Introduction
Osteoarthritis (OA) is a widely prevalent
age-associated joint disorder characterized by the progressive
destruction of cartilage and is responsible for the deterioration
of life quality and economic burden in the elderly population
worldwide (1,2). It is known that OA involves all
structures in the affected joints, resulting in joint instability,
pain, and loss of functions (3,4).
Chondrocyte is the only cell type present in articular cartilage
and plays a critical role in maintaining the dynamic equilibrium
between anabolism and catabolism of the extracellular matrix (ECM)
under physiological circumstances, which is crucial for joint
function (5).
OA has various underlying pathogenic mechanisms that
are not fully understood. Apoptosis, or programmed cell death, has
been connected with OA (6,7).
It is reported that OA cartilage has a higher proportion of
apoptotic chondrocytes than normal tissue and apoptotic cells are
located in the superficial and middle zones (6). Previous findings suggested that
chondrocyte apoptosis plays a key role in cartilage degradation and
revealed a significant correlation between apoptosis and the
severity of OA progression, indicating that inhibition of
chondrocyte apoptosis may be an important strategy for OA treatment
(7).
MicroRNAs consist of a class of single-stranded
non-coding RNAs of 18–24 nucleotides that are evolutionarily
conserved (8). MicroRNAs can
regulate gene expression by binding to the 3′-untranslated region
(3′-UTR) of their target mRNAs, leading to post-transcriptional
repression or mRNA cleavage (9,10).
MicroRNAs are involved in a number of physiological functions and
disease processes, and a growing body of evidence has elucidated
the physiologic and pathogenetic role of microRNAs in the
maintenance of joint homeostasis and the development of OA, such as
inflammation, cellular communication and cell death (11,12). MicroRNA-34a (miR-34a) is important
as a tumor suppressor gene in downregulating its target genes.
miR-34a is also involved in p53-induced cell cycle arrest, cell
senescence, apoptosis and other biological behaviors (13,14).
Silent information regulator 1 (SIRT1) is a
conserved nicotinamide adenine dinucleotide
(NAD+)-dependent deacety lase that plays a crucial role
in apoptosis, cell senescence, inflammation and tumorigenesis by
deacetylating important transcriptional factors, including p53,
FOXO and p65 (15–17).
In a previous study, silencing of miR-34a
effectively reduced rat chondrocyte apoptosis and the
downregulation of Col2a1 induced by IL-1β (18). However, the underlying mechanism
and the role of miR-34a in the progression of OA remain to be
determined. Additionally, the overexpression of SIRT1 protects
chondrocytes from osteoarthritic change induced by IL-1β (19). miR-34a is reported to induce
apoptosis in human colon cancer cells by inhibiting SIRT1 (20). Thus, in the current study, we
hypothesized whether miR-34a was able to promote apoptosis in human
chondrocyte by targeting the SIRT1/p53 signaling pathway.
First, we found an increased miR-34a expression and
a decreased level of SIRT1 in human OA chondrocytes. Second, the
overexpression of miR-34a induced apoptosis and inhibited cell
proliferation in human chondrocytes. In addition, miR-34a repressed
the expression of SIRT1 by binding 3′-UTR of SIRT1 mRNA, which
inhibited the deacetylation of p53, leading to the regulation of
downstream genes Bax and Bcl-2. Intra-articular
injection of lentivirus encoding anti-miR-34a oligonucleotide
ameliorated the progression of OA induced by surgery in rats. These
findings indicated that miR-34a is a promising gene therapeutic
target in OA.
Materials and methods
Patients
The protocol was approved by the Institutional
Review Board of Tangdu Hospital (approval ID no. TDLL-2013009)
(Shaanxi, China) and written informed consent was obtained from all
the participating patients. OA articular cartilage samples were
collected from the femoral condyles and tibial plateaus of 12
patients with primary OA undergoing total knee arthroplasty (TKA)
(mean age ± SD, 63.1±9.1 years) at the Department of Orthopedics of
Tangdu Hospital. Healthy cartilage samples were obtained from 10
trauma patients with no history of OA or other joint diseases
(46.8±12.7 years).
Isolation and primary culture of human
articular chondrocytes
To isolate primary human chondrocytes, cartilage
specimens were dissected into small sections and subjected to
sequential digestion with 0.1% trypsin (Invitrogen, Carlsbad, CA,
USA) for 30 min and then with 0.2% collagenase Type II (Millipore
Corp., Billerica, MA, USA) in Dulbecco's modified Eagle's medium
(DMEM) (Gibco) for 10 h at 37°C. Isolated chondrocytes were
filtered through 100-µm nylon filters. The cells were seeded
in culture flasks in DMEM/F12 medium (Gibco) supplemented with 10%
fetal bovine serum (FBS) (HyClone, Thermo Fisher Scientific,
Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin (Gibco), and incubated at 37°C in a humidified
atmosphere with 5% CO2.
Primary chondrocytes were used to compare gene
expression levels in normal and OA chondrocytes. The cells from
passages 1 to 2 were used for the subsequent experiments.
RNA extraction and measurement of mRNA
and microRNA expression
Total RNA was extracted from primary chondrocytes
using TRIzol reagent (Invitrogen) according to the manufacturer's
instructions. RNA was reverse-transcribed to generate first-strand
complementary DNA (cDNA) using high-capacity cDNA reverse
transcription kits (Applied Biosystems Life Technologies, Foster
City, CA, USA). Real-time PCR was performed with the Power
SYBR-Green PCR Master Mix and 7900 HT Fast Real-Time PCR system
(both from Applied Biosystems, Carlsbad, CA, USA). The data were
given as a threshold cycle (Ct). The levels of mRNA were normalized
to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA controls
using the comparative 2−ΔΔCt method. The specific
primers used for different mRNAs were: for human SIRT1,
5′-TGGCAAAGGAGCAGATTAGT AGG-3′ (forward) and 5′-CTGCCACAAGAACTAGAGG
ATAAGA-3′ (reverse); for human GAPDH, 5′-ATTCCACCC ATGGCAAATTC-3′
(forward) and 5′-TGGGATTTCCAT TGATGACAAG-3′ (reverse).
To evaluate the miR-34a expression levels, total RNA
was reverse transcribed into cDNA using the TaqMan MicroRNA Reverse
Transcription kit and TaqMan MicroRNA assays (Applied Biosystems)
according to the manufacturer's instructions. The small nuclear RNA
U6 (RNU6) was used as an endogenous control for miRNA
detection.
Transfection of microRNA
miR-34a mimic, miR-34a inhibitor and non-specific
negative control (NC) were synthesized and purified by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). There was a fluorescent FAM
label linked with the 5′ terminal of miR-34a mimic, which was used
for transfection validation. When the chondrocytes were grown to
80% confluence, miR-34a mimic, miR-34a inhibitor or non-specific
negative oligonucleotides were transfected at a working
concentration of 100 nmol/l using Lipofectamine 2000 (Invitrogen)
according to the manufacturer's instructions. Twenty-four hours
after incubation, the transfection efficiency was observed using a
fluorescence microscope (Olympus Co., Ltd., Beijing, China) and
miR-34a expression level was evaluated by quantitative PCR.
