induced calcium mobilization profiles. We employed a
stimulation protocol (Fig. 5)
designed to assess whether the CD5 modulation of Ca2+
influx is preserved despite SHP-1 deficiency. The findings
demonstrate that even in the absence of SHP-1 activity we can
elicit changes in calcium mobilization profiles consistent with
that of a functioning CD5 receptor. These results support the
involvement of CD5 in Ca2+ mobilization, as previously
shown (51). Our stimulation
protocol suggests that the method of CD5 stimulation lead to
differential effects in thymocytes at least at the level of calcium
mobilization. It is noteworthy to determine whether this
differential effect of CD5, which most likely results from whether
CD5 is recruited or sequestered away from the antigen receptor, has
a physiological impact. In this regard, it is of note that two
recent studies examining thymic selection utilizing the same
anti-CD5 antibody (53–7.3) in their studies attribute differing
roles for CD5 in the negative selection (45,52). The study by Kishimoto and Sprent
demonstrates that CD5 costimulation is required for efficient
negative selection (52), while
Page demonstrates that CD5 prevents or reduces negative selection
(45). Although these studies do
not address the mechanism for their observed results, Kishimoto and
Sprent's experimental model CD5 (52) was presented in a cross-linked form
(i.e, in precoated wells), while in Page's model CD5 was presented
in an unbound form (45).
Consistent with the negative regulatory role for CD5 in thymocytes,
the observed differences in the two studies can be explained by the
following model. In the study by Kishimoto and Sprent, CD5
crosslinking to the well possibly prevents the recruitment of CD5
to the TCR resulting in enhanced signaling leading to increased
negative selection (52). By
contrast, in Page's study, although bound to anti-CD5 antibody, CD5
remained unanchored and free to associate with the TCR and was able
to downregulate negative selection (45). Furthermore, it is possible that
the anti-CD5 antibody, by blocking the interactions of CD5 with
physiological ligands such as CD5L (53), prevents the sequestration of CD5
away from the TCR.
Given that CD5 expression is developmentally
regulated by the strength and avidity of TCR signals (3), we examined whether the thymic
expression levels of CD5 were affected in mev
compared to wild-type mice. Although we did not observe any changes
in thymic CD5 surface expression levels, the reported elevation of
CD5 in SHP-1-deficient peripheral T cells is noteworthy (34). The elevation in CD5 surface levels
in light of SHP-1 deficiency suggests that CD5 may be functioning
to provide a compensatory mechanism for maintaining steady-state
levels of TCR signaling (34).
This compensatory model is further supported by the finding that
Vav-deficient mice, in which TCR signaling is inefficient, express
low surface levels of CD5 (34,54,55).
Given the importance of CD5 during T-cell
development we determined whether the effects of CD5 overexpression
were maintained in the absence of SHP-1. The results, were in
agreement with previous studies, demonstrating a role for CD5 in
downregulating the positive and negative selection (15,16), and reveal that CD5 overexpression
reduces positive and negative selection in wild-type and
mev mice. The ability of the overexpressed CD5 to
decrease the SP CD8+ population in SHP-1-deficient H-Y
TCR females indicates that CD5 retains an inhibitory function and
is able to increase the signaling threshold for positive selection.
Although the inhibitory effects of CD5 overexpression during
negative selection in mev thymocytes is not as
apparent as that observed in wild-type or positive selection. This
is not surprising given that signaling thresholds play a vital role
during thymic selection. In this regard, the TCR signals transduced
during the negative selection were much stronger than those in the
positive selection providing a mechanism for the deletion of
self-reactive thymocytes. Therefore, given the dominant role SHP-1
occupies in downregulating TCR signals, the lack of SHP-1 reduces
the signaling threshold considerably, thereby increasing the
strength of TCR signals in general and in particular during
negative selection. It is therefore highly unlikely that in this
context, the inhibitory actions of overexpressed CD5 may be
effective to the same degree in reducing negative selection.
Therefore, our observation is likely to reflect the inability of
CD5 to compensate for the increased signaling inherent in SHP-1
deficiency rather than a lack of CD5 function (26).
In the present study, we have examined the
functional and physiological requirement of SHP-1 in the CD5
signaling pathway. The results show that although SHP-1 can
associate with CD5, the tyrosine phosphorylation profile of CD5
following TCR stimulation is no different in SHP-1-deficient viable
motheaten (mev) compared to wild-type thymocytes.
The lack of SHP-1 activity also had no impact on levels of CD5
surface expression, CD5-associated PTP activity, and intracellular
calcium mobilization profiles following TCR/CD5 co-crosslinking.
Similarly, an analysis of T-cell thymocyte populations in
mev mice expressing an H-Y transgene as well as a
construct mediating T-cell restricted CD5 overexpression, revealed
that the reduction in selection conferred by CD5 over-expression
was unaffected by SHP-1 deficiency. Cumulatively, these
observations indicate that CD5 is not a SHP-1 substrate and suggest
SHP-1 is not required for and possibly not involved in CD5-mediated
downregulation of TCR signaling. By using an in vitro
stimulation protocol that crosslinks CD5/TCR together or
separately, we have also demonstrated a differential effect of CD5,
at least on TCR-mediated calcium influx.
Acknowledgments
The authors thank Giselle Knowles and Lingli Ma for
technical assistance and Denis Bouchard for assistance with
immunofluorescence analysis.
References
|
1
|
Martínez VG, Moestrup SK, Holmskov U,
Mollenhauer J and Lozano F: The conserved scavenger receptor
cysteine-rich super-family in therapy and diagnosis. Pharmacol Rev.
63:967–1000. 2011. View Article : Google Scholar
|
|
2
|
Mandl JN, Monteiro JP, Vrisekoop N and
Germain RN: T cell-positive selection uses self-ligand binding
strength to optimize repertoire recognition of foreign antigens.
Immunity. 38:263–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Azzam HS, Grinberg A, Lui K, Shen H,
Shores EW and Love PE: CD5 expression is developmentally regulated
by T cell receptor (TCR) signals and TCR avidity. J Exp Med.
188:2301–2311. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stamou P, de Jersey J, Carmignac D,
Mamalaki C, Kioussis D and Stockinger B: Chronic exposure to low
levels of antigen in the periphery causes reversible functional
impairment correlating with changes in CD5 levels in monoclonal CD8
T cells. J Immunol. 171:1278–1284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raman C: CD5, an important regulator of
lymphocyte selection and immune tolerance. Immunol Res. 26:255–263.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klinker MW and Lundy SK: Multiple
mechanisms of immune suppression by B lymphocytes. Mol Med.
18:123–137. 2012. View Article : Google Scholar :
|
|
7
|
Mage RG and Pospisil R: CD5 and other
superantigens may select and maintain rabbit self-renewing
B-lymphocytes and human B-CLL cells. Curr Top Microbiol Immunol.
252:87–96. 2000.PubMed/NCBI
|
|
8
|
Mamonkin M, Rouce RH, Tashiro H and
Brenner MK: A T-cell-directed chimeric antigen receptor for the
selective treatment of T-cell malignancies. Blood. 126:983–992.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheng JR, Quan S and Soliven B:
CD1d(hi)CD5+ B cells expanded by GM-CSF in vivo suppress
experimental autoimmune myasthenia gravis. J Immunol.
193:2669–2677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beaudette-Zlatanova BC, Le PT, Knight KL,
Zhang S, Zakrzewski S, Parthasarathy M and Stiff PJ: A potential
role for B cells in suppressed immune responses in cord blood
transplant recipients. Bone Marrow Transplant. 48:85–93. 2013.
View Article : Google Scholar
|
|
11
|
Spertini F, Stohl W, Ramesh N, Moody C and
Geha RS: Induction of human T cell proliferation by a monoclonal
antibody to CD5. J Immunol. 146:47–52. 1991.PubMed/NCBI
|
|
12
|
Alberola-Ila J, Places L, Cantrell DA,
Vives J and Lozano F: Intracellular events involved in CD5-induced
human T cell activation and proliferation. J Immunol.
148:1287–1293. 1992.PubMed/NCBI
|
|
13
|
Ochi H and Watanabe T: Negative regulation
of B cell receptor-mediated signaling in B-1 cells through CD5 and
Ly49 co-receptors via Lyn kinase activity. Int Immunol.
12:1417–1423. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fenutría R, Martinez VG, Simões I, Postigo
J, Gil V, Martínez-Florensa M, Sintes J, Naves R, Cashman KS,
Alberola-Ila J, et al: Transgenic expression of soluble human CD5
enhances experimentally-induced autoimmune and anti-tumoral immune
responses. PLoS One. 9:e848952014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tarakhovsky A, Kanner SB, Hombach J,
Ledbetter JA, Müller W, Killeen N and Rajewsky K: A role for CD5 in
TCR-mediated signal transduction and thymocyte selection. Science.