In vitro cell proliferation assay
Cell proliferation was measured with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltet-razolium bromide (MTT)
assay. Chondrocytes were seeded in 96-well plates at a density of
5×103 cells/well containing 100 µl of culture
medium. The cells were transfected with 100 nM miR-34a mimic,
miR-34a inhibitor or non-specific NC for 6 h. After transfection,
20 µl of MTT (5 mg/ml) (Sigma-Aldrich, St. Louis, MO, USA)
was added every 24 h, followed by incubation for another 4 h at
37°C. The medium was removed, and 100 µl of dimethyl
sulfoxide was added to each well to dissolve the formazan. The
optical density (OD) was evaluated by measuring the absorbance at
the wavelength of 490 nm, with a reference wavelength of 630 nm,
using a microplate reader (Multiskan Ascent 354; Thermo Labsystems,
Vantaa, Finland).
Apoptosis assay
The apoptotic rate of chondrocytes was detected and
quantified by flow cytometry. Briefly, chondrocytes were
transfected with 100 nM miR-34a mimic, miR-34a inhibitor or NC.
Forty-eight hours after transfection, 1×105-treated
cells of each group were collected, washed and incubated with
Annexin V-FITC and propidium iodide (PI) (both from Millipore
Corp.) for 15 min at a room temperature of 20°C in the dark. The
cells were analyzed using a fluorescence-activated cell sorting
(FACS) flow cytometer (BD Biosciences, San Diego, CA, USA).
Western blot analysis
Total proteins were extracted from the cells using
RIPA buffer (Beyotime Institute of Biotechnology, Shanghai, China)
with enzyme inhibitor cocktail (Complete; Roche, Basel,
Switzerland). After being lysed on ice for 30 min, the lysate was
centrifuged at 13,000 × g for 20 min, and the supernatant was
collected for experiments. For the western blot analysis, 25
µg of extracts were separated using 10% SDS-polyacrylamide
gel electrophoresis, transferred to a polyvinylidene difluoride
membrane (Millipore Corp., Bedford, MA, USA) and incubated with
anti-SIRT1 (mouse monoclonal antibody; 1:1,000; Cat. no. 8469),
acetylated-p53 (Lys382; rabbit polyclonal antibody; 1:1,000; Cat.
no. 2525), p53 (rabbit monoclonal antibody; 1:1,000, Cat. no.
2527), Bax (rabbit monoclonal antibody; 1:1,000; Cat. no. 5023),
Bcl-2 (rabbit monoclonal antibody; 1:1,000; Cat. no. 4223) (Cell
Signaling Technology, Inc., Danvers, MA, USA) and anti-GAPDH (mouse
monoclonal antibody; 1:500; Cat. no. sc-365062; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) antibodies for 24 h at
4°C. GAPDH was used as internal control. The blots were developed
using a chemiluminescent substrate kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Intensity of the bands was detected and
analyzed using Quantity One analyzing system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Luciferase reporter assay
Total RNA (1 µg) from human chondrocytes was
reverse-transcribed into cDNA, and the SIRT1 3′-UTR was amplified
using the primers: 5′-ATAGGC CGGCATAGACGCGTTGTAATAATTGTGCAGGTAC
AGG-3′ (forward) and 5′-AAAGATCCTTTATTAAGCTTA
AGTTAACAGAAAAAAGTCAAATGAC-3′ (reverse). The 3′-UTR of SIRT1 (1,796
bp) containing the miR-34a binding sites was cloned into the
HindIII and MluI sites of the pMIR-Report Luciferase
vector (Ambion, Austin, TX, USA) downstream of the firefly
luciferase gene to develop the wild-type 3′-UTR luciferase reporter
vector. The mutant 3′-UTR luciferase reporter vector was generated
by site-directed mutagenesis using the QuikChange Site-Directed
Mutagenesis kit (Stratagene, La Jolla, CA, USA). Human chondrocytes
were plated in 24-well plates. The cells at 70–80% confluence were
co-transfected with wild-type or mutant-type 3′-UTR-Luc reporter
vector plus miR-34a mimic with Lipofectamine 2000 (Invitrogen). The
pRL-CMV Renilla luciferase vector (Promega Corp., Madison,
WI, USA) was used to normalize cell numbers and transfection
efficiency. After an additional 48 h, the luciferase activity was
measured using the dual luciferase assay (Promega Corp.) according
to the manufacturer's instructions.
Construction of lentivirus vector
Recombinant lentivirus vector encoding antisense
miR-34a (anti-miR-34a) or negative control (miR-NC) were
constructed and purchased from GeneChem Co., Ltd. (Shanghai,
China). Briefly, the oligonucleotides of antisense miR-34a
inhibitor and NC were cloned into the lentivirus expression vector
of hU6-MCS-CMV-EGFP (GeneChem Co., Ltd.). The recombinational and
packaging vectors pHelper 1.0 and 2.0 (GeneChem Co., Ltd.) were
co-transfected into 293T cells with Lipofectamine 2000 to produce
viral particles of miR-34a antisense inhibitor and NC.
Animal model of OA
Experiments were performed according to the protocol
approved by the Institutional Review Board of Tangdu Hospital and
the study was conducted in full compliance with the Declaration of
Helsinki and Institutional Animal Care Standards. Twenty-eight
10-week-old male Sprague-Dawley rats (obtained from the
Experimental Animal Center of Fourth Military Medical University)
were used in the subsequent experiments. The rats were randomly
divided into 4 groups (n=7 each): anti-miR-34a, non-specific NC,
Sham and No Surgery. For the Sham group, identical surgical
procedures were performed, although the ligaments and medial
menisci were kept intact. The No Surgery group did not undergo an
operation. The animals were anesthetized with 3% pento barbital
sodium (Cat. no. 4579; Tocris, Bristol, UK). Briefly, a 10-mm
medial parapatellar incision over the distal patella to proximal
tibial plateau was made on the right knee joint of rat.
Experimental OA was induced through anterior cruciate ligament
transection and medial meniscus resection (ACLT+MMx) as previously
described (21). The rats were
allowed to move freely within the cages after surgery. Two weeks
after surgery, the rats in the anti-miR-34a and NC groups received
an intra-articular injection of 1×109 plaque forming
units (PFU) of lentivirus vector encoding antisense miR-34a or
non-specific control diluted in 100 µl phosphate-buffered
saline (PBS), respectively. However, the Sham and No Surgery groups
received the same dose of normal saline.
Histological assessments
Ten weeks after surgery, the animals were sacrificed
by cervical dislocation under 3% pentobarbital sodium narcosis and
the whole joints were harvested. Tissues were routinely fixed in
10% buffered neutral formalin for 24 h, decalcified in 10%
ethylenediaminetetraacetic acid (EDTA) (pH 7.2) for 4 weeks,
embedded into paraffin, and coronally sectioned at 6 µm. The
sections were deparaffinized in xylene, hydrated with graded
ethanol, and stained with Safranin O and Fast Green. Histological
evaluation was performed according to the modified Mankin's score,
as previously reported (22).