269:535–537. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peña-Rossi C, Zuckerman LA, Strong J, Kwan
J, Ferris W, Chan S, Tarakhovsky A, Beyers AD and Killeen N:
Negative regulation of CD4 lineage development and responses by
CD5. J Immunol. 163:6494–6501. 1999.PubMed/NCBI
|
|
17
|
Dennehy KM, Ferris WF, Veenstra H,
Zuckerman LA, Killeen N and Beyers AD: Determination of the
tyrosine phosphorylation sites in the T cell transmembrane
glycoprotein CD5. Int Immunol. 13:149–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iwai LK, Benoist C, Mathis D and White FM:
Quantitative phosphoproteomic analysis of T cell receptor signaling
in diabetes prone and resistant mice. J Proteome Res. 9:3135–3145.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beyers AD, Spruyt LL and Williams AF:
Molecular associations between the T-lymphocyte antigen receptor
complex and the surface antigens CD2, CD4, or CD8 and CD5. Proc
Natl Acad Sci USA. 89:2945–2949. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Unkeless JC and Jin J: Inhibitory
receptors, ITIM sequences and phosphatases. Curr Opin Immunol.
9:338–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perez-Villar JJ, Whitney GS, Bowen MA,
Hewgill DH, Aruffo AA and Kanner SB: CD5 negatively regulates the
T-cell antigen receptor signal transduction pathway: Involvement of
SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol.
19:2903–2912. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carmo AM, Castro MA and Arosa FA: CD2 and
CD3 associate independently with CD5 and differentially regulate
signaling through CD5 in Jurkat T cells. J Immunol. 163:4238–4245.
1999.PubMed/NCBI
|
|
23
|
Tibaldi E, Brunati AM, Zonta F, Frezzato
F, Gattazzo C, Zambello R, Gringeri E, Semenzato G, Pagano MA and
Trentin L: Lyn-mediated SHP-1 recruitment to CD5 contributes to
resistance to apoptosis of B-cell chronic lymphocytic leukemia
cells. Leukemia. 25:1768–1781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kisielow P, Blüthmann H, Staerz UD,
Steinmetz M and von Boehmer H: Tolerance in T-cell-receptor
transgenic mice involves deletion of nonmature
CD4+8+ thymocytes. Nature. 333:742–746. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Love PE, Shores EW, Lee EJ, Grinberg A,
Munitz TI, Westphal H and Singer A: Differential effects of zeta
and eta transgenes on early alpha/beta T cell development. J Exp
Med. 179:1485–1494. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Somani AK, Yuen D, Yang Y, Love
PE and Siminovitch KA: Involvement of the SHP-1 tyrosine
phosphatase in regulation of T cell selection. J Immunol.
163:3012–3021. 1999.PubMed/NCBI
|
|
27
|
Kozlowski M, Mlinaric-Rascan I, Feng GS,
Shen R, Pawson T and Siminovitch KA: Expression and catalytic
activity of the tyrosine phosphatase PTP1C is severely impaired in
motheaten and viable motheaten mice. J Exp Med. 178:2157–2163.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pani G, Fischer KD, Mlinaric-Rascan I and
Siminovitch KA: Signaling capacity of the T cell antigen receptor
is negatively regulated by the PTP1C tyrosine phosphatase. J Exp
Med. 184:839–852. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bikah G, Carey J, Ciallella JR,
Tarakhovsky A and Bondada S: CD5-mediated negative regulation of
antigen receptor-induced growth signals in B-1 B cells. Science.
274:1906–1909. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sen G, Bikah G, Venkataraman C and Bondada
S: Negative regulation of antigen receptor-mediated signaling by
constitutive association of CD5 with the SHP-1 protein tyrosine
phosphatase in B-1 B cells. Eur J Immunol. 29:3319–3328. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gary-Gouy H, Bruhns P, Schmitt C, Dalloul
A, Daëron M and Bismuth G: The pseudo-immunoreceptor tyrosine-based
activation motif of CD5 mediates its inhibitory action on B-cell
receptor signaling. J Biol Chem. 275:548–556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dennehy KM, Broszeit R, Ferris WF and
Beyers AD: Thymocyte activation induces the association of the
proto-oncoprotein c-cbl and ras GTPase-activating protein with CD5.
Eur J Immunol. 28:1617–1625. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weiss A, Dazin PF, Shields R, Fu SM and
Lanier LL: Functional competency of T cell antigen receptors in
human thymus. J Immunol. 139:3245–3250. 1987.PubMed/NCBI
|
|
34
|
Johnson KG, LeRoy FG, Borysiewicz LK and
Matthews RJ: TCR signaling thresholds regulating T cell development
and activation are dependent upon SHP-1. J Immunol. 162:3802–3813.
1999.PubMed/NCBI
|
|
35
|
Imboden JB, Weiss A and Stobo JD: The
antigen receptor on a human T cell line initiates activation by
increasing cytoplasmic free calcium. J Immunol. 134:663–665.
1985.PubMed/NCBI
|
|
36
|
Imboden JB and Stobo JD: Transmembrane
signalling by the T cell antigen receptor. Perturbation of the
T3-antigen receptor complex generates inositol phosphates and
releases calcium ions from intracellular stores. J Exp Med.
161:446–456. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ledbetter JA, June CH, Martin PJ, Spooner
CE, Hansen JA and Meier KE: Valency of CD3 binding and
internalization of the CD3 cell-surface complex control T cell
responses to second signals: Distinction between effects on protein
kinase C, cytoplasmic free calcium, and proliferation. J Immunol.
136:3945–3952. 1986.PubMed/NCBI
|
|
38
|
Altman A, Coggeshall KM and Mustelin T:
Molecular events mediating T cell activation. Adv Immunol.
48:227–360. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hardy RR and Hayakawa K: Development and
physiology of Ly-1 B and its human homolog, Leu-1 B. Immunol Rev.
93:53–79. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
June CH, Rabinovitch PS and Ledbetter JA:
CD5 antibodies increase intracellular ionized calcium concentration
in T cells. J Immunol. 138:2782–2792. 1987.PubMed/NCBI
|
|
41
|
Gupta S: Mechanisms of transmembrane
signalling in human T cell activation. Mol Cell Biochem. 91:45–50.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vandenberghe P, Verwilghen J, Van Vaeck F
and Ceuppens JL: Ligation of the CD5 or CD28 molecules on resting
human T cells induces expression of the early activation antigen
CD69 by a calcium- and tyrosine kinase-dependent mechanism.
Immunology. 78:210–217. 1993.PubMed/NCBI
|
|
43
|
Simarro M, Calvo J, Vilà JM, Places L,
Padilla O, Alberola-Ila J, Vives J and Lozano F: Signaling through
CD5 involves acidic sphingomyelinase, protein kinase C-zeta,
mitogen-activated protein kinase kinase, and c-Jun NH2-terminal
kinase. J Immunol. 162:5149–5155. 1999.PubMed/NCBI
|
|
44
|
Osman N, Ley SC and Crumpton MJ: Evidence
for an association between the T cell receptor/CD3 antigen complex
and the CD5 antigen in human T lymphocytes. Eur J Immunol.
22:2995–3000. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Page DM: Cutting edge: Thymic selection
and autoreactivity are regulated by multiple coreceptors involved
in T cell activation. J Immunol. 163:3577–3581. 1999.PubMed/NCBI
|
|
46
|
Mageed RA, Garaud S, Taher TE, Parikh K,
Pers JO, Jamin C, Renaudineau Y and Youinou P: CD5 expression
promotes multiple intracellular signaling pathways in B lymphocyte.
Autoimmun Rev. 11:795–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Amantini C, Mosca M, Lucciarini R, Perfumi
MC and Santoni G: Thiorphan-induced survival and proliferation of
rat thymocytes by activation of Akt/survivin pathway and inhibition
of caspase-3 activity. J Pharmacol Exp Ther. 327:215–225. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dennehy KM, Broszeit R, Garnett D,
Durrheim GA, Spruyt LL and Beyers AD: Thymocyte activation induces
the association of phosphatidylinositol 3-kinase and pp120 with
CD5. Eur J Immunol. 27:679–686. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cuevas B, Lu Y, Watt S, Kumar R, Zhang J,
Siminovitch KA and Mills GB: SHP-1 regulates Lck-induced
phosphatidylinositol 3-kinase phosphorylation and activity. J Biol
Chem. 274:27583–27589. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Imani F, Rager KJ, Catipovic B and Marsh
DG: Interleukin-4 (IL-4) induces phosphatidylinositol 3-kinase
(p85) dephosphorylation. Implications for the role of SHP-1 in the
IL-4-induced signals in human B cells. J Biol Chem. 272:7927–7931.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Antony P, Petro JB, Carlesso G, Shinners
NP, Lowe J and Khan WN: B cell receptor directs the activation of
NFAT and NF-kappaB via distinct molecular mechanisms. Exp Cell Res.
291:11–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kishimoto H and Sprent J: Several
differenT cell surface molecules control negative selection of
medullary thymocytes. J Exp Med. 190:65–73. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sanjurjo L, Amézaga N, Aran G,
Naranjo-Gómez M, Arias L, Armengol C, Borràs FE and Sarrias MR: The
human CD5L/AIM-CD36 axis: A novel autophagy inducer in macrophages
that modulates inflammatory responses. Autophagy. 11:487–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Turner M, Mee PJ, Walters AE, Quinn ME,
Mellor AL, Zamoyska R and Tybulewicz VL: A requirement for the
Rho-family GTP exchange factor Vav in positive and negative
selection of thymocytes. Immunity. 7:451–460. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tanaka Y, So T, Lebedeva S, Croft M and
Altman A: Impaired IL-4 and c-Maf expression and enhanced Th1-cell
development in Vav1-deficient mice. Blood. 106:1286–1295. 2005.
View Article : Google Scholar : PubMed/NCBI
|