Statistical analysis
The continuous variables were presented as mean ±
standard deviation (SD). Statistical analysis was carried out using
SPSS 20.0 (SPSS, Inc., Chicago, IL, USA). Comparisons between
groups were made using the Student's t-test. One-way analysis of
variance (ANOVA) followed by post hoc least significant difference
(LSD) t-test was used for difference analysis in the multiple
groups. Mankin's scores were evaluated using non-parametric
statistical analyses. Results with values of p<0.05 were
considered significant.
Results
Expression of miR-34a and SIRT1 in
healthy and OA human chondrocytes
To assess the potential involvement of miR-34a and
SIRT1 in the OA process, we initially evaluated the expression
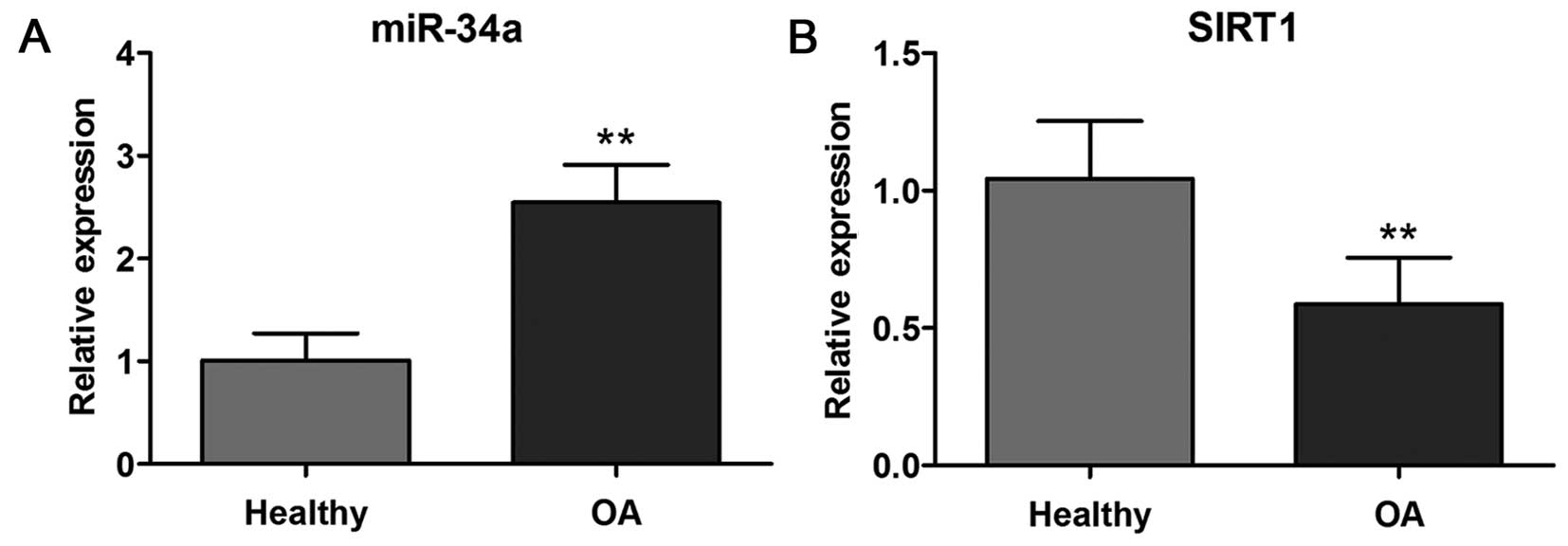
levels between healthy and OA cartilage. As shown in Fig. 1A, miR-34a expression was
significantly (p<0.01) increased in OA cartilage compared with
healthy cartilage. Conversely, the expression level of SIRT1 in
cartilage from OA patients was decreased (p<0.01) compared with
healthy cartilage confirmed by quantitative PCR (Fig. 1B).
Confirmation of miR-34a oligonucleotide
transfection into human chondrocytes
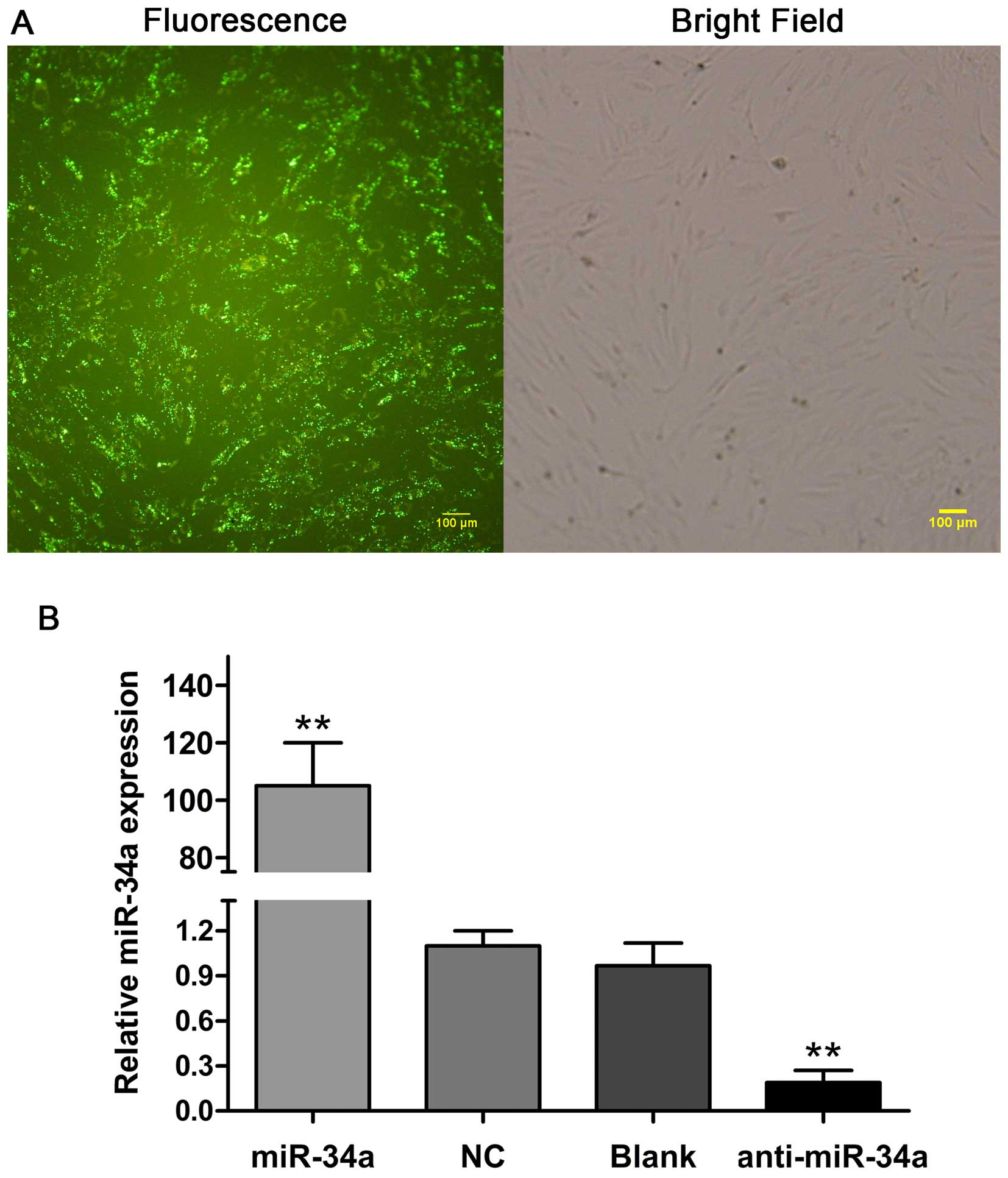
To investigate the function of miR-34a in OA, we
transfected human chondrocytes with miR-34a mimic, miR-34a
inhibitor and negative oligonucleotides, respectively. At 24 h
after transfection, the fluorescence microscopy image showed that
the transfection efficiency of miR-34a oligonucleotides into human
chondrocytes reached >80% (Fig.
2A). Compared with the NC and blank control (Blank) groups, the
miR-34a level in the miR-34a group was elevated by ~105-fold
(p<0.01). By contrast, the treatment of chondrocytes with
miR-34a inhibitor decreased miR-34a expression by 80% (p<0.01).
No statistical significance of miR-34a expression was identified
between the NC and Blank groups (Fig.
2B).
miR-34a inhibits the proliferation of
chondrocytes in vitro
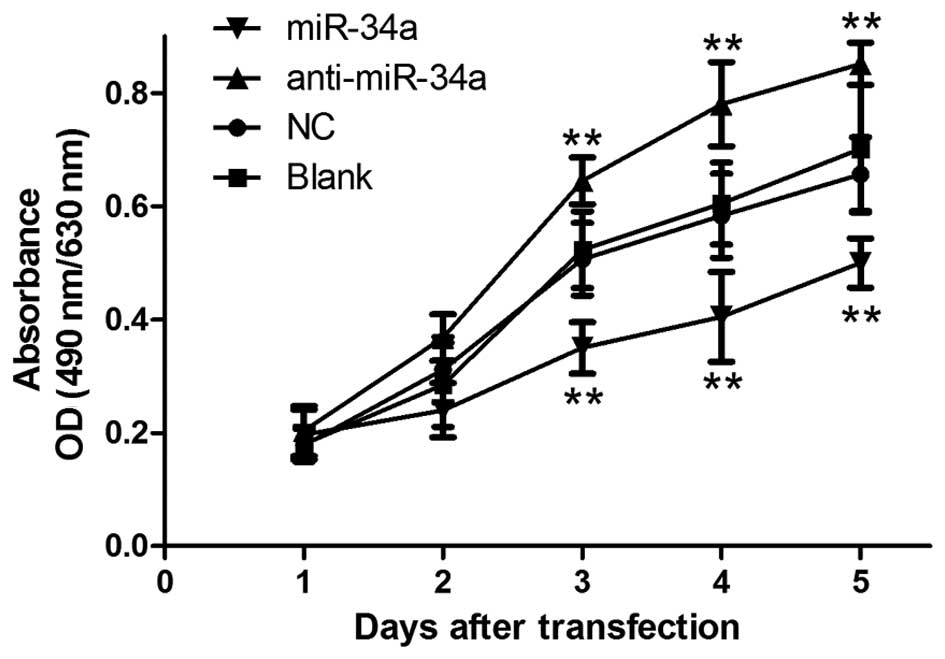
As miR-34a was markedly increased in OA chondrocyte,
it may function as a promoter of OA. Therefore, the MTT assay was
performed and proliferation curve was determined to examine the
effects of miR-34a on the proliferation of human chondrocytes.
Fig. 3 shows that chondrocytes
transfected with miR-34a mimic exhibited a significant decrease in
proliferation capacity compared with cells in the NC and Blank
groups (p<0.01). By contrast, when transfected with the miR-34a
inhibitor, chondrocytes grew at a higher rate (p<0.01). No
statistical significance was observed in the proliferation rate
between the NC and Blank groups. The results indicated that
overexpression of miR-34a inhibited the growth of human
chondrocytes in vitro.
miR-34a regulates apoptosis of human
chondrocytes in vitro
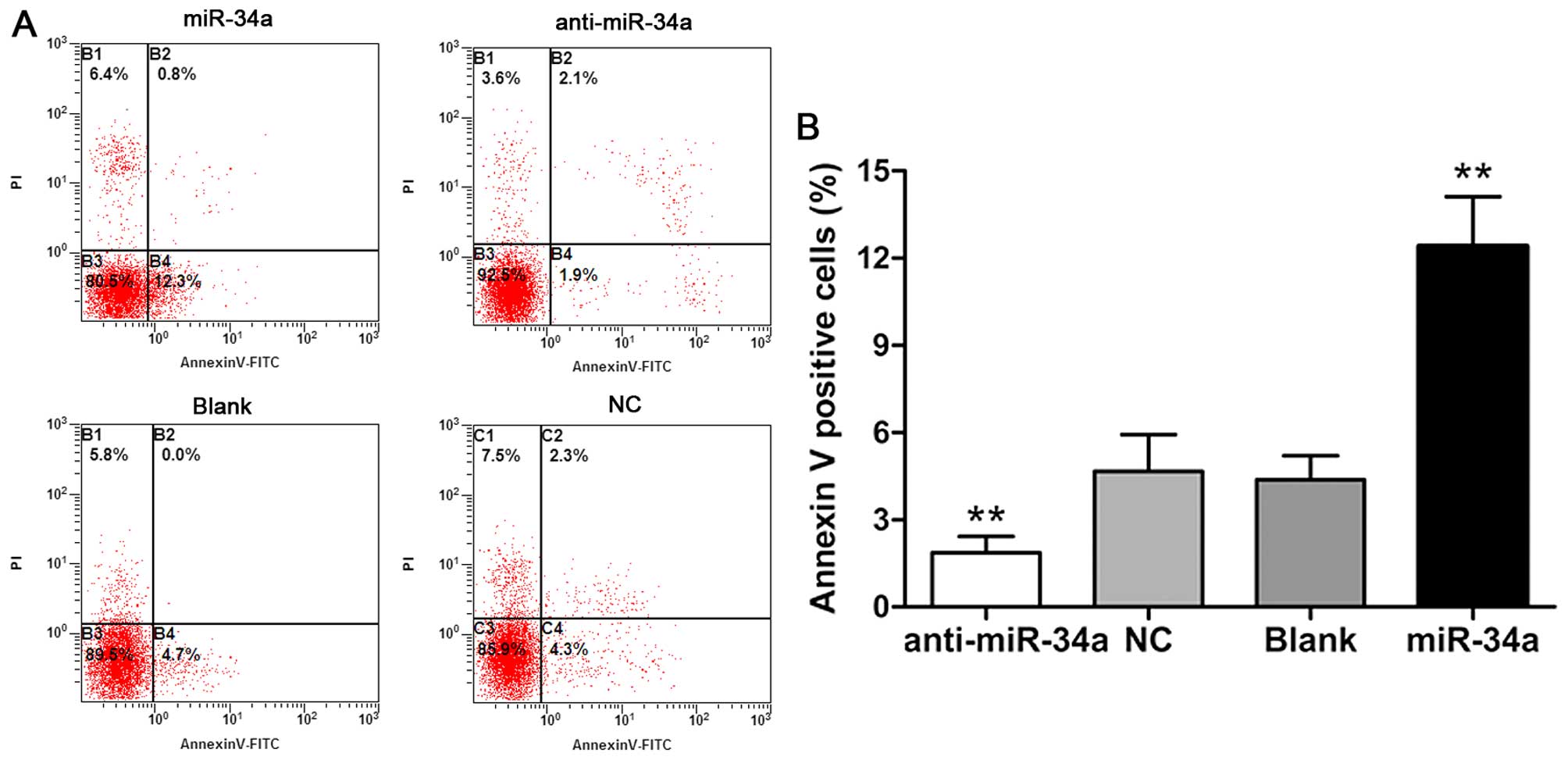
We performed Annexin V/PI staining-based flow
cytometry analysis to evaluate the effects of miR-34a on
chondrocyte apoptosis. Flow cytometry revealed that the chondro
cyte apoptotic rate was increased significantly at 48 h after
transfection with the miR-34a mimic (p<0.01). Cells in the
anti-miR-34a group showed a lower proportion of Annexin V-positive
cells (p<0.01). Cells transfected with non-specific
oligonucleotides and blank cells had similar apoptotic rates
(Fig. 4). Consequently, the
results suggested that miR-34a regulates apoptosis of human
chondrocytes in vitro.
miR-34a directly targets the SIRT1/p53
signaling pathway in human chondrocytes
miR-34a was found to directly repress the expression
of SIRT1 in HCT116 cells (20).
Our results in Fig. 1 show that
cartilage from OA patients had a higher level of miR-34a and a
lower level of SIRT1. Therefore, we further elucidated the
molecular mechanism of miR-34a-mediated biological functions. The
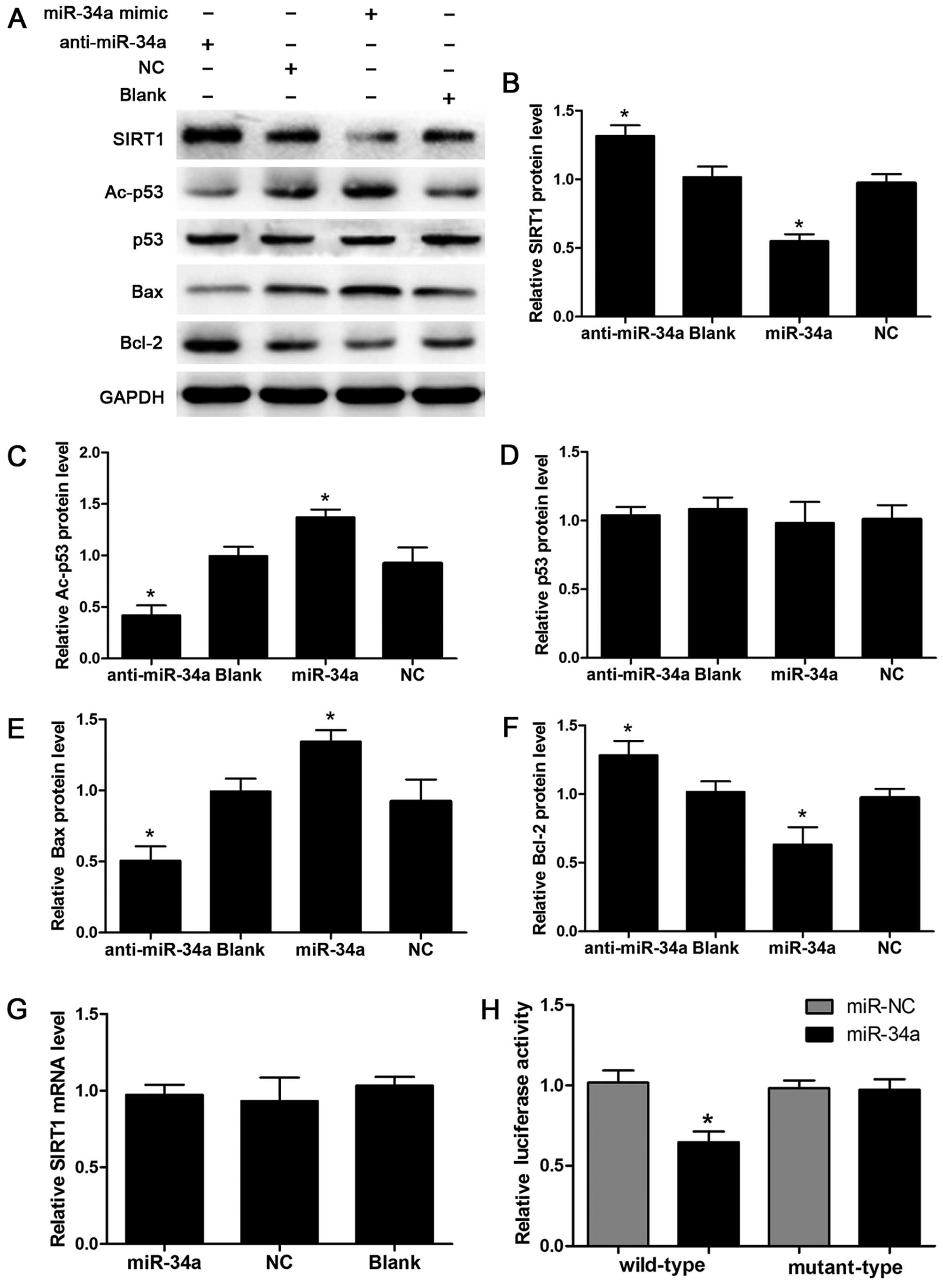
results from the western blot assay demonstrated that the SIRT1
protein level in the miR-34a group exhibited a significant decrease
as compared with the NC and Blank groups. By contrast, knockdown of
miR-34a by miR-34a inhibitor increased the protein abundance of
SIRT1 (Fig. 5A and B).
Additionally, the acetylated p53, a major target of SIRT1
deacetylation (16), was elevated
by the ectopic expression of miR-34a and decreased by knockdown of
miR-34a, while the total protein abundance of p53 was not
significantly different between the groups (Fig. 5A, C and D). The protein levels of
pro-apoptotic Bax and anti-apoptotic Bcl-2, two
SIRT1/p53 pathway downstream genes, were elevated and inhibited,
respectively, following miR-34a overexpression in human
chondrocytes. The downregulation of miR-34a significantly decreased
the Bax protein level and increased Bcl-2 (Fig. 5A, E and F). Furthermore, the
levels of SIRT1 mRNA were not altered in chondrocytes transfected
with miR-34a mimic or inhibitor (Fig.
5G).
To determine the underlying molecular mechanisms of
miR-34a-mediated regulation of SIRT1 expression in human
chondrocytes, we developed luciferase reporter vectors containing
the wild-type 3′-UTR or mutant-type 3′-UTR of miR-34a binding sites
of SIRT1 and detected the effects of miR-34a on the luciferase
activity in human chondrocytes. Luciferase analysis revealed that
the transfection of miR-34a mimic significantly suppressed the
luciferase activity of the wild-type reporter vector while mutation
of the miR-34a binding sites blocked this suppressive effect
(p<0.05) (Fig. 5H). These data
suggest that miR-34a affects SIRT1 expression by
post-transcriptional regulation and miR-34a directly targets the
SIRT1/p53 signaling pathway in human chondrocytes.
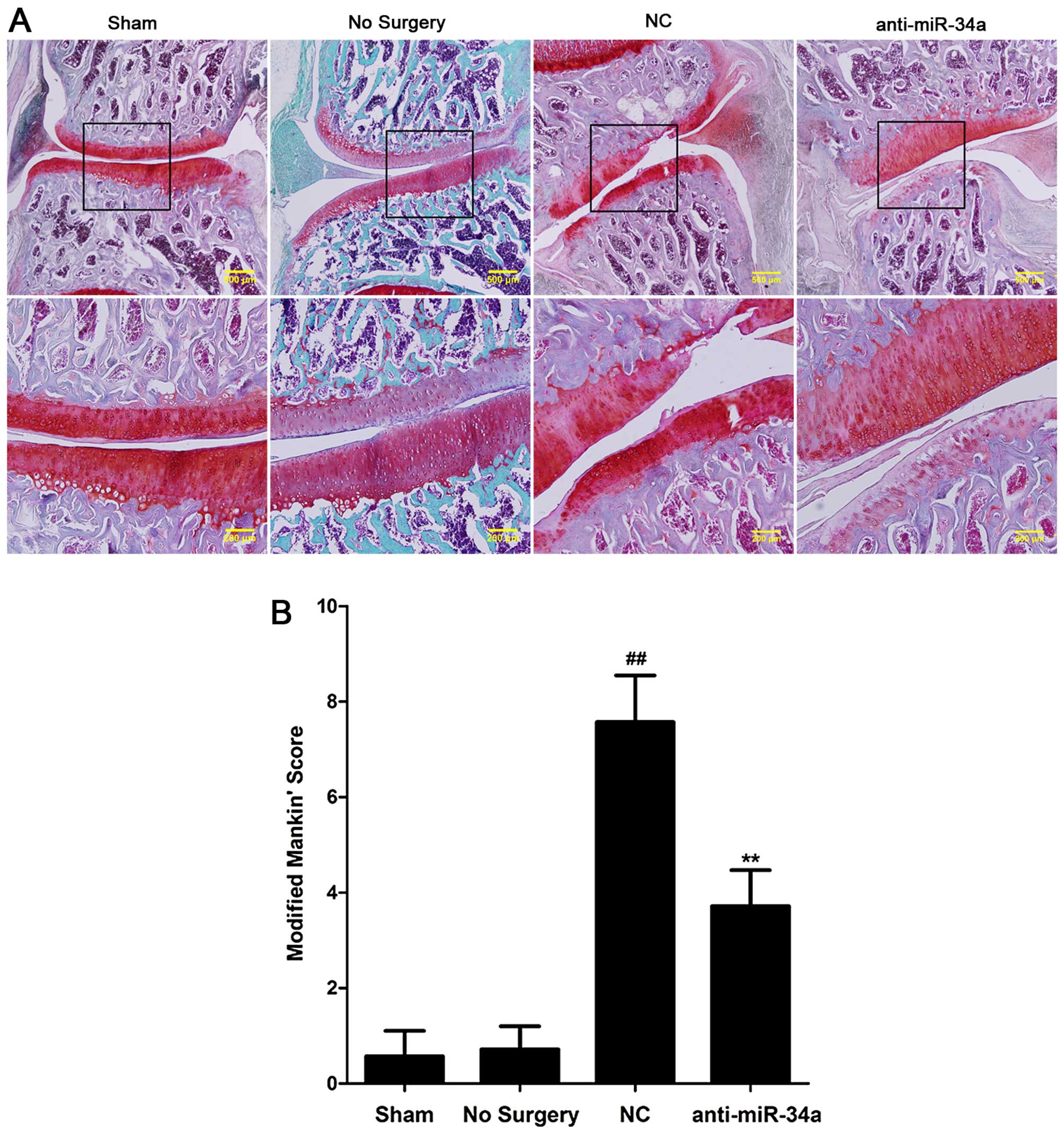
Suppression of miR-34a ameliorates the
surgery-induced progression of OA in rats
Given the findings in vitro, animal studies
were conducted to evaluate the effects of miR-34a on experimental
OA. In vivo, lentiviruses encoding miR-34a antisense
inhibitor or NC were injected into the knee joints of rats.
Cartilage was harvested for histological evaluation. The results
revealed cartilage destruction and decreased Safranin O staining in
the NC group. Intra-articular injection of miR-34a
inhibitor-expressing lentiviruses significantly attenuated
surgery-induced cartilage destruction in the anti-miR-34a group
(Fig. 6A). Fig. 6B shows the modified Mankin's score
in the miR-34a inhibitor-treated joints was significantly lower
than that for NC-treated joints (p<0.01).
Discussion
OA is a degenerative joint disorder with
multifactorial etiology caused by risk factors such as ageing,
obesity and trauma (23). Over
the past few years, microRNAs have received attention for their
essential roles in cartilage homeostasis. However, the ectopic
expression of microRNAs occurs during OA progression. It has been
reported that modulation of miR-145 affects the expression of Smad3
causing a change of its downstream target gene expression as well
as IL-1β-induced ECM degradation in OA chondrocytes (24). Jin et al have demonstrated
that miR-146a is involved in human chondrocyte apoptosis in
response to mechanical injury and contribute to the pathogenesis of
OA by modulating the VEGF and TGF-β signaling pathway (25). In a previous study, silencing of
miR-34a effectively reduced rat chondrocyte apoptosis caused by
IL-1β (18). However, the
molecular mechanism and the role of miR-34a in human chondrocyte as
well as OA progression remain to be investigated.
Members of the miR-34 family are direct
transcriptional targets of p53 while the ectopic expression of
miR-34 induces apoptosis, cell cycle arrest, senescence and other
biological behavior (14,26,27). In the present study, we observed
that the expression of miR-34a was significantly increased in
primary chondrocytes from OA patients compared with healthy
chondrocytes from traumatic amputees, which indicates involvement
of miR-34a in the pathogenesis of OA. To determine the function of
miR-34a in OA, we used chemically synthesized oligonucleotides to
manipulate the expression of miR-34a. The results showed that these
oligonucleotides were efficiently transfected into human
chondrocyte in vitro and significantly increased or
decreased miR-34a expression levels, facilitating the study of
miR-34a function.
Mounting evidence shows that chondrocyte apoptosis
plays a crucial role in the mechanisms of degeneration and
degradation of articular cartilage in OA (6,7).
Thus, the mechanism of apoptosis offers potentially useful
therapeutic targets for the management of this chronic disease
(28). In the present study,
results from the flow cytometric analysis and MTT assay revealed
that transfection of a synthetic miR-34a mimic in vitro
significantly promoted apoptosis and inhibited the proliferation in
human chondrocytes, whereas the downregulation of miR-34a led to
significant suppression of apoptosis and enhanced cell
viability.
SIRT1, a member of sirtuin family, functions as a
histone deacetylase and has been linked with age-associated
diseases such as diabetes type II, Alzheimer's and osteoporosis
(29,30). Increasing evidence suggests that
SIRT1 has a key role in OA. Inhibition of SIRT1 promoted the
development of OA by suppressing aggrecan expression and increasing
the levels of COL10A1 and ADAMTS-5 in human chondrocytes (31). Inactivation of SIRT1 promotes
apoptosis through mitochondria-related signals in chondrocytes
(32). Enhanced acetylation of
the well-known tumor suppressor gene p53 in response to
types of cell stress is crucial for p53-mediated apoptosis, cell
growth arrest and transcriptional activities (33,34). Thus, SIRT1 may regulate p53
function by deacetylating p53. Chung et al reported that the
overexpression of SIRT1 significantly protects fibroblasts from
UVB-induced cellular senescence by suppressing UVB-induced p53
acetylation and its transcriptional activity (35).
Notably, it has been reported that repression of
SIRT1 by miR-34a regulates apoptosis in colon cancer cells
(20). Thus, miR-34a induces
apoptosis in human chondrocyte by modulating SIRT1/p53 signaling
during the pathogenesis of OA. In the present study, we observed a
significantly decreased expression of SIRT1 in OA chondrocytes,
consistent with previous studies (32), showing the involvement of SIRT1 in
OA. We also found an interaction between miR-34a and SIRT1/p53
signaling. Western blot analysis revealed that the SIRT1 protein
level was considerably decreased in miR-34a-mimic-transfected
cells, leading to an increase of acetylated p53, Bax and decrease
of Bcl-2. Additionally, results from the luciferase reporter assay
demonstrated that miR-34a suppressed the luciferase activity of the
wild-type SIRT1 3′-UTR vector, while mutation of the miR-34a
binding site attenuated this suppressive effect, indicating that
miR-34a inhibits SIRT1 by directly binding to the 3′-UTR of SIRT1
mRNA.
A classical ACLT+MMx rat model is considered an
appropriate platform to verify the biological effects of miR-34a in
OA in vivo, which is feasible and reproducible.
Lentivirus-mediated gene delivery has several advantages, including
efficient transduction into a wide variety of dividing or
non-dividing cells and stable expression of transgenes (36), and has been applied to studies on
arthritis in vivo (37,38). In the current study, histological
findings showed that articular cartilage damage and severity of
disease could be attenuated by the intra-articular injection of
lentivirus encoding miR-34a antisense inhibitor. To the best of our
knowledge, the present study provides the first evidence that the
downregulation of miR-34a mediated by intra-articular injection of
lentivirus ameliorates OA progression in rat induced by surgery.
Progressive degeneration of articular cartilage has been considered
the main event underlying the pathogenesis of OA, leading to loss
of physiological function (5,39).
However, current drug therapy of OA remains unsatisfactory and may
not reverse the destruction of the articular cartilage while
end-stage patients have to resort to joint replacement surgery
(40,41). Thus, the administration of
therapeutic agents that potentially prevent degradation, inhibit
inflammation or promote cartilage self-repairing is a promising
therapeutic approach.
Taken together, we suggest that miR-34a promotes
apoptosis and inhibits proliferation by directly regulating the
SIRT1/p53 signaling pathway in primary human chon-drocytes.
Silencing miR-34a by intra-articular injection of lentivirus may
attenuate disease progression in a rat model of OA. Additional
studies examining the clinical potential of miR-34a and its target
SIRT1 in OA treatment are required. Detailed investigation into the
network of mediators involved in miR-34a regulation may accelerate
the development of a novel therapeutic approach in OA.
Acknowledgments
The present study was funded by grants from the
National Natural Science Foundation of China (nos. 81072194 and
81201633). The funders had no role in the study design.
References
|
1
|
Lawrence RC, Helmick CG, Arnett FC, Deyo
RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder
GG, et al: Estimates of the prevalence of arthritis and selected
muscu-loskeletal disorders in the United States. Arthritis Rheum.
41:778–799. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gore M, Tai KS, Sadosky A, Leslie D and
Stacey BR: Clinical comorbidities, treatment patterns, and direct
medical costs of patients with osteoarthritis in usual care: A
retrospective claims database analysis. J Med Econ. 14:497–507.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duncan R, Peat G, Thomas E, Hay E, McCall
I and Croft P: Symptoms and radiographic osteoarthritis: not as
discordant as they are made out to be? Ann Rheum Dis. 66:86–91.
2007. View Article : Google Scholar
|
|
4
|
Findlay DM and Atkins GJ:
Osteoblast-chondrocyte interactions in osteoarthritis. Curr
Osteoporos Rep. 12:127–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldring MB: The role of the chondrocyte
in osteoarthritis. Arthritis Rheum. 43:1916–1926. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blanco FJ, Guitian R, Vázquez-Martul E, de
Toro FJ and Galdo F: Osteoarthritis chondrocytes die by apoptosis.
A possible pathway for osteoarthritis pathology. Arthritis Rheum.
41:284–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hashimoto S, Ochs RL, Komiya S and Lotz M:
Linkage of chondrocyte apoptosis and cartilage degradation in human
osteoarthritis. Arthritis Rheum. 41:1632–1638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farh KK, Grimson A, Jan C, Lewis BP,
Johnston WK, Lim LP, Burge CB and Bartel DP: The widespread impact
of mammalian MicroRNAs on mRNA repression and evolution. Science.
310:1817–1821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyaki S and Asahara H: Macro view of
microRNA function in osteoarthritis. Nat Rev Rheumatol. 8:543–552.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldring MB and Marcu KB: Epigenomic and
micro-RNA-mediated regulation in cartilage development,
homeostasis, and osteoarthritis. Trends Mol Med. 18:109–118. 2012.
View Article : Google Scholar
|
|
13
|
Tazawa H, Tsuchiya N, Izumiya M and
Nakagama H: Tumor-suppressive miR-34a induces senescence-like
growth arrest through modulation of the E2F pathway in human colon
cancer cells. Proc Natl Acad Sci USA. 104:15472–15477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang TC, Wentzel EA, Kent OA,
Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M,
Ferlito M, Lowenstein CJ, et al: Transactivation of miR-34a by p53
broadly influences gene expression and promotes apoptosis. Mol
Cell. 26:745–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo J, Nikolaev AY, Imai S, Chen D, Su F,
Shiloh A, Guarente L and Gu W: Negative control of p53 by Sir2alpha
promotes cell survival under stress. Cell. 107:137–148. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaziri H, Dessain SK, Ng Eaton E, Imai SI,
Frye RA, Pandita TK, Guarente L and Weinberg RA: hSIR2(SIRT1)
functions as an NAD-dependent p53 deacetylase. Cell. 107:149–159.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kobayashi Y, Furukawa-Hibi Y, Chen C,
Horio Y, Isobe K, Ikeda K and Motoyama N: SIRT1 is critical
regulator of FOXO-mediated transcription in response to oxidative
stress. Int J Mol Med. 16:237–243. 2005.PubMed/NCBI
|
|
18
|
Abouheif MM, Nakasa T, Shibuya H, Niimoto
T, Kongcharoensombat W and Ochi M: Silencing microRNA-34a inhibits
chondrocyte apoptosis in a rat osteoarthritis model in vitro.
Rheumatology (Oxford). 49:2054–2060. 2010. View Article : Google Scholar
|
|
19
|
Matsushita T, Sasaki H, Takayama K, Ishida
K, Matsumoto T, Kubo S, Matsuzaki T, Nishida K, Kurosaka M and
Kuroda R: The overexpression of SIRT1 inhibited osteoarthritic gene
expression changes induced by interleukin-1β in human chondrocytes.
J Orthop Res. 31:531–537. 2013. View Article : Google Scholar
|
|
20
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayami T, Pickarski M, Zhuo Y, Wesolowski
GA, Rodan GA and Duong LT: Characterization of articular cartilage
and subchondral bone changes in the rat anterior cruciate ligament
transection and meniscectomized models of osteoarthritis. Bone.
38:234–243. 2006. View Article : Google Scholar
|
|
22
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II. Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971.PubMed/NCBI
|
|
23
|
Pottie P, Presle N, Terlain B, Netter P,
Mainard D and Berenbaum F: Obesity and osteoarthritis: More complex
than predicted! Ann Rheum Dis. 65:1403–1405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang B, Kang X, Xing Y, Dou C, Kang F, Li
J, Quan Y and Dong S: Effect of microRNA-145 on IL-1β-induced
cartilage degradation in human chondrocytes. FEBS Lett.
588:2344–2352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin L, Zhao J, Jing W, Yan S, Wang X, Xiao
C and Ma B: Role of miR-146a in human chondrocyte apoptosis in
response to mechanical pressure injury in vitro. Int J Mol Med.
34:451–463. 2014.PubMed/NCBI
|
|
26
|
He L, He X, Lim LP, de Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raver-Shapira N, Marciano E, Meiri E,
Spector Y, Rosenfeld N, Moskovits N, Bentwich Z and Oren M:
Transcriptional activation of miR-34a contributes to p53-mediated
apoptosis. Mol Cell. 26:731–743. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnson EO, Charchandi A, Babis GC and
Soucacos PN: Apoptosis in osteoarthritis: morphology, mechanisms,
and potential means for therapeutic intervention. J Surg Orthop
Adv. 17:147–152. 2008.PubMed/NCBI
|
|
29
|
Michan S and Sinclair D: Sirtuins in
mammals: insights into their biological function. Biochem J.
404:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng L, Chen R, Liang F, Tsuchiya H, Murai
H, Nakahashi T, Iwai K, Takahashi T, Kanda T and Morimoto S: Silent
information regulator, Sirtuin 1, and age-related diseases. Geriatr
Gerontol Int. 9:7–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujita N, Matsushita T, Ishida K, Kubo S,
Matsumoto T, Takayama K, Kurosaka M and Kuroda R: Potential
involvement of SIRT1 in the pathogenesis of osteoarthritis through
the modulation of chondrocyte gene expressions. J Orthop Res.
29:511–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takayama K, Ishida K, Matsushita T, Fujita
N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T, Fujioka H, et
al: SIRT1 regulation of apoptosis of human chondrocytes. Arthritis
Rheum. 60:2731–2740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Knights CD, Catania J, Di Giovanni S,
Muratoglu S, Perez R, Swartzbeck A, Quong AA, Zhang X, Beerman T,
Pestell RG, et al: Distinct p53 acetylation cassettes
differentially influence gene-expression patterns and cell fate. J
Cell Biol. 173:533–544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sykes SM, Mellert HS, Holbert MA, Li K,
Marmorstein R, Lane WS and McMahon SB: Acetylation of the p53
DNA-binding domain regulates apoptosis induction. Mol Cell.
24:841–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chung KW, Choi YJ, Park MH, Jang EJ, Kim
DH, Park BH, Yu BP and Chung HY: Molecular insights into SIRT1
protection against UVB-induced skin fibroblast senescence by
suppression of oxidative stress and p53 acetylation. J Gerontol A
Biol Sci Med Sci. 70:959–968. 2015. View Article : Google Scholar
|
|
36
|
Singer O and Verma IM: Applications of
lentiviral vectors for shRNA delivery and transgenesis. Curr Gene
Ther. 8:483–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen SY, Shiau AL, Li YT, Lin YS, Lee CH,
Wu CL and Wang CR: Suppression of collagen-induced arthritis by
intra-articular lentiviral vector-mediated delivery of Toll-like
receptor 7 short hairpin RNA gene. Gene Ther. 19:752–760. 2012.
View Article : Google Scholar
|
|
38
|
Shen PC, Lu CS, Shiau AL, Lee CH, Jou IM
and Hsieh JL: Lentiviral small hairpin RNA knockdown of macrophage
inflammatory protein-1γ ameliorates experimentally induced
osteoarthritis in mice. Hum Gene Ther. 24:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aigner T, Haag J, Martin J and Buckwalter
J: Osteoarthritis: aging of matrix and cells - going for a remedy.
Curr Drug Targets. 8:325–331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vangsness CT Jr, Spiker W and Erickson J:
A review of evidence-based medicine for glucosamine and chondroitin
sulfate use in knee osteoarthritis. Arthroscopy. 25:86–94. 2009.
View Article : Google Scholar
|
|
41
|
Liu XW, Zi Y, Xiang LB and Wang Y: Total
hip arthroplasty: a review of advances, advantages and limitations.
Int J Clin Exp Med. 8:27–36. 2015.
|